Prospective Audit and Feedback for Antimicrobial Treatment of Patients Receiving Renal Replacement Therapy in Community-Based University Hospitals: A before-and-after Study
Abstract
1. Introduction
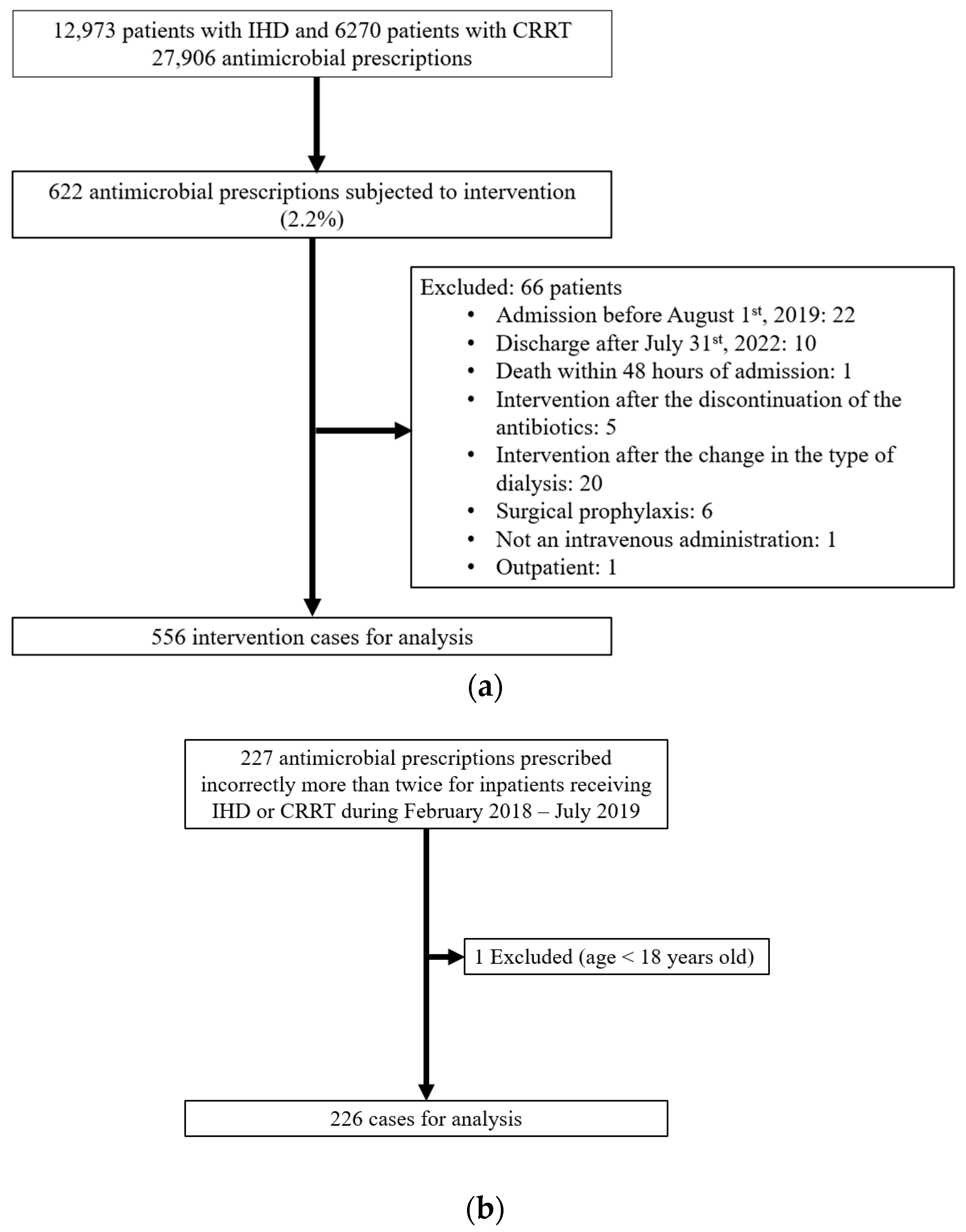
2. Results
2.1. Patient Characteristics
2.2. Outcomes
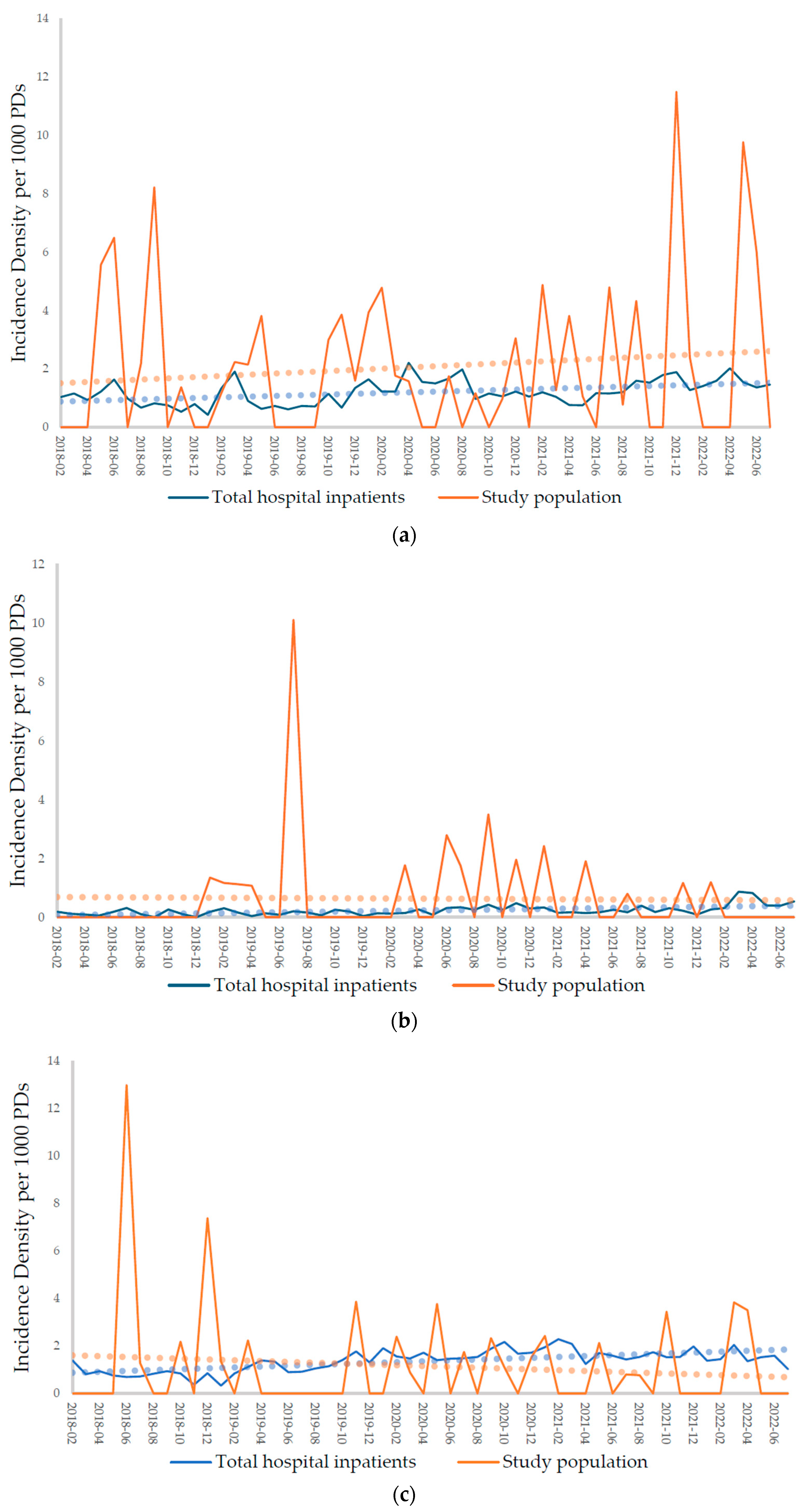
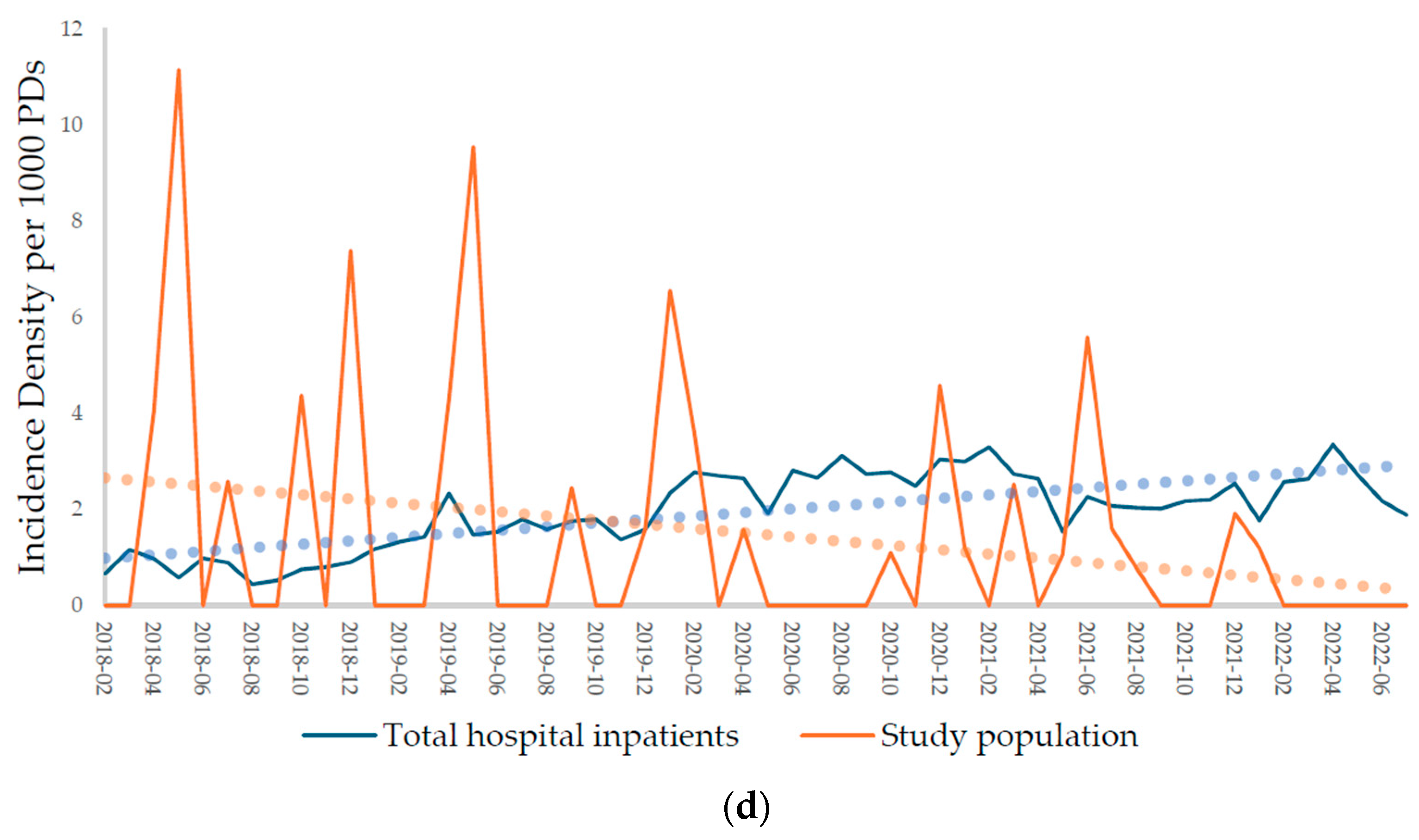
2.2.1. Incidences of Carbapenem-Resistant Acinetobacter baumannii (CRAB), Carbapenem-Resistant Enterobacterales (CRE), Methicillin-Resistant Staphylococcus aureus (MRSA), and Vancomycin-Resistant Enterococcus (VRE)
2.2.2. Comparison of Microbiological and Clinical Outcomes
2.3. Incorrect Antimicrobial Dosing Rate
3. Discussion
4. Materials and Methods
4.1. PAF Implementation
MDRO Screening Programs at the Hospitals
4.2. Study Design
4.3. Data Collection
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Spivak, E.S.; Cosgrove, S.E.; Srinivasan, A. Measuring appropriate antimicrobial use: Attempts at opening the black box. Clin. Infect. Dis. 2016, 63, 1–6. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2017; pp. 1–84. [Google Scholar]
- Howard, P.; Pulcini, C.; Levy Hara, G.; West, R.; Gould, I.; Harbarth, S.; Nathwani, D. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J. Antimicrob. Chemother. 2015, 70, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lye, D.C.; Chung, D.R.; Thamlikitkul, V.; Lu, M.; Wong, A.T.; Hsueh, P.-R.; Wang, H.; Cooper, C.; Wong, J.G. Antimicrobial stewardship capacity and manpower needs in the Asia Pacific. J. Glob. Antimicrob. Resist. 2021, 24, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.S.; Park, K.-H.; Kim, H.B.; Kim, S.-W.; Kim, B.; Moon, C.; Lee, M.S.; Yoon, Y.K.; Jeong, S.J.; Kim, Y.C. Core elements for implementing antimicrobial stewardship programs in Korean general hospitals. Infect. Chemother. 2022, 54, 637. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, M.; Moon, S.; Park, S.; Song, K.; Lee, H.; Park, J.; Lee, M.; Choi, S.; Yeom, J. Current status of antimicrobial stewardship programmes in Korean hospitals: Results of a 2018 nationwide survey. J. Hosp. Infect. 2020, 104, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Chang, H.-H.; Kim, B.; Moon, C.; Lee, M.S.; Kim, J.Y.; Jung, D.S.; Kim, S.-W.; Moon, S.M.; Kim, E.S. Human resources required for antimicrobial stewardship activities for hospitalized patients in Korea. Infect. Control Hosp. Epidemiol. 2020, 41, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.W.; Wu, J.E.; Yeo, C.L.; Chan, D.; Hsu, L.Y. Antimicrobial stewardship: A review of prospective audit and feedback systems and an objective evaluation of outcomes. Virulence 2013, 4, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Avdic, E.; Keenan, J.F.; Zhao, Y.; Anand, G.; Cooper, J.; Dezube, R.; Hsu, S.; Cosgrove, S.E. What is the more effective antibiotic stewardship intervention: Preprescription authorization or postprescription review with feedback? Clin. Infect. Dis. 2017, 64, 537–543. [Google Scholar] [PubMed]
- Lea-Henry, T.N.; Carland, J.E.; Stocker, S.L.; Sevastos, J.; Roberts, D.M. Clinical pharmacokinetics in kidney disease: Fundamental principles. Clin. J. Am. Soc. Nephrol. 2018, 13, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Xia, Y.; Chu, Y.; Zhong, H.; Li, J.; Liang, P.; Bu, Y.; Zhao, R.; Liao, Y. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front. Pharmacol. 2020, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Pea, F. Antimicrobial dose reduction in continuous renal replacement therapy: Myth or real need? A practical approach for guiding dose optimization of novel antibiotics. Clin. Pharmacokinet. 2021, 60, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Nalder, M.; Buising, K.; Pefanis, A.; Ooi, K.Y.; Pedagogos, E.; Nelson, C.; Kirkpatrick, C.M.; Kong, D.C. Patterns of use and appropriateness of antibiotics prescribed to patients receiving haemodialysis: An observational study. BMC Nephrol. 2017, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Rha, B. Vital signs: Health disparities in hemodialysis-associated staphylococcus aureus bloodstream infections—United States, 2017–2020. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, L.N.; Puls, D.L.; Mackay, K.; Forrest, G.N. Management of gram-positive coccal bacteremia and hemodialysis. Am. J. Kidney Dis. 2011, 57, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.J.; Decloe, M.; Gill, S.; Ho, G.; McCready, J.; Powis, J. Every antibiotic, every day: Maximizing the impact of prospective audit and feedback on total antibiotic use. PLoS ONE 2017, 12, e0178434. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.P.; Catrou, P.G.; Christie, J.D.; Young, P.D.; Polk, R.E. Reduction in broad-spectrum antimicrobial use associated with no improvement in hospital antibiogram. J. Antimicrob. Chemother. 2004, 53, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.J.; Majers, J.; Healy, R.; Jones, P.B.; Butler, A.M.; Snow, G.; Forsyth, S.; Lopansri, B.K.; Ford, C.D.; Hoda, D. Antimicrobial stewardship in a hematological malignancy unit: Carbapenem reduction and decreased vancomycin-resistant Enterococcus infection. Clin. Infect. Dis. 2020, 71, 960–967. [Google Scholar] [CrossRef] [PubMed]
- DeLisle, S.; Perl, T.M. Vancomycin-resistant enterococci: A road map on how to prevent the emergence and transmission of antimicrobial resistance. Chest 2003, 123, 504S–518S. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.T.; Rohde, A.M.; Schwab, F.; Märtin, N.; Kipnis, M.; Boldt, A.-C.; Behnke, M.; Denkel, L.A.; Kola, A.; Zweigner, J. Prevalence and risk factors of colonisation with vancomycin-resistant Enterococci faecium upon admission to Germany’s largest university hospital. GMS Hyg. Infect. Control 2021, 16, Doc06. [Google Scholar] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Seah, V.X.F.; Ong, R.Y.L.; Lim, A.S.Y.; Chong, C.Y.; Tan, N.W.H.; Thoon, K.C. Impact of a carbapenem antimicrobial stewardship program on patient outcomes. Antimicrob. Agents Chemother. 2017, 61, e00736-17. [Google Scholar] [CrossRef] [PubMed]
- Morrill, H.J.; Caffrey, A.R.; Gaitanis, M.M.; LaPlante, K.L. Impact of a prospective audit and feedback antimicrobial stewardship program at a veterans affairs medical center: A six-point assessment. PLoS ONE 2016, 11, e0150795. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, A.; Yao, K.; Murakami, S.; Tagashira, Y.; Hasegawa, S.; Honda, H. Barriers to adherence to antimicrobial stewardship postprescription review and feedback for broad-spectrum antimicrobial agents: A nested case-control study. Open Forum Infect. Dis. 2020, 7, ofaa298. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E.; Seo, S.K.; Bolon, M.K.; Sepkowitz, K.A.; Climo, M.W.; Diekema, D.J.; Speck, K.; Gunaseelan, V.; Noskin, G.A.; Herwaldt, L.A. Evaluation of postprescription review and feedback as a method of promoting rational antimicrobial use: A multicenter intervention. Infect. Control Hosp. Epidemiol. 2012, 33, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; Nisenbaum, R.; Brown, K.A.; Chan, A.; Downing, M. Antibiotics: Easier to start than to stop? Predictors of antimicrobial stewardship recommendation acceptance. Clin. Microbiol. Infect. 2020, 26, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Leone, M.; Madach, K.; Martin, C.; Einav, S. Antibiotic therapy in the critically ill-expert opinion of the Intensive Care Medicine Scientific Subcommittee of the European Society of Anaesthesiology. Eur. J. Anaesthesiol. EJA 2017, 34, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Bahk, H.; Lee, Y. Epidemiology of carbapenem-resistant Enterobacteriaceae in Korea between 2018 and 2019. Public Health Wkly. Rep. 2021, 14, 413–420. [Google Scholar]
- Son, K.J.; Kim, Y.A.; Park, Y.S. The trend of Clostridioides difficile infection in Korean hospitals with the analysis of nationwide sample cohort. Adv. Precis. Med. 2022, 7, 1. [Google Scholar] [CrossRef]
- Kim, J.; Bae, S.; Lee, S.; Yoo, J. Trends of antimicrobial resistance rates of major clinical pathogens isolated from general hospitals in Korea in 2016–2019: Results from Kor-GLASS. Public Health Wkly. Rep. 2021, 14, 2007–2024. [Google Scholar]
- Yoon, Y.K.; Kwon, K.T.; Jeong, S.J.; Moon, C.; Kim, B.; Kiem, S.; Kim, H.-S.; Heo, E.; Kim, S.-W.; Diseases, K.S.O.I. Guidelines on implementing antimicrobial stewardship programs in Korea. Infect. Chemother. 2021, 53, 617. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, S.H. Current status of multidrug-resistant bacteria. J. Korean Med. Assoc. 2022, 65, 468–477. [Google Scholar] [CrossRef]
- Choi, S.-M.; Lee, D.-G. Principles of selecting appropriate antimicrobial agents. J. Korean Med. Assoc. 2019, 62, 335–344. [Google Scholar] [CrossRef]
- Vonesh, E.F.; Snyder, J.J.; Foley, R.N.; Collins, A.J. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 2004, 66, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-R.; Zhu, J.-M.; Jiang, J.; Ding, X.-Q.; Fang, Y.; Shen, B.; Liu, Z.-H.; Zou, J.-Z.; Liu, L.; Wang, C.-S. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine 2015, 94, e2025. [Google Scholar] [CrossRef] [PubMed]
- Burillo, A.; Muñoz, P.; Bouza, E. Risk stratification for multidrug-resistant Gram-negative infections in ICU patients. Curr. Opin. Infect. Dis. 2019, 32, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [PubMed]
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P. Update of the treatment of nosocomial pneumonia in the ICU. Crit. Care 2020, 24, 383. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Villodres, Á.; Martín-Gandul, C.; Peñalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and risk factors for multidrug-resistant organisms colonization in long-term care facilities around the world: A review. Antibiotics 2021, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Hamed, K.H.; Rao, A.R. A modified Mann-Kendall trend test for autocorrelated data. J. Hydrol. 1998, 204, 182–196. [Google Scholar] [CrossRef]


| Characteristics | Before-PAF (n = 226) | After-PAF (n = 556) | p |
|---|---|---|---|
| Mean age: years (SD) | 67.4 (12.6) | 70.7 (12.5) | 0.01 |
| Age group: n (%) | <0.01 | ||
| ≤39 | 8 (3.5) | 4 (0.7) | |
| 40–49 | 10 (4.4) | 37 (6.7) | |
| 50–59 | 41 (18.1) | 61 (11.0) | |
| 60–69 | 58 (25.7) | 132 (23.7) | |
| 70–79 | 75 (33.2) | 174 (31.3) | |
| 80–89 | 26 (11.5) | 127 (22.8) | |
| ≥90 | 8 (3.5) | 21 (3.8) | |
| Sex: n (%) | 0.81 | ||
| Male | 138 (61.1) | 345 (62.1) | |
| Female | 88 (38.9) | 211 (37.9) | |
| Type of RRT: n (%) | 0.21 | ||
| IHD | 170 (75.2) | 392 (70.5) | |
| CRRT | 56 (24.8) | 164 (29.5) | |
| Antibiotic class: n (%) | <0.01 | ||
| Penicillin | 1 (0.4) | 2 (0.4) | |
| Cephalosporins | |||
| 1st CEPs | 8 (3.5) | 42 (7.6) | |
| 2nd CEPs | 4 (1.8) | 10 (1.8) | |
| 3rd CEPs | 12 (5.3) | 20 (3.6) | |
| 4th CEPs | 25 (11.1) | 23 (4.1) | |
| Carbapenems | 37 (16.4) | 165 (29.7) | |
| BL/BLIs | 67 (29.6) | 140 (25.2) | |
| Quinolones | 19 (8.4) | 70 (12.6) | |
| Nitroimidazole | |||
| Metronidazole | 11 (4.9) | 11 (2.0) | |
| Polymixins | |||
| Colistin | 42 (18.6) | 73 (13.1) | |
| CCI: n (%) | <0.01 | ||
| ≤2 | 134 (59.3) | 235 (42.3) | |
| 3 or 4 | 72 (31.9) | 194 (34.9) | |
| ≥5 | 20 (8.8) | 127 (22.8) | |
| Previous admission within 30 days: n (%) | 0.56 | ||
| N | 176 (77.9) | 443 (79.7) | |
| Y | 50 (22.1) | 113 (20.3) | |
| Mean (SD) | Total Hospital Inpatients | Study Population | ||
|---|---|---|---|---|
| Before-PAF | After-PAF | Before-PAF | After-PAF | |
| CRAB | 0.95 (0.37) | 1.33 (0.38) | 1.85 (2.49) | 2.17 (2.73) |
| CRE | 0.14 (0.09) | 0.28 (0.18) | 0.82 (2.30) | 0.53 (0.95) |
| MRSA | 1.12 (0.40) | 1.64 (0.28) | 1.34 (2.76) | 1.02 (1.34) |
| VRE | 1.10 (0.47) | 2.38 (0.51) | 2.41 (3.54) | 1.04 (1.66) |
| Outcome 1 | Crude IRR (95% CI) | p | Adjusted IRR (95% CI) | p |
|---|---|---|---|---|
| Microbiological | ||||
| CRAB | 1.15 (0.66–2.00) | 0.63 | 0.99 (0.56–1.76) | 0.99 |
| CRE | 1.30 (0.48–3.55) | 0.61 | 1.38 (0.50–3.87) | 0.54 |
| MRSA | 0.56 (0.28–1.09) | 0.09 | 0.56 (0.29–1.06) | 0.08 |
| VRE | 0.48 (0.29–0.82) | <0.01 | 0.53 (0.31–0.93) | 0.03 |
| CDI | 1.29 (0.67–2.46) | 0.45 | 1.49 (0.76–2.92) | 0.24 |
| Clinical | ||||
| All-cause hospital mortality | 0.77 (0.61–0.98) | 0.03 | 0.70 (0.55–0.90) | 0.01 |
| Readmission within 30 days | 1.33 (0.83–2.14) | 0.24 | 1.25 (0.76–2.05) | 0.24 |
| LOS longer than 30 days | 0.88 (0.71–1.09) | 0.25 | 0.88 (0.70–1.09) | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, N.; Bae, J.; Nam, S.Y.; Bae, J.Y.; Jun, K.-I.; Kim, J.-H.; Kim, C.-J.; Kim, K.; Kim, S.A.; Choi, H.J.; et al. Prospective Audit and Feedback for Antimicrobial Treatment of Patients Receiving Renal Replacement Therapy in Community-Based University Hospitals: A before-and-after Study. Pharmaceuticals 2024, 17, 854. https://doi.org/10.3390/ph17070854
Park N, Bae J, Nam SY, Bae JY, Jun K-I, Kim J-H, Kim C-J, Kim K, Kim SA, Choi HJ, et al. Prospective Audit and Feedback for Antimicrobial Treatment of Patients Receiving Renal Replacement Therapy in Community-Based University Hospitals: A before-and-after Study. Pharmaceuticals. 2024; 17(7):854. https://doi.org/10.3390/ph17070854
Chicago/Turabian StylePark, Namgi, Jiyeon Bae, Soo Yeon Nam, Ji Yun Bae, Kang-Il Jun, Jeong-Han Kim, Chung-Jong Kim, Kyunghee Kim, Sun Ah Kim, Hee Jung Choi, and et al. 2024. "Prospective Audit and Feedback for Antimicrobial Treatment of Patients Receiving Renal Replacement Therapy in Community-Based University Hospitals: A before-and-after Study" Pharmaceuticals 17, no. 7: 854. https://doi.org/10.3390/ph17070854
APA StylePark, N., Bae, J., Nam, S. Y., Bae, J. Y., Jun, K.-I., Kim, J.-H., Kim, C.-J., Kim, K., Kim, S. A., Choi, H. J., & Rhie, S. J. (2024). Prospective Audit and Feedback for Antimicrobial Treatment of Patients Receiving Renal Replacement Therapy in Community-Based University Hospitals: A before-and-after Study. Pharmaceuticals, 17(7), 854. https://doi.org/10.3390/ph17070854






