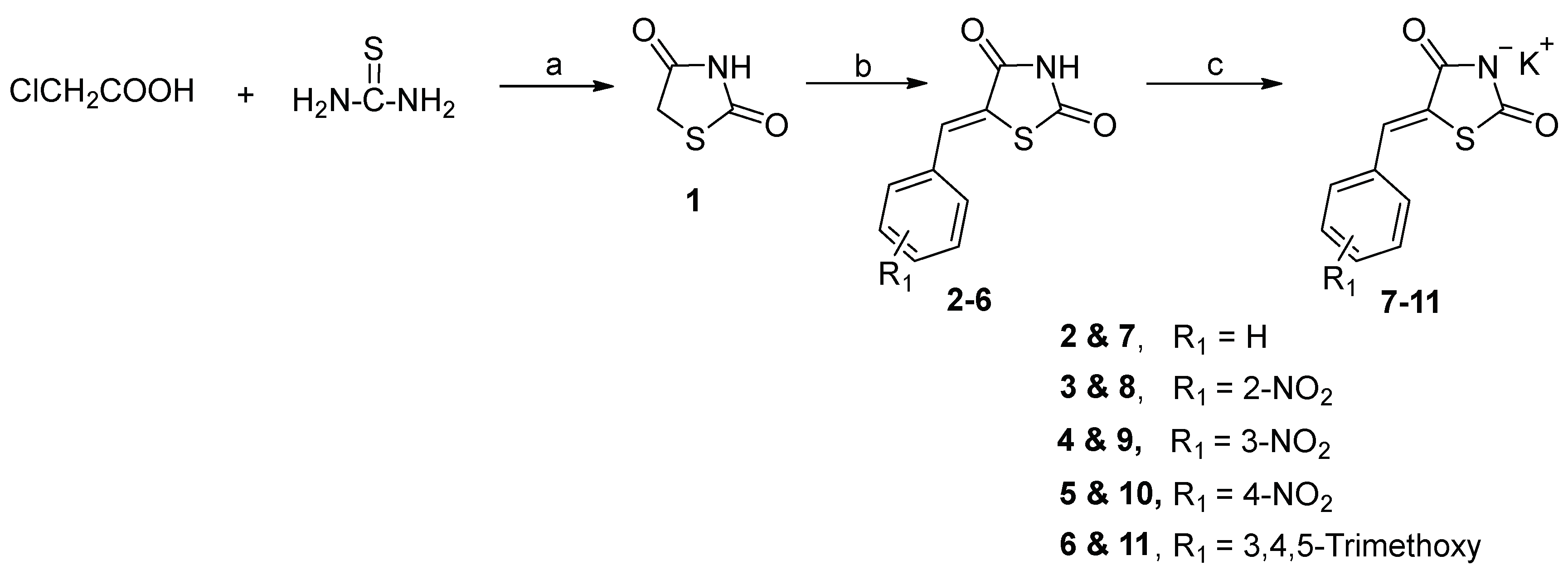
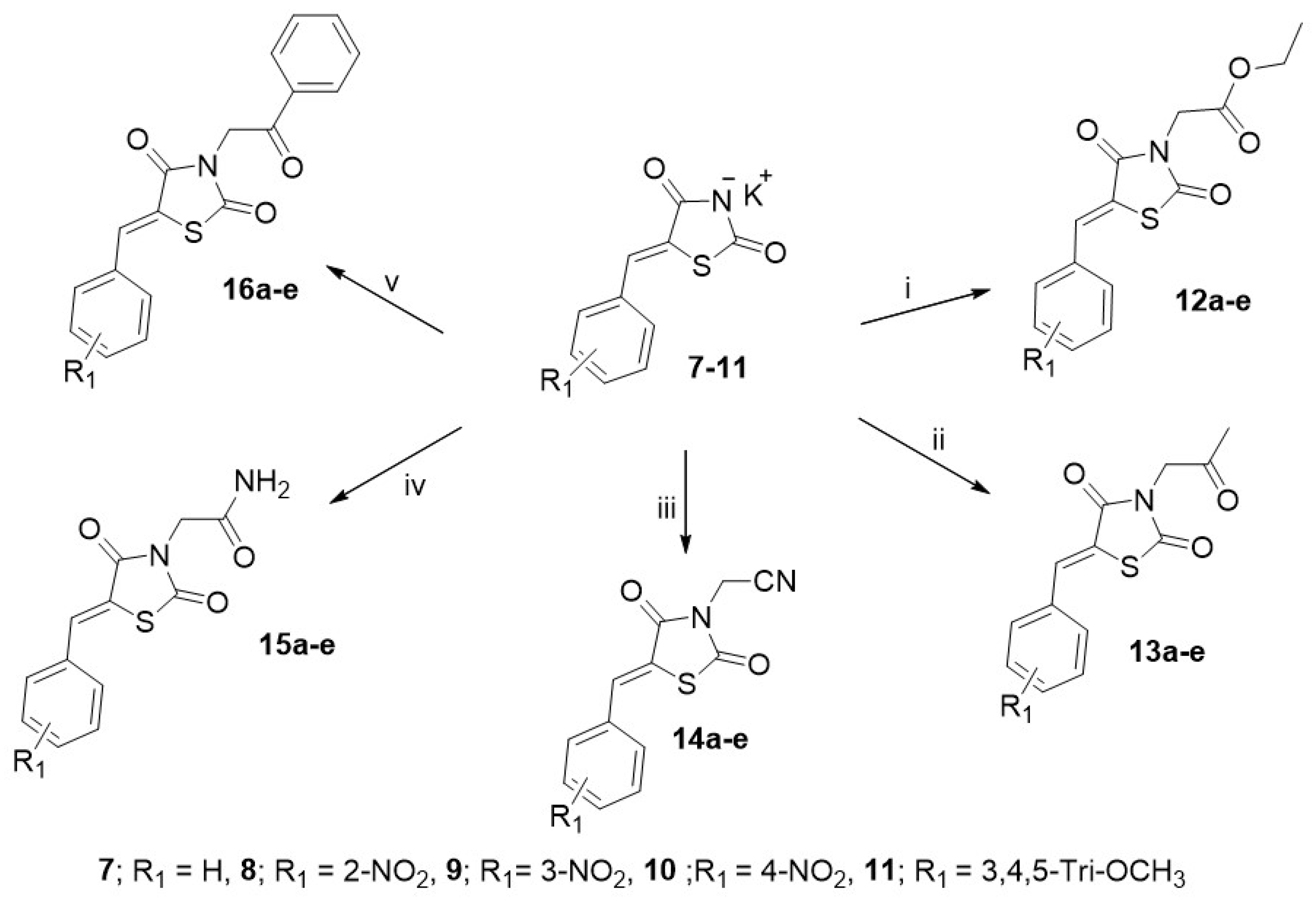
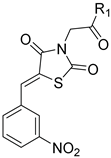
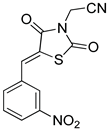
General Procedure for the Synthesis of 5-Arylidenethiazolidinediones 12b-e–16b-e
An equimolar mixture (1 mmol) of ethyl chloroacetate (0.123 g, 0.107 mL), and the potassium salts; 7 (0.234 g), 8 (0.288 g), 9 (0.288 g), 10 (0.288 g) or 11 (0.333 g) in DMF (5 mL) was heated under reflux for 6 h. After cooling, the solid products obtained were filtered, washed with water, dried, and recrystallized.
- (1)
Synthesis of compounds 12b-e
(1) Ethyl ((Z)-5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetate (12b). Brownish yellow crystals from ethanol, yield (79%), mp 81–83 °C. IR (ν cm−1): 3068 (C-H arom.), 3009 (C-H arom.), 2983 (C-H aliph.), 2937 (C-H aliph) 1741 (C=O), 1694 (C=O), 1607, 1568. 1568. 1524. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.25–8.23 (m, 2H, (-CH= + Ar-H), 7.92 (t, J = 7.5 Hz, 1H, Ar-H), 7.74–7.78 (m, 2H, Ar-H), 4.52 (s, 2H, CH2), 4.20 (q, J = 7.2 Hz, 2H, CH3CH2), 1.23 (t, J = 7.2 Hz, 3H, CH3CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.12 (C=O), 167.07 (C=O), 164.66 (C=O), 148.25 (C), 135.17 (-CH=), 131.94 (CH), 131.85 (CH), 129.81 (CH), 129.20 (C), 126.02 (CH), 125.42 (CH), 62.18 (OCH2), 42.76 (COCH2), 14.40 (CH3CH2). Anal. Calcd. for C14H12N2O6S (336.32): C, 50.00; H, 3.60; N, 8.33; S, 9.53. Found: C, 49.71; H, 3.61; N, 8.28; S, 9.55%.
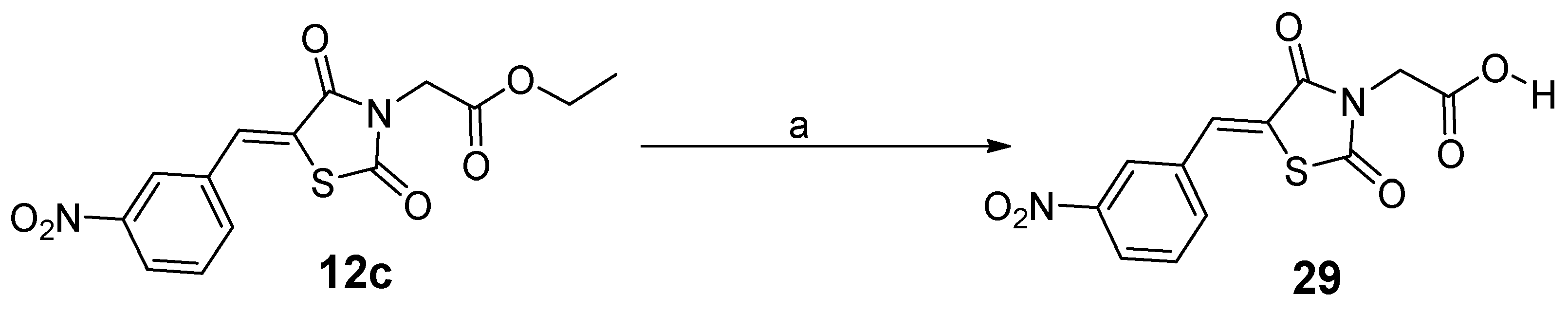
(2) Ethyl ((Z)-5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetate (12c). Yellow crystals from ethanol, yield (86%), mp 130–132 °C. IR (ν cm−1): 3067 (C-H arom.), 3000 (C-H arom.), 2963 (C-H aliph.), 2939 (C-H aliph.), 1734 (C=O), 1704 (C=O), 1689 (C=O), 1606, 1571, 1533. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.34 (ddd, J = 8.3, 2.2, 0.8 Hz, 1H, Ar-H), 8.19 (s, 1H, -CH=), 8.08 (d, J = 7.9 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 4.53 (s, 2H, -CH2-), 4.19 (q, J = 7.2 Hz, 2H, CH2CH3), 1.23 (t, J = 7.2 Hz, 3H, CH2CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.06 (C=O), 166.79 (C=O), 165.10 (C=O), 148.76 (C), 135.93 (-CH=), 134.88 (C), 132.37 (CH), 131.47 (CH), 125.41 (CH), 125.34 (CH), 123.97 (C), 62.19 (OCH2), 42.84 (COCH2), 14.42 (CH3CH2). Anal. Calcd. for C14H12N2O6S (336.32): C, 50.00; H, 3.60; N, 8.33; S, 9.53. Found: C, 49.63; H, 3.51; N, 8.17; S, 9.57%.
(3) Ethyl ((Z)-5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetate (12d). Yellow platelets from ethanol, yield (88%), mp 136–138 °C. IR (ν cm−1): 3111 (C-H arom.), 2995 (C-H aliph.), 1738 (C=O), 1698 (C=O), 1607, 1595, 1512. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.35 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.91 (d, J = 8.8 Hz, 2H, Ar-H), 4.53 (s, 2H, -CH2-), 4.19 (q, J = 7.2 Hz, 2H, CH2CH3), 1.22 (t, J = 7.2 Hz, 3H, CH2CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.03 (C=O), 166.93 (C=O), 165.07 (C=O), 148.36 (C), 139.38 (-CH=), 132.03 (C), 131.65 (2CH), 125.25 (C), 124.77 (2CH), 62.20 (O-CH2), 42.84 (CO-CH2), 14.40 (CH3). Anal. Calcd. for C14H12N2O6S (336.32): C, 50.00; H, 3.60; N, 8.33; S, 9.53. Found: C, 49.88; H, 3.51; N, 8.21; S, 9.40%.
- (2)
Synthesis of compounds 13a-e
These compounds were prepared using 1 mmol of chloroacetone (0.093 g, 0.08 mL) following the above procedure described for 12a-e.
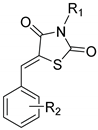
(1) (Z)-5-Benzylidene-3-(2-oxopropyl)thiazolidine-2,4-dione (13a). Pale-yellow crystals from ethanol, yield (80%), mp 139–140 °C. IR (ν cm−1): 3050 (CH arom.), 3017 (CH arom.), 2975 (CH aliph.), 2933 (CH aliph.), 1743 (C=O), 1727 (C=O), 1682 (C=O), 1608, 1597, 1570, 1573. 1H-NMR (400 MHz, DMSO) δ (ppm): 7.96 (s, 1H, -CH=), 7.65–7.63 (m, 2H, Ph-H), 7.57–7.49 (m, 3H, Ph-H), 4.68 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.77 (C=O), 167.30 (C=O), 165.67 (C=O), 133.95 (C=O), 133.27 (-CH=), 131.27 (C), 130.67 (2CH), 129.85 (2CH), 121.30 (C), 50.88 (CH2), 27.34 (CH3). Anal. Calcd. for C13H11NO3S (261.30): C, 59.76; H, 4.24; N, 5.36; S, 12.27. Found: C, 59.56; H, 4.20; N, 5.29; S, 12.21%.
(2) (Z)-5-(2-Nitrobenzylidene)-3-(2-oxopropyl)thiazolidine-2,4-dione (13b). Shiny-buff crystals from ethanol, yield (79%), mp 146–148 °C. IR (ν cm−1): 3102 (CH arom.), 3073 (CH arom.), 2984 (CH aliph.), 2945 (CH aliph.), 2861 (C-H aliph.), 1749 (C=O), 1732 (C=O), 1694 (C=O), 1615, 1603, 1570 and 1528. 1H NMR (400 MHz, DMSO) δ (ppm): 8.26–8.23 (m, 1H, Ar-H), 8.21 (s, 1H, -CH=), 7.94–7.90 (m, 1H, Ar-H), 7.76 (m, 2H, Ar-H), 4.70 (s, 2H, CH2), 2.27 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.76 (C=O), 167.10 (C=O), 164.80 (C=O), 148.26 (C), 135.16 (-CH=), 131.79 (CH), 131.47 (CH), 129.82 (CH), 129.28 (C), 126.02 (CH), 125.73 (C), 50.85 (CH2), 27.38 (CH3). Anal. Calcd. for C13H10N2O5S (306.29): C, 50.98; H, 3.29; N, 9.15; S, 10.47. Found: C, 50.81; H, 3.23; N, 9.10; S, 10.40%.
(3) (Z)-5-(3-Nitrobenzylidene)-3-(2-oxopropyl)thiazolidine-2,4-dione (13c). Small lustrous brown crystals from ethanol, yield (88%), mp 188–190 °C. IR (ν cm−1): 3073 (CH arom.), 2992 (CH aliph.), 1756 (C=O), 1729 (C=O), 1686 (C=O), 1604, 1570, 1528. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.52 (t, J = 1.9 Hz, 1H, Ar-H), 8.32 (ddd, J = 8.2, 2.2, 0.8 Hz,1H, Ar-H), 8.15 (s, 1H, -CH=), 8.07 (d, J = 8.0 Hz, 1H, Ar-H), 7.85 (t, J = 8.0 Hz, 1H, Ar-H), 4.71 (s, 2H, -CH2-), 2.27 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.73 (C=O), 166.76 (C=O), 165.23 (C=O), 148.76 (C), 135.90 (-CH=), 134.98 (C), 131.92 (CH), 131.45 (CH), 125.28 (CH), 124.29 (C), 50.93 (CH2), 27.52 (CH3). Anal. Calcd. for C13H10N2O5S (306.29): C, 50.98; H, 3.29; N, 9.15; S, 10.47. Found: C, 50.79; H, 3.23; N, 9.11; S, 10.39%.
(4) (Z)-5-(4-Nitrobenzylidene)-3-(2-oxopropyl)thiazolidine-2,4-dione (13d). Yellow crystals from ethanol-dioxane, yield (95%), mp 207–209 °C. IR (ν cm−1): 3014 (CH arom.), 2975 (CH aliph.), 2933 (CH aliph.), 1731 (C=O), 1682 br (C=O), 1608, 1514 and 1407. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.08 (s, 1H, -CH=), 7.91 (d, J = 8.8 Hz, 2H, Ar-H), 4.71 (s, 2H, CH2), 2.27 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.70 (C=O), 166.82 (C=O), 165.22 (C=O), 148.19 (C), 139.52 (-CH=), 131.60 (C + 2CH), 125.60 (C), 124.78 (2CH), 50.94 (CH2), 27.51 (CH3). Anal. Calcd. for C13H10N2O5S (306.29): C, 50.98; H, 3.29; N, 9.15; S, 10.47. Found: C, 50.91; H, 3.21; N, 9.09; S, 10.43%.
- (3)
Synthesis of compounds 14a-e
These compounds were prepared using 1 mmol of 2-chloroacetonitrile (0.076 g, 0.064 mL), following the above procedure described for 12a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)acetonitrile (14a). Brownish-yellow crystals from ethanol, yield (80%), mp 230–232 °C. IR (ν cm−1): 3000 (CH arom.), 2958 (CH arom.), 2252 (CN), 1777 (C=O), 1747 (C=O), 1697 (C=O), 1608, 1571, 1493. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.00 (s, 1H, -CH=), 7.65–7.63 (m, 2H, Ph-H), 7.58–7.50 (m, 3H, Ph-H), 4.81 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.87 (C=O), 164.70 (C=O), 134.66 (-CH=), 133.17 (C), 131.44 (CH), 130.74 (2CH), 129.91 (2CH), 120.95 (CH), 115.02 (C), 29.26 (CH2). Anal. Calcd. for C12H8N2O2S (244.27): C, 59.01; H, 3.30; N, 11.47; S, 13.12. Found: C, 59.16; H, 3.27; N, 11.41; S, 13.09%.
(2) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetonitrile (14b). Yellow crystals from ethanol, yield (85%), mp 119–120 °C. IR (ν cm−1): 3003 (CH arom.), 2963 (CH arom.), 1746 (C=O), 1694 br (C=O), 1619 (C=O), 1619, 1604, 1571. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.26–824 (m, 2H, Ar-H + -CH=), 7.93 (td, J = 7.6, 0.9 Hz, 1H, Ar-H), 7.79–7.72 (m, 2H, Ar-H), 4.82 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.73 (C=O), 164.03 (C=O), 148.24 (C), 135.24 (-CH=), 131.94 (CH), 131.90 (CH), 129.75 (CH), 129.22 (C), 126.06 (CH), 125.51 (C), 114.94 (CN), 29.32 (CH2). Anal. Calcd. for C12H7N3O4S (289.27): C, 49.83; H, 2.44; N, 14.53; S, 11.08. Found: C, 49.70; H, 2.36; N, 14.42; S, 11.12%
(3) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetonitrile (14c). Beige crystals from ethanol-dioxane, yield (95%), mp 173–175 °C. IR (ν cm−1): 3084 (CH arom.), 2998 (CH aliph.), 2957 (CH aliph.), 1751 (C=O), 1688b (C=O), 1608, 1570, 1541. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.52 (t, J = 2.0 Hz, 1H, Ar-H), 8.34 (dt, J = 10.9, 4.3 Hz, 1H, Ar-H), 8.20 (s, 1H, -CH=), 8.07 (d, J = 7.7 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 4.83 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.38 (C=O), 164.45 (C=O), 148.80 (C), 135.88 (-CH=), 134.87 (C), 132.23 (CH), 131.52 (CH), 125.43 (CH), 125.29 (CH), 124.10 (C), 114.93 (CN), 29.40 (CH2). Anal. Calcd. for C12H7N3O4S (289.27): C, 49.83; H, 2.44; N, 14.53; S, 11.08. Found: C, 49.80; H, 2.35; N, 14.27; S, 11.12%.
(4) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetonitrile (14d). Fine brownish-yellow crystals from ethanol-dioxane, yield (87%), mp 193–195 °C. IR (ν cm−1): 3079 (C-H arom.), 3029 (C-H arom.), 2997 (C-H aliph.), 2956 (C-H aliph.), 1753 (C=O), 1698 br (C=O), 1608, 1596 and 1530. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.35 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.89 (d, J = 8.8 Hz, 2H, Ar-H), 4.83 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.43 (C=O), 164.43 (C=O), 148.22 (C), 139.37 (C), 131.88 (-CH=), 131.61 (2CH), 125.38 (C), 124.80 (2CH), 114.87 (CH), 29.40 (CH2). Anal. Calcd. for C12H7N3O4S (289.27): C, 49.83; H, 2.44; N, 14.53; S, 11.08. Found: C, 49.70; H, 2.39; N, 14.50; S, 11.20%.
- (4)
Synthesis of compounds 15a-e
These compounds were prepared using 2-chloroacetamide (1 mmol, 0.093 g), following the procedure described for 12a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)acetamide (15a). Yellow crystals from ethanol, yield (76%), mp 229–231 °C. IR (ν cm−1): 3365, 3180 (NH2), 3056 (C-H arom.), 2942 (C-H aliph.), 1739 (C=O), 1693 (C=O), 1667 (C=O), 1607, 1573, 1492, 1448.1H-NMR (400 MHz, DMSO) δ (ppm): 7.97 (s, 1H, -CH=), 7.75 (s, 1H, NH), 7.66–7.65 (m, 2H, Ph-H), 7.58–7.51 (m, 3H, Ph-H), 7.34 (s, 1H, NH), 4.25 (s, 2H, CH2) 13C-NMR (100 MHz, DMSO) δ (ppm): 167.58 (C=O), 167.30 (C=O), 165.79 (C=O), 133.72 (-CH=), 133.38 (C), 131.20 (C), 130.62 (2CH), 129.88 (2CH), 121.70 (C), 43.80 (CH2). Anal. calcd. For C12H10N2O3S (262.28): C, 54.95; H, 3.84; N, 10.68; S, 12.22. Found: C, 54.87; H, 3.78; N, 10.60; S, 12.19%
(2) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (15b). Yellow crystals from ethanol, yield (89%), mp 233–235 °C. IR (ν cm−1): 3441 (N-H), 3315 (N-H), 3181 (N-H), 1747 (C=O), 1678b (C=O), 1607, 1525 and 1474. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.25–8.23 (m, 1H, Ar-H), 8.19 (s, 1H, -CH=), 7.92 (t, J = 7.5 Hz, 1H, Ar-H), 7.78–7.75 (m, 3H, Ar-H + NH), 7.36 (s, 1H, NH), 4.26 (s, 2H, CH2).13C-NMR (100 MHz, DMSO) δ (ppm): 167.35 (C=O), 167.22 (C=O), 165.05 (C=O), 148.30 (C), 135.17 (-CH=), 131.74 (CH), 130.93 (CH), 129.77 (CH), 129.32 (C), 126.08 (C), 126.05 (CH), 43.87 (CH2). Anal. calcd. for C12H9N3O5S (307.28): C, 46.91; H, 2.95; N, 13.68; S, 10.43. Found: C, 46.83; H, 2.90; N, 13.61; S, 10.32%.
(3) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (15c). Yellow crystals from ethanol-dioxane, yield (87%), mp 256–258 °C. IR (ν cm−1): 3441 (N-H), 3316 (N-H), 3190 (N-H), 3079 (C-H arom), 1747 (C=O), 1678b (C=O),1607, 1525 and 1444. 1H NMR (400 MHz, DMSO) δ (ppm): 8.52 (s, 1H, Ar-H), 8.32 (dd, J = 7.1, 1.1 Hz, 1H, Ar-H), 8.14 (s, 1H, -CH=), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.85 (t, J = 8.0 Hz, 1H, Ar-H), 7.76 (s, 1H, NH), 7.36 (s, 1H, NH), 4.26 (s, 2H, CH2).13C-NMR (100 MHz, DMSO) δ (ppm): 167.18 (C=O), 167.10 (C=O), 165.48 (C=O), 148.77 (C), 135.86 (-CH=), 135.06 (C), 131.46 (2CH), 125.23 (CH), 125.18 (CH), 124.67 (C), 43.95 (CH2). Anal. calcd. for C12H9N3O5S (307.28): C, 46.91; H, 2.95; N, 13.68; S, 10.43. Found: C, 46.85; H, 2.87; N, 13.59; S, 10.39%.
(4) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (15d). Small shiny yellow crystals from ethanol-dioxane, yield (94%), mp 281–283 °C. IR (ν cm−1): 3432, 3325, 3199 (NH2), 3026 (CH arom.), 2981 (CH aliph.), 2942 (CH aliph.), 1747 (C=O), 1683 br. (C=O), 1694 (C=O), 1607, 1509, 1442. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.07 (s, 1H, -CH=), 7.91 (d, J = 8.8 Hz, 2H, Ar-H), 7.76 (s, 1H, NH), 7.36 (s, 1H, NH), 4.26 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.17 (C=O), 167.09 (C=O), 165.47 (C=O), 148.14 (C), 139.62 (=CH-), 131.54 (C), 131.16 (C), 126.00 (C), 124.80 (2CH), 43.97 (CH2). Anal. calcd. for C12H9N3O5S (307.28): C, 46.91; H, 2.95; N, 13.68; S, 10.43. Found: C, 46.97; H, 2.88; N, 13.60; S, 10.36%.
- (5)
Synthesis of compounds 16b-e
These compounds were prepared using phenacyl bromide (1 mmol, 0.199 g), following the procedure described for 12b-e.
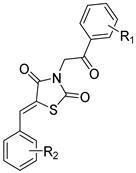
(1) (Z)-5-(2-Nitrobenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (16b). Yellow crystals from ethanol-dioxane, yield (82%), mp 156–158 °C. IR (ν cm−1): 3058 (C-H arom), 2937 (C-H arom.), 2977 (C-H aliph.), 2973 (C-H aliph.), 1746 (C=O), 1693 (C=O), 1682 (C=O), 16,204, 1595, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.27–8.25 (m, 2H, =CH- + Ar-H), 8.12–8.10 (m, 2H, Ph-H), 7.93 (t, J = 7.5 Hz, 1H, Ar-H), 7.81–7.74 (m, 3H, Ph-H), 7.62 (t, J = 7.7 Hz, 2H, Ar-H), 5.37 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 191.71 (C=O), 167.27 (C=O), 164.97 (C=O), 148.29 (C), 135.19 (-CH=), 134.99 (C), 134.21 (C), 131.82 (CH), 131.73 (C), 129.85 (CH), 129.52 (2CH), 129.29 (CH), 128.82 (2CH), 126.05 (CH), 125.69 (C), 48.46 (CH2). Anal. calcd. for C18H12N2O5S (368.36): C, 58.69; H, 3.28; N, 7.60; S, 8.70. Found: C, 58.60; H, 3.17; N, 7.54; S, 8.66%.
(2) (Z)-5-(3-Nitrobenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (16c). Yellow crystals from ethanol-dioxane, yield (85%), mp 160–162 °C. IR (ν cm−1): 3081 (C-H arom), 3067 (C-H arom.), 2934 (C-H aliph.), 1749 (C=O), 1708 (C=O), 1683 (C=O), 1599, 1535, 1449. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.33 (dd, J = 8.2, 2.1 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.11–8.08 (m, 3H, Ph-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.76 (t, J = 7.0 Hz, 1H Ar-H), 7.61 (t, J = 7.7 Hz, 2H, Ph-H), 5.37 (s, 2H, CH2). 13C-NMR (400 MHz, DMSO) δ (ppm): 191.66 (C=O), 166.91 (C=O), 165.38 (C=O), 148.74 (C), 135.93 (-CH=), 134.99 (C), 134.95 (C), 134.19 (CH), 132.14 (CH), 131.45 (CH), 129.52 (2CH), 128.82 (2CH), 125.34 (CH), 125.29 (CH), 124.23 (C), 48.54 (CH2). Anal. calcd. for C18H12N2O5S (368.36): C, 58.69; H, 3.28; N, 7.60; S, 8.70. Found: C, 58.75; H, 3.22; N, 7.51; S, 8.65%.
(3) (Z)-5-(4-Nitrobenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (16d). Brown crystals from ethanol-dioxane, yield (83%), mp 232–233 °C. IR (ν cm−1): 3073 (C-H arom), 3013 (C-H arom.), 2969 (C-H aliph.), 2933 (C-H aliph.), 1755 (C=O), 1697 (C=O), 1682 (C=O), 1607, 1595, 1517. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.38 (d, J = 8.8 Hz, 2H, Ar-H), 8.14 (s, 1H, -CH=), 8.11 (m, 2H, Ph-H), 7.95 (d, J = 8.8 Hz, 2H, Ar-H), 7.76 (t, J = 7.4 Hz, 1H, Ph-H), 7.62 (t, J = 7.8 Hz, 2H, Ph-H), 5.37 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 191.68 (C=O), 167.02 (C=O), 165.40 (C), 148.25 (CH), 139.54 (C), 135.02 (C), 134.19 (-CH=), 131.87 (CH), 131.66 (2CH), 129.54 (2CH), 128.84 (2CH), 125.59 (C), 124.82 (2CH), 48.58 (CH2). Anal. calcd. for C18H12N2O5S (368.36): C, 58.69; H, 3.28; N, 7.60; S, 8.70: Found: C, 58.59; H, 3.28; N, 7.60; S, 8.70%.
(4) (Z)-3-(2-Oxo-2-phenylethyl)-5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dione (16e). Brownish-yellow crystals from DMF-H2O, yield (62%), mp 187–189 °C. IR (ν cm−1): 3014 (C-H arom), 2969 (C-H aliph.), 2837 (C-H aliph.), 1740 (C=O), 1686b (C=O), 1605, 1577, 1504. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.14 (m, 2H, Ph-H), 8.02 (s, 1H, =CH-), 7.81 (t, J = 8.0 Hz, 1H, Ph-H), 7.66 (t, J = 7.7 Hz, 2H, Ph-H), 7.05 (s, 2H, Ar-H), 5.39 (s, 2H, CH2), 3.91 (s, 6H, 2OCH3), 3.81 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ 191.80 (C=O), 167.49 (C=O), 165.65 (C=O), 153.74 (2C), 140.24 (CH), 134.97 (C), 134.63 (=CH-), 134.24 (C), 129.53 (2CH), 128.78 (2CH), 120.24 (C), 108.25 (2CH), 60.71 (OCH3), 56.53 (2OCH3), 55.37 (CH2). Anal. calcd. for C21H19NO6S (413.44): C, 61.01; H, 4.63; N, 3.39; S, 7.75. Found C, 61.30; H, 4.54; N, 3.35; S, 7.82%.
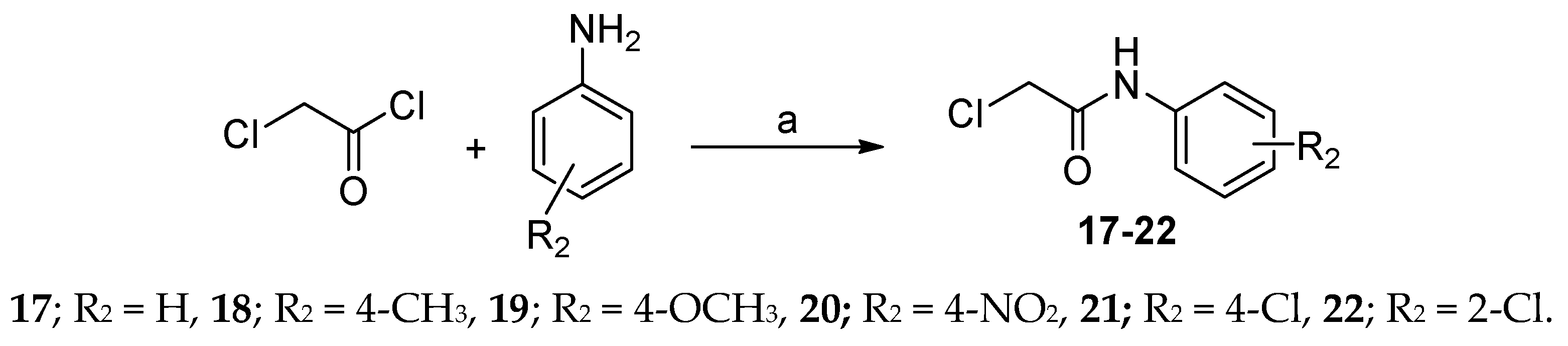
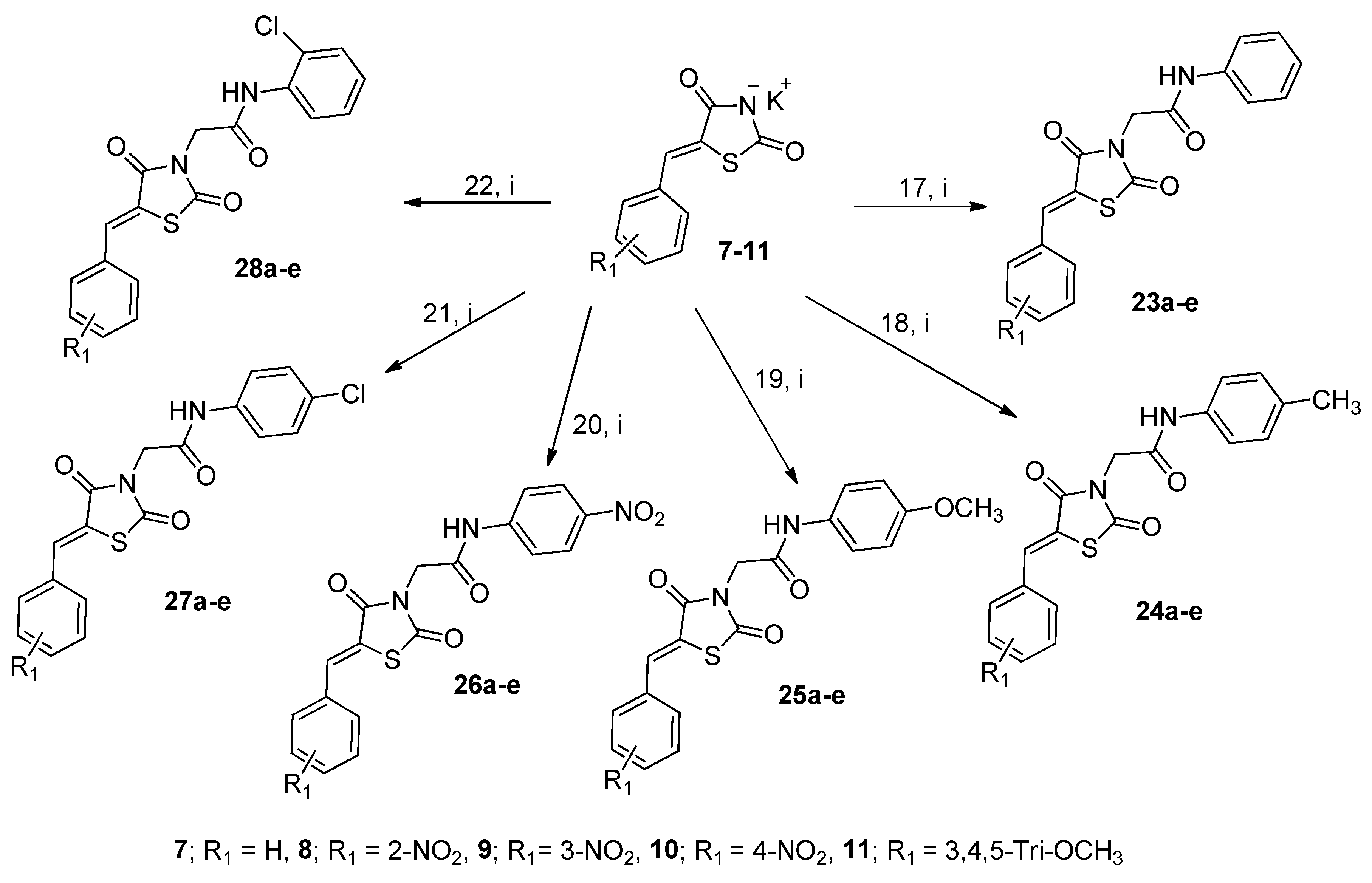
General Procedure for the Synthesis of Thiazolidine-2,4-dione Derivatives 23b-e–28b-e
- (1)
Synthesis of compounds 23b-e
A mixture of equimolar amounts of 2-chloro-N-phenylacetamide (17) (1 mmol, 0.169 g) and the potassium salt of 7 (0.234 g), 8 (0.288 g), 9 (0.288 g), 10 (0.288 g) or 11 (0.333 g) in DMF (5 mL) was heated under reflux for 6 hr. After cooling, the solid products obtained were filtered, washed with water, dried, and recrystallized.
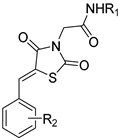
(1) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-phenylacetamide (23b). Yellow crystals from ethanol, yield (85%), mp 186–188 °C. IR (ν cm−1): 3359 (N-H), 3071 (C-H arom.) 2977 (C-H aliph.), 2938 (C-H aliph.),1748 (C=O), 1697 br (C=O), 1617, 1602, 1540, 1498. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.42 (s, 1H, NH), 8.26 (s, 1H, Ar-H), 8.24 (s, 1H, -CH=), 7.95–7.91 (m, 1H, Ar-H), 7.79–7.75 (m, 2H, Ar-H), 7.57 (d, J = 7.7 Hz, 2H, Ph-H), 7.34 (t, J = 7.9 Hz, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 4.54 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.37 (C=O), 165.03 (C=O), 164.16 (C=O), 148.30 (C), 138.85 (C), 135.20 (-CH=), 131.80 (C), 131.43 (C), 129.82 (CH), 129.37 (2CH), 129.31 (CH), 126.04 (CH), 125.82 (CH), 124.21 (CH), 119.63 (2CH), 44.60 (CH2). Anal. calcd. for: C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.45; H, 3.35; N, 10.89; S, 8.48%.
(2) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-phenylacetamide (23c). Yellow crystals from ethanol-dioxane, yield 90%, mp 255–257 °C. IR (ν cm−1): 3414 (N-H), 3067 (CH arom.), 2934 (CH aliph.), 1749 (C=O), 1683br (C=O), 1599, 1570, 1484. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.41 (s, 1H, NH), 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.33 (dd, J = 8.3, 2.2 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.57 (d, J = 8.3 Hz, 2H, Ph-H), 7.33 (m, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.02 (C=O), 165.46 (C=O), 164.12 (C=O), 148.77 (CH), 138.84 (C), 135.90 (-CH=), 135.00 (C), 131.84 (CH), 131.46 (CH), 129.36 (2CH), 125.30 (C),125.25 (CH), 124.40 (CH) 124.21 (C), 119.63 (2CH), 44.66 (CH2). Anal. calcd. for C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.42; H, 3.37; N, 11.00; S, 8.41%.
(3) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-phenylacetamide (23d). Yellow crystals from DMF-H2O, yield (75%), mp 259–261 °C. IR (ν cm−1): 3393 (N-H), 3075 (C-H arom.), 2984 (C-H aliph.), 1753 (C=O), 1697 (C=O), 1682 (C=O), 1609, 1541, 1514. 1H NMR (400 MHz, DMSO) δ (ppm): 10.41 (s, 1H, NH), 8.37 (d, J = 8.8 Hz, 2H, Ar-H), 8.12 (s, 1H, -CH=), 7.93 (d, J = 8.8 Hz, 2H, Ar-H), 7.56 (d, J = 7.6 Hz, 2H, Ph-H), 7.33 (t, J = 7.9 Hz, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.10 (C=O), 165.46 (C=O), 164.10 (C=O), 148.20 (C), 139.56 (C), 138.82 (-CH=), 131.61 (2CH), 131.56 (C), 129.37 (2CH), 125.74 (C), 124.81 (2CH), 124.22 (CH), 119.64 (2CH), 44.68 (CH2). Anal. calcd. for: C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.46; H, 3.50; N, 11.01; S, 8.45%.
(4) (Z)-N-Phenyl-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (23e). Pale yellow crystals from DMF-H2O, yield (76%), mp 226–228 °C. IR (ν cm−1): 3264 (N-H), 3147 (C-H arom.), 2992 (C-H aliph.) 2933 (C-H aliph.), 2836 (C-H aliph.),1741 (C=O), 1686 (C=O), 1670 (C=O), 1606, 1579, 1551. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.40 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.57 (d, J = 7.7 Hz, 2H, Ph-H), 7.33 (t, J = 7.9 Hz, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 6.99 (s, 2H, Ar-H), 4.53 (s, 2H, CH2), 3.86 (s, 6H, 2 OCH3), 3.75 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.57 (C=O), 165.73 (C=O), 164.25 (C=O), 153.73 (2C), 140.19 (C), 138.88 (C), 134.31 (-CH=), 129.36 (2CH), 128.82 (C), 124.17 (CH), 120.39 (C), 119.62 (2 CH), 108.21 (2CH), 60.70 (OCH3), 56.52 (2OCH3), 44.48 (CH2). Anal. calcd. for: C21H20N2O6S (428.46): C, 58.87; H, 4.71; N, 6.54; S, 7.48. Found: C, 59.01; H, 4.79; N, 6.63; S, 7.46%.
- (2)
Synthesis of compounds 24a-e.
These compounds were synthesized using 2-chloro-N-(4-methylphenyl)acetamide (18) (0.184 g, 1 mmol), following the procedure described for 23a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)-N-(4-methylphenyl) acetamide (24a). Shiny white crystals from ethanol-dioxane, yield (88%), mp 277–279 °C. IR (ν cm−1): 3266 (N-H), 3126 (C-H arom.), 3059 (C-H arom.), 2922 (C-H aliph.), 1745 (C=O), 1695 (C=O), 1659 (C=O), 1608, 1596, 1573. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.31 (s, 1H, NH), 8.00 (s, 1H, -CH=), 7.68–7.66 (m, 2H, Ph-H), 7.60–7.51 (m, 3H, Ph-H), 7.45 (d, J = 8.4 Hz, 2H, Ph-H), 7.13 (d, J = 8.4 Hz, 2H, Ph-H), 4.46 (s, 2H, CH2), 2.26 (s, 3H, CH3).13C-NMR (100 MHz, DMSO) δ (ppm): 167.60 (C=O), 165.79 (C=O), 163.97 (C=O), 136.37 (C), 134.05 (-CH=), 133.34 (C), 133.13 (C), 131.29 (C), 130.68 (2CH), 129.81 (2CH), 129.72 (2CH), (121.49) (C), 119.64 (2CH), 44.49 (CH2), 20.90 (CH3). Anal. Calcd. For C19H16N2O3S (352.41): C, 64.76; H, 4.58; N, 7.95; S, 9.10. Found: C, 64.82; H, 4.49; N, 7.87; S, 9.14%.
(2) (Z)-N-(4-Methylphenyl)-2-(5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (24b). Yellow crystals from ethanol, yield (83%), mp 181–183 °C. IR (ν cm−1): 3260 (N-H), 3196 (C-H arom.), 3128 (C-H arom.), 3077 (C-H arom.) 2991 (C-H aliph.), 1769 (C=O), 1698 (C=O), 1666 (C=O), 1607, 1551, 1523. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.33 (s, 1H, NH), 8.25 (m, 2H, Ar-H + -CH=), 7.93 (t, J = 7.5 Hz, 1H, Ar-H), 7.77 (t, J = 7.7 Hz, 2H, Ar-H), 7.45 (d, J = 8.4 Hz, 2H, Ar-H), 7.14 (d, J = 8.4 Hz, 2H, Ar-H), 4.52 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.36 (C=O), 165.04 (C=O), 163.89 (C=O), 148.30 (C), 136.35 (C), 135.18 (CH), 133.17 (C), 131.79 (C), 131.36 (CH), 129.80 (CH), 129.73 (2CH), 129.30 (CH), 126.04 (CH), 125.94 (C), 119.64 (2CH), 44.56 (CH2), 20.90 (CH3). Anal. calcd. for C19H15N3O5S (397.41): C, 57.42; H, 3.80; N, 10.57; S, 8.07. Found: C, 57.52; H, 3.89; N, 10.63; S, 8.17%.
(3) (Z)-N-(4-Methylphenyl)2-(5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (24c). Yellow crystals from ethanol-dioxane, yield (82%), mp 276–278 °C. IR (ν cm−1): 3264 (N-H), 3071 (CH arom.), 2948 (CH aliph.), 2835 (CH aliph.), 1746 (C=O), 1681 (C=O), 1664 (C=O), 1603, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.32 (s, 1H, NH), 8.52 (s, 1H, Ar-H), 8.32 (dd, J = 8.2, 1.6 Hz, 1H, Ar-H), 8.16 (s, 1H –CH=), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.85 (t, J = 8.0 Hz, 1H, Ar-H), 7.44 (d, J = 8.4 Hz, 2H, Ar-H), 7.13 (d, J = 8.4 Hz, 2H, Ar-H), 4.53 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm) 167.02 (C=O), 165.46 (C=O), 163.85 (C=O), 148.75 (C), 136.33 (C), 135.89 (–CH=), 134.99 (C), 133.17 (C), 131.80 (CH), 131.45 (CH), 129.72 (2CH), 125.38 (C) 125.23 (CH), 124.41 (CH), 119.65 (2CH), 44.61 (CH2), 20.90 (CH3). Anal. calcd. for: C19H15N3O5S (397.41): C, 57.42; H, 3.80; N, 10.57; S, 8.07. Found: C, 57.50; H, 3.88; N, 11.06; S, 8.09%.
(4) (Z)-N-(4-Methylphenyl)2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (24d). Yellow crystals from DMF-H2O, yield (80%), mp 250–252 °C. IR (ν cm−1): 3282 (N-H), 3036 (C-H arom.), 2919 (C-H aliph.), 1750 (C=O), 1704 (C=O), 1676 (C=O), 1611, 1596, 1541. 1H-NMR (400 MHz, DMSO) δ (ppm) 10.32 (s, 1H, NH), 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.92 (d, J = 8.8 Hz, 2H, Ar-H), 7.44 (d, J = 8.4 Hz, 2H, Ar-H), 7.13 (d, J = 8.4 Hz, 2H, Ar-H), 4.53 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm) 167.10 (C=O), 165.47 (C=O), 163.83 (C=O), 148.18 (C), 139.56 (C), 136.32 (-CH=), 133.18 (C), 131.60 (2CH), 131.51 (C), 129.72 (2CH), 125.76 (C), 124.80 (2CH), 119.65 (2CH), 44.45 (CH2). 20.89 (CH3). Anal. calcd. for: C19H15N3O5S (397.41): C, 57.42; H, 3.80; N, 10.57; S, 8.07. Found: C, 57.49; H, 3.90; N, 11.06; S, 8.03%.
(5) (Z)-N-(4-Methylphenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (24e). Yellow crystals from DMF-H2O, yield (81%), mp 196–198 °C. IR (ν cm−1): 3251 (N-H), 3028 (C-H arom.), 2989 (C-H aliph.) 2934 (C-H aliph.), 2836 (C-H aliph.), 1741 (C=O), 1686 (C=O), 1666 (C=O), 1606, 1579, 1546. H-NMR (400 MHz, DMSO) δ (ppm): 10.31 (s, 1H, NH), 7.94 (s, 1H, -CH=), 7.45 (d, J = 8.4 Hz, 2H, Ar-H), 7.13 (d, J = 8.4 Hz, 2H, Ar-H), 6.98 (s, 2H, Ar-H), 4.51 (s, 2H, CH2), 3.85 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.57 (C=O), 165.73 (C=O), 163.98 (C=O), 153.73 (2C), 140.18 (C), 136.38 (C), 134.28 (-CH=), 133.13 (C), 129.71 (2CH), 128.82 (C), 120.39 (C), 119.63 (2CH), 108.20 (2CH), 60.69 (CH2), 56.51 (OCH3), 44.44 (2 OCH3), 20.89 (CH3). Anal. calcd. for: C22H22N2O6S (442.49): C, 59.72; H, 5.01; N, 6.33; S, 7.25. Found: C, 57.42; H, 3.80; N, 10.57; S, 8.07%.
- (3)
Synthesis of compounds 25b-e.
These compounds were synthesized using 2-chloro-N-(4-methoxylphenyl)acetamide 19 (1 mmol, 0.2 g), following the procedure described for 23a-e.
(1) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-methoxyphenyl) acetamide (25b). Brown crystals from ethanol-dioxane, yield (86%), mp 204–205 °C. IR (ν cm−1): 3285 (N-H), 3193 (C-H arom.), 3139 (C-H arom.), 3080 (C-H arom.) 2932 (C-H aliph.), 2835 (C-H aliph.), 1750 (C=O), 1699 (C=O), 1662 (C=O), 1610, 1551, 1520.1H-NMR (400 MHz, DMSO) δ (ppm): 10.27 (s, 1H, NH), 8.25 (d, J = 8.3 Hz, 1H, Ar-H), 8.23 (s, 1H, -CH=), 7.93 (t, J = 7.6 Hz, 1H, Ar-H), 7.77 (t, J = 7.6 Hz, 2H, Ar-H), 7.48 (d, J = 9.0 Hz, 2H, Ar-H), 6.91 (d, J = 9.0 Hz, 2H, Ar-H), 4.51 (s, 2H, CH2), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.37 (C=O), 165.04 (C=O), 163.63 (C=O), 155.97 (C), 148.30 (C), 135.19 (CH), 131.97 (C), 131.79 (C), 131. 35 (CH), 129.81 (CH), 129.31 (CH), 126.04 (CH), 125.95 (C), 121.19 (2CH), 114.46 (2CH), 55.63 (OCH3), 44.49 (CH2). Anal. calcd. for: C19H15N3O6S (413.40) C, 55.20; H, 3.66; N, 10.16; S, 7.76. Found: C, 55.27; H, 3.69; N, 10.22; S, 7.78%.
(2) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-methoxyphenyl) acetamide (25c). Beige crystals from ethanol-dioxane, yield (84%), mp 252–254 °C. IR (ν cm−1): 3258 (N-H), 3069 (CH arom.), 2948 (CH aliph.), 2835 (CH aliph.), 1746 (C=O), 1682 (C=O), 1659 (C=O), 1603, 1533. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.26 (s, 1H, NH), 8.54 (t, J = 1.7 Hz, 1H, Ar-H), 8.34 (dd, J = 8.2, 2.2 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.09 (d, J = 7.8 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.47 (d, J = 9.1 Hz, 2H, Ar-H), 6.90 (d, J = 9.1 Hz, 2H, Ar-H), 4.51 (s, 2H, CH2), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.04 (C=O), 165.48 (C=O), 163.60 (C=O), 155.97 (C), 148.78 (C), 135.89 (-CH=), 135.02 (C), 131.96 (C), 131.79 (CH), 131.47 (CH), 125.30 (CH), 125.25 (CH), 124.45 (C), 121.19 (2CH), 114.46 (2CH), 55.63 (OCH3), 44.56 (CH2). Anal. calcd. for: C19H15N3O6S (413.40) C, 55.20; H, 3.66; N, 10.16; S, 7.76. Found: C, 55.32; H, 3.57; N, 10.12; S, 7.71%.
(3) (Z)-N-(4-Methoxyphenyl)-2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (25d). Yellow crystals from DMF-H2O, yield (83%), mp 254–256 °C. IR (ν cm−1): 3349 (N-H), 3073 (C-H arom.), 2937 (C-H aliph.), 1751 (C=O), 1690 br (C=O), 1611, 1593, 1512. 1H- NMR (400 MHz, DMSO) δ (ppm): 10.26 (s, 1H, NH), 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.92 (d, J = 8.8 Hz, 2H, Ar-H), 7.47 (d, J = 9.1 Hz, 2H, Ar-H), 6.90 (d, J = 9.1 Hz, 2H, Ar-H), 4.52 (s, 2H, CH2), 3.72 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.10 (C=O), 165.47 (C=O), 163.57 (C=O), 155.98 (C), 148.18 (C), 139.56 (C), 131.95 (2CH), 131.59 (CH), 131.49 (C), 125.77 (C), 124.79 (2CH), 121.20 (2CH), 114.45 (2CH), 55.62 (OCH3), 44.57 (CH2). Anal. calcd. for: C19H15N3O6S (413.40) C, 55.20; H, 3.66; N, 10.16; S, 7.76. Found: C, 55.29; H, 3.59; N, 10.00; S, 7.77%.
(4) (Z)-N-(4-Methoxyphenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (25e). Pale yellow crystals from DMF-H2O, yield (77%), mp 215–216 °C. IR (ν cm−1): 3284 (N-H), 3022 (C-H arom.), 2993 (C-H aliph.) 2934 (C-H aliph.), 2837 (C-H aliph.),1741 (C=O), 1686 (C=O), 1663 (C=O), 1606, 1579, 1550. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.25 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.47 (d, J = 9.1 Hz, 2H, Ar-H), 6.99 (s, 2H, Ar-H), 6.90 (d, J = 9.1 Hz, 2H, Ar-H), 4.49 (s, 2H, CH2), 3.86 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.58 (C=O), 165.75 (C=O), 163.72 (C=O), 155.95 (C), 153.74 (2C), 140.17 (C), 134.25 (-CH=), 132.00 (C), 128.84 (C), 121.17 (2CH), 120.45 (C), 114.45 (2CH), 108.20 (2CH), 60.70 (OCH3), 56.53 (2OCH3), 55.63 (OCH3), 44.38 (CH2). Anal. calcd. for C22H22N2O7S (458.49): C, 57.63; H, 4.84; N, 6.11; S, 6.99. Found: C, 57.57; H, 4.83; N, 6.02; S, 6.90%.
- (4)
Synthesis of compounds 26a-e.
These compounds were synthesized using 2-chloro-N-(4-nitrophenyl)acetamide 20 (1 mmol, 0.215 g), following the procedure described for 23a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)-N-(4-nitrophenyl)acetamide (26a). Fluffy off-white needles from DMF-water, yield (89%), mp 270–272 °C. IR (ν cm−1): 3273 (N-H), 3168 (C-H arom.), 3104 (C-H arom.), 2929 (C-H arom.) 2991, 1744 (C=O), 1686 br (C=O), 1619, 1595, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.03 (s, 1H, NH), 8.25 (d, J = 9.3 Hz, 2H, Ar-H), 8.01 (s, 1H, -CH=), 7.81 (d, J = 9.3 Hz, 2H, Ar-H), 7.67 (d, J = 7.1 Hz, 2H, Ph-H), 7.59–7.51 (m, 3H, Ph-H), 4.61 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.57 (C=O), 165.70 (C=O), 165.41 (C=O), 144.88 (C), 143.07 (C), 134.29 (CH), 133.19 (C), 131.35 (C), 130.70 (2CH), 129.90 (2CH), 125.57 (2CH), 121.31 (CH), 119.49 (2CH), 44.70 (CH2). Anal. Calcd. for: C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.30; H, 3.39; N, 10.90; S, 8.30%.
(2) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-nitroyphenyl) acetamide (26b). Yellow crystals from ethanol, yield (87%), mp 213–215 °C. IR (ν cm−1): 3263 (N-H), 3164 (C-H arom.), 3100 (C-H arom.), 3072 (C-H arom.) 2938 (C-H aliph.), 1750 (C=O), 1679 br (C=O), 1609, 1594, 1568, 1532. 1H NMR (400 MHz, DMSO) δ (ppm): 11.05 (s, 1H, NH), 8.26 (d, J = 9.2 Hz, 2H, Ar-H) 8.25 (s, 2H, -CH= + Ar-H ), 7.94 (t, J = 7.6 Hz, 1H), 7.82 (d, J = 9.2 Hz, 2H, Ar-H), 7.78 (t, J = 7.6 Hz, 2H, Ar-H), 4.62 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.34 (C=O), 165.35 (C=O), 164.96 (C=O), 148.30 (C), 144.87 (CH), 143.10 (C), 135.21 (-CH=), 131.86 (C), 131.68 (CH), 129.82 (CH), 129.29 (C), 126.06 (CH), 125.68 (C), 125.95 (2CH), 119.51 (2CH), 44.80 (CH2). Anal. calcd. for: C18H12N4O7S (428.38): C, 50.47; H, 2.82; N, 13.08; S, 7.48. Found: C, 50.44; H, 2.79; N, 13.01; S, 7.42%.
(3) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-nitroyphenyl) acetamide (26c). Buff crystals from ethanol-dioxane, yield (89%), mp 264–266 °C. IR (ν cm−1): 3342 (N-H), 3087 (CH arom.), 2943 (CH aliph.), 1751 (C=O), 1709 (C=O), 1686 (C=O), 1616, 1562, 1348. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.04 (s, 1H, NH), 8.52 (t, J = 1.6 Hz, 1H, Ar-H), 8.33 (dd, J = 8.2, 2.1 Hz, 1H, Ar-H), 8.25 (d, J = 9.2 Hz, 2H, Ar-H), 8.18 (s, 1H, -CH=), 8.08 (d, J = 7.8 Hz, 1H, Ar-H), 7.85 (t, J = 8.1 Hz, 1H, Ar-H), 7.81 (d, J = 9.3 Hz, 2H, Ar-H), 4.63 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.98 (C=O), 165.38 (C=O), 165.29 (C=O), 148.76 (C), 144.85 (C), 143.08 (C), 135.91 (-CH=), 134.94 (CH), 132.04 (CH), 131.46 (C), 125.57 (2CH), 125.43 (C), 125.27 (CH), 124.24 (CH), 119.49 (2CH), 44.63 (CH2). Anal. calcd. for: C18H12N4O7S (428.38): C, 50.47; H, 2.82; N, 13.08; S, 7.48. Found: C, 50.34; H, 2.78; N, 13.01; S, 7.37%.
(4) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-nitroyphenyl) acetamide (26d). Yellow crystals from DMF-H2O, yield (85%), mp 284–285 °C. IR (ν cm−1): 3264 (N-H), 3075 (C-H arom.), 2994 (C-H aliph.), 1756 (C=O), 1698 (C=O), 1609, 1593, 1517. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.03 (s, 1H, NH), 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.24 (d, J = 9.2 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.92 (d, J = 8.8 Hz, 2H, Ar-H), 7.80 (d, J = 9.3 Hz, 2H, Ar-H), 4.63 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.05 (C=O), 165.38 (C=O), 165.25 (C=O), 148.20 (C), 144.83 (-CH=), 143.08 (C), 139.47 (C), 131.73 (C), 131.62 (2CH), 125.55 (2CH + C), 124.78 (2CH), 119.49 (2CH), 44.84 (CH2). Anal. calcd. for: C18H12N4O7S (428.38): C, 50.47; H, 2.82; N, 13.08; S, 7.48. Found: C, 50.39; H, 2.77; N, 13.18; S, 7.42%.
(5) (Z)-N-(4-Nitrophenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (26e). Yellow crystals from DMF-H2O, yield (80%), mp 253–254 °C. IR (ν cm−1): 3282 (N-H), 3091 (C-H arom.), 2940 (C-H aliph.), 2893 (C-H aliph.), 1738 (C=O), 1682 (C=O), 1668, (C=O), 1607, 1596, 1507. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.03 (s, 1H, NH), 8.25 (d, J = 9.3 Hz, 2H, Ar-H), 7.96 (s, 1H, -CH=), 7.82 (d, J = 9.3 Hz, 2H, Ar-H), 6.99 (s, 2H, Ar-H), 4.61 (s, 2H, CH2), 3.86 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.54 (C=O), 165.65 (C=O), 165.43 (C=O), 153.74 (2C), 144.89 (C), 143.07 (-CH=), 140.24 (C), 134.51 (C), 128.78 (C), 125.58 (2CH), 120.24 (C), 119.48 (2CH), 108.24 (2CH), 60.71 (OCH3), 56.53 (2OCH3), 44.68 (CH2). Anal. calcd. for: C21H19N3O8S (473.46): C, 53.27; H, 4.05; N, 8.88; S, 6.77. Found: C, 53.35; H, 4.00; N, 8.79; S, 6.70%.
- (5)
Synthesis of compounds 27b-e.
These compounds were synthesized using 2-chloro-N-(4-chlorophenyl)acetamide 21 (1 mmol, 0.204 g), following the procedure described for 23a-e.
(1) (Z)-N-(4-Chlorophenyl)-2-(5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (27b). Canary yellow crystals from ethanol-dioxane, yield (85%), mp 188–190 °C. IR (ν cm−1): 3258 (N-H), 3123 (C-H arom.), 3073 (C-H arom.) 2992 (C-H aliph.), 2835 (C-H aliph.), 1756 (C=O), 1693 (C=O), 1688 (C=O), 1607, 1570, 1546. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.57 (s, 1H, NH), 8.25 (d, J = 8.5 Hz, 2H, Ar-H + -CH=), 7.93 (t, J = 7.4 Hz, 1H, Ar-H), 7.77 (t, J = 7.5 Hz, 2H, Ar-H), 7.60 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, Ar-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.35 (C=O), 165.00 (C=O), 164.39 (C=O), 148.29 (C), 137.78 (C), 135.19 (CH), 131.81 (C), 131.48 (CH), 129.80 (CH), 129.29 (C+2CH), 127.81 (C), 126.04 (CH), 125.78 (C), 121.23 (2CH), 44.61 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.65; H, 2.86; N, 10.00; S, 7.59%.
(2) (Z)-N-(4-Chlorophenyl)-2-(5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (27c). Fluffy white crystals from ethanol-dioxane, yield (90%), mp 260–261 °C. IR (ν cm−1): 3349 (N-H), 3075 (CH arom.), 2943 (CH aliph.), 1750 (C=O), 1699br (C=O), 1607 (C=O), 1603, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.56 (s, 1H, NH), 8.52 (t, J = 1.9 Hz, 1H, Ar-H), 8.34 (ddd, J = 8.3, 2.2, 0.8 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.09 (d, J = 7.8 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.59 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, Ar-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.00 (C=O), 165.43 (C=O), 164.35 (C=O), 148.76 (C), 137.77 (C), 135.90 (-CH=), 134.97 (CH), 131.90 (CH), 131.46 (CH), 129.29 (2CH), 127.82 (C), 125.31 (C), 125.25 (CH), 124.34 (C), 121.23 (2CH), 44.66 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.62; H, 2.84; N, 9.99; S, 7.55%.
(3) (Z)-N-(4-Chlorophenyl)-2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (27d). Yellow crystals from DMF-H2O, yield (90%), mp 252–254 °C. IR (ν cm−1): 3276 (N-H), 3120 (C-H arom.), 2994 (C-H aliph.), 1748 (C=O), 1704 (C=O), 1675 (C=O), 1595, 1541, 1491. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.55 (s, 1H, NH), 8.37 (d, J = 8.8 Hz, 2H, Ar-H), 8.12 (s, 1H, -CH=), 7.93 (d, J = 8.8 Hz, 2H, Ar-H), 7.59 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, Ar-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.08 (C=O), 165.43 (C=O), 164.33 (C=O), 148.20 (C), 139.54 (C), 137.76 (-CH=), 131.61 (4CH), 129.29 (2CH), 127.82 (C), 125.68 (C), 124.80 (2CH), 121.23 (C), 44.68 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.68; H, 2.88; N, 10.01; S, 7.66%.
(4) (Z)-N-(4-Chlorophenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (27e). Yellow crystals from DMF-H2O, yield (79%), mp 218–219 °C. IR (ν cm−1): 3295 (N-H), 3193 (C-H arom.), 3125 (C-H arom.), 3074 (C-H arom.) 2991 (C-H aliph.), 2838 (C-H aliph.), 1750 (C=O), 1686 (C=O), 1677 (C=O), 1603, 1577, 1507. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.55 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.59 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, 2Ar-H), 6.99 (s, 2H, Ar-H), 4.53 (s, 2H, CH2), 3.85 (s, 6H, 2 OCH3), 3.74 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.55 (C=O), 165.70 (C=O), 164.48 (C=O), 153.73 (2C), 140.20 (C), 137.81 (C), 134.37 (-CH=), 129.28 (2CH), 128.80 (C), 127.77 (C), 121.21 (2CH), 120.33 (C), 108.22 (2CH), 60.70 (OCH3), 56.52 (2OCH3), 44.49 (CH2). Anal. calcd. for: C21H19ClN2O6S (462.90): C, 54.49; H, 4.14; N, 6.05; S, 6.93. Found: C, 54.42; H, 4.13; N, 6.12; S, 6.98%.
- (6)
Synthesis of compounds 28a-e.
These compounds were synthesized using 2-chloro-N-(2-chlorophenyl)acetamide 22 (1 mmol, 0.204 g), following the procedure described for 23a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)-N-(2-chlorophenyl)acetamide (28a). White aggregate crystals from ethanol-dioxane, yield (87%), mp 243–245 °C. IR (ν cm−1): 3260 (N-H), 3020 (C-H arom.) 1747 (C=O), 1694 (C=O), 1668 (C=O), 1608, 1597, 1587. 1H-NMR (400 MHz, DMSO) δ (ppm): 1H NMR (400 MHz, DMSO) δ (ppm): 10.07 (s, 1H, NH), 8.00 (s, 1H, =CH-), 7.71–7.66 (m, 3H, Ar-H), 7.62–7.44 (m, 4H, Ar-H), 7.34 (t, J = 7.5 Hz, 1H, Ar-H), 7.22 (t, J = 7.3, 1H, Ar-H), 4.62 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.56 (C=O), 165.73 (C=O), 164.97 (C=O), 134.68 (C), 134.07 (-CH=), 133.33 (CH), 131.29 (CH), 130.68 (2CH), 130.10 (CH), 129.90 (2CH), 128.02 (CH), 127.20 (C), 126.88 (C), 126.68 (C), 121.48 (CH), 44.31 (CH2). Anal. calcd. for C18H13ClN2O3S (372.82): C, 57.99; H, 3.51; N, 7.51; S, 8.60. Found: C, 57.95; H, 3.40; N, 7.48; S, 8.53%.
(2) (Z)-N-(2-Chlorophenyl)-2-(5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (28b). Small buff crystals from ethanol-dioxane, yield (80%), mp 174–175 °C. IR (ν cm−1): 3346 (N-H), 3065 (CH arom.), 2934 (CH aliph.), 1751 (C=O), 1703 (C=O), 1697 (C=O), 1616, 1569, 1535. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.09 (s, 1H, NH), 8.25 (m, 2H, Ar-H + -CH=), 7.93 (t, J = 7.6 Hz, 1H, Ar-H), 7.79–7.75 (m, 2H, Ar-H), 7.71 (dd, J = 8.0, 1.1 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.1 Hz, 1H, Ar-H), 7.35 (td, J = 8.3, 1.2 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.2 Hz, 1H, Ar-H), 4.62 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.33 (C=O), 164.94 (C=O), 164.89 (C=O), 148.31 (C), 135.19 (-CH=), 134.65 (C), 131.80 (CH), 131.37 (CH), 130.11 (CH), 129.80 (CH), 129.30 (C), 128.03 (CH), 127.24 (CH), 127.08 (C), 126.91 (C), 126.51 (C), 126.04 (CH), 125.84 (CH), 44.39 (CH2). Anal. calcd. for C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.65; H, 2.91; N, 10.00; S, 7.77%.
(3) (Z)-N-(2-Chlorophenyl)-2-(5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (28c). White crystals from DMF-H2O, yield (69%), mp 240–242 °C. IR (ν cm−1): 3252 (NH), 3068 (C-H arom.), 3013 (C-H arom.), 2914 (C-H aliph.), 1745 (C=O), 1683 (C=O), 1668 (C=O), 1604, 1570, 1533, 1476. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.09 (s, 1H, NH), 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.33 (ddd, J = 8.3, 2.2, 0.8 Hz, 1H, Ar-H), 8.18 (s, 1H, =CH-), 8.09 (d, J = 7.9 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.70 (dd, J = 8.1, 1.5 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.4 Hz, 1H, Ar-H), 7.34 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 4.36 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.00 (C=O), 165.43 (C=O), 164.87 (C=O), 148.78 (C), 135.89 (-CH=), 135.00 (CH), 134.64 (CH), 131.82 (CH), 131.48 (CH), 130.11 (CH), 128.03 (C), 127.26 (C), 126.97 (C), 126.51 (CH), 125.28 (CH), 125.28 (CH), 124.44 (C), 44.45 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.64; H, 2.81; N, 10.01; S, 7.59%.
(4) (Z)-N-(2-Chlorophenyl)-2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (28d). Pale yellow crystals from DMF-H2O, yield (89%), mp 242–244 °C. IR (ν cm−1): 3280 (N-H), 3119 (C-H arom.), 3050 (C-H arom.) 2947 (C-H aliph.), 1757 (C=O), 1702 (C=O), 1682 (C=O), 1610, 1592, 1540. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.09 (s, 1H, NH), 8.37 (d, J = 8.8 Hz, 2H, Ar-H), 8.12 (s, 1H, -CH=), 7.93 (d, J = 8.8 Hz, 2H, Ar-H), 7.70 (dd, J = 8.8, 0.7 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H), 7.34 (td, J = 8.0, 1.3 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.3 Hz, 1H, Ar-H), 4.63 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.07 (C=O), 165.42 (C=O), 164.84 (C=O), 148.20 (C), 139.57 (-CH=), 134.63 (CH), 131.61 (2CH), 131.53 (CH), 130.11 (CH), 128.03 (CH), 127.26 (C), 126.94 (C), 126.52 (C), 125.78 (C), 124.82 (2CH), 44.47 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.67; H, 2.80; N, 10.11; S, 7.61%.
(5) (Z)-N-(2-Chlorophenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (28e). Pale yellow crystals from DMF-H2O, yield (75%), mp 222–223 °C. IR (ν cm−1): 3251 (N-H), 3047 (C-H arom.), 2938 (C-H aliph.), 2835 (C-H aliph.), 1740 (C=O), 1686 (C=O), 1671 (C=O), 1605, 1578, 1505. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.07 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.70 (dd, J = 8.1, 1.3 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H), 7.34 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 6.99 (s, 2H, Ar-H), 4.61 (s, 2H, CH2), 3.85 (s, 6H, 2 OCH3), 3.75 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.54 (C=O), 165.68 (C=O), 164.97 (C=O), 153.74 (2C), 140.18 (CH), 134.68 (C), 134.30 (-CH=), 130.26 (CH), 128.83 (CH), 128.02 (C), 127.19 (C), 126.85 (C), 126.46 (CH), 120.41 (C), 108.20 (2CH), 60.80 (OCH3), 56.53 (2OCH3), 44.27 (CH2). Anal. calcd. for: C21H19ClN2O6S (462.90): C, 54.49; H, 4.14; N, 6.05; S, 6.93. Found: C, 54.41; H, 4.09; N, 6.01; S, 6.88%.