Evaluating the Effectiveness of Phellodendron Amurense Ruprecht Extract as a Natural Anti-Caries Material
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Effect of PAR Extract over Time
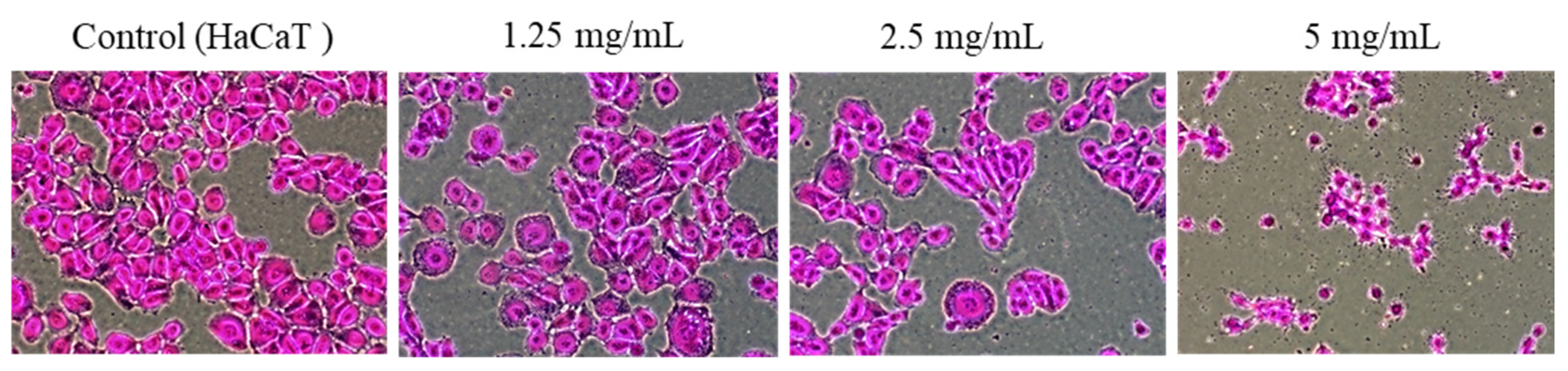
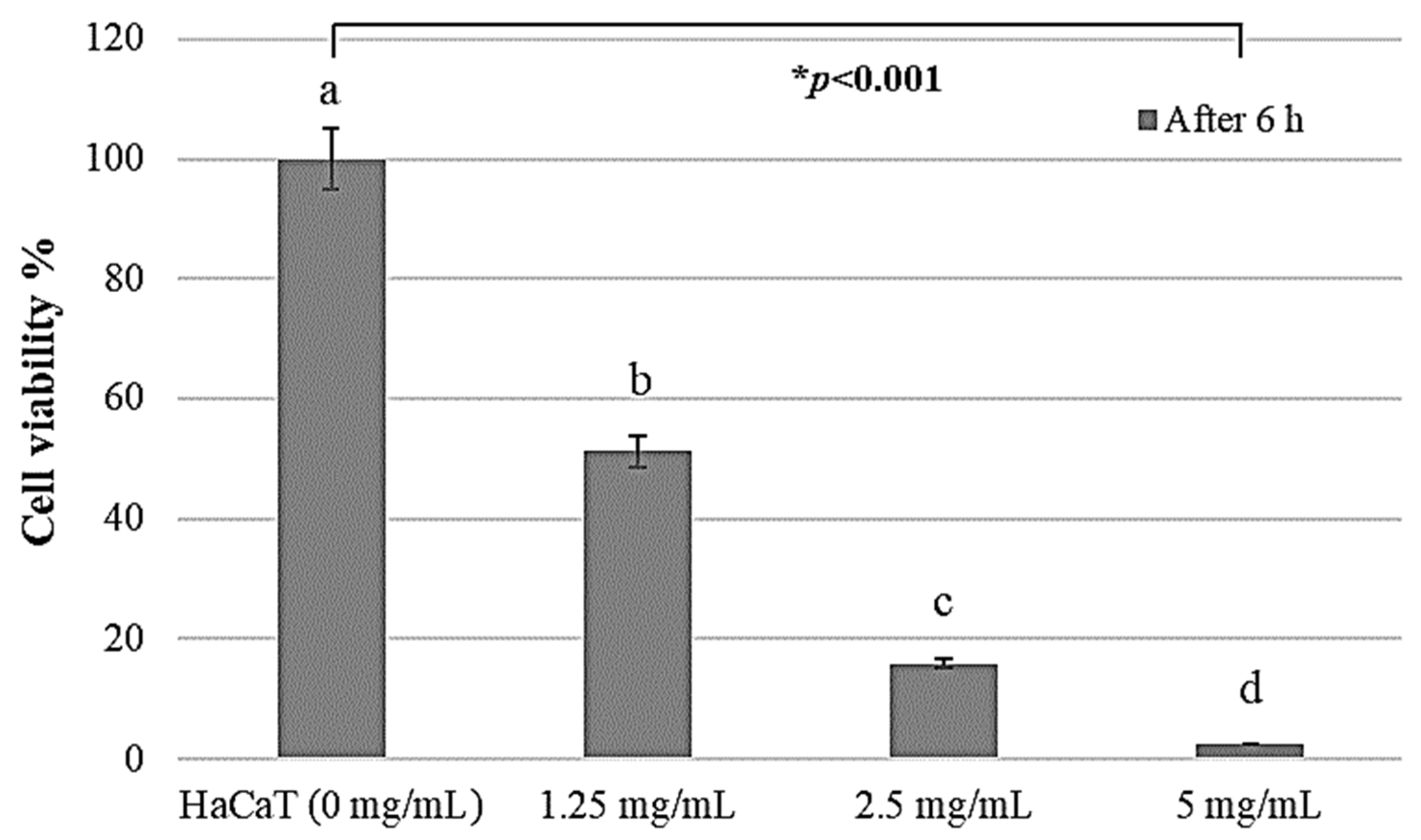
2.2. HaCaT Cell Proliferation Activity by PAR Extract
2.3. Growth-Inhibitory Effect in HaCaT Cell
3. Discussion
4. Materials and Methods
4.1. Phellodendron amurense Ruprecht (PAR) Extract
4.2. Bacterial Strain
4.3. Antibacterial Activity
4.4. Cell Culturing and Identifying the Patterns of Cell Growth
4.5. Cell Cytotoxicity Evaluation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Health and Welfare. Korean Children’s Oral Health Survey in 2015. Available online: https://www.mohw.go.kr/react/jb/sjb030301vw.jsp?PAR_MENU_ID=03&MENU_ID=0321&CONT_SEQ=332448 (accessed on 20 March 2023).
- Lee, H.J.; Choi, E.K.; Park, J.B.; Han, K.D.; Oh, S. Tooth loss predicts myocardial infarction, heart failure, stroke, and death. J. Dent. Res. 2019, 98, 164–170. [Google Scholar] [CrossRef]
- Daly, B.; Thompsell, A.; Sharpling, J.; Rooney, Y.M.; Hillman, L.; Wanyonyi, K.L.; White, S.; Gallagher, J.E. Evidence summary: The relationship between oral health and dementia. Br. Dent. J. 2018, 223, 846–853. [Google Scholar] [CrossRef]
- Limeback, H. Treating dental caries as an infectious disease. Applying the medical model in practice to prevent dental caries. Ont. Dent. 1996, 73, 23–25. [Google Scholar]
- Ayres, J.S.; Trinidad, N.J.; Vance, R.E. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat. Med. 2012, 18, 799–806. [Google Scholar] [CrossRef]
- Kahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2022, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Ain, N.; Mohd, A.; Sharaf, A.; Al-Hammadi, S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef] [PubMed]
- Ambarawati, I.G.A.D.; Sukrama, I.D.M.; Yasa, I.W.P.S. Deteksi Gen Gtf-B Streptococcus mutans Dalam Plak Dengan Gigi Karies Pada Siswa Di SD N 29 Dangin Puri. Intisari Sains Medis 2020, 11, 1049–1055. [Google Scholar] [CrossRef]
- De Oliveira, R.V.D.; Bonafé, F.S.S.; Spolidorio, D.M.P.; Koga-Ito, C.Y.; De Farias, A.L.; Kirker, K.R.; James, G.A.; Brighenti, F.L. Streptococcus mutans and Actinomyces naeslundii interaction in dual-species biofilm. Microorganisms 2020, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, Y.; Nakamura, T.; Kusumoto, Y.; Nakao, R.; Iwamoto, T.; Shinozuka, O.; Senpuku, H. Effects of pH on the properties of membrane vesicles including glucosyltransferase in Streptococcus mutans. Microorganisms 2021, 9, 2308. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef]
- Liu, Y.; Breukink, E. The membrane steps of bacterial cell wall synthesis as antibiotic targets. Antibiotics 2016, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Song, J.; Kim, J.N. Genetic mutations that confer fluoride resistance modify gene expression and virulence traits of Streptococcus mutans. Microorganisms 2021, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, E.R.H.; Ré, A.C.S.; Peixoto, M.P.G.; Ferreira, M.P.; Ricomini-Filho, A.P.; Freitas, O.; Aires, C.P. Effect of chitosan dispersion and microparticles on older Streptococcus mutans biofilms. Molecules 2019, 24, 1808. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Oliveira-Neto, J.M.; Moore, D. Chlorhexidine treatment for the prevention of dental caries in children and adolescents. Cochrane Database Syst. Rev. 2015, 4, CD008457. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Maltz, M.; Beighton, D. Multidisciplinary research agenda for novel antimicrobial agents for caries prevention and treatment. Adv. Dent. Res. 2012, 24, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.M.; Crielaard, W.; Zaura, E.; Keijser, B.J.; Brandt, B.W.; Krom, B.P. A novel compound to maintain a healthy oral plaque ecology in vitro. J. Oral Microbiol. 2016, 8, 3251. [Google Scholar] [CrossRef] [PubMed]
- Saquib, S.A.; Alqahtani, N.A.; Ahmad, I.; Kader, M.A.; Al Shahrani, S.S.; Asiri, E.A. Evaluation and comparison of antibacterial efficacy of herbal extracts in combination with antibiotics on periodontal pathobionts: An in vitro microbiological study. Antibiotics 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Suri, M.A.; Azizah, Z.; Asra, R. A review: Traditional use, phytochemical and pharmacological review of red betel leaves (Piper crocatum Ruiz & Pav). Asian J. Pharm. Res. Dev. 2021, 9, 159–163. [Google Scholar] [CrossRef]
- Li, X.; Yin, L.; Ramage, G.; Li, B.; Tao, Y.; Zhi, Q.; Lin, H.; Zhou, Y. Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. Microbiologyopen 2019, 8, e937. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef] [PubMed]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and antibiofilm activity against Streptococcus mutans of individual and mixtures of the main polyphenolic compounds found in Chilean propolis. BioMed Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef] [PubMed]
- Kole, P.L.; Jadhav, H.R.; Thakurdesai, P.; Nagappa, A.N. Cosmetics potential of herbal extracts. Nat. Prod. Radiance 2005, 4, 315–321. [Google Scholar]
- Yen, C.H. The Pharmacology of Chinese Herbs, 1st ed.; Chin-Yin Publishing: Taipei, Taiwan, 1999; pp. 376–377. [Google Scholar]
- Lee, S.H.; Lee, J.A.; Shin, M.R.; Lee, J.H.; Roh, S.S. The protective effect of water extract of Phellodendri Cortex in chronic reflux esophagitis-induced rats. Korea J. Herbol. 2020, 35, 25–36. [Google Scholar]
- Xian, Y.F.; Mao, Q.Q.; Ip, S.P.; Lin, Z.X.; Che, C.T. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phemmodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J. Ethnopharmacol. 2011, 137, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Choi, J.Y.; Oh, J.S.; Jung, H.W.; Choi, E.H.; Lee, H.S.; Kim, J.A.; Chang, T.S.; Son, J.K.; Lee, S.H. Isolation of melanin biosynthesis inhibitory compounds from the Phellodendri Cortex. Korean J. Pharmacogn. 2007, 38, 387–393. [Google Scholar]
- Kim, K.H.; Ahn, S.C.; Lee, M.S.; Kweon, O.S.; Oh, W.K.; Kim, M.S.; Sohn, C.B.; Ahn, H.S. Adipocyte differentiation inhibitor isolated from the barks of Phellodendron amurense. Korean J. Food Sci. Technol. 2003, 35, 503–509. [Google Scholar]
- Wong, R.W.K.; Hägg, U.; Samaranayake, L.; Yuen, M.K.Z.; Seneviratne, C.J.; Kao, R. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A pilot study. Int. J. Oral Maxillofac. Surg. 2010, 39, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, U.; Kunz, E.M.; Lenkeit, K.; Schaffner, W.; Meyer, J. Antimicrobial activity of Mahonia aquifolium and two of its alkaloids against oral bacteria. Schweiz. Monatsschrift Zahnmed. 2007, 117, 1126–1131. [Google Scholar]
- Okuda, T.; Jo, R.; Tsutsumi, K.; Watai, D.; Ishihara, C.; Yama, K.; Aita, Y.; Inokuchi, T.; Kimura, M.; Chikazawa, T.; et al. An in vitro study of the effects of Phellodendron bark extract and berberine chloride on periodontal pathogenic bacteria in the oral microbiome. J. Oral Biosci. 2023, 65, 72–79. [Google Scholar] [CrossRef]
- Jung, H.W.; Yang, J.Y.; Park, H.J. Research trend of health life expectancy using oral health indicators (2010–2020). J. Korean Soc. Sch. Community Health Educ. 2021, 22, 75–91. [Google Scholar] [CrossRef]
- Farva, K.; Sattar, H.; Ullah, H.; Raziq, A.; Mehmood, M.D.; Tareen, A.K.; Sultan, I.N.; Zohra, Q.; Khan, M.W. Phenotypic Analysis, Molecular Characterization, and Antibiogram of Caries-Causing Bacteria Isolated from Dental Patients. Microorganisms 2023, 11, 1952. [Google Scholar] [CrossRef]
- Kwak, D.J.; Nam, S.Y.; Lee, D.S. Antibacterial activity of Phellodendri cortex on dental caries bacteria Streptococcus sanguis. J. Technol. Dent. 2002, 24, 43–49. [Google Scholar]
- Kim, E.M.; Cho, M.J. Antimicrobial activities of oral bacteria by lichen extracts. J. Korean Soc. Dent. Hyg. 2012, 12, 81–91. [Google Scholar] [CrossRef]
- Sakaue, Y.; Domon, H.; Oda, M.; Takenaka, S.; Kubo, M.; Fukuyama, Y.; Okiji, T.; Terao, Y. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiol. Immunol. 2016, 60, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Senpuku, H.; Mohri, S.; Mihara, M.; Arai, T.; Suzuki, Y.; Saeki, Y. Effects of 7S globulin 3 derived from the adzuki bean [Vigna angularis] on the CSP- and eDNA-dependent biofilm formation of Streptococcus mutans. Arch. Oral Biol. 2019, 102, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Choi, J.H.; Song, Y.S.; Kim, G.C.; Hong, J.W. Ethanol extract of asiasari radix preferentially induces apoptosis in G361 human melanoma cells by differential regulation of p53. BMC Complement. Altern. Med. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N. The antioxidant effect of rutin in human dermal fibroblasts damaged by reactive oxygen species. Korean J. Aesthet. Cosmetol. 2014, 12, 831–836. [Google Scholar]
- Jang, G.W.; Choi, S.I.; Han, X.; Men, X.; Kwon, H.Y.; Choi, Y.E.; Kang, N.Y.; Park, B.W.; Kim, J.J.; Gang, S.; et al. Antioxidant and anti-inflammatory activities of Phellodendron amurense extract fermented with Lactobacillus plantarum CM. J. Food Hyg. Saf. 2021, 36, 196–203. [Google Scholar] [CrossRef]
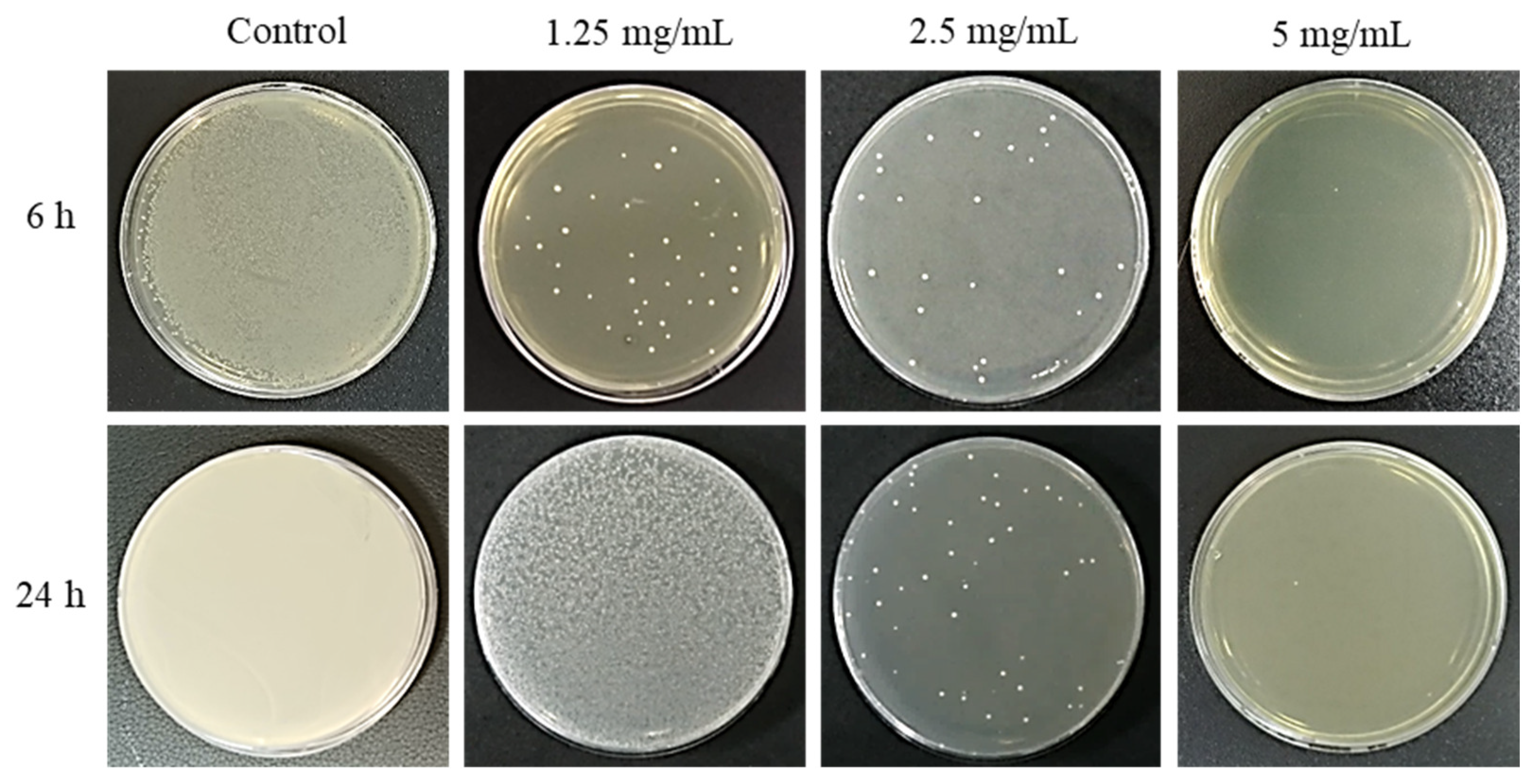

| Control (S. mutans) | 1.25 mg/mL | 2.5 mg/mL | 5 mg/mL | |
|---|---|---|---|---|
| After 6 h | 2.8 ± 0.5 × 107 | 1.6 ± 0.2 × 102 | 3.6 ± 0.3 × 101 | 0.0 |
| After 24 h | 1.6 ± 0.6 × 1010 | 4.0 ± 0.9 × 107 | 5.7 ± 0.4 × 101 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-R.; Kim, G.-C.; Nam, S.-H. Evaluating the Effectiveness of Phellodendron Amurense Ruprecht Extract as a Natural Anti-Caries Material. Pharmaceuticals 2024, 17, 603. https://doi.org/10.3390/ph17050603
Kim Y-R, Kim G-C, Nam S-H. Evaluating the Effectiveness of Phellodendron Amurense Ruprecht Extract as a Natural Anti-Caries Material. Pharmaceuticals. 2024; 17(5):603. https://doi.org/10.3390/ph17050603
Chicago/Turabian StyleKim, Yu-Rin, Gyoo-Cheon Kim, and Seoul-Hee Nam. 2024. "Evaluating the Effectiveness of Phellodendron Amurense Ruprecht Extract as a Natural Anti-Caries Material" Pharmaceuticals 17, no. 5: 603. https://doi.org/10.3390/ph17050603
APA StyleKim, Y.-R., Kim, G.-C., & Nam, S.-H. (2024). Evaluating the Effectiveness of Phellodendron Amurense Ruprecht Extract as a Natural Anti-Caries Material. Pharmaceuticals, 17(5), 603. https://doi.org/10.3390/ph17050603







