Amicis Omnia Sunt Communia: NF-κB Inhibition as an Alternative to Overcome Osteosarcoma Heterogeneity
Abstract
1. The NF-κB Pathway
2. NF-κB Dysregulation and Cancer
Osteosarcoma
3. Experimental Evidence NF-κB Inhibition
| Inhibitor | Target | IC50 | In Vivo | Antiproliferation | Anti-Motility | Chemosensitizer/Synergism | Radiosensitizer | Reference |
|---|---|---|---|---|---|---|---|---|
| Amentoflavone | IκBα degradation and p65 translocation | 50–100 μM | yes | yes | yes | yes | - | [193,196] |
| Andrographolide | Modification of p50 | 50–70 μM | yes | yes | yes | - | - | [201] |
| Bay 11-7085 | Inhibition of IκBα | 10 μM | yes | yes | yes | yes | - | [104,204] |
| Bortezomib | Proteasome | yes | yes | yes | yes | [303,304] | ||
| Caffeine | p65 and antioxidant | 1–2.80 mM | yes | yes | yes | yes | - | [322,326] |
| Curcumin | IKK activety, IkBα and p65 phosphorilation | 10–100 nM | yes | yes | yes | yes | - | [314,315] |
| DHMEQ | Inhibition of p65 | 12–48 μg/mL | - | yes | yes | yes | - | [206,207] |
| Dihydroartemisinin | IκBα degradation and DNA binding | 4.6–16 | yes | yes | yes | yes | - | [212] |
| Dihydromyricetin | Phosphorylation and degradation of IκBα | 20–60 μmol/mL | yes | yes | yes | yes | - | [216] |
| Genistein | antioxidant | 20–80 μM | yes | yes | yes | yes | - | [295,296] |
| Ginsenoside Rh2 | NF-kB degradation | 2.52–7.85 μg/mL | yes | yes | yes | yes | - | [219,220,221,222] |
| Isoalantolactone | p65 | 0–200 μM | - | yes | - | - | - | [226] |
| Isoliquiritigenin | p65 translocation | 0–100 μM | yes | yes | yes | [230,231,232] | ||
| Lapachone | p65 phosphorilation | 0–10 μM | - | yes | - | - | - | [234,235] |
| Licoricidin | p65 | 0–32 μM | yes | yes | - | yes | - | [239] |
| Magnoflorine | p65 phosphorilation, IkBα | 5–80 μM | - | yes | yes | yes | - | [241] |
| Magnolol | antioxidant | 25–41 μM | - | yes | yes | - | - | [300,301] |
| Mangostin | IkBα and p65 phosphorilation | 30–40 μM | yes | yes | yes | - | - | [291,292] |
| Matrine | p50 and p65 translocation, IkB-β | 0–1.5 mg/mL | yes | yes | yes | yes | - | [243] |
| Metformin | p65 phosphorylation | 0–50 mM | yes | yes | yes | - | - | [316,317,318,319,320] |
| Nimbolide | p-IKK-β/α, p-p65 | 0–250 µg/mL | - | yes | yes | - | - | [246,247] |
| Okadaic acid | p65 | 0–50 nM | - | yes | yes | - | - | [248,249,250] |
| Parthenolide | p-65 | 0–100 μM | yes | yes | yes | - | yes | [257] |
| PHLORETIN | IκB-α phosphorylation and p65 translocation | 100 μg | - | yes | - | yes | - | [261] |
| Punicalagin | p65 | 10–100 μM | yes | yes | yes | - | - | [262,263] |
| Raddeanin A | p65 | 1512–10.05 μM | yes | yes | yes | yes | - | [266,267] |
| Sulphoraphene | p65 | 40 μM | yes | yes | yes | - | - | [269] |
| Tetramethylpyrazine | p65 | 10.3, 24.7, 54.7 mg/mL | yes | yes | - | - | - | [271] |
| Theabrownin | p65 | 43.93 or 51.98 mg/mL | yes | yes | yes | - | - | [272,273] |
| Thymoquinone | TNF-α | 17–40 μM | yes | yes | - | yes | - | [278,280,281,282] |
| Ursolic Acid | IKK and p65 phosphorilation | 5–37 μM | yes | yes | yes | yes | - | [286,287] |
4. Final Considerations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sen, R. David Baltimore Multiple Nuclear Factors Interact with the Immunoglobulin Enhancer Sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Bours, V.; Burd, P.R.; Brown, K.; Villalobos, J.; Park, S.; Ryseck, R.P.; Bravo, R.; Kelly, K.; Siebenlist, U. A Novel Mitogen-Inducible Gene Product Related to P50/P105-NF-Kappa B Participates in Transactivation through a Kappa B Site. Mol. Cell. Biol. 1992, 12, 685–695. [Google Scholar] [CrossRef]
- Bours, V.; Villalobos, J.; Burd, P.R.; Kelly, K.; Siebenlist, U. Cloning of a Mitogen-Inducible Gene Encoding a ΚB DNA-Binding Protein with Homology to the Rel Oncogene and to Cell-Cycle Motifs. Nature 1990, 348, 76–80. [Google Scholar] [CrossRef]
- Ghosh, S.; Gifford, A.M.; Riviere, L.R.; Tempst, P.; Nolan, G.P.; Baltimore, D. Cloning of the P50 DNA Binding Subunit of NF-ΚB: Homology to Rel and Dorsal. Cell 1990, 62, 1019–1029. [Google Scholar] [CrossRef]
- Kieran, M.; Blank, V.; Logeat, F.; Vandekerckhove, J.; Lottspeich, F.; Le Bail, O.; Urban, M.B.; Kourilsky, P.; Baeuerle, P.A.; Israël, A. The DNA Binding Subunit of NF-ΚB Is Identical to Factor KBF1 and Homologous to the Rel Oncogene Product. Cell 1990, 62, 1007–1018. [Google Scholar] [CrossRef]
- Mercurio, F.; Didonato, J.; Rosette, C.; Karin, M. Molecular Cloning and Characterization of a Novel Rel/NF-ΧB Family Member Displaying Structural and Functional Homology to NF-ΧB P50/P105. DNA Cell Biol. 1992, 11, 523–537. [Google Scholar] [CrossRef]
- Meyer, R.; Hatada, E.N.; Hohmann, H.P.; Haiker, M.; Bartsch, C.; Rothlisberger, U.; Lahm, H.W.; Schlaeger, E.J.; Van Loon, A.P.G.M.; Scheidereit, C. Cloning of the DNA-Binding Subunit of Human Nuclear Factor ΚB: The Level of Its MRNA Is Strongly Regulated by Phorbol Ester or Tumor Necrosis Factor α. Proc. Natl. Acad. Sci. USA 1991, 88, 966–970. [Google Scholar] [CrossRef]
- Miyamoto, S.; Verma, I.M. REL/NF-ΚB/IkB Story. Adv. Cancer Res. 1995, 66, 255–292. [Google Scholar]
- Ruben, S.M.; Dillon, P.J.; Schreck, R.; Henkel, T.; Chen, C.H.; Maher, M.; Baeuerle, P.A.; Rosen, C.A. Isolation of a Rel-Related Human CDNA That Potentially Encodes the 65-KD Subunit of NF-ΚB. Science 1991, 251, 1490–1493. [Google Scholar] [CrossRef]
- Ryseck, R.-P.; Bull, P.; Takamiya, M.; Bours, V.; Siebenlist, U.; Dobrzanski, P.; Bravo, R. New Rel Family Transcription Activator That Can Interact with P5O-NF-ΚB. Mol. Cell Biol. 1992, 12, 674–684. [Google Scholar]
- Steward, R. Dorsal, an Embryonic Polarity Gene in Drosophila, Is Homologous to the Vertebrate Proto-Oncogene, c-Rel. Science 1987, 238, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hyden, M.S. Celebrating 25 Years of Ideas. Imunol. Rev. 2012, 49, 10–11. [Google Scholar]
- Karin, M. How NF-k B Is Activated: The Role of the I k B Kinase (IKK) Complex. Oncogene 1999, 18, 6867–6874. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, F.; Didonato, J.A.; Rosette, C.; Karin, M. P105 and P98 Precursor Protein Play an Active Role in NF-ΚB-Mediated Signal Transduction. Genes Dev. 1993, 7, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Wulczyn, F.G.; Scheidereit, C. The NF-Kappa B Precursor P105 and the Proto-Oncogene Product Bcl-3 Are I Kappa B Molecules and Control Nuclear Translocation of NF-Kappa B. EMBO J. 1993, 12, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Rice, N.R.; MacKichan, M.L.; Israël, A. The Precursor of NF-ΚB P50 Has IκB-like Functions. Cell 1992, 71, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Scheinman, R.I.; Beg, A.A.; Baldwin, A.S. NF-Kappa B P100 (Lyt-10) Is a Component of H2TF1 and Can Function as an I Kappa B-like Molecule. Mol. Cell. Biol. 1993, 13, 6089–6101. [Google Scholar] [CrossRef] [PubMed]
- Zabel, U.; Baeuerle, P.A. Purified Human IκB Can Rapidly Dissociate the Complex of the NF-ΚB Transcription Factor with Its Cognate DNA. Cell 1990, 61, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-ΚB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Li, Z.W.; Chu, W.; Hu, Y.; Delhase, M.; Deerinck, T.; Ellisman, M.; Johnson, R.; Karin, M. The IKKβ Subunit of IκB Kinase (IKK) Is Essential for Nuclear Factor ΚB Activation and Prevention of Apoptosis. J. Exp. Med. 1999, 189, 1839–1845. [Google Scholar] [CrossRef]
- Ling, L.; Cao, Z.; Goeddel, D.V. Nf-ΚB-Inducing Kinase Activates IKK-α by Phosphorylation of Ser-176. Proc. Natl. Acad. Sci. USA 1998, 95, 3792–3797. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, F.; Zhu, H.; Murray, B.W.; Shevchenko, A.; Bennett, B.L.; Li, J.W.; Young, D.B.; Barbosa, M.; Mann, M.; Manning, A.; et al. IKK-1 and IKK-2: Cytokine-Activated IκB Kinases Essential for NF-ΚB Activation. Science 1997, 278, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Alkalay, I.; Yaron, A.; Hatzubai, A.; Jung, S.; Avraham, A.; Gerlitz, O.; Pashut-Lavon, I.; Ben-Neriah, Y. In Vivo Stimulation of I Kappa B Phosphorylation Is Not Sufficient to Activate NF-Kappa B. Mol. Cell. Biol. 1995, 15, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Roff, M.; Thompson, J.; Rodriguez, M.S.; Jacque, J.M.; Baleux, F.; Arenzana-Seisdedos, F.; Hay, R.T. Role of IκBα Ubiquitination in Signal-Induced Activation of NF-ΚB in Vivo. J. Biol. Chem. 1996, 271, 7844–7850. [Google Scholar] [CrossRef] [PubMed]
- Scherer, D.C.; Brockman, J.A.; Chen, Z.; Maniatis, T.; Ballard, D.W. Signal-Induced Degradation of IκBα Requires Site-Specific Ubiquitination. Proc. Natl. Acad. Sci. USA 1995, 92, 11259–11263. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Ruben, S.M.; Scheinman, R.I.; Haskill, S.; Rosen, C.A.; Baldwin, A.S. IKB Interacts with the Nuclear Localization Sequences of the Subunits of NF-ΚB: A Mechanism for Cytoplasmic Retention. Genes Dev. 1992, 6, 2664–2665. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Baltimore, D. Inducibility of κ Immunoglobulin Enhancer-Binding Protein NF-ΚB by a Posttranslational Mechanism. Cell 1986, 47, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, H.; Hale, V.A.; Dolcet, X.; Davies, A. NF-ΚB Signalling Regulates the Growth of Neural Processes in the Developing PNS and CNS. Development 2005, 132, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-ΚB Functions in Synaptic Signaling and Behavior. Nat. Neurosci. 2003, 6, 1072–1078. [Google Scholar] [CrossRef]
- Dejardin, E. The Alternative NF-ΚB Pathway from Biochemistry to Biology: Pitfalls and Promises for Future Drug Development. Biochem. Pharmacol. 2006, 72, 1161–1179. [Google Scholar] [CrossRef]
- Sun, S.C. The Non-Canonical NF-ΚB Pathway in Immunity and Inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Harhaj, E.W.; Sun, S.-C. NF-ΚB-Inducing Kinase Regulates the Processing of NF-ΚB2 P100. Mol. Cell 2001, 7, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.; Sun, S.C. Genetic Evidence for the Essential Role of β-Transducin Repeat-Containing Protein in the Inducible Processing of NF-ΚB2/P100. J. Biol. Chem. 2002, 277, 22111–22114. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, M.; Sun, S.C. β-TrCP Binding and Processing of NF-ΚB2/P100 Involve Its Phosphorylation at Serines 866 and 870. Cell Signal 2006, 18, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKα of a Second, Evolutionary Conserved, NF-ΚB Signaling Pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Babic, I.; Nathanson, D.; Akhavan, D.; Guo, D.; Gini, B.; Dang, J.; Zhu, S.; Yang, H.; de Jesus, J.; et al. Oncogenic EGFR Signaling Activates an MTORC2-NF-ΚB Pathway That Promotes Chemotherapy Resistance. Cancer Discov. 2011, 1, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ahsan, H. Ras-Mediated Activation of NF-ΚB and DNA Damage Response in Carcinogenesis. Cancer Investig. 2020, 38, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Bonavia, R.; Inda, M.M.; Vandenberg, S.; Cheng, S.Y.; Nagane, M.; Hadwiger, P.; Tan, P.; Sah, D.W.Y.; Cavenee, W.K.; Furnari, F.B. EGFRvIII Promotes Glioma Angiogenesis and Growth through the NF-ΚB, Interleukin-8 Pathway. Oncogene 2012, 31, 4054–4066. [Google Scholar] [CrossRef]
- Puliyappadamba, V.T.; Chakraborty, S.; Chauncey, S.S.; Li, L.; Hatanpaa, K.J.; Mickey, B.; Noorani, S.; Shu, H.K.G.; Burma, S.; Boothman, D.A.; et al. Opposing Effect of EGFRWT on EGFRvIII-Mediated NF-ΚB Activation with RIP1 as a Cell Death Switch. Cell Rep. 2013, 4, 764–775. [Google Scholar] [CrossRef]
- Spiller, S.E.; Logsdon, N.J.; Deckard, L.A.; Sontheimer, H. Inhibition of Nuclear Factor Kappa-B Signaling Reduces Growth in Medulloblastoma in Vivo. BMC Cancer 2011, 11, 136. [Google Scholar] [CrossRef]
- Parker, M.; Mohankumar, K.M.; Punchihewa, C.; Weinlich, R.; Dalton, J.D.; Li, Y.; Lee, R.; Tatevossian, R.G.; Phoenix, T.N.; Thiruvenkatam, R.; et al. C11orf95-RELA Fusions Drive Oncogenic NF-ΚB Signalling in Ependymoma. Nature 2014, 506, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, T.; Wohlers, I.; Goschzik, T.; Dreschmann, V.; Denkhaus, D.; Dörner, E.; Rahmann, S.; Klein-Hitpass, L. Supratentorial Ependymomas of Childhood Carry C11orf95-RELA Fusions Leading to Pathological Activation of the NF-ΚB Signaling Pathway. Acta Neuropathol. 2014, 127, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Cherry, E.M.; Lee, D.W.; Jung, J.U.; Sitcheran, R. Tumor Necrosis Factor-like Weak Inducer of Apoptosis (TWEAK) Promotes Glioma Cell Invasion through Induction of NF-ΚB-Inducing Kinase (NIK) and Noncanonical NF-ΚB Signaling. Mol. Cancer 2015, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Friedmann-Morvinski, D.; Narasimamurthy, R.; Xia, Y.; Myskiw, C.; Soda, Y.; Verma, I.M. Targeting NF-ΚB in Glioblastoma: A Therapeutic Approach. Sci. Adv. 2016, 2, e1501292. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Ciani, Y.; Ruaro, M.E.; Isola, M.; Sorrentino, M.; Bulfoni, M.; Candotti, V.; Correcig, C.; Bourkoula, E.; Manini, I.; et al. An NF-κ B Signature Predicts Low-Grade Glioma Prognosis: A Precision Medicine Approach Based on Patient-Derived Stem Cells. Neuro Oncol. 2018, 20, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, M.; Pratap, U.P.; Viswanadhapalli, S.; Liu, J.; Venkata, P.P.; Altwegg, K.A.; Palacios, B.E.; Li, X.; Chen, Y.; et al. PELP1 Signaling Contributes to Medulloblastoma Progression by Regulating the NF-ΚB Pathway. Mol. Carcinog. 2020, 59, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Arabzade, A.; Zhao, Y.; Varadharajan, S.; Chen, H.C.; Jessa, S.; Rivas, B.; Stuckert, A.J.; Solis, M.; Kardian, A.; Tlais, D.; et al. Zfta–Rela Dictates Oncogenic Transcriptional Programs to Drive Aggressive Supratentorial Ependymoma. Cancer Discov. 2021, 11, 2200–2215. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-ΚB Signaling Pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-ΚB in Cancer: From Innocent Bystander to Major Culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Galeaz, C.; Totis, C.; Bisio, A. Radiation Resistance: A Matter of Transcription Factors. Front. Oncol. 2021, 11, 662840. [Google Scholar] [CrossRef]
- Shiloh, Y. The ATM-Mediated DNA-Damage Response: Taking Shape. Trends Biochem. Sci. 2006, 31, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Mokim Ahmed, K.; Li, J.J. NF-ΚB-Mediated Adaptive Resistance to Ionizing Radiation. Free Radic. Biol. Med. 2008, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Di Grappa, M.A.; Molyneux, S.D.; McKee, T.D.; Waterhouse, P.; Penninger, J.M.; Khokha, R. RANKL Blockade Prevents and Treats Aggressive Osteosarcomas. Sci. Transl. Med. 2015, 7, 317ra197. [Google Scholar] [CrossRef] [PubMed]
- Baud’huin, M.; Duplomb, L.; Ruiz Velasco, C.; Fortun, Y.; Heymann, D.; Padrines, M. Key Roles of the OPG–RANK–RANKL System in Bone Oncology. Expert. Rev. Anticancer Ther. 2007, 7, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Fridman, M.V.; Braga, E.A. Molecular Mechanisms and MicroRNAs in Osteosarcoma Pathogenesis. Biochemistry 2016, 81, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.S.; Chow, W.; Reed, D.R.; Lucas, D.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Budd, G.T.; Curry, W.T.; et al. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Hayashi, M.; Wagner, L.; Binitie, O.; Steppan, D.A.; Brohl, A.S.; Shinohara, E.T.; Bridge, J.A.; Loeb, D.M.; Borinstein, S.C.; et al. Treatment Pathway of Bone Sarcoma in Children, Adolescents, and Young Adults. Cancer 2017, 123, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.E. Bone Sarcoma Pathology: Diagnostic Approach for Optimal Therapy. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 794–798. [Google Scholar] [CrossRef]
- Alkazemi, B.; Ghazawi, F.M.; Lagacé, F.; Nechaev, V.; Zubarev, A.; Litvinov, I.V. Investigation of the Incidence and Geographic Distribution of Bone and Soft Tissue Sarcomas in Canada: A National Population-Based Study. Curr. Oncol. 2023, 30, 5631–5651. [Google Scholar] [CrossRef] [PubMed]
- Balmant, N.V.; de Reis, R.S.; de Santos, M.O.; Maschietto, M.; de Camargo, B. Incidence and Mortality of Bone Cancer among Children, Adolescents and Young Adults of Brazil. Clinics 2019, 74, e858. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.Y.; Bertuzzi, A.; Bielack, S.; Bjerkehagen, B.; Bonvalot, S.; Boukovinas, I.; Bruzzi, P.; Tos, A.P.D.; Dileo, P.; et al. Bone Sarcomas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25, iii113–iii123. [Google Scholar] [CrossRef]
- Zając, A.; Król, S.K.; Rutkowski, P.; Czarnecka, A.M. Biological Heterogeneity of Chondrosarcoma: From (Epi) Genetics through Stemness and Deregulated Signaling to Immunophenotype. Cancers 2021, 13, 1317. [Google Scholar] [CrossRef]
- Rajan, S.; Franz, E.M.; McAloney, C.A.; Vetter, T.A.; Cam, M.; Gross, A.C.; Taslim, C.; Wang, M.; Cannon, M.V.; Oles, A.; et al. Osteosarcoma Tumors Maintain Intra-Tumoral Transcriptional Heterogeneity during Bone and Lung Colonization. BMC Biol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Belayneh, R.; Fourman, M.S.; Bhogal, S.; Weiss, K.R. Update on Osteosarcoma. Curr. Oncol. Rep. 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21, vii320–vii325. [Google Scholar] [CrossRef]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International Osteosarcoma Incidence Patterns in Children and Adolescents, Middle Ages and Elderly Persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef]
- Abate, M.E.; Longhi, A.; Galletti, S.; Ferrari, S.; Bacci, G. Non-Metastatic Osteosarcoma of the Extremities in Children Aged 5 Years or Younger. Pediatr. Blood Cancer 2010, 55, 652–654. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone Microenvironment and Osteosarcoma Metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef]
- Kempf-Bielack, B.; Bielack, S.S.; Jürgens, H.; Branscheid, D.; Berdel, W.E.; Exner, G.U.; Göbel, U.; Helmke, K.; Jundt, G.; Kabisch, H.; et al. Osteosarcoma Relapse after Combined Modality Therapy: An Analysis of Unselected Patients in the Cooperative Osteosarcoma Study Group (COSS). J. Clin. Oncol. 2005, 23, 559–568. [Google Scholar] [CrossRef]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A Comprehensive Review of Management and Treatment Strategies. Ann. Diagn. Pathol. 2020, 49, 151654. [Google Scholar] [CrossRef]
- Yoshida, A. Osteosarcoma: Old and New Challenges. Surg. Pathol. Clin. 2021, 14, 567–583. [Google Scholar] [CrossRef]
- Chou, A.J.; Gorlick, R. Chemotherapy Resistance in Osteosarcoma: Current Challenges and Future Directions. Expert. Rev. Anticancer Ther. 2006, 6, 1075–1085. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Errani, C.; Angelini, A.; Mavrogenis, A.F. Current Treatment Considerations for Osteosarcoma Metastatic at Presentation. Orthopedics 2020, 43, E345–E358. [Google Scholar] [CrossRef]
- Lewis, I.J.; Nooij, M.A.; Whelan, J.; Sydes, M.R.; Grimer, R.; Hogendoorn, P.C.; Memon, M.A.; Weeden, S.; Uscinska, B.M.; van Glabbeke, M.; et al. Improvement in Histologic Response but Not Survival in Osteosarcoma Patients Treated with Intensified Chemotherapy: A Randomized Phase III Trial of the European Osteosarcoma Intergroup. JNCI J. Natl. Cancer Inst. 2007, 99, 112–128. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Bertoni, F.; Ruggieri, P.; Picci, P.; Longhi, A.; Casadei, R.; Fabbri, N.; Forni, C.; Versari, M.; et al. Long-Term Outcome for Patients with Nonmetastatic Osteosarcoma of the Extremity Treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/Osteosarcoma-2 Protocol: An Updated Report. J. Clin. Oncol. 2016, 18, 4016–4027. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Lari, S.; Mercuri, M.; Donati, D.; Longhi, A.; Forni, C.; Bertoni, F.; Versari, M.; Pignotti, E. Osteosarcoma of the Limb: Amputation Or Limb Salvage In Patients Treated by Neoadjuvant Chemotherapy. J. Bone Joint Surg. Br. 2002, 84, 88–92. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef]
- Ferrari, S.; Smeland, S.; Mercuri, M.; Bertoni, F.; Longhi, A.; Ruggieri, P.; Alvegard, T.A.; Picci, P.; Capanna, R.; Bernini, G.; et al. Neoadjuvant Chemotherapy with High-Dose Ifosfamide, High-Dose Methotrexate, Cisplatin, and Doxorubicin for Patients with Localized Osteosarcoma of the Extremity: A Joint Study by the Italian and Scandinavian Sarcoma Groups. J. Clin. Oncol. 2005, 23, 8845–8852. [Google Scholar] [CrossRef]
- Garcia-Ortega, D.Y.; Cabrera-Nieto, S.A.; Caro-Sánchez, H.S.; Cruz-Ramos, M. An Overview of Resistance to Chemotherapy in Osteosarcoma and Future Perspectives. Cancer Drug Resist. 2022, 5, 762. [Google Scholar] [CrossRef]
- Lézot, F.; Corre, I.; Morice, S.; Rédini, F.; Verrecchia, F. SHH Signaling Pathway Drives Pediatric Bone Sarcoma Progression. Cells 2020, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Brown, H.L. Understanding Osteosarcomas. J. Am. Acad. Physician Assist. 2018, 31, 15–19. [Google Scholar] [CrossRef]
- Kalavrezos, N.; Sinha, D. Head and Neck Sarcomas in Adulthood: Current Trends and Evolving Management Concepts. Br. J. Oral Maxillofac. Surg. 2020, 58, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. Molecular Signaling Pathways as Potential Therapeutic Targets in Osteosarcoma. Curr. Med. Chem. 2022, 29, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Zaccaria, S.; Cannon, M.V.; Cam, M.; Gross, A.C.; Raphael, B.J.; Roberts, R.D. Structurally Complex Osteosarcoma Genomes Exhibit Limited Heterogeneity within Individual Tumors and across Evolutionary Time. Cancer Res. Commun. 2023, 3, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, K.; Garnier, D.; Heymann, M.F.; Heymann, D. The Heterogeneity of Osteosarcoma: The Role Played by Cancer Stem Cells. Adv. Exp. Med. Biol. 2019, 1139, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, C.; Fang, Q.; Zhang, W.; Liu, W. Abnormal Signal Pathways and Tumor Heterogeneity in Osteosarcoma. J. Transl. Med. 2023, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.W.; Aslo, A.; Won, A.; Tan, M.; Lampkin, B.; Koeffler, H.P. Alterations of the P53, Rb and MDM2 Genes in Osteosarcoma. J. Cancer Res. Clin. Oncol. 1996, 122, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Nirala, B.K.; Yamamichi, T.; Yustein, J.T. Deciphering the Signaling Mechanisms of Osteosarcoma Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 11367. [Google Scholar] [CrossRef]
- Poos, K.; Smida, J.; Nathrath, M.; Maugg, D.; Baumhoer, D.; Korsching, E. How MicroRNA and Transcription Factor Co-Regulatory Networks Affect Osteosarcoma Cell Proliferation. PLoS Comput. Biol. 2013, 9, e1003210. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.H.; Yan, Y.G.; Wang, C.; Wang, W.J. PI3K/Akt Signaling in Osteosarcoma. Clin. Chim. Acta 2015, 444, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, H. Ras-PI3K Pathway Promotes Osteosarcoma Progression via Regulating VRK1-Mediated H2A Phosphorylation at Threonine 120. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Jin, Y.Y.; Tang, Y.L.; Yang, H.J.; Zhou, X.Q.; Lei, Z. GPNMB Silencing Suppresses the Proliferation and Metastasis of Osteosarcoma Cells by Blocking the PI3K/Akt/MTOR Signaling Pathway. Oncol. Rep. 2018, 39, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-Mediated Regulation of NFκB and the Essentialness of NFκB for the Oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Herrmann, A.; Deng, J.H.; Kujawski, M.; Niu, G.; Li, Z.; Forman, S.; Jove, R.; Pardoll, D.M.; Yu, H. Persistently Activated Stat3 Maintains Constitutive NF-ΚB Activity in Tumors. Cancer Cell 2009, 15, 283–293. [Google Scholar] [CrossRef]
- Danieau, G.; Morice, S.; Rédini, F.; Verrecchia, F.; Royer, B.B. Le New Insights about the Wnt/β-Catenin Signaling Pathway in Primary Bone Tumors and Their Microenvironment: A Promising Target to Develop Therapeutic Strategies? Int. J. Mol. Sci. 2019, 20, 3751. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-ΚB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guan, G.F.; Chen, J.; Hu, B.; Sun, C.; Ma, Q.; Wen, Y.H.; Qiu, X.C.; Zhou, Y. Aberrant CXCR4 and β-Catenin Expression in Osteosarcoma Correlates with Patient Survival. Oncol. Lett. 2015, 10, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Martins-Neves, S.R.; Corver, W.E.; Paiva-Oliveira, D.I.; van den Akker, B.E.W.M.; Briaire-de-Bruijn, I.H.; Bovée, J.V.M.G.; Gomes, C.M.F.; Cleton-Jansen, A.M. Osteosarcoma Stem Cells Have Active Wnt/β-Catenin and Overexpress SOX2 and KLF4. J. Cell. Physiol. 2016, 231, 876–886. [Google Scholar] [CrossRef]
- Spiegelman, V.S.; Slaga, T.J.; Pagano, M.; Minamoto, T.; Ronai, Z.; Fuchs, S.Y. Wnt/β-Catenin Signaling Induces the Expression and Activity of ΒTrCP Ubiquitin Ligase Receptor. Mol. Cell 2000, 5, 877–882. [Google Scholar] [CrossRef]
- Die, L.; Yan, P.; Jun Jiang, Z.; Min Hua, T.; Cai, W.; Xing, L. Glycogen Synthase Kinase-3 Beta Inhibitor Suppresses Porphyromonas Gingivalis Lipopolysaccharide-Induced CD40 Expression by Inhibiting Nuclear Factor-Kappa B Activation in Mouse Osteoblasts. Mol. Immunol. 2012, 52, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Hur, J.; Jeong, S. Beta-Catenin Binds to the Downstream Region and Regulates the Expression C-Reactive Protein Gene. Nucleic Acids Res. 2007, 35, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.; So, J.-S.; Jash, A.; Im, S.-H. Lymphoid Enhancer Binding Factor 1 Regulates Transcription through Gene Looping. J. Immunol. 2009, 183, 5129–5137. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Xie, X.B.; Wang, J.; Chen, Q.; Han, A.J.; Zou, C.Y.; Yin, J.Q.; Liu, D.W.; Liang, Y.; Zhao, Z.Q.; et al. Glycogen Synthase Kinase-3β, NF-ΚB Signaling, and Tumorigenesis of Human Osteosarcoma. JNCI J. Natl. Cancer Inst. 2012, 104, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Dong, Q.; Fu, L.; Bossler, A.; Tang, X.; Boyce, B.; Borcherding, N.; Leidinger, M.; Sardina, J.L.; Xue, H.; et al. Coactivation of NF-ΚB and Notch Signaling Is Sufficient to Induce B-Cell Transformation and Enables B-Myeloid Conversion. Blood 2020, 135, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Osipo, C.; Golde, T.E.; Osborne, B.A.; Miele, L.A. Off the Beaten Pathway: The Complex Cross Talk between Notch and NF-ΚB. Lab. Investig. 2007, 88, 11–17. [Google Scholar] [CrossRef]
- Espinosa, L.; Cathelin, S.; D’Altri, T.; Trimarchi, T.; Statnikov, A.; Guiu, J.; Rodilla, V.; Inglés-Esteve, J.; Nomdedeu, J.; Bellosillo, B.; et al. The Notch/Hes1 Pathway Sustains NF-ΚB Activation through CYLD Repression in T Cell Leukemia. Cancer Cell 2010, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Pakvasa, M.; Haravu, P.; Boachie-Mensah, M.; Jones, A.; Coalson, E.; Liao, J.; Zeng, Z.; Wu, D.; Qin, K.; Wu, X.; et al. Notch Signaling: Its Essential Roles in Bone and Craniofacial Development. Genes Dis. 2021, 8, 8–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, W.; Shao, Z. NOTCH Signaling in Osteosarcoma. Curr. Issues Mol. Biol. 2023, 45, 2266–2283. [Google Scholar] [CrossRef]
- Tang, X.F.; Cao, Y.; Peng, D.B.; Zhao, G.S.; Zeng, Y.; Gao, Z.R.; Lv, Y.F.; Guo, Q.N. Overexpression of Notch3 Is Associated with Metastasis and Poor Prognosis in Osteosarcoma Patients. Cancer Manag. Res. 2019, 11, 547–559. [Google Scholar] [CrossRef]
- López-López, S.; Monsalve, E.M.; de Ávila, M.J.R.; González-Gómez, J.; Hernández de León, N.; Ruiz-Marcos, F.; Baladrón, V.; Nueda, M.L.; García-León, M.J.; Screpanti, I.; et al. NOTCH3 Signaling Is Essential for NF-ΚB Activation in TLR-Activated Macrophages. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.W.; Pinnaduwage, D.; Gokgoz, N.; Wunder, J.S.; Andrulis, I.L. Aberrant Hedgehog Signaling and Clinical Outcome in Osteosarcoma. Sarcoma 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Han, L.; Chen, Y.; He, F.; Sun, B.; Kamar, S.; Zhang, Y.; Yang, Y.; Wang, C.; Yang, Z. Hedgehog Signalling in the Tumourigenesis and Metastasis of Osteosarcoma, and Its Potential Value in the Clinical Therapy of Osteosarcoma. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.W.; Wunder, J.S.; Dickson, B.C.; Campbell, V.; McGovern, K.; Alman, B.A.; Andrulis, I.L. Involvement and Targeted Intervention of Dysregulated Hedgehog Signaling in Osteosarcoma. Cancer 2014, 120, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, M.; Setoguchi, T.; Sasaki, H.; Matsunoshita, Y.; Gao, H.; Nagao, H.; Kunigou, O.; Komiya, S. Smoothened as a New Therapeutic Target for Human Osteosarcoma. Mol. Cancer 2010, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Dai, Y.; Kong, X.; Zhu, X.; Fan, Y.; Wang, Y.; Wu, H.; Jin, J.; Yao, W.; et al. Positive Crosstalk between Hedgehog and NF-ΚB Pathways Is Dependent on KRAS Mutation in Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021, 11, 652283. [Google Scholar] [CrossRef] [PubMed]
- Vecchiotti, D.; Verzella, D.; Di Vito Nolfi, M.; D’andrea, D.; Flati, I.; Di Francesco, B.; Cornice, J.; Alesse, E.; Capece, D.; Zazzeroni, F. Elevated NF-ΚB/SHh/GLI1 Signature Denotes a Worse Prognosis and Represent a Novel Potential Therapeutic Target in Advanced Prostate Cancer. Cells 2022, 11, 2118. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Nakamura, M.; Yamaguchi, H.; Yamanaka, N.; Akiyoshi, T.; Koga, K.; Yamaguchi, K.; Tsuneyoshi, M.; Tanaka, M.; Katano, M. Nuclear Factor-KappaB Contributes to Hedgehog Signaling Pathway Activation through Sonic Hedgehog Induction in Pancreatic Cancer. Cancer Res. 2006, 66, 7041–7049. [Google Scholar] [CrossRef] [PubMed]
- Kasperczyk, H.; Baumann, B.; Debatin, K.-M.; Fulda, S. Characterization of Sonic Hedgehog as a Novel NF-κB Target Gene That Promotes NF-κB-mediated Apoptosis Resistance and Tumor Growth in Vivo. FASEB J. 2009, 23, 21–33. [Google Scholar] [CrossRef]
- Colavito, S.A.; Zou, M.R.; Yan, Q.; Nguyen, D.X.; Stern, D.F. Significance of Glioma-Associated Oncogene Homolog 1 (GLI1) Expression in Claudin-Low Breast Cancer and Crosstalk with the Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NFκB) Pathway. Breast Cancer Res. 2014, 16, 444. [Google Scholar] [CrossRef]
- Lamora, A.; Talbot, J.; Bougras, G.; Amiaud, J.; Leduc, M.; Chesneau, J.; Taurelle, J.; Stresing, V.; Le Deley, M.C.; Heymann, M.F.; et al. Overexpression of Smad7 Blocks Primary Tumor Growth and Lung Metastasis Development in Osteosarcoma. Clin. Cancer Res. 2014, 20, 5097–5112. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, F.; Rédini, F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ Signalling Pathway in Disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine Kinase—Role and Significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef]
- Ohba, T.; Cates, J.M.M.; Cole, H.A.; Slosky, D.A.; Haro, H.; Ando, T.; Schwartz, H.S.; Schoenecker, J.G. Autocrine VEGF/VEGFR1 Signaling in a Subpopulation of Cells Associates with Aggressive Osteosarcoma. Mol. Cancer Res. 2014, 12, 1100–1111. [Google Scholar] [CrossRef]
- Daft, P.G.; Yang, Y.; Napierala, D.; Zayzafoon, M. The Growth and Aggressive Behavior of Human Osteosarcoma Is Regulated by a CaMKII-Controlled Autocrine VEGF Signaling Mechanism. PLoS ONE 2015, 10, e0121568. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Xiong, C.; Zhao, R.; Liang, H.; Luo, X. The Role of Vascular Endothelial Growth Factor as a Prognostic and Clinicopathological Marker in Osteosarcoma: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 738. [Google Scholar] [CrossRef]
- Oshiro, H.; Tome, Y.; Miyake, K.; Higuchi, T.; Sugisawa, N.; Kanaya, F.; Nishida, K.; Hoffman, R.M. An MTOR and VEGFR Inhibitor Combination Arrests a Doxorubicin Resistant Lung Metastatic Osteosarcoma in a PDOX Mouse Model. Sci. Rep. 2021, 11, 8583. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Niu, J.; Schmidt, C.; Sclabas, G.M.; Peng, B.; Uwagawa, T.; Li, Z.; Evans, D.B.; Abbruzzese, J.L.; Chiao, P.J. NF-KappaB and AP-1 Connection: Mechanism of NF-KappaB-Dependent Regulation of AP-1 Activity. Mol. Cell. Biol. 2004, 24, 7806–7819. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-ΚB, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Cao, N.; Li, S.; Wang, Z.; Ahmed, K.M.; Degnan, M.E.; Fan, M.; Dynlach, J.R.; Li, J.J. NF-KappaB-Mediated HER2 Overexpression in Radiation-Adaptive Resistance. Radiat. Res. 2009, 171, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K. The Future of HER2-Targeted Treatment for Osteosarcoma: Lessons from the Negative Trastuzumab Deruxtecan Results. Int. J. Mol. Sci. 2023, 24, 16823. [Google Scholar] [CrossRef] [PubMed]
- Sulzbacher, I.; Träxler, M.; Mosberger, I.; Lang, S.; Chott, A. Platelet-Derived Growth Factor-AA and -Alpha Receptor Expression Suggests an Autocrine and/or Paracrine Loop in Osteosarcoma. Mod. Pathol. 2000, 13, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xie, L.; Guo, W. PDGF/PDGFR Effects in Osteosarcoma and the “Add-on” Strategy. Clin. Sarcoma Res. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Ekert, P.G.; Fleuren, E.D.G. Biological and Clinical Implications of FGFR Aberrations in Paediatric and Young Adult Cancers. Oncogene 2023, 42, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Weekes, D.; Kashima, T.; Grigoriadis, A. P36. The Role of FGF-Signalling in Osteosarcoma: A Potential Therapeutic Target? Cancer Treat. Rev. 2008, 34, 26. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Zheng, H.; Du, X.L.; Yang, J.L. Characterization of FGFR Signaling Pathway as Therapeutic Targets for Sarcoma Patients. Cancer Biol. Med. 2016, 13, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Fernanda Amary, M.; Ye, H.; Berisha, F.; Khatri, B.; Forbes, G.; Lehovsky, K.; Frezza, A.M.; Behjati, S.; Tarpey, P.; Pillay, N.; et al. Fibroblastic Growth Factor Receptor 1 Amplification in Osteosarcoma Is Associated with Poor Response to Neo-Adjuvant Chemotherapy. Cancer Med. 2014, 3, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Rettew, A.N.; Getty, P.J.; Greenfield, E.M. Receptor Tyrosine Kinases in Osteosarcoma: Not Just the Usual Suspects. Adv. Exp. Med. Biol. 2014, 804, 47–66. [Google Scholar] [CrossRef]
- Greenfield, E.M.; Collier, C.D.; Getty, P.J. Receptor Tyrosine Kinases in Osteosarcoma: 2019 Update. Adv. Exp. Med. Biol. 2020, 1258, 141–155. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Versleijen-Jonkers, Y.M.H.; Boerman, O.C.; van der Graaf, W.T.A. Targeting Receptor Tyrosine Kinases in Osteosarcoma and Ewing Sarcoma: Current Hurdles and Future Perspectives. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2014, 1845, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Rettew, A.N.; Young, E.D.; Lev, D.C.; Kleinerman, E.S.; Abdul-Karim, F.W.; Getty, P.J.; Greenfield, E.M. Multiple Receptor Tyrosine Kinases Promote the in Vitro Phenotype of Metastatic Human Osteosarcoma Cell Lines. Oncogenesis 2012, 1, e34. [Google Scholar] [CrossRef]
- Abdeen, A.; Chou, A.J.; Healey, J.H.; Khanna, C.; Osborne, T.S.; Hewitt, S.M.; Kim, M.; Wang, D.; Moody, K.; Gorlick, R. Correlation between Clinical Outcome and Growth Factor Pathway Expression in Osteogenic Sarcoma. Cancer 2009, 115, 5243–5250. [Google Scholar] [CrossRef]
- Chaiyawat, P.; Klangjorhor, J.; Settakorn, J.; Champattanachai, V.; Phanphaisarn, A.; Teeyakasem, P.; Svasti, J.; Pruksakorn, D. Activation Status of Receptor Tyrosine Kinases as an Early Predictive Marker of Response to Chemotherapy in Osteosarcoma. Transl. Oncol. 2017, 10, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Niu, X.; Yao, W. Receptor Tyrosine Kinases in Osteosarcoma Treatment: Which Is the Key Target? Front. Oncol. 2020, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Chandhanayingyong, C.; Kim, Y.; Staples, J.R.; Hahn, C.; Lee, F.Y. MAPK/ERK Signaling in Osteosarcomas, Ewing Sarcomas and Chondrosarcomas: Therapeutic Implications and Future Directions. Sarcoma 2012, 2012, 404810. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.L.; Baldwin, A.S. Oncogenic Ras Enhances NF-KappaB Transcriptional Activity through Raf-Dependent and Raf-Independent Mitogen-Activated Protein Kinase Signaling Pathways. J. Biol. Chem. 1999, 274, 13841–13846. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Weberjakob Troppmair, C.K.; Whiteside, S.; Israel, A.; Rapp, U.R.; Wirth, T. Raf Induces NF-KappaB by Membrane Shuttle Kinase MEKK1, a Signaling Pathway Critical for Transformation. Proc. Natl. Acad. Sci. USA 2000, 97, 4615–4620. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, X.; Jin, J.; Feng, J.; Xu, Z.; Chen, Y.; Zhao, H.; Li, Z. A Novel Defined RAS-Related Gene Signature for Predicting the Prognosis and Characterization of Biological Function in Osteosarcoma. J. Oncol. 2022, 2022, 5939158. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef]
- Jin, J.; Cong, J.; Lei, S.; Zhang, Q.; Zhong, X.; Su, Y.; Lu, M.; Ma, Y.; Li, Z.; Wang, L.; et al. Cracking the Code: Deciphering the Role of the Tumor Microenvironment in Osteosarcoma Metastasis. Int. Immunopharmacol. 2023, 121, 110422. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Kiran, P. Tumor Microenvironment Pathways: Cross Regulation in Breast Cancer Metastasis. Genes Dis. 2022, 9, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, B.; Jiang, K.; Lao, L.; Shen, H.; Chen, Z. Activation of TNF-α/NF-ΚB Axis Enhances CRL4BDCAF11 E3 Ligase Activity and Regulates Cell Cycle Progression in Human Osteosarcoma Cells. Mol. Oncol. 2018, 12, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Deng, S.; Fang, H.S.; Yu, G.; Peng, H. Hsa-Let-7g Promotes Osteosarcoma by Reducing HOXB1 to Activate NF-ΚB Pathway. Biomed. Pharmacother. 2019, 109, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liao, Q.S.; Tang, L. MiR-155 Affects Osteosarcoma Cell Proliferation and Invasion through Regulating NF-ΚB Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7633–7639. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Gao, J.; Chen, L.; Ren, Y.; Ma, J. Targeting XIST Induced Apoptosis of Human Osteosarcoma Cells by Activation of NF-ΚB/PUMA Signal. Bioengineered 2019, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Shen, X. Long Noncoding RNAs in Osteosarcoma via Various Signaling Pathways. J. Clin. Lab. Anal. 2020, 34, e23317. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.V.; Ranjan, A.; Elias, H.K.; Parrales, A.; Sasaki, H.; Roy, B.C.; Umar, S.; Tawfik, O.W.; Iwakuma, T. Genome-Wide RNAi Screening Identifies TMIGD3 Isoform1 as a Suppressor of NF-ΚB and Osteosarcoma Progression. Nat. Commun. 2016, 7, 13561. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Chen, P.C.; Chang, T.M.; Hou, C.H. Thrombospondin-2 Stimulates MMP-9 Production and Promotes Osteosarcoma Metastasis via the PLC, PKC, c-Src and NF-ΚB Activation. J. Cell. Mol. Med. 2020, 24, 12826–12839. [Google Scholar] [CrossRef]
- Feng, Z.M.; Guo, S.M. Tim-3 Facilitates Osteosarcoma Proliferation and Metastasis through the NF-ΚB Pathway and Epithelial-Mesenchymal Transition. Genet. Mol. Res. 2016, 1–9. [Google Scholar] [CrossRef]
- Gong, T.; Su, X.; Xia, Q.; Wang, J.; Kan, S. Expression of NF-ΚB and PTEN in Osteosarcoma and Its Clinical Significance. Oncol. Lett. 2017, 14, 6744–6748. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Chilton-MacNeill, S.; Koti, M.; Van Wijnen, A.J.; Squire, J.A.; Zielenska, M. Digital Expression Profiling Identifies RUNX2, CDC5L, MDM2, RECQL4, and CDK4 as Potential Predictive Biomarkers for Neo-Adjuvant Chemotherapy Response in Paediatric Osteosarcoma. PLoS ONE 2014, 9, e95843. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, Y.; Zhu, H.; Kuang, Z.; Chen, D.; Fan, T. Prognostic Significance of β-Catenin Expression in Osteosarcoma: A Meta-Analysis. Front. Oncol. 2020, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.C.; Cam, H.; Phelps, D.A.; Saraf, A.J.; Bid, H.K.; Cam, M.; London, C.A.; Winget, S.A.; Arnold, M.A.; Brandolini, L.; et al. IL-6 and CXCL8 Mediate Osteosarcoma-Lung Interactions Critical to Metastasis. JCI Insight 2018, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hong, C.S.; Hu, X.; Yang, C.; Wang, H.; Zhu, D.; Moon, S.; Dmitriev, P.; Lu, J.; Chiang, J.; et al. Inhibition of Protein Phosphatase 2A with the Small Molecule LB100 Overcomes Cell Cycle Arrest in Osteosarcoma after Cisplatin Treatment. Cell Cycle 2015, 14, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, G.; Li, H.; Lai, F.; Duan, P.; Cheng, M. Epithelial to Mesenchymal Transition Relevant Subtypes with Distinct Prognosis and Responses to Chemo- or Immunotherapies in Osteosarcoma. J. Immunol. Res. 2022, 2022, 1377565. [Google Scholar] [CrossRef] [PubMed]
- Bakkalci, D.; Al-Badri, G.; Yang, W.; Nam, A.; Liang, Y.; Fisher, J.; Cheema, U. Engineering a Metastatic Stroma Directs the Osteosarcoma Tumour Transcriptome in a Spatially Specific Manner. Appl. Mater. Today 2023, 35, 101994. [Google Scholar] [CrossRef]
- Zu, D.; Dong, Q.; Chen, S.; Chen, Y.; Yao, J.; Zou, Y.; Lin, J.; Fang, B.; Wu, B. MiRNA-331-3p Affects the Proliferation, Metastasis, and Invasion of Osteosarcoma through SOCS1/JAK2/STAT3. J. Oncol. 2022, 2022, 6459029. [Google Scholar] [CrossRef]
- Li, T.; Tang, Z.; Li, S.; Lu, M. Development of a Novel Six DNA Damage Response-Related Prognostic Signature in Osteosarcoma. Cell Mol. Biol. 2024, 70, 110–115. [Google Scholar] [CrossRef]
- Tu, B.; Du, L.; Fan, Q.M.; Tang, Z.; Tang, T.T. STAT3 Activation by IL-6 from Mesenchymal Stem Cells Promotes the Proliferation and Metastasis of Osteosarcoma. Cancer Lett. 2012, 325, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Squire, J.A.; Pei, J.; Marrano, P.; Beheshti, B.; Bayani, J.; Lim, G.; Moldovan, L.; Zielenska, M. High-Resolution Mapping of Amplifications and Deletions in Pediatric Osteosarcoma by Use of CGH Analysis of CDNA Microarrays. Genes Chromosomes Cancer 2003, 38, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Qu, X.; Xia, J.; Li, D.; Li, X.; Wang, Y.; He, Z.; Li, S.; Zhou, Y.; et al. Identification of Differentially Expressed Genes in the Development of Osteosarcoma Using RNA-Seq. Oncotarget 2016, 7, 87194–87205. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, R.; Morelli, M.; Lessi, F.; Mazzanti, C.M.; Menicagli, M.; Capanna, R.; Andreani, L.; Coccoli, L.; Aretini, P.; Franchi, A. Identification of New Potential Prognostic and Predictive Markers in High-Grade Osteosarcoma Using Whole Exome Sequencing. Int. J. Mol. Sci. 2023, 24, 86. [Google Scholar] [CrossRef] [PubMed]
- Sonaglio, V.; de Carvalho, A.C.; Toledo, S.R.C.; Salinas-Souza, C.; Carvalho, A.L.; Petrilli, A.S.; De Camargo, B.; Vettore, A.L. Aberrant DNA Methylation of ESR1 and P14ARF Genes Could Be Useful as Prognostic Indicators in Osteosarcoma. Onco Targets Ther. 2013, 6, 713–723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Q.; Yin, X.; Zhang, Y. MicroRNA-221 Promotes Cell Proliferation and Inhibits Apoptosis in Osteosarcoma Cells by Directly Targeting FBXW11 and Regulating Wnt Signaling. Arch. Med. Res. 2021, 52, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Gomez Lira, M.; Minoia, A.; Bertacco, J.; Orsi, S.; Lauriola, A.; Li Vigni, V.; Gandini, A.; Antoniazzi, F.; Zipeto, D.; et al. Expression of FBXW11 in Normal and Disease-Associated Osteogenic Cells. J. CellMol. Med. 2023, 27, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sethi, G. Targeting Transcription Factor NF-KappaB to Overcome Chemoresistance and Radioresistance in Cancer Therapy. Biochim. Biophys. Acta 2010, 1805, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-ΚB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View. Int. J. Mol. Sci. 2020, 21, 70. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals Targeting NF-ΚB Signaling: Potential Anti-Cancer Interventions. J. Pharm. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bai, L.; Chen, W.; Xu, S. The NF-KappaB Activation Pathways, Emerging Molecular Targets for Cancer Prevention and Therapy. Expert Opin. Ther. Targets 2010, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Letícia de Castro Barbosa, M.; Alves da Conceicao, R.; Guerra Manssour Fraga, A.; Dias Camarinha, B.; Cristina de Carvalho Silva, G.; Gilcler Ferreira Lima, A.; Azevedo Cardoso, E.; de Oliveira Freitas Lione, V. NF-ΚB Signaling Pathway Inhibitors as Anticancer Drug Candidates. Anticancer Agents Med. Chem. 2017, 17, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D.; Herscovitch, M. Inhibitors of NF-ΚB Signaling: 785 and Counting. Oncogene 2006, 25, 6887–6899. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-ΚB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-KappaB a Good Target for Cancer Therapy? Hopes and Pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Carvalho, G.; Fabre, C.; Grosjean, J.; Fenaux, P.; Kroemer, G. Targeting NF-KappaB in Hematologic Malignancies. Cell Death Differ. 2006, 13, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, B.; Verzella, D.; Capece, D.; Vecchiotti, D.; Di Vito Nolfi, M.; Flati, I.; Cornice, J.; Di Padova, M.; Angelucci, A.; Alesse, E.; et al. NF-ΚB: A Druggable Target in Acute Myeloid Leukemia. Cancers 2022, 14, 3557. [Google Scholar] [CrossRef] [PubMed]
- Rasmi, R.R.; Sakthivel, K.M.; Guruvayoorappan, C. NF-ΚB Inhibitors in Treatment and Prevention of Lung Cancer. Biomed. Pharmacother. 2020, 130, 110569. [Google Scholar] [CrossRef]
- Zakaria, N.; Yusoff, N.M.; Zakaria, Z.; Widera, D.; Yahaya, B.H. Inhibition of NF-ΚB Signaling Reduces the Stemness Characteristics of Lung Cancer Stem Cells. Front. Oncol. 2018, 8, 366363. [Google Scholar] [CrossRef]
- Amentoflavone|C30H18O10|CID 5281600—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amentoflavone (accessed on 27 April 2024).
- Banerjee, T.; Valacchi, G.; Ziboh, V.A.; Van Der Vliet, A. Inhibition of TNFalpha-Induced Cyclooxygenase-2 Expression by Amentoflavone through Suppression of NF-KappaB Activation in A549 Cells. Mol. Cell. Biochem. 2002, 238, 105–110. [Google Scholar] [CrossRef]
- Su, C.M.; Li, C.H.; Huang, M.C.; Yueh, P.F.; Hsu, F.T.; Lin, R.F.; Hsu, L.C. Reinforcement of Sorafenib Anti-Osteosarcoma Effect by Amentoflavone Is Associated with the Induction of Apoptosis and Inactivation of ERK/NF-ΚB. In Vivo 2022, 36, 1136–1143. [Google Scholar] [CrossRef]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef]
- Pan, P.J.; Tsai, J.J.; Liu, Y.C. Amentoflavone Inhibits Metastatic Potential through Suppression of ERK/NF-ΚB Activation in Osteosarcoma U2OS Cells. Anticancer Res. 2017, 37, 4911–4918. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chung, J.G.; Chien, Y.T.; Lin, S.S.; Hsu, F.T. Suppression of ERK/NF-ΚB Activation Is Associated with Amentoflavone-Inhibited Osteosarcoma Progression In Vivo. Anticancer Res. 2019, 39, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Andrographolide|C20H30O5|CID 5318517—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Andrographolide (accessed on 27 April 2024).
- Xia, Y.-F.; Ye, B.-Q.; Li, Y.-D.; Wang, J.-G.; He, X.-J.; Lin, X.; Yao, X.; Ma, D.; Slungaard, A.; Hebbel, R.P.; et al. Andrographolide Attenuates Inflammation by Inhibition of NF-Kappa B Activation through Covalent Modification of Reduced Cysteine 62 of P50. J. Immunol. 2004, 173, 4207–4217. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, L.; Gao, R.; Jiang, F.; Cao, J.; Liu, H. Andrographolide Alleviates Acute Brain Injury in a Rat Model of Traumatic Brain Injury: Possible Involvement of Inflammatory Signaling. Front. Neurosci. 2018, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zou, J.; Yan, L.; Yu, X.; Lu, P.; Wu, X.; Li, Q.; Gu, R.; Zhu, D. Andrographolide Induces Autophagic Cell Death and Inhibits Invasion and Metastasis of Human Osteosarcoma Cells in An Autophagy-Dependent Manner. Cell. Physiol. Biochem. 2017, 44, 1396–1410. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Chen, S.; Wang, Z.; Yao, Y.; Chen, T.; Ye, Z.; Lin, P. Andrographolide Induces Apoptosis in Human Osteosarcoma Cells via the ROS/JNK Pathway. Int. J. Oncol. 2020, 56, 1417–1428. [Google Scholar] [CrossRef]
- Huang, H.; Lu, Q.; Yuan, X.; Zhang, P.; Ye, C.; Wei, M.; Yang, C.; Zhang, L.; Huang, Y.; Luo, X.; et al. Andrographolide Inhibits the Growth of Human Osteosarcoma Cells by Suppressing Wnt/β-Catenin, PI3K/AKT and NF-ΚB Signaling Pathways. Chem. Biol. Interact. 2022, 365, 110068. [Google Scholar] [CrossRef]
- Pierce, J.W.; Schoenleber, R.; Jesmok, G.; Best, J.; Moore, S.A.; Collins, T.; Gerritsen, M.E. Novel Inhibitors of Cytokine-Induced IκBα Phosphorylation and Endothelial Cell Adhesion Molecule Expression Show Anti-Inflammatory Effects in Vivo. J. Biol. Chem. 1997, 272, 21096–21103. [Google Scholar] [CrossRef]
- Tan, B.; Yuan, Z.; Zhang, Q.; Xiqiang, X.; Dong, J. The NF-ΚB Pathway Is Critically Implicated in the Oncogenic Phenotype of Human Osteosarcoma Cells. J. Appl. Biomed. 2021, 19, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Kawaguchi, M.; Yorita, K.; Tanaka, H. Antitumor Effect of Dehydroxymethylepoxyquinomicin, a Small Molecule Inhibitor of Nuclear Factor-KB, on Glioblastoma. Neuro Oncol. 2012, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Katsman, A.; Umezawa, K.; Bonavida, B. Reversal of Resistance to Cytotoxic Cancer Therapies: DHMEQ as a Chemo-Sensitizing and Immuno-Sensitizing Agent. Drug Resist. Updates 2007, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gamero, A.M.; Borges, K.S.; Silveira, V.d.S.; Lira, R.C.P.; Queiroz, R.d.P.G.; Valera, F.C.P.; Scrideli, C.A.; Umezawa, K.; Tone, L.G. Inhibition of Nuclear Factor-ΚB by Dehydroxymethylepoxyquinomicin Induces Schedule-Dependent Chemosensitivity to Anticancer Drugs and Enhances Chemoinduced Apoptosis in Osteosarcoma Cells. Anticancer Drugs 2012, 23, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, X.; Chen, W.; Chen, Y.; Zhang, Q.; Mo, S.; Lu, J. Dihydroartemisinin: A Potential Natural Anticancer Drug. Int. J. Biol. Sci. 2021, 17, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, S.S.; Zhang, J.L.; Lou, X.E.; Zhou, H.J. Dihydroartemisinin Induces Autophagy by Suppressing NF-ΚB Activation. Cancer Lett. 2014, 343, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Xu, J.; Li, L.; Dong, Q.; Shi, Q.; Zuo, G.; Zhou, L.; Weng, Y.; Tang, M.; et al. Dihydroartemisinin Inhibits Tumor Growth of Human Osteosarcoma Cells by Suppressing Wnt/β-Catenin Signaling. Oncol. Rep. 2013, 30, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, B.; Su, Y.; Badshah, S.A.; Wang, X.; Li, X.; Xue, Y.; Xie, L.; Wang, Z.; Yang, Z.; et al. Iron Promotes Dihydroartemisinin Cytotoxicity via ROS Production and Blockade of Autophagic Flux via Lysosomal Damage in Osteosarcoma. Front. Pharmacol. 2020, 11, 493314. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.C.; Pei, L.B.; Shi, L.L.; Yan, J.L.; Ma, X.H. Anti-Tumor Effects of Dihydroartemisinin on Human Osteosarcoma. Mol. Cell Biochem. 2011, 351, 99–108. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.; Liang, J.; Li, Q.; Hu, H.; Zhou, Y.; Zhang, B. Dihydroartemisinin Potentiates VEGFR-TKIs Antitumorigenic Effect on Osteosarcoma by Regulating Loxl2/VEGFA Expression and Lipid Metabolism Pathway. J. Cancer 2023, 14, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, P.; Zhang, Y.; Han, S.; Wang, G.; Wang, H.; Song, H.; Li, S. Dynamic Nanoassemblies Derived from Small-Molecule Homodimeric Prodrugs for in Situ Drug Activation and Safe Osteosarcoma Treatment. iScience 2023, 26, 107409. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Ma, J.; Wang, K.S.; Mi, C.; Lv, Y.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Dihydromyricetin Suppresses TNF-α-Induced NF-ΚB Activation and Target Gene Expression. Mol. Cell Biochem. 2016, 422, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yin, J.Q.; Wu, M.S.; Song, G.; Xie, X.B.; Zou, C.; Tang, Q.; Wu, Y.; Lu, J.; Wang, Y.; et al. Dihydromyricetin Activates AMP-Activated Protein Kinase and P38(MAPK) Exerting Antitumor Potential in Osteosarcoma. Cancer Prev. Res. 2014, 7, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Lu, K.H.; Yang, J.S.; Hsieh, Y.H.; Lin, C.W.; Yang, S.F. Dihydromyricetin Suppresses Cell Metastasis in Human Osteosarcoma through SP-1- and NF-ΚB-Modulated Urokinase Plasminogen Activator Inhibition. Phytomedicine 2021, 90, 153642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Qiu, E. Protection of Oxidative Stress Induced Apoptosis in Osteosarcoma Cells by Dihydromyricetin through Down-Regulation of Caspase Activation and up-Regulation of BcL-2. Saudi J. Biol. Sci. 2017, 24, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, D.H.; Han, S.J.; Hyun, J.W.; Kim, H.S. Repression of Matrix Metalloproteinase Gene Expression by Ginsenoside Rh2 in Human Astroglioma Cells. Biochem. Pharmacol. 2007, 74, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.Y.; Hwang, J.Y.; Cho, J.Y. Ginsenosides from Panax Ginseng as Key Modulators of NF-ΚB Signaling Are Powerful Anti-Inflammatory and Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 6119. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, H.; Zhou, Z.; Mao, X.; Ye, Z.; Zhang, Z.; Tu, S.; Zhang, Y.; Cai, X.; Lan, X.; et al. Integrated Bioinformatic Analysis and Experiment Confirmation of the Antagonistic Effect and Molecular Mechanism of Ginsenoside Rh2 in Metastatic Osteosarcoma. J. Pharm. Biomed. Anal. 2021, 201, 114088. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, W.; Zhou, X.; Fu, J.; He, C. Tumor Cell Membrane-Camouflaged Responsive Nanoparticles Enable MRI-Guided Immuno-Chemodynamic Therapy of Orthotopic Osteosarcoma. Bioact. Mater. 2022, 17, 221–233. [Google Scholar] [CrossRef]
- Rasul, A.; Khan, M.; Ali, M.; Li, J.; Li, X. Targeting Apoptosis Pathways in Cancer with Alantolactone and Isoalantolactone. ScientificWorldJournal 2013, 2013, 248532. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.I.; Ju, R.K.; Hyun, A.L.; Chan, H.J.; Young, K.K.; Konishi, T.; Eun, J.K.; Park, J.H.Y.; Kim, J.S. Induction of Detoxifying Enzyme by Sesquiterpenes Present in Inula Helenium. J. Med. Food 2007, 10, 503–510. [Google Scholar] [CrossRef]
- Konishi, T.; Shimada, Y.; Nagao, T.; Okabe, H.; Konoshima, T. Antiproliferative Sesquiterpene Lactones from the Roots of Inula Helenium. Biol. Pharm. Bull. 2002, 25, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Khan, M.; Rasul, A.; Sun, M.; Sui, Y.; Zhong, L.; Yang, L.; Zhu, Q.; Feng, L.; Ma, T. Isoalantolactone Inhibits Constitutive NF-ΚB Activation and Induces Reactive Oxygen Species-Mediated Apoptosis in Osteosarcoma U2OS Cells through Mitochondrial Dysfunction. Oncol. Rep. 2014, 32, 1585–1593. [Google Scholar] [CrossRef]
- Wang, K.L.; Yu, Y.C.; Hsia, S.M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef]
- Zhang, Z.; Yung, K.K.L.; Ko, J.K.S. Therapeutic Intervention in Cancer by Isoliquiritigenin from Licorice: A Natural Antioxidant and Redox Regulator. Antioxidants 2022, 11, 1349. [Google Scholar] [CrossRef]
- Blockade of Cytokine-Induced Endothelial Cell Adhesion Molecule Expression by Licorice Isoliquiritigenin through NF-KappaB Signal Disruption—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17259331/ (accessed on 27 April 2024).
- Jian, M.; Sun, X.; Cheng, G.; Zhang, H.; Li, X.; Song, F.; Liu, Z.; Wang, Z. Discovery of Phenolic Matrix Metalloproteinase Inhibitors by Peptide Microarray for Osteosarcoma Treatment. J. Nat. Prod. 2022, 85, 2424–2432. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Yang, Q.Q.; Ma, R.B.; Ke, Y.; Dong, F.; Wu, X.E. Isoliquiritigenin Suppresses Osteosarcoma U2OS Cell Proliferation and Invasion by Regulating the PI3K/Akt Signalling Pathway. Chemotherapy 2018, 63, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, X.; Sun, C.; Liu, X.; Shi, X.; Wu, S. Isoliquiritigenin Inhibits the Proliferation, Apoptosis and Migration of Osteosarcoma Cells. Oncol. Rep. 2019, 41, 2502–2510. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Rangel, L.P.; Duarte da Cunha Martins-Dinis, M.M.; da Silva Ferretti, G.D.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules 2020, 25, 893. [Google Scholar] [CrossRef]
- Liu, T.J.; Lin, S.Y.; Chau, Y.P. Inhibition of Poly(ADP-Ribose) Polymerase Activation Attenuates β-Lapachone-Induced Necrotic Cell Death in Human Osteosarcoma Cells. Toxicol. Appl. Pharmacol. 2002, 182, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Kondo, T.; Lee, H.; Song, C.W.; Park, H.J. Hyperthermia Enhances the Effect of β-Lapachone to Cause ΓH2AX Formations and Cell Death in Human Osteosarcoma Cells. Int. J. Hyperth. 2011, 27, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Saitoh, T. The Chemical Studies on the Oriental Plant Drugs. XIX. Some New Constituents of Licorice Root. 1. The Structure of Licoricidin. Chem. Pharm. Bull. 1968, 16, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wu, F.; Bai, Y.; Wang, T.; Ma, S.; Guo, L.; Liu, G.; Leng, G.; Kong, Y.; Zhang, Y. Licoricidin Combats Gastric Cancer by Targeting the ICMT/Ras Pathway in Vitro and in Vivo. Front. Pharmacol. 2022, 13, 972825. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Tang, S.; Li, K.; Li, Z.; Liang, W.; Qiao, X.; Wang, Q.; Yu, S.; Ye, M. Licoricidin Inhibits the Growth of SW480 Human Colorectal Adenocarcinoma Cells in Vitro and in Vivo by Inducing Cycle Arrest, Apoptosis and Autophagy. Toxicol. Appl. Pharmacol. 2017, 326, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Liu, J.; Lu, Y.; Li, D. Licoricidin Enhances Gemcitabine-Induced Cytotoxicity in Osteosarcoma Cells by Suppressing the Akt and NF-ΚB Signal Pathways. Chem. Biol. Interact. 2018, 290, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Kuang, T.; Du, H.; Li, Q.; Feng, T.; Zhang, Y.; Fan, G. Magnoflorine: A Review of Its Pharmacology, Pharmacokinetics and Toxicity. Pharmacol. Res. 2020, 152, 104632. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, G.; Wang, W.; Qiu, E.; Pei, Y.; Zhang, X. Magnoflorine Inhibits the Malignant Phenotypes and Increases Cisplatin Sensitivity of Osteosarcoma Cells via Regulating MiR-410-3p/HMGB1/NF-ΚB Pathway. Life Sci. 2020, 256, 117967. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, F.; Wu, L.; Xu, Y.; Du, Q. Matrine Exerts Pharmacological Effects through Multiple Signaling Pathways: A Comprehensive Review. Drug Des. Devel. Ther. 2022, 16, 533–569. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.N.; Zhao, H.M.; Tong, Z.C.; Yang, J.; Wang, H.; Liang, X.J. Matrine Inhibits the Invasive Properties of Human Osteosarcoma Cells by Downregulating the ERK-NF-ΚB Pathway. Anticancer Drugs 2014, 25, 1035–1043. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, S.; Yang, Y.; Yang, C.; Chen, D.; Yao, Z.; Sun, B. Matrine Enhances the Efficacy of Adriamycin Chemotherapy in Osteosarcoma Cells by the STAT3 Pathway. Anticancer Drugs 2019, 30, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, M.A. Therapeutics Role of Azadirachta Indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid. Based Complement. Alternat Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Quistad, G.B.; Casida, J.E. Cytotoxicity of Nimbolide, Epoxyazadiradione and Other Limonoids from Neem Insecticide. Life Sci. 1996, 58, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Hou, C.H.; Lin, F.L.; Tsao, Y.T.; Hou, S.M. Nimbolide Induces ROS-Regulated Apoptosis and Inhibits Cell Migration in Osteosarcoma. Int. J. Mol. Sci. 2015, 16, 23405–23424. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Martín, H.; Mariño, C.; Rossignoli, A.E. Okadaic Acid Depuration from the Cockle Cerastoderma Edule. Toxins 2022, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhao, X.Y.; Ji, L.D.; Xu, J. Okadaic Acid (OA): Toxicity, Detection and Detoxification. Toxicon 2019, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Okamura, H.; Morimoto, H.; Teramachi, J.; Haneji, T. Protein Phosphatase 2A Cα Regulates Proliferation, Migration, and Metastasis of Osteosarcoma Cells. Lab. Investig. 2016, 96, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.D. Advances in Chemistry and Bioactivity of Parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum Parthenium L.): A Systematic Review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef]
- Kwok, B.H.B.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The Anti-Inflammatory Natural Product Parthenolide from the Medicinal Herb Feverfew Directly Binds to and Inhibits IκB Kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Emanuele, S.; Di Fiore, R.; Vento, R.; Tesoriere, G. Parthenolide Induces Caspase-Independent and AIF-Mediated Cell Death in Human Osteosarcoma and Melanoma Cells. J. Cell Physiol. 2013, 228, 952–967. [Google Scholar] [CrossRef]
- Kishida, Y.; Yoshikawa, H.; Myoui, A. Parthenolide, a Natural Inhibitor of Nuclear Factor-KappaB, Inhibits Lung Colonization of Murine Osteosarcoma Cells. Clin. Cancer Res. 2007, 13, 59–67. [Google Scholar] [CrossRef]
- Zuch, D.; Giang, A.H.; Shapovalov, Y.; Schwarz, E.; Rosier, R.; O’Keefe, R.; Eliseev, R.A. Targeting Radioresistant Osteosarcoma Cells with Parthenolide. J. Cell Biochem. 2012, 113, 1282–1291. [Google Scholar] [CrossRef]
- Sugiyasu, K.; Nanno, K.; Tamai, N.; Hashimoto, N.; Kishida, Y.; Yoshikawa, H.; Myoui, A. Radio-Sensitization of the Murine Osteosarcoma Cell Line LM8 with Parthenolide, a Natural Inhibitor of NF-ΚB. Oncol. Lett. 2011, 2, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, R.; Yan, X.; Liu, H.; Zhang, Y.; Mu, D.; Han, J.; Li, X. Phloretin Induces Apoptosis of Human Esophageal Cancer via a Mitochondria-Dependent Pathway. Oncol. Lett. 2017, 14, 6763–6768. [Google Scholar] [CrossRef]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Ramniwas, S.; Vashishth, K.; Varol, M.; Jaswal, V.S.; Haque, S.; Sak, K. Phloretin, as a Potent Anticancer Compound: From Chemistry to Cellular Interactions. Molecules 2022, 27, 8819. [Google Scholar] [CrossRef]
- Huang, W.C.; Dai, Y.W.; Peng, H.L.; Kang, C.W.; Kuo, C.Y.; Liou, C.J. Phloretin Ameliorates Chemokines and ICAM-1 Expression via Blocking of the NF-ΚB Pathway in the TNF-α-Induced HaCaT Human Keratinocytes. Int. Immunopharmacol. 2015, 27, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.S.; Yang, R.S.; Fu, W.M. Osteopontin Upregulates the Expression of Glucose Transporters in Osteosarcoma Cells. PLoS ONE 2014, 9, e109550. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, X.; Wang, H. Punicalagin Inhibited Proliferation, Invasion and Angiogenesis of Osteosarcoma through Suppression of NF-κB Signaling. Mol. Med. Rep. 2020, 22, 2386–2394. [Google Scholar] [CrossRef]
- Wang, X.Z.; Zhang, S.F.; Yang, Z.H.; Ye, Z.W.; Liu, J. Punicalagin Suppresses Osteosarcoma Growth and Metastasis by Regulating NF-ΚB Signaling. J. Biol. Regul. Homeost. Agents 2020, 5, 1699–1708. [Google Scholar] [CrossRef]
- Naz, I.; Ramchandani, S.; Khan, M.R.; Yang, M.H.; Ahn, K.S. Anticancer Potential of Raddeanin A, a Natural Triterpenoid Isolated from Anemone Raddeana Regel. Molecules 2020, 25, 1035. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhu, J.; Zhao, A.; Zhang, J.; Wang, Y.; Zhang, H.; Zhang, L.; Zhang, Q. Raddeanin A, a Natural Triterpenoid Saponin Compound, Exerts Anticancer Effect on Human Osteosarcoma via the ROS/JNK and NF-ΚB Signal Pathway. Toxicol. Appl. Pharmacol. 2018, 353, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, J.; Sun, W.; Zhang, T.; Zuo, D.; Wang, H.; Wang, G.; Xu, J.; Yin, F.; Mao, M.; et al. Antitumor Activity of Raddeanin A Is Mediated by Jun Amino-Terminal Kinase Activation and Signal Transducer and Activator of Transcription 3 Inhibition in Human Osteosarcoma. Cancer Sci. 2019, 110, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Zuo, D.; Zhang, T.; Yin, F.; Zhou, Z.; Wang, H.; Xu, J.; Mao, M.; Wang, G.; et al. Attenuation of STAT3 Phosphorylation Promotes Apoptosis and Chemosensitivity in Human Osteosarcoma Induced by Raddeanin A. Int. J. Biol. Sci. 2019, 15, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, C.; Zhu, Y.; Fu, F.; Wang, G.; Li, S. Sulforaphene Inhibits the Progression of Osteosarcoma via Regulating FSTL1/NF-ΚB Pathway. Life Sci. 2020, 263, 118485. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, Q.; Zhou, S.; Xu, S.; Yao, K. Tetramethylpyrazine: A Review on Its Mechanisms and Functions. Biomed. Pharmacother. 2022, 150, 113005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, Q.; Zhao, W. Tetramethylpyrazine Inhibits Osteosarcoma Cell Proliferation via Downregulation of NF-ΚB in Vitro and in Vivo. Mol. Med. Rep. 2013, 8, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhou, L.; Yan, B.; Yan, L.; Liu, F.; Tong, P.; Yu, W.; Dong, X.; Xie, L.; Zhang, J.; et al. Theabrownin Triggers DNA Damage to Suppress Human Osteosarcoma U2OS Cells by Activating P53 Signalling Pathway. J. Cell Mol. Med. 2018, 22, 4423–4436. [Google Scholar] [CrossRef]
- Jin, W.; Gu, C.; Zhou, L.; Yang, X.; Gui, M.; Zhang, J.; Chen, J.; Dong, X.; Yuan, Q.; Shan, L. Theabrownin Inhibits the Cytoskeleton-dependent Cell Cycle, Migration and Invasion of Human Osteosarcoma Cells through NF-κB Pathway-related Mechanisms. Oncol. Rep. 2020, 44, 2621–2633. [Google Scholar] [CrossRef]
- Alhmied, F.; Alammar, A.; Alsultan, B.; Alshehri, M.; Pottoo, F.H. Molecular Mechanisms of Thymoquinone as Anticancer Agent. Comb. Chem. High. Throughput Screen. 2021, 24, 1644–1653. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Asemi, Z.; Yousefi, B. Potential Anticancer Properties and Mechanisms of Thymoquinone in Osteosarcoma and Bone Metastasis. Cell. Mol. Biol. Lett. 2022, 27, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Effects of Thymoquinone and Selenium on the Proliferation of Mg 63 Cells in Tissue Culture—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19141954/ (accessed on 27 April 2024).
- Sarman, H.; Bayram, R.; Benek, S.B. Anticancer Drugs with Chemotherapeutic Interactions with Thymoquinone in Osteosarcoma Cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1263–1270. [Google Scholar] [PubMed]
- Ahmadzadeh, H.; Ahmadi, M.; Golchin, A.; Malakoti, F.; Maleki, M.; Alemi, F.; Bazavar, M.; Yousefi, B. The Effect of TQ and Cis in OS. Drug Res. 2022, 72, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Sanapour, N.; Malakoti, F.; Shanebandi, D.; Targhazeh, N.; Yousefi, B.; Soleimanpour, J.; Majidinia, M. Thymoquinone Augments Methotrexate-Induced Apoptosis on Osteosarcoma Cells. Drug Res. 2022, 72, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Khyavi, P.A.; Valizadeh, A.; Shanehbandi, D.; Yousefi, B.; Soleimanpour, J. Thymoquinone Potentiates Methotrexate Mediated-Apoptosis in Saos-2 Osteosarcoma Cell Line. Drug Res. 2022, 72, 390–395. [Google Scholar] [CrossRef]
- Roepke, M.; Diestel, A.; Bajbouj, K.; Walluscheck, D.; Schonfeld, P.; Roessner, A.; Schneider-Stock, R.; Gali-Muhtasib, H. Lack of P53 Augments Thymoquinone-Induced Apoptosis and Caspase Activation in Human Osteosarcoma Cells. Cancer Biol. Ther. 2007, 6, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.Z.; Yang, S.Z.; Ying, X.Z.; Liao, W.; Liu, H.X.; Lin, Z.Q.; Chen, Q.Y.; et al. Antitumor and Anti-Angiogenesis Effects of Thymoquinone on Osteosarcoma through the NF-ΚB Pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Kuttan, G. Ursolic Acid Induces Apoptosis by Activating P53 and Caspase-3 Gene Expressions and Suppressing NF-KappaB Mediated Activation of Bcl-2 in B16F-10 Melanoma Cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Proliferative Inhibition, Cell-Cycle Dysregulation, and Induction of Apoptosis by Ursolic Acid in Human Non-Small Cell Lung Cancer A549 Cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef]
- Zhang, R.X.; Li, Y.; Tian, D.D.; Liu, Y.; Nian, W.; Zou, X.; Chen, Q.Z.; Zhou, L.Y.; Deng, Z.L.; He, B.C. Ursolic Acid Inhibits Proliferation and Induces Apoptosis by Inactivating Wnt/β-Catenin Signaling in Human Osteosarcoma Cells. Int. J. Oncol. 2016, 49, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Cheng, C.H.; Lee, Y.H.; Chang, I.L.; Chen, H.Y.; Hsieh, C.P.; Chueh, P.J. Ursolic Acid Triggers Apoptosis in Human Osteosarcoma Cells via Caspase Activation and the ERK1/2 MAPK Pathway. J. Agric. Food Chem. 2016, 64, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fu, H.; Wang, Z.; Yin, F.; Li, J.; Hua, Y.; Cai, Z. A New Synthetic Ursolic Acid Derivative IUA with Anti-Tumor Efficacy Against Osteosarcoma Cells via Inhibition of JNK Signaling Pathway. Cell. Physiol. Biochem. 2014, 34, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Huang, Y.F.; Hsieh, C.P.; Chueh, P.J.; Chen, Y.L. Combined Use of Zoledronic Acid Augments Ursolic Acid-Induced Apoptosis in Human Osteosarcoma Cells through Enhanced Oxidative Stress and Autophagy. Molecules 2016, 21, 1640. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Ni, Z.; Wu, K.; Cheng, P.; Ji, X.; Li, G.; Shang, X. A Novel Redox-Responsive Ursolic Acid Polymeric Prodrug Delivery System for Osteosarcoma Therapy. Drug Deliv. 2021, 28, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, G.; Zhou, X.; Huang, L.; Meng, J.; He, B.; Qi, Y. α-Mangostin Inhibits LPS-Induced Bone Resorption by Restricting Osteoclastogenesis via NF-ΚB and MAPK Signaling. Chin. Med. 2022, 17, 34. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Dong, Y.; Ren, F. α-Mangostin Induces Apoptosis in Human Osteosarcoma Cells Through ROS-Mediated Endoplasmic Reticulum Stress via the WNT Pathway. Cell Transplant. 2021, 30, 09636897211035080. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, B.S.; Yu, S.B.; Kang, H.M.; Kim, H.J.; Kim, I.R. Induction of Apoptosis and Inhibition of Epithelial Mesenchymal Transition by α-Mangostin in MG-63 Cell Lines. Evid.-Based Complement. Altern. Med. 2018, 2018, 3985082. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Z.L.; Liu, B.; Yan, X.B.; Feng, J.; Tao, H.M. Enhanced Anticancer Effect of Gemcitabine by Genistein in Osteosarcoma: The Role of Akt and Nuclear Factor-KappaB. Anticancer Drugs 2010, 21, 288–296. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Shen, C.; Lai, J.; Shi, Z.; Liu, B.; Tao, H. Genistein Potentiates the Anti-Cancer Effects of Gemcitabine in Human Osteosarcoma via the Downregulation of Akt and Nuclear Factor-ΚB Pathway. Anticancer Agents Med. Chem. 2012, 12, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Aizawa, J.; Sakayama, K.; Kidani, T.; Takata, T.; Norimatsu, Y.; Miura, H.; Masuno, H. Genistein Inhibits Cell Invasion and Motility by Inducing Cell Differentiation in Murine Osteosarcoma Cell Line LM8. BMC Cell Biol. 2012, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Wako, H.; Nakata, K.; Aida, R. Genistein Induces Antiproliferative Activity and Apoptosis in Human Osteosarcoma Saos-2 Cells. Anticancer Res. 2023, 43, 5387–5392. [Google Scholar] [CrossRef] [PubMed]
- Kidani, T.; Nakamura, A.; Kamei, S.; Norimatsu, Y.; Miura, H.; Masuno, H. Overexpression of Cytoplasmic β-Catenin Inhibits the Metastasis of the Murine Osteosarcoma Cell Line LM8. Cancer Cell Int. 2014, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Lee, S.; Hong, S.; Park, J.; Park, E.; Kim, J.; Park, S.; et al. Anti-Inflammatory Effects of Magnolol and Honokiol Are Mediated through Inhibition of the Downstream Pathway of MEKK-1 in NF-KappaB Activation Signaling. Planta Med. 2005, 71, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Ku, M.C.; Lee, K.C.; Yueh, P.F.; Hsu, F.T.; Lin, R.F.; Yang, C.C.; Wang, W.C.; Chen, J.H.; Hsu, L.C.; et al. Magnolol Suppresses ERK/NF-ΚB Signaling and Triggers Apoptosis Through Extrinsic/Intrinsic Pathways in Osteosarcoma. Anticancer Res. 2022, 42, 4403–4410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wen, H.; Li, H. Magnolol Induces Apoptosis in Osteosarcoma Cells via G0/G1 Phase Arrest and P53-Mediated Mitochondrial Pathway. J. Cell Biochem. 2019, 120, 17067–17079. [Google Scholar] [CrossRef]
- Van Stiphout, C.M.; Luu, A.K.; Viloria-Petit, A.M. Proteasome Inhibitors and Their Potential Applicability in Osteosarcoma Treatment. Cancers 2022, 14, 4544. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Nakamura, T.; Nakamura, K.; Hagi, T.; Okamoto, T.; Kita, K.; Matsuyama, Y.; Yoshida, K.; Asanuma, Y.; Sudo, A. Compound Library Screening for Synergistic Drug Combinations: MTOR Inhibitor and Proteasome Inhibitor Effective against Osteosarcoma Cells. Anticancer Res. 2022, 42, 4319–4328. [Google Scholar] [CrossRef]
- Nakamura, K.; Asanuma, K.; Okamoto, T.; Iino, T.; Hagi, T.; Nakamura, T.; Sudo, A. Combination of Everolimus and Bortezomib Inhibits the Growth and Metastasis of Bone and Soft Tissue Sarcomas via JNK/P38/ERK MAPK and AKT Pathways. Cancers 2023, 15, 2468. [Google Scholar] [CrossRef]
- Shishodia, S.; Amin, H.M.; Lai, R.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibits Constitutive NF-ΚB Activation, Induces G1/S Arrest, Suppresses Proliferation, and Induces Apoptosis in Mantle Cell Lymphoma. Biochem. Pharmacol. 2005, 70, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; An, F.; He, X.; Cao, X. Curcumin Inhibits the Proliferation and Invasion of Human Osteosarcoma Cell Line MG-63 by Regulating MiR-138. Int. J. Clin. Exp. Pathol. 2015, 8, 14946. [Google Scholar] [PubMed]
- Aziz, M.N.M.; Rahim, N.F.C.; Hussin, Y.; Yeap, S.K.; Masarudin, M.J.; Mohamad, N.E.; Akhtar, M.N.; Osman, M.A.; Cheah, Y.K.; Alitheen, N.B. Anti-Metastatic and Anti-Angiogenic Effects of Curcumin Analog DK1 on Human Osteosarcoma Cells In Vitro. Pharmaceuticals 2021, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Lu, P.W.A.; Lin, C.W.; Yang, S.F. Curcumin in Human Osteosarcoma: From Analogs to Carriers. Drug Discov. Today 2023, 28, 103437. [Google Scholar] [CrossRef] [PubMed]
- Maran, A.; Yaszemski, M.J.; Kohut, A.; Voronov, A. Curcumin and Osteosarcoma: Can Invertible Polymeric Micelles Help? Materials 2016, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, M.; Zandieh Doulabi, B.; Sun, Y.; Liu, Y. Paradox: Curcumin, a Natural Antioxidant, Suppresses Osteosarcoma Cells via Excessive Reactive Oxygen Species. Int. J. Mol. Sci. 2023, 24, 11975. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Fan, R.; Zhu, K.; Wang, Y.; Xie, W.; Liang, Y. Curcumin Induces Ferroptosis and Apoptosis in Osteosarcoma Cells by Regulating Nrf2/GPX4 Signaling Pathway. Exp. Biol. Med. 2023, 248, 2183. [Google Scholar] [CrossRef]
- Zahedipour, F.; Bolourinezhad, M.; Teng, Y.; Sahebkar, A. The Multifaceted Therapeutic Mechanisms of Curcumin in Osteosarcoma: State-of-the-Art. J. Oncol. 2021, 2021, 3006853. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, M.; Guo, W.; Sun, W.; Liu, Y. Curcumin in Osteosarcoma Therapy: Combining with Immunotherapy, Chemotherapeutics, Bone Tissue Engineering Materials and Potential Synergism with Photodynamic Therapy. Front. Oncol. 2021, 11, 672490. [Google Scholar] [CrossRef]
- Dhule, S.S.; Penfornis, P.; He, J.; Harris, M.R.; Terry, T.; John, V.; Pochampally, R. The Combined Effect of Encapsulating Curcumin and C6 Ceramide in Liposomal Nanoparticles against Osteosarcoma. Mol. Pharm. 2014, 11, 417–427. [Google Scholar] [CrossRef]
- Ma, D.; Tremblay, P.; Mahngar, K.; Collins, J.; Hudlicky, T.; Pandey, S. Selective Cytotoxicity against Human Osteosarcoma Cells by a Novel Synthetic C-1 Analogue of 7-Deoxypancratistatin Is Potentiated by Curcumin. PLoS ONE 2011, 6, e28780. [Google Scholar] [CrossRef] [PubMed]
- Lamoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Xu, Y.; Wang, X.; Han, W.; Pan, H.; Xiao, M. Metformin: A Novel but Controversial Drug in Cancer Prevention and Treatment. Mol. Pharm. 2015, 12, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Choi, A.; Lee, M.; Lee, J.A. Metformin Displays in Vitro and in Vivo Antitumor Effect against Osteosarcoma. Korean J. Pediatr. 2016, 59, 374. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, P.; Xu, K.; Chen, T.; Jiao, J.; Wei, H.; Yang, X.; Xu, W.; Wan, W.; Xiao, J. Metformin Induces Cell Cycle Arrest, Apoptosis and Autophagy through ROS/JNK Signaling Pathway in Human Osteosarcoma. Int. J. Biol. Sci. 2020, 16, 74–84. [Google Scholar] [CrossRef]
- Metts, J.L.; Trucco, M.; Weiser, D.A.; Thompson, P.; Sandler, E.; Smith, T.; Crimella, J.; Sansil, S.; Thapa, R.; Fridley, B.L.; et al. A Phase I Trial of Metformin in Combination with Vincristine, Irinotecan, and Temozolomide in Children with Relapsed or Refractory Solid and Central Nervous System Tumors: A Report from the National Pediatric Cancer Foundation. Cancer Med. 2023, 12, 4270–4281. [Google Scholar] [CrossRef]
- Miwa, S.; Sugimoto, N.; Yamamoto, N.; Shirai, T.; Nishida, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Igarashi, K.; Yachie, A.; et al. Caffeine Induces Apoptosis of Osteosarcoma Cells by Inhibiting AKT/MTOR/S6K, NF-ΚB and MAPK Pathways. Anticancer Res. 2012, 32, 3643–3649. [Google Scholar] [PubMed]
- Miwa, S.; Sugimoto, N.; Shirai, T.; Hayashi, K.; Nishida, H.; Ohnari, I.; Takeuchi, A.; Yachie, A.; Tsuchiya, H. Caffeine Activates Tumor Suppressor PTEN in Sarcoma Cells. Int. J. Oncol. 2011, 39, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ii, S.; Ueda, Y.; Shimazaki, M.; Katsuta, S.; Takazawa, K.; Kanazawa, Y.; Tomita, K.; Tsuchiya, H. Identification of Novel Genes Involved in the Synergistic Antitumor Effect of Caffeine in Osteosarcoma Cells Using CDNA Macroarray. Anticancer Res. 2008, 28, 645–653. [Google Scholar]
- Kawahara, M.; Takahashi, Y.; Takazawa, K.; Tsuchiya, H.; Tomita, K.; Yokogawa, K.; Miyamoto, K.I. Caffeine Dose-Dependently Potentiates the Antitumor Effect of Cisplatin on Osteosarcomas. Anticancer Res. 2008, 28, 1681–1685. [Google Scholar]
- Abe, K.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Tsuchiya, H. Caffeine Citrate Enhanced Cisplatin Antitumor Effects in Osteosarcoma and Fibrosarcoma in Vitro and in Vivo. BMC Cancer 2019, 19, 689. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Oshiro, H.; Miyake, K.; Sugisawa, N.; Han, Q.; Tan, Y.; Park, J.; Zhang, Z.; Razmjooei, S.; Yamamoto, N.; et al. Oral Recombinant Methioninase, Combined with Oral Caffeine and Injected Cisplatinum, Overcome Cisplatinum-Resistance and Regresses Patient-Derived Orthotopic Xenograft Model of Osteosarcoma. Anticancer Res. 2019, 39, 4653–4657. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Murakami, T.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Tsuchiya, H.; Hoffman, R.M. Antimetastatic Efficacy of the Combination of Caffeine and Valproic Acid on an Orthotopic Human Osteosarcoma Cell Line Model in Nude Mice. Anticancer Res. 2017, 37, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
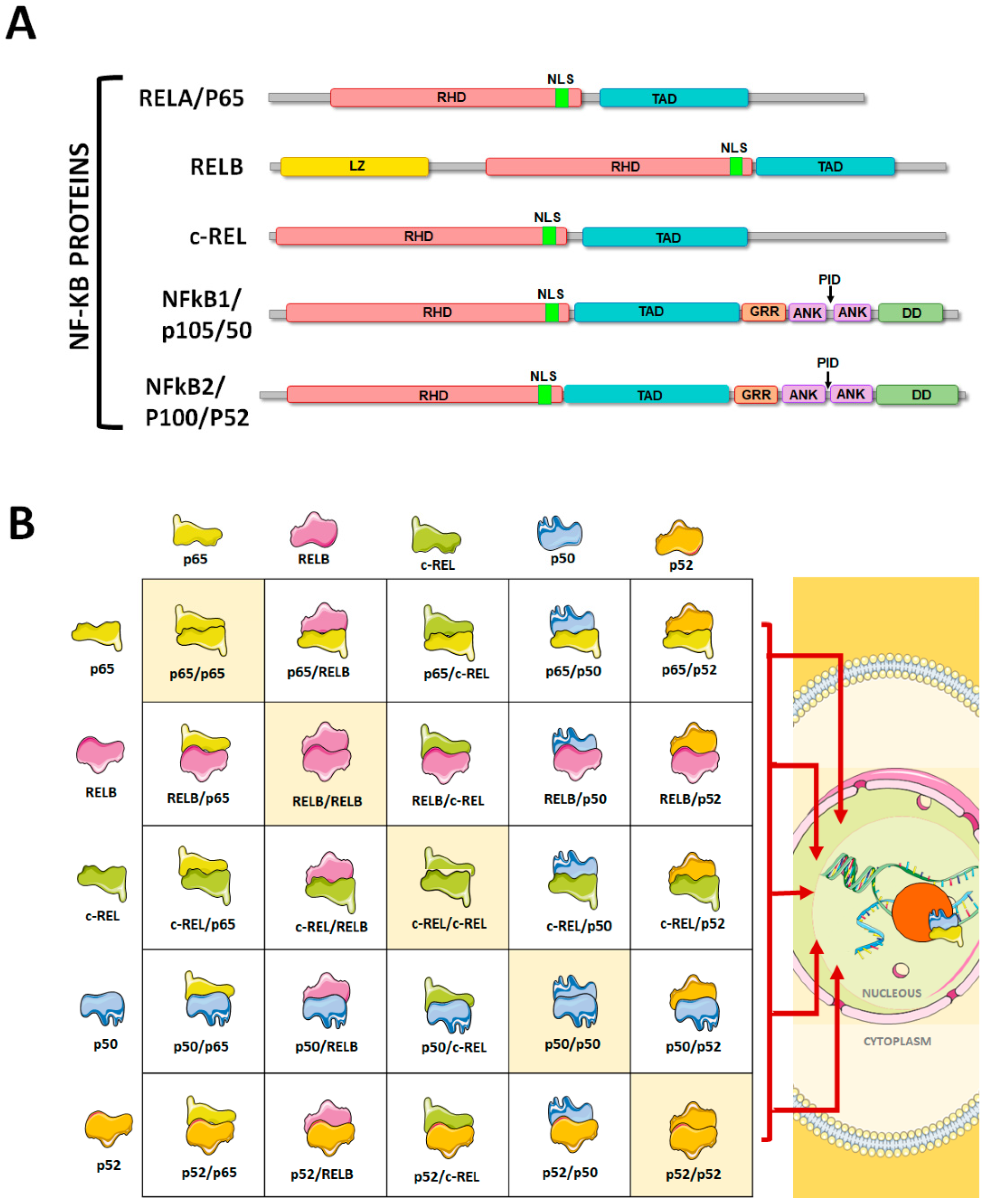
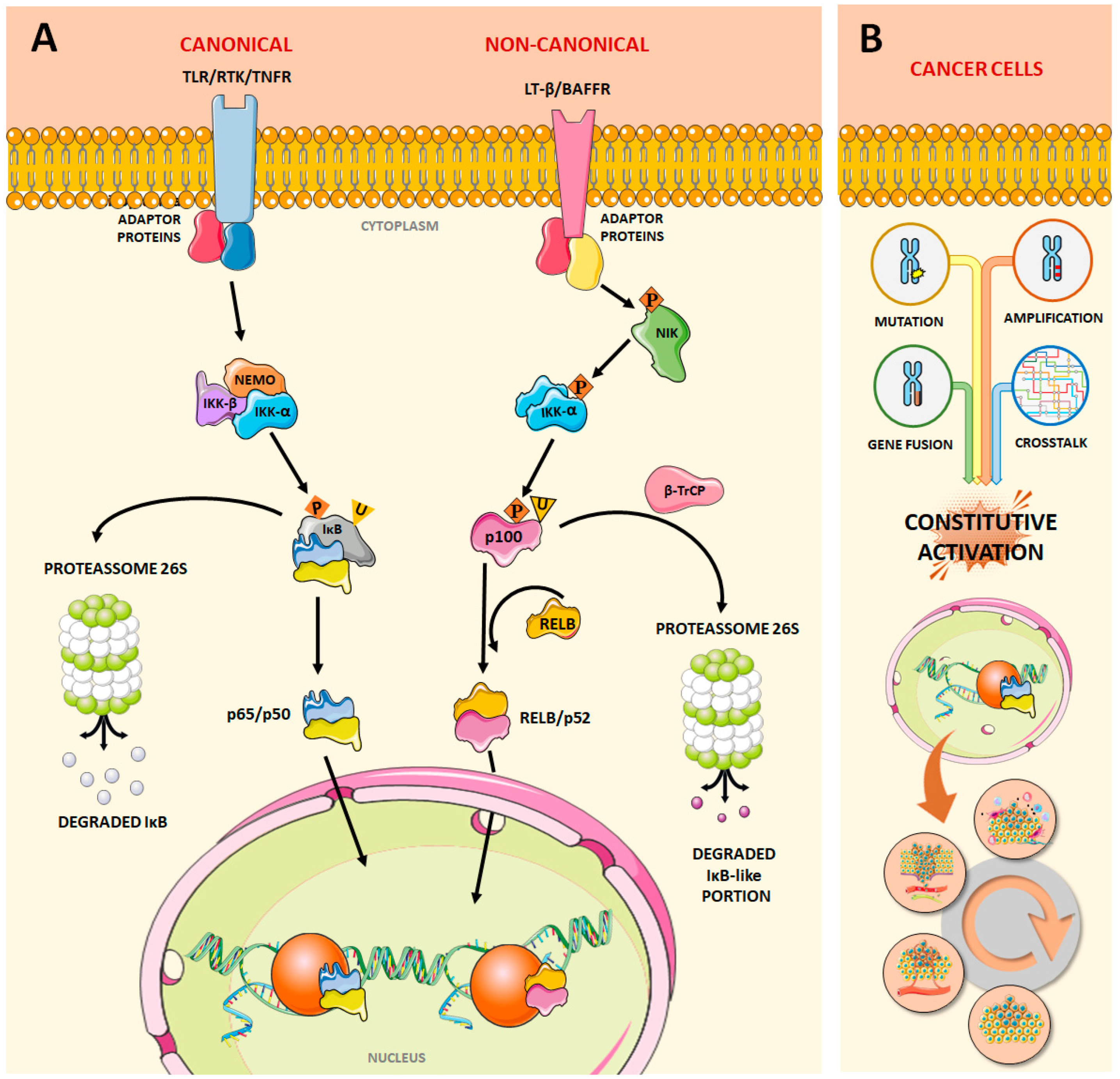


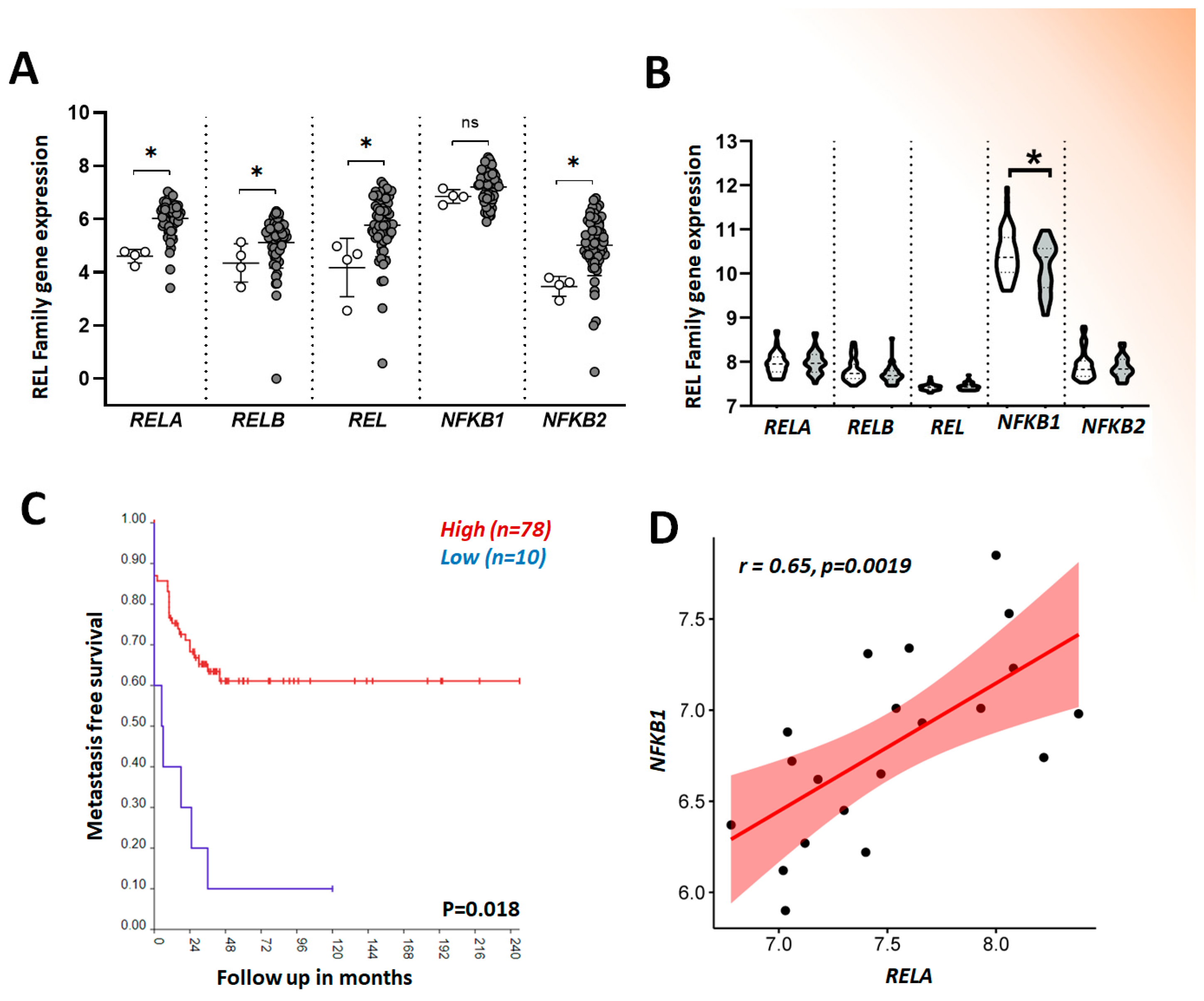

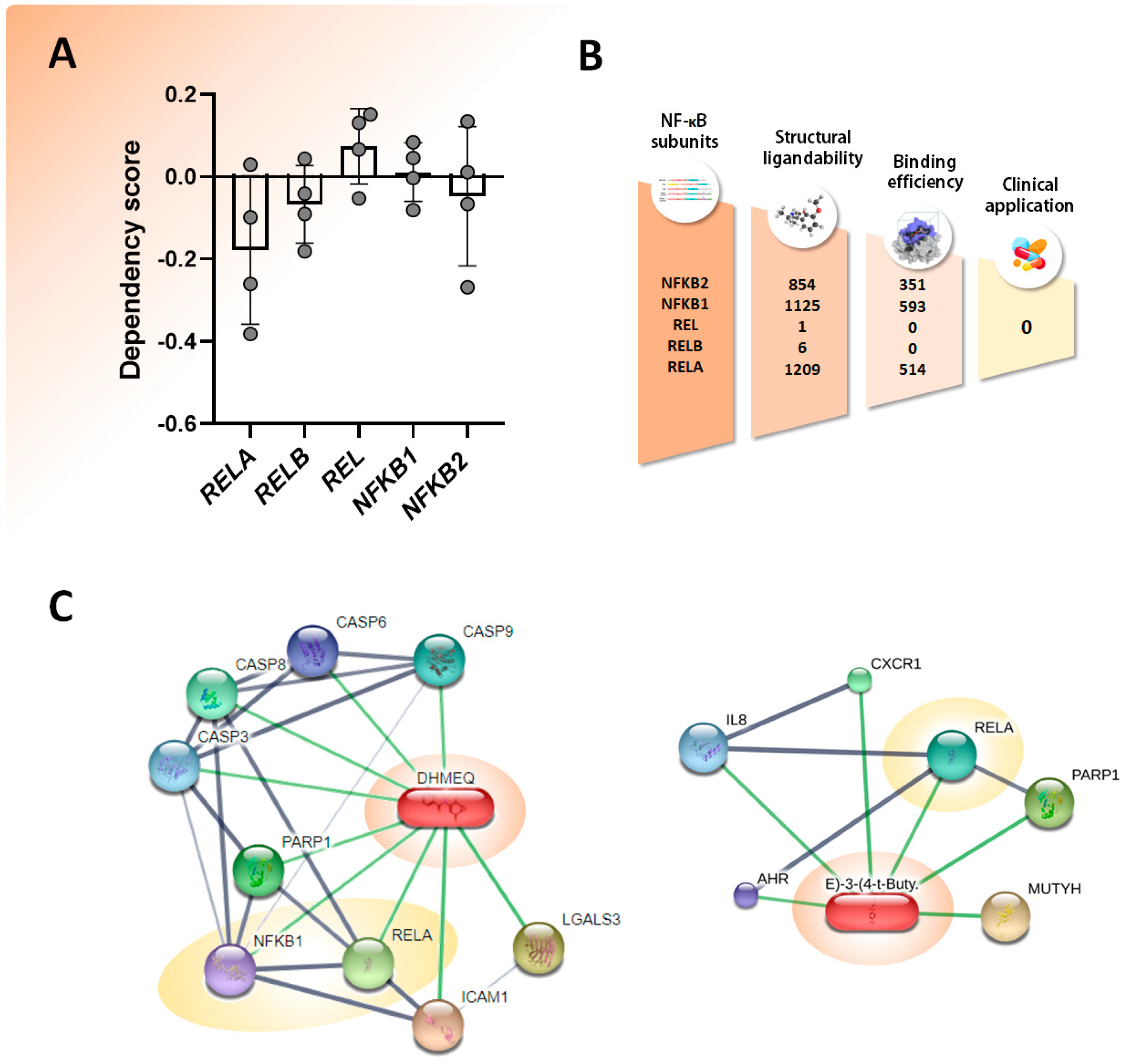
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, M.; Guenka, S.; Bastos, D.; Oliveira, K.L.; Brassesco, M.S. Amicis Omnia Sunt Communia: NF-κB Inhibition as an Alternative to Overcome Osteosarcoma Heterogeneity. Pharmaceuticals 2024, 17, 734. https://doi.org/10.3390/ph17060734
Medeiros M, Guenka S, Bastos D, Oliveira KL, Brassesco MS. Amicis Omnia Sunt Communia: NF-κB Inhibition as an Alternative to Overcome Osteosarcoma Heterogeneity. Pharmaceuticals. 2024; 17(6):734. https://doi.org/10.3390/ph17060734
Chicago/Turabian StyleMedeiros, Mariana, Sophia Guenka, David Bastos, Karla Laissa Oliveira, and María Sol Brassesco. 2024. "Amicis Omnia Sunt Communia: NF-κB Inhibition as an Alternative to Overcome Osteosarcoma Heterogeneity" Pharmaceuticals 17, no. 6: 734. https://doi.org/10.3390/ph17060734
APA StyleMedeiros, M., Guenka, S., Bastos, D., Oliveira, K. L., & Brassesco, M. S. (2024). Amicis Omnia Sunt Communia: NF-κB Inhibition as an Alternative to Overcome Osteosarcoma Heterogeneity. Pharmaceuticals, 17(6), 734. https://doi.org/10.3390/ph17060734






