Glycyrrhizic Acid Nanoparticles Subside the Activity of Methicillin-Resistant Staphylococcus aureus by Suppressing PBP2a
Abstract
1. Introduction
2. Results
2.1. Preparation and Characterization of GA-NPs
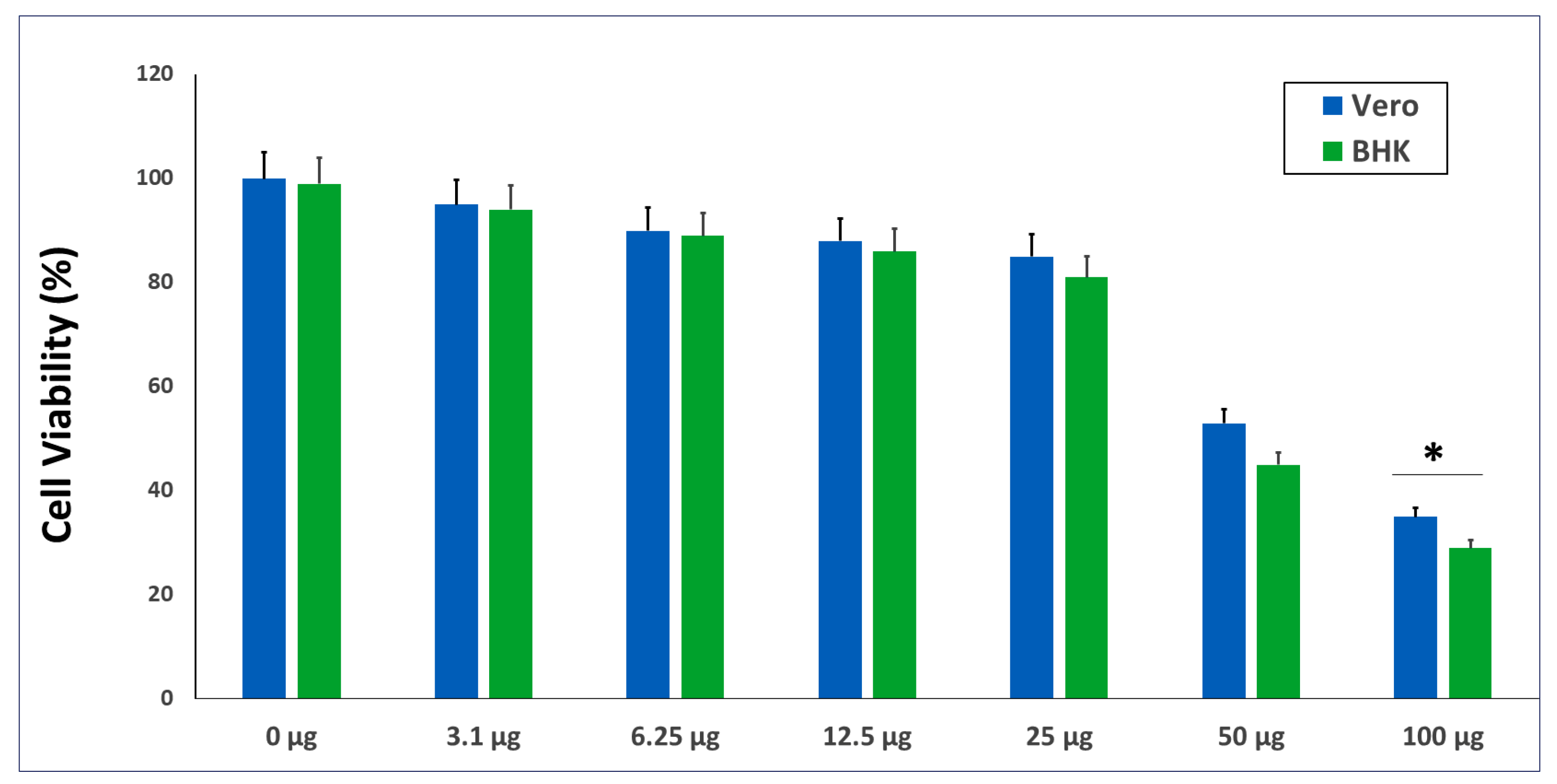
2.2. The In Vitro Cytotoxicity of the GANPs
2.3. In Vitro Susceptibility Test
2.3.1. Disk Diffusion Method
2.3.2. Minimum Inhibitory Concentration (MIC) Evaluation for Antibacterial Activity
2.3.3. Time-Kill Assay
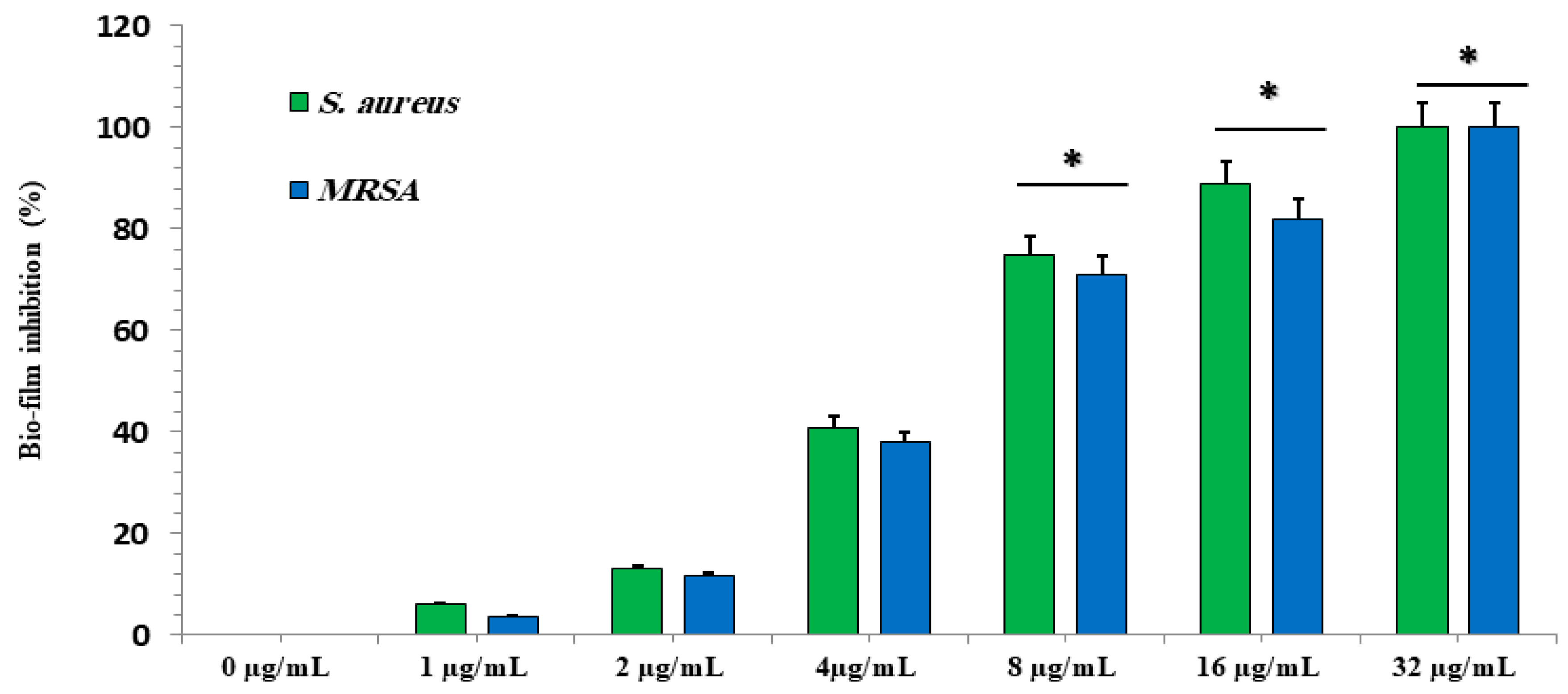
2.3.4. Effect of Different GA-NP Concentrations on Biofilms
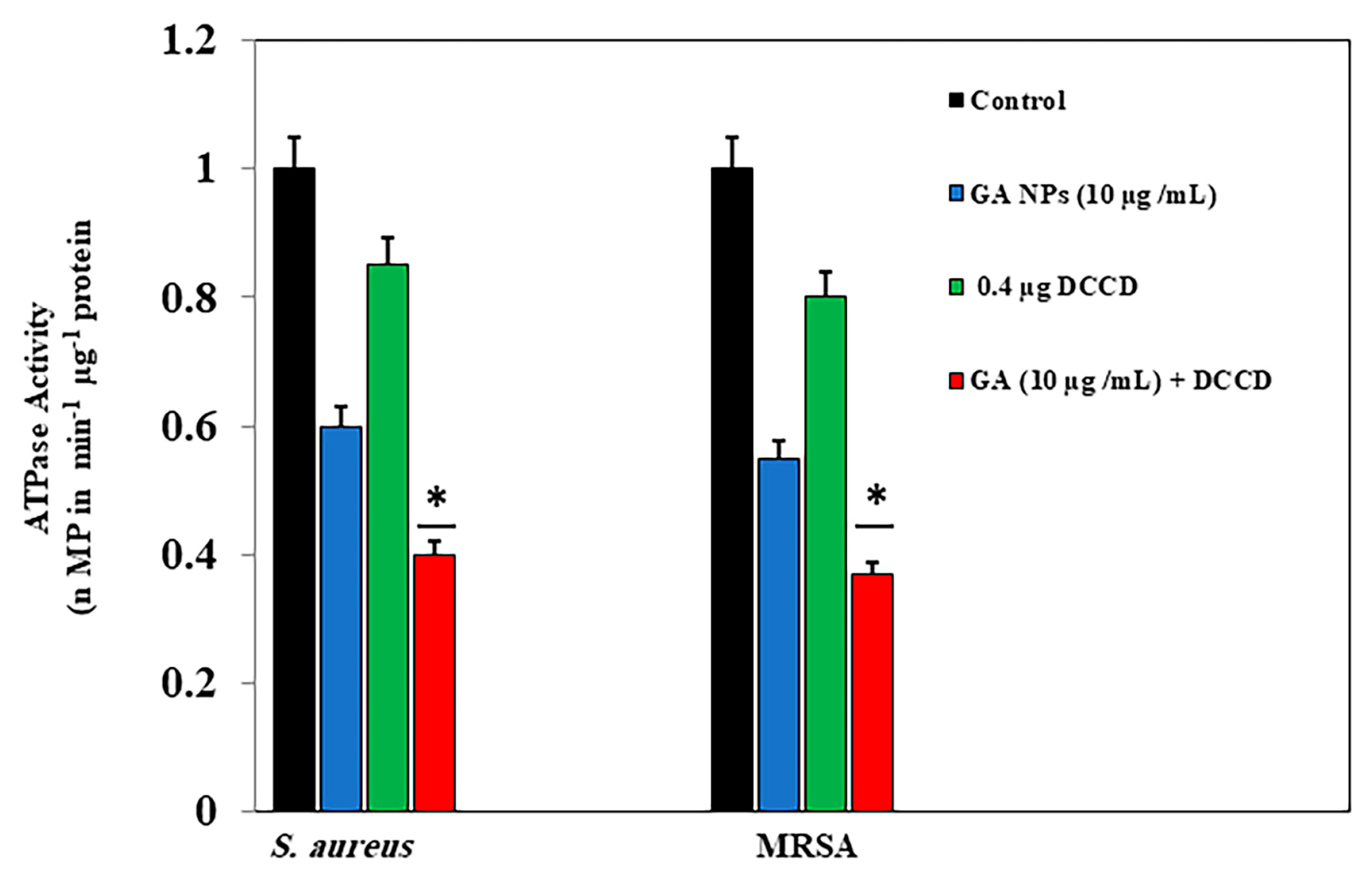
2.3.5. ATPase Activity Assay
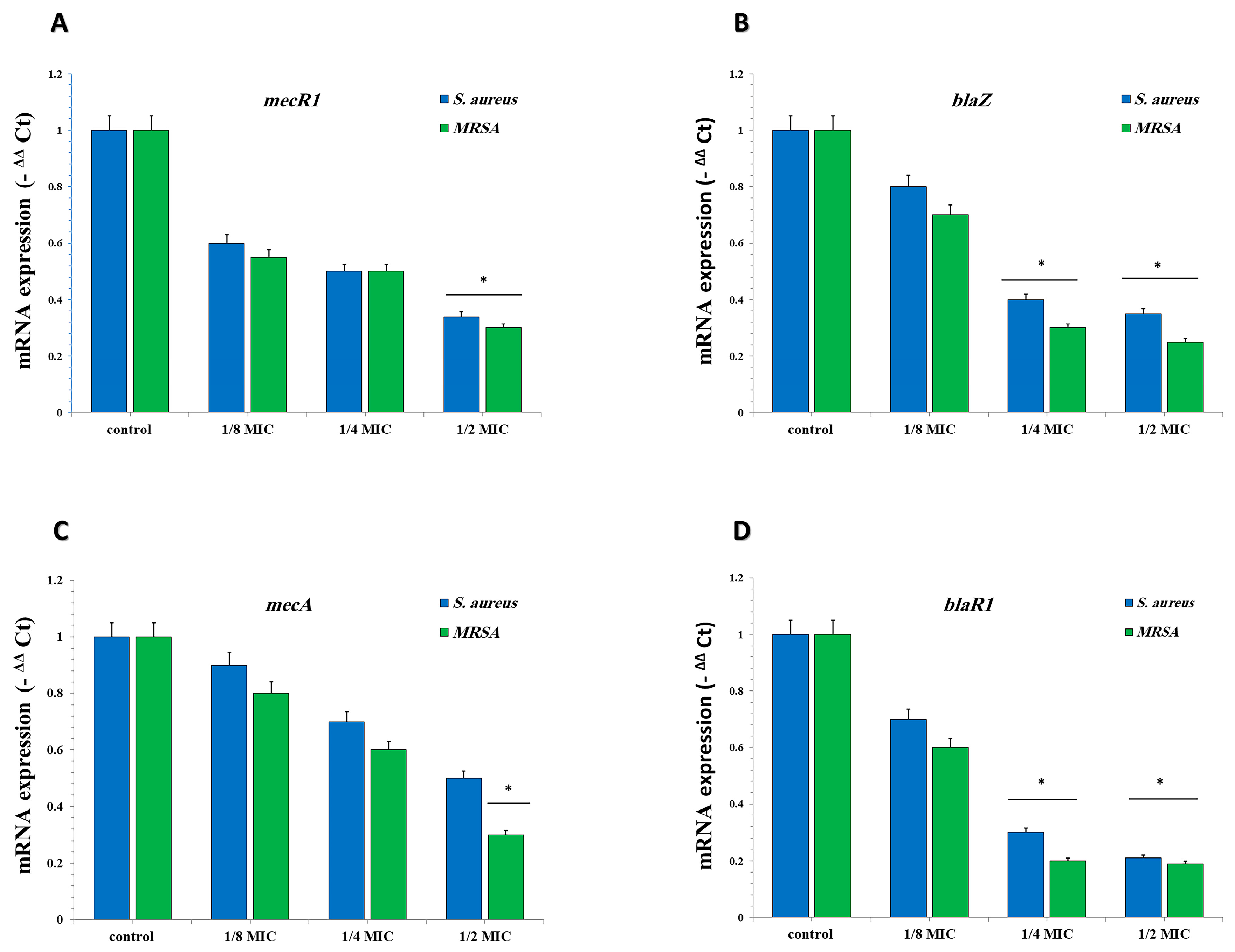
2.3.6. GA-NPs Represses the Transcription of mecA, blaZ, blaR1, and mecR1 in S. aureus and MRSA
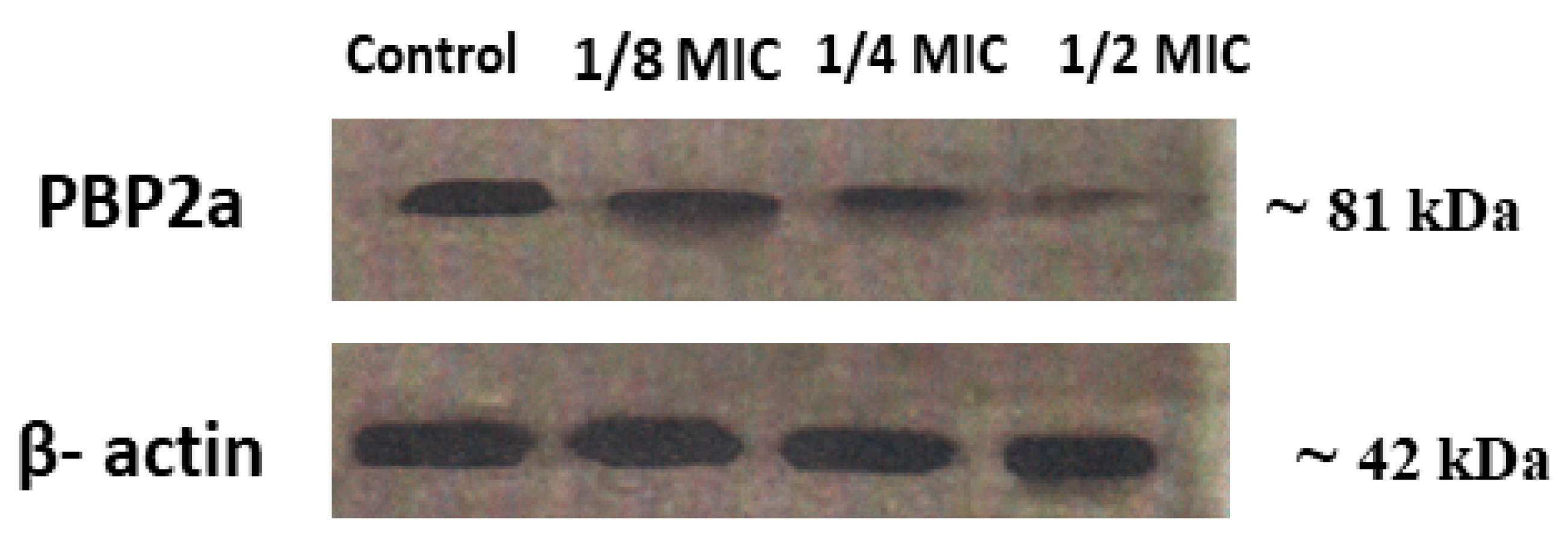
2.3.7. Expression of PBP2a in S. aureus and MRSA
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization
4.2. Characterization
4.3. The Viability of GA-NPs
4.4. Bacteria Strain Preparation
4.5. In Vitro Susceptibility Test
4.5.1. Disk Diffusion Method
4.5.2. MIC Assay
4.5.3. Time-Kill Assay
4.5.4. ATPase Activity Assay
4.5.5. Anti-Biofilm Activity of GA-NPs
4.5.6. Reverse Transcription qPCR
4.5.7. Western Blot Analysis
4.5.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, A.; Al-Salmi, F.A.; Saleh, M.A.; Sabatier, J.-M.; Alatawi, F.A.; Alenezi, M.A.; Albalwe, F.M.; Albalawi, H.M.R.; Darwish, D.B.E.; Sharaf, E.M. Inhibition Mechanism of Methicillin-Resistant Staphylococcus aureus by Zinc Oxide Nanorods via Suppresses Penicillin-Binding Protein 2a. ACS Omega 2023, 8, 9969–9977. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, E.M.; Hassan, A.; Al-Salmi, F.A.; Albalwe, F.M.; Albalawi, H.M.R.; Darwish, D.B.; Fayad, E. Synergistic antibacterial activity of compact silver/magnetite core-shell nanoparticles core-shell against Gram-negative foodborne pathogens. Front. Microbiol. 2022, 13, 929491. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Elebeedy, D.; Matar, E.R.; Elsayed, A.F.M.; El Maksoud, A.I.A. Investigation of angiogenesis and wound healing potential mechanisms of zinc oxide nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef]
- Hassan, A.; Al-Salmi, F.A.; Abuamara, T.M.M.; Matar, E.R.; Amer, M.E.; Fayed, E.M.M.; Hablas, M.G.A.; Mohammed, T.S.; Ali, H.E.; El-Fattah, F.M.A.; et al. Ultrastructural analysis of zinc oxide nanospheres enhances anti-tumor efficacy against Hepatoma. Front. Oncol. 2022, 12, 933750. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Yeh, W.W.; Wennersten, C.B.; Venkataraman, L.; Albano, E.; Alyea, E.P.; Gold, H.S.; Baden, L.R.; Pillai, S.K. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 2006, 44, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.M.; Clewell, D.B.; Gill, S.R.; Clark, N.C.; McDougal, L.K.; Flannagan, S.E.; Kolonay, J.F.; Shetty, J.; Killgore, G.E.; Tenover, F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003, 302, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Kawada-Matsuo, M.; Oogai, Y.; Hayashi, T.; Nakamura, N.; Komatsuzawa, H. Antibacterial effects of glycyrrhetinic acid and its derivatives on Staphylococcus aureus. PLoS ONE 2016, 11, e0165831. [Google Scholar] [CrossRef]
- Green, A.E.; Rowlands, R.S.; Cooper, R.A.; Maddocks, S.E. The effect of the flavonol morin on adhesion and aggregation of Streptococcus pyogenes. FEMS Microbiol. Lett. 2012, 333, 54–58. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhang, Y.; Kang, Y.; Xu, W.; Jiang, L.; Guo, T.; Huan, C. Glycyrrhizin inhibits PEDV infection and proinflammatory cytokine secretion via the HMGB1/TLR4-MAPK p38 pathway. Int. J. Mol. Sci. 2020, 21, 2961. [Google Scholar] [CrossRef]
- Ganjhu, R.K.; Mudgal, P.P.; Maity, H.; Dowarha, D.; Devadiga, S.; Nag, S.; Arunkumar, G. Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease 2015, 26, 225–236. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Hayashi, H.; Yokoshima, K.; Chiba, R.; Fujii, I.; Fattokhov, I.; Saidov, M. Field Survey of Glycyrrhiza Plants in Central Asia (5). Chemical Characterization of G. bucharica Collected in Tajikistan. Chem. Pharm. Bull. 2019, 67, 534–539. [Google Scholar] [CrossRef]
- Li, J.-Y.; Cao, H.-Y.; Liu, P.; Cheng, G.-H.; Sun, M.-Y. Glycyrrhizic acid in the treatment of liver diseases: Literature review. BioMed Res. Int. 2014, 2014, 872139. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Han, H.-W.; Kwak, J.-H.; Jang, T.-S.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. Grapefruit Seed Extract as a Natural Derived Antibacterial Substance against Multidrug-Resistant Bacteria. Antibiotics 2021, 10, 85. [Google Scholar] [CrossRef]
- Hassan, A.; Mohsen, R.; Rezk, A.; Bangay, G.; Rijo, P.; Soliman, M.F.; Hablas, M.G.A.; Swidan, K.A.K.; Mohammed, T.S.; Zoair, M.A.; et al. Enhancement of Vitamin C’s Protective Effect against Thimerosal-Induced Neurotoxicity in the Cerebral Cortex of Wistar Albino Rats: An In Vivo and Computational Study. ACS Omega 2024, 9, 8973–8984. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Rijo, P.; Abuamara, T.M.M.; Lashin, L.S.A.; Kamar, S.A.; Bangay, G.; Al-Sawahli, M.M.; Fouad, M.K.; Zoair, M.A.; Abdalrhman, T.I.; et al. Synergistic Differential DNA Demethylation Activity of Danshensu (Salvia miltiorrhiza) Associated with Different Probiotics in Nonalcoholic Fatty Liver Disease. Biomedicines 2024, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, R.; Vink, C.; Kalenic, S.; Friedrich, A.; Bruggeman, C.; Stobberingh, E. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2007, 13, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.-J.; Sit, N.-W.; Ooi, P.A.-C.; Ee, K.-Y.; Lim, T.-M. The antibacterial potential of honeydew honey produced by stingless bee (Heterotrigona itama) against antibiotic resistant bacteria. Antibiotics 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Carrera, B.; Clares, B.; Noé, V.; Mallandrich, M.; Calpena, A.C.; García, M.L.; Garduño-Ramírez, M.L. Cytotoxic evaluation of (2S)-5, 7-dihydroxy-6-prenylflavanone derivatives loaded PLGA nanoparticles against MiaPaCa-2 cells. Molecules 2017, 22, 1553. [Google Scholar] [CrossRef]
- Bălășoiu, R.M.; Biță, A.; Stănciulescu, E.C.; Bălășoiu, M.; Bejenaru, C.; Bejenaru, L.E.; Pisoschi, C.G. In vitro Antimicrobial Activity of Some Extracts of Salvia spp Harvested from the Oltenia Flora Using Different Solvents. Curr. Health Sci. J. 2023, 49, 397. [Google Scholar]
- Fiore, C.; Salvi, M.; Palermo, M.; Sinigaglia, G.; Armanini, D.; Toninello, A. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004, 1658, 195–201. [Google Scholar] [CrossRef]
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa, T.-O.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, Y.J.; Yu, M.H.; Bo, M.H.H.; Seo, H.J.; Lee, S.P.; Lee, I.S. Antimicrobial effect and resistant regulation of Glycyrrhiza uralensis on methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 2009, 23, 101–111. [Google Scholar] [CrossRef]
- Long, D.R.; Mead, J.; Hendricks, J.M.; Hardy, M.E.; Voyich, J.M. 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob. Agents Chemother. 2013, 57, 241–247. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, L.; et al. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 2020, 16, e1906206. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Senok, A.; Nassar, R.; Kaklamanos, E.G.; Belhoul, K.; Abu Fanas, S.; Nassar, M.; Azar, A.J.; Müller, E.; Reissig, A.; Gawlik, D.; et al. Molecular characterization of Staphylococcus aureus isolates associated with nasal colonization and environmental contamina-tion in academic dental clinics. Microb. Drug Resist. 2020, 26, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Tiwari, R.; Singh, V.K.; Singh, P.; Khadim, S.R.; Singh, U.; Laxmi; Srivastava, V.; Hasan, S.; Asthana, R.K. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J. Drug Deliv. Sci. Technol. 2019, 51, 164–176. [Google Scholar] [CrossRef]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: Growth inhibition, cell injury, oxidative stress and internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar] [CrossRef]
- Mun, S.-H.; Kang, O.-H.; Joung, D.-K.; Kim, S.-B.; Choi, J.-G.; Shin, D.-W.; Kwon, D.-Y. In vitro anti-MRSA activity of carvone with gentamicin. Exp. Ther. Med. 2014, 7, 891–896. [Google Scholar] [CrossRef]
- Blbulyan, S.; Trchounian, A. Impact of membrane-associated hydrogenases on the FOF1-ATPase in Escherichia coli during glycerol and mixed carbon fermentation: ATPase activity and its inhibition by N,N′-dicyclohexylcarbodiimide in the mutants lacking hydrogenases. Arch. Biochem. Biophys. 2015, 579, 67–72. [Google Scholar] [CrossRef]



| Test Material | Inhibition Zone of S. aureus | Inhibition Zone of MRSA |
|---|---|---|
| GA-NPs | 25 ± 0.04 | 16 ± 0.1 |
| GA | 16 ± 0.02 | 13 ± 0.05 |
| Linezolid (LZD) | 35 ± 0.01 | 23 ± 0.12 |
| Test Material | MIC (µg/mL) | |
|---|---|---|
| S. aureus | MRSA | |
| GA-NPs | 10.9 ± 0.01 | 9 ± 0.01 |
| GA | 13.9 ± 0.08 | 12 ± 0.03 |
| Linezolid (LZD) | 8.2 ± 0.01 | 7.4 ± 0.01 |
| Primer | Sequence (5′-3′) |
|---|---|
| 16S RNA | F:ACTCCTACGGGAGGCAGCAG |
| R:ATTACCGCGGCTGCTGG | |
| mecA | F:CAATGCCAAAATCTCAGGTAAAGTG |
| R:AACCATCGTTACGGATTGCTTC | |
| mecR1 | F:GTGCTCGTCTCCACGTTAATTCCA |
| R:GACTAACCGAAGAAGTCGTGTCAG | |
| blaR1 | F:CACTATTCTCAGAATGACTTGGT |
| R:GACTAACCGAAGAAGTCGTGTCAG | |
| blaZ | F:GCTTTAAAAGAACTTATTGAGGCTTC |
| R:CCACCGATYTCKTTTATAATTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijo, P.; Abuamara, T.M.M.; Ali Lashin, L.S.; Kamar, S.A.; Isca, V.M.S.; Mohammed, T.S.; Abdrabo, M.S.M.; Amin, M.A.; Abd El Maksoud, A.I.; Hassan, A. Glycyrrhizic Acid Nanoparticles Subside the Activity of Methicillin-Resistant Staphylococcus aureus by Suppressing PBP2a. Pharmaceuticals 2024, 17, 589. https://doi.org/10.3390/ph17050589
Rijo P, Abuamara TMM, Ali Lashin LS, Kamar SA, Isca VMS, Mohammed TS, Abdrabo MSM, Amin MA, Abd El Maksoud AI, Hassan A. Glycyrrhizic Acid Nanoparticles Subside the Activity of Methicillin-Resistant Staphylococcus aureus by Suppressing PBP2a. Pharmaceuticals. 2024; 17(5):589. https://doi.org/10.3390/ph17050589
Chicago/Turabian StyleRijo, Patricia, Tamer M. M. Abuamara, Lashin Saad Ali Lashin, Sherif A. Kamar, Vera M. S. Isca, Tahseen S. Mohammed, Mohamed S. M. Abdrabo, Mohamed A. Amin, Ahmed I. Abd El Maksoud, and Amr Hassan. 2024. "Glycyrrhizic Acid Nanoparticles Subside the Activity of Methicillin-Resistant Staphylococcus aureus by Suppressing PBP2a" Pharmaceuticals 17, no. 5: 589. https://doi.org/10.3390/ph17050589
APA StyleRijo, P., Abuamara, T. M. M., Ali Lashin, L. S., Kamar, S. A., Isca, V. M. S., Mohammed, T. S., Abdrabo, M. S. M., Amin, M. A., Abd El Maksoud, A. I., & Hassan, A. (2024). Glycyrrhizic Acid Nanoparticles Subside the Activity of Methicillin-Resistant Staphylococcus aureus by Suppressing PBP2a. Pharmaceuticals, 17(5), 589. https://doi.org/10.3390/ph17050589









