Anti-Diabetic Activities and Molecular Docking Studies of Aryl-Substituted Pyrazolo[3,4-b]pyridine Derivatives Synthesized via Suzuki Cross-Coupling Reaction
Abstract
1. Introduction
2. Result and Discussion
2.1. Anti-Diabetic Activities of Arylated carboxylate Derivatives (6a–i)
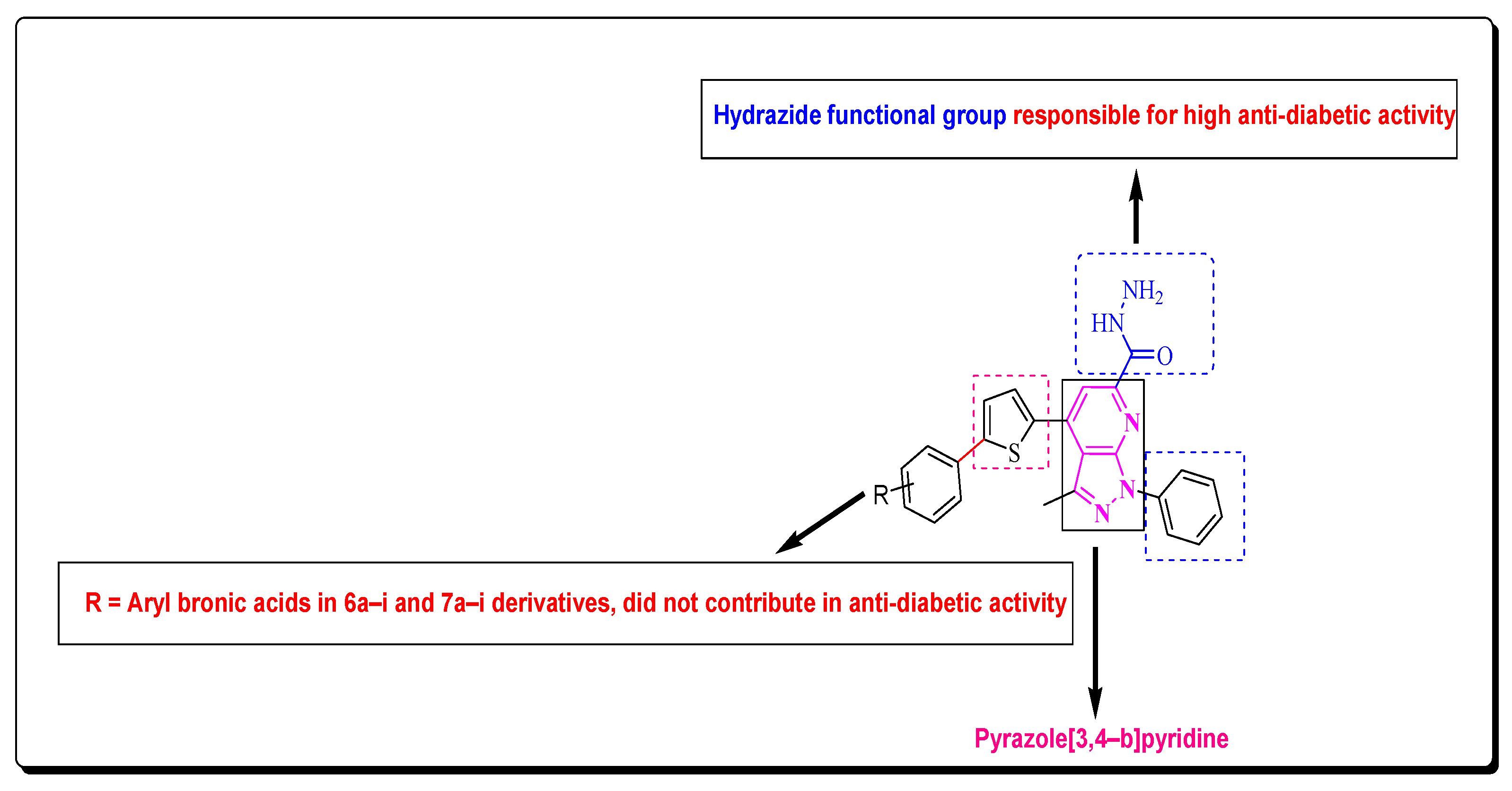
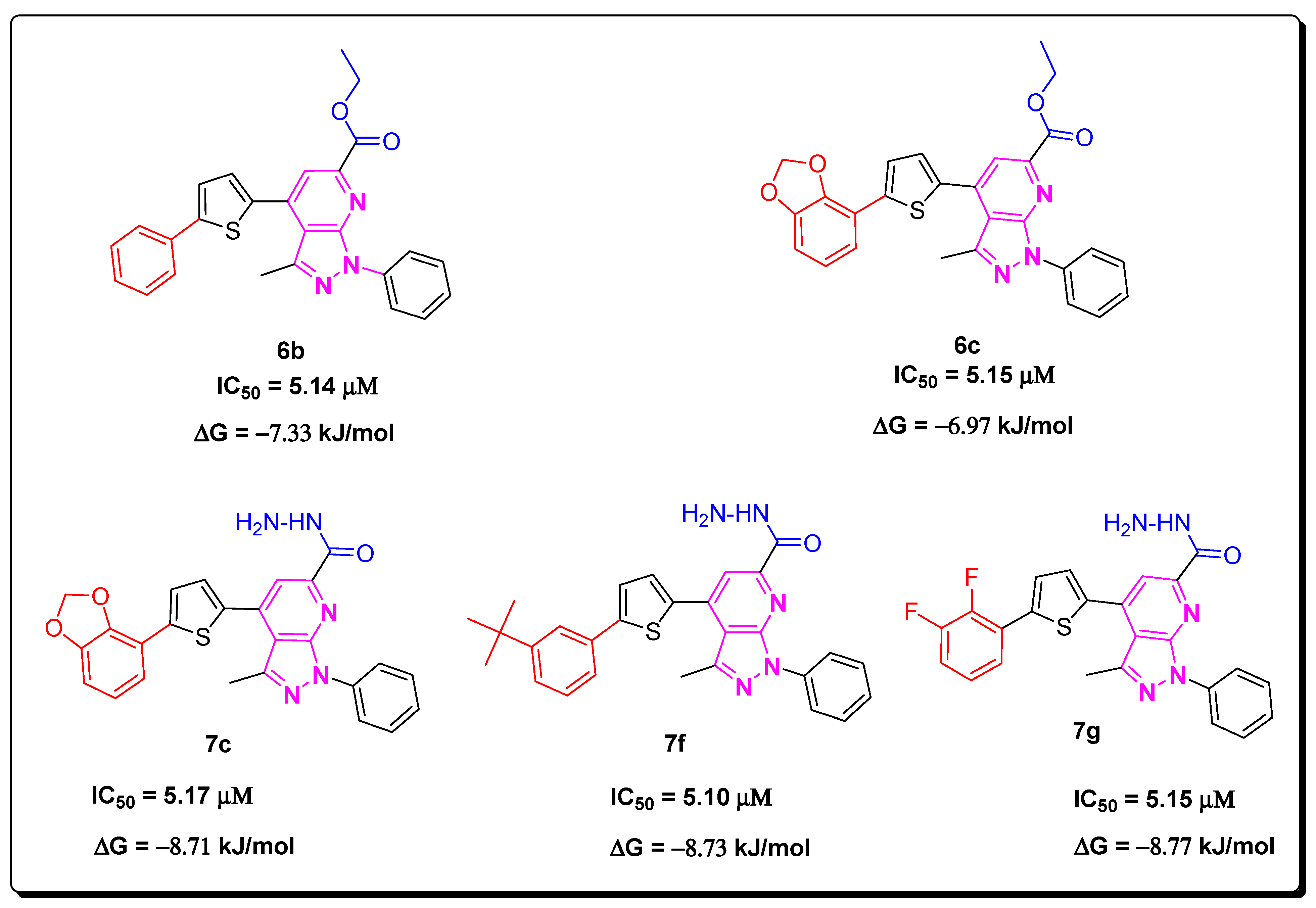
2.2. Structure–Activity Relationship
2.3. Anti-Diabetic Activities of Arylated Hydrazide Derivatives (7a–i)
2.4. Structure–Activity Relationship of (7a–i)
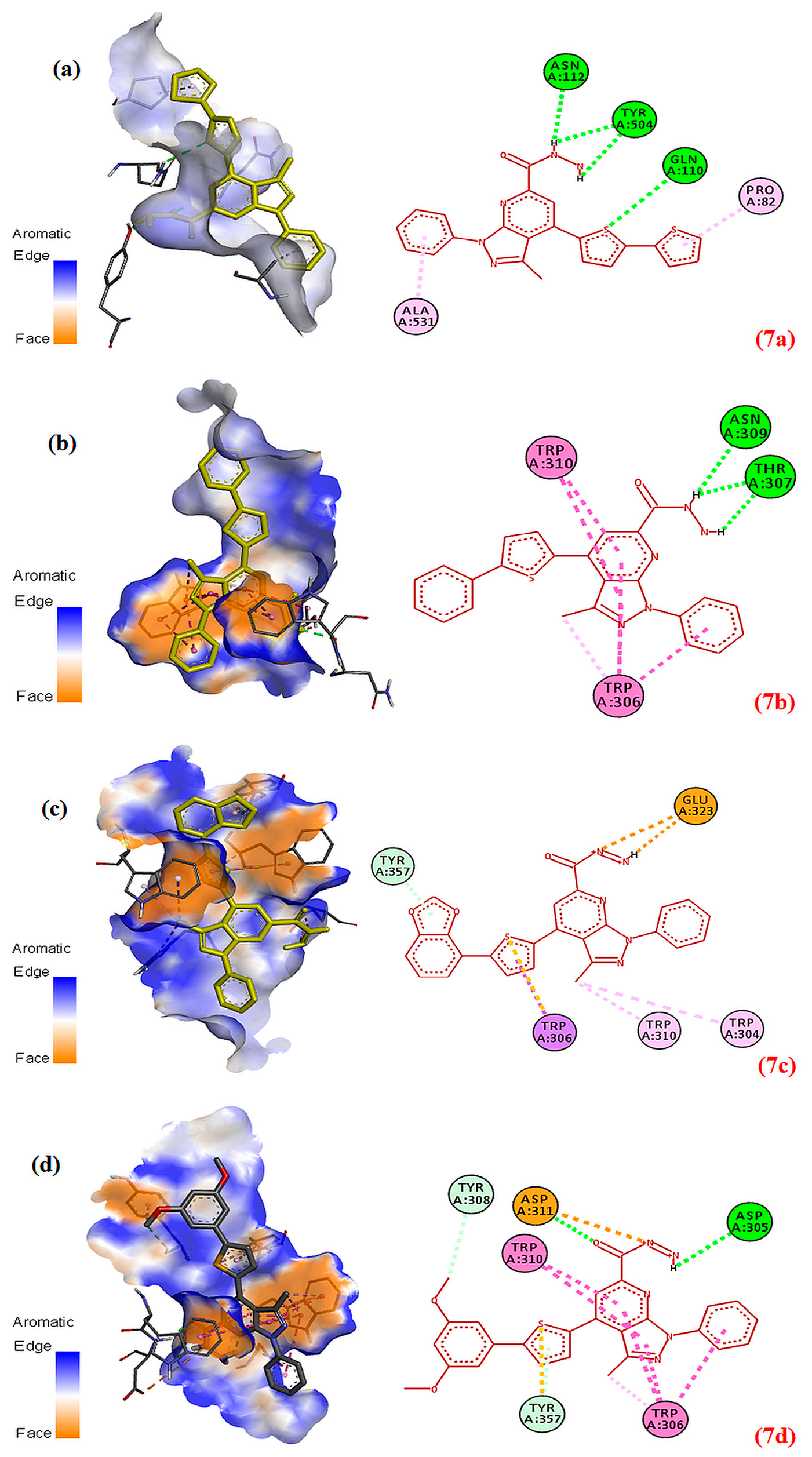
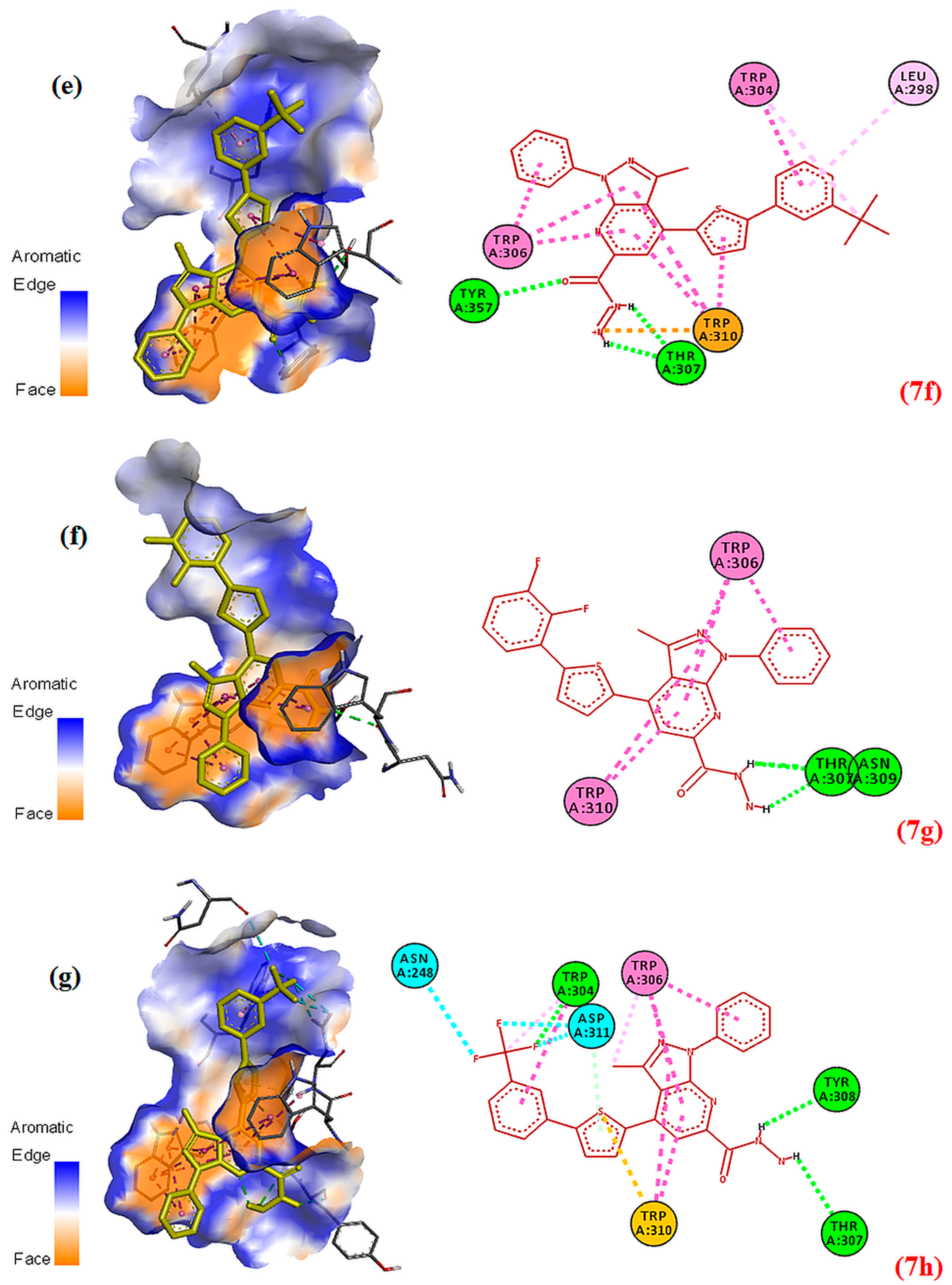
2.5. Docking Studies
2.6. Structure–Activity Relationship (SAR) of the Most Potent Derivatives and Their In Silico Studies
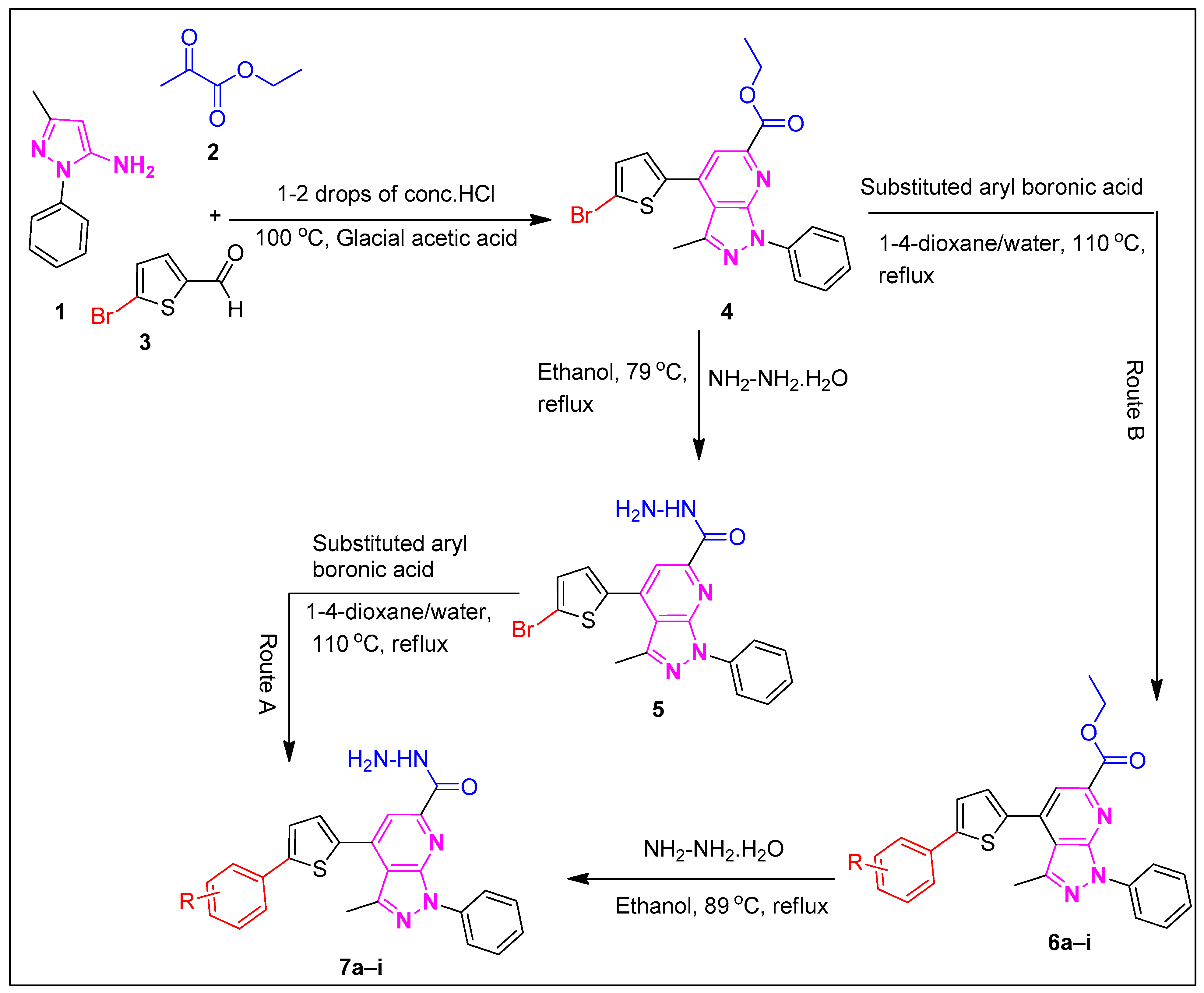
3. Materials and Methods
3.1. Synthesis of Ethyl 7-(6-Bromothiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (4)
3.2. Synthesis of 7-(6-Bromothiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (5)
3.2.1. Synthesis of Biaryl Derivatives (6a–i) via Suzuki Cross-Coupling
3.2.2. Synthesis of Aryl-substituted pyrazolo[3,4-b]pyridine-6-carbohydrazide (7a–i) Derivatives
3.3. Characterization Data
3.3.1. Ethyl 4-(5-Bromothiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (4)
3.3.2. 4-(5-Bromothiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (5)
3.3.3. Ethyl 4-([2,2′-Bithiophen]-5-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6a)
3.3.4. Ethyl 3-Methyl-1-phenyl-4-(5-phenylthiophen-2-yl)-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6b)
3.3.5. Ethyl 4-(5-(Benzo[d][1,3]dioxol-4-yl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6c)
3.3.6. Ethyl 4-(5-(3,5-Dimethoxyphenyl)thiophene-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6d)
3.3.7. Ethyl 4-(5-(3,5-Dimethylphenyl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6e)
3.3.8. Ethyl 4-(5-(3-(Tert-butyl)phenyl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6f)
3.3.9. Ethyl 4-(5-(2,3-Difluorophenyl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6g)
3.3.10. Ethyl 3-Methyl-1-phenyl-4-(5-(3-(trifluoromethyl)phenyl)thiophene-2-yl)-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6h)
3.3.11. Ethyl 4-(5′-Chloro-[2,2′-bithiophen]-5-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylate (6i)
3.3.12. 4-([2,2′-Bithiophen]-5-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7a)
3.3.13. 3-Methyl-1-phenyl-4-(5-phenylthiophen-2-yl)-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7b)
3.3.14. 4-(5-(Benzo[d][1,3]dioxol-4-yl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7c)
3.3.15. 4-(5-(3,5-Dimethoxyphenyl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7d)
3.3.16. 4-(5-(2,4-Dimethylphenyl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7e)
3.3.17. 4-(5-(3-(Tert-butyl)phenyl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7f)
3.3.18. 4-(5-(2,3-Fluorophenyl)thiophen-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7g)
3.3.19. 3-Methyl-1-phenyl-4-(5-(3-(trifluoromethyl)phenyl)thiophen-2-yl)-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7h)
3.3.20. 4-(5′-Chloro-[2,2′-bithiophen]-5-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carbohydrazide (7i)
3.4. Procedure for Anti-Diabetic Activity Analysis
3.5. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelhafeez, S.; Al-Swat, H.; Babiker, S.; Nasir, O.; Eisa, B. The prevalence of Diabetes Mellitus in Taif Region, Saudi Arabia during 2013–2014. Int. J. Sci. Res. 2018, 8, 1603–1607. [Google Scholar]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications; 3rd Completely Revised and Enlarged Edition; Wiley: Hoboken, NJ, USA, 2012; Volume 12, p. 69469. [Google Scholar]
- Tafesse, T.B.; Moghadam, E.S.; Bule, M.H.; Abadian, N.; Abdollahi, M.; Faramarzi, M.A.; Amini, M. Synthesis and biological evaluation of 2-(2-methyl-1H-pyrrol-3-yl)-2-oxo-N-(pyridine-3-yl) acetamide derivatives: In vitro α-glucosidase inhibition, and kinetic and molecular docking study. Chem. Pap. 2020, 74, 1583–1596. [Google Scholar]
- Taha, M.; Irshad, M.; Imran, S.; Rahim, F.; Selvaraj, M.; Almandil, N.B.; Mosaddik, A.; Chigurupati, S.; Nawaz, F.; Ismail, N.H.; et al. Thiazole Based Carbohydrazide Derivatives as α-Amylase Inhibitor and Their Molecular Docking Study. Heterocycl. Chem. 2019, 1, 7502347. [Google Scholar] [CrossRef]
- Hussain, R.; Rehman, W.; Khan, S.; Maalik, A.; Hefnawy, M.; Alanazi, A.S.; Khan, Y.; Rasheed, L. Imidazopyridine-Based Thiazole Derivatives as Potential Antidiabetic Agents: Synthesis, In Vitro Bioactivity, and In Silico Molecular Modeling Approach. Pharmaceuticals 2023, 16, 1288. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Echeta, C.K.; Igwe, V.S. Diabetes and the nutrition and diets for ts prevention and treatment: A systematic review and dietetic perspective. Health Sci. Res. 2020, 6, 5–19. [Google Scholar]
- Gohar, N.A.; Fayed, E.A.; Ammar, Y.A.; Abu Ali, O.A.; Ragab, A.; Mahfoz, A.M.; Abusaif, M.S. Fluorinated indeno-quinoxaline bearing thiazole moieties as hypoglycaemic agents targeting α-amylase, and α-glucosidase: Synthesis, molecular docking, and ADMET studies. J. Enzyme Inhib. Med. Chem. 2024, 39, 2367128. [Google Scholar] [CrossRef]
- Kurumurthy, C.; Veeraswamy, B.; Rao, P.S.; Kumar, G.S.; Rao, P.S.; Reddy, V.L.; Rao, J.V.; Narsaiah, B. Synthesis of novel 1,2,3-triazole tagged pyrazolo [3,4-b]pyridine derivatives and their cytotoxic activity. Bioorg. Med. Chem. Lett. 2014, 24, 746–749. [Google Scholar] [CrossRef]
- Chavva, K.; Pillalamarri, S.; Banda, V.; Gautham, S.; Gaddamedi, J.; Yedla, P.; Kumar, C.G.; Banda, N. Synthesis and biological evaluation of novel alkyl amide functionalized trifluoromethyl substituted pyrazolo [3,4-b]pyridine derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 5893–5895. [Google Scholar] [CrossRef]
- Aggarwal, R.; Kumar, S.; Sadana, R.; Guzman, A.; Kumar, V. Multicomponent synthesis, in vitro cytotoxic evaluation and molecular modelling studies of polyfunctionalized pyrazolo [3,4-b]pyridine derivatives against three human cancer cell lines. Synth. Commun. 2021, 51, 3308–3324. [Google Scholar] [CrossRef]
- Ibrahim, D. Synthesis and biological evaluation of 3,6-disubstituted [1,2,4]triazolo [3,4-b][1,3,4]thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur. J. Med. Chem. 2009, 44, 2776–2781. [Google Scholar]
- El-Borai, M.A.; Rizk, H.F.; Abd-Aal, M.F.; El-Deeb, I.Y. Synthesis of Pyrazolo [3,4-b]pyridines under Microwave Irradiation in Multi-Component Reactions and Their Antitumor and Antimicrobial Activities. Part 1. Eur. J. Med. Chem. 2012, 48, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.S.; Ali, M.A. Novel Pyrazolo [3,4-b]pyridine Derivatives: Synthesis, Characterization, Antimicrobial and Antiproliferative Profile. Biol. Pharm. Bull. 2016, 39, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sayed Ali, T.E. Synthesis of some novel pyrazolo [3,4-b]pyridine and pyrazolo [3,4-d]pyrimidine derivatives bearing 5,6-diphenyl-1,2,4-triazine moiety as potential antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Nagender, P.; Reddy, G.M.; Kumar, R.N.; Poornachandra, Y.; Kumar, C.G.; Narsaiah, B. Synthesis, cytotoxicity, antimicrobial and anti-biofilm activities of novel pyrazolo [3,4-b]pyridine and pyrimidine functionalized 1,2,3-triazole derivatives. Med. Chem. Lett. 2014, 24, 2905–2908. [Google Scholar] [CrossRef] [PubMed]
- Fathy, U.; Younis, A.; Awad, H. Ultrasonic assisted synthesis, anticancer and antioxidant activity of some novel pyrazolo [3,4-b]pyridine derivatives. J. Chem. Pharm. Res. 2015, 7, 4–12. [Google Scholar]
- Gouda, M.A. Synthesis and Antioxidant Evaluation of Some New Pyrazolopyridine Derivatives. Arch. Pharm. 2012, 345, 155–162. [Google Scholar] [CrossRef]
- Bilavendran, J.D.; Manikandan, A.; Thangarasu, P.; Sivakumar, K. Synthesis, Biochemical Characterization, and Theoretical Studies of Novelβ-Keto-enol Pyridine and Furan Derivatives as Potent Antifungal Agents. Bioorg. Chem. 2020, 94, 103–484. [Google Scholar]
- Fichez, J.; Soulie, C.; Le Corre, L.; Sayon, S.; Priet, S.; Alvarez, K.; Delelis, O.; Gizzi, P.; Prestat, G.; Gravier-Pelletier, C. Discovery, SAR study and ADME properties of methyl 4-amino-3-cyano-1-(2-benzyloxyphenyl)-1H-pyrazole-5-carboxylate as an HIV-1 replication inhibitor. RSC Med. Chem. 2020, 11, 577–582. [Google Scholar] [CrossRef]
- Hawas, S.S.; El-Gohary, N.S.; Gabr, M.T.; Shaaban, M.I.; El-Ashmawy, M.B. Synthesis, molecular docking, antimicrobial, antiquorum-sensing and antiproliferative activities of new series of pyrazolo [3,4-b]pyridine analogs. Synth. Commun. 2019, 49, 2466–2487. [Google Scholar] [CrossRef]
- Bernardino, A.M.; de Azevedo, A.R.; Pinheiro, L.C.; Borges, J.C.; Carvalho, V.L.; Miranda, M.D.; de Meneses, M.D.; Nascimento, M.; Ferreira, D.; Rebello, M.A.; et al. Synthesis and antiviral activity of new 4-(phenylamino)/4-[(methylpyridin-2-yl)amino]-1-phenyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acids derivatives. Med. Chem. Res. 2007, 16, 352–369. [Google Scholar] [CrossRef]
- Attaby, F.A.; Elghandour, A.H.; Ali, M.A.; Ibrahem, Y.M. Synthesis, Reactions, and Antiviral Activity of 1-(1H-Pyrazolo[3,4-b]pyridin-5-yl)ethanone and Pyrido [2′,3′:3,4]pyrazolo[5,1-c][1,2,4]triazine Derivatives. Phosphorus Sulfur Silicon 2006, 181, 1087–1102. [Google Scholar] [CrossRef]
- Gudmundsson, K.S.; Johns, B.A.; Wang, Z.; Turner, E.M.; Allen, S.H.; Freeman, G.A.; Boyd, F.L., Jr.; Sexton, C.J.; Selleseth, D.W.; Moniri, K.R.; et al. Synthesis of novel substituted 2-phenylpyrazolopyridines with potent activity against herpesviruses. Bioorg. Med. Chem. 2005, 13, 5346–5361. [Google Scholar] [CrossRef] [PubMed]
- Yuen, O.Y.; Ng, S.S.; Pang, W.H.; So, C.M. Palladium-catalyzed chemoselective Suzuki–Miyaura cross-coupling reaction of poly (pseudo) halogenated arenes. J. Organomet. Chem. 2024, 1005, 122–983. [Google Scholar] [CrossRef]
- Düfert, M.A.; Billingsley, K.L.; Buchwald, S.L. Suzuki-Miyaura cross-coupling of unprotected, nitrogen-rich heterocycles: Substrate scope and mechanistic investigation. J. Am. Chem. Soc. 2013, 13, 12877–12885. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, S.; Padusha, M.S.; Sajith, A.M. Application of Palladium Based Precatalytic Systems in the Suzuki-Miyaura Cross-Coupling Reactions of Chloro-Heterocycles. Chem. Sel. 2020, 5, 9005–9016. [Google Scholar] [CrossRef]
- Mahmood, N.; Rasool, N.; Ikram, H.M.; Hashmi, M.A.; Mahmood, T.; Zubair, M.; Ahmad, G.; Rizwan, K.; Rashid, T.; Rashid, U. Synthesis of 3,4-Biaryl-2,5-Dichlorothiophenethrough Suzuki Cross-Coupling and Theoretical Exploration of Their Potential Applications as Nonlinear Optical Materials. Symmetry 2018, 10, 766. [Google Scholar] [CrossRef]
- Nawaz, M.; Taha, M.; Qureshi, F.; Ullah, N.; Selvaraj, M.; Shahzad, S.; Chigurupati, S.; Waheed, A.; Almutairi, F.A. Structural elucidation, molecular docking, α-amylase and α-glucosidase inhibition studies of 5-amino-nicotinic acid derivatives. BMC Chem. 2020, 14, 43. [Google Scholar] [CrossRef]
- Reddy, M.U.; Reddy, M.S. Synthesis, Characterization and Anti-diabetic activity of vanillin based acetohydrazide-hydrazone derivatives. World J. Pharm. Res. 2017, 6, 814–825. [Google Scholar]
- Rafique, I.; Maqbool, T.; Javed, S. Synthesis of Pyrazolo[3,4-b]pyridine Derivatives and Their In-Vitro and In-Silico Antidiabetic Activities. J. Cell. Biochem. 2024, 10, 30646. [Google Scholar] [CrossRef]
- Eseyin, O.A.; Edem, E.; Johnson, E.; Ahmad, A.; Afzal, S. Synthesis and in vitro anti-diabetic activity of some alkyl carbazole compounds. Trop. J. Pharm. Res. 2018, 17, 537–541. [Google Scholar] [CrossRef]
- Zarren, G.; Shafiq, N.; Arshad, U.; Rafiq, N.; Parveen, S.; Ahmad, Z. Copper-catalyzed one-pot relay synthesis of anthraquinone based pyrimidine derivative as a probe for antioxidant and antidiabetic activity. J. Mol. Struct. 2021, 1227, 129668. [Google Scholar] [CrossRef]
- Available online: www.rcsb.org (accessed on 3 December 2023).
- Elhady, S.S.; Alshobaki, N.M.; Elfaky, M.A.; Koshak, A.E.; Alharbi, M.; Abdelhameed, R.F.; Darwish, K.M. Deciphering molecular aspects of potential α-glucosidase inhibitors within Aspergillus terreus: A computational odyssey of molecular docking-coupled dynamics simulations and pharmacokinetic profiling. Metabolites 2023, 13, 942. [Google Scholar] [CrossRef] [PubMed]




| Entry | Catalyst | Base | Solvent | % Yield |
|---|---|---|---|---|
| 1 | Pd(PPh3)2 | K2CO3 | Dioxane/water | 70 |
| Toluene/water | 16 | |||
| Dioxane | Unsuccessful | |||
| 2 | Pd(PPh3)2 | K2CO3 | Dioxane/water | 50 |
| Pd(dppf3)2 | Traces | |||
| Cu(OAC)2 | Unsuccessful | |||
| 3 | Pd(PPh3)2 | CH3CO2K | Dioxane/water | 10 |
| K2CO3 | 66 |
| Compound | IC50 Value (µm) |
|---|---|
| 4 | 7.26 |
| 6a | 8.38 |
| 6b | 5.14 |
| 6c | 5.15 |
| 6d | 7.06 |
| 6e | 9.01 |
| 6f | 58.56 |
| 6g | 5.56 |
| 6h | 5.20 |
| 6i | 7.40 |
| Acarbose | 200.1 |
| Compound | IC50 Value (µM) |
|---|---|
| 5 | 7.91 |
| 7a | 5.21 |
| 7b | 5.18 |
| 7c | 5.17 |
| 7d | 5.12 |
| 7e | 8.94 |
| 7f | 5.10 |
| 7g | 5.15 |
| 7h | 5.19 |
| 7i | 8.45 |
| Acarbose | 200.1 |
| Ester Derivatives | ∆G (kcal/mol) | Hydrazide Derivatives | ∆G (kcal/mol) |
|---|---|---|---|
| 5 | −6.70 | ||
| 6a | −6.87 | 7a | −8.13 |
| 6b | −7.33 | 7b | −8.68 |
| 6c | −6.97 | 7c | −8.71 |
| 6d | −6.87 | 7d | −8.61 |
| 6e | −6.07 | 7e | −7.31 |
| 6f | −6.8 | 7f | −8.73 |
| 6g | −6.67 | 7g | −8.77 |
| 6h | −6.92 | 7h | −8.18 |
| 6i | −6.06 | 7i | −7.92 |
| Acarbose | −5.57 |
| Ligand (∆G) | Type of Bonding | Interacting Amino Acids |
|---|---|---|
| 6b (−7.33) | H-bonding, π–donor H-bonding *, π alkyl | LYS A:227, ARG A:373, ARG A:373, ASP A:373 |
| 6c (−6.97) | H-bonding, π–alkyl, alkyl | LYS A:227, ARG A:373, GLN A:119, ARG A:373, ASN A:372 |
| 6g (−6.67) | H-bonding, π-sigma, | HIS A:123, LYS A:227, ARG A:373, HIS A:219 |
| 6h (−6.92) | Fluorine, π–sigma, amide–π–π stacked, π–alkyl | GLU A:168, GLU A:167, PRO A:167, ALA A:163, |
| Ligand (∆G) | Type of Bonding | Interacting Amino Acids |
|---|---|---|
| 7a (−8.13) | H-bonding, π–alkyl | ASN A:112, TYR A:507, GLN A:110, PRO A:82, ALA A:631 |
| 7b (−8.68) | H-bonding, π–alkyl, π–π stacked | ACN A:302, THR A:307, TPR A:310, TPR A:306 |
| 7c (−8.71) | π–alkyl, π–π stacked, attractive charge | TPR A:306, TPR A:310, TPR A:307, GLU A:323 |
| 7d (−8.61) | H-bonding, attractive charge, carbon–hydrogen bond | ACN A:309, THR A:307, ASP A:311, ASP A:303, TRP A:307 |
| 7f (−8.73) | H-bonding, attractive charge, π–π stacked | THR A:307, TYR A:367, TRP A:310, TRP A:306, TRP A:307, LEU A:298 |
| 7g (−8.77) | H-bonding, π–π stacked | THR A:307,ASN A:309, TPR A:306, TPR A:310 |
| 7h (−8.18) | H-bonding, π–sulfur, halogen bonding, π–π T-shaped | THR A:307, TYR A:308, TPR A:310, ASN A:278, ASP A:311, TPR A:306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafique, I.; Maqbool, T.; Rutjes, F.P.J.T.; Irfan, A.; Jardan, Y.A.B. Anti-Diabetic Activities and Molecular Docking Studies of Aryl-Substituted Pyrazolo[3,4-b]pyridine Derivatives Synthesized via Suzuki Cross-Coupling Reaction. Pharmaceuticals 2024, 17, 1326. https://doi.org/10.3390/ph17101326
Rafique I, Maqbool T, Rutjes FPJT, Irfan A, Jardan YAB. Anti-Diabetic Activities and Molecular Docking Studies of Aryl-Substituted Pyrazolo[3,4-b]pyridine Derivatives Synthesized via Suzuki Cross-Coupling Reaction. Pharmaceuticals. 2024; 17(10):1326. https://doi.org/10.3390/ph17101326
Chicago/Turabian StyleRafique, Iqra, Tahir Maqbool, Floris P. J. T. Rutjes, Ali Irfan, and Yousef A. Bin Jardan. 2024. "Anti-Diabetic Activities and Molecular Docking Studies of Aryl-Substituted Pyrazolo[3,4-b]pyridine Derivatives Synthesized via Suzuki Cross-Coupling Reaction" Pharmaceuticals 17, no. 10: 1326. https://doi.org/10.3390/ph17101326
APA StyleRafique, I., Maqbool, T., Rutjes, F. P. J. T., Irfan, A., & Jardan, Y. A. B. (2024). Anti-Diabetic Activities and Molecular Docking Studies of Aryl-Substituted Pyrazolo[3,4-b]pyridine Derivatives Synthesized via Suzuki Cross-Coupling Reaction. Pharmaceuticals, 17(10), 1326. https://doi.org/10.3390/ph17101326










