Synthesis and Antiviral Activity of Novel β-D-N4-Hydroxycytidine Ester Prodrugs as Potential Compounds for the Treatment of SARS-CoV-2 and Other Human Coronaviruses
Abstract
:1. Introduction
2. Results and Discussion
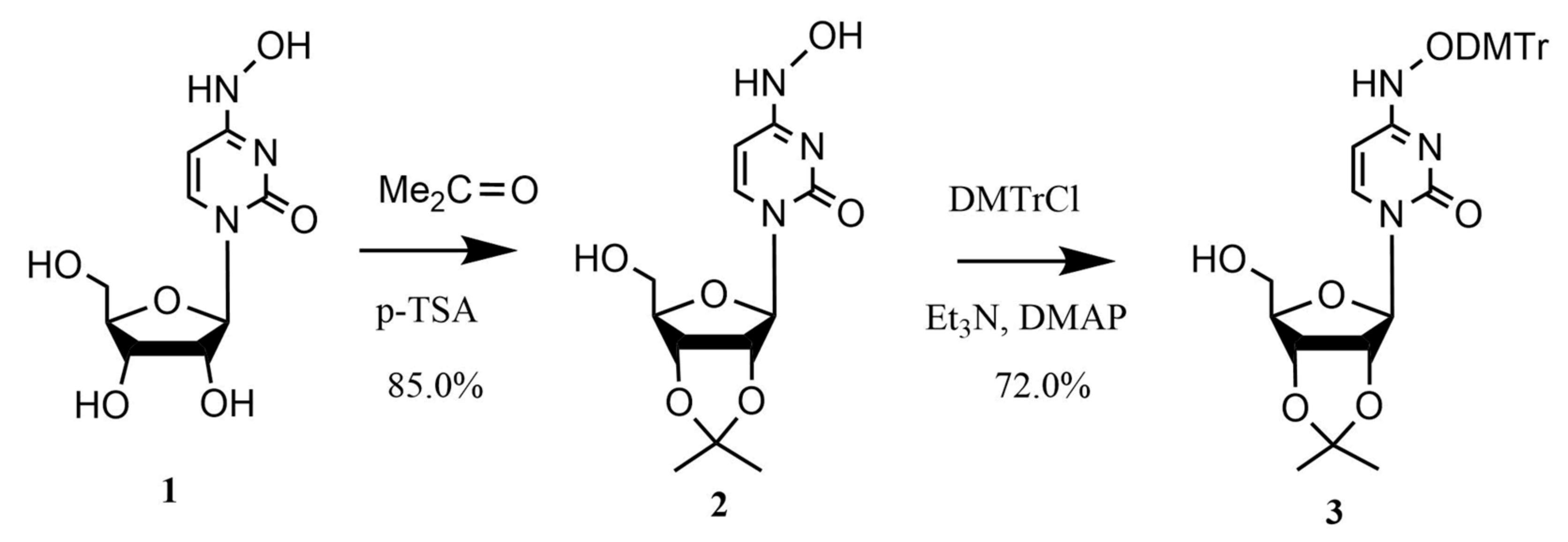
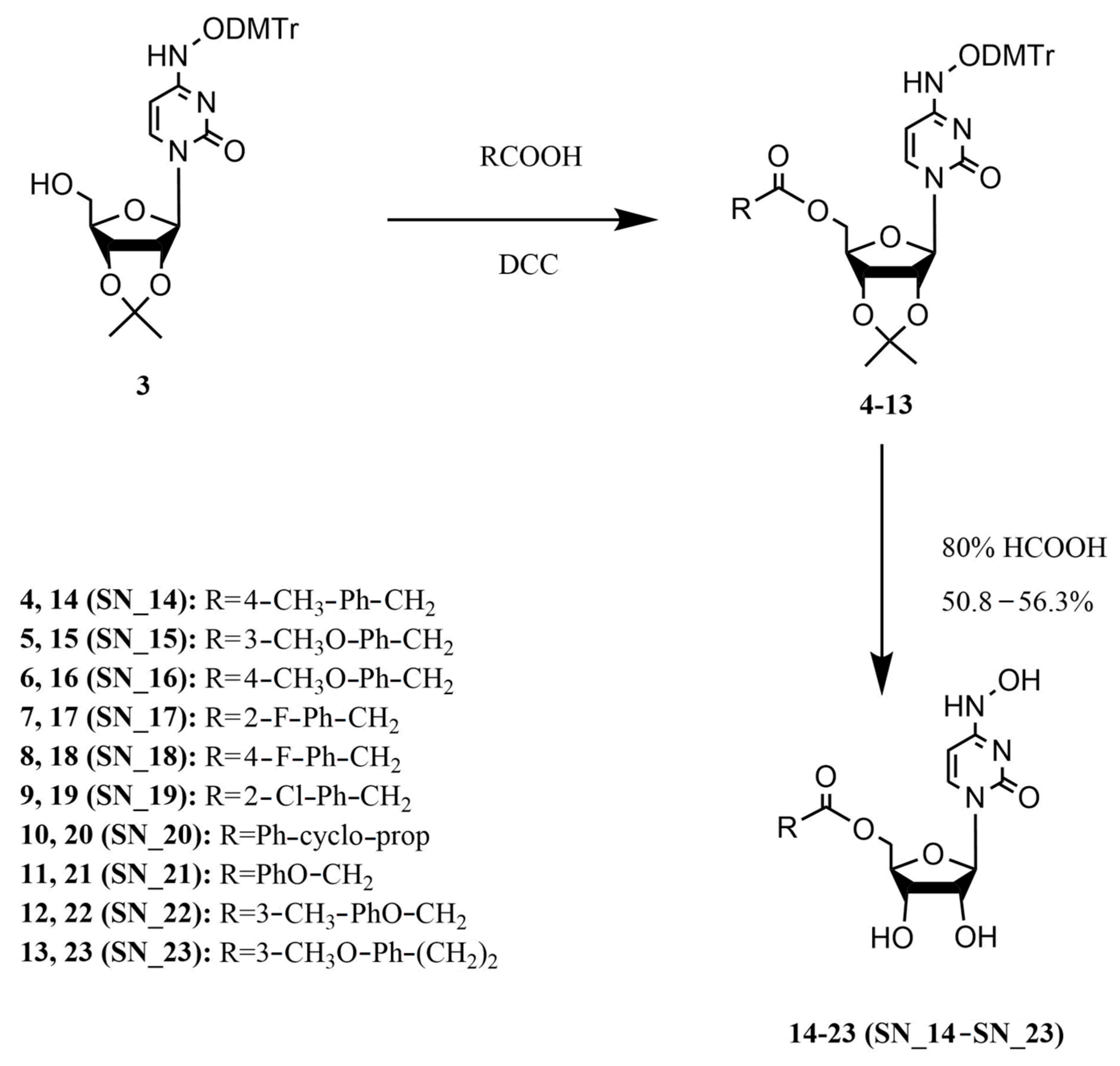
2.1. Chemistry
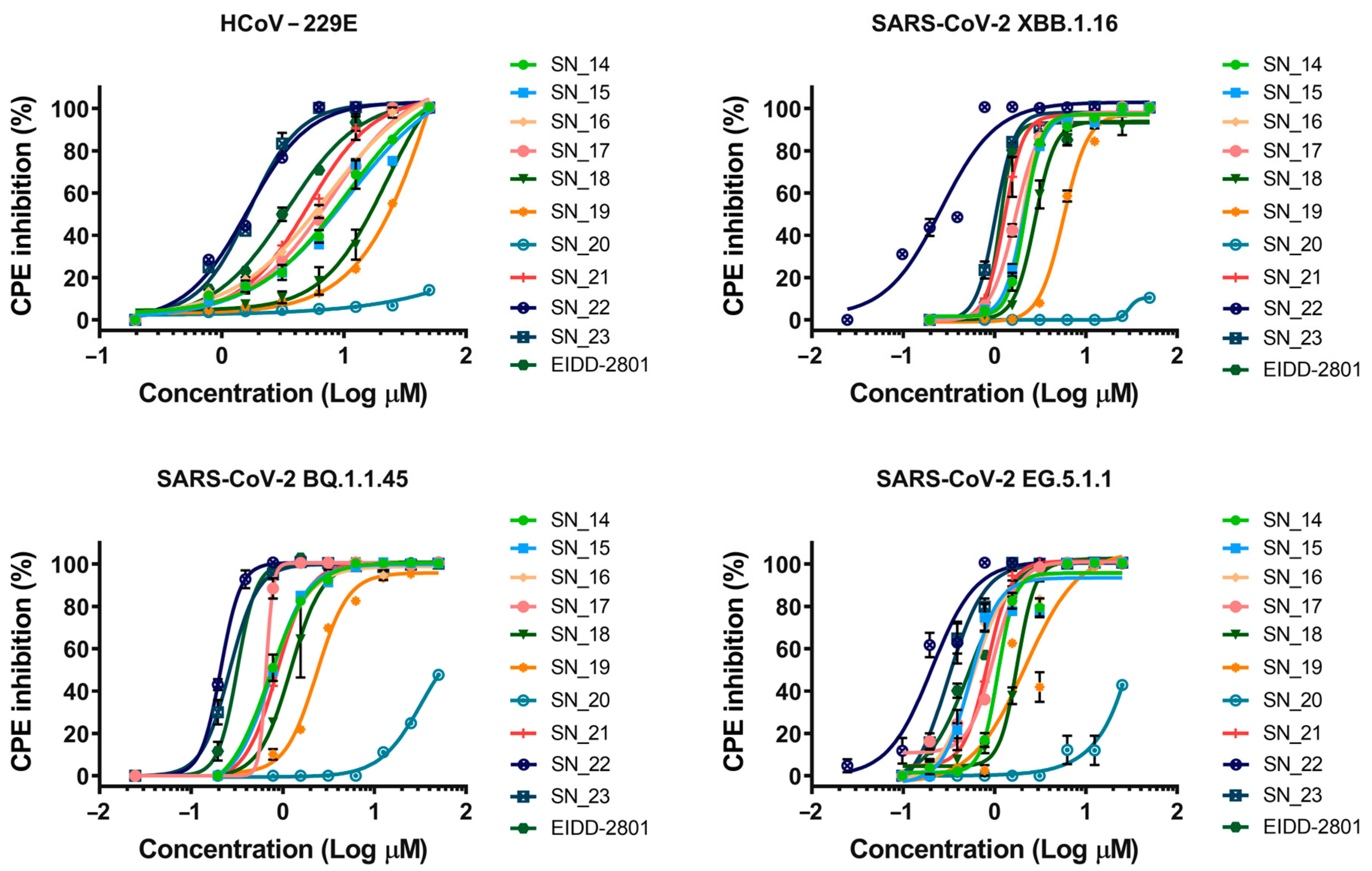
2.2. Antiviral Activity
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis of 5′-O-(4-Methylphenyl)acetyl-N4-hydroxycytidine (14, SN_14)
4.1.2. Synthesis of 5′-O-(3-Methoxyphenyl)acetyl-N4-hydroxycytidine (15, SN_15)
4.1.3. Synthesis of 5′-O-(4-Methoxyphenyl)acetyl-N4-hydroxycytidine (16, SN_16)
4.1.4. Synthesis of 5′-O-(2-Fluorophenyl)acetyl-N4-hydroxycytidine (17, SN_17)
4.1.5. Synthesis of 5′-O-(4-Fluorophenyl)acetyl-N4-hydroxycytidine (18, SN_18)
4.1.6. Synthesis of 5′-O-(2-Chlorophenyl)acetyl-N4-hydroxycytidine (19, SN_19)
4.1.7. Synthesis of 5′-O-(1-Phenylcyclopropanoyl-1-carbonyl)-N4-hydroxycytidine (20, SN_20)
4.1.8. Synthesis of 5′-O-Phenoxyacetyl-N4-hydroxycytidine (21, SN_21)
4.1.9. Synthesis of 5′-O-(3-Methylphenoxy)acetyl-N4-hydroxycytidine (22, SN_22)
4.1.10. Synthesis of 5′-O-3-(3-Methoxyphenyl)propanoyl-N4-hydroxycytidine (23, SN_23)
4.2. Antiviral Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 28 October 2023).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, M.; Lin, W.; Dong, W.; Xu, J. “Mutation Blacklist” and “Mutation Whitelist” of SARS-CoV-2. J. Biosaf. Biosecurity 2022, 4, 114–120. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.; Canard, B. Kill or Corrupt: Mechanisms of Action and Drug-Resistance of Nucleotide Analogues against SARS-CoV-2. Antivir. Res. 2023, 210, 105501. [Google Scholar] [CrossRef] [PubMed]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; André, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir Remain Active against SARS-CoV-2 Omicron and Other Variants of Concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef]
- Shen, Y.; Eades, W.; Liu, W.; Yan, B. The COVID-19 Oral Drug Molnupiravir Is a CES2 Substrate: Potential Drug-Drug Interactions and Impact of CES2 Genetic Polymorphism In Vitro. Drug Metab. Dispos. 2022, 50, 1151–1160. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Marzi, M. Review on Molnupiravir as a Promising Oral Drug for the Treatment of COVID-19. Med. Chem. Res. 2022, 31, 232–243. [Google Scholar] [CrossRef]
- Agostini, M.L.; Pruijssers, A.J.; Chappell, J.D.; Gribble, J.; Lu, X.; Andres, E.L.; Bluemling, G.R.; Lockwood, M.A.; Sheahan, T.P.; Sims, A.C.; et al. Small-Molecule Antiviral β-d-N4-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance. J. Virol. 2019, 93, e01348-19. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schafer, A.; Dinnon, K.H.; Stevens, L.J.; et al. An Orally Bioavailable Broad-Spectrum Antiviral Inhibits SARS-CoV-2 in Human Airway Epithelial Cell Cultures and Multiple Coronaviruses in Mice. Sci. Transl. Med. 2020, 12, 5883. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Toots, M.; Lee, S.; Lee, M.E.; Ludeke, B.; Luczo, J.M.; Ganti, K.; Cox, R.M.; Sticher, Z.M.; Edpuganti, V.; et al. Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Foo, C.S.; Kaptein, S.J.F.; Zhang, X.; Do, T.N.D.; Langendries, L.; Vangeel, L.; Breuer, J.; Pang, J.; Williams, R.; et al. The Combined Treatment of Molnupiravir and Favipiravir Results in a Potentiation of Antiviral Efficacy in a SARS-CoV-2 Hamster Infection Model. eBioMedicine 2021, 72, 103595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Y.; Wen, X.; Ma, H. Current Prodrug Strategies for Improving Oral Absorption of Nucleoside Analogues. Asian J. Pharm. Sci. 2014, 9, 65–74. [Google Scholar] [CrossRef]
- Pruijssers, A.J.; Denison, M.R. Nucleoside Analogues for the Treatment of Coronavirus Infections. Curr. Opin. Virol. 2019, 35, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Bonilla, H.; Jagannathan, P.; Shafer, R.W. SARS-CoV-2 Antiviral Therapy. Clin. Microbiol. Rev. 2021, 34, e0010921. [Google Scholar] [CrossRef]
- Wen, Z.-H.; Wang, M.-M.; Li, L.-Y.; Herdewijn, P.; Snoeck, R.; Andrei, G.; Liu, Z.-P.; Liu, C. Synthesis and Anti-SARS-CoV-2 Evaluation of Lipid Prodrugs of β-D-N4-Hydroxycytidine (NHC) and a 3′-Fluoro-Substituted Analogue of NHC. Bioorg. Chem. 2023, 135, 106527. [Google Scholar] [CrossRef]
- Ford, A.; Mullins, N.D.; Balzarini, J.; Maguire, A.R. Synthesis and Evaluation of Prodrugs of α-Carboxy Nucleoside Phosphonates. J. Org. Chem. 2022, 87, 14793–14808. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Jureka, A.S.; Edwards, M.R.; Lohan, S.; Williams, C.G.; Keiser, P.T.; Davey, R.A.; Totonchy, J.; Tiwari, R.K.; Basler, C.F.; et al. Synthesis and Antiviral Activity of Fatty Acyl Conjugates of Remdesivir against Severe Acute Respiratory Syndrome Coronavirus 2 and Ebola Virus. Eur. J. Med. Chem. 2021, 226, 113862. [Google Scholar] [CrossRef]
- Aggarwal, S.K.; Gogu, S.R.; Rangan, S.R.S.; Agrawal, K.C. Synthesis and Biological Evaluation of Prodrugs of Zidovudine. J. Med. Chem. 1990, 33, 1505–1510. [Google Scholar] [CrossRef]
- Cao, L.; Li, Y.; Yang, S.; Li, G.; Zhou, Q.; Sun, J.; Xu, T.; Yang, Y.; Liao, R.; Shi, Y.; et al. The Adenosine Analog Prodrug ATV006 Is Orally Bioavailable and Has Preclinical Efficacy against Parental SARS-CoV-2 and Variants. Sci. Transl. Med. 2022, 14, eabm7621. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.; Marasco, C.J.; Morris-Natschke, S.L.; Meyer, K.L.; Gumus, F.; Surles, J.R.; Ishaq, K.S.; Kucera, L.S.; Iyer, N.; Wallen, C.A.; et al. Synthesis and Evaluation of Novel Ether Lipid Nucleoside Conjugates for Anti-HIV-1 Activity. J. Med. Chem. 1991, 34, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.M. Rationale and Applications of Lipids as Prodrug Carriers. Eur. J. Pharm. Sci. 2000, 11, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, A.J.; Wiemer, D.F. Prodrugs of Phosphonates and Phosphates: Crossing the Membrane Barrier. Top. Curr. Chem. 2015, 360, 115. [Google Scholar] [CrossRef] [PubMed]
- Sinokrot, H.; Smerat, T.; Najjar, A.; Karaman, R. Advanced Prodrug Strategies in Nucleoside and Non-Nucleoside Antiviral Agents: A Review of the Recent Five Years. Molecules 2017, 22, 1736. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.E.; Kashemirov, B.A.; Zakharova, V.M.; Krylov, I.S.; Williams, M.; Krečmerová, M.; Drach, J.C.; Hilfinger, J.M. Amido Tyrosine Esters: A Promising New Approach to Antiviral Nucleoside Phosphonate Prodrugs. Antivir. Res. 2011, 90, A23–A24. [Google Scholar] [CrossRef]
- Beaumont, K.; Webster, R.; Gardner, I.; Dack, K. Design of Ester Prodrugs to Enhance Oral Absorption of Poorly Permeable Compounds: Challenges to the Discovery Scientist. Curr. Drug Metab. 2003, 4, 461–485. [Google Scholar] [CrossRef]
- Vale, N.; Ferreira, A.; Matos, J.; Fresco, P.; Gouveia, M.J. Amino Acids in the Development of Prodrugs. Molecules 2018, 23, 2318. [Google Scholar] [CrossRef]
- Chan, O.H.; Stewart, B.H. Physicochemical and Drug-Delivery Considerations for Oral Drug Bioavailability. Drug Discov. Today 1996, 1, 461–473. [Google Scholar] [CrossRef]
- Huttunen, K.M.; Raunio, H.; Rautio, J. Prodrugs—From Serendipity to Rational Design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Imai, T. Human Carboxylesterase Isozymes: Catalytic Properties and Rational Drug Design. Drug Metab. Pharmacokinet. 2006, 21, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Hosokawa, M. Structure, Function and Regulation of Carboxylesterases. Chem. Biol. Interact. 2006, 162, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Liederer, B.M.; Borchardt, R.T. Enzymes Involved in the Bioconversion of Ester-Based Prodrugs. J. Pharm. Sci. 2006, 95, 1177–1195. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.K.; Chhikara, B.S.; Hanley, M.J.; Ye, G.; Doncel, G.F.; Parang, K. Synthesis and Biological Evaluation of Fatty Acyl Ester Derivatives of (-)-2′,3′-Dideoxy-3′-Thiacytidine. J. Med. Chem. 2012, 55, 4861–4871. [Google Scholar] [CrossRef] [PubMed]
- Parang, K.; Knaus, E.E.; Wiebe, L.I. Synthesis, in Vitro Anti-HIV Activity, and Biological Stability of 5’-O-Myristoyl Analogue Derivatives of 3’-Fluoro-2’,3’-Dideoxythymidine (FLT) as Potential Bifunctional Prodrugs of FLT. Nucleosides Nucleotides 1998, 17, 987–1008. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Barreiro, E. Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef]
- Ballatore, C.; Huryn, D.M.; Smith, A.B. Carboxylic Acid (Bio)Isosteres in Drug Design. Chem. Med. Chem. 2013, 8, 385–395. [Google Scholar] [CrossRef]
- Jayashree, B.S.; Nikhil, P.S.; Paul, S. Bioisosterism in Drug Discovery and Development—An Overview. Med. Chem. 2022, 18, 915–925. [Google Scholar] [CrossRef]
- Thornber, C.W. Isosterism and Molecular Modification in Drug Design. Chem. Soc. Rev. 1979, 8, 563–580. [Google Scholar] [CrossRef]
- Patani, G.A.; LaVoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef] [PubMed]
- Smart, B.E. Fluorine Substituent Effects (on Bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Shastina, N.S.; Sinyavin, A.E.; Luiksaar, S.I.; Zolotov, S.A. Use of 5′-O-(3-phenylpropionyl)-N4-hydroxycytidine to Inhibit Influenza Virus Replication In Vitro and In Vivo. Patent No. 2791806, 13 March 2023. 14p. Available online: https://yandex.ru/patents/doc/RU2791523C1_20230309 (accessed on 8 December 2022).
- Bender, D.M.; Peterson, J.A.; McCarthy, J.R.; Gunaydin, H.; Takano, Y.; Houk, K.N. Cyclopropanecarboxylic Acid Esters as Potential Prodrugs with Enhanced Hydrolytic Stability. Org. Lett. 2008, 10, 509–511. [Google Scholar] [CrossRef] [PubMed]
- De Meijere, A. Bonding Properties of Cyclopropane and Their Chemical Consequences. Angew. Chemie Int. Ed. 1979, 18, 809–826. [Google Scholar] [CrossRef]
- Fuchs, R.; Kaplan, C.A.; Bloomfield, J.J.; Hatch, L.F. Transmission of Electronic Effects by the Cyclopropane Ring. Ionization Constants of m- and p-Substituted 2α-Phenylpropionic, Cis- and Trans-2-Phenylcyclopropanecarboxylic Acids in 50% Ethanol. J. Org. Chem. 1962, 27, 733–736. [Google Scholar] [CrossRef]
- Chung, M.C.; Ferreira, E.I.; Santos, J.L.; Giarolla, J.; Rando, D.G.; Almeida, A.E.; Bosquesi, P.L.; Menegon, R.F.; Blau, L. Prodrugs for the Treatment of Neglected Diseases. Molecules 2008, 13, 616–677. [Google Scholar] [CrossRef] [PubMed]
- Landowski, C.P.; Lorenzi, P.L.; Song, X.; Amidon, G.L. Nucleoside ester prodrug substrate specificity of liver carboxylesterase. J. Pharmacol. Exp. Ther. 2006, 316, 572–580. [Google Scholar] [CrossRef]
- Takahashi, M.; Hirota, I.; Nakano, T.; Kotani, T.; Takani, D.; Shiratori, K.; Choi, Y.; Haba, M.; Hosokawa, M. Effects of Steric Hindrance and Electron Density of Ester Prodrugs on Controlling the Metabolic Activation by Human Carboxylesterase. Drug Metab. Pharmacokinet. 2021, 38, 100391. [Google Scholar] [CrossRef]
- Mizoi, K.; Takahashi, M.; Sakai, S.; Ogihara, T.; Haba, M.; Hosokawa, M. Structure-Activity Relationship of Atorvastatin Derivatives for Metabolic Activation by Hydrolases. Xenobiotica 2020, 50, 261–269. [Google Scholar] [CrossRef]
- Siniavin, A.E.; Streltsova, M.A.; Nikiforova, M.A.; Kudryavtsev, D.S.; Grinkina, S.D.; Gushchin, V.A.; Mozhaeva, V.A.; Starkov, V.G.; Osipov, A.V.; Lummis, S.C.R.; et al. Snake Venom Phospholipase A2s Exhibit Strong Virucidal Activity against SARS-CoV-2 and Inhibit the Viral Spike Glycoprotein Interaction with ACE2. Cell. Mol. Life Sci. 2021, 1, 7777–7794. [Google Scholar] [CrossRef]

| Compound, R | CC50, µM (Vero E6 and huh-7) | HCoV-229E (IC50, µM) | SARS-CoV-2 (IC50, µM) | Selectivity Index (SI) for SARS-CoV-2 | ||
|---|---|---|---|---|---|---|
| XBB.1.16 | BQ.1.1.45 | EG.5.1.1 | CC50/IC50 (Mean) | |||
SN_14  | ≥50 | 10.54 | 2.16 | 0.70 | 1.09 | 38 |
SN_15 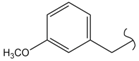 | 11.09 | 2.09 | 0.74 | 0.53 | 45 | |
SN_16 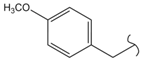 | 7.29 | 2.02 | 0.71 | 0.56 | 46 | |
SN_17 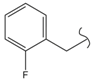 | 7.86 | 1.69 | 0.65 | 0.98 | 45 | |
SN_18  | 20.97 | 2.76 | 1.21 | 1.78 | 26 | |
SN_19 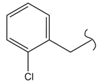 | 23.8 | 5.71 | 2.34 | 2.16 | 15 | |
SN_20 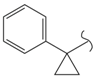 | >100 | >100 | ≥50 | >100 | - | |
SN_21 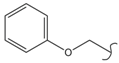 | 5.13 | 1.28 | 0.86 | 0.83 | 51 | |
SN_22 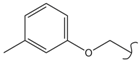 | 1.62 | 0.25 | 0.21 | 0.20 | 227 | |
SN_23 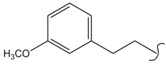 | 1.68 | 1.02 | 0.26 | 0.30 | 95 | |
EIDD-2801 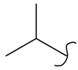 | 3.44 | 1.51 | 0.31 | 0.53 | 64 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darnotuk, E.S.; Siniavin, A.E.; Shastina, N.S.; Luyksaar, S.I.; Inshakova, A.M.; Bondareva, N.E.; Zolotov, S.A.; Lubenec, N.L.; Sheremet, A.B.; Logunov, D.Y.; et al. Synthesis and Antiviral Activity of Novel β-D-N4-Hydroxycytidine Ester Prodrugs as Potential Compounds for the Treatment of SARS-CoV-2 and Other Human Coronaviruses. Pharmaceuticals 2024, 17, 35. https://doi.org/10.3390/ph17010035
Darnotuk ES, Siniavin AE, Shastina NS, Luyksaar SI, Inshakova AM, Bondareva NE, Zolotov SA, Lubenec NL, Sheremet AB, Logunov DY, et al. Synthesis and Antiviral Activity of Novel β-D-N4-Hydroxycytidine Ester Prodrugs as Potential Compounds for the Treatment of SARS-CoV-2 and Other Human Coronaviruses. Pharmaceuticals. 2024; 17(1):35. https://doi.org/10.3390/ph17010035
Chicago/Turabian StyleDarnotuk, Elizaveta S., Andrei E. Siniavin, Natal’ya S. Shastina, Sergey I. Luyksaar, Anna M. Inshakova, Natalia E. Bondareva, Sergey A. Zolotov, Nadezhda L. Lubenec, Anna B. Sheremet, Denis Y. Logunov, and et al. 2024. "Synthesis and Antiviral Activity of Novel β-D-N4-Hydroxycytidine Ester Prodrugs as Potential Compounds for the Treatment of SARS-CoV-2 and Other Human Coronaviruses" Pharmaceuticals 17, no. 1: 35. https://doi.org/10.3390/ph17010035
APA StyleDarnotuk, E. S., Siniavin, A. E., Shastina, N. S., Luyksaar, S. I., Inshakova, A. M., Bondareva, N. E., Zolotov, S. A., Lubenec, N. L., Sheremet, A. B., Logunov, D. Y., Zigangirova, N. A., Gushchin, V. A., & Gintsburg, A. L. (2024). Synthesis and Antiviral Activity of Novel β-D-N4-Hydroxycytidine Ester Prodrugs as Potential Compounds for the Treatment of SARS-CoV-2 and Other Human Coronaviruses. Pharmaceuticals, 17(1), 35. https://doi.org/10.3390/ph17010035





