Non-Apoptotic Programmed Cell Death as Targets for Diabetic Retinal Neurodegeneration
Abstract
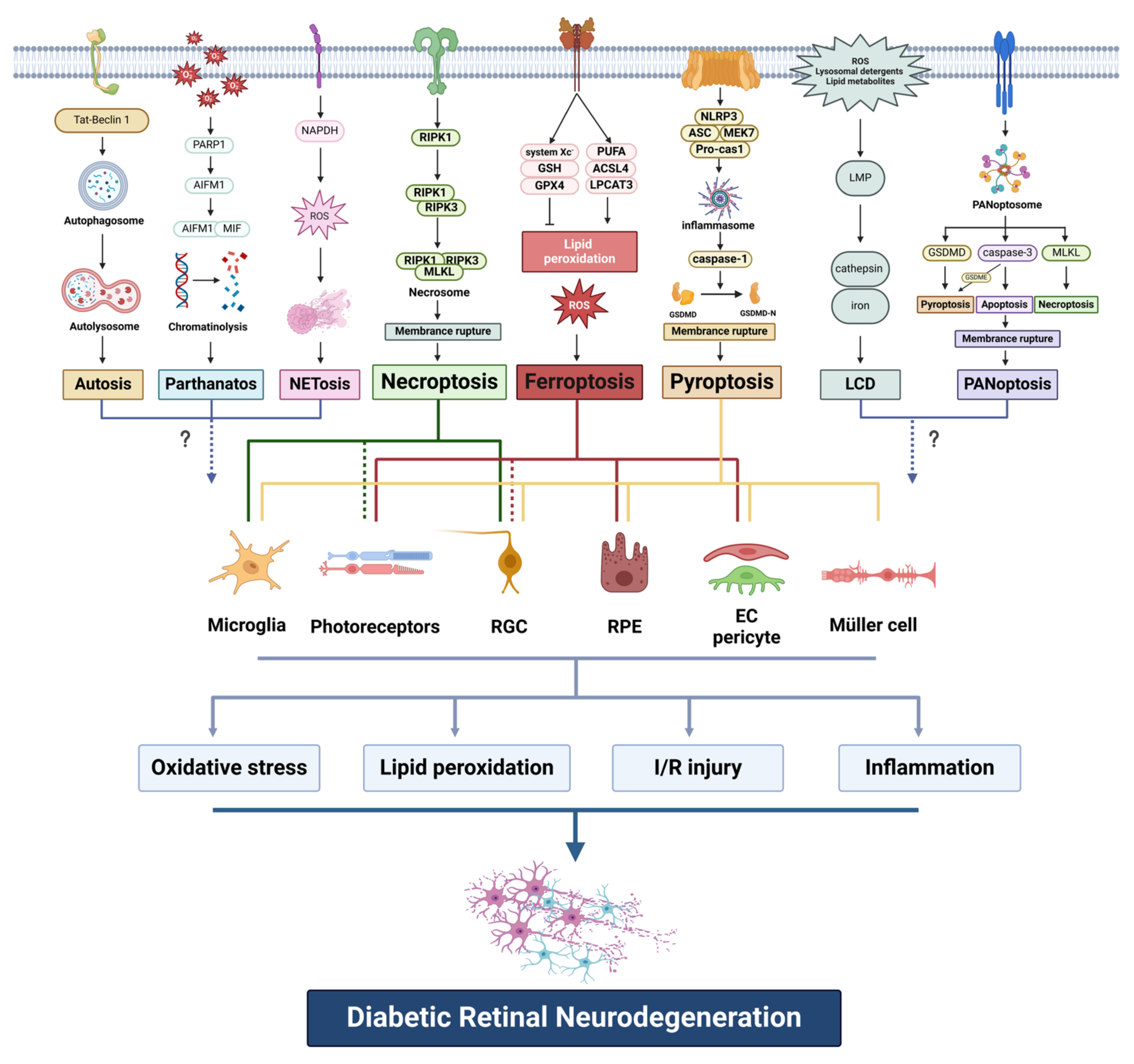
1. Introduction
2. Ferroptosis
2.1. Overview of Ferroptosis
2.2. Ferroptosis in Diabetic Retinal Neurodegeneration
2.2.1. Retinal Pigment Epithelium
2.2.2. Photoreceptors
2.2.3. Retinal Capillary Endothelial Cells
2.2.4. Retinal Ganglion Cells
2.2.5. Other Retinal Cells
3. Pyroptosis
3.1. Overview of Pyroptosis
3.2. Pyroptosis in Diabetic Retinal Neurodegeneration
3.2.1. Retinal Pigment Epithelium
3.2.2. Retinal Ganglion Cells
3.2.3. Glia
3.2.4. Retinal Vascular Cells
4. Necroptosis
4.1. Overview of Necroptosis
4.2. Necroptosis in Diabetic Retinal Neurodegeneration
4.2.1. Retinal Ganglion Cells
4.2.2. Microglia
4.2.3. Photoreceptors
5. Other Programmed Cell Death Types
5.1. Parthanatos
5.2. PANoptosis
5.3. NETosis
5.4. Other Non-Apoptotic PCD
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, C.; Pu, M. High glucose levels impact visual response properties of retinal ganglion cells in C57 mice-An in vitro physiological study. Sci. China Life Sci. 2017, 60, 1428–1435. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Li, Q.; Zhao, S.; Gao, Y. MiR-192 attenuates high glucose-induced pyroptosis in retinal pigment epithelial cells via inflammasome modulation. Bioengineered 2022, 13, 10362–10372. [Google Scholar] [CrossRef]
- Zha, X.; Xi, X.; Fan, X.; Ma, M.; Zhang, Y.; Yang, Y. Overexpression of METTL3 attenuates high-glucose induced RPE cell pyroptosis by regulating miR-25-3p/PTEN/Akt signaling cascade through DGCR8. Aging 2020, 12, 8137–8150. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Sachdeva, M.M. Retinal Neurodegeneration in Diabetes: An Emerging Concept in Diabetic Retinopathy. Curr. Diab. Rep. 2021, 21, 65. [Google Scholar] [CrossRef]
- Zafar, S.; Sachdeva, M.; Frankfort, B.J.; Channa, R. Retinal Neurodegeneration as an Early Manifestation of Diabetic Eye Disease and Potential Neuroprotective Therapies. Curr. Diab. Rep. 2019, 19, 17. [Google Scholar] [CrossRef]
- Simó, R.; Simó-Servat, O.; Bogdanov, P.; Hernández, C. Diabetic Retinopathy: Role of Neurodegeneration and Therapeutic Perspectives. Asia Pac. J. Ophthalmol. 2022, 11, 160–167. [Google Scholar] [CrossRef]
- Pillar, S.; Moisseiev, E.; Sokolovska, J.; Grzybowski, A. Recent Developments in Diabetic Retinal Neurodegeneration: A Literature Review. J. Diabetes Res. 2020, 2020, 5728674. [Google Scholar] [CrossRef]
- Sohn, E.H.; Han, I.C.; Abramoff, M.D. Diabetic Retinal Neurodegeneration-Should We Redefine Retinopathy from Diabetes? JAMA Ophthalmol. 2019, 137, 1132–1133. [Google Scholar] [CrossRef]
- Lim, H.B.; Shin, Y.I.; Lee, M.W.; Park, G.S.; Kim, J.Y. Longitudinal Changes in the Peripapillary Retinal Nerve Fiber Layer Thickness of Patients with Type 2 Diabetes. JAMA Ophthalmol. 2019, 137, 1125–1132. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Liu, X.; Zhuang, L.; Gan, B. Disulfidptosis: Disulfide stress-induced cell death. Trends Cell Biol. 2023, 34, 327–337. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Shen, J.; San, W.; Zheng, Y.; Zhang, S.; Cao, D.; Chen, Y.; Meng, G. Different types of cell death in diabetic endothelial dysfunction. Biomed. Pharmacother. 2023, 168, 115802. [Google Scholar] [CrossRef]
- Christgen, S.; Tweedell, R.E.; Kanneganti, T.D. Programming inflammatory cell death for therapy. Pharmacol. Ther. 2022, 232, 108010. [Google Scholar] [CrossRef]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Gregolin, C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta 1985, 839, 62–70. [Google Scholar] [CrossRef]
- Conrad, M.; Pratt, D.A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Qi, H.; Kan, K.; Sticht, C.; Bennewitz, K.; Li, S.; Qian, X.; Poschet, G.; Kroll, J. Acrolein-inducing ferroptosis contributes to impaired peripheral neurogenesis in zebrafish. Front. Neurosci. 2022, 16, 1044213. [Google Scholar] [CrossRef]
- Mu, L.; Wang, D.; Dong, Z.; Wu, J.; Wu, X.; Su, J.; Zhang, Y. Abnormal Levels of Serum Ferroptosis-Related Biomarkers in Diabetic Retinopathy. J. Ophthalmol. 2022, 2022, 3353740. [Google Scholar] [CrossRef]
- Cao, D.; Wang, C.; Zhou, L. Identification and comprehensive analysis of ferroptosis-related genes as potential biomarkers for the diagnosis and treatment of proliferative diabetic retinopathy by bioinformatics methods. Exp. Eye Res. 2023, 232, 109513. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, J.; Liang, Q. Identification of key ferroptosis genes in diabetic retinopathy based on bioinformatics analysis. PLoS ONE 2023, 18, e0280548. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Cheng, Y.; Liu, K.; Zou, H.; You, Z. Identification of potential ferroptosis-related biomarkers and a pharmacological compound in diabetic retinopathy based on machine learning and molecular docking. Front. Endocrinol. 2022, 13, 988506. [Google Scholar] [CrossRef]
- Lu, C.; Lan, Q.; Song, Q.; Yu, X. Identification and validation of ferroptosis-related genes for diabetic retinopathy. Cell Signal. 2024, 113, 110955. [Google Scholar] [CrossRef]
- Yu, F.; Wang, C.; Su, Y.; Chen, T.; Zhu, W.; Dong, X.; Ke, W.; Cai, L.; Yang, S.; Wan, P. Comprehensive analysis of ferritinophagy-related genes and immune infiltration landscape in diabetic retinopathy. Front. Endocrinol. 2023, 14, 1177488. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, Y.; Liang, J.J.; Zhuang, X.; Ng, T.K. Longitudinal and simultaneous profiling of 11 modes of cell death in mouse retina post-optic nerve injury. Exp. Eye Res. 2022, 222, 109159. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium ARPE-19 cells. Cell Death Dis. 2021, 12, 230. [Google Scholar] [CrossRef]
- Ryan, S.K.; Zelic, M.; Han, Y.; Teeple, E.; Chen, L.; Sadeghi, M.; Shankara, S.; Guo, L.; Li, C.; Pontarelli, F.; et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2022, 26, 12–26. [Google Scholar] [CrossRef]
- Bielmeier, C.B.; Schmitt, S.I.; Kleefeldt, N.; Boneva, S.K.; Schlecht, A.; Vallon, M.; Tamm, E.R.; Hillenkamp, J.; Ergun, S.; Neueder, A.; et al. Deficiency in Retinal TGFbeta Signaling Aggravates Neurodegeneration by Modulating Pro-Apoptotic and MAP Kinase Pathways. Int. J. Mol. Sci. 2022, 23, 2626. [Google Scholar] [CrossRef]
- Shao, J.; Bai, Z.; Zhang, L.; Zhang, F. Ferrostatin-1 alleviates tissue and cell damage in diabetic retinopathy by improving the antioxidant capacity of the Xc(-)-GPX4 system. Cell Death Discov. 2022, 8, 426. [Google Scholar] [CrossRef]
- Xi, X.; Chen, Q.; Ma, J.; Wang, X.; Zhang, J.; Li, Y. Sestrin2 ameliorates diabetic retinopathy by regulating autophagy and ferroptosis. J. Mol. Histol. 2024, 55, 169–184. [Google Scholar] [CrossRef]
- Liu, C.; Sun, W.; Zhu, T.; Shi, S.; Zhang, J.; Wang, J.; Gao, F.; Ou, Q.; Jin, C.; Li, J.; et al. Glia maturation factor-β induces ferroptosis by impairing chaperone-mediated autophagic degradation of ACSL4 in early diabetic retinopathy. Redox Biol. 2022, 52, 102292. [Google Scholar] [CrossRef]
- Singh, L.P.; Yumnamcha, T.; Devi, T.S. Mitophagy, Ferritinophagy and Ferroptosis in Retinal Pigment Epithelial Cells Under High Glucose Conditions: Implications for Diabetic Retinopathy and Age-Related Retinal Diseases. JOJ Ophthalmol. 2021, 8, 77–85. [Google Scholar]
- Zhu, Z.; Duan, P.; Song, H.; Zhou, R.; Chen, T. Downregulation of Circular RNA PSEN1 ameliorates ferroptosis of the high glucose treated retinal pigment epithelial cells via miR-200b-3p/cofilin-2 axis. Bioengineered 2021, 12, 12555–12567. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, C.; Dong, X.; Wang, H. A novel miR-338-3p/SLC1A5 axis reprograms retinal pigment epithelium to increases its resistance to high glucose-induced cell ferroptosis. J. Mol. Histol. 2022, 53, 561–571. [Google Scholar] [CrossRef]
- Fan, X.; Xu, M.; Ren, Q.; Fan, Y.; Liu, B.; Chen, J.; Wang, Z.; Sun, X. Downregulation of fatty acid binding protein 4 alleviates lipid peroxidation and oxidative stress in diabetic retinopathy by regulating peroxisome proliferator-activated receptor gamma-mediated ferroptosis. Bioengineered 2022, 13, 10540–10551. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, S.; Fu, L.; Xu, Y.; Wang, S.; Zhang, G.; Pan, D.; Tao, L.; Shen, X. 1,8-Cineole ameliorates diabetic retinopathy by inhibiting retinal pigment epithelium ferroptosis via PPAR-γ/TXNIP pathways. Biomed. Pharmacother. 2023, 164, 114978. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.; Zhang, D.; Han, W. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered 2022, 13, 8240–8254. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Jian, W.; Han, X.; Zhang, Y.; Zeng, Y.; Liu, R.; Wang, Q.; Song, Q. Protective benefits of salvianic acid A against retinal iron overload by inhibition of ferroptosis. Biomed. Pharmacother. 2023, 165, 115140. [Google Scholar] [CrossRef]
- Azuma, K.; Koumura, T.; Iwamoto, R.; Matsuoka, M.; Terauchi, R.; Yasuda, S.; Shiraya, T.; Watanabe, S.; Aihara, M.; Imai, H.; et al. Mitochondrial glutathione peroxidase 4 is indispensable for photoreceptor development and survival in mice. J. Biol. Chem. 2022, 298, 101824. [Google Scholar] [CrossRef] [PubMed]
- Moos, W.H.; Faller, D.V.; Glavas, I.P.; Harpp, D.N.; Kamperi, N.; Kanara, I.; Kodukula, K.; Mavrakis, A.N.; Pernokas, J.; Pernokas, M.; et al. Treatment and prevention of pathological mitochondrial dysfunction in retinal degeneration and in photoreceptor injury. Biochem. Pharmacol. 2022, 203, 115168. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gao, S.; Wang, Y.; Li, N.; Yang, Z.; Yao, H.; Chen, Y.; Cheng, Y.; Zhong, Y.; Shen, X. Inhibition of Ferroptosis Ameliorates Photoreceptor Degeneration in Experimental Diabetic Mice. Int. J. Mol. Sci. 2023, 24, 16946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, C.Q.; Yi, W.Z.; Ouyang, P.W.; Yang, B.F.; Liu, Q.; Liu, J.M.; Wu, Y.N.; Liang, A.R.; Cui, Y.H.; et al. Ferroptosis Contributes to Microvascular Dysfunction in Diabetic Retinopathy. Am. J. Pathol. 2024, 194, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Cai, Y.; Jiang, Y.; Gong, Y.; Cai, C.; Lai, D.; Jin, X.; Guan, Z.; Qiu, Q. Pipecolic acid mitigates ferroptosis in diabetic retinopathy by regulating GPX4-YAP signaling. Biomed. Pharmacother. 2023, 169, 115895. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Gong, Q.; Zhang, J.; Wang, H.; Qiu, Q.; Zhang, J.; Luo, D. TRIM46 aggravated high glucose-induced hyper permeability and inflammatory response in human retinal capillary endothelial cells by promoting IkappaBalpha ubiquitination. Eye Vis. 2022, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, Q.; Wang, H.; Chen, C.; Luo, D. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp. Cell Res. 2021, 407, 112800. [Google Scholar] [CrossRef] [PubMed]
- Zhan, D.; Zhao, J.; Shi, Q.; Lou, J.; Wang, W. 25-hydroxyvitamin D3 inhibits oxidative stress and ferroptosis in retinal microvascular endothelial cells induced by high glucose through down-regulation of miR-93. BMC Ophthalmol. 2023, 23, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Yang, J.; Wang, J.; Wu, Y.; Zhu, R.; Liu, Q.; Xie, P. lncRNA ZFAS1 Positively Facilitates Endothelial Ferroptosis via miR-7-5p/ACSL4 Axis in Diabetic Retinopathy. Oxid. Med. Cell Longev. 2022, 2022, 9004738. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, S.; Wang, L.; Liu, S.; Zhang, L.; Du, J.; Wu, Z.; Huang, X. Effects of amygdalin on ferroptosis and oxidative stress in diabetic retinopathy progression via the NRF2/ARE signaling pathway. Exp. Eye Res. 2023, 234, 109569. [Google Scholar] [CrossRef]
- Qin, Q.; Yu, N.; Gu, Y.; Ke, W.; Zhang, Q.; Liu, X.; Wang, K.; Chen, M. Inhibiting multiple forms of cell death optimizes ganglion cells survival after retinal ischemia reperfusion injury. Cell Death Dis. 2022, 13, 507. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, B.; Huang, S.; Wu, P.; Zhou, M.; Zhao, J.; Hei, X.; Ke, Y.; Zhang, Y.; Huang, D. Melatonin Alleviates Retinal Ischemia-Reperfusion Injury by Inhibiting p53-Mediated Ferroptosis. Antioxidants 2023, 12, 1173. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.O.; Behl, B.; Rathinam, V.A. Pyroptosis-induced inflammation and tissue damage. Semin. Immunol. 2023, 69, 101781. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrín, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Kayagaki, N.; Kornfeld, O.S.; Lee, B.L.; Stowe, I.B.; O’Rourke, K.; Li, Q.; Sandoval, W.; Yan, D.; Kang, J.; Xu, M.; et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, J.; Song, Y.; Liang, T.; Liu, S.; Ren, C.; Song, X.; Cui, L.; Sun, Y. Pyroptosis and degenerative diseases of the elderly. Cell Death Dis. 2023, 14, 94. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Xue, W.; Cui, D.; Qiu, Y. Research Progress of Pyroptosis in Alzheimer’s Disease. Front. Mol. Neurosci. 2022, 15, 872471. [Google Scholar] [CrossRef]
- Shi, M.; Liu, L.; Min, X.; Mi, L.; Chai, Y.; Chen, F.; Wang, J.; Yue, S.; Zhang, J.; Deng, Q.; et al. Activation of Sigma-1 Receptor Alleviates ER-Associated Cell Death and Microglia Activation in Traumatically Injured Mice. J. Clin. Med. 2022, 11, 2348. [Google Scholar] [CrossRef]
- Chen, M.; Rong, R.; Xia, X. Spotlight on pyroptosis: Role in pathogenesis and therapeutic potential of ocular diseases. J. Neuroinflammation 2022, 19, 183. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, X.; Gao, S.; Zhou, N.; Duan, J.; Zhang, M. Pyroptosis: A New Insight into Eye Disease Therapy. Front. Pharmacol. 2021, 12, 797110. [Google Scholar] [CrossRef]
- Bucolo, C.; Drago, F. Effects of neurosteroids on ischemia-reperfusion injury in the rat retina: Role of sigma1 recognition sites. Eur. J. Pharmacol. 2004, 498, 111–114. [Google Scholar] [CrossRef]
- Bucolo, C.; Drago, F.; Lin, L.R.; Reddy, V.N. Sigma receptor ligands protect human retinal cells against oxidative stress. Neuroreport 2006, 17, 287–291. [Google Scholar] [CrossRef]
- Xi, X.; Wang, M.; Chen, Q.; Ma, J.; Zhang, J.; Li, Y. DNMT1 regulates miR-20a/TXNIP-mediated pyroptosis of retinal pigment epithelial cells through DNA methylation. Mol. Cell Endocrinol. 2023, 577, 112012. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, M.; Wang, Y.; Ma, H.; Zhou, Y.; Jiang, X.; Liu, Y. Updates on RPE cell damage in diabetic retinopathy (Review). Mol. Med. Rep. 2023, 28, 185. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qi, P.; Cui, H.; Lu, Q.; Gao, X. CircFAT1 regulates retinal pigment epithelial cell pyroptosis and autophagy via mediating m6A reader protein YTHDF2 expression in diabetic retinopathy. Exp. Eye Res. 2022, 222, 109152. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.H.; Luo, Y.N.; Wei, R.Z.; Yin, J.Y.; Qin, Z.L.; Lu, L.L.; Ma, W.H. CircZNF532 knockdown protects retinal pigment epithelial cells against high glucose-induced apoptosis and pyroptosis by regulating the miR-20b-5p/STAT3 axis. J. Diabetes Investig. 2022, 13, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, L.; Han, Q.; Fu, J.; Xiao, F. HAGLR, stabilized by m6A modification, triggers PTEN-Akt signaling cascade-mediated RPE cell pyroptosis via sponging miR-106b-5p. J. Biochem. Mol. Toxicol. 2024, 38, e23596. [Google Scholar] [CrossRef] [PubMed]
- Yumnamcha, T.; Devi, T.S.; Singh, L.P. Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Li, N.; Xu, M.; Chen, J.L.; He, H.; Liu, J.; Wang, T.H.; Zuo, Z.F. Salidroside protects RGC from pyroptosis in diabetes-induced retinopathy associated with NLRP3, NFEZL2 and NGKB1, revealed by network pharmacology analysis and experimental validation. Eur. J. Med. Res. 2024, 29, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, X.L.; Xu, M.; Chen, J.L.; Wang, Y.F.; Jie, S.; Xiao, Y.G.; Gao, A.S.; Zhang, L.C.; Liu, X.Z.; et al. Network pharmacology mechanism of Scutellarin to inhibit RGC pyroptosis in diabetic retinopathy. Sci. Rep. 2023, 13, 6504. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Poly(ADP-Ribose) polymerase-1 in acute neuronal death and inflammation: A strategy for neuroprotection. Ann. N. Y. Acad. Sci. 2003, 993, 217–228, discussion 287–218. [Google Scholar] [CrossRef] [PubMed]
- Au, N.P.B.; Ma, C.H.E. Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy. Front. Immunol. 2022, 13, 860070. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; You, J.; Yao, Y.; Xie, M. High glucose induces pyroptosis of retinal microglia through NLPR3 inflammasome signaling. Arq. Bras. Oftalmol. 2021, 84, 67–73. [Google Scholar] [CrossRef]
- Xie, Z.; Ying, Q.; Luo, H.; Qin, M.; Pang, Y.; Hu, H.; Zhong, J.; Song, Y.; Zhang, Z.; Zhang, X. Resveratrol Alleviates Retinal Ischemia-Reperfusion Injury by Inhibiting the NLRP3/Gasdermin D/Caspase-1/Interleukin-1β Pyroptosis Pathway. Investig. Opthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, S.; Li, C.; Tang, M.; Sun, T.; Zheng, Z. Transient receptor potential channel 6 knockdown prevents high glucose-induced Müller cell pyroptosis. Exp. Eye Res. 2023, 227, 109381. [Google Scholar] [CrossRef]
- Kong, H.; Zhao, H.; Chen, T.; Song, Y.; Cui, Y. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022, 13, 336. [Google Scholar] [CrossRef]
- Yang, K.; Liu, J.; Zhang, X.; Ren, Z.; Gao, L.; Wang, Y.; Lin, W.; Ma, X.; Hao, M.; Kuang, H. H3 Relaxin Alleviates Migration, Apoptosis and Pyroptosis Through P2X7R-Mediated Nucleotide Binding Oligomerization Domain-Like Receptor Protein 3 Inflammasome Activation in Retinopathy Induced by Hyperglycemia. Front. Pharmacol. 2020, 11, 603689. [Google Scholar] [CrossRef]
- You, H.; Li, H.; Gou, W. lncRNA HOTAIR promotes ROS generation and NLRP3 inflammasome activation by inhibiting Nrf2 in diabetic retinopathy. Medicine 2023, 102, e35155. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Chen, G.; He, S. MiR-200c-3p regulates pyroptosis by targeting SLC30A7 in diabetic retinopathy. Hum. Exp. Toxicol. 2022, 41, 9603271221099589. [Google Scholar] [CrossRef]
- Gu, C.; Draga, D.; Zhou, C.; Su, T.; Zou, C.; Gu, Q.; Lahm, T.; Zheng, Z.; Qiu, Q. miR-590-3p Inhibits Pyroptosis in Diabetic Retinopathy by Targeting NLRP1 and Inactivating the NOX4 Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4215–4223. [Google Scholar] [CrossRef]
- Wang, J.J.; Chen, Z.L.; Wang, D.D.; Wu, K.F.; Huang, W.B.; Zhang, L.Q. linc00174 deteriorates the pathogenesis of diabetic retinopathy via miR-26a-5p/PTEN/Akt signalling cascade-mediated pyroptosis. Biochem. Biophys. Res. Commun. 2022, 630, 92–100. [Google Scholar] [CrossRef]
- Gan, J.; Huang, M.; Lan, G.; Liu, L.; Xu, F. High Glucose Induces the Loss of Retinal Pericytes Partly via NLRP3-Caspase-1-GSDMD-Mediated Pyroptosis. Biomed. Res. Int. 2020, 2020, 4510628. [Google Scholar] [CrossRef]
- Yu, X.; Ma, X.; Lin, W.; Xu, Q.; Zhou, H.; Kuang, H. Long noncoding RNA MIAT regulates primary human retinal pericyte pyroptosis by modulating miR-342-3p targeting of CASP1 in diabetic retinopathy. Exp. Eye Res. 2021, 202, 108300. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The Release of Damage-Associated Molecular Patterns and Its Physiological Relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2018, 20, 19–33. [Google Scholar] [CrossRef]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Sridharan, H.; Huang, C.; Mandal, P.; Upton, J.W.; Gough, P.J.; Sehon, C.A.; Marquis, R.W.; Bertin, J.; Mocarski, E.S. Toll-like Receptor 3-mediated Necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013, 288, 31268–31279. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, T.; Sun, X.; Zheng, Y.; Cheng, B.; Li, M.; Liu, X.; He, C. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 2017, 25, 180–189. [Google Scholar] [CrossRef]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. DAI/ZBP1/DLM-1 Complexes with RIP3 to Mediate Virus-Induced Programmed Necrosis that Is Targeted by Murine Cytomegalovirus vIRA. Cell Host Microbe 2012, 11, 290–297. [Google Scholar] [CrossRef]
- Newton, K.; Wickliffe, K.E.; Dugger, D.L.; Maltzman, A.; Roose-Girma, M.; Dohse, M.; Kőműves, L.; Webster, J.D.; Dixit, V.M. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 2019, 574, 428–431. [Google Scholar] [CrossRef]
- Yan, J.; Wan, P.; Choksi, S.; Liu, Z.-G. Necroptosis and tumor progression. Trends Cancer 2022, 8, 21–27. [Google Scholar] [CrossRef]
- Richard, R.; Mousa, S. Necroptosis in Alzheimer’s disease: Potential therapeutic target. Biomed. Pharmacother. 2022, 152, 113203. [Google Scholar] [CrossRef] [PubMed]
- Kolbrink, B.; von Samson-Himmelstjerna, F.A.; Murphy, J.M.; Krautwald, S. Role of necroptosis in kidney health and disease. Nat. Rev. Nephrol. 2023, 19, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Neyra, K.; Galindo-Cabello, N.; Hernández-Rodríguez, L.A.; González-Pérez, F.; Rodríguez-Cabello, J.C.; González-Sarmiento, R.; Pastor, J.C.; Usategui-Martín, R.; Fernandez-Bueno, I. Programmed Cell Death and Autophagy in an in vitro Model of Spontaneous Neuroretinal Degeneration. Front. Neuroanat. 2022, 16, 812487. [Google Scholar] [CrossRef]
- Ma, H.; Yang, F.; York, L.R.; Li, S.; Ding, X.-Q. Excessive Thyroid Hormone Signaling Induces Photoreceptor Degeneration in Mice. eNeuro 2023, 10, ENEURO.0058-23.2023. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; Puertas-Neyra, K.; Galindo-Cabello, N.; Hernández-Rodríguez, L.A.; González-Pérez, F.; Rodríguez-Cabello, J.C.; González-Sarmiento, R.; Pastor, J.C.; Fernandez-Bueno, I. Retinal Neuroprotective Effect of Mesenchymal Stem Cells Secretome Through Modulation of Oxidative Stress, Autophagy, and Programmed Cell Death. Investig. Opthalmol. Vis. Sci. 2022, 63, 27. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Sosunov, S.A.; Shakerley, N.L.; Ten, V.S.; Ratner, A.J. Hyperglycemic Conditions Prime Cells for RIP1-dependent Necroptosis. J. Biol. Chem. 2016, 291, 13753–13761. [Google Scholar] [CrossRef]
- Gao, S.; Huang, X.; Zhang, Y.; Bao, L.; Wang, X.; Zhang, M. Investigation on the expression regulation of RIPK1/RIPK3 in the retinal ganglion cells (RGCs) cultured in high glucose. Bioengineered 2021, 12, 3947–3956. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, C.; Cui, K.; Fan, M.; Xiang, W.; Ye, D.; Shi, Y.; Ye, H.; Bai, X.; Wei, Y.; et al. GSK840 Alleviates Retinal Neuronal Injury by Inhibiting RIPK3/MLKL-Mediated RGC Necroptosis after Ischemia/Reperfusion. Investig. Opthalmol. Vis. Sci. 2023, 64, 42. [Google Scholar] [CrossRef]
- Ding, W.; Shang, L.; Huang, J.-F.; Li, N.; Chen, D.; Xue, L.-X.; Xiong, K. Receptor interacting protein 3-induced RGC-5 cell necroptosis following oxygen glucose deprivation. BMC Neurosci. 2015, 16, 49. [Google Scholar] [CrossRef]
- Cai, R.; Xue, W.; Liu, S.; Petersen, R.B.; Huang, K.; Zheng, L. Overexpression of glyceraldehyde 3-phosphate dehydrogenase prevents neurovascular degeneration after retinal injury. Faseb J. 2015, 29, 2749–2758. [Google Scholar] [CrossRef]
- Kim, C.R.; Kim, J.H.; Park, H.-Y.L.; Park, C.K. Ischemia Reperfusion Injury Triggers TNFα Induced-Necroptosis in Rat Retina. Curr. Eye Res. 2016, 42, 771–779. [Google Scholar] [CrossRef]
- Gao, S.; Andreeva, K.; Cooper, N.G. Ischemia-reperfusion injury of the retina is linked to necroptosis via the ERK1/2-RIP3 pathway. Mol. Vis. 2014, 20, 1374–1387. [Google Scholar] [PubMed]
- Lee, Y.S.; Dayma, Y.; Park, M.Y.; Kim, K.I.; Yoo, S.E.; Kim, E. Daxx is a key downstream component of receptor interacting protein kinase 3 mediating retinal ischemic cell death. FEBS Lett. 2013, 587, 266–271. [Google Scholar] [CrossRef]
- Huang, Z.; Liang, J.; Chen, S.; Ng, T.K.; Brelén, M.E.; Liu, Q.; Yang, R.; Xie, B.; Ke, S.; Chen, W.; et al. RIP3-mediated microglial necroptosis promotes neuroinflammation and neurodegeneration in the early stages of diabetic retinopathy. Cell Death Dis. 2023, 14, 227. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Huang, Z.; Yang, Z.; Zhou, T.; Liu, S.; Hao, Z.; Wang, J.; Feng, Q.; Liu, Y.; et al. A specific RIP3(+) subpopulation of microglia promotes retinopathy through a hypoxia-triggered necroptotic mechanism. Proc. Natl. Acad. Sci. USA 2021, 118, e2023290118. [Google Scholar] [CrossRef]
- He, Y.; Xu, Y.; Chen, Z.; He, B.; Quan, Z.; Zhang, R.; Ren, Y. Protective Effect of Mitochondrially Targeted Peptide Against Oxidant Injury of Cone Photoreceptors Through Preventing Necroptosis Pathway. J. Biomed. Nanotechnol. 2021, 17, 279–290. [Google Scholar] [CrossRef]
- Trichonas, G.; Murakami, Y.; Thanos, A.; Morizane, Y.; Kayama, M.; Debouck, C.M.; Hisatomi, T.; Miller, J.W.; Vavvas, D.G. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 21695–21700. [Google Scholar] [CrossRef]
- Sato, K.; Li, S.; Gordon, W.C.; He, J.; Liou, G.I.; Hill, J.M.; Travis, G.H.; Bazan, N.G.; Jin, M. Receptor Interacting Protein Kinase-Mediated Necrosis Contributes to Cone and Rod Photoreceptor Degeneration in the Retina Lacking Interphotoreceptor Retinoid-Binding Protein. J. Neurosci. 2013, 33, 17458–17468. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Mitochondrial and nuclear cross talk in cell death: Parthanatos. Ann. N. Y. Acad. Sci. 2008, 1147, 233–241. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.I.; Dawson, T.M.; Dawson, V.L. Poly (ADP-ribose) (PAR)-dependent cell death in neurodegenerative diseases. Int. Rev. Cell Mol. Biol. 2020, 353, 1–29. [Google Scholar] [CrossRef]
- Pan, Y.R.; Song, J.Y.; Fan, B.; Wang, Y.; Che, L.; Zhang, S.M.; Chang, Y.X.; He, C.; Li, G.Y. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun. Signal 2020, 18, 27. [Google Scholar] [CrossRef]
- Sun, J.; Liu, G.; Chen, R.; Zhou, J.; Chen, T.; Cheng, Y.; Lou, Q.; Wang, H. PARP1 Is Upregulated by Hyperglycemia Via N6-methyladenosine Modification and Promotes Diabetic Retinopathy. Discov. Med. 2022, 34, 115–129. [Google Scholar]
- Xu, J.C.; Fan, J.; Wang, X.; Eacker, S.M.; Kam, T.I.; Chen, L.; Yin, X.; Zhu, J.; Chi, Z.; Jiang, H.; et al. Cultured networks of excitatory projection neurons and inhibitory interneurons for studying human cortical neurotoxicity. Sci. Transl. Med. 2016, 8, 333ra348. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Y.; Yu, Y.; Wang, G.; Yu, Y. Hydrogen-rich medium alleviates high glucose-induced oxidative stress and parthanatos in rat Schwann cells in vitro. Mol. Med. Rep. 2019, 19, 338–344. [Google Scholar] [CrossRef]
- Gu, L.; Xu, H.; Wang, F.; Xu, G.; Sinha, D.; Wang, J.; Xu, J.Y.; Tian, H.; Gao, F.; Li, W.; et al. Erythropoietin exerts a neuroprotective function against glutamate neurotoxicity in experimental diabetic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8208–8222. [Google Scholar] [CrossRef]
- Jung, K.I.; Han, J.S.; Park, C.K. Neuroprotective Effects of Nicotinamide (Vitamin B(3)) on Neurodegeneration in Diabetic Rat Retinas. Nutrients 2022, 14, 1162. [Google Scholar] [CrossRef]
- Malireddi, R.K.S.; Kesavardhana, S.; Kanneganti, T.D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front. Cell Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef]
- Ye, D.; Xu, Y.; Shi, Y.; Fan, M.; Lu, P.; Bai, X.; Feng, Y.; Hu, C.; Cui, K.; Tang, X.; et al. Anti-PANoptosis is involved in neuroprotective effects of melatonin in acute ocular hypertension model. J. Pineal Res. 2022, 73, e12828. [Google Scholar] [CrossRef]
- Zeng, Z.; You, M.; Fan, C.; Rong, R.; Li, H.; Xia, X. Pathologically high intraocular pressure induces mitochondrial dysfunction through Drp1 and leads to retinal ganglion cell PANoptosis in glaucoma. Redox Biol. 2023, 62, 102687. [Google Scholar] [CrossRef]
- Yan, W.T.; Zhao, W.J.; Hu, X.M.; Ban, X.X.; Ning, W.Y.; Wan, H.; Zhang, Q.; Xiong, K. PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons. Neural Regen. Res. 2023, 18, 357–363. [Google Scholar] [CrossRef]
- Xu, X.; Lan, X.; Fu, S.; Zhang, Q.; Gui, F.; Jin, Q.; Xie, L.; Xiong, Y. Dickkopf-1 exerts protective effects by inhibiting PANoptosis and retinal neovascularization in diabetic retinopathy. Biochem. Biophys. Res. Commun. 2022, 617, 69–76. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Brinkmann, V.; Laube, B.; Abu Abed, U.; Goosmann, C.; Zychlinsky, A. Neutrophil extracellular traps: How to generate and visualize them. J. Vis. Exp. 2010, 36, e1724. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, X.; He, Q.; Xiao, Q.A.; Wang, D.; Huang, M.; Zhang, X. Diabetes-associated neutrophil NETosis: Pathogenesis and interventional target of diabetic complications. Front. Endocrinol. 2023, 14, 1202463. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front. Immunol. 2018, 9, 3076. [Google Scholar] [CrossRef]
- Barliya, T.; Dardik, R.; Nisgav, Y.; Dachbash, M.; Gaton, D.; Kenet, G.; Ehrlich, R.; Weinberger, D.; Livnat, T. Possible involvement of NETosis in inflammatory processes in the eye: Evidence from a small cohort of patients. Mol. Vis. 2017, 23, 922–932. [Google Scholar]
- Binet, F.; Cagnone, G.; Crespo-Garcia, S.; Hata, M.; Neault, M.; Dejda, A.; Wilson, A.M.; Buscarlet, M.; Mawambo, G.T.; Howard, J.P.; et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020, 369, 934. [Google Scholar] [CrossRef]
- Thakur, V.; Gonzalez, M.A.; Parada, M.; Martinez, R.D.; Chattopadhyay, M. Role of Histone Deacetylase Inhibitor in Diabetic Painful Neuropathy. Mol. Neurobiol. 2023, 61, 2283–2296. [Google Scholar] [CrossRef]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M., Jr.; Wei, Y.; Ginet, V.; Zhang, L.; Posner, B.; Tran, K.A.; Green, D.R.; Xavier, R.J.; et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef]
- Pivtoraiko, V.N.; Stone, S.L.; Roth, K.A.; Shacka, J.J. Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid. Redox Signal 2009, 11, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Bell, C.M.; Berlinicke, C.A.; Marsh-Armstrong, N.; Zack, D.J. Programmed switch in the mitochondrial degradation pathways during human retinal ganglion cell differentiation from stem cells is critical for RGC survival. Redox Biol. 2020, 34, 101465. [Google Scholar] [CrossRef]
- White, E. Entosis: It’s a cell-eat-cell world. Cell 2007, 131, 840–842. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. Mechanisms of alkaliptosis. Front. Cell Dev. Biol. 2023, 11, 1213995. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Ke, S.; Ye, W.; Xie, B.; Huang, Z. Non-Apoptotic Programmed Cell Death as Targets for Diabetic Retinal Neurodegeneration. Pharmaceuticals 2024, 17, 837. https://doi.org/10.3390/ph17070837
Lin Y, Ke S, Ye W, Xie B, Huang Z. Non-Apoptotic Programmed Cell Death as Targets for Diabetic Retinal Neurodegeneration. Pharmaceuticals. 2024; 17(7):837. https://doi.org/10.3390/ph17070837
Chicago/Turabian StyleLin, Yingjia, Shuping Ke, Weiqing Ye, Biyao Xie, and Zijing Huang. 2024. "Non-Apoptotic Programmed Cell Death as Targets for Diabetic Retinal Neurodegeneration" Pharmaceuticals 17, no. 7: 837. https://doi.org/10.3390/ph17070837
APA StyleLin, Y., Ke, S., Ye, W., Xie, B., & Huang, Z. (2024). Non-Apoptotic Programmed Cell Death as Targets for Diabetic Retinal Neurodegeneration. Pharmaceuticals, 17(7), 837. https://doi.org/10.3390/ph17070837





