Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Analysis of the Russian Database of Spontaneous Reports
Abstract
1. Introduction
2. Results
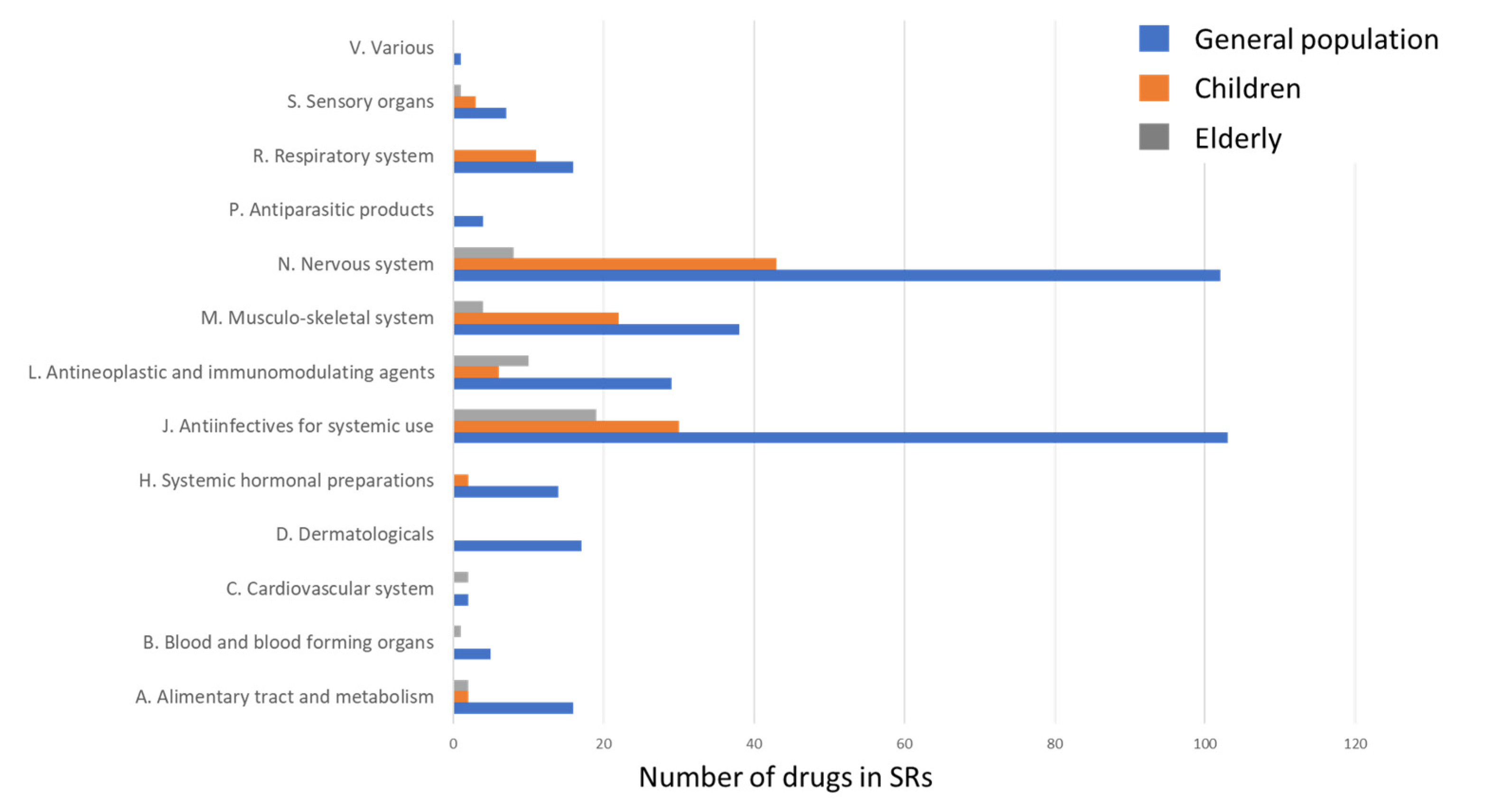
2.1. The Structure of Drugs Involved in SJS and TEN Development
2.1.1. ATC Level 1 Group J Drugs Involved in SJS and TEN
2.1.2. ATC Level 1 Group N Drugs Involved in SJS and TEN
2.2. SJS and TEN in Pediatric SRs
2.3. SJS and TEN in the Elderly SRs
2.4. Lethal Cases
3. Discussion
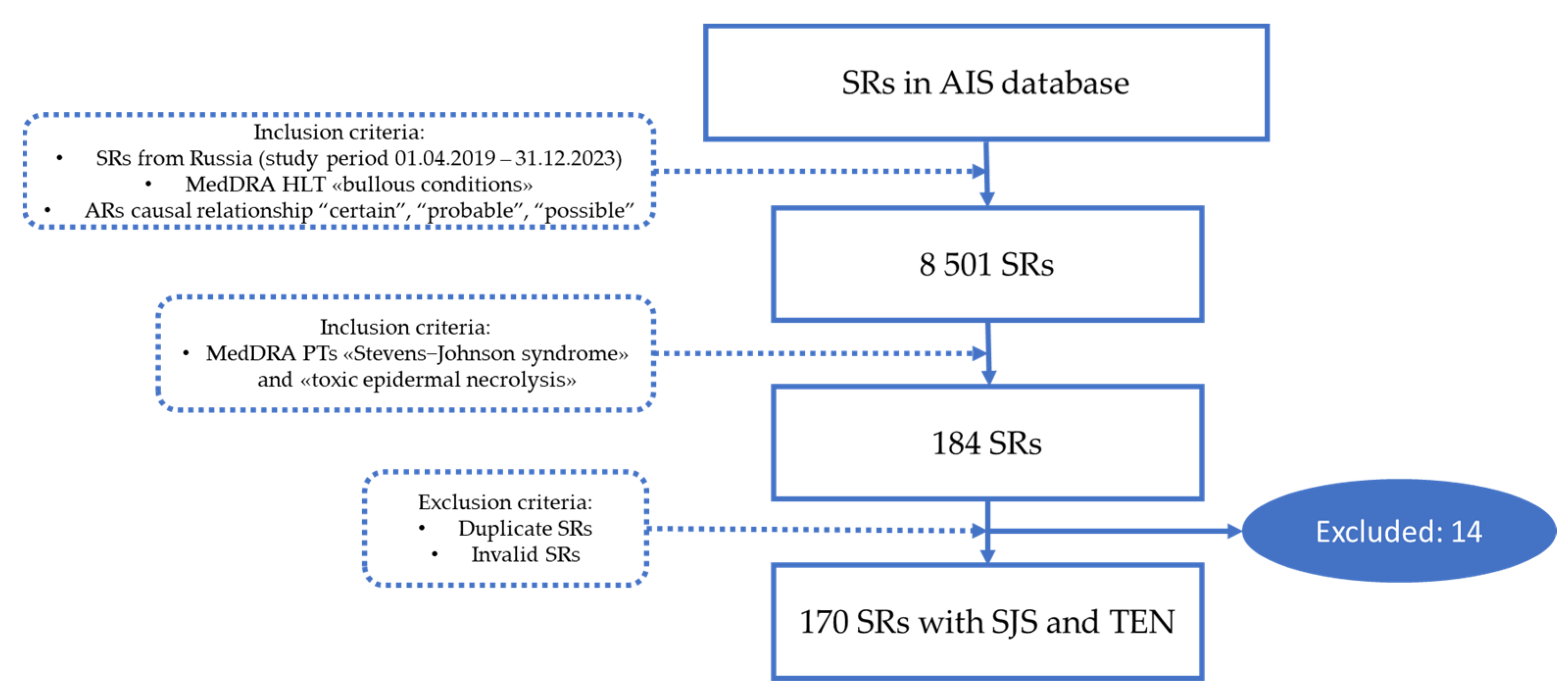
4. Materials and Methods
“Adverse reaction—A response to a medicinal product, which is noxious and unintended. Adverse reaction may arise from use of the product within or outside the terms of the marketing authorization or from occupational exposure. Use outside the marketing authorization includes off-label use, overdose, misuse, abuse, and medication errors.”
“Causality—In accordance with ICH-E2A, the definition of an adverse reaction implies at least a reasonable possibility of a causal relationship between a suspected medicinal product and an adverse event. An adverse reaction, in contrast to an adverse event, is characterized by the fact that a causal relationship between a medicinal product and an occurrence is suspected. For regulatory reporting purposes, as detailed in ICH-E2D, if an event is spontaneously reported, even if the relationship is unknown or unstated, it meets the definition of an adverse reaction. Therefore, all spontaneous reports notified by healthcare professionals or consumers are considered suspected adverse reactions, since they convey the suspicions of the primary sources, unless the reporters specifically state that they believe the events to be unrelated or that a causal relationship can be excluded.”
“A spontaneous report is an unsolicited communication by a healthcare professional, or consumer to a competent authority, marketing authorisation holder or other organization (e.g., regional pharmacovigilance center, poison control center) that describes one or more suspected adverse reactions in a patient who was given one or more medicinal products. It does not derive from a study or any organized data collection systems.”
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duong, T.A.; Valeyrie-Allanore, L.; Wolkenstein, P.; Chosidow, O. Severe cutaneous adverse reactions to drugs. Lancet 2017, 390, 1996–2011. [Google Scholar] [CrossRef]
- Thong, B.Y.-H. Drug-induced Stevens Johnson syndrome and toxic epidermal necrolysis: Interpreting the systematic reviews on immunomodulatory therapies. Asia Pac. Allergy 2023, 13, 72–76. [Google Scholar] [CrossRef]
- Dobry, A.S.; Himed, S.; Waters, M.; Kaffenberger, B.H. Scoring Assessments in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Front. Med. 2022, 9, 883121. [Google Scholar] [CrossRef]
- Marks, M.E.; Botta, R.K.; Abe, R.; Beachkofsky, T.M.; Boothman, I.; Carleton, B.C.; Chung, W.-H.; Cibotti, R.R.; Dodiuk-Gad, R.P.; Grimstein, C.; et al. Updates in SJS/TEN: Collaboration, innovation, and community. Front. Med. 2023, 10, 1213889. [Google Scholar] [CrossRef]
- Frantz, R.; Huang, S.; Are, A.; Motaparthi, K. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina 2021, 57, 895. [Google Scholar] [CrossRef]
- Pan, R.-Y.; Chu, M.-T.; Wang, C.-W.; Lee, Y.-S.; Lemonnier, F.; Michels, A.W.; Schutte, R.; Ostrov, D.A.; Chen, C.-B.; Phillips, E.J.; et al. Identification of drug-specific public TCR driving severe cutaneous adverse reactions. Nat. Commun. 2019, 10, 3569. [Google Scholar] [CrossRef]
- Chen, C.-B.; Abe, R.; Pan, R.-Y.; Wang, C.-W.; Hung, S.-I.; Tsai, Y.-G.; Chung, W.-H. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. J. Immunol. Res. 2018, 2018, 6431694. [Google Scholar] [CrossRef]
- Aptula, A.O.; Roberts, D.W.; Pease, C.K. Haptens, prohaptens and prehaptens, or electrophiles and proelectrophiles. Contact Dermat. 2007, 56, 54–56. [Google Scholar] [CrossRef]
- Kridin, K.; Brüggen, M.-C.; Chua, S.-L.; Bygum, A.; Walsh, S.; Nägeli, M.C.; Kucinskiene, V.; French, L.; Tétart, F.; Didona, B.; et al. Assessment of Treatment Approaches and Outcomes in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. JAMA Dermatol. 2021, 157, 1182–1190. [Google Scholar] [CrossRef]
- Wasuwanich, P.; So, J.M.; Chakrala, T.S.; Chen, J.; Motaparthi, K. Epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the United States and factors predictive of outcome. JAAD Int. 2023, 13, 17–25. [Google Scholar] [CrossRef]
- Wang, L.; Varghese, S.; Bassir, F.; Lo, Y.-C.; Ortega, C.A.; Shah, S.; Blumenthal, K.G.; Phillips, E.J.; Zhou, L. Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review of PubMed/MEDLINE case reports from 1980 to 2020. Front. Med. 2022, 9, 949520. [Google Scholar] [CrossRef]
- Stanley, E.A.; Zhang, L.; O’hara, J.; Haertsch, P.; Maitz, P. The seven-fold rise in incidence of Stevens-Johnson syndrome & toxic epidermal necrolysis: Associations with COVID-19 and the vaccine. Burns 2024, 50, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W.; Kim, H.-Y.; Shin, K.; Kim, S.H. Clinical characteristics of drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: A single-center study. Asia Pac. Allergy 2022, 12, e17. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shou, Y.-H.; Li, F.; Zhu, X.-H.; Yang, Y.-S.; Xu, J.-H. Retrospective study of 213 cases of Stevens–Johnson syndrome and toxic epidermal necrolysis from China. Burns 2020, 46, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Techasatian, L.; Panombualert, S.; Uppala, R.; Jetsrisuparb, C. Drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in children: 20 years study in a tertiary care hospital. World J. Pediatr. 2017, 13, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N.; Maman, D.; Stein, N.; Saliba, W. Culprit Medications and Risk Factors Associated with Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Population-Based Nested Case–Control Study. Am. J. Clin. Dermatol. 2022, 23, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Knox, C.; Phillips, E.J. Worldwide Prevalence of Antibiotic-Associated Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2023, 159, 384–392. [Google Scholar] [CrossRef] [PubMed]
- de Bustros, P.; Baldea, A.; Sanford, A.; Joyce, C.; Adams, W.; Bouchard, C. Review of culprit drugs associated with patients admitted to the burn unit with the diagnosis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Syndrome. Burns 2022, 48, 1561–1573. [Google Scholar] [CrossRef]
- Abulatan, I.T.; Ben-David, S.G.; Morales-Colon, L.A.; Beason, E.; Fakoya, A.O.; Morales-Colon, L.A.; Fakoya, A.O. A Compilation of Drug Etiologies of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Cureus 2023, 15, e48728. [Google Scholar] [CrossRef]
- Neagu, T.P.; Tiglis, M.; Peride, I.; Niculae, A. Toxic Epidermal Necrolysis, A Serious Side Effect of Tenoxicam Use: A Case Report. Healthcare 2023, 11, 2195. [Google Scholar] [CrossRef]
- Shao, Q.-H.; Yin, X.-D.; Zeng, N.; Zhou, Z.-X.; Mao, X.-Y.; Zhu, Y.; Zhao, B.; Li, Z.-L. Stevens-Johnson Syndrome Following Non-steroidal Anti-inflammatory Drugs: A Real-World Analysis of Post-marketing Surveillance Data. Front. Pediatr. 2022, 10, 896867. [Google Scholar] [CrossRef] [PubMed]
- Fabian, I.M.; Maddox, K.; Robicheaux, C.; Islam, R.K.; Anwar, A.; Dorius, B.; Robinson, C.L.; Kaye, A.M.; Varrassi, G.; Ahmadzadeh, S.; et al. Stevens-Johnson Syndrome from Combined Allopurinol and Angiotensin-Converting Enzyme Inhibitors: A Narrative Review. Cureus 2024, 16, e51899. [Google Scholar] [CrossRef]
- Anis, T.R.; Meher, J. Allopurinol-Induced Stevens–Johnson Syndrome (SJS). Clin. Pharmacol. Adv. Appl. 2023, 15, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Sabharwal, N.; Patti, R.; Kupfer, Y. Allopurinol-Induced Stevens-Johnson Syndrome. Am. J. Med. Sci. 2019, 357, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Sampath, A.; Zaman, S.U.; Pati, A.K.; Atal, S. Genetic predisposition for the development of lamotrigine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: A systematic review and meta-analysis. Pers. Med. 2023, 20, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Rajan, A.K.; Chhabra, M.; Kashyap, A.; Chandran, V.P.; Venkataraman, R.; Nair, S.; Thunga, G. Role of human leukocyte antigen in anti-epileptic drugs-induced Stevens–Johnson Syndrome/toxic epidermal necrolysis: A meta-analysis. Seizure 2022, 102, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Tangamornsuksan, W.; Chanprasert, S.; Nadee, P.; Rungruang, S.; Meesilsat, N.; Ueta, M.; Lohitnavy, M. HLA genotypes and cold medicine-induced Stevens–Johnson syndrome/toxic epidermal necrolysis with severe ocular complications: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 10589. [Google Scholar] [CrossRef] [PubMed]
- Fouchard, N.; Bertocchi, M.; Roujeau, J.-C.; Revuz, J.; Wolkenstein, P.; Bastuji-Garin, S. SCORTEN: A Severity-of-Illness Score for Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2000, 115, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Wickner, P.G.; Lau, J.J.; Zhou, L. Stevens-Johnson syndrome and toxic epidermal necrolysis: A cross-sectional analysis of patients in an integrated allergy repository of a large health care system. J. Allergy Clin. Immunol. Pract. 2015, 3, 277–280.e1. [Google Scholar] [CrossRef]
- Oshikoya, K.A.; Ogunyinka, I.A.; Ogar, C.K.; Abiola, A.; Ibrahim, A.; Oreagba, I.A. Severe cutaneous adverse drug reactions manifesting as Stevens-Johnson syndrome and toxic epidermal necrolysis reported to the national pharmacovigilance center in Nigeria: A database review from 2004 to 2017. Ther. Adv. Drug Saf. 2020, 11, 2042098620905998. [Google Scholar] [CrossRef]
- Yang, M.-S.; Lee, J.Y.; Kim, J.; Kim, G.-W.; Kim, B.-K.; Kim, J.-Y.; Park, H.-W.; Cho, S.-H.; Min, K.-U.; Kang, H.-R. Incidence of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Nationwide Population-Based Study Using National Health Insurance Database in Korea. PLoS ONE 2016, 11, e0165933. [Google Scholar] [CrossRef] [PubMed]
- Kourouma, S.; Sangaré, A.; Kaloga, M.; Kouassi, I.; Ecra, E.; Gbery, I.; Ahogo, C.; Kassi, K.; Camara, B. Stevens-Johnson syndrome and toxic epidermal necrolysis: Retrospective study of 185 cases in Abidjan (Côte d’Ivoire). Médecine Santé Trop. 2014, 24, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Hu, S.; Zhang, S.-Z.; Huang, J.-W.; Zhang, J.; Ji, C.; Cheng, B. The Epidemiology of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in China. J. Immunol. Res. 2018, 2018, 4320195. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.B.; Reis, C.S.; Novaes, A.G.; de Carvalho, M.R.; Göttems, L.B.D.; Novaes, M.R.C.G. Stevens-Johnson syndrome and toxic epidermal necrolysis: Epidemiological and clinical outcomes analysis in public hospitals. An. Bras. Dermatol. 2017, 92, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.; Yoldas, A.; Turk, B.; Karaarslan, I.; Sagduyu, I.; Ceylan, C.; Unal, I.; Ozturk, G. Stevens–Johnson syndrome and toxic epidermal necrolysis: 11-year demographic clinical and prognostic characteristics. Indian J. Dermatol. 2022, 67, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Alajaji, A.; Shekaran, J.C.; Aldhabbah, O.M.; Alhindi, H.A.; Almazyad, N.S.; Aljutayli, Z.A.; Abaalkhail, S.; Alfouzan, S. Toxic Epidermal Necrolysis (TEN)/Stevens-Johnson Syndrome (SJS) Epidemiology and Mortality Rate at King Fahad Specialist Hospital (KFSH) in Qassim Region of Saudi Arabia: A Retrospective Study. Dermatol. Res. Pract. 2020, 2020, 7524726. [Google Scholar] [CrossRef] [PubMed]
- Kanagarajan, A.; Murthy, A.B.; Moni, P.K.; Palanivel, N. Clinicoetiological study of Stevens-Johnson syndrome and toxic epidermal necrolysis spectrum and the correlation of SCORTEN with prognosis. Indian J. Dermatol. 2023, 68, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, N.; Nakatani, E.; Hashizume, H.; Sasaki, H.; Miyachi, Y. Risk factors and drugs that trigger the onset of Stevens–Johnson syndrome and toxic epidermal necrolysis: A population-based cohort study using the Shizuoka Kokuho database. JAAD Int. 2022, 11, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kaimal, S.; Lobo, C.; Narayan, G.; Augustine, M. Stevens-Johnson syndrome and toxic epidermal necrolysis: A fresh look at an old foe. Indian J. Dermatol. 2023, 68, 34–40. [Google Scholar] [CrossRef]
- Sunaga, Y.; Kurosawa, M.; Ochiai, H.; Watanabe, H.; Sueki, H.; Azukizawa, H.; Asada, H.; Watanabe, Y.; Yamaguchi, Y.; Aihara, M.; et al. The nationwide epidemiological survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan, 2016-2018. J. Dermatol. Sci. 2020, 100, 175–182. [Google Scholar] [CrossRef]
- Diphoorn, J.; Cazzaniga, S.; Gamba, C.; Schroeder, J.; Citterio, A.; Rivolta, A.L.; Vighi, G.D.; Naldi, L.; The REACT-Lombardia Study Group. Incidence, causative factors and mortality rates of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in northern Italy: Data from the REACT registry. Pharmacoepidemiol. Drug Saf. 2016, 25, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Go, H.; Saigusa, Y.; Takamura, N.; Watanabe, Y.; Yamane, Y.; Totsuka, M.; Ishikawa, H.; Nakamura, K.; Matsukura, S.; et al. Mortality and risk factors on admission in toxic epidermal necrolysis: A cohort study of 59 patients. Allergol. Int. 2021, 70, 229–234. [Google Scholar] [CrossRef]
- Fei, W.; Shen, J.; Cai, H. Causes of Drug-Induced Severe Cutaneous Adverse Reaction Epidermal Necrolysis (EN): An Analysis Using FDA Adverse Event Reporting System (FAERS) Database. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2249–2257. [Google Scholar] [CrossRef]
- Noe, M.H.; Rosenbach, M.; Hubbard, R.A.; Mostaghimi, A.; Cardones, A.R.; Chen, J.K.; Cotliar, J.; Davis, M.D.P.; Dominguez, A.; Fox, L.P.; et al. Development and Validation of a Risk Prediction Model for In-Hospital Mortality Among Patients with Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis—ABCD-10. JAMA Dermatol. 2019, 155, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Falotico, J.M.; Desai, A.D.; Lipner, S.R. Pediatric Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A national analysis of 2016 Kids’ Inpatient Database. Arch. Dermatol. Res. 2023, 315, 653–656. [Google Scholar] [CrossRef]
- Lipovy, B.; Holoubek, J.; Hanslianova, M.; Cvanova, M.; Klein, L.; Grossova, I.; Zajicek, R.; Bukovcan, P.; Koller, J.; Baran, M.; et al. Impact of Antibiotics Associated with the Development of Toxic Epidermal Necrolysis on Early and Late-Onset Infectious Complications. Microorganisms 2021, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Chiang, V.; Kan, A.K.C.; Saha, C.; Au, E.Y.L.; Li, P.H. Identifying the most at-risk age-group and longitudinal trends of drug allergy labeling amongst 7.3 million individuals in Hong Kong. BMC Med. 2024, 22, 30. [Google Scholar] [CrossRef]
- Fukasawa, T.; Urushihara, H.; Takahashi, H.; Okura, T.; Kawakami, K. Risk of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Antibiotic Use: A Case-Crossover Study. J. Allergy Clin. Immunol. Pract. 2023, 11, 3463–3472. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, R.; Kuzmenkov, A. The Dynamics of Antimicrobial Resistance among Enterobacteriaceae Isolates in Russia: Results of the 2012–2018 INFORM and ATLAS International Program Studies. Antibiotics 2022, 11, 790. [Google Scholar] [CrossRef]
- Ivanova, O.; Blumenkrants, D.; Krylova, E.; Soltynskaya, I.; Goncharova, A.; Chaikin, E.; Akhmetzyanova, A.; Panin, A. Founding of the culture collection of antibiotic-resistant strains of zoonotic bacteria in the Russian Federation. Vet. World 2023, 16, 1451–1460. [Google Scholar] [CrossRef]
- Borrelli, E.P.; Lee, E.Y.; Descoteaux, A.M.; Kogut, S.J.; Caffrey, A.R. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia 2018, 59, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-S.; Lee, J.Y.; Kim, J.; Kim, G.-W.; Kim, B.-K.; Kim, J.Y.; Park, H.-W.; Cho, S.-H.; Min, K.-U.; Kang, H.-R. Searching for the Culprit Drugs for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis from a Nationwide Claim Database in Korea. J. Allergy Clin. Immunol. Pract. 2020, 8, 690–695.e2. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, T.; Takahashi, H.; Takahashi, K.; Tanemura, N.; Amagai, M.; Urushihara, H. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with anticonvulsants in a Japanese population: Matched case–control and cohort studies. Allergol. Int. 2021, 70, 335–342. [Google Scholar] [CrossRef]
- Abtahi-Naeini, B.; Dehghan, M.-S.; Paknazar, F.; Shahmoradi, Z.; Faghihi, G.; Sabzghabaee, A.M.; Akbari, M.; Hadian, M.; Momen, T. Clinical and Epidemiological Features of Patients with Drug-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Iran: Different Points of Children from Adults. Int. J. Pediatr. 2022, 2022, 8163588. [Google Scholar] [CrossRef] [PubMed]
- Gleghorn, K.L.; Voigt, C.; Kelly, B. Toxic epidermal necrolysis and Stevens-Johnson syndrome/toxic epidermal necrolysis overlap in pediatric patients with a focus on newer antiepileptic drugs: A 25-year retrospective study at a single tertiary care center. Pediatr. Dermatol. 2021, 38, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Chatproedprai, S.; Wutticharoenwong, V.; Tempark, T.; Wananukul, S. Clinical Features and Treatment Outcomes among Children with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A 20-Year Study in a Tertiary Referral Hospital. Dermatol. Res. Pract. 2018, 2018, 3061084. [Google Scholar] [CrossRef]
- Sibbald, C.; Putterman, E.; Micheletti, R.; Treat, J.; Castelo-Soccio, L. Retrospective review of drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis cases at a pediatric tertiary care institution. Pediatr. Dermatol. 2020, 37, 461–466. [Google Scholar] [CrossRef]
- Chen, C.-B.; Wu, M.-Y.; Ng, C.Y.; Lu, C.-W.; Wu, J.; Kao, P.-H.; Yang, C.-K.; Peng, M.-T.; Huang, C.-Y.; Chang, W.-C.; et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag. Res. 2018, 10, 1259–1273. [Google Scholar] [CrossRef]
- Tanaka, R.; Yonemori, K.; Hirakawa, A.; Kinoshita, F.; Kobayashi, Y.; Yamazaki, N.; Fujimoto, M.; Tamura, K.; Fujiwara, Y. Anticancer Agent-Induced Life-Threatening Skin Toxicities: A Database Study of Spontaneous Reporting Data. Oncologist 2019, 24, 266–272. [Google Scholar] [CrossRef]
- Frey, N.; Bodmer, M.; Bircher, A.; Jick, S.S.; Meier, C.R.; Spoendlin, J. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis in Association with Commonly Prescribed Drugs in Outpatient Care Other than Anti-Epileptic Drugs and Antibiotics: A Population-Based Case–Control Study. Drug Saf. 2019, 42, 55–66. [Google Scholar] [CrossRef]
- Cheng, L. Current Pharmacogenetic Perspective on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Front. Pharmacol. 2021, 12, 588063. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Abe, R.; Anderson, P.; Anderson, W.; Ardern-Jones, M.R.; Beachkofsky, T.M.; Bellon, T.; Biala, A.K.; Bouchard, C.; Cavalleri, G.L.; et al. SJS/TEN 2019: From science to translation. J. Dermatol. Sci. 2020, 98, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Rai, S.K.; Terkeltaub, R.; Kim, S.C.; Menendez, M.E.; Choi, H.K. Racial disparities in the risk of Stevens–Johnson Syndrome and toxic epidermal necrolysis as urate-lowering drug adverse events in the United States. Semin. Arthritis Rheum. 2016, 46, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Dastgheib, L.; Rostami, F.; Gharesi-Fard, B.; Asadi-Pooya, A.A.; Namjoo, S.; Tahmasebi, F.; Hadibarhaghtalab, M. Association of Human Leukocyte Antigen Alleles with Carbamazepine- or Lamotrigine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in an Iranian Population: A Case-control Study. Iran. J. Med. Sci. 2023, 48, 70–76. [Google Scholar] [CrossRef]
- Phillips, E.J. Defining Regional Differences in Drug-Induced Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis: A Tool to Improve Drug Safety? Clin. Pharmacol. Ther. 2019, 105, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.G.; Lehloenya, R.; Dlamini, S.; Risma, K.; White, K.D.; Konvinse, K.C.; Phillips, E.J. Severe Delayed Cutaneous and Systemic Reactions to Drugs: A Global Perspective on the Science and Art of Current Practice. J. Allergy Clin. Immunol. Pract. 2017, 5, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M. Findings by an International Collaboration on SJS/TEN With Severe Ocular Complications. Front. Med. 2021, 8, 649661. [Google Scholar] [CrossRef] [PubMed]
- Stancil, S.L.; Sandritter, T.; Strawn, J.R. Pharmacogenetics and Oxcarbazepine in Children and Adolescents: Beyond HLA-B*15:02. J. Child. Adolesc. Psychopharmacol. 2024, 34, 61–66. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH E2B (R3) Electronic Transmission of Individual Case Safety Reports (ICSRs)—Data Elements and Message Specification—Implementation Guide—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-e2b-r3-electronic-transmission-individual-case-safety-reports-icsrs-data-elements-message (accessed on 1 February 2024).
- MedDRA. Available online: https://www.meddra.org/how-to-use/support-documentation/english (accessed on 1 February 2024).
- Butranova, O.; Zyryanov, S.; Gorbacheva, A.; Asetskaya, I.; Polivanov, V. Drug-Induced Anaphylaxis: National Database Analysis. Pharmaceuticals 2024, 17, 90. [Google Scholar] [CrossRef]
- Rozhdestvensky, D.A. New Edition of the EAEU Good Pharmacovigilance Practice: What Has Changed? Saf. Risk Pharmacother. 2023, 11, 7–13. (In Russian) [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP) Module VI—Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev 2). Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (accessed on 1 February 2024).


| Criterion | N (Total—170) | % |
|---|---|---|
| AEs resulted in death | 12 | 7.1 |
| Life-threatening AEs + AEs requiring or prolonging hospitalization (both criteria chosen) | 36 | 21.2 |
| Life-threatening AEs | 28 | 16.5 |
| AEs causing persistent or significant disability or incapacity + AEs requiring or prolonging hospitalization (both criteria chosen) | 1 | 0.6 |
| AEs requiring or prolonging hospitalization | 69 | 40.5 |
| Other conditions judged to represent significant hazards | 24 | 14.1 |
| Age Group | N (Total—170) | % | SJS N (Total—56) (%) | TEN N (Total—114) (%) |
|---|---|---|---|---|
| 0–1 (infants) | 4 | 2.4 | 1 (1.8) | 3 (2.6) |
| >1–3 (toddlers) | 8 | 4.7 | 5 (8.9) | 3 (2.6) |
| 4–12 (childhood) | 23 | 13.5 | 7 (12.5) | 16 (14.0) |
| 13–18 years (adolescence) | 16 | 9.4 | 5 (8.9) | 11 (9.6) |
| 19–59 years (adults) | 77 | 45.3 | 23 (41.1) | 54 (47.4) |
| 60–74 years (youngest-old) | 29 | 17.1 | 8 (14.3) | 21 (18.4) |
| 75–89 years (middle-old) | 5 | 2.9 | 1 (1.8) | 4 (3.5) |
| ≥85 years (oldest-old) | 2 | 1.2 | 1 (1.8) | 1 (0.9) |
| No data | 6 | 3.5 | 5 (8.9) | 1 (0.9) |
| ATC 1 Level Group | N (Total—354) | % |
|---|---|---|
| A. Alimentary tract and metabolism | 16 | 4.5 |
| B. Blood and blood-forming organs | 5 | 1.4 |
| C. Cardiovascular system | 2 | 0.6 |
| D. Dermatologicals | 17 | 4.8 |
| H. Systemic hormonal preparations | 14 | 3.9 |
| J. Anti-infectives for systemic use | 103 | 29.1 |
| L. Antineoplastic and immunomodulating agents | 29 | 8.2 |
| M. Musculo-skeletal system | 38 | 10.7 |
| N. Nervous system | 102 | 28.8 |
| P. Antiparasitic products | 4 | 1.1 |
| R. Respiratory system | 16 | 4.5 |
| S. Sensory organs | 7 | 2.0 |
| V. Various | 1 | 0.3 |
| Drugs | N (Total—75) | % |
|---|---|---|
| J01A Tetracyclines (Tigecycline) | 2 | 2.7 |
| J01B Amphenicols (Chloramphenicol) | 3 | 4.0 |
| J01C Beta-lactams, penicillins | 16 | 21.3 |
| Amoxicillin | 8 | 10.7 |
| Ampicillin + sulbactam | 1 | 1.3 |
| Amoxicillin + sulbactam | 1 | 2.7 |
| Amoxicillin + clavulanic acid | 6 | 8 |
| J01D Other beta-lactam antibiotics | 30 | 40.0 |
| J01DB First-generation cephalosporins (Cefazolin) | 1 | 1.3 |
| J01DC Second-generation cephalosporins (Cefuroxime) | 1 | 1.3 |
| J01DD Third-generation cephalosporins | 24 | 32.0 |
| Cefotaxime | 2 | 2.7 |
| Ceftriaxone | 15 | 20.0 |
| Cefixime | 4 | 5.3 |
| Cefoperazone + sulbactam | 3 | 4.0 |
| J01DH Carbapenems | 4 | 5.3 |
| Meropenem | 3 | 4.0 |
| Imipenem + cilastatin | 1 | 1.3 |
| J01E Sulfonamides and trimethoprim | 2 | 2.7 |
| J01EB Short-acting sulfonamides (Sulfanilamide) | 1 | 1.3 |
| J01EE Combinations of sulfonamides and trimethoprim, including derivatives (Trimethoprim/sulfamethoxazole) | 1 | 1.3 |
| J01FA Macrolides | 11 | 14.7 |
| Erythromycin | 2 | 2.7 |
| Clarithromycin | 2 | 2.7 |
| Azithromycin | 7 | 9.3 |
| J01GB Other aminoglycosides (Kanamycin) | 1 | 1.3 |
| J01MA Fluoroquinolones | 6 | 8.0 |
| Ciprofloxacin | 2 | 2.7 |
| Levofloxacin | 3 | 4.0 |
| Moxifloxacin | 1 | 1.3 |
| J01X Other antibacterials | 4 | 5.3 |
| J01XA Glycopeptide antibacterials (Vancomycin) | 3 | 4.0 |
| J01XB Polymyxins (Polymyxin B) | 1 | 1.3 |
| Drugs | N (Total—21) | % |
|---|---|---|
| J05AB Nucleosides and nucleotides excluding reverse transcriptase inhibitors | 5 | 23.8 |
| Acyclovir | 4 | 19 |
| Valgancyclovir | 1 | 4.8 |
| J05AC Cyclic amines (Rimantadine) | 1 | 4.8 |
| J05AE Protease inhibitors | 2 | 9.5 |
| Ritonavir | 1 | 4.8 |
| Darunavir | 1 | 4.8 |
| J05AF Nucleoside and nucleotide reverse transcriptase inhibitors | 6 | 28.6 |
| Lamivudine | 3 | 14.3 |
| Tenofovir disoproxil | 3 | 14.3 |
| J05AG Non-nucleoside reverse transcriptase inhibitors | 2 | 9.5 |
| Nevirapine | 1 | 4.8 |
| Efavirenz | 1 | 4.8 |
| J05AX Other antivirals | 5 | 23.8 |
| Riamilovir | 1 | 4.8 |
| Umifenovir | 1 | 4.8 |
| Favipiravir | 3 | 14.3 |
| Drugs | N (Total—102) | % |
|---|---|---|
| N01B Anesthetics, local | 2 | 2.0 |
| N01BA Esters of aminobenzoic acid (Procaine) | 1 | 1.0 |
| N01BB Amides (Lidocaine) | 1 | 1.0 |
| N02B Other analgesics and antipyretics | 19 | 18.6 |
| N02BA Salicylic acid and derivatives (Acetylsalicylic acid) | 2 | 2.0 |
| N02BE Anilides | 17 | 16.7 |
| N02BE01 Paracetamol | 13 | 12.7 |
| N02BE51 Paracetamol, combinations excluding psycholeptics | 4 | 3.9 |
| N03 Antiepileptics | 65 | 63.7 |
| N03AA Barbiturates and derivatives (Phenobarbital) | 2 | 2.0 |
| N03AF Carboxamide derivatives (Carbamazepine) | 10 | 9.8 |
| N03AG Fatty acid derivatives (Valproic acid) | 7 | 6.9 |
| N03AX Other antiepileptics | 46 | 45.1 |
| Lamotrigine | 40 | 39.2 |
| Levetiracetam | 6 | 5.9 |
| N04AA Tertiary amines (Trihexyphenidyl) | 1 | 1.0 |
| N04BB Adamantane derivatives (Amantadine) | 1 | 1.0 |
| N05 Psycholeptics | 8 | 7.8 |
| N05A Antipsychotics | 6 | 5.9 |
| N05AD Butyrophenone derivatives | 2 | 2.0 |
| Haloperidol | 1 | 1.0 |
| Droperidol | 1 | 1.0 |
| N05AH Diazepines, oxazepines, thiazepines, and oxepines (Quetiapine) | 2 | 2.0 |
| N05AL Benzamides (Tiapride) | 1 | 1.0 |
| N05AX Other antipsychotics (Paliperidone) | 1 | 1.0 |
| N05B Anxiolytics | 2 | 2.0 |
| N05BA Benzodiazepine derivatives (Alprazolam) | 1 | 1.0 |
| N05BX Other anxiolytics (Phenazepam) | 1 | 1.0 |
| N06 Psychoanaleptics | 4 | 3.9 |
| N06A Antidepressants | 3 | 2.9 |
| N06AA Non-selective monoamine reuptake inhibitors (Amitriptyline) | 1 | 1.0 |
| N06AB Selective serotonin reuptake inhibitors (Sertraline) | 2 | 2.0 |
| N06BX Other psychostimulants and nootropics (bovine cerebral cortex polypeptides) | 1 | 1.0 |
| N07XX Other nervous system drugs (Ethylmethylhydroxypyridine succinate) | 2 | 2.0 |
| Drugs | N (Total—119) | % |
|---|---|---|
| A. Alimentary tract and metabolism | 2 | 1.7 |
| A02 Drugs for acid-related disorders (Omeprazole) | 1 | 0.8 |
| A12 Mineral supplements (magnesium (different salts in combination)) | 1 | 0.8 |
| H Systemic hormonal preparations, excluding sex hormones and insulins | 2 | 1.7 |
| H02 Corticosteroids for systemic use (Prednisolone) | 1 | 0.8 |
| H03 Thyroid therapy (Potassium iodide) | 1 | 0.8 |
| J. Anti-infectives for systemic use | 30 | 25.2 |
| J01 Antibacterials for systemic use | 25 | 21 |
| J01B Amphenicols (Chloramphenicol) | 3 | 2.5 |
| J01C Beta-lactam antibiotics, penicillins | 9 | 7.6 |
| Amoxicillin | 4 | 3.4 |
| Amoxicillin + clavulanic acid | 5 | 4.2 |
| J01D Other beta-lactam antibiotics | 10 | 8.4 |
| J01DD Third-generation cephalosporins | 10 | 8.4 |
| Cefotaxime | 1 | 0.8 |
| Ceftriaxone | 5 | 4.2 |
| Cefixime | 3 | 2.5 |
| Cefoperazone + sulbactam | 1 | 0.8 |
| J01FA Macrolides (Azithromycin) | 2 | 1.7 |
| J01MA Fluoroquinolones (Levofloxacin) | 1 | 0.8 |
| J05 Antivirals for systemic use | 3 | 2.5 |
| J05AB Nucleosides and nucleotides excluding reverse transcriptase inhibitors (Acyclovir) | 2 | 1.7 |
| J05AX Other antivirals (Umifenovir) | 1 | 0.8 |
| J07 Vaccines | 2 | 1.7 |
| L. Antineoplastic and immunomodulating agents | 6 | 5.0 |
| L01A Alkylating agents (Temozolomide) | 1 | 0.8 |
| L03 Immunostimulants (Interferon alfa-2b) | 3 | 2.5 |
| L04 Immunosuppressants (Belimumab) | 2 | 1.7 |
| M. Musculo-skeletal system | 24 | 20.2 |
| M01 Anti-inflammatory and antirheumatic products (Ibuprofen) | 22 | 18.5 |
| M02 Topical products for joint and muscular pain (Benzydamine) | 2 | 1.7 |
| N. Nervous system | 43 | 36.1 |
| N01B Anesthetics, local (Procaine) | 1 | 0.8 |
| N02 Analgesics (Paracetamol) | 9 | 7.6 |
| N03 Antiepileptics | 32 | 26.9 |
| N03AF Carboxamide derivatives (Carbamazepine) | 4 | 3.4 |
| N03AG Fatty acid derivatives (Valproic acid) | 5 | 4.2 |
| N03AX Other antiepileptics | 23 | 19.3 |
| Lamotrigine | 19 | 16.0 |
| Levetiracetam | 4 | 3.4 |
| N06 Psychoanaleptics | 1 | 0.8 |
| N06BX Other psychostimulants and nootropics (bovine cerebral cortex polypeptides) | 1 | 0.8 |
| R Respiratory system | 9 | 7.6 |
| R05 Cough and cold preparations | ||
| Butamirate | 1 | 0.8 |
| R06 Antihistamines for systemic use | 8 | 6.7 |
| R06AB Substituted alkylamines (Dimetindene) | 1 | 0.8 |
| R06AD Phenothiazine derivatives (Alimemazine) | 1 | 0.8 |
| R06AE Piperazine derivatives (Cetirizine) | 5 | 4.2 |
| R06AX Other antihistamines for systemic use (Loratadine) | 1 | 0.8 |
| S Sensory organs | 3 | 2.5 |
| S02 Otologicals (Lidocaine + phenazone) | 1 | 0.8 |
| Drugs | N (Total—48) | % |
|---|---|---|
| A. Alimentary tract and metabolism | 2 | 4.2 |
| A04A Antiemetics and Antinauseants (Granisetron) | 1 | 2.1 |
| A11G Ascorbic acid (Vitamin C), including combinations | 1 | 2.1 |
| B Blood and blood-forming organs | 1 | 2.1 |
| B05BA Solutions for parenteral nutrition (Dextrose) | 1 | 2.1 |
| C. Cardiovascular system | 2 | 4.2 |
| C03C High-ceiling diuretics (Furosemide) | 1 | 2.1 |
| C09 Agents acting on the renin–angiotensin system (Perindopril) | 1 | 2.1 |
| J. Anti-infectives for systemic use | 19 | 39.6 |
| J01 Antibacterials for systemic use | 17 | 35.4 |
| J01A Tetracyclines (Tigecycline) | 1 | 2.1 |
| J01C Beta-lactam antibiotics, penicillins (Ampicillin + sulbactam) | 1 | 2.1 |
| J01D Other beta-lactam antibiotics | 8 | 16.7 |
| J01DB First-generation cephalosporins (Cefazolin) | 1 | 2.1 |
| J01DD Third-generation cephalosporins (Ceftriaxone) | 4 | 8.3 |
| J01DH Carbapenems (Meropenem) | 3 | 6.3 |
| J01FA Macrolides (Azithromycin) | 2 | 4.2 |
| J01MA Fluoroquinolones | 3 | 6.3 |
| Ciprofloxacin | 1 | 2.1 |
| Levofloxacin | 1 | 2.1 |
| Moxifloxacin | 1 | 2.1 |
| J01XA Glycopeptide antibacterials (Vancomycin) | 2 | 4.2 |
| J02 Antimycotics for systemic use (Voriconazole) | 1 | 2.1 |
| J07B Viral vaccines | 1 | 2.1 |
| L. Antineoplastic and immunomodulating agents | 10 | 20.8 |
| L01A Alkylating agents (Ifosfamide) | 1 | 2.1 |
| L01B Antimetabolites | 2 | 4.2 |
| Gemcitabine | 1 | 2.1 |
| Capecitabine | 1 | 2.1 |
| L01D Cytotoxic antibiotics and related substances (Doxorubicin) | 1 | 2.1 |
| L01E Protein kinase inhibitors | 3 | 6.3 |
| Ibrutinib | 2 | 4.2 |
| Afatinib | 1 | 2.1 |
| L01F Monoclonal antibodies and antibody–drug conjugates (Pembrolizumab) | 1 | 2.1 |
| L02B Hormone antagonists and related agents | 2 | 4.2 |
| Enzalutamide | 1 | 2.1 |
| Apalutamide | 1 | 2.1 |
| M. Musculo-skeletal system | 5 | 10.4 |
| M01 Anti-inflammatory and antirheumatic products | 3 | 6.3 |
| M01A—Anti-inflammatory and antirheumatic products, non-steroids | 3 | 6.3 |
| Tenoxicam | 1 | 2.1 |
| Meloxicam | 1 | 2.1 |
| Ibuprofen | 1 | 2.1 |
| M02 Topical products for joint and muscular pain | 1 | 2.1 |
| M02AA Anti-inflammatory preparations, non-steroids for topical use (Ketoprofen) | 2 | 4.2 |
| N. Nervous system | 8 | 16.7 |
| N02 Analgesics | 2 | 4.2 |
| N02BA Salicylic acid and derivatives (Acetylsalicylic acid) | 1 | 2.1 |
| N02BE Anilides (Paracetamol) | 1 | 2.1 |
| N03 Antiepileptics | 4 | 8.3 |
| N03AF Carboxamide derivatives (Carbamazepine) | 1 | 2.1 |
| N03AX Other antiepileptics (Lamotrigine) | 3 | 6.3 |
| N05 Psycholeptics | 2 | 4.2 |
| N05AD Butyrophenone derivatives (Droperidol) | 1 | 2.1 |
| N05BX Other anxiolytics (Phenazepam) | 1 | 2.1 |
| S Sensory organs | 1 | 2.1 |
| S01 Ophthalmologicals (Levofloxacin) | 1 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyryanov, S.; Asetskaya, I.; Butranova, O.; Terekhina, E.; Polivanov, V.; Yudin, A.; Samsonova, K. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Analysis of the Russian Database of Spontaneous Reports. Pharmaceuticals 2024, 17, 675. https://doi.org/10.3390/ph17060675
Zyryanov S, Asetskaya I, Butranova O, Terekhina E, Polivanov V, Yudin A, Samsonova K. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Analysis of the Russian Database of Spontaneous Reports. Pharmaceuticals. 2024; 17(6):675. https://doi.org/10.3390/ph17060675
Chicago/Turabian StyleZyryanov, Sergey, Irina Asetskaya, Olga Butranova, Elizaveta Terekhina, Vitaly Polivanov, Alexander Yudin, and Kristina Samsonova. 2024. "Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Analysis of the Russian Database of Spontaneous Reports" Pharmaceuticals 17, no. 6: 675. https://doi.org/10.3390/ph17060675
APA StyleZyryanov, S., Asetskaya, I., Butranova, O., Terekhina, E., Polivanov, V., Yudin, A., & Samsonova, K. (2024). Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Analysis of the Russian Database of Spontaneous Reports. Pharmaceuticals, 17(6), 675. https://doi.org/10.3390/ph17060675





