Jingzhi Guanxin Oral Liquids Attenuate Atherosclerotic Coronary Heart Disease via Modulating Lipid Metabolism and PPAR-Related Targets
Abstract
1. Introduction
2. Results
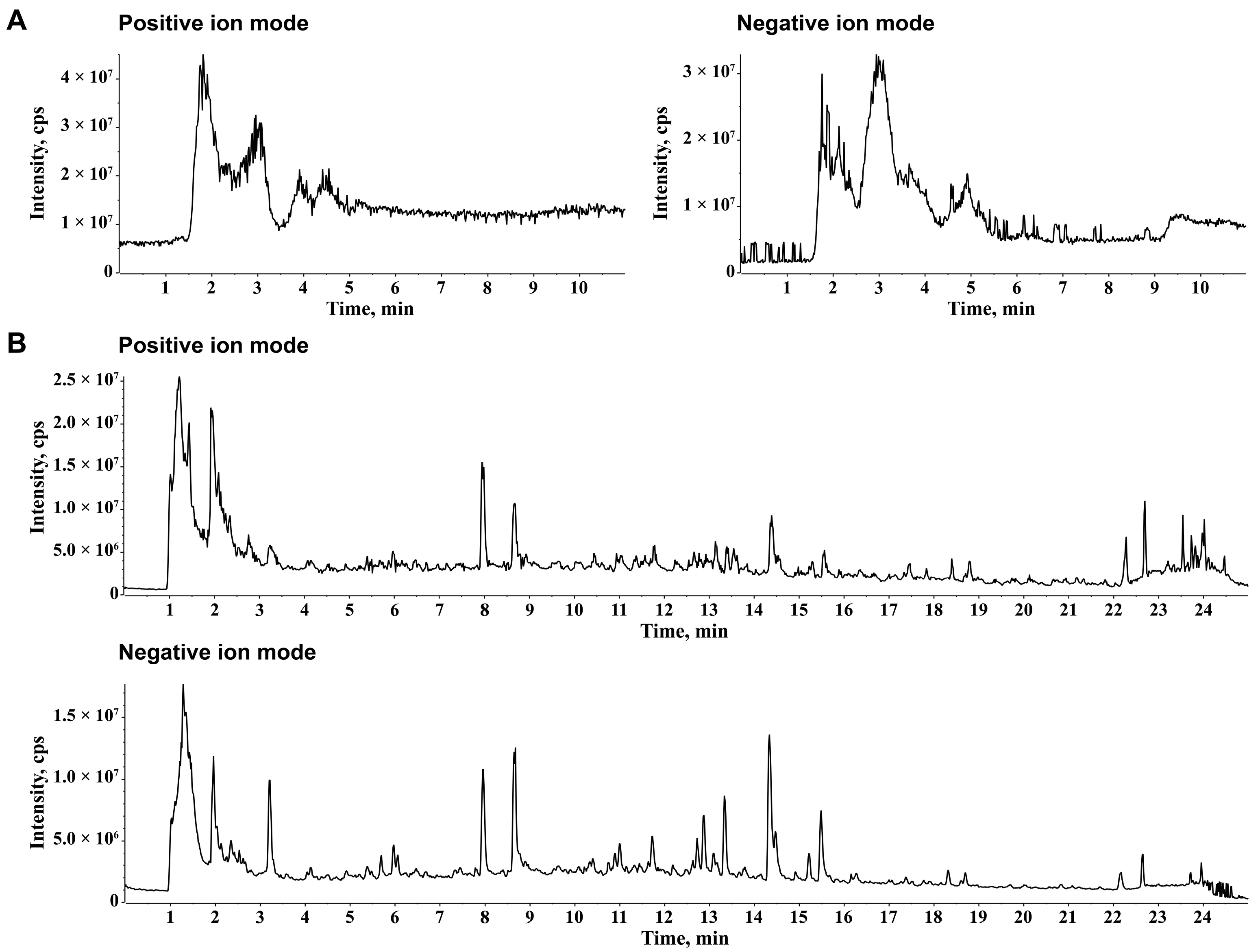
2.1. Chemical Ingredients of JZGX
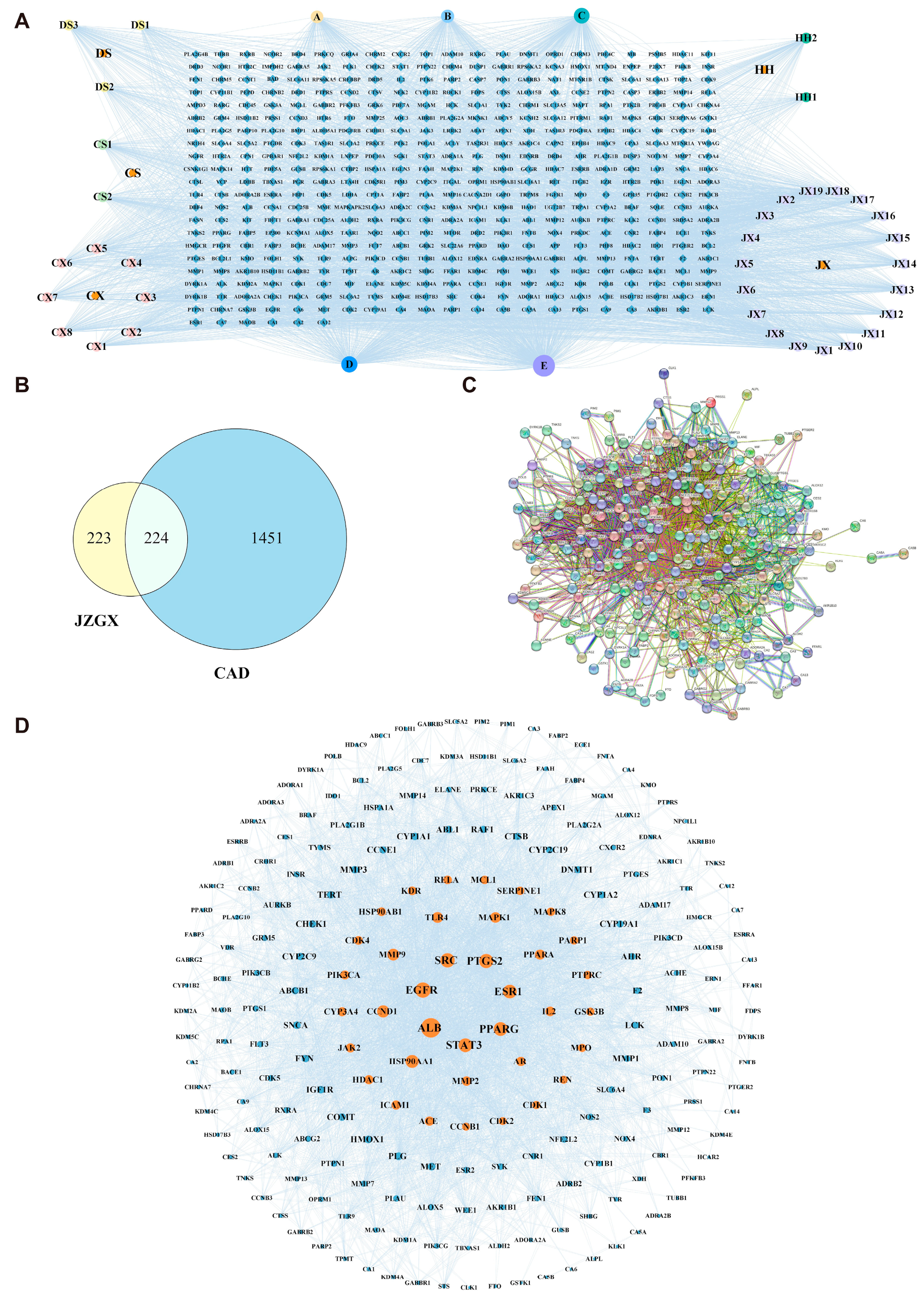
2.2. Key Targets for JZGX in the Treatment of CAD
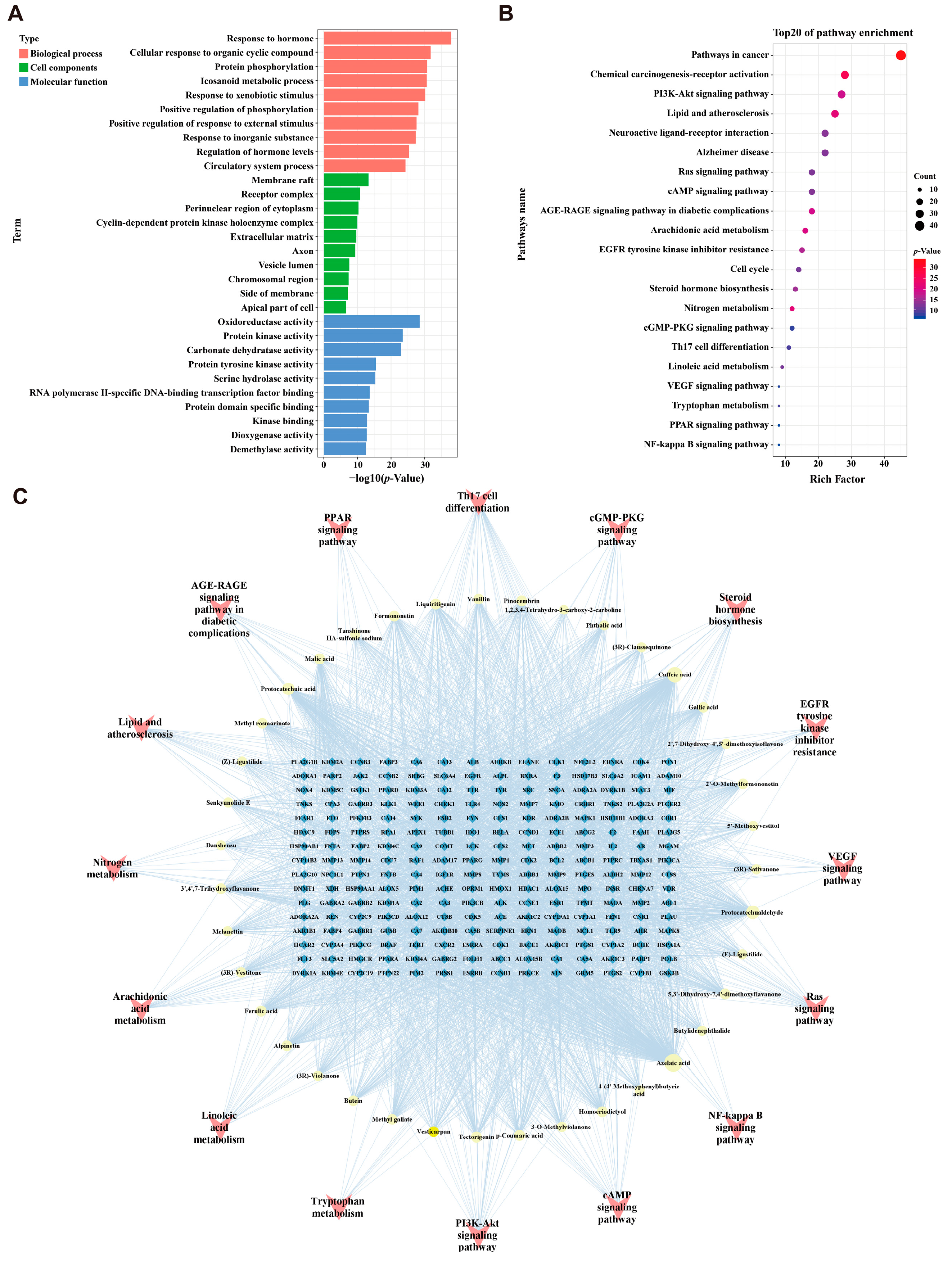
2.3. Enrichment Analysis and “Components-Targets-Pathways” Network Construction
2.4. JZGX Inhibited the HFD-Induced Formation of Atherosclerotic Plaques
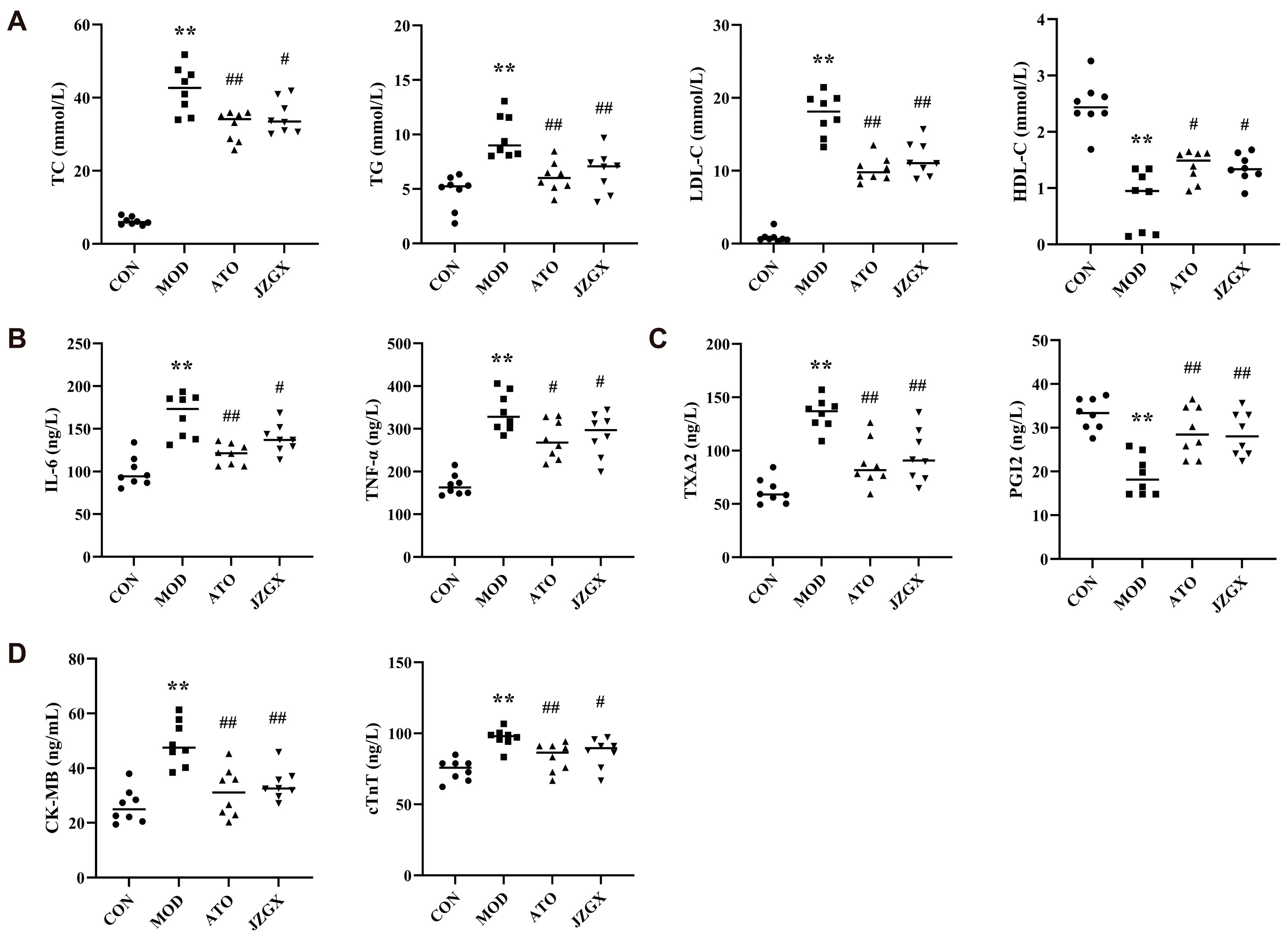
2.5. Effects of JZGX Treatments on Cardiovascular Biomarkers
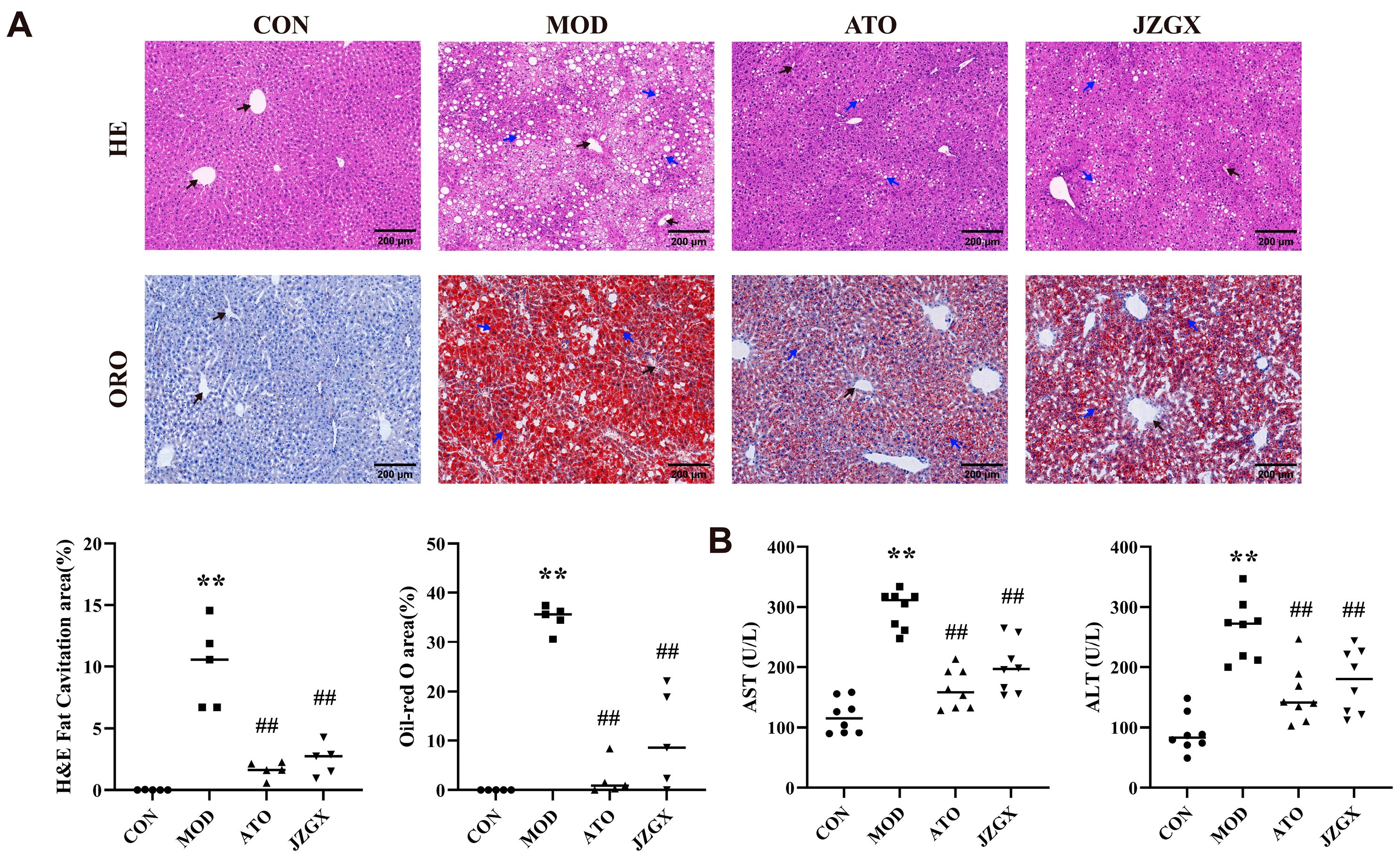
2.6. JZGX Alleviated HFD-Induced Hepatic Lipid Accumulation
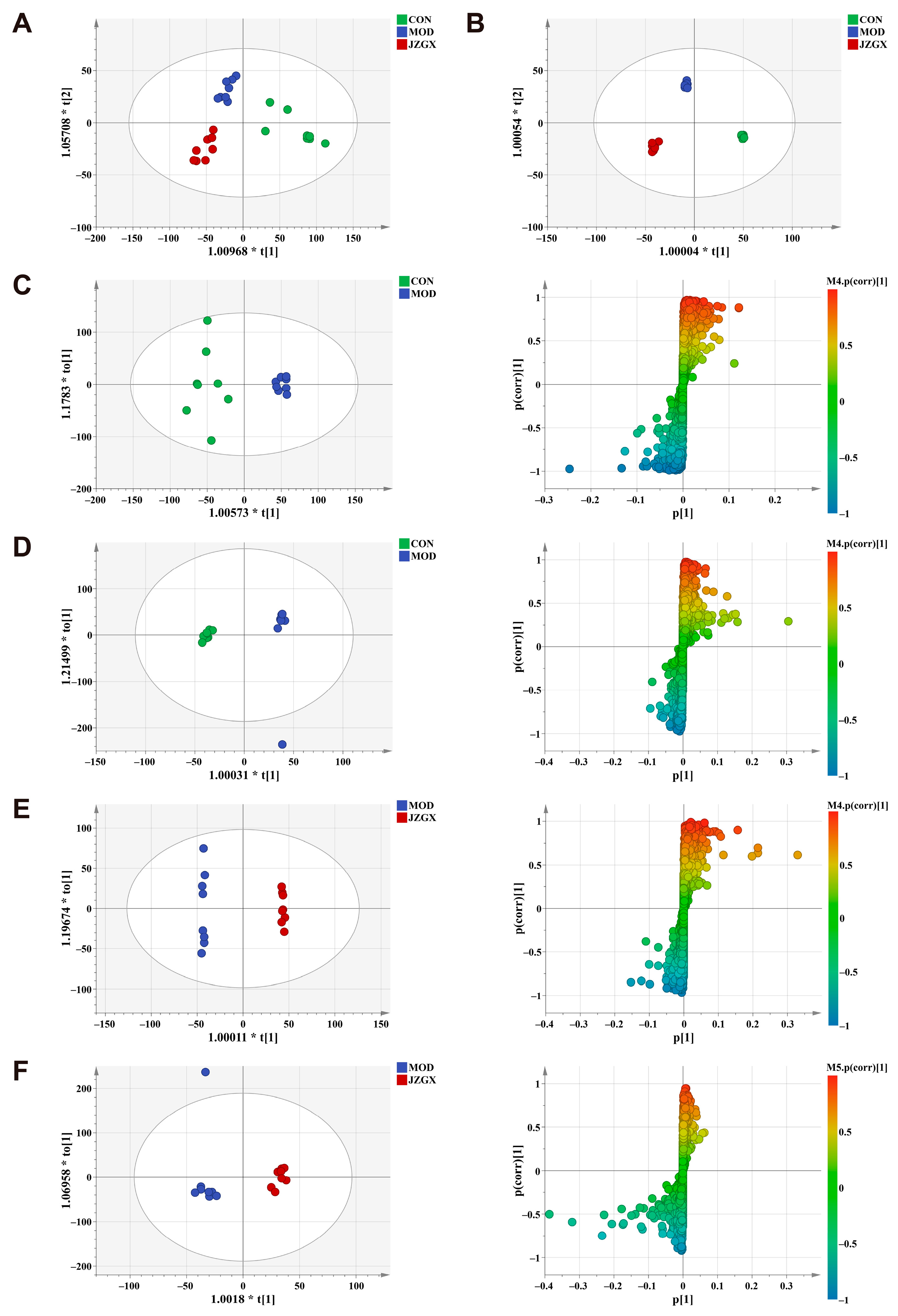
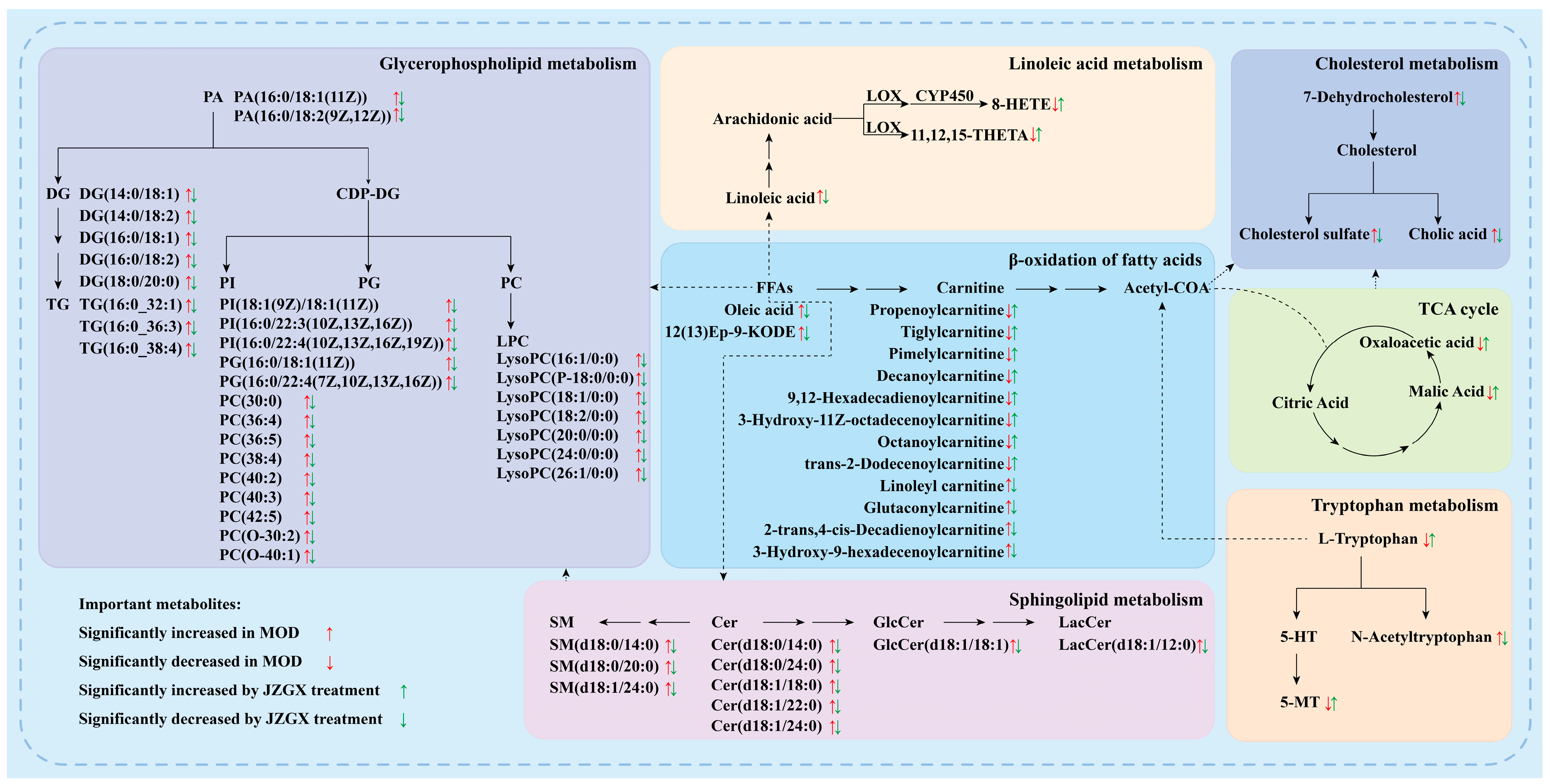
2.7. Serum Metabolomic Biomarkers for the JZGX Administration on CAD
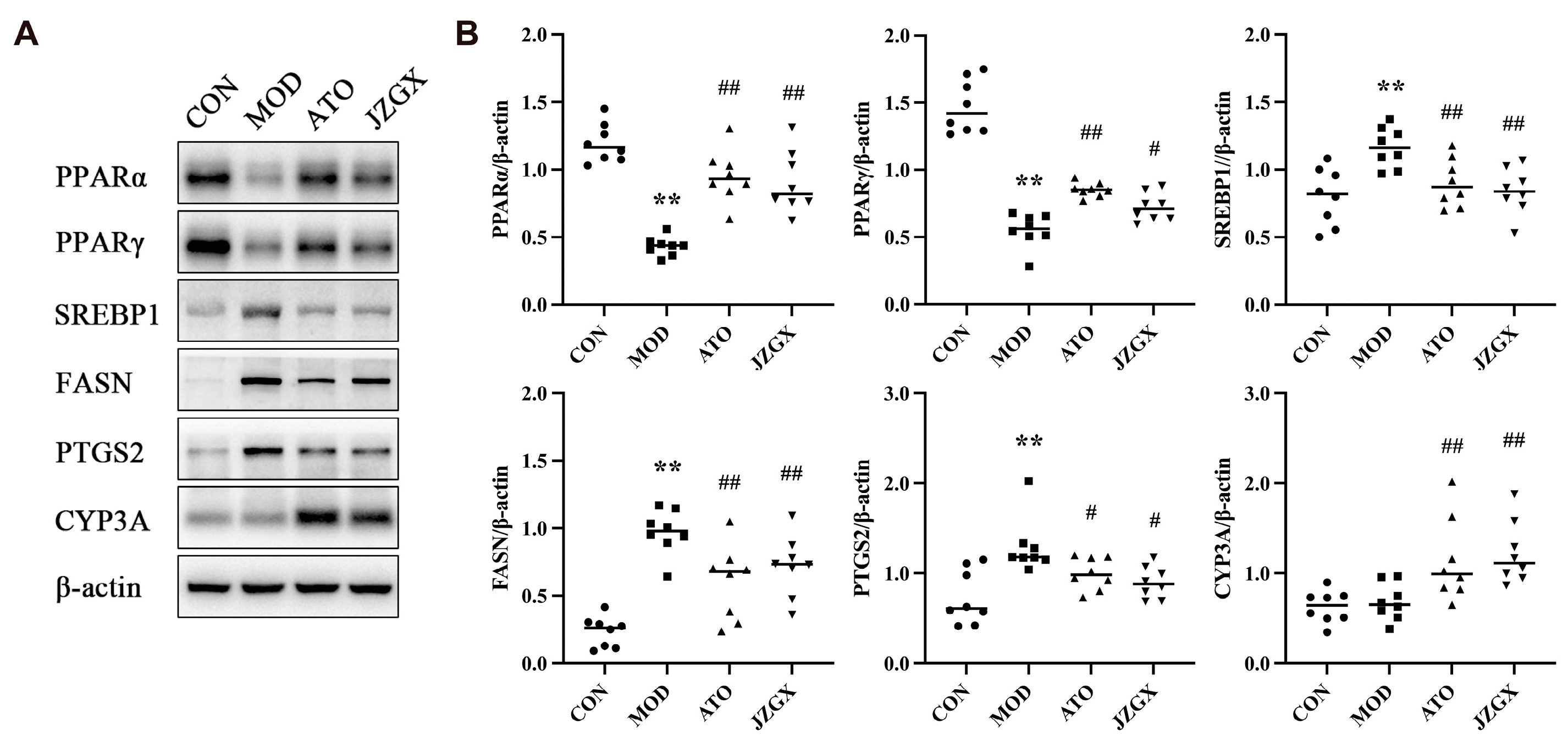
2.8. JZGX Influenced the Expression of PPAR-Related Lipid Metabolism Proteins
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. UPLC-Q-TOF-MS/MS Analysis for JZGX Ingredient Identification
4.3. Construction of the JZGX Active Component and CAD-related Target Network
4.4. Animals and Drug Administration
4.5. Histological Measurement
4.6. Detection of Biochemical Indexes
4.7. Untargeted Serum Metabolomics Profiling
4.8. Western Blotting
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: A narrative review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on Cardiovascular Health and Diseases in China 2022: An Updated Summary. Biomed. Environ. Sci. 2023, 36, 669–701. [Google Scholar]
- Davies, M.J.; Thomas, A.C. Plaque fissuring—The cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br. Heart J. 1985, 53, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, M.; Winslow, B.T.; Mills, K.; Dauber, I.M. Medical management of stable coronary artery disease. Am. Fam. Physician 2011, 83, 819–826. [Google Scholar] [PubMed]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-associated side effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Tsui, L.; Ye, P.; Xu, S.; Lin, Y.; Chen, B.; Chen, S.; Cheng, R. Adverse drug reactions of statin therapy in China from 1989 to 2019: A national database analysis. Eur. J. Hosp. Pharm. 2023, 30, e82–e89. [Google Scholar] [CrossRef]
- Pan, S.; Chen, S.; Dong, H.; Yu, Z.; Dong, J.; Long, Z.; Fong, W.; Han, Y.; Ko, K. New perspectives on Chinese herbal medicine (Zhong-Yao) research and development. Evid. Based Complement. Alternat Med. 2011, 2011, 403709. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Q.; Li, L.; Huang, S.; Yi, S.; Hu, Z. Effect of traditional Chinese medicine on the cardiovascular diseases. Front. Pharmacol. 2022, 13, 806300. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Liu, P. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Xie, P.; Cui, L.; Shan, Y.; Kang, W. Antithrombotic effect and mechanism of radix paeoniae rubra. Biomed. Res. Int. 2017, 2017, 9475074. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, C.; Gao, F.; Fu, Q.; Fu, C.; He, Y.; Zhang, J. A systematic review on the rhizome of Ligusticum chuanxiong Hort. (Chuanxiong). Food Chem. Toxicol. 2018, 119, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Xue, Y.; Guo, D.; Sun, L.; Guo, M. Carthami flos: A review of its ethnopharmacology, pharmacology and clinical applications. Rev. Bras. Farmacogn. 2015, 25, 553–566. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Meng, H.; Yu, Z.; Yang, M.; Wei, J. Dalbergia odorifera: A review of its traditional uses, phytochemistry, pharmacology, and quality control. J. Ethnopharmacol. 2020, 248, 112328. [Google Scholar] [CrossRef]
- Xin, W.; Zi-Yi, W.; Zheng, J.-H.; Shao, L. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar]
- Zhao, S.; Liu, Z.; Wang, M.; He, D.; Liu, L.; Shu, Y.; Song, Z.; Li, H.; Liu, Y.; Lu, A. Anti-inflammatory effects of Zhishi and Zhiqiao revealed by network pharmacology integrated with molecular mechanism and metabolomics studies. Phytomedicine 2018, 50, 61–72. [Google Scholar] [CrossRef]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.-M.; Ricard, S.; Nicaud, V.; Mallet, C.; Arveiler, D.; Evans, A.; Ruidavets, J.B.; Luc, G.; Bara, L.; Parra, H.J.; et al. Polymorphisms of the tumour necrosis factor-α gene, coronary heart disease and obesity. Eur. J. Clin. Invest. 1998, 28, 59–66. [Google Scholar] [CrossRef]
- Gryglewski, R.; Dembínska-Kieć, A.; Korbut, R. A possible role of thromboxane A2 (TXA2) and prostacyclin (PGI2) in circulation. Acta Biol. Med. Ger. 1978, 37, 715–723. [Google Scholar] [PubMed]
- Peela, J.; Jarari, A.; Hai, A.; Rawal, A.; Kolla, S.; Sreekumar, S.; Khurana, L.; Sidhanathi, N. Cardiac biomarkers: The troponins and CK-MB. J. Medical Biomed. Sci. 2010, 2, 190–197. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc’h, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Caturano, A.; Vetrano, E.; Cesaro, A.; Rinaldi, L.; Salvatore, T.; Marfella, R.; Sardu, C.; Moscarella, E.; Gragnano, F. Pathophysiological mechanisms and clinical evidence of relationship between Nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Rev. Cardiovasc. Med. 2021, 22, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet 2000, 355, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Apekey, T.A.; Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 2014, 236, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.C. Chemometrics methods and strategies in metabolomics. In Metabolomics: From Fundamentals to Clinical Applications; Springer: Cham, Switzerland, 2017; pp. 163–190. [Google Scholar]
- Zandbergen, F.; Plutzky, J. PPARα in atherosclerosis and inflammation. Biochim. Biophys. Acta 2007, 1771, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shen, W.-J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017, 13, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Wootton, R.; Lewis, B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 534–542. [Google Scholar] [CrossRef]
- McLaren, J.E.; Michael, D.R.; Ashlin, T.G.; Ramji, D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011, 50, 331–347. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Pecere, S.; Gasbarrini, A.; Ojetti, V. Physiology and pathophysiology of liver lipid metabolism. Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 1055–1067. [Google Scholar] [CrossRef]
- Huang, N.K.; Bůžková, P.; Matthan, N.R.; Djoussé, L.; Hirsch, C.H.; Kizer, J.R.; Longstreth, W., Jr.; Mukamal, K.J.; Lichtenstein, A.H. Associations of serum nonesterified fatty acids with coronary heart disease mortality and nonfatal myocardial infarction: The CHS (cardiovascular health study) cohort. J. Am. Heart Assoc. 2021, 10, e019135. [Google Scholar] [CrossRef]
- Nomura, S.O.; Karger, A.B.; Weir, N.L.; Duprez, D.A.; Tsai, M.Y. Free fatty acids, cardiovascular disease, and mortality in the Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 2020, 14, 531–541. [Google Scholar] [CrossRef]
- Hennig, B.; Toborek, M.; McClain, C.J. High-Energy Diets, Fatty Acids and Endothelial Cell Function: Implications for Atherosclerosis. J. Am. Coll. Nutr. 2001, 20, 97–105. [Google Scholar] [CrossRef]
- Jira, W.; Spiteller, G.; Carson, W.; Schramm, A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem. Phys. Lipids 1998, 91, 1–11. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Litvinov, D.; Selvarajan, K.; Garelnabi, M. Lipid peroxidation and decomposition—Conflicting roles in plaque vulnerability and stability. Biochim. Biophys. Acta 2008, 1781, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, Patterns of Recognition, and Pathophysiology. Antioxid. Redox Signal 2010, 13, 39–75. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Neyen, C.; Gordon, S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 2012, 217, 492–502. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Yu, K.; Bayona, W.; Kallen, C.B.; Harding, H.P.; Ravera, C.P.; McMahon, G.; Brown, M.; Lazar, M.A. Differential Activation of Peroxisome Proliferator-activated Receptors by Eicosanoids. J. Biol. Chem. 1995, 270, 23975–23983. [Google Scholar] [CrossRef]
- Poulsen, L.l.C.; Siersbæk, M.; Mandrup, S. PPARs: Fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012, 23, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.B.; Spitzbarth, N.; Gauthier, K.M.; Pfister, S.L. 11,12,15-Trihydroxyeicosatrienoic acid mediates ACh-induced relaxations in rabbit aorta. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2648–H2656. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.R.; Lee, J.Y.; Park, H.S.; Heo, H.J.; Park, J.Y.; Bae, S.S.; Hong, K.W.; Sung, S.M.; Kim, C.D. Oleic acid enhances vascular smooth muscle cell proliferation via phosphatidylinositol 3-kinase/Akt signaling pathway. Pharmacol. Res. 2006, 54, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, D.; Li, D.; Tang, B.; Yang, Y. Oleic acid induces smooth muscle foam cell formation and enhances atherosclerotic lesion development via CD36. Lipids Health Dis. 2011, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kukharenko, A.; Brito, A.; Kozhevnikova, M.V.; Moskaleva, N.; Markin, P.A.; Bochkareva, N.; Korobkova, E.O.; Belenkov, Y.N.; Privalova, E.V.; Larcova, E.V.; et al. Relationship between the plasma acylcarnitine profile and cardiometabolic risk factors in adults diagnosed with cardiovascular diseases. Clin. Chim. Acta 2020, 507, 250–256. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [PubMed]
- Talayero, B.G.; Sacks, F.M. The Role of Triglycerides in Atherosclerosis. Curr. Cardiol. Rep. 2011, 13, 544–552. [Google Scholar] [CrossRef]
- Chatterjee, S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1523–1533. [Google Scholar] [CrossRef]
- Merten, M.; Dong, J.F.; Lopez, J.A.; Thiagarajan, P. Cholesterol sulfate: A new adhesive molecule for platelets. Circulation 2001, 103, 2032–2034. [Google Scholar] [CrossRef]
- Murphy, C.; Parini, P.; Wang, J.; Björkhem, I.; Eggertsen, G.; Gåfvels, M. Cholic acid as key regulator of cholesterol synthesis, intestinal absorption and hepatic storage in mice. Biochim. Biophys. Acta 2005, 1735, 167–175. [Google Scholar] [CrossRef]
- Chirala, S.S.; Wakil, S.J. Structure and function of animal fatty acid synthase. Lipids 2004, 39, 1045–1053. [Google Scholar] [CrossRef]
- Semple, R.K.; Chatterjee, V.K.K.; O’Rahilly, S. PPARγ and human metabolic disease. J. Clin. Investig. 2006, 116, 581–589. [Google Scholar] [CrossRef]
- Janani, C.; Kumari, B.R. PPAR gamma gene–a review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Siva, D.; Abinaya, S.; Rajesh, D.; Archunan, G.; Padmanabhan, P.; Gulyás, B.; Achiraman, S. Mollification of doxorubicin (DOX)-mediated cardiotoxicity using conjugated chitosan nanoparticles with supplementation of propionic acid. Nanomaterials 2022, 12, 502. [Google Scholar] [CrossRef]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Chawla, A.; Boisvert, W.A.; Lee, C.-H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Takeuchi, Y.; Nakagawa, Y.; Takahashi, H.; Okazaki, H.; Iizuka, Y.; Ohashi, K.; Gotoda, T. SREBP-1-independent regulation of lipogenic gene expression in adipocytes. J. Lipid Res. 2007, 48, 1581–1591. [Google Scholar] [CrossRef]
- Latasa, M.-J.; Griffin, M.J.; Moon, Y.S.; Kang, C.; Sul, H.S. Occupancy and function of the− 150 sterol regulatory element and− 65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol. Cell Biol. 2003, 23, 5896–5907. [Google Scholar] [CrossRef]
- Gosmain, Y.; Dif, N.; Berbe, V.; Loizon, E.; Rieusset, J.; Vidal, H.; Lefai, E. Regulation of SREBP-1 expression and transcriptional action on HKII and FAS genes during fasting and refeeding in rat tissues. J. Lipid Res. 2005, 46, 697–705. [Google Scholar] [CrossRef]
- Schroeder, F.; Petrescu, A.D.; Huang, H.; Atshaves, B.P.; McIntosh, A.L.; Martin, G.G.; Hostetler, H.A.; Vespa, A.; Landrock, D.; Landrock, K.K.; et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 2008, 43, 1–17. [Google Scholar] [CrossRef]
- Klyushova, L.S.; Perepechaeva, M.L.; Grishanova, A.Y. The Role of CYP3A in Health and Disease. Biomedicines 2022, 10, 2686. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kobayashi, K.; Watanabe, M.; Kazuki, Y.; Takehara, S.; Inaba, A.; Nitta, S.I.; Senda, N.; Oshimura, M.; Chiba, K. Knockout of mouse Cyp3a gene enhances synthesis of cholesterol and bile acid in the liver. J. Lipid Res. 2013, 54, 2060–2068. [Google Scholar] [CrossRef]
- Jamwal, R.; de la Monte, S.M.; Ogasawara, K.; Adusumalli, S.; Barlock, B.B.; Akhlaghi, F. Nonalcoholic Fatty Liver Disease and Diabetes Are Associated with Decreased CYP3A4 Protein Expression and Activity in Human Liver. Mol. Pharm. 2018, 15, 2621–2632. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Schriemer, R.; Robertson, G.R. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J. Hepatol. 2002, 37, 206–213. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Chitturi, S.; Farrell, G.C. Etiopathogenesis of nonalcoholic steatohepatitis. Semin. Liver Dis. 2001, 21, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Mei, J.; Yang, J.; Wu, Z.; Liu, J.; Miao, P.; Chen, Y.; Wen, Z.; Zhao, Z.; Kong, H.; et al. ApoE deficiency promotes non-alcoholic fatty liver disease in mice via impeding AMPK/mTOR mediated autophagy. Life Sci. 2020, 252, 117601. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, T.; Jiang, Y.; He, Y.; Li, P.; Peng, W.; Wang, Y.; Su, W. Jingzhi Guanxin Oral Liquids Attenuate Atherosclerotic Coronary Heart Disease via Modulating Lipid Metabolism and PPAR-Related Targets. Pharmaceuticals 2024, 17, 784. https://doi.org/10.3390/ph17060784
Wang X, Hu T, Jiang Y, He Y, Li P, Peng W, Wang Y, Su W. Jingzhi Guanxin Oral Liquids Attenuate Atherosclerotic Coronary Heart Disease via Modulating Lipid Metabolism and PPAR-Related Targets. Pharmaceuticals. 2024; 17(6):784. https://doi.org/10.3390/ph17060784
Chicago/Turabian StyleWang, Xinning, Tao Hu, Yuliang Jiang, Yan He, Peibo Li, Wei Peng, Yonggang Wang, and Weiwei Su. 2024. "Jingzhi Guanxin Oral Liquids Attenuate Atherosclerotic Coronary Heart Disease via Modulating Lipid Metabolism and PPAR-Related Targets" Pharmaceuticals 17, no. 6: 784. https://doi.org/10.3390/ph17060784
APA StyleWang, X., Hu, T., Jiang, Y., He, Y., Li, P., Peng, W., Wang, Y., & Su, W. (2024). Jingzhi Guanxin Oral Liquids Attenuate Atherosclerotic Coronary Heart Disease via Modulating Lipid Metabolism and PPAR-Related Targets. Pharmaceuticals, 17(6), 784. https://doi.org/10.3390/ph17060784






