Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy
Abstract
1. Introduction
2. Current Clinical Applications of Chemotherapy
3. Gut Microbiota and Chemotherapy
3.1. Gut Microbiota in Tumorigenesis and Development
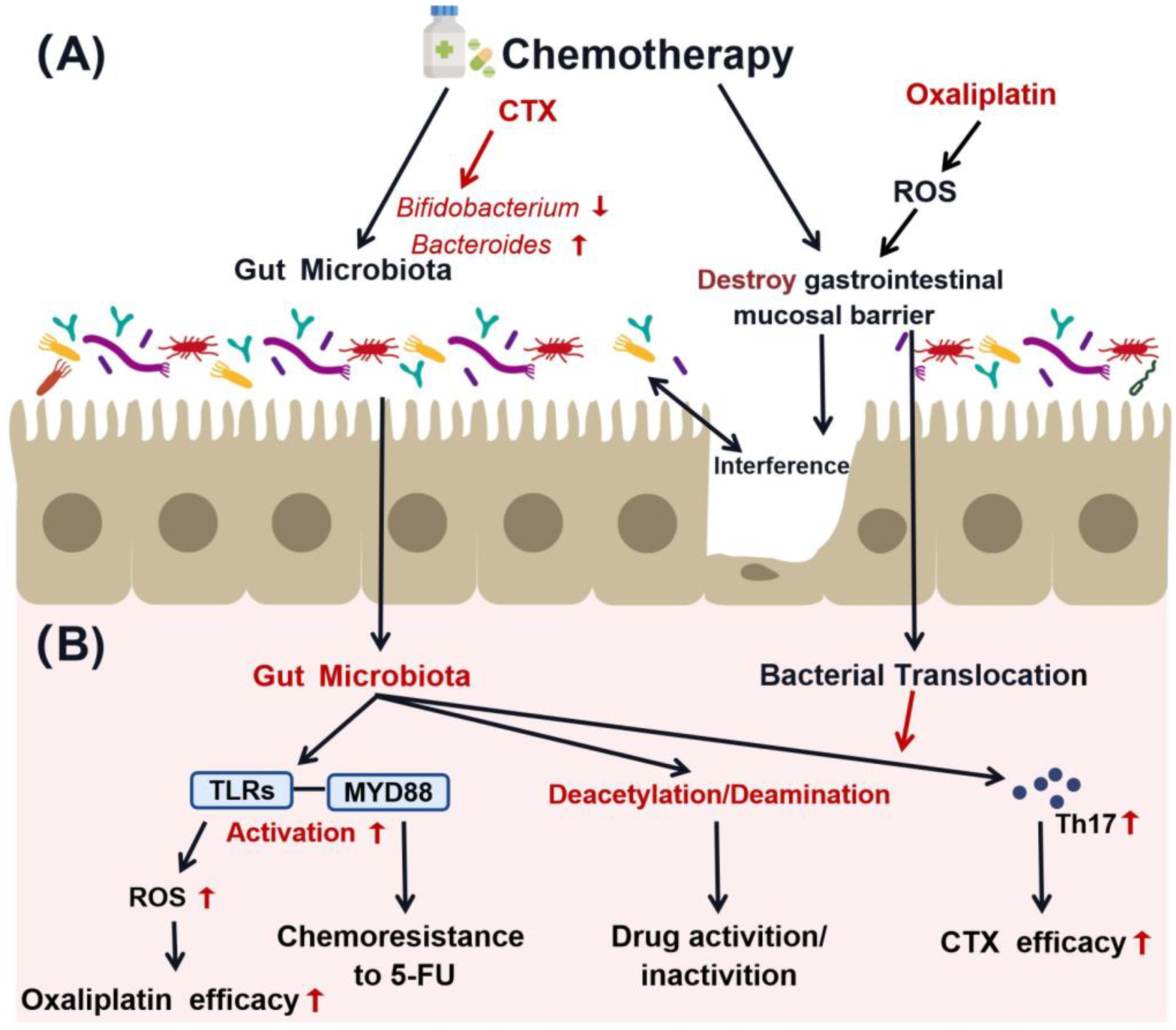
3.2. Interaction between Gut Microbiota and Chemotherapy
4. Gut Microbiota-Mediated Immunomodulation in Chemotherapy Sensitivity
4.1. Effects of Immune Modulation on Chemotherapy Response
4.2. Interaction of Gut Microbiota and Related Metabolites with Immune System
4.3. Interactions among Gut Microbiota, Immune System, and Chemotherapy
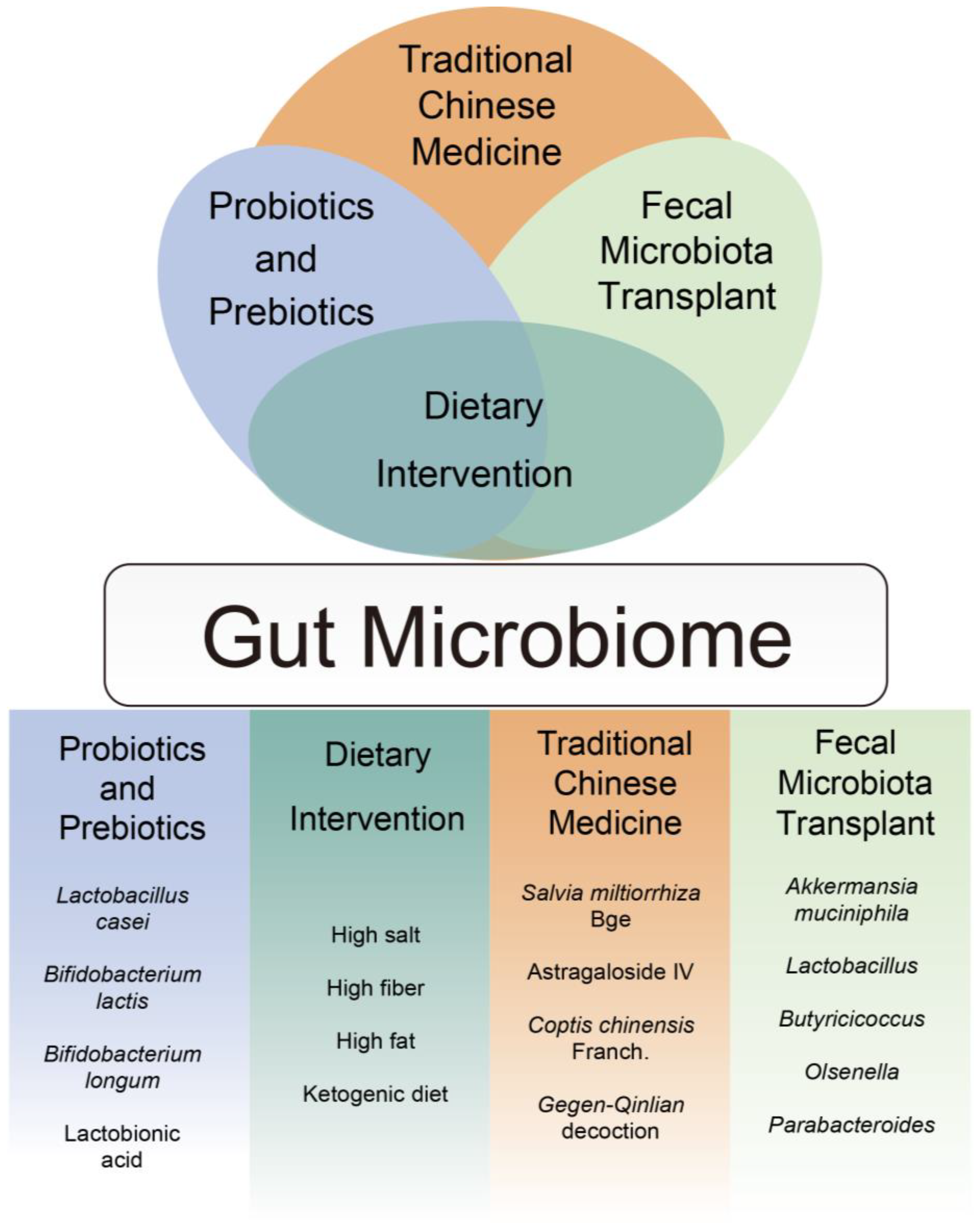
5. Clinical Application
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Society of Clinical Oncology (CSCO) Diagnosis and Treatment Guidelines for Colorectal Cancer Working Group. Guidelines of Chinese Society of Clinical Oncology (CSCO) for Colorectal Cancer; People’s Medical Publishing House (PMPH): Beijing, China, 2020; pp. 63–68. [Google Scholar]
- Ervin, S.M.; Ramanan, S.V.; Bhatt, A.P. Relationship Between the Gut Microbiome and Systemic Chemotherapy. Dig. Dis. Sci. 2020, 65, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Jaye, K.; Li, C.G.; Bhuyan, D.J. The complex interplay of gut microbiota with the five most common cancer types: From carcinogenesis to therapeutics to prognoses. Crit. Rev. Oncol. Hematol. 2021, 165, 103429. [Google Scholar] [CrossRef] [PubMed]
- Yixia, Y.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. The alterations of microbiota and pathological conditions in the gut of patients with colorectal cancer undergoing chemotherapy. Anaerobe 2021, 68, 102361. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Ruff, W.E.; Longbrake, E.E. Influence of immunomodulatory drugs on the gut microbiota. Transl. Res. 2021, 233, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, X.; Guo, Y.; Yan, J.; Abuduwaili, A.; Aximujiang, K.; Yan, J.; Wu, M. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Inno, A.; Belluomini, L.; Bocus, P.; Bisoffi, Z.; Russo, A.; Arcaro, G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, X. Evaluation of the Efficacy of Neoadjuvant Chemotherapy for Breast Cancer. Drug Des. Devel. Ther. 2020, 14, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ke, K.; Jin, W.; Zhu, Q.; Zhu, Q.; Mei, R.; Zhang, R.; Yu, S.; Shou, L.; Sun, X.; et al. Identification of Genes Related to 5-Fluorouracil Based Chemotherapy for Colorectal Cancer. Front. Immunol. 2022, 13, 887048. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Zhang, A.Q.; Cheng, S.X.; Rong, L.; Zhang, X.Z. Drug self-delivery systems for cancer therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Han, K.; Chen, S.; Chen, W.H.; Lei, Q.; Liu, Y.; Zhuo, R.X.; Zhang, X.Z. Synergistic gene and drug tumor therapy using a chimeric peptide. Biomaterials 2013, 34, 4680–4689. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, L.R.; Adkins, J.C.; Plosker, G.L.; Goa, K.L. Oxaliplatin: A review of its use in the management of metastatic colorectal cancer. Drugs Aging 1999, 14, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16 (Suppl. S1), 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.V.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav. Immun. 2021, 95, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Boyle, F.; Pavlakis, N.; Clarke, S.; Guminski, A.; Eade, T.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; et al. Emerging Evidence of the Gut Microbiome in Chemotherapy: A Clinical Review. Front. Oncol. 2021, 11, 706331. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.M.; Kim, H.; Bennett, D.; Calle, R.A.; Collins, S.; Esquejo, R.M.; He, T.; Joaquim, S.; Joyce, A.; Lambert, M.; et al. GDF-15 Neutralization Alleviates Platinum-Based Chemotherapy-Induced Emesis, Anorexia, and Weight Loss in Mice and Nonhuman Primates. Cell Metab. 2020, 32, 938–950.e936. [Google Scholar] [CrossRef] [PubMed]
- Secombe, K.R.; Van Sebille, Y.Z.A.; Mayo, B.J.; Coller, J.K.; Gibson, R.J.; Bowen, J.M. Diarrhea Induced by Small Molecule Tyrosine Kinase Inhibitors Compared With Chemotherapy: Potential Role of the Microbiome. Integr. Cancer Ther. 2020, 19, 1534735420928493. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Al-Dasooqi, N.; Bowen, J.M.; Tan, T.H.; Radzuan, M.; Logan, R.M.; Mayo, B.; Keefe, D.M.; Gibson, R.J. Biomarkers of chemotherapy-induced diarrhoea: A clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support Care Cancer 2013, 21, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Kalasabail, S.; Engelman, J.; Zhang, L.Y.; El-Omar, E.; Yim, H.C.H. A Perspective on the Role of Microbiome for Colorectal Cancer Treatment. Cancers 2021, 13, 4623. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Huang, Y.L.; Kuo, L.M.; Chen, Y.T.; Hung, C.F.; Hsieh, P.W. Protective role of casuarinin from Melastoma malabathricum against a mouse model of 5-fluorouracil-induced intestinal mucositis: Impact on inflammation and gut microbiota dysbiosis. Phytomedicine 2022, 101, 154092. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Hershman, D.L.; Martin, P.; Leonard, J.P.; Cheung, Y.K. Toxicity burden score: A novel approach to summarize multiple toxic effects. Ann. Oncol. 2012, 23, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Delord, J.P.; Raymond, E.; Chaouche, M.; Ruffie, P.; Ducreux, M.; Faivre, S.; Boige, V.; Le Chevalier, T.; Rixe, O.; Baudin, E.; et al. A dose-finding study of gemcitabine and vinorelbine in advanced previously treated malignancies. Ann. Oncol. 2000, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Holmes, F.A. Combination chemotherapy with Taxol (paclitaxel) in metastatic breast cancer. Ann. Oncol. 1994, 5 (Suppl. S6), S23–S27. [Google Scholar] [PubMed]
- Lyman, G.H. Impact of chemotherapy dose intensity on cancer patient outcomes. J. Natl. Compr. Canc. Netw. 2009, 7, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Hekmatshoar, Y.; Rahbar Saadat, Y.; Hosseiniyan Khatibi, S.M.; Ozkan, T.; Zununi Vahed, F.; Nariman-Saleh-Fam, Z.; Pourghassem Gargari, B.; Sunguroglu, A.; Zununi Vahed, S. The impact of tumor and gut microbiotas on cancer therapy: Beneficial or detrimental? Life Sci. 2019, 233, 116680. [Google Scholar] [CrossRef]
- Chadha, J.; Nandi, D.; Atri, Y.; Nag, A. Significance of human microbiome in breast cancer: Tale of an invisible and an invincible. Semin. Cancer Biol. 2021, 70, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Iannone, L.F.; Bersanelli, M.; Mini, E.; Catalano, M. The gut microbiome and efficacy of cancer immunotherapy. Pharmacol. Ther. 2022, 231, 107973. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Greten, T.F. Gut microbiome in HCC-Mechanisms, diagnosis and therapy. J. Hepatol. 2020, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.G.; Cowley, E.S.; Breister, A.; Matatov, S.; Lucio, L.; Polak, P.; Ridlon, J.M.; Gaskins, H.R.; Anantharaman, K. Diversity and distribution of sulfur metabolic genes in the human gut microbiome and their association with colorectal cancer. Microbiome 2022, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Schwabkey, Z.I.; Wiesnoski, D.H.; Chang, C.C.; Tsai, W.B.; Pham, D.; Ahmed, S.S.; Hayase, T.; Ortega Turrubiates, M.R.; El-Himri, R.K.; Sanchez, C.A.; et al. Diet-derived metabolites and mucus link the gut microbiome to fever after cytotoxic cancer treatment. Sci. Transl. Med. 2022, 14, eabo3445. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mitchell, S.A.; Jacobsen, P.B.; Pirl, W.F. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA Cancer J. Clin. 2015, 65, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K. Practical aspects of pain control in the patient with cancer. CA Cancer J. Clin. 1988, 38, 327–352. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.F. Thrombosis in cancer patients. Ann. Oncol. 2000, 11 (Suppl. S3), 273–276. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Song, Y.; Sun, M.; Wang, A.; Jiang, S.; Mu, G.; Tuo, Y. Exopolysaccharide Produced by Lactiplantibacillus plantarum-12 Alleviates Intestinal Inflammation and Colon Cancer Symptoms by Modulating the Gut Microbiome and Metabolites of C57BL/6 Mice Treated by Azoxymethane/Dextran Sulfate Sodium Salt. Foods 2021, 10, 3060. [Google Scholar] [CrossRef]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of Gut Microbiota in the Development and Treatment of Colorectal Cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, G.; Cao, H.; Yu, D.; Fang, X.; de Vos, W.M.; Wu, H. Gut dysbacteriosis and intestinal disease: Mechanism and treatment. J. Appl. Microbiol. 2020, 129, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, X.; Chen, T. Exploring the Modulatory Effects of Gut Microbiota in Anti-Cancer Therapy. Front. Oncol. 2021, 11, 644454. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wu, K.; Long, Z.; Zhou, X.; Zhong, C.; Wang, S.; Lai, H.; Guo, Y.; Lv, D.; Lu, J.; et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome 2022, 10, 94. [Google Scholar] [CrossRef]
- Singh, A.; Nayak, N.; Rathi, P.; Verma, D.; Sharma, R.; Chaudhary, A.; Agarwal, A.; Tripathi, Y.B.; Garg, N. Microbiome and host crosstalk: A new paradigm to cancer therapy. Semin. Cancer Biol. 2021, 70, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Baylatry, M.T.; Joly, A.C.; Sokol, H. Gut microbiota: What impact on colorectal carcinogenesis and treatment? Bull Cancer 2018, 105, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, H.; Xu, S.; Liu, D.; Cheng, Y.; Gao, B.; Li, X.; Chen, J. The gut microbiome can be used to predict the gastrointestinal response and efficacy of lung cancer patients undergoing chemotherapy. Ann. Palliat Med. 2020, 9, 4211–4227. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, C.; Zhao, L.; Sun, Z.; Ge, Y.; Li, X. The Relationship Between Gut Microbiome Features and Chemotherapy Response in Gastrointestinal Cancer. Front. Oncol. 2021, 11, 781697. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Liu, M.T.; Tao, T.; Zhu, X.; Fei, F.Q. The role of gut microbiota in tumorigenesis and treatment. Biomed. Pharmacother. 2021, 138, 111444. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, Z.; Xue, Y.; Zhang, J.; Zhu, J.; Gao, R.; Yao, S.; Ye, Y.; Wang, S.; Lin, C.; et al. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes 2020, 11, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Pandya, G.; Kirtonia, A.; Singh, A.; Goel, A.; Mohan, C.D.; Rangappa, K.S.; Pandey, A.K.; Kapoor, S.; Tandon, S.; Sethi, G.; et al. A comprehensive review of the multifaceted role of the microbiota in human pancreatic carcinoma. Semin. Cancer Biol. 2021, 86, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xia, X.; Wang, P.; Chen, S.; Yu, C.; Huang, R.; Zhang, R.; Wang, Y.; Lu, L.; Yuan, F.; et al. Induction and Amelioration of Methotrexate-Induced Gastrointestinal Toxicity are Related to Immune Response and Gut Microbiota. EBioMedicine 2018, 33, 122–133. [Google Scholar] [CrossRef]
- Gonzalez-Mercado, V.J.; Henderson, W.A.; Sarkar, A.; Lim, J.; Saligan, L.N.; Berk, L.; Dishaw, L.; McMillan, S.; Groer, M.; Sepehri, F.; et al. Changes in Gut Microbiome Associated With Co-Occurring Symptoms Development During Chemo-Radiation for Rectal Cancer: A Proof of Concept Study. Biol. Res. Nurs. 2021, 23, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol. Res. 2015, 171, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.R.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer 2018, 124, 3990–3999. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Shen, L.; Shi, W.; Xia, F.; Zhang, H.; Wang, Y.; Zhang, J.; Wang, Y.; Sun, X.; Zhang, Z.; et al. Gut Microbiome Components Predict Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Prospective, Longitudinal Study. Clin. Cancer Res. 2021, 27, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chu, R.; Wang, C.; Li, Y.; Wu, B.; Wan, J. Microbiome characteristics and Bifidobacterium longum in colorectal cancer patients pre- and post-chemotherapy. Transl. Cancer Res. 2020, 9, 2178–2190. [Google Scholar] [CrossRef] [PubMed]
- Caridis, D.T.; Reinhold, R.B.; Woodruff, P.W.; Fine, J. Endotoxaemia in man. Lancet 1972, 1, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.D.; Garlington, A.W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect. Immun. 1979, 23, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, D.; Lennernäs, H. Review on the effect of chemotherapy on the intestinal barrier: Epithelial permeability, mucus and bacterial translocation. Biomed. Pharmacother. 2023, 162, 114644. [Google Scholar] [CrossRef] [PubMed]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Smet, A.; Kupcinskas, J.; Link, A.; Hold, G.L.; Bornschein, J. The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse? Cell Mol. Gastroenterol. Hepatol. 2022, 13, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nakagawa, S.; Sawayama, H.; Ishimoto, T.; Imai, K.; Iwatsuki, M.; Hashimoto, D.; Baba, Y.; Yamashita, Y.I.; Yoshida, N.; et al. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett. 2017, 402, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Goubet, A.G.; Daillere, R.; Routy, B.; Derosa, L.; P, M.R.; Zitvogel, L. The impact of the intestinal microbiota in therapeutic responses against cancer. C R Biol. 2018, 341, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Wu, C.J.; Hung, Y.W.; Lee, C.J.; Chi, C.T.; Lee, I.C.; Yu-Lun, K.; Chou, S.H.; Luo, J.C.; Hou, M.C.; et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004779. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.Y.; Zhang, P.; Du, H.Z.; Gao, Y.Q.; Sun, R.Q.; Qin, S.Y.; Tian, Y.; Li, J.; Zhang, Y.X.; Chu, W.H.; et al. Prevotella contributes to individual response of FOLFOX in colon cancer. Clin. Transl. Med. 2021, 11, e512. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.F.; Lu, H.F.; Zhang, C.X.; Xu, Z.R.; Yang, Y.H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xing, C.; Sun, W.; Hou, G.; Yang, G.; Yuan, L. Lactobacillus supplementation prevents cisplatin-induced cardiotoxicity possibly by inflammation inhibition. Cancer Chemother. Pharmacol. 2018, 82, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Vande Voorde, J.; Sabuncuoğlu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, L.; Kelly, L. Bringing microbiome-drug interaction research into the clinic. EBioMedicine 2019, 44, 708–715. [Google Scholar] [CrossRef]
- Maier, L.; Typas, A. Systematically investigating the impact of medication on the gut microbiome. Curr. Opin. Microbiol. 2017, 39, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Daillere, R.; Boneca, I.G.; Lepage, P.; Langella, P.; Chamaillard, M.; Pittet, M.J.; Ghiringhelli, F.; Trinchieri, G.; Goldszmid, R.; et al. Gut microbiome and anticancer immune response: Really hot Sh*t! Cell Death Differ. 2015, 22, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Cario, E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr. Opin. Support Palliat. Care 2016, 10, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.; Sarfraz, M.; Mousa, W.K. The Interplay between Gut Microbiota and Oral Medications and Its Impact on Advancing Precision Medicine. Metabolites 2023, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kamada, N.; Moon, J.J. Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv. Drug Deliv. Rev. 2021, 179, 114021. [Google Scholar] [CrossRef] [PubMed]
- Gil Del Alcazar, C.R.; Alečković, M.; Polyak, K. Immune Escape during Breast Tumor Progression. Cancer Immunol. Res. 2020, 8, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Liu, P.; Han, Z.; Ying, W.; Li, C.; Yang, Y.; Wang, S.; Yang, J.; Cao, F.; Shen, J.; et al. Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8(+) T cell effector functions. Immunity 2024, 57, 876–889.e11. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Wang, Q.; Qi, Y.; Jiang, Y.; He, J.; Lin, Y.; Sun, Y.; Xu, J.; Chen, W.; Fan, L.; et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 2024, 187, 1651–1665.e1621. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, H.; Huang, J. The tumor immune microenvironment and immune-related signature predict the chemotherapy response in patients with osteosarcoma. BMC Cancer 2021, 21, 581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bastian, I.N.; Long, M.D.; Dow, M.; Li, W.; Liu, T.; Ngu, R.K.; Antonucci, L.; Huang, J.Y.; Phung, Q.T.; et al. Activation of NF-κB and p300/CBP potentiates cancer chemoimmunotherapy through induction of MHC-I antigen presentation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025840118. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, W.; Gong, X.; Ren, S.; Su, C.; Jiang, T.; Zhou, C. Immune-checkpoint inhibitors plus chemotherapy versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer. J. Immunother. Cancer 2020, 8, e001300. [Google Scholar] [CrossRef] [PubMed]
- Sougiannis, A.T.; VanderVeen, B.N.; Enos, R.T.; Velazquez, K.T.; Bader, J.E.; Carson, M.; Chatzistamou, I.; Walla, M.; Pena, M.M.; Kubinak, J.L.; et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav. Immun. 2019, 80, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The role of gut microbiome in cancer genesis and cancer prevention. Health Sci. Rev. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Wei, Y.; Kuang, D.M. Influence of gut and intratumoral microbiota on the immune microenvironment and anti-cancer therapy. Pharmacol. Res. 2021, 174, 105966. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.B.; Zhou, Y.L.; Fang, J.Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.W.; Ologun, G.; Arora, R.; McQuade, J.L.; Wargo, J.A. Gut Microbiome Modulates Response to Cancer Immunotherapy. Dig. Dis. Sci. 2020, 65, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, T.B.; Okereke, C.; Seamon, M.; Purohit, S.; Wakade, C.; Sharma, A. Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, L.; Shen, S.; Guo, Y.; Cao, Q.; Cai, X.; Feng, J.; Yan, Y.; Hu, T.; Luo, S.; et al. Intestinal dysbacteriosis-induced IL-25 promotes development of HCC via alternative activation of macrophages in tumor microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 303. [Google Scholar] [CrossRef] [PubMed]
- Heng, X.; Jiang, Y.; Chu, W. Influence of Fluconazole Administration on Gut Microbiome, Intestinal Barrier, and Immune Response in Mice. Antimicrob. Agents Chemother. 2021, 65, 10-1128. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Nandi, D.; Nag, A. The pint- sized powerhouse: Illuminating the mighty role of the gut microbiome in improving the outcome of anti- cancer therapy. Semin. Cancer Biol. 2021, 70, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Lyu, W.; Hirota, K.; Saito, E.; Miyoshi, M.; Hohjoh, H.; Furukawa, K.; Saito, K.; Haritani, M.; Taguchi, A.; et al. Eggshell membrane modulates gut microbiota to prevent murine pre-cachexia through suppression of T helper cell differentiation. J. Cachexia Sarcopenia Muscle 2022, 13, 2088–2101. [Google Scholar] [CrossRef] [PubMed]
- Roberti, M.P.; Yonekura, S.; Duong, C.P.M.; Picard, M.; Ferrere, G.; Tidjani Alou, M.; Rauber, C.; Iebba, V.; Lehmann, C.H.K.; Amon, L.; et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat. Med. 2020, 26, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Yang, H.; Chen, L. Metabolic regulation on the immune environment of glioma through gut microbiota. Semin. Cancer Biol. 2021, 86, 990–997. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.D.; Hong, H.; Ahmad, S.; Holloway, R.W. The gut microbiome and cancer immunotherapeutics: A review of emerging data and implications for future gynecologic cancer research. Crit. Rev. Oncol. Hematol. 2021, 157, 103165. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Liu, B.; Yu, D.; Sun, J.; Ge, L.; Tang, W.; Liu, S. Gut microbiota contributes to the methionine metabolism in host. Front. Microbiol. 2022, 13, 1065668. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity. Front. Immunol. 2023, 14, 1127743. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chen, D.; Zhang, K.; Zhang, W.; Liu, T.; Wang, S.; Dai, X.; Wang, B.; Zhong, W.; Cao, H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 2022, 526, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Kato, I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016, 3, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Gut microbiota contributes towards immunomodulation against cancer: New frontiers in precision cancer therapeutics. Semin. Cancer Biol. 2021, 70, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhou, Z.; Liang, Y.; Cheng, X.; Li, Y.; Teng, W.; Zhao, M.; Liu, C.; Guan, M.; Zhao, C. Targeting strategies for chemotherapy-induced peripheral neuropathy: Does gut microbiota play a role? Crit. Rev. Microbiol. 2019, 45, 369–393. [Google Scholar] [CrossRef]
- Singh, K.P.; Dhruva, A.; Flowers, E.; Paul, S.M.; Hammer, M.J.; Wright, F.; Cartwright, F.; Conley, Y.P.; Melisko, M.; Levine, J.D.; et al. Alterations in Patterns of Gene Expression and Perturbed Pathways in the Gut-Brain Axis Are Associated With Chemotherapy-Induced Nausea. J. Pain Symptom Manag. 2020, 59, 1248–1259.e1245. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Flament, C.; Zoubir, M.; Pautier, P.; LeCesne, A.; Ribrag, V.; Soria, J.C.; Marty, V.; Vielh, P.; Robert, C.; et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011, 71, 661–665. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef]
- Raza, M.H.; Gul, K.; Arshad, A.; Riaz, N.; Waheed, U.; Rauf, A.; Aldakheel, F.; Alduraywish, S.; Rehman, M.U.; Abdullah, M.; et al. Microbiota in cancer development and treatment. J. Cancer Res. Clin. Oncol. 2019, 145, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Coppola, G.; Palma, G.; Barbieri, A.; Luciano, A.; Del Prete, P.; Rossetti, S.; Berretta, M.; Facchini, G.; Perdonà, S.; et al. Microbiota effects on cancer: From risks to therapies. Oncotarget 2018, 9, 17915–17927. [Google Scholar] [CrossRef] [PubMed]
- Picardo, S.L.; Coburn, B.; Hansen, A.R. The microbiome and cancer for clinicians. Crit. Rev. Oncol. Hematol. 2019, 141, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Ciardiello, D.; Villani, A.; Maiorano, B.A.; Latiano, T.P.; Maiello, E.; Perri, F.; Pazienza, V. Insights into the role of gut and intratumor microbiota in pancreatic ductal adenocarcinoma as new key players in preventive, diagnostic and therapeutic perspective. Semin. Cancer Biol. 2021, 86, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Kwong, T.N.Y.; Wu, C.Y.; Yu, J. Clinical applications of gut microbiota in cancer biology. Semin. Cancer Biol. 2019, 55, 28–36. [Google Scholar] [CrossRef]
- Chen, S.M.; Chieng, W.W.; Huang, S.W.; Hsu, L.J.; Jan, M.S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 20319. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Hennenberg, E.M.; Eyking, A.; Rünzi, M.; Gerken, G.; Scott, P.; Parkhill, J.; Walker, A.W.; Cario, E. TLR signaling modulates side effects of anticancer therapy in the small intestine. J. Immunol. 2015, 194, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Liu, C.Y.; Lee, H.C.; Huang, Y.H.; Li, L.H.; Chiau, J.C.; Wang, T.E.; Chu, C.H.; Shih, S.C.; Tsai, T.H.; et al. Lactobacillus casei Variety rhamnosus Probiotic Preventively Attenuates 5-Fluorouracil/Oxaliplatin-Induced Intestinal Injury in a Syngeneic Colorectal Cancer Model. Front. Microbiol. 2018, 9, 983. [Google Scholar] [CrossRef]
- Zhu, H.; Lu, C.; Gao, F.; Qian, Z.; Yin, Y.; Kan, S.; Chen, D. Selenium-enriched Bifidobacterium longum DD98 attenuates irinotecan-induced intestinal and hepatic toxicity in vitro and in vivo. Biomed. Pharmacother. 2021, 143, 112192. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Wang, Y.; Wang, D.; Ke, Y.; Zeng, X. Propionate and Butyrate Produced by Gut Microbiota after Probiotic Supplementation Attenuate Lung Metastasis of Melanoma Cells in Mice. Mol. Nutr. Food Res. 2021, 65, e2100096. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Meynet, O.; Zunino, B.; Happo, L.; Pradelli, L.A.; Chiche, J.; Jacquin, M.A.; Mondragón, L.; Tanti, J.F.; Taillan, B.; Garnier, G.; et al. Caloric restriction modulates Mcl-1 expression and sensitizes lymphomas to BH3 mimetic in mice. Blood 2013, 122, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e132. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zeng, T.; Wang, Y.; Cui, H.; Wu, J.; Zou, B.; Tao, Z.; Zhang, L.; Garside, G.B.; Tao, S. Dietary restriction increases protective gut bacteria to rescue lethal methotrexate-induced intestinal toxicity. Gut Microbes 2020, 12, 1714401. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Luo, W.; Shi, Y.; Fan, Z.; Ji, G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1755, author reply pp. 1755–1756. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qian, X.; Chen, S.; Fu, X.; Ma, G.; Zhang, A. Akkermansia muciniphila Enhances the Antitumor Effect of Cisplatin in Lewis Lung Cancer Mice. J. Immunol. Res. 2020, 2020, 2969287. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Lee, H.C.; Li, L.H.; Chiang Chiau, J.S.; Wang, T.E.; Chuang, W.H.; Chen, M.J.; Wang, H.Y.; Shih, S.C.; Liu, C.Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, G.; Zhou, H.; Wu, W.K.K.; Wong, S.H.; Coker, O.O.; Dai, Z.; Li, X.; Szeto, C.H.; Sugimura, N.; Lam, T.Y.; et al. Alterations in Enteric Virome Are Associated With Colorectal Cancer and Survival Outcomes. Gastroenterology 2018, 155, 529–541.e525. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, X.L.; Zhang, Y.J.; Nie, Y.Z.; Wu, K.C.; Shi, Y.Q. Efficacy and safety of fecal microbiota transplantation by washed preparation in patients with moderate to severely active ulcerative colitis. J. Dig. Dis. 2020, 21, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Sun, R.; Wang, Q.; Liu, F.; Tang, D.; Chang, X. Standardized Astragalus Mongholicus Bunge-Curcuma Aromatica Salisb. Extract Efficiently Suppresses Colon Cancer Progression Through Gut Microbiota Modification in CT26-Bearing Mice. Front. Pharmacol. 2021, 12, 714322. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Jia, Y.; Li, J.; Kuai, W.; Li, Y.; Guo, F.; Xu, X.; Zhao, Z.; Lv, J.; Li, Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huang, G.; Khan, I.; Su, L.; Xia, W.; Law, B.Y.K.; Wong, V.K.W.; Wu, Q.; Wang, J.; Leong, W.K.; et al. Poria cocos polysaccharides exert prebiotic function to attenuate the adverse effects and improve the therapeutic outcome of 5-FU in Apc(Min/+) mice. Chin. Med. 2022, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yue, R.; Chen, Y.; Yang, M.; Huang, X.; Shui, J.; Peng, Y.; Chin, J. Gut Microbiota, a Potential New Target for Chinese Herbal Medicines in Treating Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2019, 2019, 2634898. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Lu, C.C.; Lai, W.F.; Wu, T.S.; Lu, J.J.; Chen, Y.M.; Tzeng, C.M.; Liu, H.T.; Wei, H.; Lai, H.C. Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell 2021, 12, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Gil, A.; Pérez-Sanz, F.; García-Solano, J.; Alburquerque-González, B.; Parreño-González, M.A.; Legaz-García, M.D.C.; Fernández-Breis, J.T.; Rodriguez-Braun, E.; Pimentel, P.; Tuomisto, A.; et al. ColPortal, an integrative multiomic platform for analysing epigenetic interactions in colorectal cancer. Sci. Data 2019, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Gao, Y.; Chai, X.; Pi, R.; Chan, G.; Hu, Y. Translating traditional herbal formulas into modern drugs: A network-based analysis of Xiaoyao decoction. Chin. Med. 2020, 15, 25. [Google Scholar] [CrossRef] [PubMed]


| Cancer Species | Model | Drug | Gut Microbiota | Metabolites | Mechanism | Toxicity/Efficacy | References |
|---|---|---|---|---|---|---|---|
| EL4 lymphoma, MC38 colon carcinoma, and B16 melanoma | Subcutaneous injection in mice | Oxaliplatin and Cisplatin | Ruminococcus ↑ and Alistipes ↑ | - | ROS production | Enhance efficacy | [114] |
| Metastasizing B16F10 melanomas and non-metastasizing MCA205 sarcomas | Subcutaneous injection in mice | CTX | Lactobacillus johnsonii ↑ and E. hirae ↑ | - | Promotes IL-17 production by CD4 T-cells | Enhance efficacy | [111] |
| - | Mice | Irinotecan | Bacteroides vulgatus ↑ and Clostridium ramosum ↑ | - | β-glucuronidase activates SN-38 | Enhance toxicity | [120] |
| - | Mice | MTX | Overall abundance of gut microbiota ↓ | - | Gut microbiota containsTLR2 agonist PCSK | Enhance toxicity | [121] |
| Mice colon cancer cell line CT-26 | Subcutaneous injected in mice | FOLFOX | Prevotella ↑ | 3-Oxocholic acid ↑ | Significantly improved the expression of P-EGFR/P-ERK/c-MYC and LOX | Reduce efficacy | [67] |
| - | Mice | Paclitaxel | Tyzzerella ↑, Romboutsia ↑ and Turicibacter ↑ | SCFAs ↑ | Increased anxiety-like behavior | Enhance toxicity | [15] |
| Pancreatic cancer | Induction of pancreatitis by intraperitoneal injection into the right lower quadrant of KC transgenic mice for 7 h | Gemcitabine | Lactobacillus paracasei ↑ | - | Increase IFN-γ levels to suppress Th2 cytokine production and regulate Th1/Th2 immune balance | Enhance efficacy | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Hou, X.; Wang, H.; Du, H.; Liu, Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals 2024, 17, 604. https://doi.org/10.3390/ph17050604
Deng Y, Hou X, Wang H, Du H, Liu Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals. 2024; 17(5):604. https://doi.org/10.3390/ph17050604
Chicago/Turabian StyleDeng, Yufei, Xiaoying Hou, Haiping Wang, Hongzhi Du, and Yuchen Liu. 2024. "Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy" Pharmaceuticals 17, no. 5: 604. https://doi.org/10.3390/ph17050604
APA StyleDeng, Y., Hou, X., Wang, H., Du, H., & Liu, Y. (2024). Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals, 17(5), 604. https://doi.org/10.3390/ph17050604







