Impact of Cannabidiol and Exercise on Clinical Outcomes and Gut Microbiota for Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors: A Case Report
Abstract
1. Introduction
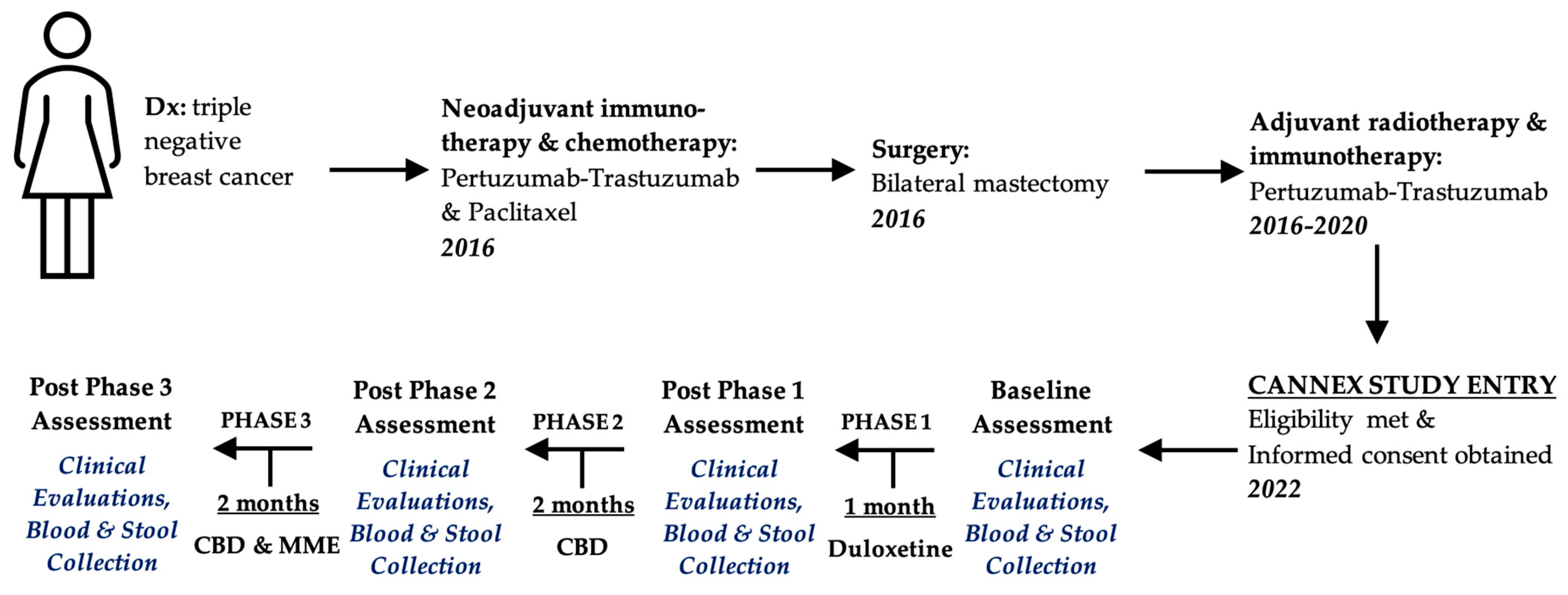
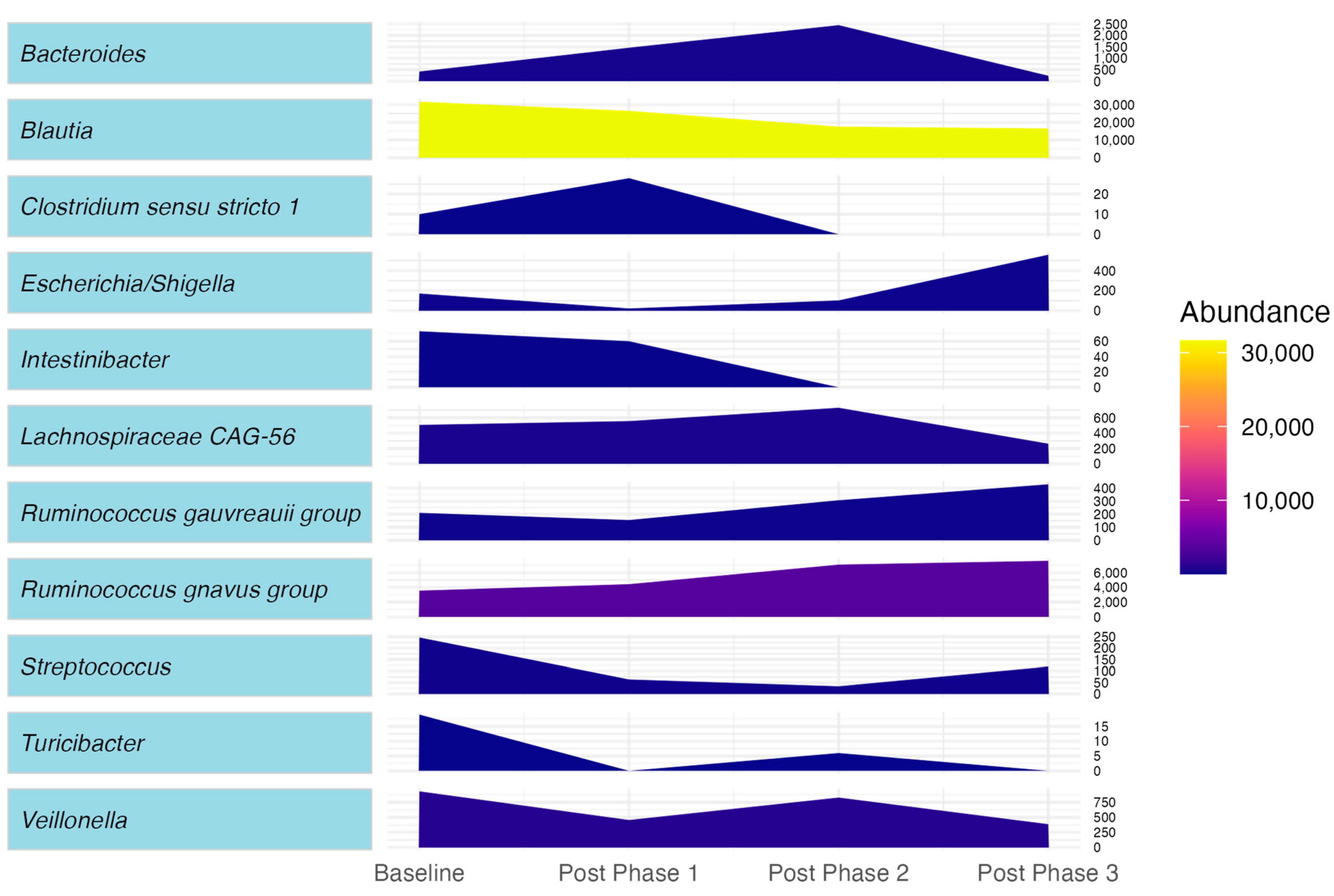
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hershman, D.L.; Weimer, L.H.; Wang, A.; Kranwinkel, G.; Brafman, L.; Fuentes, D.; Awad, D.; Crew, K.D. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res. Treat. 2011, 125, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Kautio, A.L.; Haanpää, M.; Kautiainen, H.; Kalso, E.; Saarto, T. Burden of chemotherapy-induced neuropathy—A cross-sectional study. Support. Care Cancer 2011, 19, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Reeves, B.N.; Dakhil, S.R.; Sloan, J.A.; Wolf, S.L.; Burger, K.N.; Kamal, A.; Le-Lindqwister, N.A.; Soori, G.S.; Jaslowski, A.J.; et al. Natural History of Paclitaxel-Associated Acute Pain Syndrome: Prospective Cohort Study NCCTG N08C1. J. Clin. Oncol. 2011, 29, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Bland, K.A.; Kirkham, A.A.; Bovard, J.; Shenkier, T.; Zucker, D.; McKenzie, D.C.; Davis, M.K.; Gelmon, K.A.; Campbell, K.L. Effect of Exercise on Taxane Chemotherapy-Induced Peripheral Neuropathy in Women with Breast Cancer: A Randomized Controlled Trial. Clin. Breast Cancer 2019, 19, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Bruna, J. Taxane-Induced Peripheral Neurotoxicity. Toxics 2015, 3, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, L.; d’Angelo, M.; Antonosante, A.; Allegretti, M.; Cimini, A. Chemokine Signaling in Chemotherapy-Induced Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 2904. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Huh, B.; Kim, H.K.; Kim, K.H.; Abdi, S. Treatment of Chemotherapy-Induced Peripheral Neuropathy: Systematic Review and Recommendations. Pain Physician 2018, 21, 571–592. [Google Scholar]
- Hu, L.Y.; Mi, W.L.; Wu, G.C.; Wang, Y.Q.; Mao-Ying, Q.L. Prevention and Treatment for Chemotherapy-Induced Peripheral Neuropathy: Therapies Based on CIPN Mechanisms. Curr. Neuropharmacol. 2019, 17, 184–196. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- King, K.M.; Myers, A.M.; Soroka-Monzo, A.J.; Tuma, R.F.; Tallarida, R.J.; Walker, E.A.; Ward, S.J. Single and combined effects of Δ9-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br. J. Pharmacol. 2017, 174, 2832–2841. [Google Scholar] [CrossRef]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Giaddui, M.; Kjelstrom, S.; Erebor, E.; Meske, S.; Saeed, L.; Ruiz, K.; Ghaneie, A.; Hibbs, J.; Hong, J.; et al. Safety and efficacy of cannabidiol in the management of chemotherapy-induced peripheral neuropathy. J. Clin. Oncol. 2023, 41, 12020. [Google Scholar] [CrossRef]
- Guo, S.; Han, W.; Wang, P.; Wang, X.; Fang, X. Effects of exercise on chemotherapy-induced peripheral neuropathy in cancer patients: A systematic review and meta-analysis. J. Cancer Surviv. 2023, 17, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Streckmann, F.; Zopf, E.M.; Lehmann, H.C.; May, K.; Rizza, J.; Zimmer, P.; Gollhofer, A.; Bloch, W.; Baumann, F.T. Exercise intervention studies in patients with peripheral neuropathy: A systematic review. Sports Med. 2014, 44, 1289–1304. [Google Scholar] [CrossRef]
- Bristot, V.; Poletto, G.; Pereira, D.M.R.; Hauck, M.; Schneider, I.J.C.; Aguiar, A.S. The effects of exercise on circulating endocannabinoid levels—A protocol for a systematic review and meta-analysis. Syst. Rev. 2022, 11, 98. [Google Scholar] [CrossRef]
- Bouchenaki, H.; Danigo, A.; Sturtz, F.; Hajj, R.; Magy, L.; Demiot, C. An overview of ongoing clinical trials assessing pharmacological therapeutic strategies to manage chemotherapy-induced peripheral neuropathy, based on preclinical studies in rodent models. Fundam. Clin. Pharmacol. 2021, 35, 506–523. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids--at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Di Marzo, V. The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 2021, 248, R83–R97. [Google Scholar] [CrossRef]
- Panee, J.; Gerschenson, M.; Chang, L. Associations Between Microbiota, Mitochondrial Function, and Cognition in Chronic Marijuana Users. J. Neuroimmune Pharmacol. 2018, 13, 113–122. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Koduru, V.; Bienen, E.J.; Sadosky, A. Characterizing neuropathic pain profiles: Enriching interpretation of painDETECT. Patient Relat. Outcome Meas. 2016, 7, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, J.V.; Leivas, E.G.; de Sá Ferreira, A.; Nogueira, L.A.C. Does the painDETECT questionnaire identify impaired conditioned pain modulation in people with musculoskeletal pain?—A diagnostic accuracy study. Arch. Physiother. 2023, 13, 17. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: A systematic review. BMC Med. Res. Methodol. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Hile, E.; Levangie, P.; Ryans, K.; Gilchrist, L. Oncology Section Task Force on Breast Cancer Outcomes: Clinical Measures of Chemotherapy-induced Peripheral Neuropathy—A Systematic Review. Rehabil. Oncol. 2015, 33, 32–41. [Google Scholar] [CrossRef]
- Calhoun, E.A.; Welshman, E.E.; Chang, C.H.; Lurain, J.R.; Fishman, D.A.; Hunt, T.L.; Cella, D. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int. J. Gynecol. Cancer 2003, 13, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.; Cella, D.; Yost, K. The F unctional A ssessment of C hronic I llness T herapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Lopez, V.; Lam, S.C.; Leung, A.K.T.; Li, Y.C.; Wong, K.H.; Au, J.S.K.; Sundar, R.; Chan, A.; De Ng, T.R.; et al. Psychometric testing of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) subscale in a longitudinal study of cancer patients treated with chemotherapy. Health Qual. Life Outcomes 2020, 18, 246. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-h dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Liu, A.; Shi, J.; Xu, Y.-J.; Liu, Y. Multi-Omics Reveals the Effects of Cannabidiol on Gut Microbiota and Metabolic Phenotypes. Cannabis Cannabinoid Res. 2023, 9, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Jay, C.A.; Shade, S.B.; Vizoso, H.; Reda, H.; Press, S.; Kelly, M.E.; Rowbotham, M.C.; Petersen, K.L. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007, 68, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.S.; Marcotte, T.D.; Umlauf, A.; Gouaux, B.; Atkinson, J.H. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J. Pain 2015, 16, 616–627. [Google Scholar] [CrossRef]
- Lynch, M.E.; Cesar-Rittenberg, P.; Hohmann, A.G. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J. Pain Symptom Manag. 2014, 47, 166–173. [Google Scholar] [CrossRef]
- Waissengrin, B.; Mirelman, D.; Pelles, S.; Bukstein, F.; Blumenthal, D.T.; Wolf, I.; Geva, R. Effect of cannabis on oxaliplatin-induced peripheral neuropathy among oncology patients: A retrospective analysis. Ther. Adv. Med. Oncol. 2021, 13, 1758835921990203. [Google Scholar] [CrossRef]
- Nielsen, S.W.; Hasselsteen, S.D.; Dominiak, H.S.H.; Labudovic, D.; Reiter, L.; Dalton, S.O.; Herrstedt, J. Oral cannabidiol for prevention of acute and transient chemotherapy-induced peripheral neuropathy. Support. Care Cancer 2022, 30, 9441–9451. [Google Scholar] [CrossRef]
- De Vita, M.J.; Moskal, D.; Maisto, S.A.; Ansell, E.B. Association of Cannabinoid Administration with Experimental Pain in Healthy Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 1118–1127. [Google Scholar] [CrossRef]
- Cluny, N.L.; Keenan, C.M.; Reimer, R.A.; Le Foll, B.; Sharkey, K.A. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Δ9-Tetrahydrocannabinol. PLoS ONE 2015, 10, e0144270. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Grüter, T.; Mohamad, N.; Rilke, N.; Blusch, A.; Sgodzai, M.; Demir, S.; Pedreiturria, X.; Lemhoefer, K.; Gisevius, B.; Haghikia, A.; et al. Propionate exerts neuroprotective and neuroregenerative effects in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2023, 120, e2216941120. [Google Scholar] [CrossRef] [PubMed]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Dawin, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef]
- Corriero, A.; Giglio, M.; Inchingolo, F.; Moschetta, A.; Varrassi, G.; Puntillo, F. Gut Microbiota Modulation and Its Implications on Neuropathic Pain: A Comprehensive Literature Review. Pain Ther. 2024, 13, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.J.; Kim, Y.M.; Kim, K.S.; Choi, J.O.; Cho, S.H.; An, S.; Park, S.H.; Cho, Y.J.; Park, J.H.; Seo, S.U.; et al. Protective role of colitis in inflammatory arthritis via propionate-producing Bacteroides in the gut. Front. Immunol. 2023, 14, 1064900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, X.; Ding, D.; Lu, Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J. Int. Med. Res. 2020, 48, 300060520936806. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhi, F. Lower Level of Bacteroides in the Gut Microbiota Is Associated with Inflammatory Bowel Disease: A Meta-Analysis. Biomed Res. Int. 2016, 2016, 5828959. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Varsha, K.K.; Nagarkatti, M.; Nagarkatti, P. Role of Gut Microbiota in Cannabinoid-Mediated Suppression of Inflammation. Adv. Drug Alcohol Res. 2022, 2, 10550. [Google Scholar] [CrossRef] [PubMed]
- Jessup, D.; Woods, K.; Thakker, S.; Damaj, M.I.; Akbarali, H.I. Short-chain fatty acid, butyrate prevents morphine-and paclitaxel-induced nociceptive hypersensitivity. Sci. Rep. 2023, 13, 17805. [Google Scholar] [CrossRef] [PubMed]
- Sayols-Baixeras, S.; Dekkers, K.F.; Baldanzi, G.; Jönsson, D.; Hammar, U.; Lin, Y.T.; Ahmad, S.; Nguyen, D.; Varotsis, G.; Pita, S.; et al. Streptococcus Species Abundance in the Gut Is Linked to Subclinical Coronary Atherosclerosis in 8973 Participants From the SCAPIS Cohort. Circulation 2023, 148, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Akinsuyi Oluwamayowa, S.; Roesch Luiz, F.W. Meta-Analysis Reveals Compositional and Functional Microbial Changes Associated with Osteoporosis. Microbiol. Spectr. 2023, 11, e00322–e00323. [Google Scholar] [CrossRef]
- McCrary, J.M.; Goldstein, D.; Wyld, D.; Henderson, R.; Lewis, C.R.; Park, S.B. Mobility in survivors with chemotherapy-induced peripheral neuropathy and utility of the 6-min walk test. J. Cancer Surviv. Res. Pract. 2019, 13, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef]
| Variable (Unit; Normal Range) | Baseline | Post Phase 1 | Post Phase 2 | Post Phase 3 |
|---|---|---|---|---|
| Weight (kg) | 92.1 | 92.7 | 93.7 | 95.3 |
| Height (cm) | 166 | 166 | 166 | 166 |
| BMI (kg/m2) | 33.43 | 33.65 | 34.01 | 34.59 |
| ASMI (kg/m2) | 7.40 | 7.37 | 7.22 | 7.46 |
| PBMC Count (106/mL; 0.5–3) | 3.1 * | 2.8 | 5.4 * | 4.4 * |
| Lymphocytes (%; 22–44) | 32.53 | 45.20 * | 38.48 | 37.27 |
| Monocytes (%; 3–10) | 12.69 * | 9.23 | 9.78 | 8.64 |
| Monocytes (absolute counts; 109/L; 0–0.8) | 0.84 * | 0.53 | 0.58 | 0.53 |
| CRP (mg/L; 0–5) | 14.00 * | 3.60 | 3.80 | 4 |
| SII | 420.28 | 206.68 | 323.96 | 334.19 |
| PLR | 123.61 | 88.33 | 111.71 | 107.46 |
| MLR | 0.39 | 0.21 | 0.26 | 0.23 |
| NLR | 1.57 | 0.91 | 1.31 | 1.36 |
| Bilirubin (total; μmol/L; 1.7–18.9) | 7.9 | 6.4 | 5.7 | 6.6 |
| Alkaline phosphatase (U/L; 42–98) | 68 | 60 | 68 | 62 |
| Variable/Measure (Unit) | Baseline | Post Phase 1 | Post Phase 2 | Post Phase 3 |
|---|---|---|---|---|
| PainDETECT Score | 27 | 24 | 26 | 18 |
| FACT-GOG-Ntx Score | 37 | 34 | 44 | 48 |
| FACT-GOG-Ntx TOI Score | 74 | 78 | 84 | 93 |
| FACT-GOG Total Score | 67 | 77 | 73 | 81.3 |
| GSLPA Score | 22 | 30 | 40 | 46 |
| GS (m/s) | 0.91 | 1.00 | 1.08 | 1.16 |
| 5× STS (seconds) | 14.65 | 9.8 | 11.09 | 10.69 |
| HGS (kg) | 27 | 28 | 29 | 30 |
| 9-HPT (seconds) | 19.3 | 17.8 | 18.34 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigano, M.; Kubal, S.; Lu, Y.; Habib, S.; Samarani, S.; Cama, G.; Viau, C.; Farzin, H.; Koudieh, N.; Xia, J.; et al. Impact of Cannabidiol and Exercise on Clinical Outcomes and Gut Microbiota for Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors: A Case Report. Pharmaceuticals 2024, 17, 834. https://doi.org/10.3390/ph17070834
Vigano M, Kubal S, Lu Y, Habib S, Samarani S, Cama G, Viau C, Farzin H, Koudieh N, Xia J, et al. Impact of Cannabidiol and Exercise on Clinical Outcomes and Gut Microbiota for Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors: A Case Report. Pharmaceuticals. 2024; 17(7):834. https://doi.org/10.3390/ph17070834
Chicago/Turabian StyleVigano, MariaLuisa, Sarah Kubal, Yao Lu, Sarah Habib, Suzanne Samarani, Georgina Cama, Charles Viau, Houman Farzin, Nebras Koudieh, Jianguo Xia, and et al. 2024. "Impact of Cannabidiol and Exercise on Clinical Outcomes and Gut Microbiota for Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors: A Case Report" Pharmaceuticals 17, no. 7: 834. https://doi.org/10.3390/ph17070834
APA StyleVigano, M., Kubal, S., Lu, Y., Habib, S., Samarani, S., Cama, G., Viau, C., Farzin, H., Koudieh, N., Xia, J., Ahmad, A., Vigano, A., & Costiniuk, C. T. (2024). Impact of Cannabidiol and Exercise on Clinical Outcomes and Gut Microbiota for Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors: A Case Report. Pharmaceuticals, 17(7), 834. https://doi.org/10.3390/ph17070834






