Blood–Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience
Abstract
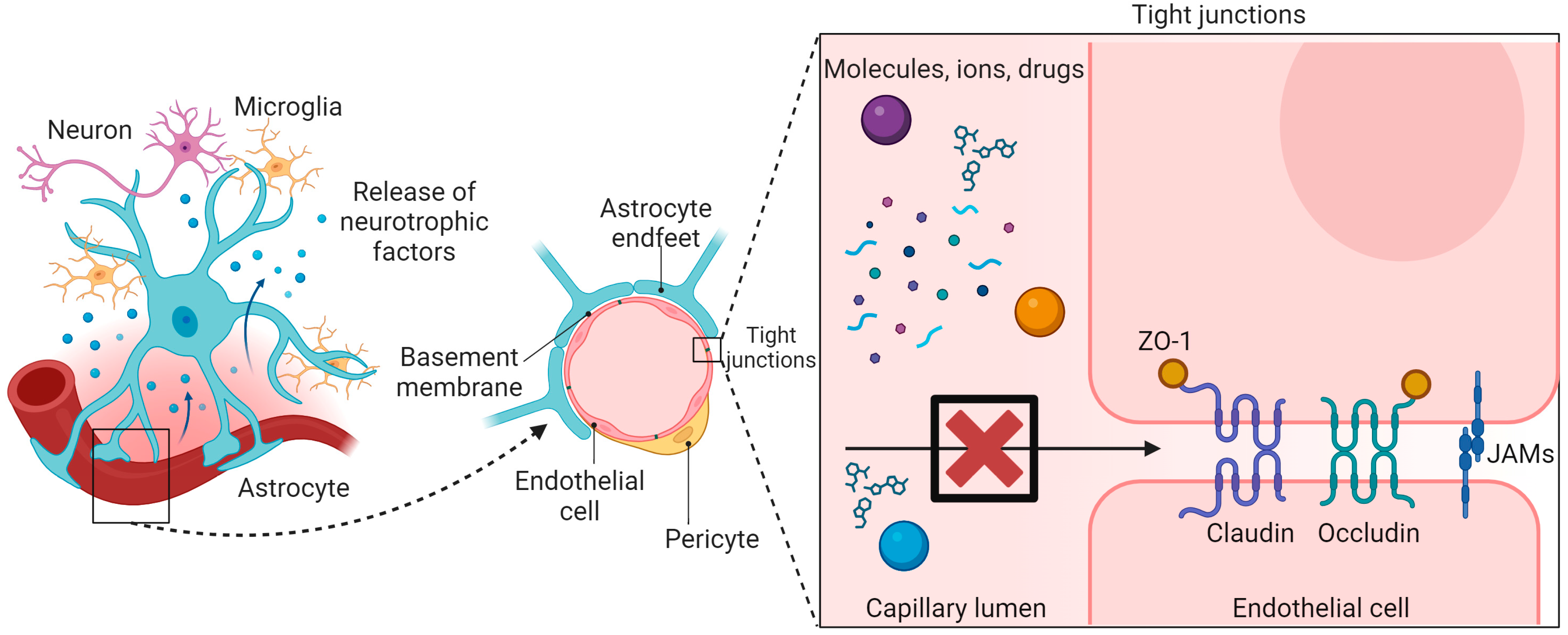
1. Introduction
2. Factors for Optimization of Nanoparticles to Enhance BBB Penetration
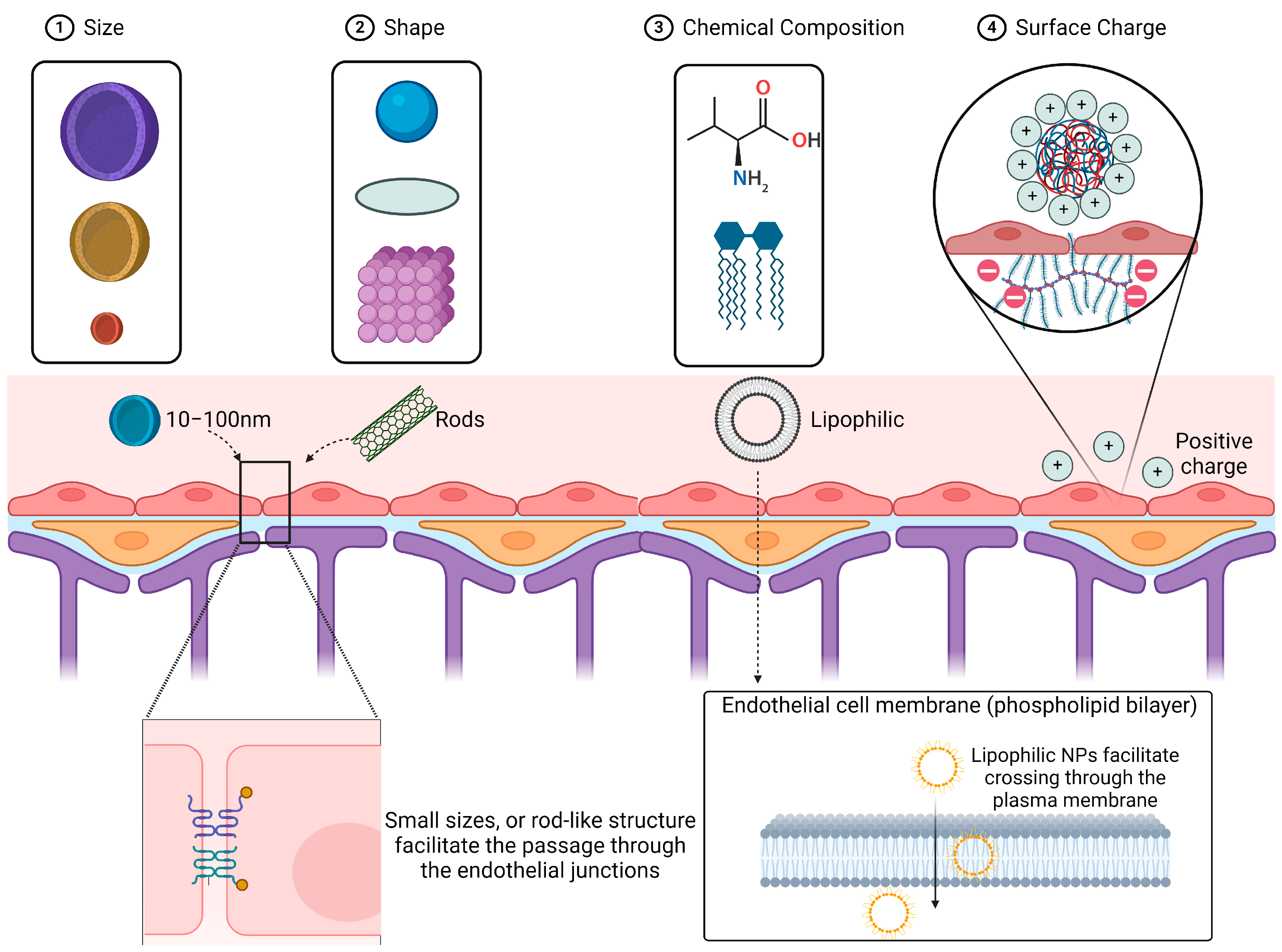
2.1. Size and Shape
2.2. Chemical Composition (Lipophilicity, Biodegradability)
2.3. Surface Charge
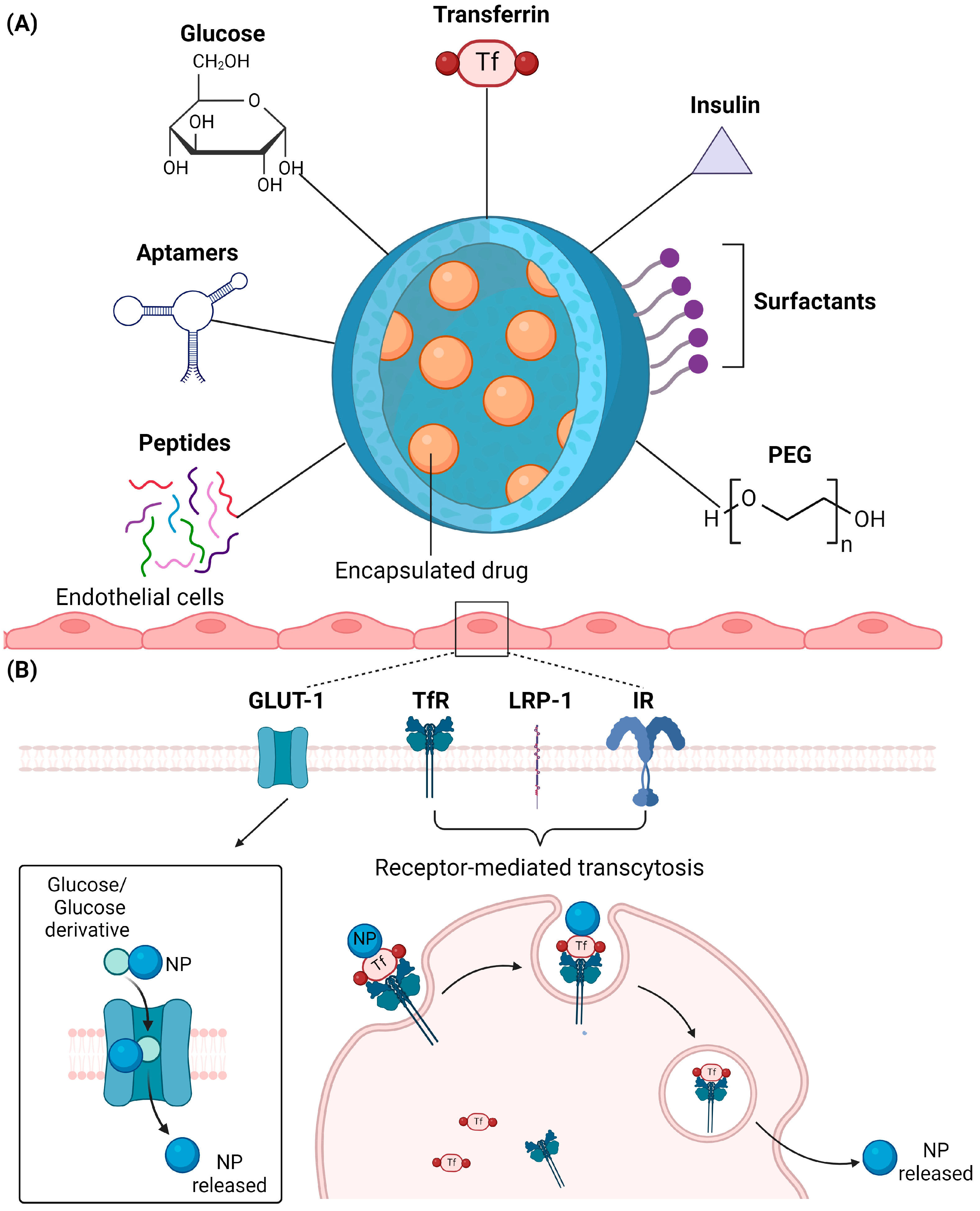
2.4. Surface Modification
2.4.1. Glucose
2.4.2. Transferrin
2.4.3. Insulin
2.4.4. Polyethylene Glycol (PEG)
2.4.5. Peptides
2.4.6. Aptamers
2.4.7. Surfactants
3. In Vitro and In Vivo Models of BBB and Ways to Detect BBB Penetration
3.1. In Vitro Transwell Models
3.2. In Vitro Microfluidic Models
3.3. In Vivo Models
3.4. Clinical Detection of BBB Penetration
4. Route of NP Administration and Uptake under Normal BBB Conditions
5. Effect of BBB Breakdown on NP-Mediated Drug Delivery
6. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of Nanotechnology in Drug Delivery to the Central Nervous System. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Pitt, D.; Lo, C.H.; Gauthier, S.A.; Hickman, R.A.; Longbrake, E.; Airas, L.M.; Mao-Draayer, Y.; Riley, C.; De Jager, P.L.; Wesley, S.; et al. Toward Precision Phenotyping of Multiple Sclerosis. Neurol.-Neuroimmunol. Neuroinflamm. 2022, 9, e200025. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H. Heterogeneous Tau Oligomers as Molecular Targets for for Alzheimer’s Disease and Related Tauopathies. Biophysica 2022, 2, 440–451. [Google Scholar] [CrossRef]
- Lo, C.H.; Sachs, J.N. The Role of Wild-Type Tau in Alzheimer’s Disease and Related Tauopathies. J. Life Sci. 2020, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging Therapies in Parkinson Disease—Repurposed Drugs and New Approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Demetzos, C. Promising Nanotechnology Approaches in Treatment of Autoimmune Diseases of Central Nervous System. Brain Sci. 2020, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug Transport across the Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, F.; Wang, Y. The Blood—Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood-Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood-Brain Barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Yang, Y.; Ju, W.N.; Wang, X.; Zhang, H.L. Emerging Roles of Astrocytes in Neuro-Vascular Unit and the Tripartite Synapse with Emphasis on Reactive Gliosis in the Context of Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Macvicar, B.A.; Newman, E.A. Astrocyte Regulation of Blood Flow in the Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.B.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for Delivering Therapeutics across the Blood—Brain Barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a Highway to the Brain: A Review on Nose-to-Brain Drug Delivery Using Nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Duzagac, F.; Canzonieri, V.; Rizzolio, F. Self-Therapeutic Nanomaterials for Cancer Therapy: A Review. ACS Appl. Nano Mater. 2020, 3, 4962–4971. [Google Scholar] [CrossRef]
- Han, R.; Xiao, Y.; Bai, Q.; Choi, C.H.J. Self-Therapeutic Metal-Based Nanoparticles for Treating Inflammatory Diseases. Acta Pharm. Sin. B 2023, 13, 1847–1865. [Google Scholar] [CrossRef]
- Lo, C.H.; Zeng, J. Application of Polymersomes in Membrane Protein Study and Drug Discovery: Progress, Strategies, and Perspectives. Bioeng. Transl. Med. 2023, 8, e10350. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Veroniaina, H.; Qi, X.; Chen, P.; Li, F.; Ke, P.C. Soft and Condensed Nanoparticles and Nanoformulations for Cancer Drug Delivery and Repurpose. Adv. Ther. 2020, 3, 1900102. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural Biodegradable Polymers Based Nano-Formulations for Drug Delivery: A Review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-Formulations of Drugs: Recent Developments, Impact and Challenges. Biochimie 2016, 128–129, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Langer, R.; Wechsler, M.E.; Peppas, N.A. Engineering Precision Nanoparticles. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Martin, A.; Han, X.; Shirihai, O.; Grinstaff, M. Biodegradable PLGA Nanoparticles Restore Lysosomal Acidity and Protect Neural PC-12 Cells against Mitochondrial Toxicity. Ind. Eng. Chem. Res. 2019, 58, 13910–13917. [Google Scholar] [CrossRef]
- Zeng, J.; Acin-Perez, R.; Assali, E.A.; Martin, A.; Brownstein, A.J.; Petcherski, A.; Fernández-del-Rio, L.; Xiao, R.; Lo, C.H.; Shum, M.; et al. Restoration of Lysosomal Acidification Rescues Autophagy and Metabolic Dysfunction in Non-Alcoholic Fatty Liver Disease. Nat. Commun. 2023, 14, 2573. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Li, X.; Ji, R.; Hao, Z.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases. Polymers 2023, 15, 2196. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C. Particle Size-Dependent Organ Distribution of Gold Nanoparticles after Intravenous Administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Cruz, L.J.; Stammes, M.A.; Que, I.; Van Beek, E.R.; Knol-blankevoort, V.T.; Snoeks, T.J.A.; Chan, A.; Kaijzel, E.L.; Löwik, C.W.G.M. Effect of PLGA NP Size on Ef Fi Ciency to Target Traumatic Brain Injury. J. Control. Release 2016, 223, 31–41. [Google Scholar] [CrossRef]
- Gao, K.; Jiang, X. Influence of Particle Size on Transport of Methotrexate across Blood Brain Barrier by Polysorbate 80-Coated Polybutylcyanoacrylate Nanoparticles. Int. J. Pharm. 2006, 310, 213–219. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of Nanoparticle Size, Shape and Surface Chemistry in Oral Drug Delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Nowak, M.; Brown, T.D.; Graham, A.; Helgeson, M.E.; Mitragotri, S. Size, Shape, and Flexibility Influence Nanoparticle Transport across Brain Endothelium under Flow. Bioeng. Transl. Med. 2020, 5, e10153. [Google Scholar] [CrossRef]
- Kolhar, P.; Anselmo, A.C.; Gupta, V.; Pant, K.; Prabhakarpandian, B.; Ruoslahti, E.; Mitragotri, S. Using Shape Effects to Target Antibody-Coated Nanoparticles to Lung and Brain Endothelium. Proc. Natl. Acad. Sci. USA 2013, 110, 10753–10758. [Google Scholar] [CrossRef]
- Zhu, X.; Vo, C.; Taylor, M.; Smith, B.R. Non-Spherical Micro- and Nanoparticles in Nanomedicine. Mater. Horiz. 2019, 6, 1094–1121. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood-Brain Barrier-Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Khaledian, S.; Dayani, M.; Fatahian, A.; Fatahian, R.; Martinez, F. Efficiency of Lipid-Based Nano Drug Delivery Systems in Crossing the Blood–Brain Barrier: A Review. J. Mol. Liq. 2022, 346, 118278. [Google Scholar] [CrossRef]
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Lipid Nanoparticles Strategies to Modify Pharmacokinetics of Central Nervous System Targeting Drugs: Crossing or Circumventing the Blood–Brain Barrier (BBB) to Manage Neurological Disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Akhtar, B. Biodegradable Nanoparticles as Drug Delivery Devices. J. Drug Deliv. Sci. Technol. 2021, 64, 102638. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable Nanoparticles Are Excellent Vehicle for Site Directed In-Vivo Delivery of Drugs and Vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Kempe, K.; Nicolazzo, J.A. Biodegradable Polymeric Nanoparticles for Brain-Targeted Drug Delivery BT—Nanomedicines for Brain Drug Delivery; Morales, J.O., Gaillard, P.J., Eds.; Springer: New York, NY, USA, 2021; pp. 1–27. ISBN 978-1-0716-0838-8. [Google Scholar]
- Wilson, B.; Mohamed Alobaid, B.N.; Geetha, K.M.; Jenita, J.L. Chitosan Nanoparticles to Enhance Nasal Absorption and Brain Targeting of Sitagliptin to Treat Alzheimer’s Disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102176. [Google Scholar] [CrossRef]
- Cunha, A.; Gaubert, A.; Latxague, L.; Dehay, B. PLGA-Based Nanoparticles for Neuroprotective Drug Delivery in Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1042. [Google Scholar] [CrossRef]
- Gao, S.; Xu, Y.; Asghar, S.; Chen, M.; Zou, L.; Eltayeb, S.; Huo, M.; Ping, Q.; Xiao, Y. Polybutylcyanoacrylate Nanocarriers as Promising Targeted Drug Delivery Systems. J. Drug Target. 2015, 23, 481–496. [Google Scholar] [CrossRef]
- Nanoparticles, C.; Yue, Z.; Wei, W.; Lv, P.; Yue, H.; Wang, L.; Su, Z.; Ma, G. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar]
- Ribeiro, M.M.B.; Domingues, M.M.; Freire, J.M.; Santos, N.C.; Castanho, M.A.R.B. Translocating the Blood-Brain Barrier Using Electrostatics. Front. Cell. Neurosci. 2012, 6, 44. [Google Scholar] [CrossRef]
- Ribeiro, M.M.B.; Pinto, R.T.; Domingues, M.M.; Serrano, I.; Bardaji, E.R.; Tavares, I.; Castanho, M.A. Chemical Conjugation of the Neuropeptide Kyotorphin and Ibuprofen Enhances Brain Targeting and Analgesia. Mol. Pharm. 2011, 8, 1929–1940. [Google Scholar] [CrossRef]
- Kim, B.; Han, G.; Toley, B.J.; Kim, C.; Rotello, V.M.; Forbes, N.S. Tuning Payload Delivery in Tumour Cylindroids Using Gold Nanoparticles. Nat. Nanotechnol. 2010, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D.; Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle Surface Charges Alter Blood—Brain Barrier Integrity and Permeability Nanoparticle Surface Charges Alter Blood—Brain Barrier Integrity and Permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef]
- Wu, J.R.; Hernandez, Y.; Miyasaki, K.F.; Kwon, E.J. Engineered Nanomaterials That Exploit Blood-Brain Barrier Dysfunction for Delivery to the Brain. Adv. Drug Deliv. Rev. 2023, 197, 114820. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R.R. The Effect of Nanoparticle Size on the Ability to Cross the Blood-Brain Barrier: An in Vivo Study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Kang, Y.-S.; Buciak, J.L.; Yang, J. Human Insulin Receptor Monoclonal Antibody Undergoes High Affinity Binding to Human Brain Capillaries in Vitro and Rapid Transcytosis Through the Blood–Brain Barrier in Vivo in the Primate. Pharm. Res. 1995, 12, 807–816. [Google Scholar] [CrossRef]
- Joseph, A.; Simo, G.M.; Gao, T.; Alhindi, N.; Xu, N.; Graham, D.J.; Gamble, L.J.; Nance, E. Surfactants Influence Polymer Nanoparticle Fate within the Brain. Biomaterials 2021, 277, 121086. [Google Scholar] [CrossRef]
- Deng, H.; Dutta, P.; Liu, J. Stochastic Modeling of Nanoparticle Internalization and Expulsion through Receptor-Mediated Transcytosis. Nanoscale 2019, 11, 11227–11235. [Google Scholar] [CrossRef]
- Nance, E.A.; Woodworth, G.F.; Sailor, K.A.; Shih, T.; Swaminathan, G.; Xiang, D.; Eberhart, C. A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles within Brain Tissue. Sci. Transl. Med. 2012, 4, 149ra119. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Boado, R.J.; Farrell, C.R. Brain-Type Glucose Transporter the Blood-Brain Barrier (GLUT-1) Is Selectively Localized to the Blood-Brain Barrier. Studies with Quantitative Western Blotting and In Situ Hybridization. J. Biol. Chem. 1990, 265, 18035–18040. [Google Scholar] [CrossRef]
- Gromnicova, R.; Davies, H.A.; Sreekanthreddy, P.; Romero, I.A.; Lund, T.; Roitt, I.M.; Phillips, J.B.; Male, D.K. Glucose-Coated Gold Nanoparticles Transfer across Human Brain Endothelium and Enter Astrocytes In Vitro. PLoS ONE 2013, 8, e81043. [Google Scholar] [CrossRef]
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Biomaterials Nanoparticles of 2-Deoxy-D-Glucose Functionalized Poly (Ethylene Glycol)-Co-Poly (Trimethylene Carbonate) for Dual-Targeted Drug Delivery in Glioma Treatment. Biomaterials 2014, 35, 518–529. [Google Scholar] [CrossRef]
- Wu, H.; Lu, H.; Xiao, W.; Yang, J.; Du, H.; Shen, Y.; Qu, H.; Jia, B.; Manna, S.K.; Ramachandran, M.; et al. Sequential Targeting in Crosslinking Nanotheranostics for Tackling the Multibarriers of Brain Tumors. Adv. Mater. 2020, 31, e1903759. [Google Scholar] [CrossRef]
- Kyrtata, N.; Emsley, H.C.A.; Sparasci, O.; Parkes, L.M.; Dickie, B.R. A Systematic Review of Glucose Transport Alterations in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 626636. [Google Scholar] [CrossRef]
- Talasila, K.M.; Røsland, G.V.; Hagland, H.R.; Eskilsson, E.; Flønes, I.H.; Fritah, S.; Azuaje, F.; Atai, N.; Harter, P.N.; Mittelbronn, M.; et al. The Angiogenic Switch Leads to a Metabolic Shift in Human Glioblastoma. Neuro. Oncol. 2017, 19, 383–393. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L. Progress in Neurobiology Targeting the Transferrin Receptor for Brain Drug Delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Mishra, V.; Mahor, S.; Rawat, A.; Gupta, P.N.; Dubey, P.; Khatri, K.; Vyas, S.P. Targeted Brain Delivery of AZT via Transferrin Anchored Pegylated Albumin Nanoparticles. J. Drug Target. 2006, 14, 45–53. [Google Scholar] [CrossRef]
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and Brain Uptake of Transferrin-Containing Nanoparticles by Tuning Avidity to Transferrin Receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef]
- Clark, A.J.; Davis, M.E. Increased Brain Uptake of Targeted Nanoparticles by Adding an Acid-Cleavable Linkage between Transferrin and the Nanoparticle Core. Proc. Natl. Acad. Sci. USA 2015, 112, 12486–12491. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, Y.; Cai, R.; Wang, W.; Cui, H.; Liu, M.; Zhang, B.; Mei, Q. The Enhancement of SiPLK1 Penetration across BBB and Its Anti Glioblastoma Activity in Vivo by Magnet and Transferrin Co-Modified Nanoparticle. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 991–1003. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the Insulin Receptor: Nanoparticles for Drug Delivery across the Blood-Brain Barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Ko, H.-F. Targeting Delivery of Saquinavir to the Brain Using 83-14 Monoclonal Antibody-Grafted Solid Lipid Nanoparticles. Biomaterials 2013, 34, 4818–4830. [Google Scholar] [CrossRef]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of Nanoparticles through the Blood–Brain Barrier for Imaging and Therapeutic Applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Pawar, A.; Mahadik, K.R.; Gajbhiye, V. PEGylated Nanocarriers: A Promising Tool for Targeted Delivery to the Brain. Colloids Surf. B Biointerfaces 2020, 187, 110770. [Google Scholar] [CrossRef]
- Huang, J.Y.; Lu, Y.M.; Wang, H.; Liu, J.; Liao, M.H.; Hong, L.J.; Tao, R.R.; Ahmed, M.M.; Liu, P.; Liu, S.S.; et al. The Effect of Lipid Nanoparticle PEGylation on Neuroinflammatory Response in Mouse Brain. Biomaterials 2013, 34, 7960–7970. [Google Scholar] [CrossRef]
- Parrasia, S.; Szabo, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
- Temming, K.; Schiffelers, R.M.; Molema, G.; Kok, R.J. RGD-Based Strategies for Selective Delivery of Therapeutics and Imaging Agents to the Tumour Vasculature. Drug Resist. Updat. 2005, 8, 381–402. [Google Scholar] [CrossRef]
- Lou, B.; Connor, K.; Sweeney, K.; Miller, I.S.; Farrell, A.O.; Ruiz-hernandez, E.; Murray, D.M.; Duffy, G.P.; Wolfe, A.; Mastrobattista, E.; et al. RGD-Decorated Cholesterol Stabilized Polyplexes for Targeted SiRNA Delivery to Glioblastoma Cells. Drug Deliv. Transl. Res. 2019, 9, 679–693. [Google Scholar] [CrossRef]
- Ruan, H.; Chen, X.; Xie, C.; Li, B.; Ying, M.; Liu, Y.; Zhang, M.; Zhang, X.; Zhan, C.; Lu, W.; et al. Stapled RGD Peptide Enables Glioma-Targeted Drug Delivery by Overcoming Multiple Barriers. ACS Appl. Mater. Interfaces 2017, 9, 17745–17756. [Google Scholar] [CrossRef] [PubMed]
- Di Polidoro, A.C.; Zambito, G.; Haeck, J.; Mezzanotte, L.; Lamfers, M.; Netti, P.A.; Torino, E. Theranostic Design of Angiopep-2 Conjugated Hyaluronic Acid Nanoparticles (Thera-ANG-cHANPs) for Dual Targeting and Boosted Imaging of Glioma Cells. Cancers 2021, 13, 503. [Google Scholar] [CrossRef]
- Khan, N.U.; Ni, J.; Ju, X.; Miao, T.; Chen, H.; Han, L. Escape from Abluminal LRP1-Mediated Clearance for Boosted Nanoparticle Brain Delivery and Brain Metastasis Treatment. Acta Pharm. Sin. B 2021, 11, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Brinkhuis, R.P.; Stojanov, K.; Weijers, C.A.G.M.; Zuilhof, H.; Rutjes, F.P.J.T.; Hoekstra, D.; Van Hest, J.C.M.; Zuhorn, I.S. Peptide-Mediated Blood—Brain Barrier Transport of Polymersomes. Angew. Chem. Int. Ed. Engl. 2012, 51, 8339–8342. [Google Scholar] [CrossRef]
- Zhang, H.; Van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Biomaterials Science Nanoparticles for Transport across the Blood—Brain. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zheng, X.; Guo, Q.; Yang, P.; Pang, X.; Qian, K.; Lu, W. Systemic Delivery of BACE1 SiRNA through Neuron-Targeted Nanocomplexes for Treatment of Alzheimer’s Disease. J. Control. Release 2018, 279, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Rotman, M.; Welling, M.M.; Bunschoten, A.; De Backer, M.E.; Rip, J.; Nabuurs, R.J.A.; Gaillard, P.J.; Van Buchem, M.A.; Van Der Maarel, S.M.; Van Der Weerd, L. Enhanced Glutathione PEGylated Liposomal Brain Delivery of an Anti-Amyloid Single Domain Antibody Fragment in a Mouse Model for Alzheimer’s Disease. J. Control. Release 2015, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- McCully, M.; Sanchez-Navarro, M.; Teixido, M.; Giralt, E. Peptide Mediated Brain Delivery of Nano- and Submicroparticles: A Synergistic Approach. Curr. Pharm. Des. 2018, 24, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Seo, M.; Kim, K.; Kim, A.R.; Lee, H.; Kim, H.S.; Park, C.G.; Cho, S.W.; Kang, J.H.; Joo, J.; et al. Blood−Brain Barrier Discovered via Aptamer Nanoconstructs Crossing Human Technology Microphysiological System-Based SELEX. ACS Nano 2023, 17, 8153–8166. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S. Aptamer-Conjugated Gold Nanoparticles Targeting Epidermal Growth Factor Receptor Variant III for the Treatment of Glioblastoma Aptamer-Conjugated Gold Nanoparticles Targeting Epidermal Growth Factor Receptor Variant III for the Treatment of Glioblastoma. Int. J. Nanomed. 2020, 15, 1363–1372. [Google Scholar] [CrossRef]
- Monaco, I.; Camorani, S.; Colecchia, D.; Locatelli, E.; Calandro, P.; Oudin, A.; Niclou, S.; Arra, C.; Chiariello, M.; Cerchia, L.; et al. Aptamer Functionalization of Nanosystems for Glioblastoma Targeting through the Blood—Brain Barrier. J. Med. Chem. 2017, 60, 4510–4516. [Google Scholar] [CrossRef]
- Murakami, K.; Izuo, N.; Bitan, G. Aptamers Targeting Amyloidogenic Proteins and Their Emerging Role in Neurodegenerative Diseases. J. Biol. Chem. 2022, 298, 101478. [Google Scholar] [CrossRef]
- Madani, F.; Esnaashari, S.S.; Bergonzi, M.C.; Webster, T.J.; Younes, H.M.; Khosravani, M.; Adabi, M. Paclitaxel/Methotrexate Co-Loaded PLGA Nanoparticles in Glioblastoma Treatment: Formulation Development and in Vitro Antitumor Activity Evaluation. Life Sci. 2020, 256, 117943. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Nowak, M.; Bayles, A.V.; Prabhakarpandian, B.; Karande, P.; Lahann, J.; Helgeson, M.E.; Mitragotri, S. A Microfluidic Model of Human Brain (ΜHuB) for Assessment of Blood Brain Barrier. Bioeng. Transl. Med. 2019, 4, e10126. [Google Scholar] [CrossRef] [PubMed]
- Shawahna, R.; Decleves, X.; Scherrmann, J.-M. Hurdles with Using in Vitro Models to Predict Human Blood-Brain Barrier Drug Permeability: A Special Focus on Transporters and Metabolizing Enzymes. Curr. Drug Metab. 2013, 14, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Katt, M.E.; Shusta, E.V. In Vitro Models of the Blood-Brain Barrier: Building in Physiological Complexity. Curr. Opin. Chem. Eng. 2020, 30, 42–52. [Google Scholar] [CrossRef]
- Jagtiani, E.; Yeolekar, M.; Naik, S.; Patravale, V. In Vitro Blood Brain Barrier Models: An Overview. J. Control. Release 2022, 343, 13–30. [Google Scholar] [CrossRef]
- Pérez-López, A.; Torres-Suárez, A.I.; Martín-Sabroso, C.; Aparicio-Blanco, J. An Overview of in Vitro 3D Models of the Blood-Brain Barrier as a Tool to Predict the in Vivo Permeability of Nanomedicines. Adv. Drug Deliv. Rev. 2023, 196, 114816. [Google Scholar] [CrossRef] [PubMed]
- Petrovskaya, A.V.; Barykin, E.P.; Tverskoi, A.M.; Varshavskaya, K.B.; Mitkevich, V.A.; Petrushanko, I.Y.; Makarov, A.A. Blood–Brain Barrier Transwell Modeling. Mol. Biol. 2022, 56, 1020–1027. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. Capture, Crawl, Cross: The T Cell Code to Breach the Blood-Brain Barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef]
- Ding, H.; Sagar, V.; Agudelo, M.; Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Raymond, A.; Samikkannu, T.; Nair, M.P. Enhanced Blood-Brain Barrier Transmigration Using a Novel Transferrin Embedded Fluorescent Magneto-Liposome Nanoformulation. Nanotechnology 2014, 25, 55101. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. In-Vitro Blood-Brain Barrier Modeling: A Review of Modern and Fast-Advancing Technologies. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef]
- Ye, D.; Raghnaill, M.N.; Bramini, M.; Mahon, E.; Åberg, C.; Salvati, A.; Dawson, K.A. Nanoparticle Accumulation and Transcytosis in Brain Endothelial Cell Layers. Nanoscale 2013, 5, 11153–11165. [Google Scholar] [CrossRef]
- De Jong, E.; Williams, D.S.; Abdelmohsen, L.K.E.A.; Van Hest, J.C.M.; Zuhorn, I.S. A Filter-Free Blood-Brain Barrier Model to Quantitatively Study Transendothelial Delivery of Nanoparticles by Fluorescence Spectroscopy. J. Control. Release 2018, 289, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent Progress in Translational Engineered in Vitro Models of the Central Nervous System. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef]
- Linville, R.M.; Sklar, M.B.; Grifno, G.N.; Nerenberg, R.F.; Zhou, J.; Ye, R.; DeStefano, J.G.; Guo, Z.; Jha, R.; Jamieson, J.J.; et al. Three-Dimensional Microenvironment Regulates Gene Expression, Function, and Tight Junction Dynamics of IPSC-Derived Blood–Brain Barrier Microvessels. Fluids Barriers CNS 2022, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.R.; Gromnicova, R.; Brachner, A.; Kraev, I.; Romero, I.A.; Neuhaus, W.; Male, D. A Hydrogel Model of the Human Blood-Brain Barrier Using Differentiated Stem Cells. PLoS ONE 2023, 18, e0283954. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chung, M.; Lee, S.-R.; Jeon, N.L. 3D Brain Angiogenesis Model to Reconstitute Functional Human Blood–Brain Barrier in Vitro. Biotechnol. Bioeng. 2020, 117, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Kumar, A.; Dev, A.; Gupta, V.K.; Han, S.S. Advances in Hydrogel-Based Microfluidic Blood-Brain-Barrier Models in Oncology Research. Pharmaceutics 2022, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.R.; Pentelute, B.L.; Lawler, S.E. Blood-Brain-Barrier Spheroids as an in Vitro Screening Platform for Brain-Penetrating Agents. Nat. Commun. 2017, 8, 15623. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, C.; Duschmalé, M.; Gavrilov, A.; Brandenberg, N.; Hoehnel, S.; Ceroni, C.; Lassalle, E.; Kassianidou, E.; Knoetgen, H.; Niewoehner, J.; et al. Investigating Receptor-Mediated Antibody Transcytosis Using Blood–Brain Barrier Organoid Arrays. Fluids Barriers CNS 2021, 18, 43. [Google Scholar] [CrossRef]
- Sokolova, V.; Nzou, G.; van der Meer, S.B.; Ruks, T.; Heggen, M.; Loza, K.; Hagemann, N.; Murke, F.; Giebel, B.; Hermann, D.M.; et al. Ultrasmall Gold Nanoparticles (2 Nm) Can Penetrate and Enter Cell Nuclei in an In Vitro 3D Brain Spheroid Model. Acta Biomater. 2020, 111, 349–362. [Google Scholar] [CrossRef]
- Kumarasamy, M.; Sosnik, A. Heterocellular Spheroids of the Neurovascular Blood-Brain Barrier as a Platform for Personalized Nanoneuromedicine. iScience 2021, 24, 102183. [Google Scholar] [CrossRef] [PubMed]
- Heymans, M.; Sevin, E.; Gosselet, F.; Lundquist, S.; Culot, M. Mimicking Brain Tissue Binding in an in Vitro Model of the Blood-Brain Barrier Illustrates Differences between in Vitro and in Vivo Methods for Assessing the Rate of Brain Penetration. Eur. J. Pharm. Biopharm. 2018, 127, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, B.; Xiang, M.; Yang, X.; Liu, Y.; Liu, X.; Shen, Y. Advances on Fluid Shear Stress Regulating Blood-Brain Barrier. Microvasc. Res. 2020, 128, 103930. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.R.; Valkai, S.; Kincses, A.; Petneházi, A.; Czeller, T.; Veszelka, S.; Ormos, P.; Deli, M.A.; Dér, A. A Versatile Lab-on-a-Chip Tool for Modeling Biological Barriers. Sensors Actuators B Chem. 2016, 222, 1209–1219. [Google Scholar] [CrossRef]
- Wang, J.D.; Khafagy, E.-S.; Khanafer, K.; Takayama, S.; ElSayed, M.E.H. Organization of Endothelial Cells, Pericytes, and Astrocytes into a 3D Microfluidic in Vitro Model of the Blood-Brain Barrier. Mol. Pharm. 2016, 13, 895–906. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered Human Blood–Brain Barrier Platform for Understanding Nanoparticle Transport Mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef]
- Falanga, A.P.; Pitingolo, G.; Celentano, M.; Cosentino, A.; Melone, P.; Vecchione, R.; Guarnieri, D.; Netti, P.A. Shuttle-Mediated Nanoparticle Transport across an in Vitro Brain Endothelium under Flow Conditions. Biotechnol. Bioeng. 2017, 114, 1087–1095. [Google Scholar] [CrossRef]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.F.; Ingber, D.E. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, H.N.; Im, S.-K.; Chung, S.; Kang, J.Y.; Choi, N. Collagen-Based Brain Microvasculature Model in Vitro Using Three-Dimensional Printed Template. Biomicrofluidics 2015, 9, 24115. [Google Scholar] [CrossRef]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G. A 3D Real-Scale, Biomimetic, and Biohybrid Model of the Blood-Brain Barrier Fabricated through Two-Photon Lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef]
- van Rooy, I.; Cakir-Tascioglu, S.; Hennink, W.E.; Storm, G.; Schiffelers, R.M.; Mastrobattista, E. In Vivo Methods to Study Uptake of Nanoparticles into the Brain. Pharm. Res. 2011, 28, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Meng, H.; Pan, Y.; Liu, J.; Li, J.; Qi, Y.; Huang, Y. Influence of Nanoparticle Size on Blood–Brain Barrier Penetration and the Accumulation of Anti-Seizure Medicines in the Brain. J. Mater. Chem. B 2022, 10, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the Optimum Size of Nanoparticles for Their Delivery into the Brain Assisted by Focused Ultrasound-Induced Blood–Brain Barrier Opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Zhong, G.; Long, H.; Zhou, T.; Liu, Y.; Zhao, J.; Han, J.; Yang, X.; Yu, Y.; Chen, F.; Shi, S. Blood-Brain Barrier Permeable Nanoparticles for Alzheimer’s Disease Treatment by Selective Mitophagy of Microglia. Biomaterials 2022, 288, 121690. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Ho, W.; Chen, C.; Chen, Q.; Li, F.; Tang, M.; Fan, Q.; Wan, J.; Yu, W.; et al. Targeted MRNA Nanoparticles Ameliorate Blood–Brain Barrier Disruption Postischemic Stroke by Modulating Microglia Polarization. ACS Nano 2024, 18, 3260–3275. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Biolchi Mayer, A.; Lima, M.R.; Geraldes, L.R.; Zanotto, L.N.; Moreira, K.G.; Martins, O.P.; Piva, H.L.; Felipe, M.S.S.; Amaral, A.C.; et al. Dopamine-Loaded Nanoparticle Systems Circumvent the Blood–Brain Barrier Restoring Motor Function in Mouse Model for Parkinson’s Disease. Sci. Rep. 2021, 11, 15185. [Google Scholar] [CrossRef]
- Groeneveld, G.J.; Hay, J.L. Drug Discovery and the BBB Measuring Blood—Brain Barrier Penetration Using the NeuroCart, a CNS Test Battery. Drug Discov. Today Technol. 2016, 20, 27–34. [Google Scholar] [CrossRef]
- Weji, B.G.; Obsa, M.S.; Melese, K.G.; Azeze, G.A. Incidence and Risk Factors of Postdural Puncture Headache: Prospective Cohort Study Design. Perioper. Med. 2020, 9, 32. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and Clinical Potential of Nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech 2011, 12, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Effinger, A.; Driscoll, C.M.O.; Mcallister, M.; Fotaki, N. Impact of Gastrointestinal Disease States on Oral Drug Absorption—Implications for Formulation Design—A PEARRL Review. J. Pharm. Pharmacol. 2019, 71, 674–698. [Google Scholar] [CrossRef]
- Rai, G.; Gauba, P.; Dang, S. Recent Advances in Nanotechnology for Intra-Nasal Drug Delivery and Clinical Applications. J. Drug Deliv. Sci. Technol. 2023, 86, 104726. [Google Scholar] [CrossRef]
- Hussain, A.A. Intranasal Drug Delivery. Adv. Drug Deliv. Rev. 1998, 29, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Sun, I.-C.; Ahn, C.-H.; Lee, S.; Kim, K. Recent Trend of Ultrasound-Mediated Nanoparticle Delivery for Brain Imaging and Treatment. ACS Appl. Mater. Interfaces 2023, 15, 120–137. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G. Nanoparticle Transport across the Blood Brain Barrier. Tissue Barriers 2016, 4, e1153568. [Google Scholar] [CrossRef]
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the Blood—Brain Barrier for the Therapy of Malignant Brain Tumor: Current Status and Prospects of Drug Delivery Approaches. J. Nanobiotechnol. 2022, 20, 412. [Google Scholar] [CrossRef]
- Gao, H.; Chen, H.; Cui, G.-Y.; Hu, J.-X. Damage Mechanism and Therapy Progress of the Blood-Brain Barrier after Ischemic Stroke. Cell Biosci. 2023, 13, 196. [Google Scholar] [CrossRef]
- Cash, A.; Theus, M.H. Mechanisms of Blood–Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Perriot, S.; Gastfriend, B.D.; Steinfort, M.; Cibien, C.; Soldati, S.; Matsuo, K.; Guimbal, S.; Mathias, A.; Palecek, S.P.; et al. Intrinsic Blood—Brain Barrier Dysfunction Contributes to Multiple Sclerosis Pathogenesis. Brain 2022, 145, 4334–4348. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Xiong, N.; Xu, H.; Chai, S.; Wang, H.; Wang, J.; Zhao, H.; Jiang, X.; Fu, P.; et al. Remodelling and Treatment of the Blood-Brain Barrier in Glioma. Cancer Manag. Res. 2021, 13, 4217–4232. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Piacentino, G.; Constantin, G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The Contribution of Inflammatory Astrocytes to BBB Impairments in a Brain-Chip Model of Parkinson’s Disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The Blood-Brain Barrier in Health and Disease: Important Unanswered Questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.; Tiram, G.; Sousa-Herves, A.; Eldar-Boock, A.; Krivitsky, A.; Scomparin, A.; Yeini, E.; Ofek, P.; Ben-Shushan, D.; Vossen, L.I.; et al. Co-targeting Co-Targeting the Tumor Endothelium and P-Selectin-Expressing Glioblastoma Cells Leads to a Remarkable Therapeutic Outcome. eLife 2017, 6, 25281. [Google Scholar] [CrossRef]
- Marcos-contreras, O.A.; Brenner, J.S.; Kiseleva, R.Y.; Zuluaga-ramirez, V.; Greineder, C.F.; Villa, C.H.; Hood, E.D.; Myerson, J.W.; Muro, S.; Persidsky, Y.; et al. Combining Vascular Targeting and the Local Fi Rst Pass Provides 100-Fold Higher Uptake of ICAM-1-Targeted vs Untargeted Nanocarriers in the in Fl Amed Brain. J. Control. Release 2019, 301, 54–61. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Q.; Chow, P.K.; Wang, D.; Wang, C. Biomaterials Transferrin-Conjugated Magnetic Silica PLGA Nanoparticles Loaded with Doxorubicin and Paclitaxel for Brain Glioma Treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef]
- Demeule, M.; Che, C.; Lavalle, I. Antitumour Activity of ANG1005, a Conjugate between Paclitaxel and the New Brain Delivery Vector Angiopep-2. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, X.R.F.; Andjelkovic, X.A.V. Claudin-1-Dependent Destabilization of the Blood—Brain Barrier in Chronic Stroke. Neurobiol. Dis. 2019, 39, 743–757. [Google Scholar] [CrossRef]
- Bony, B.A.; Tarudji, A.W.; Miller, H.A.; Gowrikumar, S.; Roy, S.; Curtis, E.T.; Gee, C.C.; Vecchio, A.; Dhawan, P.; Kievit, F.M. Claudin-1-Targeted Nanoparticles for Delivery to Aging-Induced Alterations in the Blood—Brain Barrier. ACS Nano 2021, 15, 18520–18531. [Google Scholar] [CrossRef]
- Pang, L.; Zhu, Y.; Qin, J.; Zhao, W.; Wang, J. Primary M1 Macrophages as Multifunctional Carrier Combined with PLGA Nanoparticle Delivering Anticancer Drug for Efficient Glioma Therapy. Drug Deliv. 2018, 25, 1922–1931. [Google Scholar] [CrossRef]
- Hou, J.; Yang, X.; Li, S.; Cheng, Z.; Wang, Y.; Zhao, J.; Zhang, C. Accessing Neuroinflammation Sites: Monocyte/Neutrophil-Mediated Drug Delivery for Cerebral Ischemia. Sci. Adv. 2019, 5, eaau8301. [Google Scholar] [CrossRef]
- Chai, Z.; Hu, X.; Wei, X.; Zhan, C.; Lu, L.; Jiang, K.; Lu, W. A Facile Approach to Functionalizing Cell Membrane-Coated Nanoparticles with Neurotoxin-Derived Peptide for Brain-Targeted Drug Delivery. J. Control. Release 2017, 264, 102–111. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B. V Blood–Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Wang, Y.; Koivisto, O.; Zhou, J.; Shu, Y.; Zhang, H. Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment. Adv. Drug Deliv. Rev. 2021, 176, 113891. [Google Scholar] [CrossRef]
- Mukai, H.; Ogawa, K.; Kato, N.; Kawakami, S. Recent Advances in Lipid Nanoparticles for Delivery of Nucleic Acid, MRNA, and Gene Editing-Based Therapeutics. Drug Metab. Pharmacokinet. 2022, 44, 100450. [Google Scholar] [CrossRef]
- Glass, Z.; Li, Y.; Xu, Q. Nanoparticles for CRISPR-Cas9 Delivery. Nat. Biomed. Eng. 2017, 1, 854–855. [Google Scholar] [CrossRef]
- Lee, B.; Lee, K.; Panda, S.; Gonzales-Rojas, R.; Chong, A.; Bugay, V.; Park, H.M.; Brenner, R.; Murthy, N.; Lee, H.Y. Nanoparticle Delivery of CRISPR into the Brain Rescues a Mouse Model of Fragile X Syndrome from Exaggerated Repetitive Behaviours. Nat. Biomed. Eng. 2018, 2, 497–507. [Google Scholar] [CrossRef]
- Wang, M.; Zuris, J.A.; Meng, F.; Rees, H.; Sun, S.; Deng, P.; Han, Y.; Gao, X.; Pouli, D.; Wu, Q.; et al. Efficient Delivery of Genome-Editing Proteins Using Bioreducible Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 2868–2873. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, X.; Yang, Q.; Zheng, M.; Shimoni, O.; Ruan, W.; Wang, Y.; Zhang, D.; Yin, J.; Huang, X.; et al. Blood-Brain Barrier-Penetrating Single CRISPR-Cas9 Nanocapsules for Effective and Safe Glioblastoma Gene Therapy. Sci. Adv. 2022, 8, eabm8011. [Google Scholar] [CrossRef]
- Ralvenius, W.T.; Andresen, J.L.; Huston, M.M.; Penney, J.; Bonner, J.M.; Fenton, O.S.; Langer, R.; Tsai, L.-H. Nanoparticle-Mediated Delivery of Anti-PU.1 SiRNA via Localized Intracisternal Administration Reduces Neuroinflammation. Adv. Mater. 2024, 36, 2309225. [Google Scholar] [CrossRef]
- Asimakidou, E.; Reynolds, R.; Barron, A.M.; Lo, C.H. Autolysosomal Acidification Impairment as a Mediator for TNFR1 Induced Neuronal Necroptosis in Alzheimer’s Disease. Neural Regen. Res. 2024, 19, 1869–1870. [Google Scholar] [CrossRef]
- Lo, C.H.; Zeng, J. Defective Lysosomal Acidification: A New Prognostic Marker and Therapeutic Target for Neurodegenerative Diseases. Transl. Neurodegener. 2023, 12, 29. [Google Scholar] [CrossRef]
- Quick, J.D.; Silva, C.; Wong, J.H.; Lim, K.L.; Reynolds, R.; Barron, A.M.; Zeng, J.; Lo, C.H. Lysosomal Acidification Dysfunction in Microglia: An Emerging Pathogenic Mechanism of Neuroinflammation and Neurodegeneration. J. Neuroinflamm. 2023, 20, 185. [Google Scholar] [CrossRef]
- O’Connor, L.M.; O’Connor, B.A.; Bin Lim, S.; Zeng, J.; Lo, C.H. Integrative Multi-Omics and Systems Bioinformatics in Translational Neuroscience: A Data Mining Perspective. J. Pharm. Anal. 2023, 13, 836–850. [Google Scholar] [CrossRef]
- O’Connor, L.M.; O’Connor, B.A.; Zeng, J.; Lo, C.H. Data Mining of Microarray Datasets in Translational Neuroscience. Brain Sci. 2023, 13, 1318. [Google Scholar] [CrossRef]


| BBB Model | Features | Advantages | Limitations |
|---|---|---|---|
| In vitro BBB models | |||
| Transwell | Two-compartment model comprising BBB endothelial cells grown as a monolayer on the porous membrane insert, while other astrocytes, pericytes, and other neural cells are grown in the basolateral compartment. | Simple to set up and allows for co-culturing of different cells in the neurovascular unit. | Monolayer of cells fails to replicate the organization and architecture of the BBB. NPs can be trapped in the membrane filter. Absence of fluid shear. |
| Hydrogel | Generated by co-culturing cells from the neurovascular unit within hydrogels that emulate the extracellular matrix, through self-assembly in culture media, or by employing precision-engineered microstructures designed to simulate the 3D physiological architecture. | The extracellular matrix enables cell–cell and cell–ECM interactions in a 3D environment, with possible vasculogenesis. Affordable procedure with minimal need for specialized equipment. | Difficulty in optimizing the mechanical properties of the hydrogel for suitable cell growth. Limited contact between various cell types. Absence of fluid shear. |
| Spheroid/organoid | Formed by a collection of organ-specific cell types capable of self-assembly and self-organization akin to in vivo conditions. | Direct cell–cell contact among neurovascular unit cells. Ease of multiplexing using spheroid/organoid arrays. | No current demonstration of direct quantification of NP transport. Absence of fluid shear. |
| Microfluidic | Typically comprises a main flow channel lined with cultured BBB endothelial cells flanked by a channel containing the supporting cell types, interfaced by a porous polymeric membrane. 3D upgrades are available with biomimetic cylindrical blood vessels. | Inclusion of fluid shear to reproduce physiological conditions. Direct visualization of NP localization is possible. | Microfabrication process is tedious and resource-intensive. Sophisticated skillset required for microchannel assembly and cell culture. |
| In vivo BBB models | |||
| Mice (Balb/c, Kunming, ICR, FVB/Ntac) | NPs are administered and assessed qualitatively via direct fluorescence visualization or behavioral tests or quantitatively using autoradiography, pharmacokinetic blood plasma measurements, microdialysis, or histological biodistribution studies | Reproduces the true physiological complexity in terms of architecture and cellular composition. Available disease models such as AD, PD, and stroke. | Large biological variability between animals reduces reproducibility of findings. Tedious and expensive to maintain animals, requiring stringent study planning and animal welfare considerations. |
| Rat (P18, Sprague Dawley, Wistar, Fischer-344) | |||
| Human studies | |||
| Clinical trials | NP penetrance and entry into the BBB parenchyma can be evaluated based on clinical, electrophysiological, and imaging techniques such as PET, SPECT, and CSF sampling. | Circumvents model fidelity limitations. Direct visualization of localization of radiolabelled drugs in the brain possible. | Radiological imaging techniques are expensive and have limited accuracy in quantifying drug concentrations in the CNS. Lumbar puncture for CSF sampling can be distressing and cannot conclude whether a compound has reached its target. |
| NP Used | Identifier (Start Year) | Study Scope | Phase/Status |
|---|---|---|---|
| Curcumin NP | NCT02104752 (2014) | Test whether curcumin NPs will improve behavioral measures and biomarkers of cognition and neuroplasticity in patients with schizophrenia who have been prescribed antipsychotics. | 1,2/Completed |
| Ultrasmall superparamagnetic iron oxide NP | NCT02549898 (2015) | Investigate inflammation of cranial and meningeal arteries during pharmacologically induced migraine attacks using black blood imaging MRI. | -/Completed |
| Ultrasmall superparamagnetic iron oxide NP | NCT02511028 (2015) | Investigate the safety of ferumoxytol and examine the spatial and temporal enhancement patterns of ferumoxytol compared to patterns seen with gradient-echo imaging and gadolinium contrast in multiple sclerosis lesions. | 1/Completed |
| NU-0129 spherical nucleic acid gold NP | NCT03020017 (2017) | Assess the safety of intravenous NU-0129 in patients with recurrent glioblastoma multiforme or gliosarcoma. | 1/Completed |
| MTX110 NP | NCT03566199 (2018) | Study the side effects of panobinostat nanoparticle formulation MTX110 in treating participants with newly-diagnosed diffuse intrinsic pontine glioma. | 1,2/Completed |
| CNM-Au8 gold nanocrystals | NCT03993171 (2019) | Assess the CNS metabolic effects, safety, pharmacokinetics, and pharmacodynamics of CNM-Au8 in patients who have been diagnosed with multiple sclerosis. | 2/Recruiting |
| CNM-Au8 gold nanocrystals | NCT04098406 (2019) | Assess the efficacy, safety, and pharmacokinetics/pharmacodynamics effects of CNM-Au8 as a disease-modifying agent for the treatment of amyotrophic lateral sclerosis. | 2/Completed |
| CNM-Au8 gold nanocrystals | NCT03815916 (2019) | Assess the CNS metabolic effects, safety, pharmacokinetics, and pharmacodynamics of CNM-Au8 in patients with PD. | 2/Completed |
| MTX110 NP | NCT04264143 (2020) | Find the maximum tolerated dose of MTX110 (a water-soluble panobinostat nanoparticle formulation) and gadolinium that can be given safely in children with newly diagnosed diffuse midline gliomas. | 1/Completed |
| NTLA-2001 lipid NP | NCT04601051 (2020) | Evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of NTLA-2001 in participants with hereditary transthyretin amyloidosis. | 1/Active, not recruiting |
| AGuIX Gd-based NP | NCT04899908 (2021) | Determine whether AGuIX Gd-based nanoparticles make radiation work more effectively in the treatment of patients with brain metastases. | 2/Recruiting |
| AGuIX Gd-based NP | NCT04881032 (2022) | Evaluate the association of AGuIX nanoparticles with radiotherapy plus concomitant temozolomide in the treatment of newly diagnosed glioblastoma. | 1,2/Recruiting |
| Ferumoxytol superparamagnetic iron oxide | NCT06146751 (2023) | Evaluate the effectiveness of a novel ferumoxytol-enhanced cardiac magnetic resonance in detecting intracardiac thrombus in patients with ventricular aneurysm and after percutaneous ventricular reconstruction. | -/Recruiting |
| NanoTherm ASI iron oxide NP | NCT06271421 (2024) | Evaluate the efficacy and tolerance of using the NanoTherm therapy system in recurrent glioblastoma multiforme | -/Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asimakidou, E.; Tan, J.K.S.; Zeng, J.; Lo, C.H. Blood–Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience. Pharmaceuticals 2024, 17, 612. https://doi.org/10.3390/ph17050612
Asimakidou E, Tan JKS, Zeng J, Lo CH. Blood–Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience. Pharmaceuticals. 2024; 17(5):612. https://doi.org/10.3390/ph17050612
Chicago/Turabian StyleAsimakidou, Evridiki, Justin Kok Soon Tan, Jialiu Zeng, and Chih Hung Lo. 2024. "Blood–Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience" Pharmaceuticals 17, no. 5: 612. https://doi.org/10.3390/ph17050612
APA StyleAsimakidou, E., Tan, J. K. S., Zeng, J., & Lo, C. H. (2024). Blood–Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience. Pharmaceuticals, 17(5), 612. https://doi.org/10.3390/ph17050612






