1. Introduction
Infections in the intestinal and respiratory tracts caused by antibiotic-resistant bacteria are among the top 10 leading causes of death and represent a challenge to public health and the global economy [
1,
2,
3]. Intestinal infections caused more than 2.648 million deaths in 2020, while respiratory infections caused more than 3.051 million deaths, causing high levels of morbidity and hospitalization [
1,
4,
5,
6]. These infections have been of concern to healthcare professionals and the scientific community, especially nowadays, due to cases of resistant hospital-acquired infections and bacterial coinfection with SARS-CoV-2, the etiologic agent of COVID-19 [
2,
7].
The bacteria that cause these infections include
Pseudomonas aeruginosa and
Klebsiella pneumoniae.
P. aeruginosa is an opportunistic Gram-negative pathogen that is associated with intestinal infections, pneumonia, and other diseases, such as irritable bowel syndrome or ulcerative colitis in hospitalized, immunocompromised patients. This bacterium is associated with structural lung diseases, such as cystic fibrosis, and is a common coinfectant in patients with COVID-19 [
3,
8,
9].
K. pneumoniae is related to urinary tract infections, intestinal infections, and pneumonia, as well as infections affecting elderly patients with chronic diseases and/or respiratory diseases or the immunosuppressed. It causes secondary coinfection during hospitalization in patients with COVID-19, aggravating the clinical picture and causing longer hospital stays, poor prognosis, and mortality [
8,
10,
11].
Infections caused by
P. aeruginosa and
K. pneumoniae are difficult to treat, especially when they are resistant to antimicrobials and biofilm producers [
12,
13]. These infections are common in hospitals, especially among long-term hospitalized patients exposed to invasive devices, thus requiring a strategic therapeutic regimen [
3,
14]. Furthermore, the ability of
P. aeruginosa and
K. pneumoniae to form biofilms is a major cause of therapeutic failure [
9,
13,
14].
Research into new antibiotics is essential, due to the increase in bacterial resistance that limits the use of existing medicines and treatments. The infections caused by
K. pneumoniae and
P. aeruginosa are recognized worldwide for causing high rates of morbidity and mortality, especially when these infections are secondary and opportunistic [
15,
16]. To treat these infections, polymyxins, ceftazidime–avibactam, tigecycline, sulbactam and sulbactam-containing combinations, aminoglycosides, fosfomycin, and especially those combination therapies containing these antimicrobial agents, are used [
17,
18].
The diversity in response to treatment demonstrates the complexity of this scenario, mainly with infections promoted by bacteria with drug resistance profiles that may vary between patients, including resistance to carbapenems, fluoroquinolones, and other classes of antibiotics. Thus, bacterial resistance, the formation of biofilms, and the lack of correct treatment are the limitations of the use of conventional antibiotics, requiring new antibacterial therapeutic strategies in the treatment of serious infections [
19,
20].
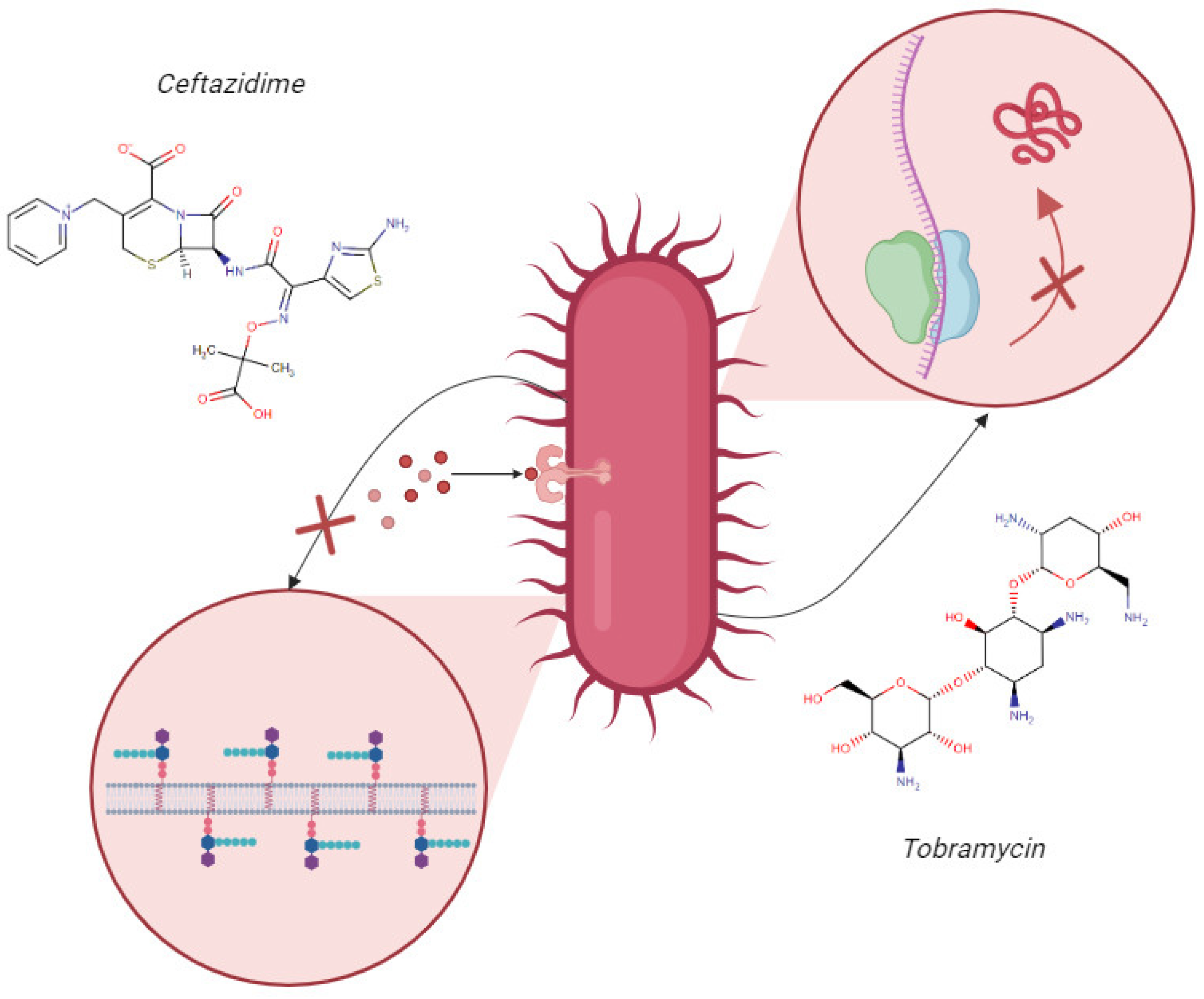
Therefore, the use of combined antimicrobial therapy of an aminoglycoside, such as tobramycin (TOB), associated with a β-lactam, such as ceftazidime (CAZ), for treating infections caused by resistant strains and biofilm formers is an alternative option [
10,
21,
22]. The therapeutic combination of ceftazidime and tobramycin has emerged as a promising strategy in the treatment of multidrug-resistant bacterial infections. This approach is widely used in clinical practice, due to its potential for synergistic interaction [
23,
24]. The synergistic potential is attributed to the distinct mechanisms of action of these two molecules. CAZ binds to bacterial cell wall proteins and inhibits bacterial cell wall synthesis, promoting the disruption of the cell wall, due to instability, and bacterial death (
Figure 1). Meanwhile, TOB enters more easily into the bacterial cell, due to the instability of the cell wall, binding to the 30S subunit of the bacterial ribosome, interfering with protein synthesis, and causing bacterial death (
Figure 1) [
24,
25].
Therefore, when combining ceftazidime and tobramycin, the synergistic potential of their complementary mechanisms of action favors the possibility of a more effective therapy against bacterial infections, especially in cases of multidrug resistance and in patients with cystic fibrosis [
23,
24,
25]. However, these drugs are unstable, due to pH variation, are sensitive to degradation by digestive enzymes, and have low oral and nasal bioavailability, limiting their administration by these routes [
21,
22].
Encapsulating these drugs in chitosan-coated zein nanoparticles is a proposed antimicrobial therapy that can be used to overcome these limitations, increase bioavailability, and enable the delivery of the antimicrobials within biofilms [
26,
27]. Zein is a protein that forms a non-toxic, biocompatible, gastro-resistant, mucoadhesive, easily obtained, and low-cost polymer with hydrophilic and hydrophobic regions [
27,
28]. Chitosan is a natural polysaccharide derived from chitin that has non-toxic, biocompatible, mucoadhesive, biodegradable, and antimicrobial characteristics. Coating zein nanoparticles with this polymer can improve the stability and efficiency of the drug encapsulation, making it an advantageous alternative for the controlled release of antimicrobials [
26,
27,
29].
Thus, this research presents an innovative character as it proposes, as goals, encapsulating and co-encapsulating CAZ and TOB in chitosan-coated zein nanoparticles for oral and nasal administration and evaluating the antibacterial activity of these nanocarriers against multidrug-resistant and biofilm-producing P. aeruginosa and K. pneumoniae.
3. Discussion
In this study, ZNP–CH, CAZ–ZNP–CH, TOB–ZNP–CH, and CAZ–TOB–ZNP–CH showed particle sizes smaller than 340 nm and PDI values smaller than 0.3. A small difference in the average diameter was observed between ZNP–CH, CAZ–ZNP–CH, TOB–ZNP–CH, and CAZ–TOB–ZNP–CH. As it is a nanosphere, chemical interaction with the encapsulated drugs can lead to the formation of more compact nanoparticles, changing the density and the size of the nanoparticles [
30,
31]. For this study, these small size variations are not relevant, since we designed these nanoparticles to be absorbed or bound to the mucosa, being developed as therapeutic strategies for oral and nasal applications [
32,
33].
A Ø value between 100 and 700 nm is ideal for nasal and oral applications, since NPs with this particle size can be transported intracellularly through the nasal epithelium [
32], while NPs with sizes between 300 and 500 nm can be endocytosed in the intestine, mainly by M cells, which are specialized epithelial cells that line the lymphoid follicles of Peyer’s plates and can reach more specific biological targets due to their size and relative mobility in the intestine [
33]. The PDI sets the homogeneity profile of dispersions, and values equal to or less than 0.3 indicate a monodisperse profile and are ideal for in vivo applications [
30].
ZNP–CH, CAZ–ZNP–CH, TOB–ZNP–CH, and CAZ–TOB–ZNP–CH showed a positive surface charge with ζ above +35 mV. The ζ is a parameter that indicates nanoparticle stability, since values greater than +30 mV promote nanoparticle repulsion, minimizing the aggregation between them [
33,
34]. Positively charged nanocarriers exhibit interactions with airway mucosa, as strong electrostatic interactions occur with the sialic and acidic anionic parts of the glycosaminoglycans contained in mucin and on the surface of the airway cells [
35,
36]. Similarly, these positively charged surface-active nanostructures can prolong their residence time in the small intestine and exhibit strong adhesion to the intestinal mucosa due to their electrostatic interactions with negative charges in the mucus layer of this environment. Therefore, they may be more likely to be captured by Peyer’s patches [
36,
37].
Since parameters such as Ø, PDI, ζ, and pH are essential for determining the administration route, formulation stability, and applications for in vivo testing, the chitosan-coated zein nanoparticles developed in this study are suitable for administration through the nasal and oral routes. The pH values of the nanoparticles were around 5. The pH of the nasal fluid is slightly acidic, and is between 5.5 and 6.5, while the stomach pH can range between 1.5 and 2.0, and the intestinal pH between 6.0 and 8.0 [
38,
39].
The %EE of the CAZ in the nanoparticles ranged between 73.68 ± 0.7 and 87.28 ± 0.2%, and that of the TOB ranged between 57.47 ± 0.5 and 63.38 ± 0.7% (
Table 1). The literature indicates a wide %EE variation of β-lactams and aminoglycosides in polymeric nanoparticles due to the chemical affinity of the drugs with the constituent polymers of NPs [
39,
40,
41,
42,
43].
A reduction of approximately 10% occurred regarding the %EE of CAZ and TOB in CAZ–TOB–ZNP–CH. Drug co-encapsulation is a challenge, since encapsulating two drugs in the same nanoparticle can generate competition for binding sites [
44,
45]. However, co-encapsulation can allow for synergism, enhanced antimicrobial activity, a broadening of the antimicrobial spectrum, a decreased stimulation of resistance development, a relative dosage adjustment of multiple drugs, reduced toxic effects, and cost effectiveness [
44,
45].
Coating zein nanoparticles with carbohydrates increases encapsulation and improves the physical and photochemical stability of the drug, promoting greater molecular interaction between the drugs and their biological targets and facilitating the formation of nanoparticles with ideal physicochemical features for in vitro and in vivo applications [
27,
46,
47]. Furthermore, chitosan has been shown to interact with the mucosal surfaces of the nasal, pulmonary, and gastrointestinal tracts [
48,
49]. Thus, its bioadhesiveness and resistance to degradation make this polymer convenient for coating particles for nasal and oral applications [
29].
In the images, small aggregates of ZNP–CH and CAZ–ZNP–CH can be seen that do not interfere with the PDI of the formulations, as seen in
Figure S2. It has been highlighted that no aggregates are observed in CAZ–TOB–ZNP–CH, this being the final formulation proposed in the treatment of
K. pneumoniae and
P. aeruginosa infections [
31,
50,
51,
52].
In the FTIR spectrum, absorption bands between 1600–1700 and 1510–1570 cm
−1 representing the characteristics of amide I and amide II are present in all of the nanoparticles [
53,
54]. An absorption band ranging from 3500 to 3000 cm
−1, referring to the NH
3+ groups of the protonated side chain of amino acid lysine, was also observed in all of the nanoparticles [
55]. It was also observed that symmetric C–H strain-related vibration at 1375 cm
−1, the antisymmetric C–O–C stretching and C–N stretching at 1150 cm
−1, the C–O stretching vibration at 1026 cm
−1, and the peaks between 896 and 1154 cm
−1 correspond to the saccharide structure of chitosan [
56,
57]. For CAZ, another stretching at around 1670 cm
−1 was evident, due to the axial deformation of the amide group C=O at 1450 cm
−1 by the C–N bond and between the bands 600–800 cm
−1 by the adjacent hydrogen deformations [
58,
59]. The TOB spectrum, in turn, presents absorption bands at 3400–3200 cm
−1, due to N–H or O–H stretching; at 2910 cm
−1, due to aliphatic C–H stretching; at 1588 cm
−1, due to N–H bending; at 1461 cm
−1, due to CH2 grouping; at 1349–1380 cm
−1, due to in-plane O–H bending vibration; and at 1032 cm
−1, due to C–N or C–O stretching [
60,
61].
In the XRD figure, the peaks of 9.6° and 19.4° correspond to zein’s presence, indicating the molecule’s amorphous character [
27,
62]. However, through some intermolecular interactions with other compounds, one can have new diffraction of crystalline character [
62]. The 20.4° peak corresponds to the chitosan presence, since two peaks are found in the diffractogram of this molecule at around 2θ = 10.5° and 20°, due to its high degree of crystallinity [
63,
64]. In
Figure 4, other peaks (2θ = 21°, 22°, 24°, 25°, 36°, 40°, 43°, and 44°) of varying intensity were identified in the nanoparticles; however, they do not correspond to the characteristic peaks of CAZ (2θ at 20.2°, 21.5°, and 22.3°) [
58,
59,
65,
66] or TOB (2θ at 17, 7°, 18.3°, and 18.8°) [
61,
65], indicating only a small degree of crystallinity of these nanoparticles and possible hydrogen bonds or electrostatic interactions between the drugs, the carrier polymer, and the coating polymer.
In the TGA thermogram, this mass loss profile presented by the nanoparticles developed in this study is different from the zein degradation profile because, at 83.7 °C, there is a 5.6% loss, and, at 385.2 °C, there is a 73.8% loss [
67]. Lysine has two weight losses in the temperature ranges of 255–365 °C (57%) and 478–589 °C (24%) [
68], and chitosan presents three weight loss ranges, the first being between 50 and 100 °C (5%), the second between 280 and 390 °C (70%), and the third above 390 °C (15%) [
69,
70].
The zein mass loss is attributed to the breakdown of amino acid residues and the cleavage of the peptide bonds of the protein [
67]. Meanwhile, chitosan mass loss occurs at the first point due to the evaporation of the water adsorbed to the chitosan polymer by hydrogen bonds, at the second by deacetylation depolymerization and cleavage of glycosidic bonds, and at the third by the decomposition of the residual carbon [
69].
CAZ has mass loss at intervals of 50–100 °C of around 10%, and, above 190 °C, loss of around 75% occurs [
71,
72]. TOB shows a mass loss of around 90% between 25 °C and 125 °C [
60,
73]. The thermogram results show no significant mass loss between 25 °C and 200 °C, evidencing CAZ and TOB encapsulation and protection against heat degradation for the drugs and the thermal stability of the nanoparticles (
Figure 5). In the DSC thermogram, zein demonstrates endothermic peaks in the range of 50–100 °C [
74,
75]. Chitosan, in turn, is characterized by an endothermic peak near 100 °C and an exothermic peak at around 270 °C [
74,
76]. Lysine shows two endothermic peaks at 74.1 °C and 259.3 °C [
77,
78]. CAZ shows an endothermic peak at around 120 °C [
71], while TOB shows three endothermic peaks at 115.64 °C, 176.70 °C, and 230 °C, and an exothermic peak at 206.94 °C [
65]. We can observe that the peaks corresponding to zein, lysine, and chitosan have been shifted, and the peaks corresponding to the drugs are not evident, indicating CAZ and TOB encapsulation in the nanoparticles and an improved thermal stability of these antibiotics.
In a stable state at gastrointestinal pH, there was an increase in the nanoparticle size, a decrease in PDI, and a reduction in zeta potential at pH 1.2 and pH 6.8. Other studies in the literature have also proven the changes in these parameters after the exposure of chitosan-coated zein NPs to different pHs, such as the research of Wang et al. [
79], who performed zein nanoparticle coating with carboxymethyl chitosan encapsulating β-carotene with antioxidant potential, and Khan et al. [
80], who developed alginate/chitosan-coated zein nanoparticles containing resveratrol.
With values of pH below 6.0, chitosan undergoes a protonation process of the NH
2 groups and, consequently, the expansion of the polymer chain and an increase in particle size [
79,
80]. Thus, it can be observed that the change in the diameter of the nanoparticles with the decrease in pH is related to the phenomenon of the protonation of the amino groups present in the molecular structure of chitosan [
81,
82]. The decrease in the zeta potential happens as an electrostatic effect of the adsorption of the other molecules and ions present in the simulated gastric fluid [
76,
83]. Furthermore, studies show that the presence of ions in the medium that are used to form gastric solutions, as mentioned above, can partially neutralize these positive charges, leading to less electrostatic repulsion between the particles and less aggregation [
84,
85].
When increasing the pH to 6.8, deprotonation of the ionized groups (-NH3+) occurs, and, thus, there is a reduction in the zeta potential, leading to less electrostatic repulsion between the nanoparticles and the promotion of colloidal aggregation and increased particle size [
32,
77]. The reduction in the surface charge may be an indication of nanoparticle destabilization, and, for application in intestinal infections, it is relevant that there is a destabilization and erosion of the nanoparticles in order to release the medicines into the intestinal region, promoting the fight against the resistant bacterial strains [
82,
83,
85]. CAZ–ZNP–CH, TOB–ZNP–CH, CAZ–TOB–ZNP–CH, and ZNP–CH demonstrate colloidal stability by electrostatic and steric repulsion under simulated gastrointestinal conditions, demonstrating their potential for future applications as oral antimicrobial delivery vehicles for intestinal delivery.
As for stability in months, some studies have evaluated the stability of carbohydrate-coated zein nanoparticles. Yuan et al. [
86], Cai et al. [
87], and Zhang et al. [
54] developed zein nanoparticles containing curcumin coated with dextran, pectin, and fucan, respectively. The authors evaluated the stability for 28 days and observed no significant macroscopic, particle size, or PDI changes. Other studies have already shown the long-term stability of chitosan-coated zein nanoparticles, such as Chen et al. [
88], who encapsulated curcumin and piperine in chitosan-coated zein nanoparticles, and Xiao et al. [
89], who produced zein nanoparticles associated with carboxymethyl chitosan containing genistein. These authors observed that Ø, PDI, and ζ showed no significant changes after a 60-day storage period.
In the release kinetics tests, the results show that the formulations showed a kinetic profile with a rapid initial release, followed by a controlled release for up to 24 h in the simulated gastrointestinal fluids. The controlled release profile of antibiotics in simulated gastric and intestinal pH is influenced by coating with chitosan, as reported in the studies of Pauluk et al. [
90], Chen et al. [
91], Zhou et al. [
92], and Ruan et al. [
93], who encapsulated resveratrol, β-carotene, quercetin, and astilbine, respectively, in chitosan-coated zein nanoparticles. The chitosan coating allows for controlled drug release because it forms a thick, dense layer around the zein nanoparticles that promotes the slower release of the active ingredients [
77,
93].
P. aeruginosa and
K. pneumoniae are bacteria that cause intestinal and respiratory infections affecting a large portion of the world’s population and are on the list of bacteria that pose the greatest risk to human health [
94,
95]. The
P. aeruginosa isolates used in this study present a resistance to quinolones, polymyxins, cephalosporins, carbapenems, and other β-lactams [
96]. The
K. pneumoniae isolates used in this study carry the
blaKPC-2 gene encoding the Ambler class A carbapenemase and the
acrB and
acrF genes encoding efflux pumps, conferring their resistance to carbapenems, cephalosporins, aminoglycosides, and quinolones [
30]. The accumulation of various resistance mechanisms results in infections with high mortality rates due to the scarcity and inefficiency of therapies. Thus, is necessary to develop new therapeutic options [
96,
97]. Some studies have developed therapeutic strategies to combat bacterial infections, testing the antibacterial activity of CAZ or TOB encapsulated alone in nanocarriers, especially liposomes, against bacterial strains with and without an antibiotic resistance profile [
98,
99,
100,
101].
Torres et al. [
98] encapsulated CAZ in liposomes (LIPO–CAZ) and tested the antimicrobial potential against
P. aeruginosa strain SPM-1 (clinical isolate resistant to cefepime and ceftazidime). CAZ and LIPO–CAZ showed MICs of 1024 µg/mL and 512 µg/mL against
P. aeruginosa SPM-1, respectively. Hedayati Ch et al. [
101] tested the activity of TOB encapsulated in niosomes against
P. aeruginosa strains resistant to β-lactams, aminoglycosides, and quinolones. The MIC values for the TOB ranged from 2 to 8 µg/mL, and, for the TOB-containing niosomes, the range was observed from 0.125 to 2 µg/mL. The MBC values varied from 2 to 8 µg/mL for the TOB and from 0.125 to 4 µg/mL for the niosomes containing TOB.
The data show that the CAZ or TOB encapsulated in the nanocarriers exhibit antibacterial potential. However, the nanocarriers developed by Torres et al. [
98] and Hedayati Ch et al. [
95] are lipidic and without a polymeric coating; therefore, they are unstable, because they undergo rapid enzymatic digestion and bile salt actions that interact with the liposomes, such as surfactants in the gastrointestinal tract [
102]. Thus, these liposomes cannot be administered orally, unlike CAZ–ZNP–CH and TOB–ZNP–CH.
Nevertheless, CAZ–TOB–ZNP–CH presented more in vitro antibacterial activity than CAZ–ZNP–CH or TOB–ZNP–CH, evidencing that antibiotic combination potentiates the antimicrobial effect. Some studies have performed the co-encapsulation of antibacterial agents and have evaluated their action. Schiffelers et al. [
103] developed liposomes encapsulating gentamicin and ceftazidime (LE–GN–CZ), testing their antibacterial activity in vivo in mice with lung infections caused by resistant strains of
K. pneumoniae. A single dose of LE–GN–CZ (2.5/1.6 mg/kg) applied for 14 days increased the animal survival rate compared to the LE–GN (20 mg/kg) and LE–CZ (12.5 mg/kg) formulations applied alone, showing that the synergistic interaction was effective in overcoming infections promoted by resistant
K. pneumoniae.
Ye et al. [
99] encapsulated clarithromycin (CLA) and TOB in liposomes (TOB/CLA–CPROLips) and tested the antibacterial activity against
P. aeruginosa strain PAO1. CLA, TOB, and TOB/CLA–CPROLips had MIC values of >16 µg/mL, 16 µg/mL, 1 µg/mL, respectively. Wang et al. [
100] developed liposomes containing colistin and ciprofloxacin to treat infections caused by multidrug-resistant
P. aeruginosa H131300444 and
P. aeruginosa H133880624. The MIC value of colistin was 128 µg/mL against both of the strains, while ciprofloxacin was 16 µg/mL for
P. aeruginosa H133880624 and 8 µg/mL for
P. aeruginosa H131300444. The combination of the two drugs co-encapsulated in the liposomes at a concentration of 8 µg/mL eradicated the growth of both of the strains within 24 h.
The co-encapsulation of these drugs in the nanocarriers developed by Schiffelers et al. [
97], Ye et al. [
99], and Wang et al. [
100] showed antibacterial potential, as well as CAZ–TOB–ZNP–CH. However, the use of CAZ–TOB–ZNP–CH has become promising for oral and nasal administration, unlike the liposomes developed by the above authors.
In this study, we have observed the antibacterial potential of all of the formulations encapsulating CAZ and TOB, especially the formulation containing both drugs. This potentiation of antibacterial action happened because of the association of the two different action mechanisms, since CAZ acts by inhibiting the synthesis of the peptidoglycan of the bacteria cell wall and TOB induces the formation of non-functional proteins compromising the bacterial metabolism, leading to bacterial death [
104,
105].
Thus, combination therapy is considered an effective strategy to treat multidrug-resistant bacterial infections. The administration of combined drugs in a single vehicle enables the synergistic action of the different mechanisms of action, the delivery of the drugs to the infection sites, the proper exposure of patients to the drug, and the reduced stimulus for developing bacterial resistance [
46,
106], making co-encapsulation in chitosan-coated zein nanoparticles a promising alternative for resistant respiratory and intestinal infections.
Biofilm is a virulence factor with clinical relevance, as it is associated with healthcare infections, causing concern for healthcare professionals and the general population [
107]. Poor antibiotic penetration through the biofilm matrix and the presence of persistent cells contribute to the resistance of biofilm-forming bacteria to antibiotics, leading to persistent infections that cause hospitalization, patient suffering, and reduced quality of life [
103,
104]. Biofilms are constantly associated with human diseases, including surgical implant infections, gum disease, and digestive, urinary, and respiratory tract infections induced by catheters and other invasive devices [
107,
108]. The infections caused by these bacteria become serious when they colonize the GIT of hospitalized and immunocompromised patients [
107,
108,
109]. Moreover, biofilms have been associated with the initiation and development of stomach, small intestine, and colon cancer by producing genotoxins. They are also associated with inflammatory bowel disease, especially the development of ulcerative colitis and Crohn’s disease caused by
P. Aeruginosa and
K. pneumoniae [
110,
111].
In the respiratory tract,
P. aeruginosa produces biofilms in the sinuses, becoming a reservoir in lung abscesses in ventilator-associated pneumonia, in bronchiectasis, and in chronic lung infections associated with cystic fibrosis [
112,
113]. Meanwhile,
K. pneumoniae is associated with biofilm production in pneumonia, promoting the pathogenicity and chronicity of respiratory infections [
114,
115]. The biofilm-producing ability of
P. aeruginosa and
K. pneumoniae is associated with increased morbidity and mortality in patients, especially those with other infections, such as COVID-19 [
9,
11]. Thus, a treatment with the potential to eliminate this microorganism, inhibit biofilm formation, and/or eradicate biofilms already formed is needed [
107,
108,
109,
110].
Biofilm production is an important factor in the survival and virulence of
P. aeruginosa and
K. pneumoniae in adverse environmental conditions, including hospital environments, especially in intensive care units (ICU) and surgical centers, facilitating the establishment and maintenance of chronic and persistent infections [
116]. The aim of inhibiting biofilm formation has led to some studies proposing the co-encapsulation of antimicrobial agents in nanocarriers. Mahdiun et al. [
117] encapsulated bismuth-ethanediol (BiEDT) and TOB in niosomes (Nio–BiEDT–TOB) and tested the antibiofilm activity at subinhibitory concentrations (MIC/2, MIC/4, MIC/8, and MIC/16) against
P. aeruginosa ATCC 27853. The inhibition of BiEDT, TOB, and Nio–BiEDT–TOB against this bacterium ranged from 35% to 60%, 45% to 63%, and 45% to 80%, respectively.
Ye et al. [
99] encapsulated CLA and TOB in liposomes (TOB/CLA–CPROLips) and tested the inhibition of biofilm formation against
P. aeruginosa strain PAO1. At subinhibitory concentrations, CLA, TOB, and TOB/CLA–CPROLips inhibited the biofilm formation by 2% to 5%, 5% to 15%, and 15% to 30%, respectively. The studies by Mahdiun et al. [
111] and Ye et al. [
99] show lower percentages of biofilm formation inhibition than those observed for CAZ–ZNP–CH, TOB–ZNP–CH, and CAZ–TOB–ZNP–CH, indicating that the encapsulation of CAZ and TOB alone, or in combination, in chitosan-coated zein nanoparticles have greater potential for antibiofilm activity and can be administered orally and nasally.
The physicochemical properties of the formulations influence the antibiofilm activity. The coating of zein nanoparticles by chitosan imparts a positive surface charge onto CAZ–ZNP–CH, TOB–ZNP–CH, and CAZ–TOB–ZNP–CH, enabling the electrostatic interaction of these nanocarriers with the surface of the negatively charged bacterial cells. This interaction can reduce the adhesion of the bacteria to surfaces, preventing biofilm formation [
118,
119]. The literature has proven the antibiofilm activity of CAZ and TOB. CAZ reduces the expression of the
ibpA gene, decreasing bacterial motility; reduces the expression of adhesion genes
fimG,
csgA, and
ybgD; reduces the expression of the motility gene
flgA and the genes regulating quorum sensing (QS); reduces the communication process between bacterial cells
luxS and
luxR; increases the expression of the indole synthesis gene
tnaA, negatively modulating biofilm production; and inhibits the production of bis-(3′,5′)-cyclic dimeric guanosine monophosphate (c-di-GMP), which promotes the biosynthesis of exopolysaccharides, such as Pel and alginate, thereby negatively modulating biofilm formation [
120,
121,
122]. As for TOB’s antibiofilm activity, this drug suppresses the gene expression of
pelA and
pslA, genes encoding synthesis of the exopolysaccharides Pel and Psl, which promote the attachment of bacteria to surfaces, thus inhibiting biofilm formation [
101]. Thus, this study suggests that chitosan-coated zein nanoparticles containing CAZ and/or TOB inhibit biofilm formation by the electrostatic interaction of the nanoparticle surface with the bacterial surface and by modulating the gene expression promoted by the drugs.
The biofilms produced are associated with antibacterial therapy failure, especially in healthcare-related infections (HAIs), causing longer hospital stays, high morbidity and mortality rates, and economic burden; therefore, treating these infections is a challenge, due to the scarcity of drugs that can eradicate biofilms [
123,
124].
From the perspective of eradicating biofilms, Halwani et al. [
125] developed liposomes encapsulating bismuth-thiol and tobramycin (LipoBiEDT–TOB) and evaluated the antibiofilm activity against aminoglycoside-resistant
P. aeruginosa strains (PA-48912-1, PA-4892-2, and PA-48913) isolated from patients with cystic fibrosis. The formulations encapsulating the antimicrobials in isolation and with free drugs did not eradicate biofilms at the tested concentrations; however, LipoBiEDT–TOB showed MBEC values of 64 µg/mL for PA-48912-1, 256 µg/mL for PA-4892-2, and 512 µg/mL for PA-48913. A study by Halwani et al. [
125] showed no MBEC values for the formulations encapsulating only one drug, unlike the results for CAZ–ZNP–CH and TOB–ZNP–CH (12.5 to 50 µg/mL), which eradicated the biofilm. Moreover, the MBEC values of CAZ–TOB–ZNP–CH show that this formulation has a higher potential to eradicate biofilms than the formulation developed by Halwani et al. [
125].
Some of the physicochemical aspects of the formulations, such as the particle size and zeta potential, are critical for biofilm eradication. The size of the nanoparticles collaborates in the penetration of the drugs through the exopolysaccharide matrix, since NPs between 10 and 500 nm penetrate through the water channels and biofilm pores [
126,
127]. In the present study, the average diameter of the nanoparticles ranged from 314 to 336 nm.
In this study, the zeta potential of the nanoparticles ranged between +39 and +50 mV. The surface charge of the nanoparticles is another important parameter for penetrating biofilms, as positively charged nanocarriers are more attracted to biofilm surfaces (negative charge) and are more likely to penetrate and accumulate drugs inside biofilms, possibly eradicating them [
126,
128].
Furthermore, to eradicate biofilms, the direct interaction of nanocarriers with bacterial cells in the biofilm and/or bacteria detaching from the polymeric matrix, the interaction or denaturation of the EPS matrix, and cell death induction by action of antimicrobials become essential [
120,
126,
127]. Thus, the chitosan-coated zein nanoparticles containing CAZ and TOB developed in this study are candidates for therapies for infections caused by biofilm-forming
P. aeruginosa and
K. pneumoniae [
108,
128,
129].