Euiin-Tang Attenuates Obesity-Induced Asthma by Resolving Metaflammation
Abstract
1. Introduction
2. Results
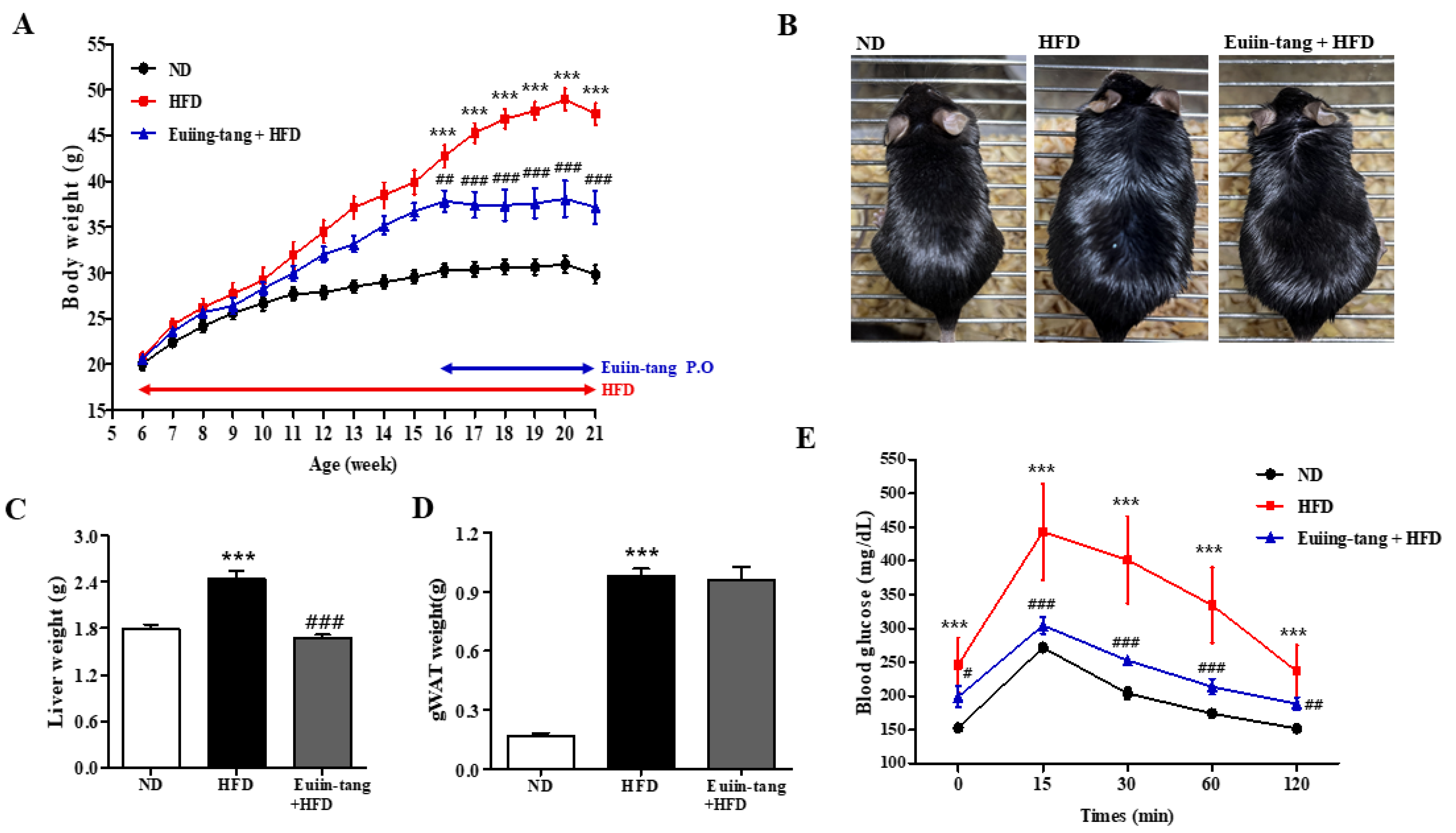
2.1. Euiin-Tang Suppressed HFD-Induced Obesity in C57BL/6 Mice
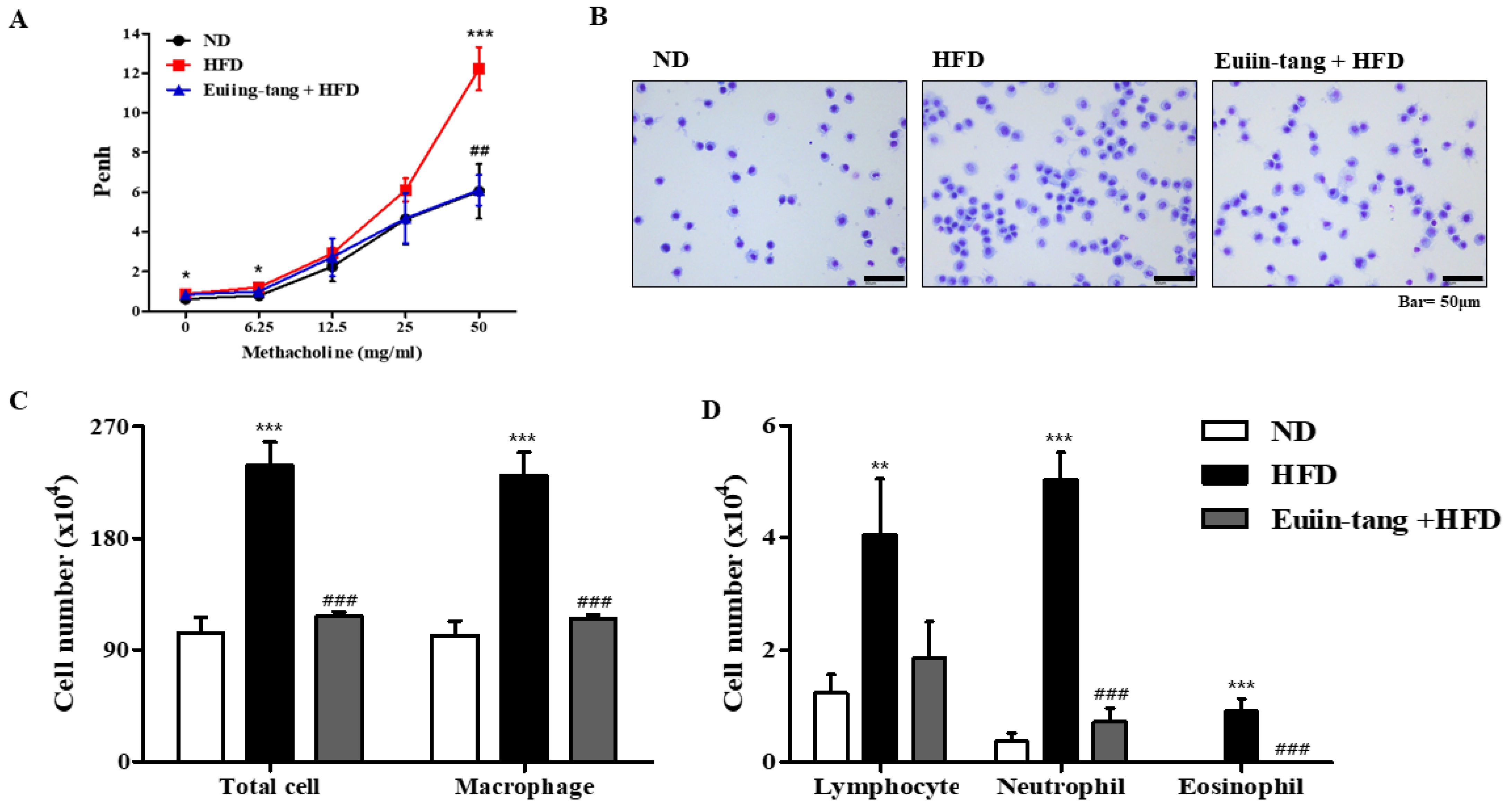
2.2. Euiin-Tang Suppressed AHR and Immune Cell Infiltration in BALF of C57BL/6 Mice
2.3. Euiin-Tang Suppressed Pathological Changes in the Lungs of C57BL/6 Mice
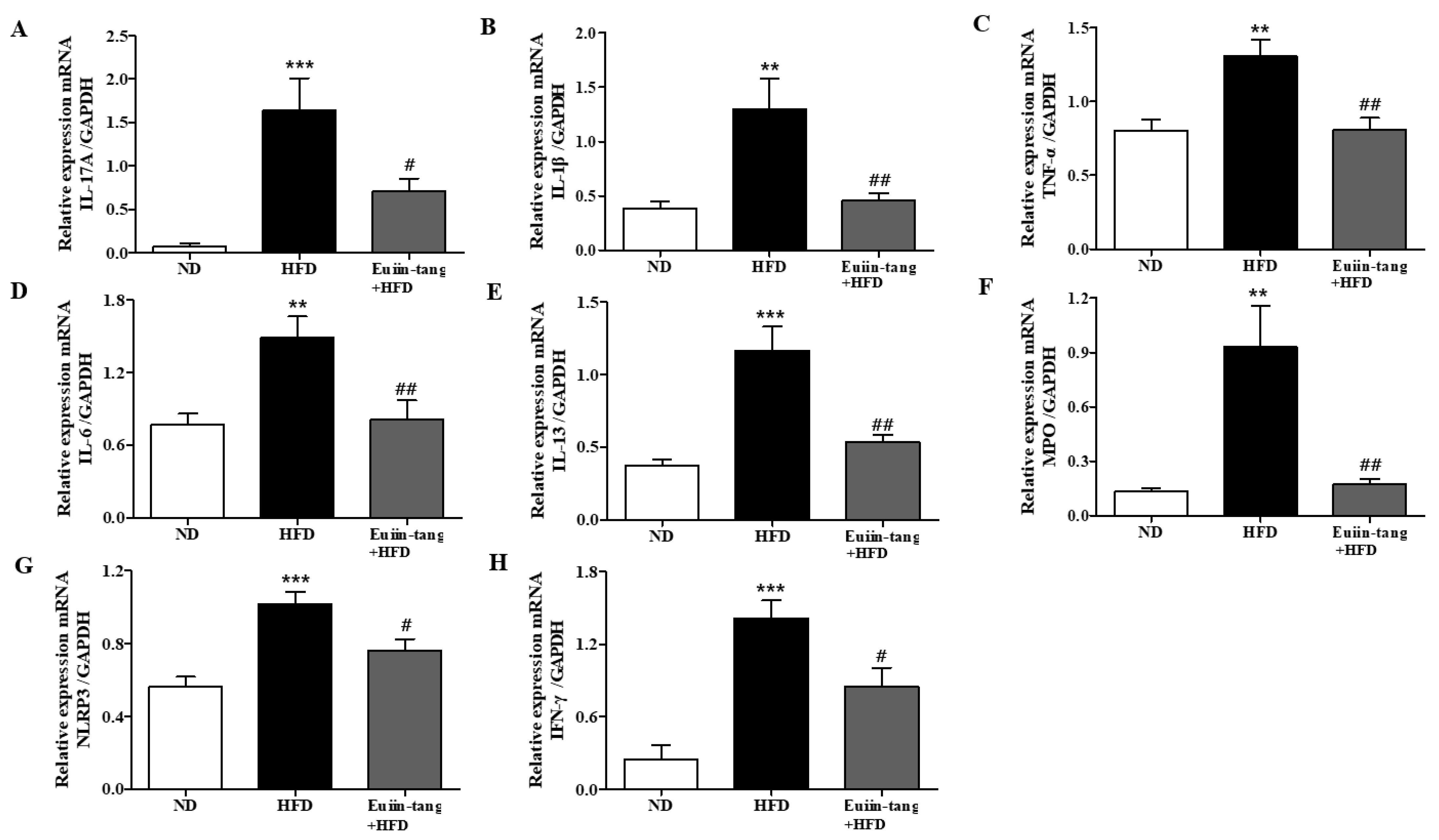
2.4. Euiin-Tang Suppressed Inflammatory Cytokine Levels in the Lungs and Gonadal White Adipose Tissue in C57BL/6 Mice
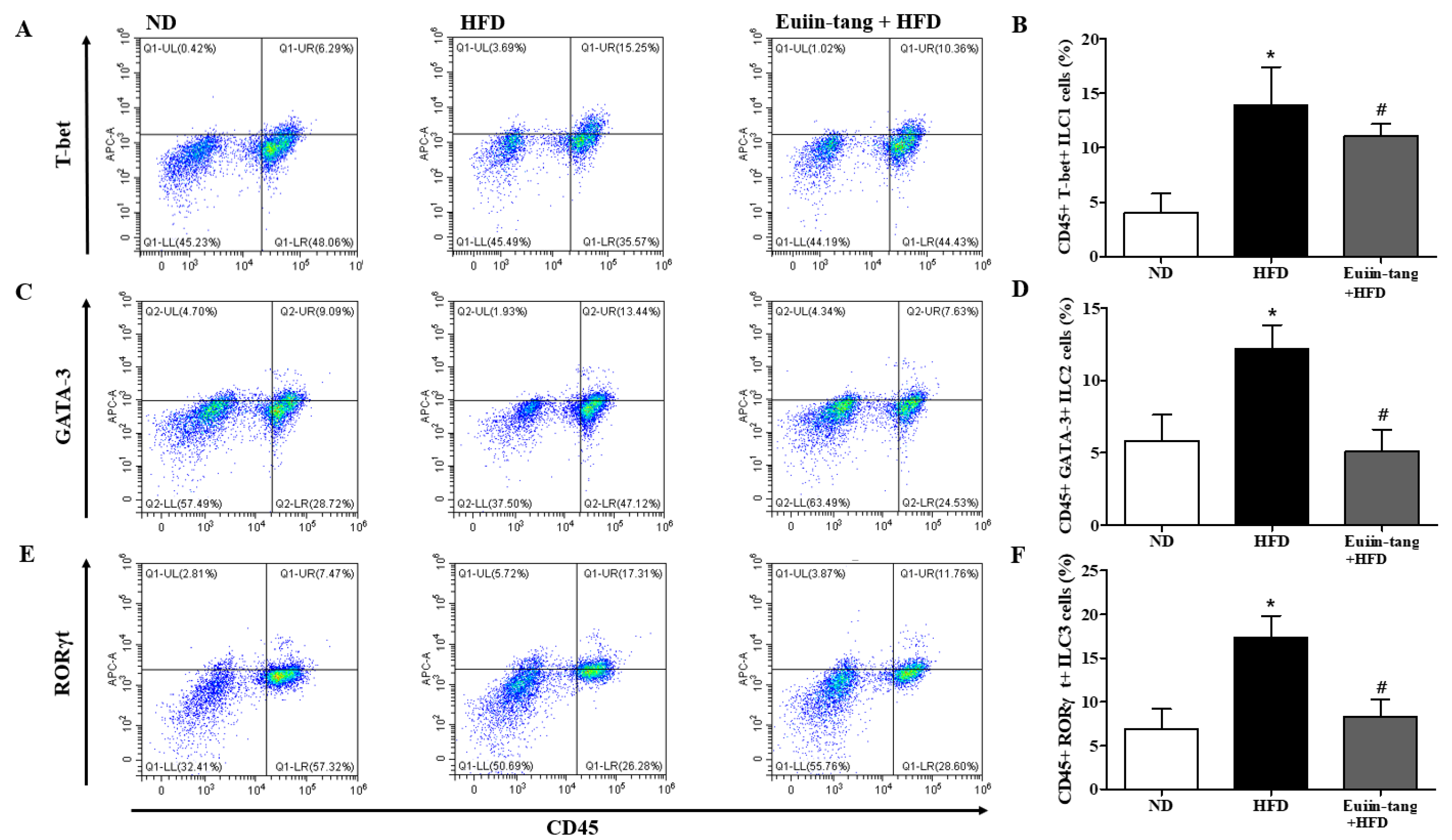
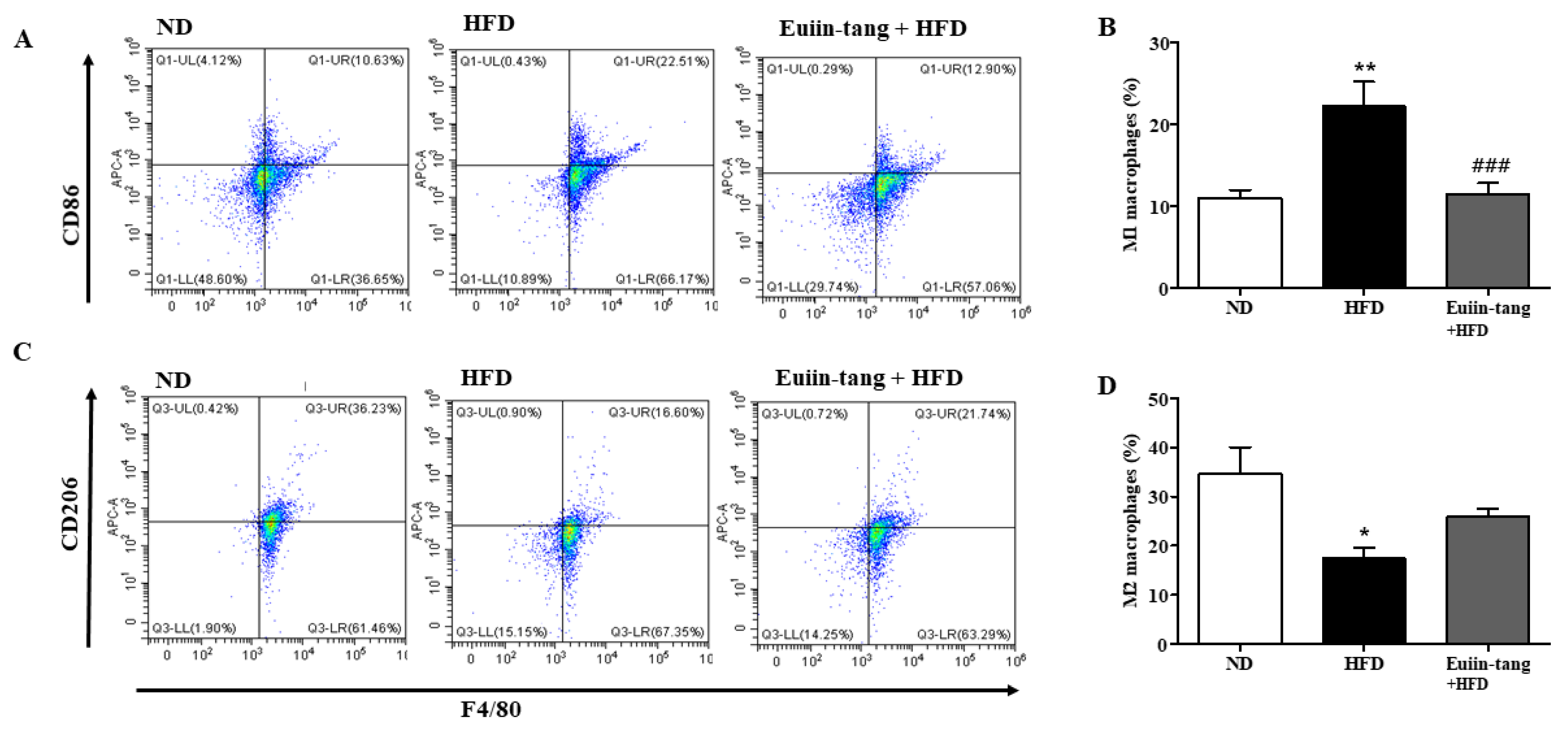
2.5. Euiin-Tang Suppressed ILCs and Increased Anti-Inflammatory M2 Macrophages in the Lungs of Mice
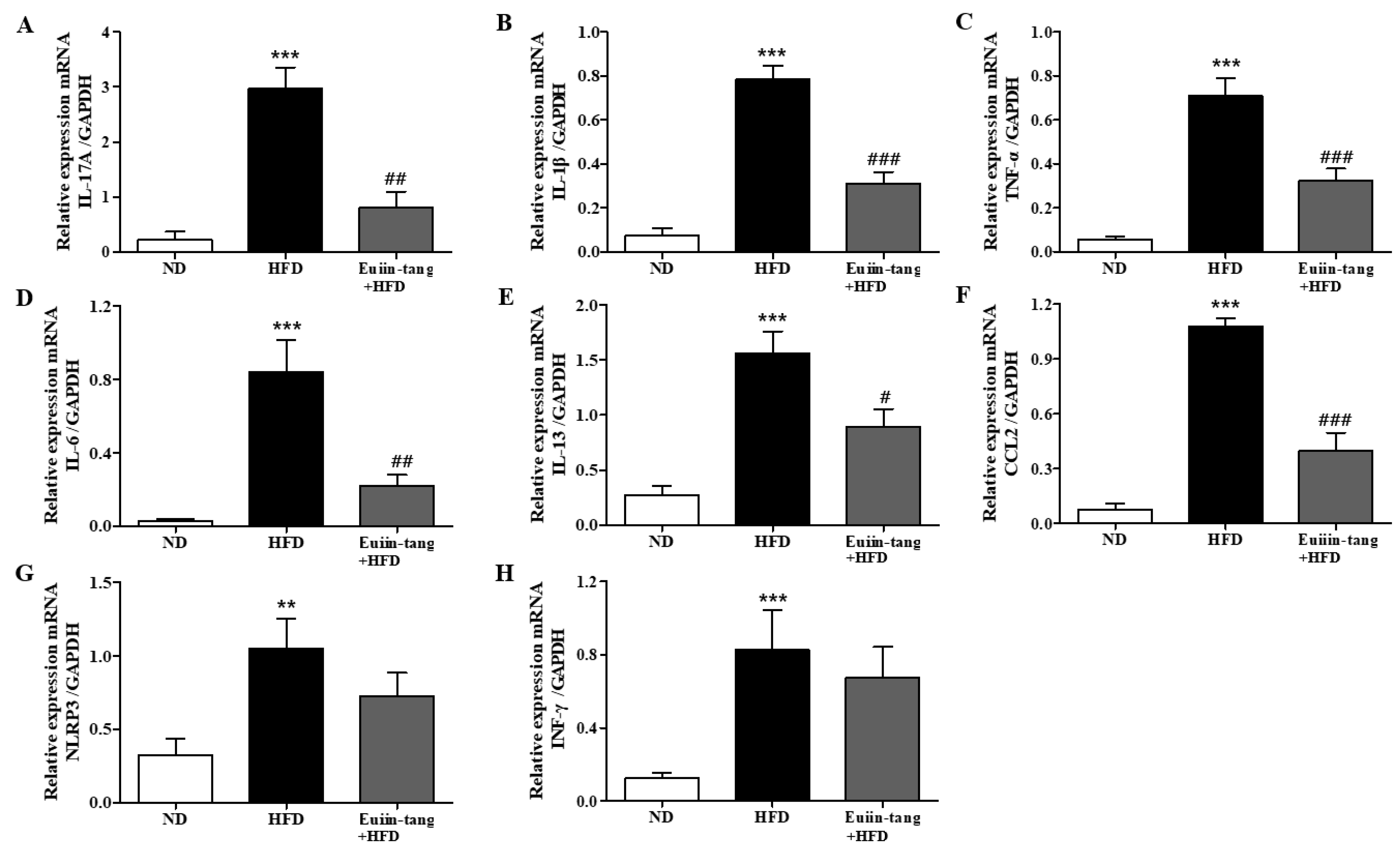
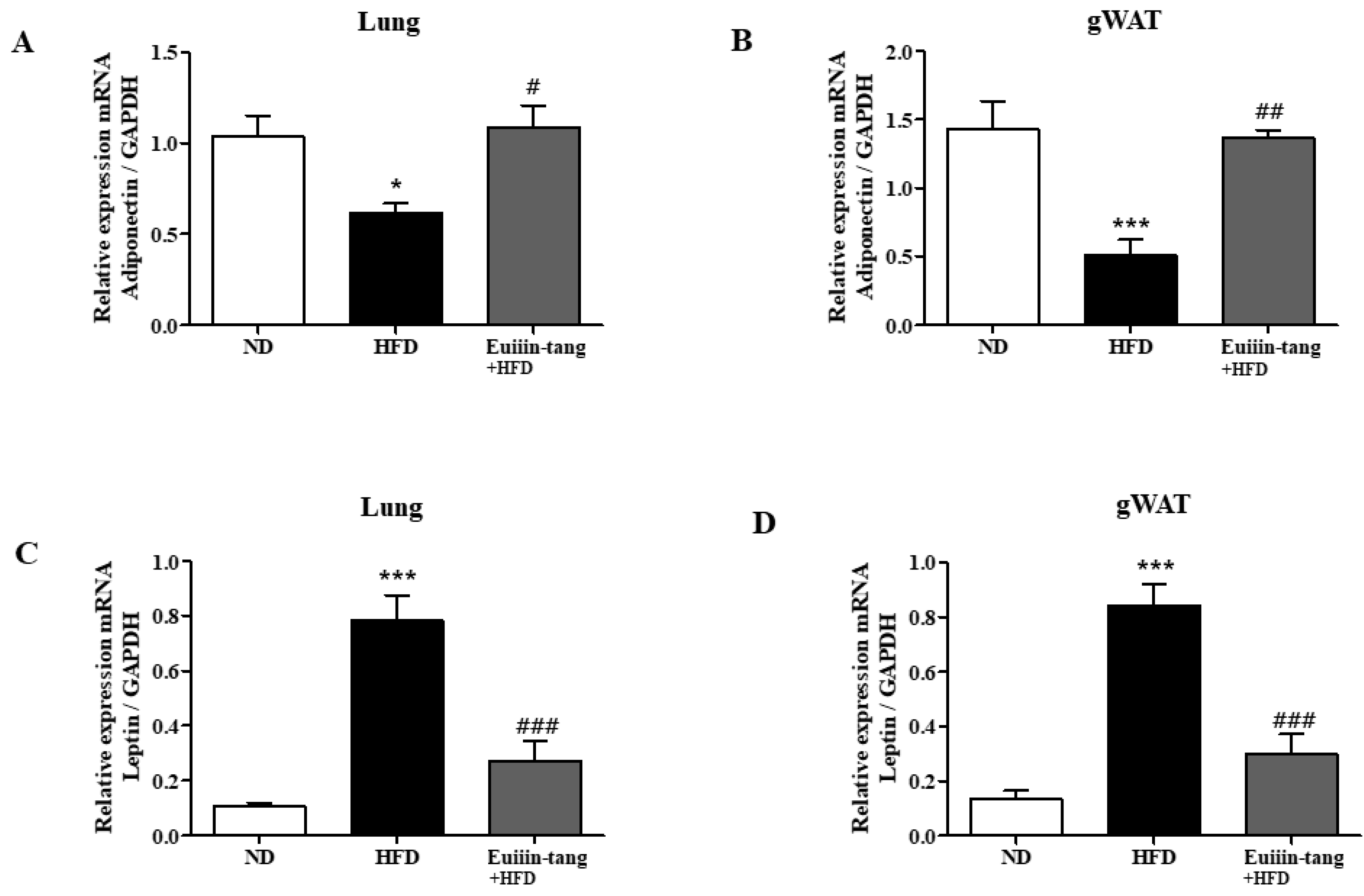
2.6. Euiin-Tang Increased Adiponectin Levels and Suppressed Leptin Levels in the Lungs and Gonadal White Adipose Tissue of Mice
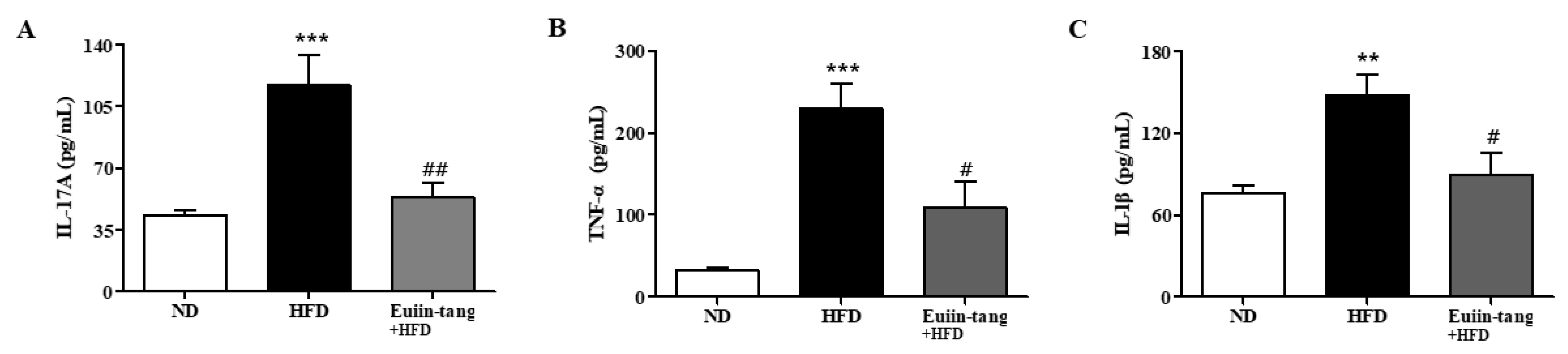
2.7. Euiin-Tang Suppressed Inflammatory Cytokine Levels in the BALF of Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mouse Strain
4.3. High-Fat Diet (HFD) Feeding
4.4. Oral Glucose Tolerance Test
4.5. Measuring Airway Hyperresponsiveness (AHR) to Methacholine
4.6. Bronchoalveolar Lavage Fluid (BALF) Cell Counting and Analysis
4.7. Histological Examination of the Lung
4.8. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
4.9. Flow Cytometry
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Chapter 1—Worldwide epidemic of obesity. In Obesity and Obstetrics, 2nd ed.; Mahmood, T.A., Arulkumaran, S., Chervenak, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- Pathak, M.P.; Patowary, P.; Goyary, D.; Das, A.; Chattopadhyay, P. β-caryophyllene ameliorated obesity-associated airway hyperresponsiveness through some non-conventional targets. Phytomedicine 2021, 89, 153610. [Google Scholar] [CrossRef] [PubMed]
- Peerboom, S.; Graff, S.; Seidel, L.; Paulus, V.; Henket, M.; Sanchez, C.; Guissard, F.; Moermans, C.; Louis, R.; Schleich, F. Predictors of a good response to inhaled corticosteroids in obesity-associated asthma. Biochem. Pharmacol. 2020, 179, 113994. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liu, L.; Han, L.; Yu, Y. Leptin promotes IL-18 secretion by activating the NLRP3 inflammasome in RAW 264.7 cells. Mol. Med. Rep. 2017, 16, 9770–9776. [Google Scholar] [CrossRef][Green Version]
- Salvator, H.; Grassin-Delyle, S.; Brollo, M.; Couderc, L.J.; Abrial, C.; Victoni, T.; Naline, E.; Devillier, P. Adiponectin Inhibits the Production of TNF-α, IL-6 and Chemokines by Human Lung Macrophages. Front. Pharmacol. 2021, 12, 718929. [Google Scholar] [CrossRef]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’Oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Pathak, M.P.; Pathak, K.; Saikia, R.; Gogoi, U. Herbal medicine for the treatment of obesity-associated asthma: A comprehensive review. Front. Pharmacol. 2023, 14, 1186060. [Google Scholar] [CrossRef]
- Kim, S.-J.; Seo, Y.-H.; Lee, H.-S.; Chang, H.-K.; Cho, J.-H.; Kim, K.-W.; Song, M.-Y. Research trends of herbal medicines for obesity: Mainly since 2015 to 2019. J. Korean Med. Rehabil. 2020, 30, 89–103. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Ryu, E.-K. The change of weight loss of oriental obesity treatment. J. Korean Med. Obes. Res. 2009, 9, 53–58. [Google Scholar]
- Kim, K.-S.; Song, J.-C. Effects of Chegameyiin-tang extract on the change of the weight, tissue in epididymal fat, blood, leptin and uncoupled protein in visceral fat of obesity rats induced by high fat diet. J. Korean Med. Obes. Res. 2001, 1, 85–100. [Google Scholar]
- Park, K.; Ho, L.H.; Kyung, S.Y. Anti-inflammation and anti-obesity effects of Euiiin-tang granules on high fat diet-induced obese C57BL/6J mice. J. Korean Med. Rehabil. 2012, 22, 47–66. [Google Scholar]
- Jovicic, N.; Jeftic, I.; Jovanovic, I.; Radosavljevic, G.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS ONE 2015, 10, e0134089. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, H.J.; Chang, Y.J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.A. Obesity, airway hyperresponsiveness, and inflammation. J. Appl. Physiol. 2010, 108, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.A.; Johnston, R.A. Obesity and asthma. Pharmacol. Ther. 2006, 110, 83–102. [Google Scholar] [CrossRef]
- Hsu, A.T.; Gottschalk, T.A.; Tsantikos, E.; Hibbs, M.L. The Role of Innate Lymphoid Cells in Chronic Respiratory Diseases. Front. Immunol. 2021, 12, 733324. [Google Scholar] [CrossRef] [PubMed]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, M.; Kim, S.J.; Yoo, H.J.; Kim, S.H.; Park, H.S. Metabolic shift favoring C18:0 ceramide accumulation in obese asthma. Allergy 2020, 75, 2858–2866. [Google Scholar] [CrossRef]
- Chong, L.; Liu, L.; Zhu, L.; Li, H.; Shao, Y.; Zhang, H.; Yu, G. Expression Levels of Predominant Adipokines and Activations of STAT3, STAT6 in an Experimental Mice Model of Obese Asthma. Iran. J. Allergy Asthma Immunol. 2019, 18, 62–71. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.; Li, Y.-Z.; Zhao, Z.-Y.; Liu, C.-X. Research and application of adlay in medicinal field. Chin. Herb. Med. 2017, 9, 126–133. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, J.; Chen, J.; Pu, X.; Li, X.; Yang, X.; Yang, L.; Ding, Y.; Nong, M.; Zhang, S.; et al. Actional Mechanisms of Active Ingredients in Functional Food Adlay for Human Health. Molecules 2022, 27, 4808. [Google Scholar] [CrossRef]
- Zhao, L.; Rao, S.; Zhu, X.; Liu, S.; Tao, Q.; Yang, X.; Zhu, Y.; Hu, J. Coicis Semen formula treating monosodium glutamate-induced obesity in mice by alleviating hypothalamic injury. Food Agric. Immunol. 2020, 31, 84–99. [Google Scholar] [CrossRef]
- Kim, S.O.; Yun, S.J.; Jung, B.; Lee, E.H.; Hahm, D.H.; Shim, I.; Lee, H.J. Hypolipidemic effects of crude extract of adlay seed (Coix lachrymajobi var. mayuen) in obesity rat fed high fat diet: Relations of TNF-a and leptin mRNA expressions and serum lipid levels. Life Sci. 2004, 75, 1391–1404. [Google Scholar] [CrossRef]
- Chang, M.S.; Oh, M.S.; Jung, K.J.; Park, S.; Choi, S.B.; Ko, B.-S.; Park, S.K. Effects of Okchun-San, a herbal formulation, on blood glucose levels and body weight in a model of Type 2 diabetes. J. Ethnopharmacol. 2006, 103, 491–495. [Google Scholar] [CrossRef]
- Kim, S.O.; Yun, S.J.; Lee, E.H. The water extract of adlay seed (Coix lachrymajobi var. mayuen) exhibits anti-obesity effects through neuroendocrine modulation. Am. J. Chin. Med. 2007, 35, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.T.; Nam Trung, T.; Bich Thu, N.; Van On, T.; Hai Nam, N.; Van Men, C.; Thi Phuong, T.; Bae, K. Adlay seed extract (Coix lachryma-jobi L.) decreased adipocyte differentiation and increased glucose uptake in 3T3-L1 cells. J. Med. Food 2010, 13, 1331–1339. [Google Scholar] [CrossRef]
- Jang, J.-W.; Lim, D.-W.; Chang, J.-U.; Kim, J.-E. The Combination of Ephedrae herba and Coicis semen in Gambihwan Attenuates Obesity and Metabolic Syndrome in High-Fat Diet–Induced Obese Mice. Evid. Based Complement. Altern. Med. 2018, 2018, 5614091. [Google Scholar] [CrossRef]
- Song, Y.-K.; Cha, Y.-Y.; Ko, S.-G. Analysis of the obesity-related research for each constituent herb of Euiiin-tang. J. Soc. Korean Med. Obes. Res. 2014, 14, 72–79. [Google Scholar] [CrossRef][Green Version]
- Yu, G.R.; Kim, J.E.; Lim, D.W.; Park, W.H. The combination of Ephedrae herba and coixol from Coicis semen attenuate adiposity via glucocorticoid receptor regulation. Sci. Rep. 2023, 13, 20324. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Williams, E.J.; Negewo, N.A.; Baines, K.J. Role of the NLRP3 inflammasome in asthma: Relationship with neutrophilic inflammation, obesity, and therapeutic options. J. Allergy Clin. Immunol. 2021, 147, 2060–2062. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Li, Q.; Scott, H.A.; Rutting, S.; Berthon, B.S.; Gibson, P.G.; Hansbro, P.M.; Williams, E.; Horvat, J.; Simpson, J.L.; et al. Saturated fatty acids, obesity, and the nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome in asthmatic patients. J. Allergy Clin. Immunol. 2019, 143, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Gainsford, T.; Willson, T.A.; Metcalf, D.; Handman, E.; McFarlane, C.; Ng, A.; Nicola, N.A.; Alexander, W.S.; Hilton, D.J. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 14564–14568. [Google Scholar] [CrossRef]
- Aleffi, S.; Petrai, I.; Bertolani, C.; Parola, M.; Colombatto, S.; Novo, E.; Vizzutti, F.; Anania, F.A.; Milani, S.; Rombouts, K.; et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology 2005, 42, 1339–1348. [Google Scholar] [CrossRef]
- Sánchez-Ortega, H.; Jiménez-Cortegana, C.; Novalbos-Ruiz, J.P.; Gómez-Bastero, A.; Soto-Campos, J.G.; Sánchez-Margalet, V. Role of Leptin as a Link between Asthma and Obesity: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 24, 546. [Google Scholar] [CrossRef]
- Machado, M.E.; Porto, L.C.; Alves Galvão, M.G.; Sant’Anna, C.C.; Lapa, E.S.J.R. SNPs, adipokynes and adiposity in children with asthma. J. Asthma 2023, 60, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Cui, X.; Qualls, C.; Beckett, W.S.; Gross, M.D.; Steffes, M.W.; Smith, L.J.; Jacobs, D.R., Jr. Association between asthma and serum adiponectin concentration in women. Thorax 2008, 63, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ran, N.; Xiong, L.; Wang, G.; Guan, X.; Wang, Z.; Guo, Y.; Pang, Z.; Fang, K.; Lu, J.; et al. Obesity-Related Asthma: Immune Regulation and Potential Targeted Therapies. J. Immunol. Res. 2018, 2018, 1943497. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.C.; Chang, B.H.; Tien, H.H.; Cai, Y.L.; Fan, Y.C.; Chen, W.J.; Chu, H.F.; Chen, Y.H.; Huang, C. Synbiotic Intervention with an Adlay-Based Prebiotic and Probiotics Improved Diet-Induced Metabolic Disturbance in Mice by Modulation of the Gut Microbiota. Nutrients 2021, 13, 3161. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, F.; Zhang, X. Structural modulation of gut microbiota reveals Coix seed contributes to weight loss in mice. Appl. Microbiol. Biotechnol. 2019, 103, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.; Ju, Y.; Park, S.; Lee, M.; Kim, H.; Kim, H.; Ko, B. Effect of insulin-like action and insulin sensitizing on 3T3-L1 adipocytes from coicis semen. J. Korean Orient. Med. 2002, 23, 83–91. [Google Scholar]
- Takahashi, M.; Konno, C.; Hikino, H. Isolation and Hypoglycemic Activity of Coixans A, B and C, Glycans of Coix lachryma-jobi var. ma-yuen Seeds1. Planta Med. 1986, 52, 64–65. [Google Scholar] [CrossRef]
- Zihui, X.; Shiwen, Z.; Linqin, H. Effect of coixan on insulin resistance in the experimental II model diabetic rats. Chin. J. Diabetes 2002, 1, 11. [Google Scholar]
- Lee, Y.-J.; Im, D.-S. Efficacy Comparison of LPA2 Antagonist H2L5186303 and Agonist GRI977143 on Ovalbumin-Induced Allergic Asthma in BALB/c Mice. Int. J. Mol. Sci. 2022, 23, 9745. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-E.; Im, D.-S. Euiin-Tang Attenuates Obesity-Induced Asthma by Resolving Metaflammation. Pharmaceuticals 2024, 17, 853. https://doi.org/10.3390/ph17070853
Lee Y-E, Im D-S. Euiin-Tang Attenuates Obesity-Induced Asthma by Resolving Metaflammation. Pharmaceuticals. 2024; 17(7):853. https://doi.org/10.3390/ph17070853
Chicago/Turabian StyleLee, Ye-Eul, and Dong-Soon Im. 2024. "Euiin-Tang Attenuates Obesity-Induced Asthma by Resolving Metaflammation" Pharmaceuticals 17, no. 7: 853. https://doi.org/10.3390/ph17070853
APA StyleLee, Y.-E., & Im, D.-S. (2024). Euiin-Tang Attenuates Obesity-Induced Asthma by Resolving Metaflammation. Pharmaceuticals, 17(7), 853. https://doi.org/10.3390/ph17070853






