Preventing High Fat Diet-Induced Obesity and Related Hepatic Steatosis by Chlorin e6-Mediated Photodynamic Therapy
Abstract
1. Introduction
2. Results
2.1. Ce6-PDT Decreased HFD-Induced Obesity in OVX Female Beagle Dogs
2.2. Ce6-PDT Ameliorated the AST/ALT Ratio and Blood Lipid Levels in HFD-Induced OVX Female Beagle Dogs
2.3. Histopathological Examinations of Adipose Tissues in HFD-Induced OVX Female Beagle Dogs
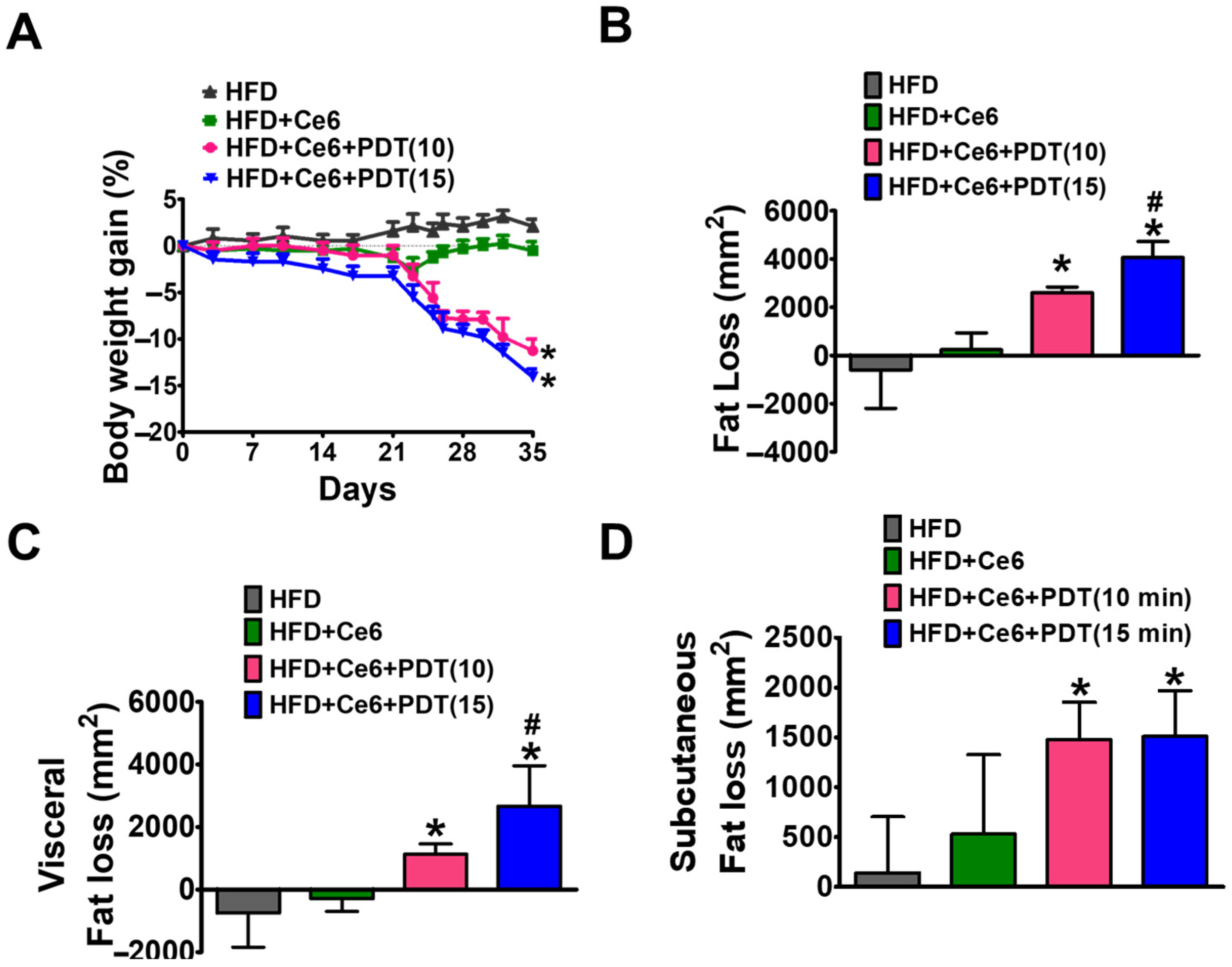
2.4. Study of Ce6-PDT in C57BL6 Male Mice with HFD-Induced Obesity
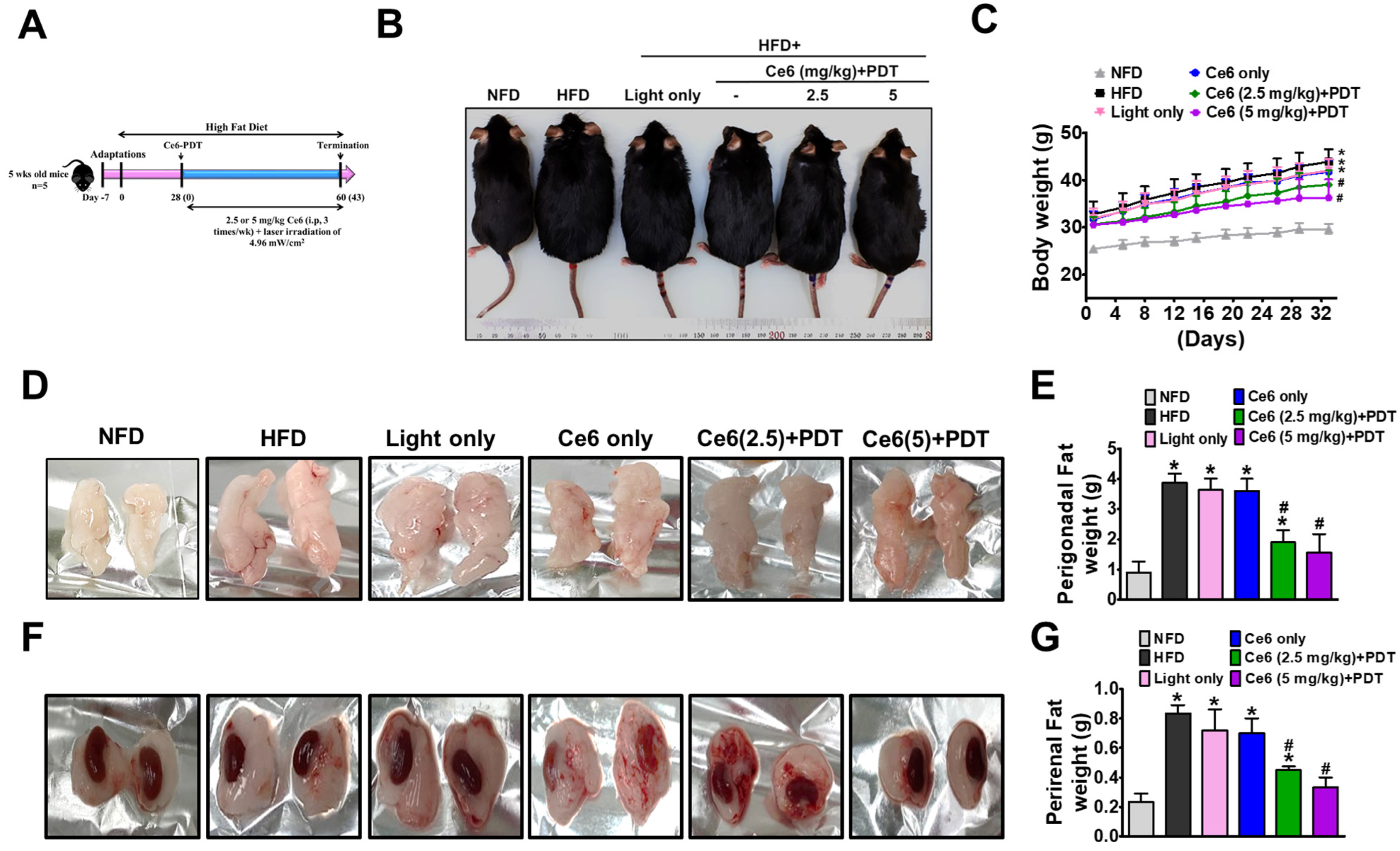
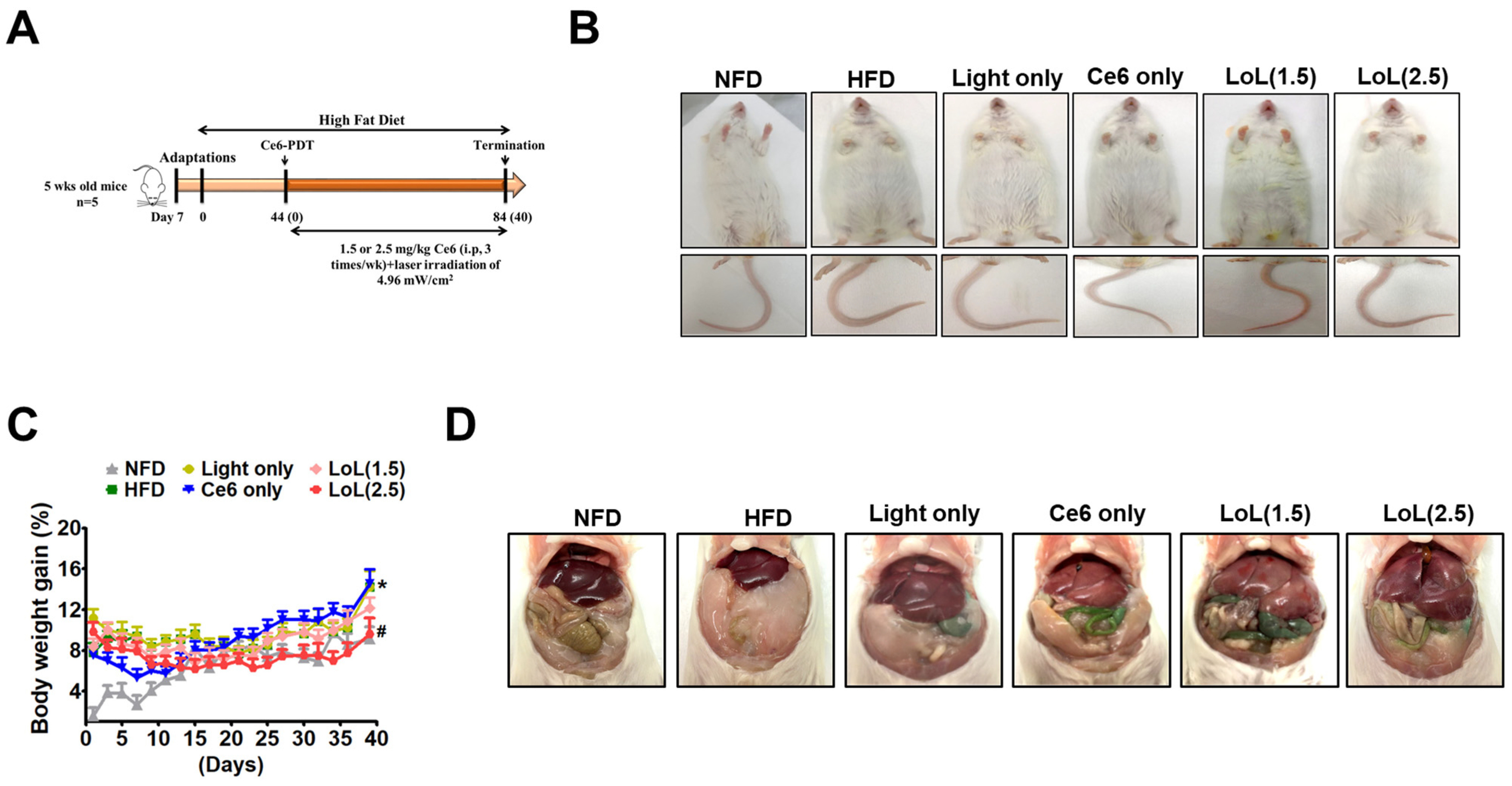
2.5. Ce6-PDT Improved Body Weight and Fat Accumulation in the HFD-Induced Obese Balb/c Mice
2.6. Ce6-PDT Suppressed Adipose Tissue Size and Distribution of White Adipose Tissue in Balb/c Mice with HFD-Induced Obesity
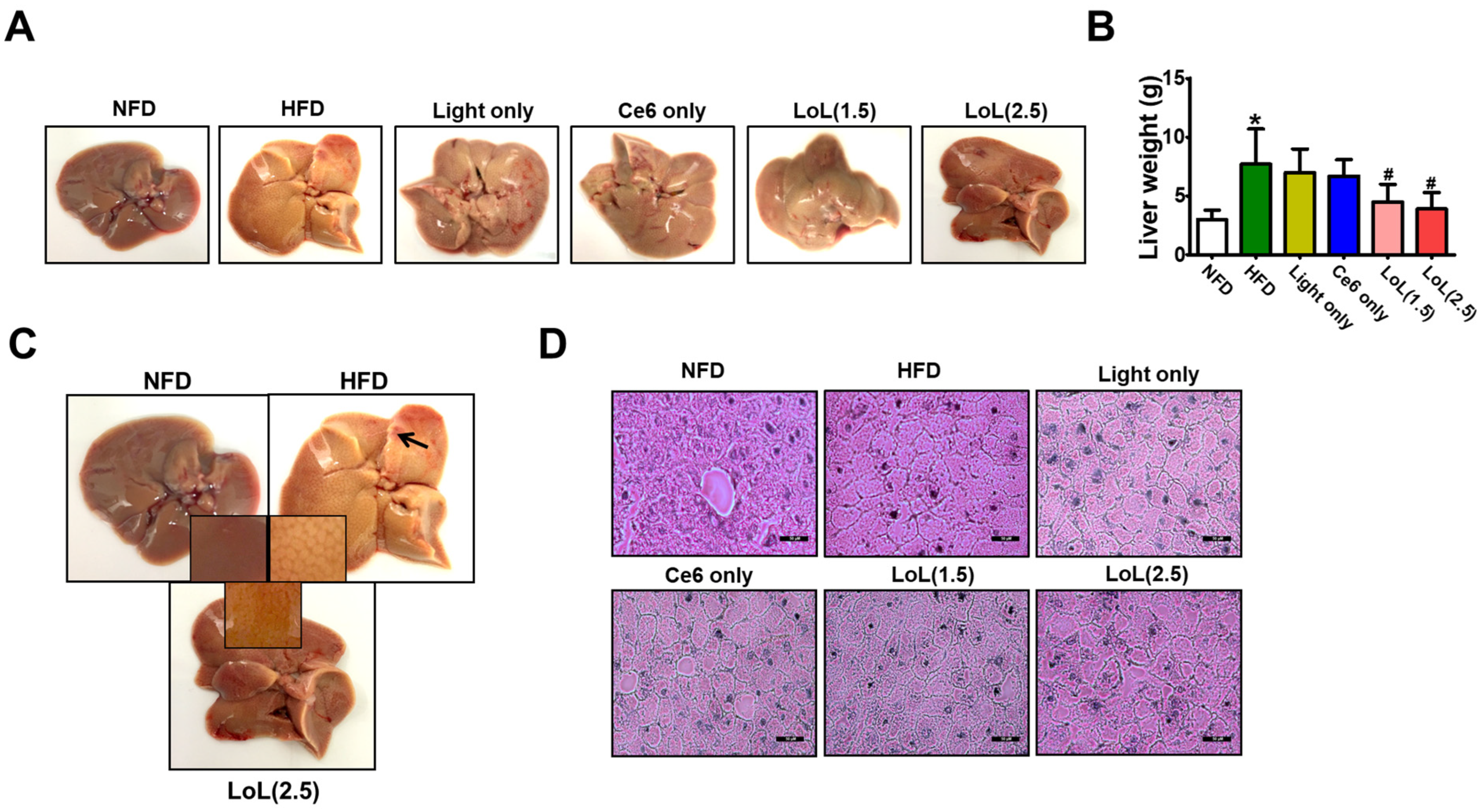
2.7. Ce6-PDT Improved Hepatic Steatosis in HFD-Induced Obese Balb/c Mice
3. Discussion
4. Materials and Methods
4.1. Study Dogs
4.2. Body Weight Increase Regimen
4.3. CT Scan
4.4. Blood Biochemical Test
4.5. Autopsy
4.6. Mice Model 1
4.7. Mice Model 2
4.8. Histopathological Examination
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, W.; Liu, F. Obesity: Causes, Consequences, Treatments, and Challenges; Oxford University Press: Oxford, UK, 2021; Volume 13, pp. 463–465. [Google Scholar]
- Shah, M.; Jeffery, R.W. Is obesity due to overeating and inactivity, or to a defective metabolic rate? A review. Ann. Behav. Med. 1991, 13, 73–81. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Zoran, D.L. Obesity in dogs and cats: A metabolic and endocrine disorder. Vet. Clin. Small Anim. Pract. 2010, 40, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.; Cunningham, S.; Lund, E.; Khanna, C.; Naramore, R.; Patel, A.; Day, M. Obesity and associated comorbidities in people and companion animals: A one health perspective. J. Comp. Pathol 2017, 156, 296–309. [Google Scholar] [CrossRef]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Al-Jawaldeh, A.; Abbass, M.M. Unhealthy dietary habits and obesity: The major risk factors beyond non-communicable diseases in the eastern mediterranean region. Front. Nutr. 2022, 9, 817808. [Google Scholar] [CrossRef]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Eggers, S.; Ohnesorg, T.; Sinclair, A. Genetic regulation of mammalian gonad development. Nat. Rev. Endocrinol. 2014, 10, 673–683. [Google Scholar] [CrossRef]
- Ylli, D.; Sidhu, S.; Parikh, T.; Burman, K.D. Endocrine changes in obesity. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Unger, C.A.; Aladhami, A.K.; Hope III, M.C.; Pourhoseini, S.; Nagarkatti, M.; McGuinness, O.P.; Murphy, E.A.; Velázquez, K.T.; Enos, R.T. Congenital adiponectin deficiency mitigates high-fat-diet-induced obesity in gonadally intact male and female, but not in ovariectomized mice. Sci. Rep. 2022, 12, 16668. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef]
- Dumitrascu, D.L.; Neuman, M.G. Non-alcoholic fatty liver disease: An update on diagnosis. Clujul Med. 2018, 91, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Chen, S.; Chen, X.; Ren, Q.; Yue, L.; Pan, X.; Zhao, H.; Li, Z.; Chen, X. Semaglutide ameliorates metabolism and hepatic outcomes in an NAFLD mouse model. Front. Endocrinol. 2022, 13, 1046130. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Ruiqi, W.; Hongji, Y.; Ning, M.; Chenzuo, J.; Yu, Z.; Zhixuan, X.; Qiang, L.; Qibing, L.; Weiying, L. Mangiferin ameliorates HFD-induced NAFLD through regulation of the AMPK and NLRP3 inflammasome signal pathways. J. Immunol. 2021, 2021, 4084566. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem.-Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef]
- Lee, K.C.; Wu, P.S.; Lin, H.C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 2023, 29, 77. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Cannella, L.; De Martin, S. The role of oxidative stress in NAFLD–NASH–HCC transition—Focus on NADPH oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F. Hepatic steatosis as a marker of metabolic dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef]
- Tchang, B.G.; Aras, M.; Kumar, R.B.; Aronne, L.J. Pharmacologic Treatment of Overweight and Obesity in Adults. 2015. Available online: https://europepmc.org/article/NBK/nbk279038 (accessed on 2 August 2021).
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed. Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.-Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Gurung, P.; Lim, J.; Shrestha, R.; Kim, Y.-W. Chlorin e6-associated photodynamic therapy enhances abscopal antitumor effects via inhibition of PD-1/PD-L1 immune checkpoint. Sci. Rep 2023, 13, 4647. [Google Scholar] [CrossRef]
- Chen, R.; Huang, S.; Lin, T.; Ma, H.; Shan, W.; Duan, F.; Lv, J.; Zhang, J.; Ren, L.; Nie, L. Photoacoustic molecular imaging-escorted adipose photodynamic–browning synergy for fighting obesity with virus-like complexes. Nat. Nanotechnol. 2021, 16, 455–465. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; De Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Neuberger, W.; Albrecht, V. Removal of Fat Cells by PDT. U.S. Patent US20070154538A1, 5 July 2007. [Google Scholar]
- Wanner, M.; Mihm Jr, M.C.; Farinelli, W.A.; Doukas, A.; Zurakowski, D.; Piris, A.; Avram, M.M.; Klein, J.A.; Anderson, R.R. Use of photodynamic therapy and sterile water to target adipose tissue. Dermatol. Surg. 2015, 41, 803–811. [Google Scholar] [CrossRef]
- Li, Q.; Hagberg, C.E.; Silva Cascales, H.; Lang, S.; Hyvönen, M.T.; Salehzadeh, F.; Chen, P.; Alexandersson, I.; Terezaki, E.; Harms, M.J. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat. Med. 2021, 27, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.L.; Bang, H.T.; Ji, S.Y.; Jeong, J.Y.; Kim, M.; Kim, B.; Lee, S.D.; Lee, Y.K.; Reddy, K.E.; Kim, K.H. A simple method to evaluate body condition score to maintain the optimal body weight in dogs. J. Anim. Sci. Technol. 2019, 61, 366. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Singh, J.; Boparai, R.K.; Zhu, J.; Mantri, S.; Khare, P.; Khardori, R.; Kondepudi, K.K.; Chopra, K.; Bishnoi, M. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol. Res. 2017, 123, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Shliakhtsin, S.; Trukhachova, T.; Isakau, H.; Istomin, Y. Pharmacokinetics and biodistribution of Photolon® (Fotolon®) in intact and tumor-bearing rats. Photodiagnosis Photodyn. Ther. 2009, 6, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Gurung, P.; Lim, J.; Thapa Magar, T.B.; Kim, C.-W.; Lee, H.Y.; Kim, Y.-W. Anti-Obesity Effect of Chlorin e6-Mediated Photodynamic Therapy on Mice with High-Fat-Diet-Induced Obesity. Pharmaceuticals 2023, 16, 1053. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Oh, D.; Lee, J.H.; Park, J.H.; Kim, K.P.; Lee, S.S.; Park, D.H. Initial human experience of endoscopic ultrasound-guided photodynamic therapy with a novel photosensitizer and a flexible laser-light catheter. Endoscopy 2015, 47, 1035–1038. [Google Scholar] [CrossRef]
- Shrestha, R.; Lee, H.J.; Lim, J.; Gurung, P.; Thapa Magar, T.B.; Kim, Y.-T.; Lee, K.; Bae, S.; Kim, Y.-W. Effect of Photodynamic Therapy with Chlorin e6 on Canine Tumors. Life 2022, 12, 2102. [Google Scholar] [CrossRef] [PubMed]
- Murtaj, V.; Penati, S.; Belloli, S.; Foti, M.; Coliva, A.; Papagna, A.; Gotti, C.; Toninelli, E.; Chiaffarelli, R.; Mantero, S.; et al. Brain sex-dependent alterations after prolonged high fat diet exposure in mice. Commun. Biol. 2022, 5, 1276. [Google Scholar] [CrossRef]
- Ryu, A.-R.; Kim, Y.-W.; Lee, M.-Y. Chlorin e6-mediated photodynamic therapy modulates adipocyte differentiation and lipogenesis in 3T3-L1 cells. Photodiagnosis Photodyn. Ther. 2020, 31, 101917. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in combination with organic photosensitizers: Beyond the light penetration depth limit of photodynamic therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-M.; Lee, H.-S.; Jeong, D.; Oh, H.-K.; Ra, K.-H.; Lee, M.-Y. Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life Sci. 2015, 124, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Cmaj 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F. Nonalcoholic fatty liver disease: Another leap forward. Nat. Rev. Gastroenterol. 2021, 18, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory links between high fat diets and diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castro, C.; Garvey, W.T. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol. Metab. Clin. N. Am. 2008, 37, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Pontes-da-Silva, R.M.; de Souza Marinho, T.; de Macedo Cardoso, L.E.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Obese mice weight loss role on nonalcoholic fatty liver disease and endoplasmic reticulum stress treated by a GLP-1 receptor agonist. Int. J. Obes. 2022, 46, 21–29. [Google Scholar]
- Lee, H.; Ahn, J.; Shin, S.S.; Yoon, M. Ascorbic acid inhibits visceral obesity and nonalcoholic fatty liver disease by activating peroxisome proliferator-activated receptor α in high-fat-diet-fed C57BL/6J mice. Int. J. Obes. 2019, 43, 1620–1630. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Lee, S.; Chung, M.J.; Ahn, M.; Park, H.J.; Wang, E.K.; Guon, T.; Kee, H.J.; Ku, C.R.; Na, K. Surfactant-like photosensitizer for endoscopic duodenal ablation: Modulating meal-stimulated incretin hormones in obese and type 2 diabetes. Biomaterials 2023, 302, 122336. [Google Scholar] [CrossRef]



| Component | High Fat Diet (60% kcal) | Normal Diet (10% kcal) |
|---|---|---|
| g | g | |
| Casein 80-mesh | 200 | 200 |
| L-Cystine | 3 | 3 |
| Corn Starch | 0 | 315 |
| Maltodextrin 10 | 125 | 35 |
| Sucrose | 68.8 | 350 |
| Cellulose BW200 | 50 | 50 |
| Soyabean Oil | 25 | 25 |
| Lard | 245 | 20 |
| Mineral Mix S10026 | 10 | 10 |
| Dicalcium | 13 | 13 |
| Phosphate | ||
| Calcium Carbonate | 5.5 | 5.5 |
| Potassium Citrate | 16.5 | 16.5 |
| Vitamin Mix 10001 | 10 | 10 |
| Choline tartrate | 2 | 2 |
| FD and C dye | 0.05 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurung, P.; Lim, J.; Kim, Y.-W. Preventing High Fat Diet-Induced Obesity and Related Hepatic Steatosis by Chlorin e6-Mediated Photodynamic Therapy. Pharmaceuticals 2024, 17, 729. https://doi.org/10.3390/ph17060729
Gurung P, Lim J, Kim Y-W. Preventing High Fat Diet-Induced Obesity and Related Hepatic Steatosis by Chlorin e6-Mediated Photodynamic Therapy. Pharmaceuticals. 2024; 17(6):729. https://doi.org/10.3390/ph17060729
Chicago/Turabian StyleGurung, Pallavi, Junmo Lim, and Yong-Wan Kim. 2024. "Preventing High Fat Diet-Induced Obesity and Related Hepatic Steatosis by Chlorin e6-Mediated Photodynamic Therapy" Pharmaceuticals 17, no. 6: 729. https://doi.org/10.3390/ph17060729
APA StyleGurung, P., Lim, J., & Kim, Y.-W. (2024). Preventing High Fat Diet-Induced Obesity and Related Hepatic Steatosis by Chlorin e6-Mediated Photodynamic Therapy. Pharmaceuticals, 17(6), 729. https://doi.org/10.3390/ph17060729





