NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders
Abstract
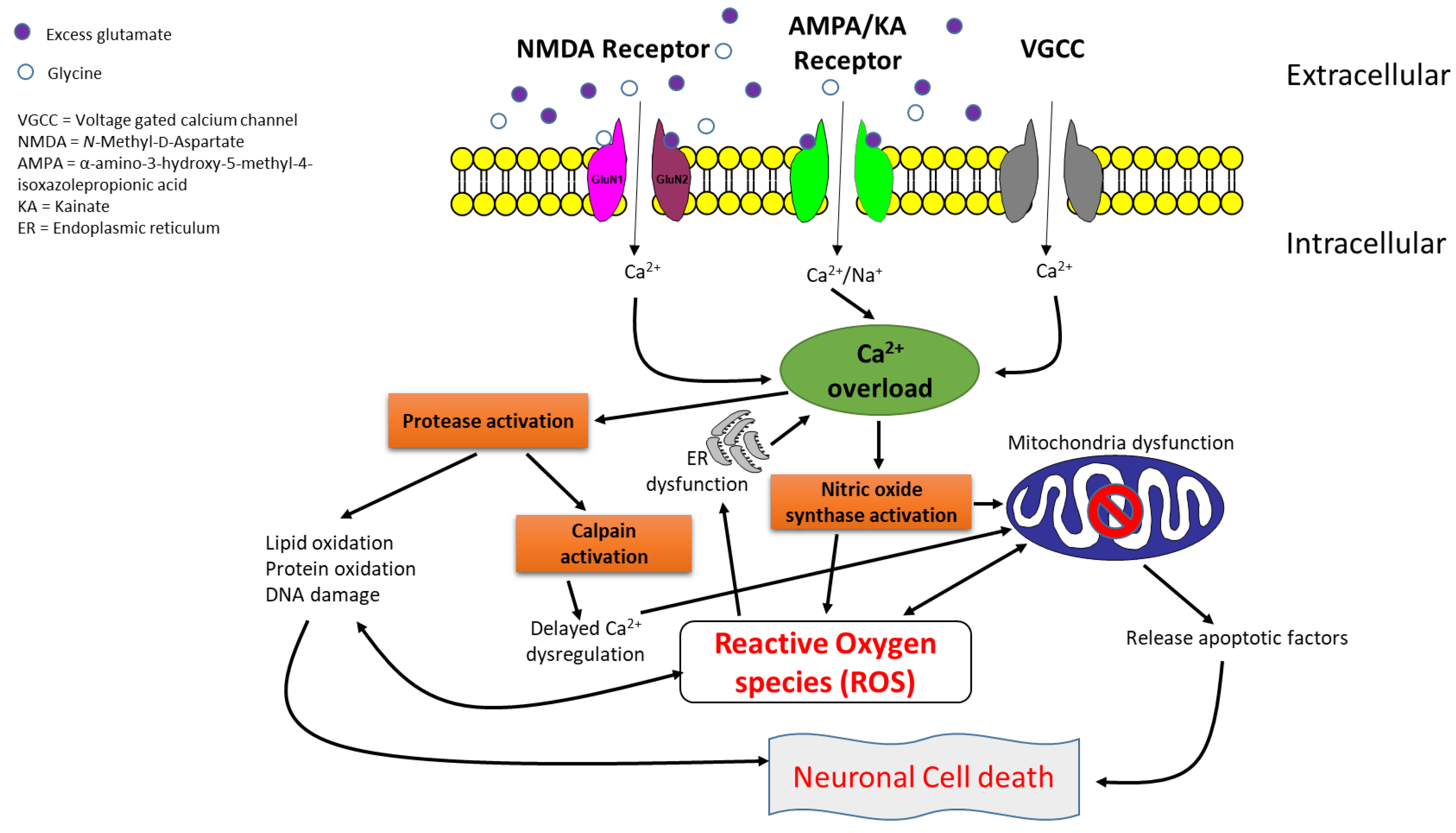
1. Introduction
2. Structure and Functions of NMDA Receptors
3. Types and Molecular Mechanisms of NMDA Receptor Antagonists
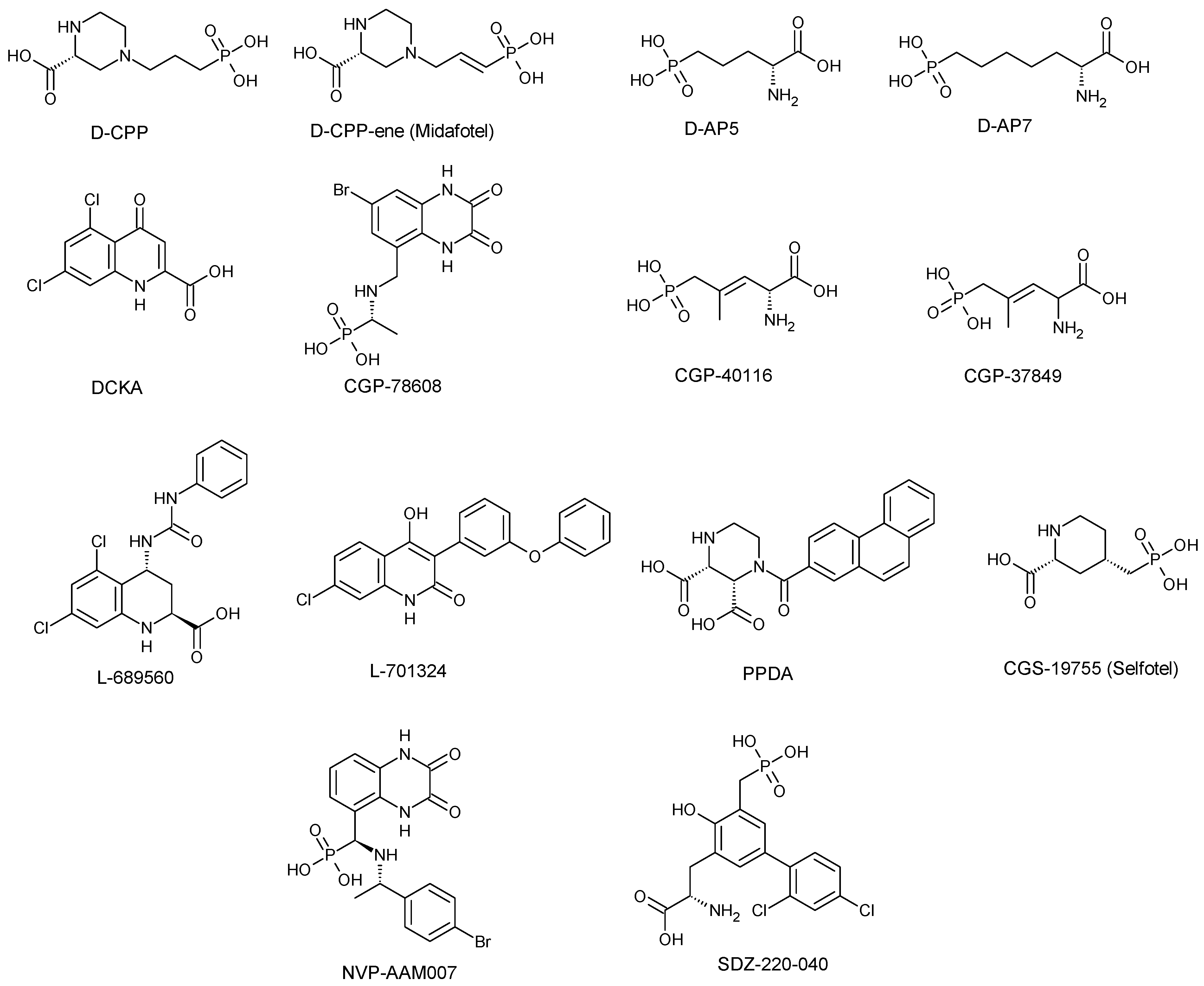
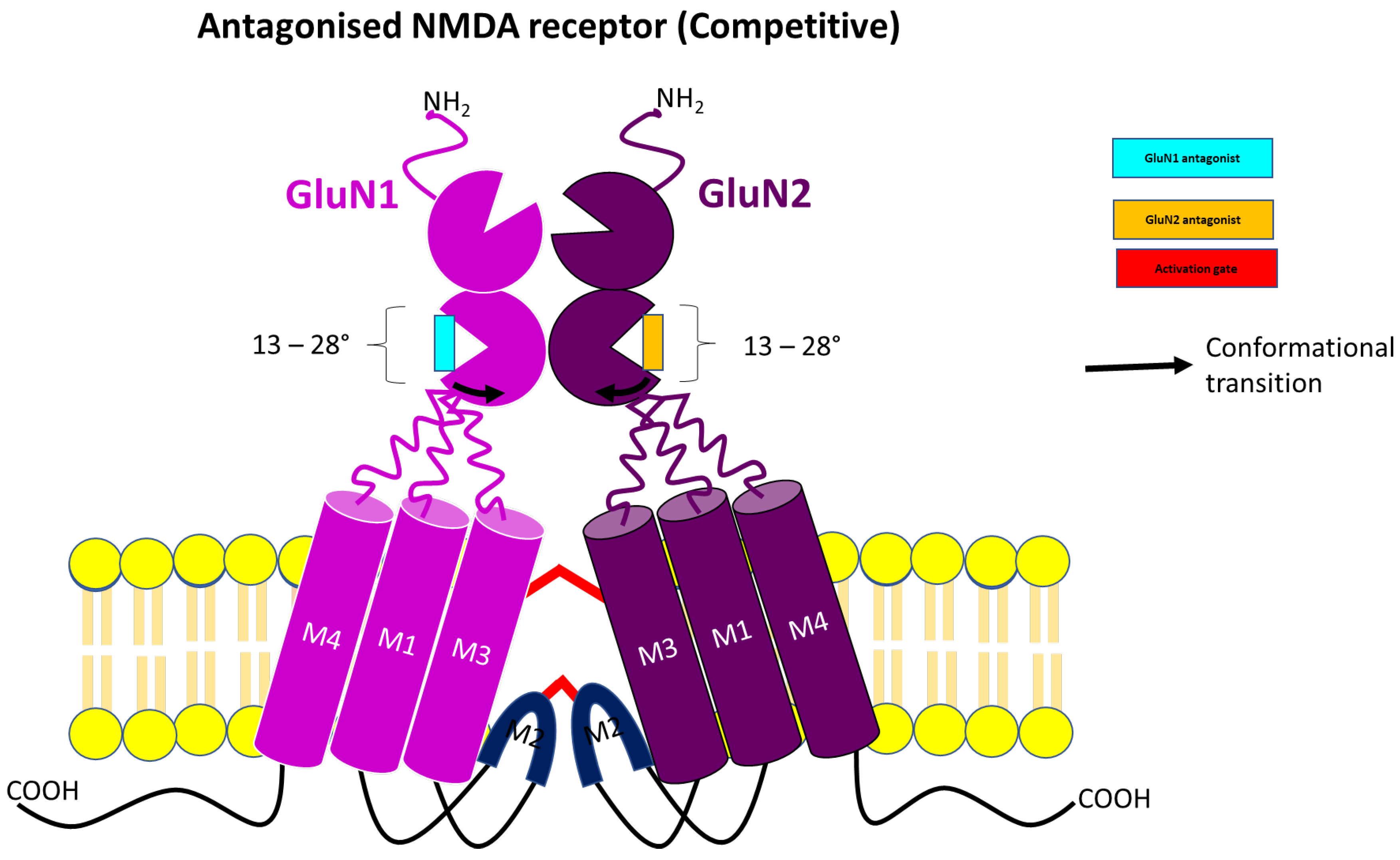
3.1. Competitive NMDA Receptor Antagonist
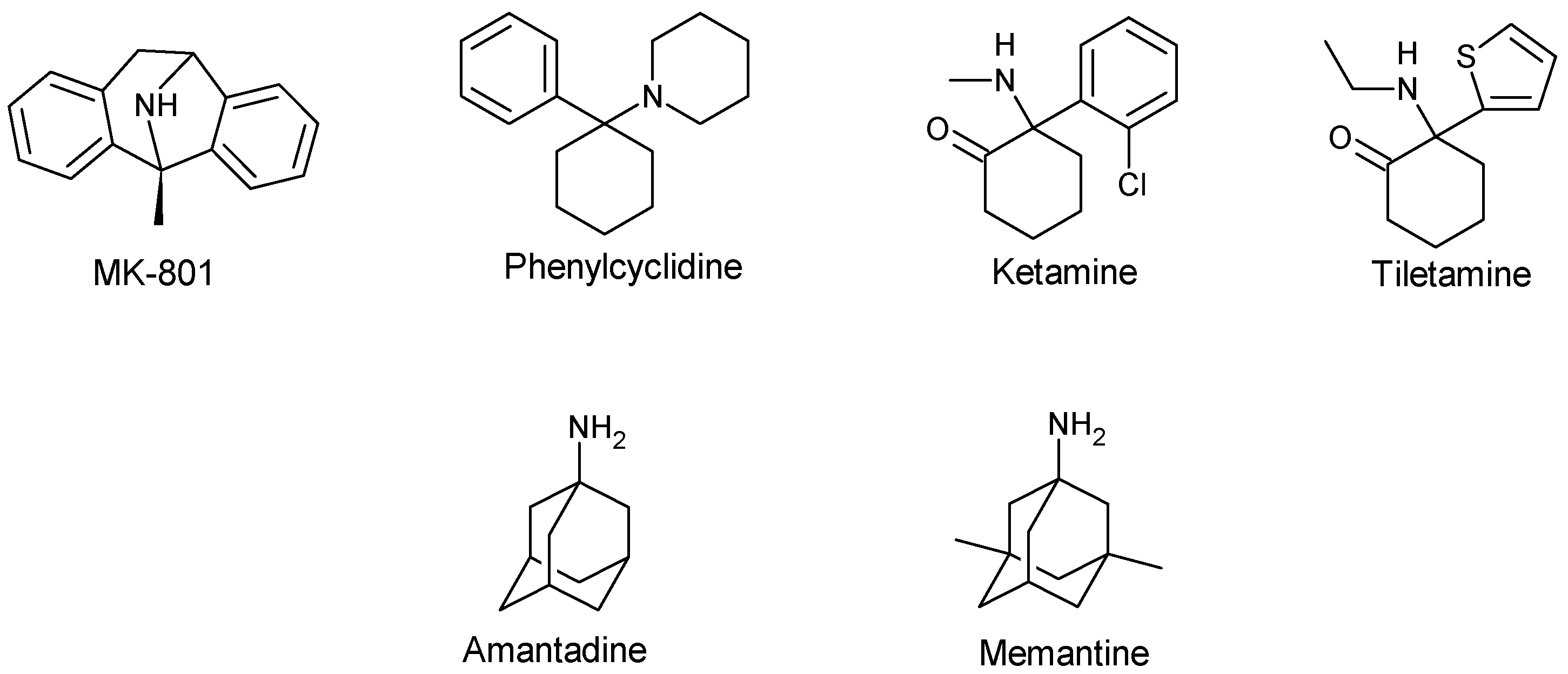
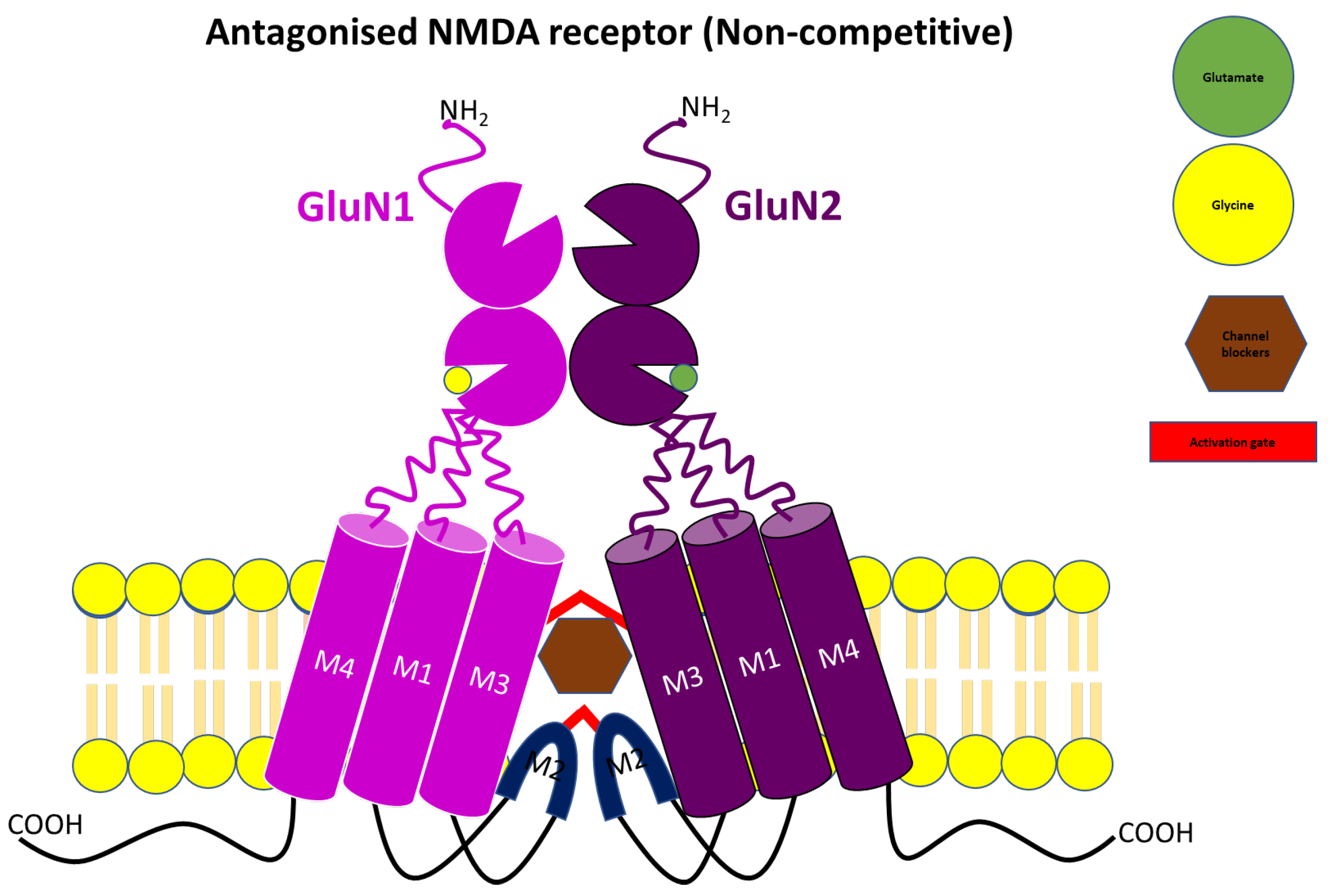
3.2. Uncompetitive or Non-Competitive NMDA Receptor Antagonists
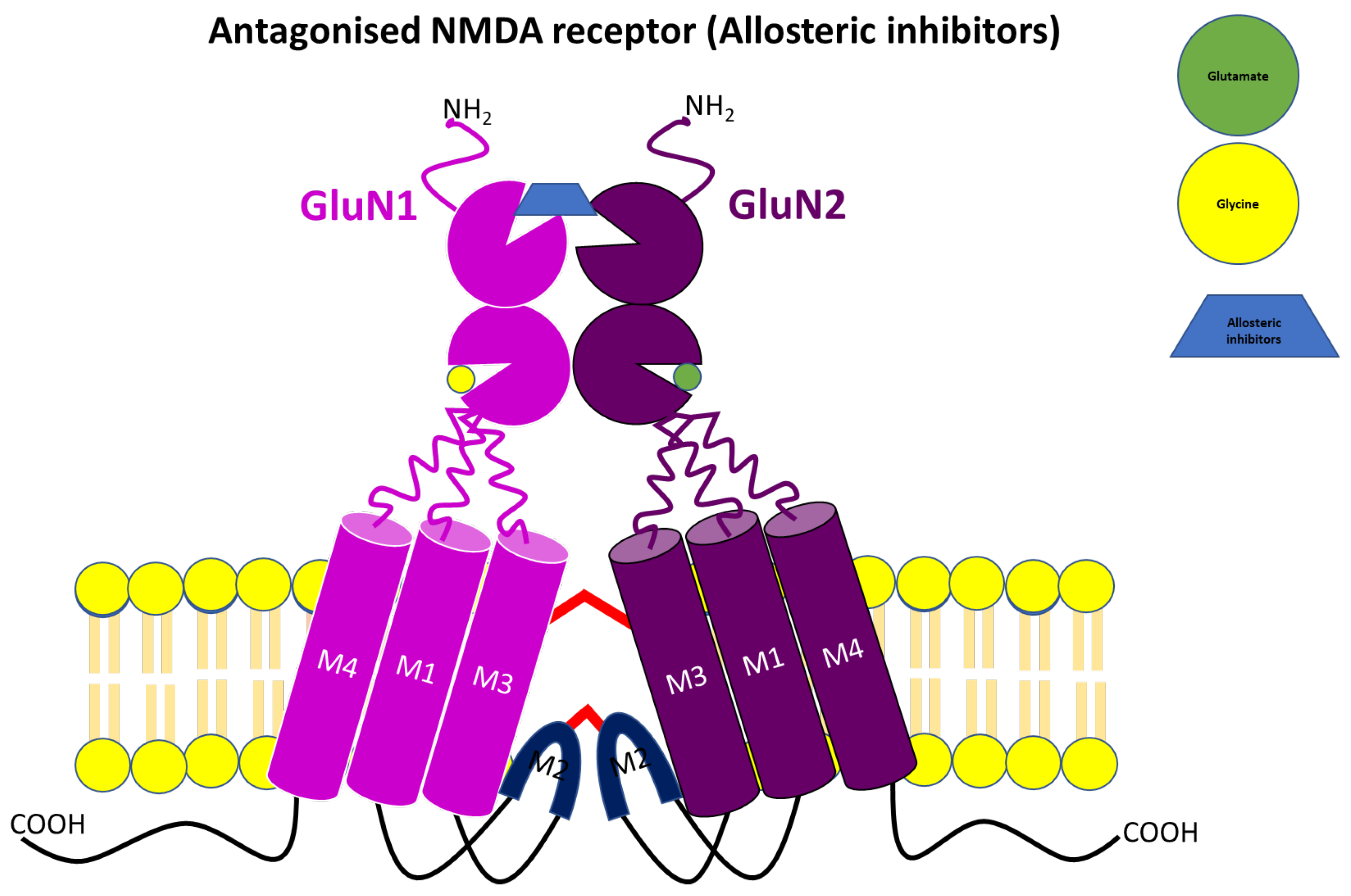
3.3. Allosteric NMDA Receptor Antagonists
| Antagonist Type | Compounds | Receptor Subunits/Subtypes | Developmental Stage | Pharmacological Profiles | Side-Effect Profiles | References |
|---|---|---|---|---|---|---|
| Competitive antagonists | D-CPP/D-CPP-ene (Midafotel) | GluN2A | Terminated at Phase 11 clinical trials | Antiepileptic and neuroprotective effects against head injury, cerebral ischemia and stroke Alteration of acute behavioural response to cocaine. Stimulate short-term increase in NREM (non-rapid eye movement) sleep | Hallucinations Poor concentration Confusion Gait ataxia Sedation Depression | [28,39,42,54,55,68,107] |
| D-AP5/D-AP7 | Non-subunit selective | Preclinical or experimental studies | Block fear acquisition and expression Block or interfere with acute response to psychostimulants such as cocaine amphetamine, or methamphetamine | Similar to those of D-CPP-ene/D-CPP | [28,55] | |
| DCKA | GluN1 | Preclinical or experimental studies | Anxiolytic effect Neuroprotective against NMDA/glycine-induced toxicity | Lack of psychotomimetic effects or side effects associated with dopaminergic transmission | [28,56,108,109,110] | |
| CGP-78608, CGP-37849 & CGP-40116 | GluN1 | Preclinical or Experimental studies | Anticonvulsant effect | Lack of side effects associated with dopaminergic transmission | [28,45,64,68,111] | |
| CGS-19755 (Selfotel) | GluN2A | Terminated at Phase III clinical trials | Neuroprotective effect against global and focal ischemia, trauma and stroke | Psychotomimetic side effects like Hallucination Confusion Paranoia Delirium Lack of side effects associated with dopaminergic transmission | [28,44,46,52,53] | |
| L689-560 & L701-324 | GluN1 | Preclinical or experimental studies | Anticonvulsant effects Anxiolytic Antidepressant-like effect in mice | Sedation Lack of neuronal vacuolisation and psychotomimetic potential Ataxia at a high dose Modest impairment of reference memory, but no negative effect on working memory | [28,49,56,112,113,114,115,116] | |
| PPDA | GluN2A, GluN2C &GluN2D | Preclinical or experimental studies | Prevent the complete worsening effect of tissue-type plasminogen activator on NMDA-induced neuronal death in both cultured cortical and hippocampal neurons Anti-allynic and anti-hyperalgesic effects in rat | Motor dysfunction at high dose | [28,41,58,60,117,118,119,120,121] | |
| NVP-AAMO77 (PEAQX) | GluN2A GluN2C & GluN2B | Preclinical or experimental studies | Produce anti-compulsive behaviour in a rat model Impairment of contextual and temporal fear responses Antidepressant-like effect in rodents | Affect motor coordination stamina and motivation run in a rat dyskinesia model Motor memory impairment or learning memory deficit | [28,36,117,119,122,123,124,125,126] | |
| SDZ-220-040 | GluN2B | Preclinical or experimental studies | Design to readily cross the BBB. Effectively disrupt prepulse inhibition in rats Anticonvulsant effect Protection against focal ischemia Attenuate neuropathic pain | Sedation Ataxia Psychotomimetic effects | [28,127,128,129] | |
| Non-competitive antagonist | MK-801 | Open-Channel blocker | Preclinical or experimental studies | Reverse mild stress-induced anhedonia in male Wistar rats Neuroprotective effect in several animal models of cerebral ischaemia Block L-Dopa-induced dyskinesia in a rat preclinical model, but only at concentrations that worsen parkinsonism Anti-convulsant effect | Weight loss Hypothermia Death Hallucination Ataxia Hyperlocomotion | [53,61,63,66,130,131] |
| Memantine | Open-channel blocker | Approved for AD in human | Neuroprotective effect in AD, vascular dementia and prodromal stages of psychosis Antidepressant-like effect Antinociceptive effect in rats Anticonvulsant effect | Occasional restlessness Headache Hypertension Drowsiness Constipation Diarrhoea Nausea Anorexia Dyspnea Slight dizziness at a high dose | [45,62,71,72,75,132,133,134] | |
| Amantadine | Open-channel blocker | Approved for PD in human | Anti-dyskinetic effect Effectively reduce L-Dopa-induced abnormal involuntary movement Anti-convulsant effect Neuroprotective effect | Visual hallucination Confusion Blurred vision Leg oedema Dry mouth Constipation Urinary retention | [62,63,66,131,135] | |
| PCP | Open-channel blocker | Preclinical/experimental studies | Anticonvulsant effects in NMDA- or quinolate-induced seizure model Anaesthetic effect | Hallucination Ataxia Hyperlocomotion Emergency delirium | [45,136] | |
| Ketamine | Open-channel blocker | Approved as an anaesthetic agent | Anticonvulsant effect in NMDA- or quinolate-induced seizure model Anaesthetic effect Antidepressant effect in resistant major depressive disorder | Induce cognitive deficits and psychotic symptoms Hallucination Abuse Psychological and physiological dependences Possible neurotoxicity Nystagmus Drowsiness Nausea and vomiting Blood pressure elevation Liver and bladder damage | [45,130,132,133,136,137,138,139] | |
| Tiletamine | Channel blocker | Approved for veterinary use | Anaesthetic effect Anticonvulsant effects in NMDA- or quinolate-induced seizure model | Robust Sedation in human and animal Ataxia Feeling of dissociation Hallucination | [136] | |
| Negative allosteric modulator | Ifenprodil | GluN2B | Phase III clinical trials completed | Neuroprotective effect in both in vitro and in vivo models of cerebral ischemia Anticonvulsant effects in rodent Rapid antidepressant effect Alleviate neuropathic pain | Impair cognitive behavioural tasks | [28,81,85,93,100,134,140,141,142] |
| Radiprodil | GluN2B | Terminated at Phase II clinical trials | Anticonvulsant effect in rate model (stronger in young rat pups than adult animals) Decrease epileptic spasms in infants | Vomiting Pyrexia | [100] | |
| Ro25-6981 | GluN2B | Preclinical or experimental studies | Rapid antidepressant effect and counteract depressive-like behaviour in chronically stressed rodent Neuroprotective effect against glutamate-induced toxicity in a cultured cortical neuron Improve anxiety and compulsive behaviour in obsessive-compulsive disorder rat Alleviate cerebral ischemia-reperfusion and oxidative damage in male Sprague Dawley rats Antipakinsonian effect in 6-OHDA-lesioned and MPTP PD rat model | Reduced memory in early life stress mice | [28,88,143,144,145,146] | |
| DQP-1105 | GluN2C & GluN2D | Preclinical or experimental studies | Neuroprotective effects in GluN2D-rich substantia nigra compacta dopaminergic neurons | Motor dysfunction | [28,103,147,148,149,150,151] |
4. Current Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Li, T.; Wang, Z.; Yin, Y.; Zhang, S.; Wang, C.; Hu, X.; Lu, S. Bibliometric Analysis of Research on Neurodegenerative Diseases and Single-Cell RNA Sequencing: Opportunities and Challenges. iScience 2023, 26, 107833. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Qiu, C. Prevention of Common Neurodegenerative Disorders in the Elderly. Exp. Gerontol. 2009, 44, 46–50. [Google Scholar] [CrossRef]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Abu-Duhier, F.M.; Barreto, G.E.; Ashraf, G.M. Impact of Sex Differences and Gender Specificity on Behavioral Characteristics and Pathophysiology of Neurodegenerative Disorders. Neurosci. Biobehav. Rev. 2019, 102, 95–105. [Google Scholar] [CrossRef]
- Maselli, F.; D’Antona, S.; Utichi, M.; Arnaudi, M.; Castiglioni, I.; Porro, D.; Papaleo, E.; Gandellini, P.; Cava, C. Computational Analysis of Five Neurodegenerative Diseases Reveals Shared and Specific Genetic Loci. Comput. Struct. Biotechnol. J. 2023, 21, 5395–5407. [Google Scholar] [CrossRef]
- Joshi, R.; Missong, H.; Mishra, J.; Kaur, S.; Saini, S.; Kandimalla, R.; Reddy, P.H.; Babu, A.; Bhatti, G.K.; Bhatti, J.S. Nanotheranostics Revolutionizing Neurodegenerative Diseases: From Precision Diagnosis to Targeted Therapies. J. Drug Deliv. Sci. Technol. 2023, 89, 105067. [Google Scholar] [CrossRef]
- Arbo, B.D.; Schimith, L.E.; Goulart dos Santos, M.; Hort, M.A. Repositioning and Development of New Treatments for Neurodegenerative Diseases: Focus on Neuroinflammation. Eur. J. Pharmacol. 2022, 919, 174800. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Zhao, T.; Gong, M.; Wang, X.; Zhang, Y.; Xu, L.; Li, W.; Li, Y.; Jia, J. The Role of N-Methyl-D-Aspartate Glutamate Receptors in Alzheimer’s Disease: From Pathophysiology to Therapeutic Approaches. Prog. Neurobiol. 2023, 231, 102534. [Google Scholar] [CrossRef]
- Agid, Y. Neurodegenerative Disorders: Are We Wrong? Rev. Neurol. 2022, 178, 407–413. [Google Scholar] [CrossRef]
- Mendes, D.; Peixoto, F.; Oliveira, M.M.; Andrade, P.B.; Videira, R.A. Mitochondria Research and Neurodegenerative Diseases: On the Track to Understanding the Biological World of High Complexity. Mitochondrion 2022, 65, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Ontario, M.L.; Siracusa, R.; Modafferi, S.; Scuto, M.; Sciuto, S.; Greco, V.; Bertuccio, M.P.; Trovato Salinaro, A.; Crea, R.; Calabrese, E.J.; et al. Potential Prevention and Treatment of Neurodegenerative Disorders by Olive Polyphenols and Hidrox. Mech. Ageing Dev. 2022, 203, 111637. [Google Scholar] [CrossRef]
- Awad, R.; Avital, A.; Sosnik, A. Polymeric Nanocarriers for Nose-to-Brain Drug Delivery in Neurodegenerative Diseases and Neurodevelopmental Disorders. Acta Pharm. Sin. B 2023, 13, 1866–1886. [Google Scholar] [CrossRef] [PubMed]
- Thukral, P.; Chowdhury, R.; Sable, H.; Kaushik, A.; Chaudhary, V. Sustainable Green Synthesized Nanoparticles for Neurodegenerative Diseases Diagnosis and Treatment. Mater. Today Proc. 2023, 73, 323–328. [Google Scholar] [CrossRef]
- Rose, C.R.; Ziemens, D.; Untiet, V.; Fahlke, C. Molecular and Cellular Physiology of Sodium-Dependent Glutamate Transporters. Brain Res. Bull. 2018, 136, 3–16. [Google Scholar] [CrossRef] [PubMed]
- van Onselen, R.; Downing, T.G. Uptake of β-N-Methylamino-L-Alanine (BMAA) into Glutamate-Specific Synaptic Vesicles: Exploring the Validity of the Excitotoxicity Mechanism of BMAA. Neurosci. Lett. 2024, 821, 137593. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, D.; Xu, X.; Jia, Q.; Li, Z.; Xu, R.; Chen, Z.; Zhao, Y. Synthesis and Neuroprotective Effects of New Genipin Derivatives against Glutamate-Induced Oxidative Damage. Fitoterapia 2023, 169, 105616. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Salazar, I.L.; Almeida, R.D.; Silva, R.M. Molecular Mechanisms of Ischemia and Glutamate Excitotoxicity. Life Sci. 2023, 328, 121814. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Niu, L.; Yan, J.; Liu, H.; Wang, D.; Hui, J.; Dai, H.; Song, J.; Zhang, Z. High-Frequency Repetitive Transcranial Magnetic Stimulation Improves Depressive-like Behaviors in CUMS-Induced Rats by Modulating Astrocyte GLT-1 to Reduce Glutamate Toxicity. J. Affect. Disord. 2024, 348, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate Excitotoxicity: Potential Therapeutic Target for Ischemic Stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef] [PubMed]
- Velu, L.; Pellerin, L.; Julian, A.; Paccalin, M.; Giraud, C.; Fayolle, P.; Guillevin, R.; Guillevin, C. Early Rise of Glutamate-Glutamine Levels in Mild Cognitive Impairment: Evidence for Emerging Excitotoxicity. J. Neuroradiol. 2024, 51, 168–175. [Google Scholar] [CrossRef]
- Khesmakhi, M.V.; Salimi, Z.; Pourmotabbed, A.; Moradpour, F.; Rezayof, A.; Nedaei, S.E. The Role of Glutamate NMDA Receptors of the Mediodorsal Thalamus in Scopolamine-Induced Amnesia in Rats. Neurosci. Lett. 2024, 820, 137595. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-Induced Excitotoxicity in Parkinson’s Disease: The Role of Glial Cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Burada, A.P.; Vinnakota, R.; Bharti, P.; Dutta, P.; Dubey, N.; Kumar, J. Emerging Insights into the Structure and Function of Ionotropic Glutamate Delta Receptors. Br. J. Pharmacol. 2022, 179, 3612–3627. [Google Scholar] [CrossRef]
- Mori, H.; Mishina, M. Structure and Function of the NMDA Receptor Channel. Neuropharmacology 1995, 34, 1219–1237. [Google Scholar] [CrossRef]
- Murthy, S.E. Glycine-Bound NMDA Receptors Are Stretch-Activated. Trends Neurosci. 2022, 45, 794–795. [Google Scholar] [CrossRef]
- Olivero, G.; Grilli, M.; Marchi, M.; Pittaluga, A. Metamodulation of Presynaptic NMDA Receptors: New Perspectives for Pharmacological Interventions. Neuropharmacology 2023, 234, 109570. [Google Scholar] [CrossRef]
- Li, W.; Kutas, M.; Gray, J.A.; Hagerman, R.H.; Olichney, J.M. The Role of Glutamate in Language and Language Disorders—Evidence from ERP and Pharmacologic Studies. Neurosci. Biobehav. Rev. 2020, 119, 217–241. [Google Scholar] [CrossRef]
- Yi, F.; Mou, T.-C.; Dorsett, K.N.; Volkmann, R.A.; Menniti, F.S.; Sprang, S.R.; Hansen, K.B. Structural Basis for Negative Allosteric Modulation of GluN2A-Containing NMDA Receptors. Neuron 2016, 91, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tajima, N. Structural Insights into NMDA Receptor Pharmacology. Biochem. Soc. Trans. 2023, 51, 1713–1731. [Google Scholar] [CrossRef]
- Gawande, D.Y.; Shelkar, G.P.; Narasimhan, K.K.S.; Liu, J.; Dravid, S.M. GluN2D Subunit-Containing NMDA Receptors Regulate Reticular Thalamic Neuron Function and Seizure Susceptibility. Neurobiol. Dis. 2023, 181, 106117. [Google Scholar] [CrossRef]
- Mony, L.; Paoletti, P. Mechanisms of NMDA Receptor Regulation. Curr. Opin. Neurobiol. 2023, 83, 102815. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Shuaib, A. NMDA/NR2BA Selective Antagonists in the Treatment of Ischemic Brain Injury. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Rong, Y.; Chen, J.; Dang, S.; Wang, Z.; Baudry, M. Calpain-Mediated Regulation of NMDA Receptor Structure and Function. Brain Res. 1998, 790, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, N.; Zhao, F.; Huang, B.; Kang, D.; Zhan, P.; Liu, X. Discovery of GluN2A Subtype-Selective N-Methyl-d-Aspartate (NMDA) Receptor Ligands. Acta Pharm. Sin. B 2024, 14, 1987–2005. [Google Scholar] [CrossRef]
- Wu, E.; Zhang, J.; Zhang, J.; Zhu, S. Structural Insights into Gating Mechanism and Allosteric Regulation of NMDA Receptors. Curr. Opin. Neurobiol. 2023, 83, 102806. [Google Scholar] [CrossRef] [PubMed]
- Nikam, S.; Meltzer, L. NR2B Selective NMDA Receptor Antagonists. Curr. Pharm. Des. 2005, 8, 845–855. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Fu, P.; Zhang, Z.; Lin, K.; Ko, J.K.-S.; Yung, K.K.-L. Roles of Glutamate Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4391. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Huang, S.M.; Peng, H.H.; Lin, S.W.; Lin, S.R.; Chin, T.Y.; Huang, S.M. Imbalance of Synaptic and Extrasynaptic NMDA Receptors Induced by the Deletion of CRMP1 Accelerates Age-Related Cognitive Decline in Mice. Neurobiol. Aging 2024, 135, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, L.; Pinto, A.; Mastronardi, F.; Iannuzzi, M.C.; Cullia, G.; Nielsen, B.; De Micheli, C.; Conti, P. 3-Carboxy-Pyrazolinalanine as a New Scaffold for Developing Potent and Selective NMDA Receptor Antagonists. Eur. J. Med. Chem. 2013, 68, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Geter-Douglass, B.; Witkin, J.M. Dizocilpine-like Discriminative Stimulus Effects of Competitive NMDA Receptor Antagonists in Mice. Psychopharmacology 1997, 133, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kvist, T.; Steffensen, T.B.; Greenwood, J.R.; Mehrzad Tabrizi, F.; Hansen, K.B.; Gajhede, M.; Pickering, D.S.; Traynelis, S.F.; Kastrup, J.S.; Bräuner-Osborne, H. Crystal Structure and Pharmacological Characterization of a Novel N-Methyl-d-Aspartate (NMDA) Receptor Antagonist at the GluN1 Glycine Binding Site. J. Biol. Chem. 2013, 288, 33124–33135. [Google Scholar] [CrossRef]
- Jespersen, A.; Tajima, N.; Fernandez-Cuervo, G.; Garnier-Amblard, E.C.; Furukawa, H. Structural Insights into Competitive Antagonism in NMDA Receptors. Neuron 2014, 81, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Wlaź, P.; Ebert, U.; Potschka, H.; Löscher, W. Electrical but Not Chemical Kindling Increases Sensitivity to Some Phencyclidine-like Behavioral Effects Induced by the Competitive NMDA Receptor Antagonist d-CPPene in Rats. Eur. J. Pharmacol. 1998, 353, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Lind, G.E.; Mou, T.-C.; Tamborini, L.; Pomper, M.G.; De Micheli, C.; Conti, P.; Pinto, A.; Hansen, K.B. Structural Basis of Subunit Selectivity for Competitive NMDA Receptor Antagonists with Preference for GluN2A over GluN2B Subunits. Proc. Natl. Acad. Sci. USA 2017, 114, E6942–E6951. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Kagaya, T.; Ogura, H.; Nishizawa, Y. Competitive NMDA Receptor Antagonists Disrupt Prepulse Inhibition without Reduction of Startle Amplitude in a Dopamine Receptor-Independent Manner in Mice. Eur. J. Pharmacol. 1999, 364, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Driver, C.; Jackson, T.N.W.; Lagopoulos, J.; Hermens, D.F. Molecular Mechanisms Underlying the N-Methyl-d-Aspartate Receptor Antagonists: Highlighting Their Potential for Transdiagnostic Therapeutics. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 119, 110609. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.; Hogan, M.; Hakim, A.M. The Effects of a Competitive NMDA Receptor Antagonist (CGS-19755) on Cerebral Blood Flow and PH in Focal Ischemia. J. Cereb. Blood Flow Metab. 1991, 11, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Willmore, C.B.; Bespalov, A.Y.; Beardsley, P.M. Competitive and Noncompetitive NMDA Antagonist Effects in Rats Trained to Discriminate Lever-Press Counts. Pharmacol. Biochem. Behav. 2001, 69, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Avenet, P.; Léonardon, J.; Besnard, F.; Graham, D.; Depoortere, H.; Scatton, B. Antagonist Properties of Eliprodil and Other NMDA Receptor Antagonists at Rat NR1A/NR2A and NR1A/NR2B Receptors Expressed in Xenopus Oocytes. Neurosci. Lett. 1997, 223, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Danysz, W.; Parsons, C.G.; Karcz-Kubicha, M.; Schwaier, A.; Popik, P.; Wedzony, K.; Lazarewicz, J.; Quack, G. GlycineB Antagonists as Potential Therapeutic Agents. Previous Hopes and Present Reality. Amino Acids 1998, 14, 235–239. [Google Scholar] [CrossRef]
- Mugnaini, M.; Dal Forno, G.; Corsi, M.; Bunnemann, B. Receptor Binding Characteristics of the Novel NMDA Receptor Glycine Site Antagonist [3H]GV150526A in Rat Cerebral Cortical Membranes. Eur. J. Pharmacol. 2000, 391, 233–241. [Google Scholar] [CrossRef]
- Jansen, M.; Dannhardt, G. Antagonists and Agonists at the Glycine Site of the NMDA Receptor for Therapeutic Interventions. Eur. J. Med. Chem. 2003, 38, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Péez-Pinzón, M.A.; Steinberg, G.K. CGS 19755 (Selfotel): A Novel Neuroprotective Agent Against CNS Injury. CNS Drug Rev. 1996, 2, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, J.; Ritchie, I.M.; Butcher, S.P.; Kelly, J.S. Comparison of the Patterns of Altered Cerebral Glucose Utilisation Produced by Competitive and Non-Competitive NMDA Receptor Antagonists. Brain Res. 1996, 735, 67–82. [Google Scholar] [CrossRef]
- Bespalov, A.; Kudryashova, M.; Zvartau, E. Prolongation of Morphine Analgesia by Competitive NMDA Receptor Antagonist D-CPPene (SDZ EAA 494) in Rats. Eur. J. Pharmacol. 1998, 351, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Carmack, S.A.; Kim, J.S.; Sage, J.R.; Thomas, A.W.; Skillicorn, K.N.; Anagnostaras, S.G. The Competitive NMDA Receptor Antagonist CPP Disrupts Cocaine-Induced Conditioned Place Preference, but Spares Behavioral Sensitization. Behav. Brain Res. 2013, 239, 155–163. [Google Scholar] [CrossRef]
- Riaza Bermudo-Soriano, C.; Perez-Rodriguez, M.M.; Vaquero-Lorenzo, C.; Baca-Garcia, E. New Perspectives in Glutamate and Anxiety. Pharmacol. Biochem. Behav. 2012, 100, 752–774. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, T.; Chen, Q.; Li, C.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; Chen, H.; Zhou, Z.; et al. Biomimetic Dendrimer–Peptide Conjugates for Early Multi-Target Therapy of Alzheimer’s Disease by Inflammatory Microenvironment Modulation. Adv. Mater. 2021, 33, 2100746. [Google Scholar] [CrossRef] [PubMed]
- Jullienne, A.; Montagne, A.; Orset, C.; Lesept, F.; Jane, D.E.; Monaghan, D.T.; Maubert, E.; Vivien, D.; Ali, C. Selective Inhibition of GluN2D-Containing N-Methyl-D-Aspartate Receptors Prevents Tissue Plasminogen Activator-Promoted Neurotoxicity Both in Vitro and in Vivo. Mol. Neurodegener. 2011, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, O.S.; Mela, F.; Calcagno, M.; Budri, M.; Viaro, R.; Dekundy, A.; Parsons, C.G.; Auberson, Y.P.; Morari, M. GluN2A and GluN2B NMDA Receptor Subunits Differentially Modulate Striatal Output Pathways and Contribute to Levodopa-Induced Abnormal Involuntary Movements in Dyskinetic Rats. ACS Chem. Neurosci. 2013, 4, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Mikics, E.; Toth, M.; Biro, L.; Bruzsik, B.; Nagy, B.; Haller, J. The Role of GluN2B-Containing NMDA Receptors in Short- and Long-Term Fear Recall. Physiol. Behav. 2017, 177, 44–48. [Google Scholar] [CrossRef]
- Papp, M.; Moryl, E. Antidepressant Activity of Non-Competitive and Competitive NMDA Receptor Antagonists in a Chronic Mild Stress Model of Depression. Eur. J. Pharmacol. 1994, 263, 1–7. [Google Scholar] [CrossRef]
- Szakács, R.; Weiczner, R.; Mihály, A.; Krisztin-Péva, B.; Zádor, Z.; Zádor, E. Non-Competitive NMDA Receptor Antagonists Moderate Seizure-Induced c-Fos Expression in the Rat Cerebral Cortex. Brain Res. Bull. 2003, 59, 485–493. [Google Scholar] [CrossRef]
- Tóth, Z.; Mihály, A.; Mátyás, A.; Krisztin-Péva, B. Non-Competitive Antagonists of NMDA and AMPA Receptors Decrease Seizure-Induced c-Fos Protein Expression in the Cerebellum and Protect against Seizure Symptoms in Adult Rats. Acta Histochem. 2018, 120, 236–241. [Google Scholar] [CrossRef]
- Bubser, M.; Keseberg, U.; Notz, P.K.; Schmidt, W.J. Differential Behavioural and Neurochemical Effects of Competitive and Non-Competitive NMDA Receptor Antagonists in Rats. Eur. J. Pharmacol. 1992, 229, 75–82. [Google Scholar] [CrossRef]
- Sun, W.L.; Wessinger, W.D. Characterization of the Non-Competitive Antagonist Binding Site of the NMDA Receptor in Dark Agouti Rats. Life Sci. 2004, 75, 1405–1415. [Google Scholar] [CrossRef]
- Flores, A.J.; Bartlett, M.J.; So, L.Y.; Laude, N.D.; Parent, K.L.; Heien, M.L.; Sherman, S.J.; Falk, T. Differential Effects of the NMDA Receptor Antagonist MK-801 on Dopamine Receptor D1- and D2-Induced Abnormal Involuntary Movements in a Preclinical Model. Neurosci. Lett. 2014, 564, 48–52. [Google Scholar] [CrossRef]
- Berger, M.L.; Rebernik, P. Differential Influence of 7 Cations on 16 Non-Competitive NMDA Receptor Blockers. Bioorg. Med. Chem. Lett. 2015, 25, 4131–4135. [Google Scholar] [CrossRef]
- Marcus, M.M.; Mathé, J.M.; Nomikos, G.G.; Svensson, T.H. Effects of Competitive and Non-Competitive NMDA Receptor Antagonists on Dopamine Output in the Shell and Core Subdivisions of the Nucleus Accumbens. Neuropharmacology 2001, 40, 482–490. [Google Scholar] [CrossRef]
- Blot, K.; Bai, J.; Otani, S. The Effect of Non-Competitive NMDA Receptor Antagonist MK-801 on Neuronal Activity in Rodent Prefrontal Cortex: An Animal Model for Cognitive Symptoms of Schizophrenia. J. Physiol. Paris 2013, 107, 448–451. [Google Scholar] [CrossRef]
- Stuchlík, A.; Vales, K. Systemic Administration of MK-801, a Non-Competitive NMDA-Receptor Antagonist, Elicits a Behavioural Deficit of Rats in the Active Allothetic Place Avoidance (AAPA) Task Irrespectively of Their Intact Spatial Pretraining. Behav. Brain Res. 2005, 159, 163–171. [Google Scholar] [CrossRef]
- Lipton, S.A. Failures and Successes of NMDA Receptor Antagonists: Molecular Basis for the Use of Open-Channel Blockers like Memantine in the Treatment of Acute and Chronic Neurologic Insults. NeuroRx 2004, 1, 101–110. [Google Scholar] [CrossRef]
- Raghavendra Rao, V.L.; Dogan, A.; Todd, K.G.; Bowen, K.K.; Dempsey, R.J. Neuroprotection by Memantine, a Non-Competitive NMDA Receptor Antagonist after Traumatic Brain Injury in Rats. Brain Res. 2001, 911, 96–100. [Google Scholar] [CrossRef]
- Losi, G.; Lanza, M.; Makovec, F.; Artusi, R.; Caselli, G.; Puia, G. Functional in Vitro Characterization of CR 3394: A Novel Voltage Dependent N-Methyl-D-Aspartate (NMDA) Receptor Antagonist. Neuropharmacology 2006, 50, 277–285. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Van der Schyf, C.J. Rationally Designed Multi-Targeted Agents Against Neurodegenerative Diseases. Curr. Med. Chem. 2013, 20, 1662–1672. [Google Scholar] [CrossRef]
- Shafiei-Irannejad, V.; Abbaszadeh, S.; Janssen, P.M.L.; Soraya, H. Memantine and Its Benefits for Cancer, Cardiovascular and Neurological Disorders. Eur. J. Pharmacol. 2021, 910, 174455. [Google Scholar] [CrossRef]
- Lin, J.-C.; Chan, M.-H.; Lee, M.-Y.; Chen, Y.-C.; Chen, H.-H. N,N-Dimethylglycine Differentially Modulates Psychotomimetic and Antidepressant-like Effects of Ketamine in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 71, 7–13. [Google Scholar] [CrossRef]
- Suzuki, A.; Murakami, K.; Tajima, Y.; Hara, H.; Kunugi, A.; Kimura, H. TAK-137, an AMPA Receptor Potentiator with Little Agonistic Effect, Produces Antidepressant-like Effect without Causing Psychotomimetic Effects in Rats. Pharmacol. Biochem. Behav. 2019, 183, 80–86. [Google Scholar] [CrossRef]
- Subramanian, S.; Haroutounian, S.; Palanca, B.J.A.; Lenze, E.J. Ketamine as a Therapeutic Agent for Depression and Pain: Mechanisms and Evidence. J. Neurol. Sci. 2022, 434, 120152. [Google Scholar] [CrossRef]
- Costa, B.M.; Irvine, M.W.; Fang, G.; Eaves, R.J.; Mayo-Martin, M.B.; Skifter, D.A.; Jane, D.E.; Monaghan, D.T. A Novel Family of Negative and Positive Allosteric Modulators of NMDA Receptors. J. Pharmacol. Exp. Ther. 2010, 335, 614–621. [Google Scholar] [CrossRef]
- Sirrieh, R.E.; MacLean, D.M.; Jayaraman, V. A Conserved Structural Mechanism of NMDA Receptor Inhibition: A Comparison of Ifenprodil and Zinc. J. Gen. Physiol. 2015, 146, 173–181. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Fan, C.; Mao, L.; Xie, R.; Wang, S.; Yang, M.; Yuan, H.; Yang, X.; Sun, J.; et al. The GluN1/GluN2B NMDA Receptor and Metabotropic Glutamate Receptor 1 Negative Allosteric Modulator Has Enhanced Neuroprotection in a Rat Subarachnoid Hemorrhage Model. Exp. Neurol. 2018, 301, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Paoletti, P. Allosteric Modulators of NMDA Receptors: Multiple Sites and Mechanisms. Curr. Opin. Pharmacol. 2015, 20, 14–23. [Google Scholar] [CrossRef]
- Zhu, S.; Stein, R.A.; Yoshioka, C.; Lee, C.-H.; Goehring, A.; Mchaourab, H.S.; Gouaux, E. Mechanism of NMDA Receptor Inhibition and Activation. Cell 2016, 165, 704–714. [Google Scholar] [CrossRef]
- Quan, J.; Yang, H.; Qin, F.; He, Y.; Liu, J.; Zhao, Y.; Ma, C.; Cheng, M. Discovery of Novel Tryptamine Derivatives as GluN2B Subunit-Containing NMDA Receptor Antagonists via Pharmacophore-Merging Strategy with Orally Available Therapeutic Effect of Cerebral Ischemia. Eur. J. Med. Chem. 2023, 253, 115318. [Google Scholar] [CrossRef] [PubMed]
- Mony, L.; Kew, J.N.C.; Gunthorpe, M.J.; Paoletti, P. Allosteric Modulators of NR2B-containing NMDA Receptors: Molecular Mechanisms and Therapeutic Potential. Br. J. Pharmacol. 2009, 157, 1301–1317. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, L.H.; Chen, L.; Hao, D.; Chen, J. Neuroprotection by Tetrahydroxystilbene Glucoside in the MPTP Mouse Model of Parkinson’s Disease. Toxicol. Lett. 2013, 222, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Irvine, M.W.; Fang, G.; Sapkota, K.; Burnell, E.S.; Volianskis, A.; Costa, B.M.; Culley, G.; Collingridge, G.L.; Monaghan, D.T.; Jane, D.E. Investigation of the Structural Requirements for N-Methyl-D-Aspartate Receptor Positive and Negative Allosteric Modulators Based on 2-Naphthoic Acid. Eur. J. Med. Chem. 2019, 164, 471–498. [Google Scholar] [CrossRef]
- Gibb, A.J. Allosteric Antagonist Action at Triheteromeric NMDA Receptors. Neuropharmacology 2022, 202, 108861. [Google Scholar] [CrossRef]
- Markus, A.; Schreiber, J.A.; Goerges, G.; Frehland, B.; Schepmann, D.; Daniliuc, C.; Fröhlich, R.; Seebohm, G.; Wünsch, B. Phenol-Benzoxazolone Bioisosteres of GluN2B-NMDA Receptor Antagonists: Unexpected Rearrangement during Reductive Alkylation with Phenylcyclohexanone. Arch. Pharm. 2022, 355, 2200225. [Google Scholar] [CrossRef]
- Temme, L.; Bechthold, E.; Schreiber, J.A.; Gawaskar, S.; Schepmann, D.; Robaa, D.; Sippl, W.; Seebohm, G.; Wünsch, B. Negative Allosteric Modulators of the GluN2B NMDA Receptor with Phenylethylamine Structure Embedded in Ring-Expanded and Ring-Contracted Scaffolds. Eur. J. Med. Chem. 2020, 190, 112138. [Google Scholar] [CrossRef]
- Hanson, J.E.; Ma, K.; Elstrott, J.; Weber, M.; Saillet, S.; Khan, A.S.; Simms, J.; Liu, B.; Kim, T.A.; Yu, G.-Q.; et al. GluN2A NMDA Receptor Enhancement Improves Brain Oscillations, Synchrony, and Cognitive Functions in Dravet Syndrome and Alzheimer’s Disease Models. Cell Rep. 2020, 30, 381–396.e4. [Google Scholar] [CrossRef]
- Sapkota, K.; Burnell, E.S.; Irvine, M.W.; Fang, G.; Gawande, D.Y.; Dravid, S.M.; Jane, D.E.; Monaghan, D.T. Pharmacological Characterization of a Novel Negative Allosteric Modulator of NMDA Receptors, UBP792. Neuropharmacology 2021, 201, 108818. [Google Scholar] [CrossRef]
- Hanson, J.E.; Yuan, H.; Perszyk, R.E.; Banke, T.G.; Xing, H.; Tsai, M.C.; Menniti, F.S.; Traynelis, S.F. Therapeutic Potential of N-Methyl-D-Aspartate Receptor Modulators in Psychiatry. Neuropsychopharmacology 2024, 49, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhou, Q. Enhancing NMDA Receptor Function: Recent Progress on Allosteric Modulators. Neural Plast. 2017, 2017, 2875904. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; O’Connor, M.J.; Masukawa, L.M.; McGonigle, P. Polyamine Effects on the NMDA Receptor in Human Brain. Exp. Neurol. 1994, 130, 323–330. [Google Scholar] [CrossRef]
- Monaghan, D.T.; Irvine, M.W.; Costa, B.M.; Fang, G.; Jane, D.E. Pharmacological Modulation of NMDA Receptor Activity and the Advent of Negative and Positive Allosteric Modulators. Neurochem. Int. 2012, 61, 581–592. [Google Scholar] [CrossRef]
- Kane, L.T.; Costa, B.M. Identification of Novel Allosteric Modulator Binding Sites in NMDA Receptors: A Molecular Modeling Study. J. Mol. Graph. Model. 2015, 61, 204–213. [Google Scholar] [CrossRef]
- Perszyk, R.; Katzman, B.M.; Kusumoto, H.; Kell, S.A.; Epplin, M.P.; Tahirovic, Y.A.; Moore, R.L.; Menaldino, D.; Burger, P.; Liotta, D.C.; et al. An NMDAR Positive and Negative Allosteric Modulator Series Share a Binding Site and Are Interconverted by Methyl Groups. eLife 2018, 7, e34711. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Kumata, K.; Chen, Z.; Zhang, Y.D.; Chen, J.H.; Hatori, A.; Fu, H.L.; Rong, J.; Deng, X.Y.; Yamasaki, T.; et al. Synthesis and Preliminary Evaluation of Novel 11C-Labeled GluN2B-Selective NMDA Receptor Negative Allosteric Modulators. Acta Pharmacol. Sin. 2021, 42, 491–498. [Google Scholar] [CrossRef]
- Auvin, S.; Dozières-Puyravel, B.; Avbersek, A.; Sciberras, D.; Collier, J.; Leclercq, K.; Mares, P.; Kaminski, R.M.; Muglia, P. Radiprodil, a NR2B Negative Allosteric Modulator, from Bench to Bedside in Infantile Spasm Syndrome. Ann. Clin. Transl. Neurol. 2020, 7, 343–352. [Google Scholar] [CrossRef]
- Montastruc, J.L.; Rascol, O.; Senard, J.M. Glutamate Antagonists and Parkinson’s Disease: A Review of Clinical Data. Neurosci. Biobehav. Rev. 1997, 21, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hashimoto, K.; Niitsu, T.; Hosoda, Y.; Oda, Y.; Shiko, Y.; Ozawa, Y.; Kawasaki, Y.; Kanahara, N.; Shiina, A.; et al. Ifenprodil Tartrate Treatment of Adolescents with Post-Traumatic Stress Disorder: A Double-Blind, Placebo-Controlled Trial. Psychiatry Res. 2022, 311, 114486. [Google Scholar] [CrossRef]
- Swanger, S.A.; Vance, K.M.; Acker, T.M.; Zimmerman, S.S.; DiRaddo, J.O.; Myers, S.J.; Bundgaard, C.; Mosley, C.A.; Summer, S.L.; Menaldino, D.S.; et al. A Novel Negative Allosteric Modulator Selective for GluN2C/2D-Containing NMDA Receptors Inhibits Synaptic Transmission in Hippocampal Interneurons. ACS Chem. Neurosci. 2018, 9, 306–319. [Google Scholar] [CrossRef]
- Rajan, R.; Schepmann, D.; Schreiber, J.A.; Seebohm, G.; Wünsch, B. Synthesis of GluN2A-Selective NMDA Receptor Antagonists with an Electron-Rich Aromatic B-Ring. Eur. J. Med. Chem. 2021, 209, 112939. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.M.; Irvine, M.W.; Fang, G.; Eaves, R.J.; Mayo-Martin, M.B.; Laube, B.; Jane, D.E.; Monaghan, D.T. Structure-Activity Relationships for Allosteric NMDA Receptor Inhibitors Based on 2-Naphthoic Acid. Neuropharmacology 2012, 62, 1730–1736. [Google Scholar] [CrossRef]
- Sapkota, K.; Dore, K.; Tang, K.; Irvine, M.; Fang, G.; Burnell, E.S.; Malinow, R.; Jane, D.E.; Monaghan, D.T. The NMDA Receptor Intracellular C-Terminal Domains Reciprocally Interact with Allosteric Modulators. Biochem. Pharmacol. 2019, 159, 140–153. [Google Scholar] [CrossRef]
- Campbell, I.G.; Gustafson, L.M.; Feinberg, I. The Competitive NMDA Receptor Antagonist CPPene Stimulates NREM Sleep and Eating in Rats. Neuropsychopharmacology 2002, 26, 348–357. [Google Scholar] [CrossRef]
- Baron, B.M.; Siegel, B.W.; Slone, A.L.; Harrison, B.L.; Palfreyman, M.G.; Hurt, S.D. [3H]5,7-Dichlorokynurenic Acid, a Novel Radioligand Labels NMDA Receptor-Associated Glycine Binding Sites. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 206, 149–154. [Google Scholar] [CrossRef]
- Corbett, R.; Dunn, R.W. Effects of 5,7 Dichlorokynurenic Acid on Conflict, Social Interaction and plus Maze Behaviors. Neuropharmacology 1993, 32, 461–466. [Google Scholar] [CrossRef]
- Frankiewicz, T.; Pilc, A.; Parsons, C.G. Differential Effects of NMDA–Receptor Antagonists on Long-Term Potentiation and Hypoxic/Hypoglycaemic Excitotoxicity in Hippocampal Slices. Neuropharmacology 2000, 39, 631–642. [Google Scholar] [CrossRef]
- Hauber, W.; Waldenmeier, M.T. The AMPA Receptor Antagonist GYKI 52466 Reverses the Anti-Cataleptic Effects of the Competitive NMDA Receptor Antagonist CGP 37849. Eur. J. Pharmacol. 1994, 256, 339–342. [Google Scholar] [CrossRef]
- Obrenovitch, T.P.; Hardy, A.M.; Zilkha, E. Effects of L-701,324, a High-Affinity Antagonist at the N-Methyl-D-Aspartate (NMDA) Receptor Glycine Site, on the Rat Electroencephalogram. Naunyn. Schmiedebergs. Arch. Pharmacol. 1997, 355, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Wlaź, P.; Ebert, U.; Löscher, W. Anticonvulsant Effects of Eliprodil Alone or Combined with the Glycine(B) Receptor Antagonist L-701,324 or the Competitive NMDA Antagonist CGP 40116 in the Amygdala Kindling Model in Rats. Neuropharmacology 1999, 38, 243–251. [Google Scholar] [CrossRef]
- Konieczny, J.; Ossowska, K.; Schulze, G.; Coper, H.; Wolfarth, S. L-701,324, a Selective Antagonist at the Glycine Site of the NMDA Receptor, Counteracts Haloperidol-Induced Muscle Rigidity in Rats. Psychopharmacology 1999, 143, 235–243. [Google Scholar] [CrossRef]
- Stone, T.W. Development and Therapeutic Potential of Kynurenic Acid and Kynurenine Derivatives for Neuroprotection. Trends Pharmacol. Sci. 2000, 21, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ji, C.-H.; Wang, Y.; Zhao, J.; Liu, Y.; Tang, W.-Q.; Gu, J.-H.; Jiang, B. Antidepressant-like Activity of L-701324 in Mice: A Behavioral and Neurobiological Characterization. Behav. Brain Res. 2021, 399, 113038. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Tse, H.W.; Skifter, D.A.; Morley, R.; Jane, D.E.; Monaghan, D.T. Structure—Activity Analysis of a Novel NR2C/NR2D-Preferring NMDA Receptor Antagonist: 1-(Phenanthrene-2-Carbonyl) Piperazine-2,3-Dicarboxylic Acid. Br. J. Pharmacol. 2004, 141, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Morley, R.M.; Jane, D.E.; Monaghan, D.T. The Effect of Competitive Antagonist Chain Length on NMDA Receptor Subunit Selectivity. Neuropharmacology 2005, 48, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, T.E.; Bannister, N.J.; Collett, V.J.; Dargan, S.L.; Massey, P.V.; Bortolotto, Z.A.; Fitzjohn, S.M.; Bashir, Z.I.; Collingridge, G.L.; Lodge, D. Differential Roles of NR2A and NR2B-Containing NMDA Receptors in LTP and LTD in the CA1 Region of Two-Week Old Rat Hippocampus. Neuropharmacology 2007, 52, 60–70. [Google Scholar] [CrossRef]
- Costa, B.M.; Feng, B.; Tsintsadze, T.S.; Morley, R.M.; Irvine, M.W.; Tsintsadze, V.; Lozovaya, N.A.; Jane, D.E.; Monaghan, D.T. N-Methyl-D-Aspartate (NMDA) Receptor NR2 Subunit Selectivity of a Series of Novel Piperazine-2,3-Dicarboxylate Derivatives: Preferential Blockade of Extrasynaptic NMDA Receptors in the Rat Hippocampal CA3-CA1 Synapse. J. Pharmacol. Exp. Ther. 2009, 331, 618–626. [Google Scholar] [CrossRef]
- Baron, A.; Montagne, A.; Cassé, F.; Launay, S.; Maubert, E.; Ali, C.; Vivien, D. NR2D-Containing NMDA Receptors Mediate Tissue Plasminogen Activator-Promoted Neuronal Excitotoxicity. Cell Death Differ. 2010, 17, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Kinarsky, L.; Feng, B.; Skifter, D.A.; Morley, R.M.; Sherman, S.; Jane, D.E.; Monaghan, D.T. Identification of Subunit- and Antagonist-Specific Amino Acid Residues in the N -Methyl-d-Aspartate Receptor Glutamate-Binding Pocket. J. Pharmacol. Exp. Ther. 2005, 313, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Zhuo, M. Targeting the NMDA Receptor Subunit NR2B for the Treatment of Neuropathic Pain. Neurotherapeutics 2009, 6, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Lemay-Clermont, J.; Robitaille, C.; Auberson, Y.P.; Bureau, G.; Cyr, M. Blockade of NMDA Receptors 2A Subunit in the Dorsal Striatum Impairs the Learning of a Complex Motor Skill. Behav. Neurosci. 2011, 125, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; D’Amico, D.; Dierssen, M. From Neural to Genetic Substrates of Panic Disorder: Insights from Human and Mouse Studies. Eur. J. Pharmacol. 2015, 759, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, J.; Guo, L.; Yue, R.; Li, S.; Cui, B.; Guo, S.; Niu, Q.; Yu, Y.; Wang, H.; et al. Amorphous Selenium Inhibits Oxidative Stress Injury of Neurons in Vascular Dementia Rats by Activating NMDAR Pathway. Eur. J. Pharmacol. 2023, 955, 175874. [Google Scholar] [CrossRef] [PubMed]
- Urwyler, S.; Campbell, E.; Fricker, G.; Jenner, P.; Lemaire, M.; McAllister, K.H.; Neijt, H.C.; Park, C.K.; Perkins, M.; Rudin, M.; et al. Biphenyl-Derivatives of 2-Amino-7-Phosphono-Heptanoic Acid, a Novel Class of Potent Competitive N-Methyl-D-Aspartate Receptor Antagonists—II. Pharmacological Characterization in Vivo. Neuropharmacology 1996, 35, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Urwyler, S. Biphenyl-Derivatives of 2-Amino-7-Phosphono-Heptanoic Acid, a Novel Class of Potent Competitive N-Methyl-?-Aspartate Receptor Antagonists—I. Pharmacological Characterization in Vitro. Neuropharmacology 1996, 35, 643–654. [Google Scholar] [CrossRef]
- Olsson, S.K.; Linderholm, K.; Erhardt, S.; Engberg, G. P.1.c.039 Increased Midbrain Dopaminergic Firing by the Competitive N-Methyl-D-Aspartate Receptor Antagonist SDZ 220–581. Eur. Neuropsychopharmacol. 2007, 17, S264–S265. [Google Scholar] [CrossRef]
- Bender, C.; de Olmos, S.; Bueno, A.; de Olmos, J.; Lorenzo, A. Comparative Analyses of the Neurodegeneration Induced by the Non-Competitive NMDA-Receptor-Antagonist Drug MK801 in Mice and Rats. Neurotoxicol. Teratol. 2010, 32, 542–550. [Google Scholar] [CrossRef]
- Mellone, M.; Gardoni, F. Modulation of NMDA Receptor at the Synapse: Promising Therapeutic Interventions in Disorders of the Nervous System. Eur. J. Pharmacol. 2013, 719, 75–83. [Google Scholar] [CrossRef]
- Ghasemi, M.; Phillips, C.; Fahimi, A.; McNerney, M.W.; Salehi, A. Mechanisms of Action and Clinical Efficacy of NMDA Receptor Modulators in Mood Disorders. Neurosci. Biobehav. Rev. 2017, 80, 555–572. [Google Scholar] [CrossRef]
- Czarnecka, K.; Chuchmacz, J.; Wójtowicz, P.; Szymański, P. Memantine in Neurological Disorders—Schizophrenia and Depression. J. Mol. Med. 2021, 99, 327–334. [Google Scholar] [CrossRef]
- Hedegaard, M.; Hansen, K.B.; Andersen, K.T.; Bräuner-Osborne, H.; Traynelis, S.F. Molecular Pharmacology of Human NMDA Receptors. Neurochem. Int. 2012, 61, 601–609. [Google Scholar] [CrossRef]
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the Treatment of Parkinson’s Disease and Other Movement Disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef]
- Kolesnikova, T.O.; Khatsko, S.L.; Shevyrin, V.A.; Morzherin, Y.Y.; Kalueff, A.V. Effects of a Non-Competitive N-Methyl-d-Aspartate (NMDA) Antagonist, Tiletamine, in Adult Zebrafish. Neurotoxicol. Teratol. 2017, 59, 62–67. [Google Scholar] [CrossRef]
- Cadinu, D.; Grayson, B.; Podda, G.; Harte, M.K.; Doostdar, N.; Neill, J.C. NMDA Receptor Antagonist Rodent Models for Cognition in Schizophrenia and Identification of Novel Drug Treatments, an Update. Neuropharmacology 2018, 142, 41–62. [Google Scholar] [CrossRef]
- Kalmoe, M.C.; Janski, A.M.; Zorumski, C.F.; Nagele, P.; Palanca, B.J.; Conway, C.R. Ketamine and Nitrous Oxide: The Evolution of NMDA Receptor Antagonists as Antidepressant Agents. J. Neurol. Sci. 2020, 412, 116778. [Google Scholar] [CrossRef]
- Kolcheva, M.; Ladislav, M.; Netolicky, J.; Kortus, S.; Rehakova, K.; Krausova, B.H.; Hemelikova, K.; Misiachna, A.; Kadkova, A.; Klima, M.; et al. The Pathogenic N650K Variant in the GluN1 Subunit Regulates the Trafficking, Conductance, and Pharmacological Properties of NMDA Receptors. Neuropharmacology 2023, 222, 109297. [Google Scholar] [CrossRef]
- Kew, J.N.C.; Trube, G.; Kemp, J.A. State-dependent NMDA Receptor Antagonism by Ro 8-4304, a Novel NR2B Selective, Non-competitive, Voltage-independent Antagonist. Br. J. Pharmacol. 1998, 123, 463–472. [Google Scholar] [CrossRef]
- Volgraf, M.; Sellers, B.D.; Jiang, Y.; Wu, G.; Ly, C.Q.; Villemure, E.; Pastor, R.M.; Yuen, P.W.; Lu, A.; Luo, X.; et al. Discovery of GluN2A-Selective NMDA Receptor Positive Allosteric Modulators (PAMs): Tuning Deactivation Kinetics via Structure-Based Design. J. Med. Chem. 2016, 59, 2760–2779. [Google Scholar] [CrossRef]
- Jimenez, E.C. Peptide Antagonists of NMDA Receptors: Structure-Activity Relationships for Potential Therapeutics. Peptides 2022, 153, 170796. [Google Scholar] [CrossRef]
- Löschmann, P.A.; De Groote, C.; Smith, L.; Wüllner, U.; Fischer, G.; Kemp, J.A.; Jenner, P.; Klockgether, T. Antiparkinsonian Activity of Ro 25-6981, a NR2B Subunit Specific NMDA Receptor Antagonist, in Animal Models of Parkinson’s Disease. Exp. Neurol. 2004, 187, 86–93. [Google Scholar] [CrossRef]
- Stan, T.L.; Alvarsson, A.; Branzell, N.; Sousa, V.C.; Svenningsson, P. NMDA Receptor Antagonists Ketamine and Ro25-6981 Inhibit Evoked Release of Glutamate in Vivo in the Subiculum. Transl. Psychiatry 2014, 4, e395. [Google Scholar] [CrossRef]
- Lesuis, S.L.; Lucassen, P.J.; Krugers, H.J. Early Life Stress Impairs Fear Memory and Synaptic Plasticity; a Potential Role for GluN2B. Neuropharmacology 2019, 149, 195–203. [Google Scholar] [CrossRef]
- Gao, X.; Chen, F.; Xu, X.; Liu, J.; Dong, F.; Liu, Y. Ro25-6981 Alleviates Neuronal Damage and Improves Cognitive Deficits by Attenuating Oxidative Stress via the Nrf2/ARE Pathway in Ischemia/Reperfusion Rats. J. Stroke Cerebrovasc. Dis. 2023, 32, 106971. [Google Scholar] [CrossRef]
- Acker, T.M.; Yuan, H.; Hansen, K.B.; Vance, K.M.; Ogden, K.K.; Jensen, H.S.; Burger, P.B.; Mullasseril, P.; Snyder, J.P.; Liotta, D.C.; et al. Mechanism for Noncompetitive Inhibition by Novel GluN2C/D N-Methyl-D-Aspartate Receptor Subunit-Selective Modulators. Mol. Pharmacol. 2011, 80, 782–795. [Google Scholar] [CrossRef]
- Wyllie, D.J.A.; Livesey, M.R.; Hardingham, G.E. Influence of GluN2 Subunit Identity on NMDA Receptor Function. Neuropharmacology 2013, 74, 4–17. [Google Scholar] [CrossRef]
- Pearlstein, E.; Gouty-Colomer, L.A.; Michel, F.J.; Cloarec, R.; Hammond, C. Glutamatergic Synaptic Currents of Nigral Dopaminergic Neurons Follow a Postnatal Developmental Sequence. Front. Cell. Neurosci. 2015, 9, 210. [Google Scholar] [CrossRef]
- Morris, P.G.; Mishina, M.; Jones, S. Altered Synaptic and Extrasynaptic NMDA Receptor Properties in Substantia Nigra Dopaminergic Neurons from Mice Lacking the GluN2D Subunit. Front. Cell. Neurosci. 2018, 12, 354. [Google Scholar] [CrossRef]
- Pálfi, E.; Lévay, G.; Czurkó, A.; Lendvai, B.; Kiss, T. Acute Blockade of NR2C/D Subunit-Containing N-Methyl-D-Aspartate Receptors Modifies Sleep and Neural Oscillations in Mice. J. Sleep Res. 2021, 30, e13257. [Google Scholar] [CrossRef]
- Chizh, B.A.; Headley, P.M.; Tzschentke, T.M. NMDA Receptor Antagonists as Analgesics: Focus on the NR2B Subtype. Trends Pharmacol. Sci. 2001, 22, 636–642. [Google Scholar] [CrossRef]
- He, H.; Yao, J.; Zhang, Y.; Chen, Y.; Wang, K.; Lee, R.J.; Yu, B.; Zhang, X. Solid Lipid Nanoparticles as a Drug Delivery System to across the Blood-Brain Barrier. Biochem. Biophys. Res. Commun. 2019, 519, 385–390. [Google Scholar] [CrossRef]
- Inês Teixeira, M.; Lopes, C.M.; Gonçalves, H.; Catita, J.; Margarida Silva, A.; Rodrigues, F.; Helena Amaral, M.; Costa, P.C. Riluzole-Loaded Lipid Nanoparticles for Brain Delivery: Preparation, Optimization and Characterization. J. Mol. Liq. 2023, 388, 122749. [Google Scholar] [CrossRef]
- Ortega Martínez, E.; Morales Hernández, M.E.; Castillo-González, J.; González-Rey, E.; Ruiz Martínez, M.A. Dopamine-Loaded Chitosan-Coated Solid Lipid Nanoparticles as a Promise Nanocarriers to the CNS. Neuropharmacology 2024, 249, 109871. [Google Scholar] [CrossRef]
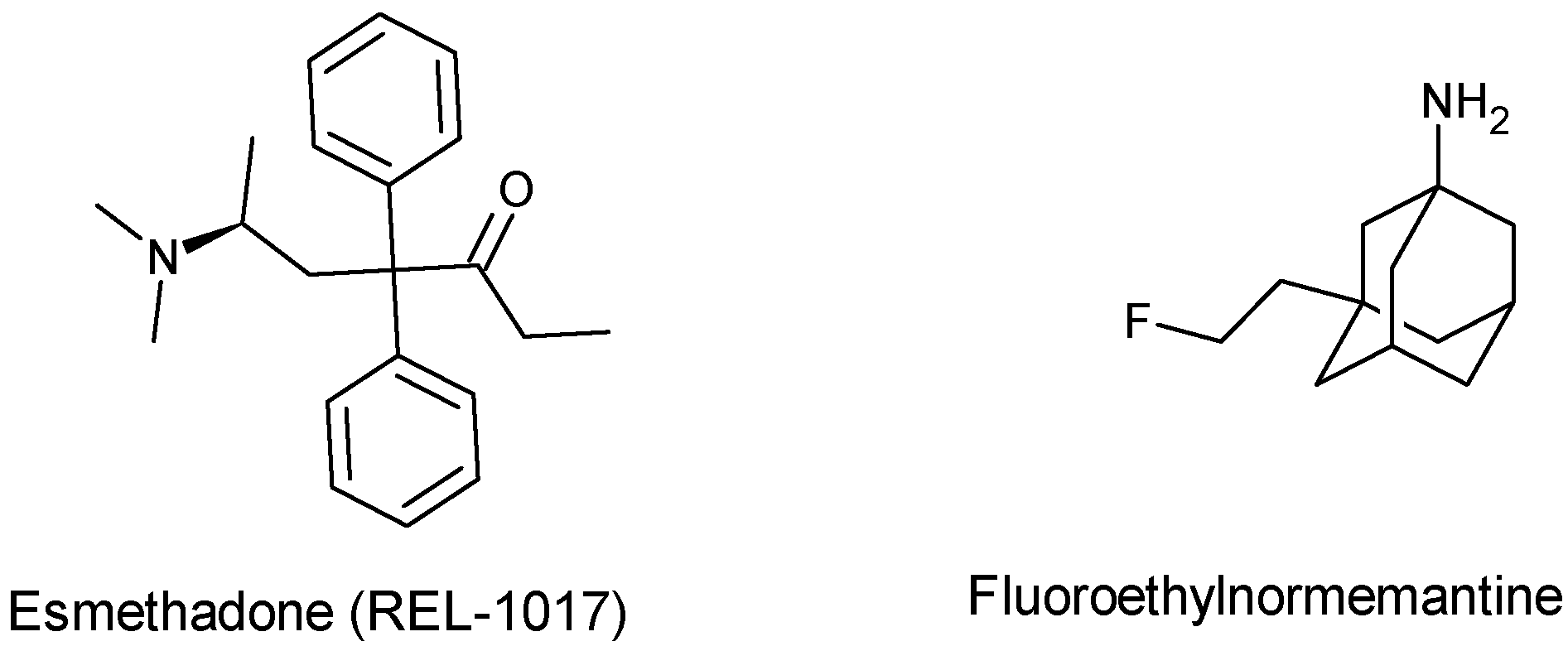
- Chen, B.K.; Luna, V.M.; Shannon, M.E.; Hunsberger, H.C.; Mastrodonato, A.; Stackmann, M.; McGowan, J.C.; Rubinstenn, G.; Denny, C.A. Fluoroethylnormemantine, a Novel NMDA Receptor Antagonist, for the Prevention and Treatment of Stress-Induced Maladaptive Behavior. Biol. Psychiatry 2021, 90, 458–472. [Google Scholar] [CrossRef]
- Salabert, A.S.; Fonta, C.; Fontan, C.; Adel, D.; Alonso, M.; Pestourie, C.; Belhadj-Tahar, H.; Tafani, M.; Payoux, P. Radiolabeling of [18F]-Fluoroethylnormemantine and Initial in Vivo Evaluation of This Innovative PET Tracer for Imaging the PCP Sites of NMDA Receptors. Nucl. Med. Biol. 2015, 42, 643–653. [Google Scholar] [CrossRef]
- ReST Therapeutics. First-In-Human (FIH), Single Ascending Dose (SAD) Study of FluoroEthylNorMemantine (FENM). ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05921929?tab=table (accessed on 8 April 2024).
- Shram, M.J.; Henningfield, J.E.; Apseloff, G.; Gorodetzky, C.W.; De Martin, S.; Vocci, F.L.; Sapienza, F.L.; Kosten, T.R.; Huston, J.; Buchhalter, A.; et al. The Novel Uncompetitive NMDA Receptor Antagonist Esmethadone (REL-1017) Has No Meaningful Abuse Potential in Recreational Drug Users. Transl. Psychiatry 2023, 13, 192. [Google Scholar] [CrossRef]
- Egunlusi, A.O.; Malan, S.F.; Palchykov, V.A.; Joubert, J. Calcium Modulating Effect of Polycyclic Cages: A Suitable Therapeutic Approach Against Excitotoxic-Induced Neurodegeneration. Mini-Rev. Med. Chem. 2024, 24, 1277–1292. [Google Scholar] [CrossRef]
- Gutti, G.; Leifeld, J.; Kakarla, R.; Bajad, N.G.; Ganeshpurkar, A.; Kumar, A.; Krishnamurthy, S.; Klein-Schmidt, C.; Tapken, D.; Hollmann, M.; et al. Discovery of Triazole-Bridged Aryl Adamantane Analogs as an Intriguing Class of Multifunctional Agents for Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2023, 259, 115670. [Google Scholar] [CrossRef]
- Zindo, F.T.; Barber, Q.R.; Joubert, J.; Bergh, J.J.; Petzer, J.P.; Malan, S.F. Polycyclic Propargylamine and Acetylene Derivatives as Multifunctional Neuroprotective Agents. Eur. J. Med. Chem. 2014, 80, 122–134. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent Advance on Carbamate-Based Cholinesterase Inhibitors as Potential Multifunctional Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef]
- Knez, D.; Diez-Iriepa, D.; Chioua, M.; Gottinger, A.; Denic, M.; Chantegreil, F.; Nachon, F.; Brazzolotto, X.; Skrzypczak-Wiercioch, A.; Meden, A.; et al. 8-Hydroxyquinolylnitrones as Multifunctional Ligands for the Therapy of Neurodegenerative Diseases. Acta Pharm. Sin. B 2023, 13, 2152–2175. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Liu, R.; Huang, Y.; Zhang, N.; Zhang, R. Memantine, Donepezil, or Combination Therapy—What Is the Best Therapy for Alzheimer’s Disease? A Network Meta-Analysis. Brain Behav. 2020, 10, e01831. [Google Scholar] [CrossRef]
- Iosifescu, D.V.; Jones, A.; O’Gorman, C.; Streicher, C.; Feliz, S.; Fava, M.; Tabuteau, H. Efficacy and Safety of AXS-05 (Dextromethorphan-Bupropion) in Patients With Major Depressive Disorder. J. Clin. Psychiatry 2022, 83, 22–27. [Google Scholar] [CrossRef]
- Akbar, D.; Rhee, T.G.; Ceban, F.; Ho, R.; Teopiz, K.M.; Cao, B.; Subramaniapillai, M.; Kwan, A.T.H.; Rosenblat, J.D.; McIntyre, R.S. Dextromethorphan-Bupropion for the Treatment of Depression: A Systematic Review of Efficacy and Safety in Clinical Trials. CNS Drugs 2023, 37, 867–881. [Google Scholar] [CrossRef]
- Yu, G.; Shi, Y.; Cong, S.; Wu, C.; Liu, J.; Zhang, Y.; Liu, H.; Liu, X.; Deng, H.; Tan, Z.; et al. Synthesis and Evaluation of Butylphthalide-Scutellarein Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2024, 265, 116099. [Google Scholar] [CrossRef]
- McKay, S.; Ryan, T.J.; McQueen, J.; Indersmitten, T.; Marwick, K.F.M.; Hasel, P.; Kopanitsa, M.V.; Baxter, P.S.; Martel, M.A.; Kind, P.C.; et al. The Developmental Shift of NMDA Receptor Composition Proceeds Independently of GluN2 Subunit-Specific GluN2 C-Terminal Sequences. Cell Rep. 2018, 25, 841–851.e4. [Google Scholar] [CrossRef]
- Sun, W.; Hansen, K.B.; Jahr, C.E. Allosteric Interactions between NMDA Receptor Subunits Shape the Developmental Shift in Channel Properties. Neuron 2017, 94, 58–64.e3. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egunlusi, A.O.; Joubert, J. NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders. Pharmaceuticals 2024, 17, 639. https://doi.org/10.3390/ph17050639
Egunlusi AO, Joubert J. NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders. Pharmaceuticals. 2024; 17(5):639. https://doi.org/10.3390/ph17050639
Chicago/Turabian StyleEgunlusi, Ayodeji Olatunde, and Jacques Joubert. 2024. "NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders" Pharmaceuticals 17, no. 5: 639. https://doi.org/10.3390/ph17050639
APA StyleEgunlusi, A. O., & Joubert, J. (2024). NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders. Pharmaceuticals, 17(5), 639. https://doi.org/10.3390/ph17050639






