Ethnomedicinal Study and Evaluation of the Anxiolytic-like and Diuretic Effects of the Orchid Stanhopea tigrina Bateman ex Lindl—(Orchidaceae)
Abstract
1. Introduction
2. Results
2.1. Ethnomedicinal Study
2.2. Chemical Characterization of S. tigrina Extracts
2.3. Determination of In Vitro Antioxidant Capacity Using ABTS and DPPH
2.4. Acute Toxicity Assessment
2.5. Test to Determine the Anxiolytic and Sedative Activity of Extracts of S. tigrina
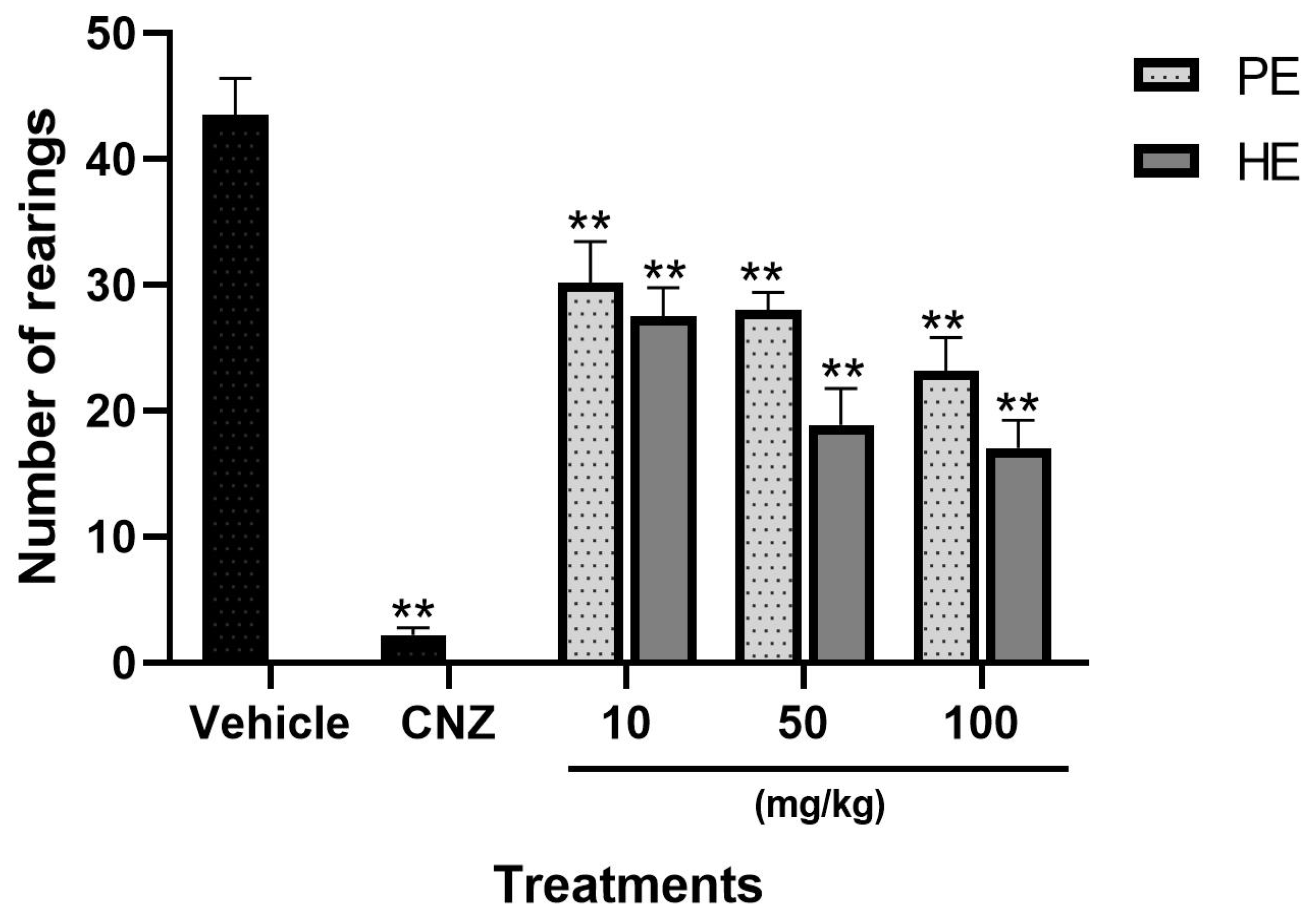
2.5.1. Exploratory Cylinder Test
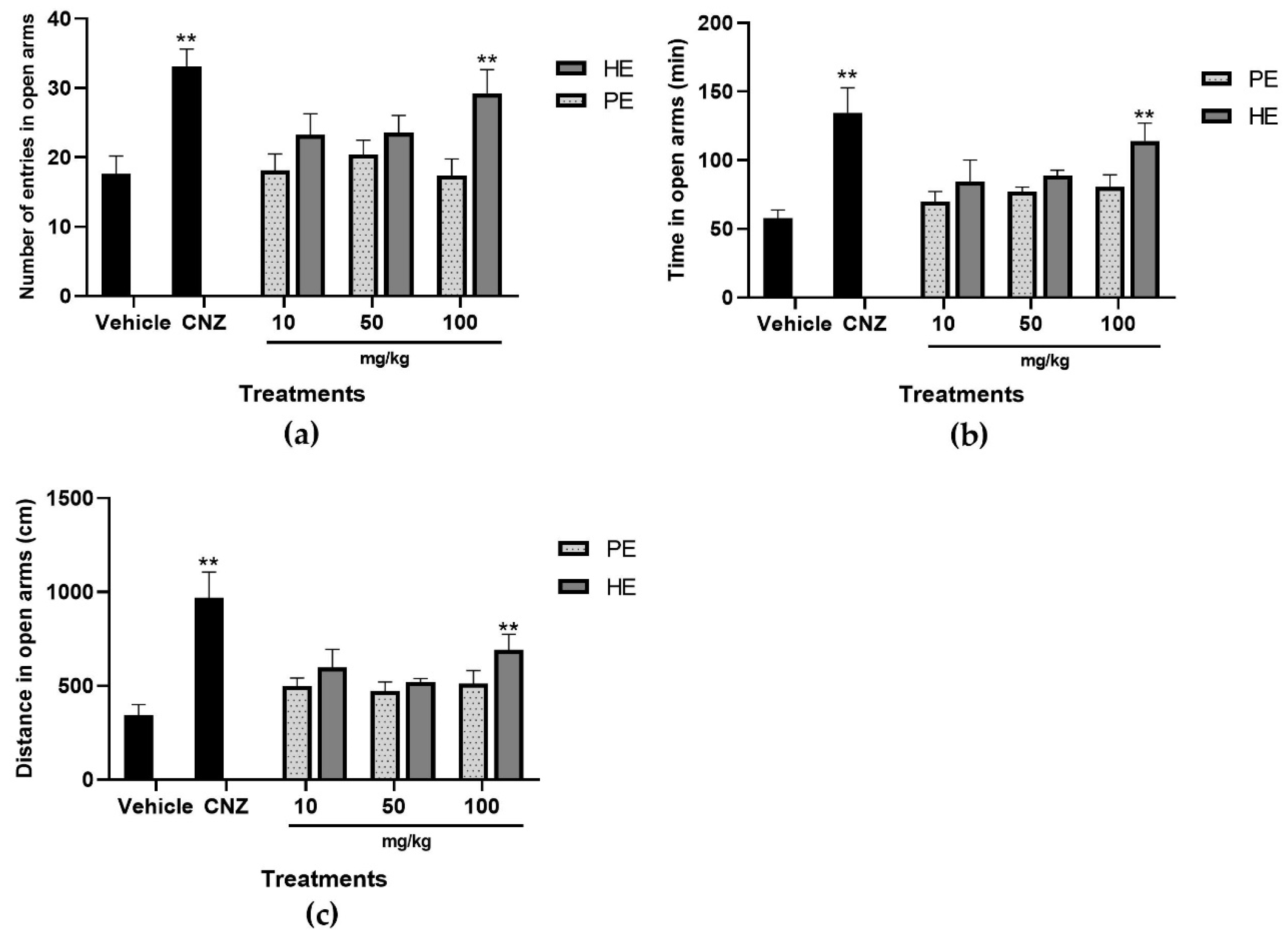
2.5.2. Elevated plus Maze
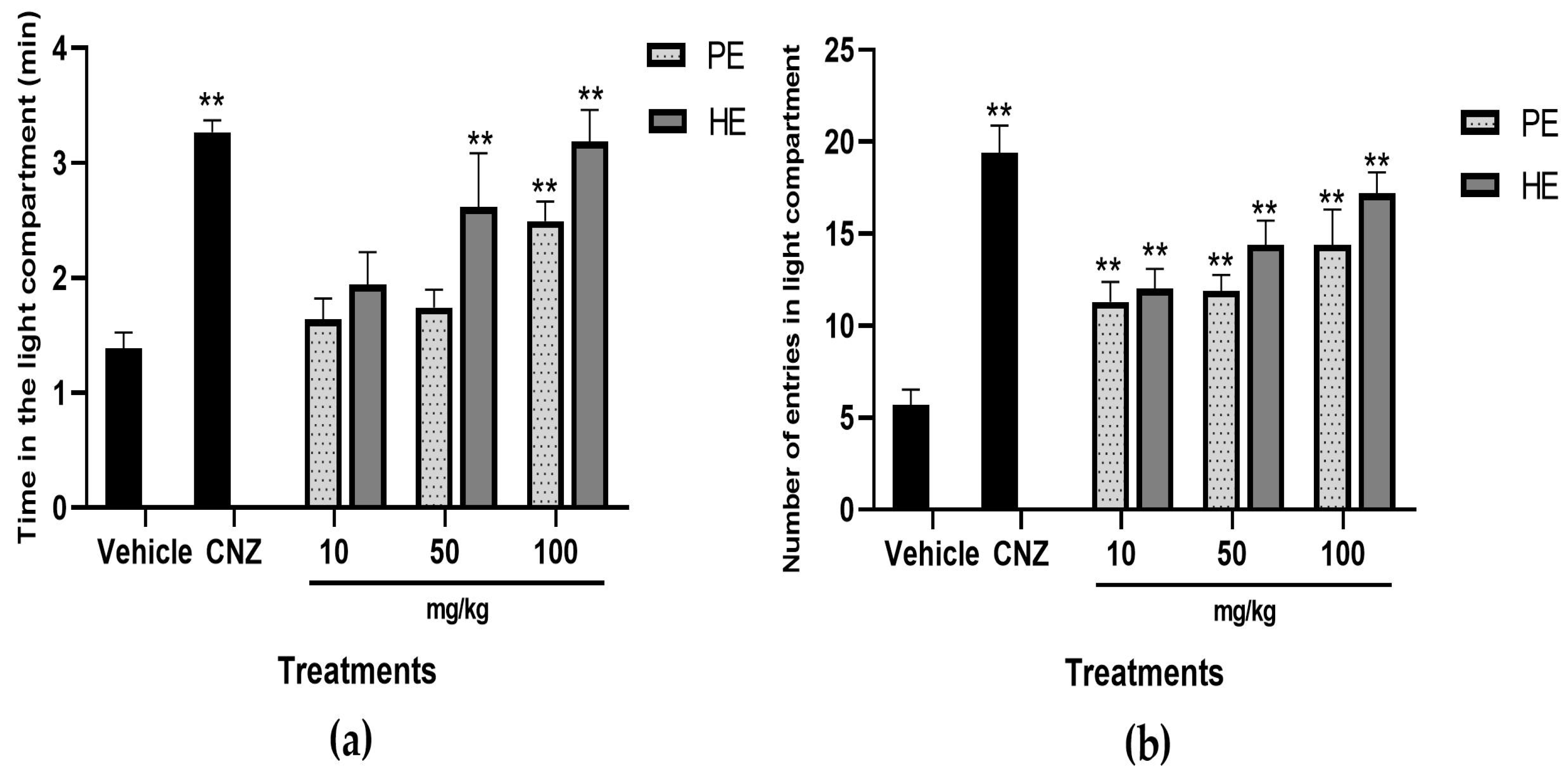
2.5.3. Light–Dark Box Test
2.5.4. Open-Field Test
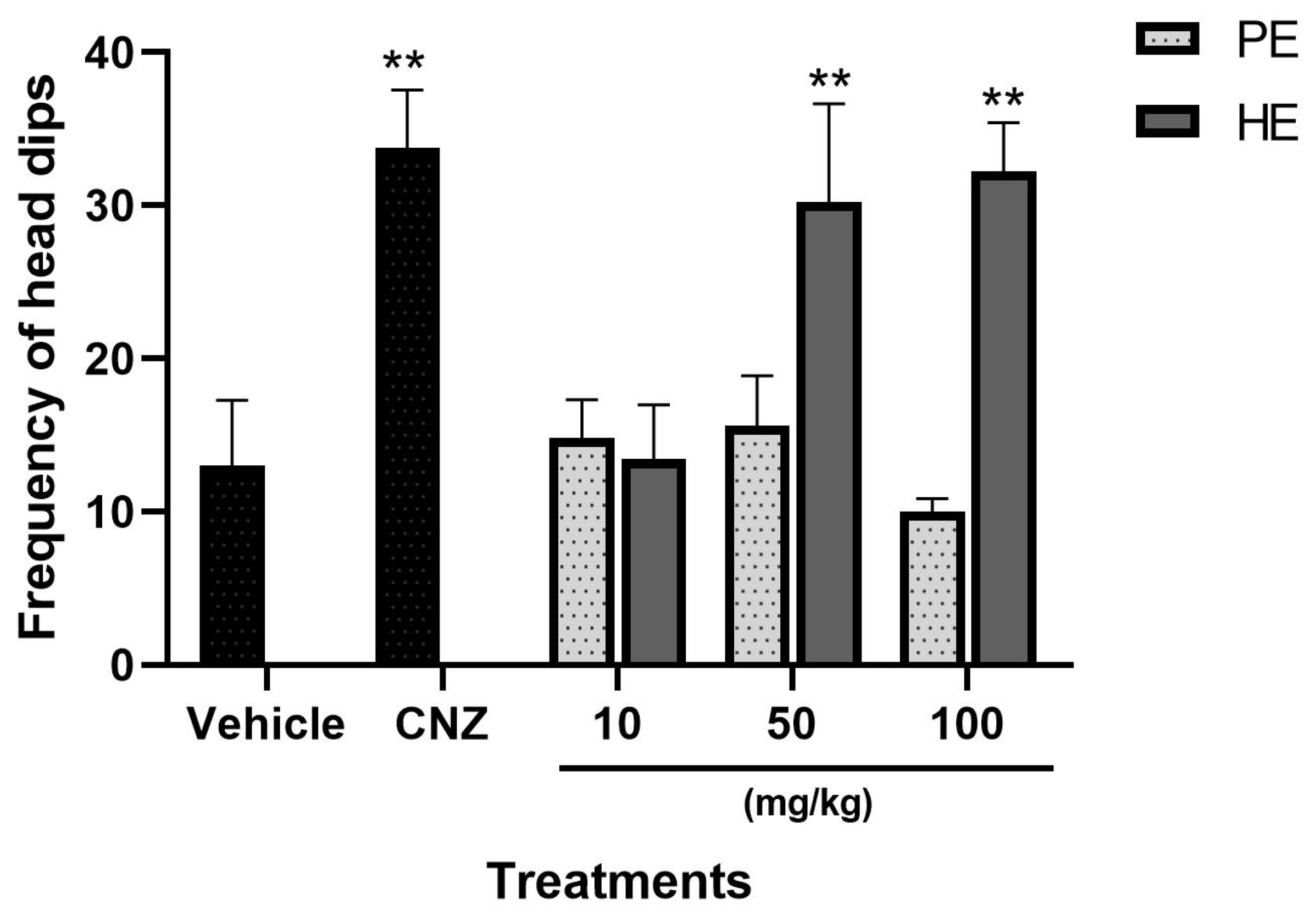
2.5.5. Hole Board Test
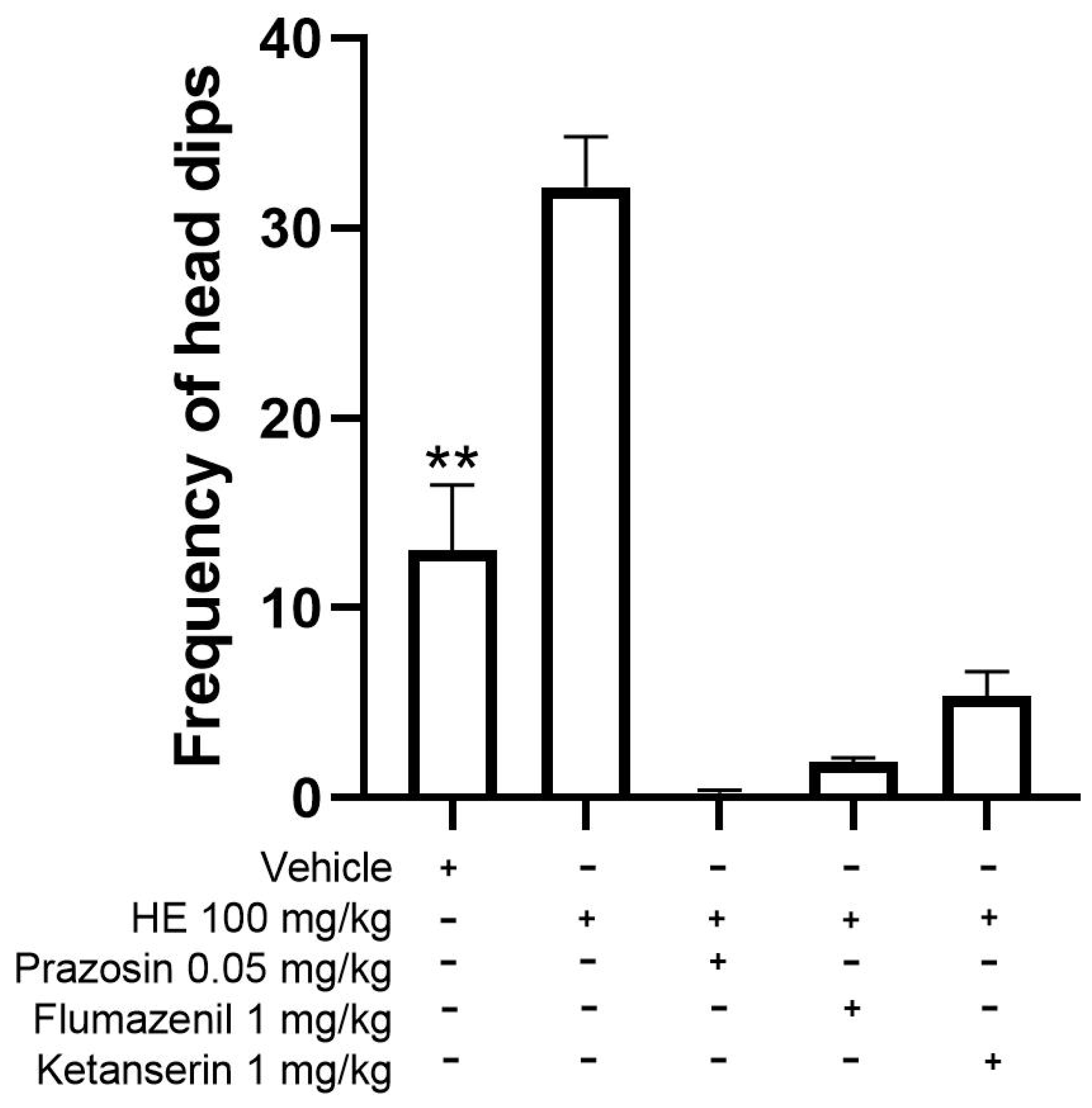
2.5.6. Mechanism of Action for Anxiolytic-like Behavior
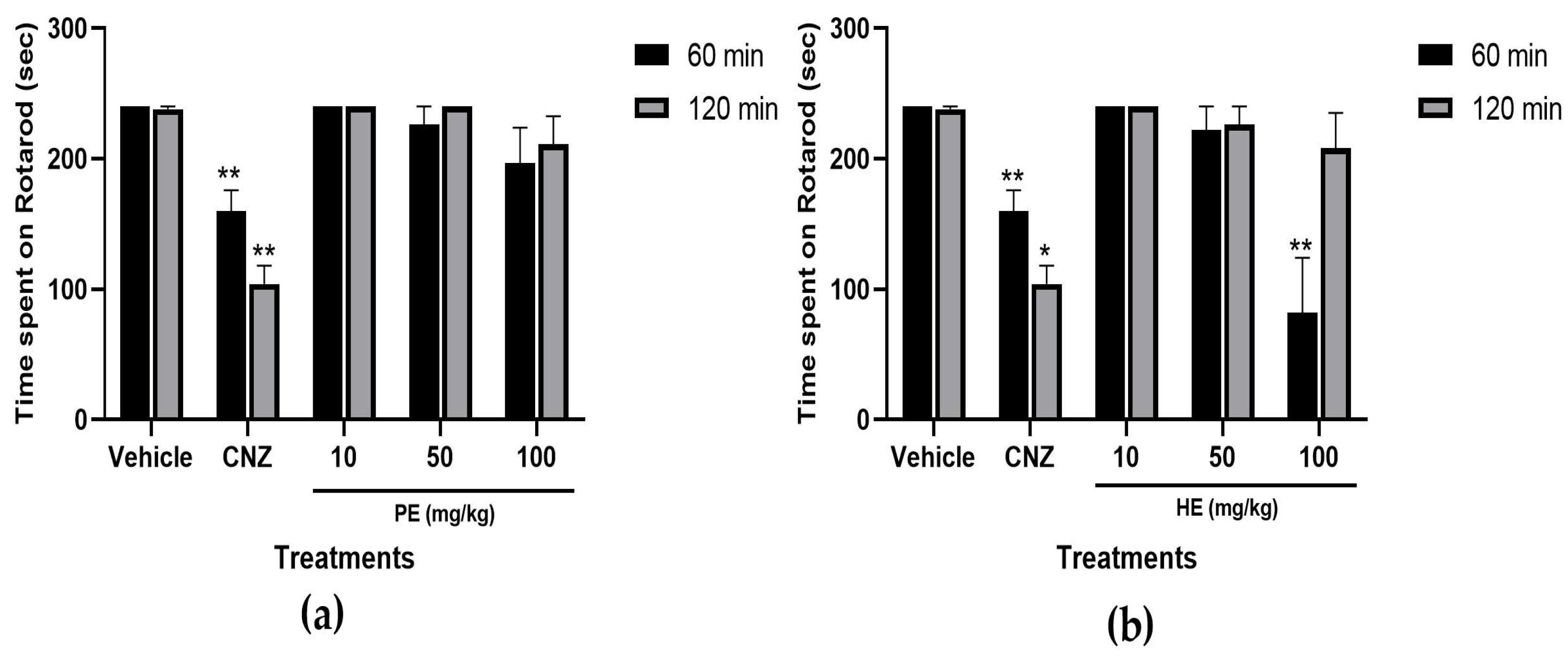
2.5.7. Rotarod Test
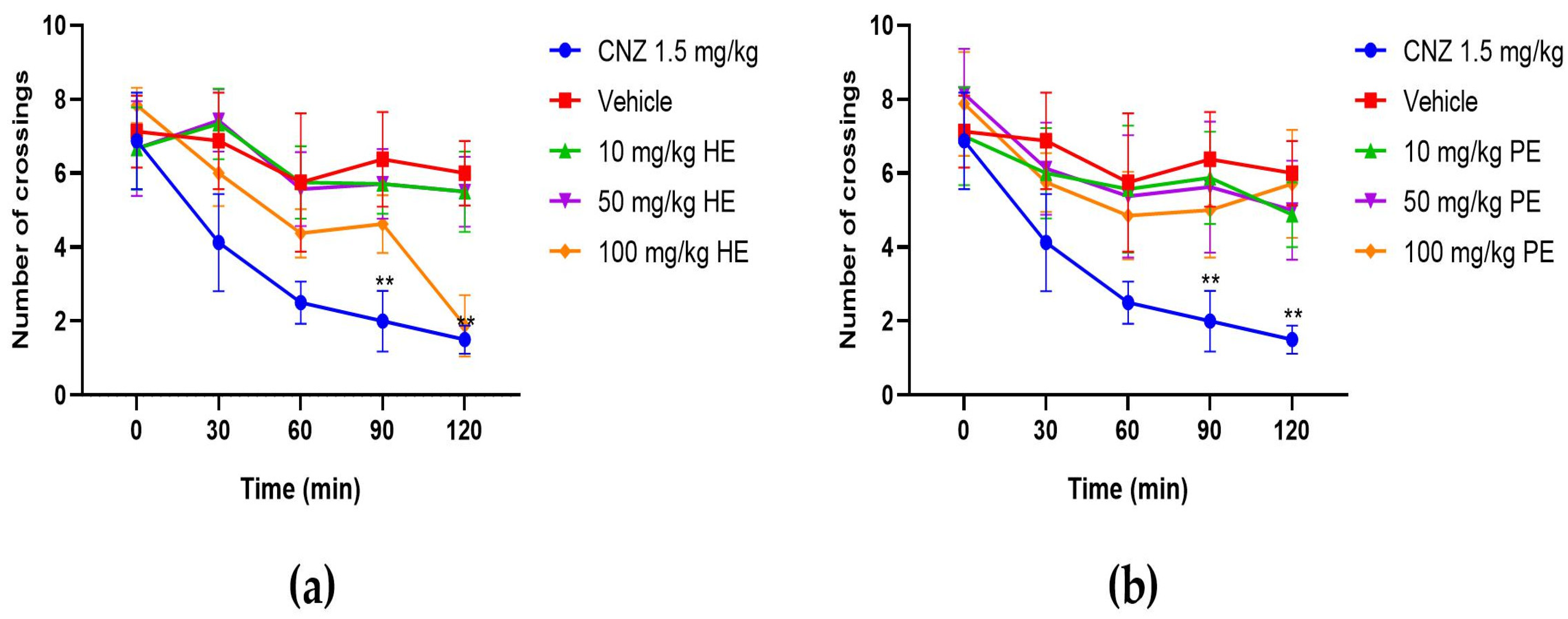
2.5.8. Hole Cross Test
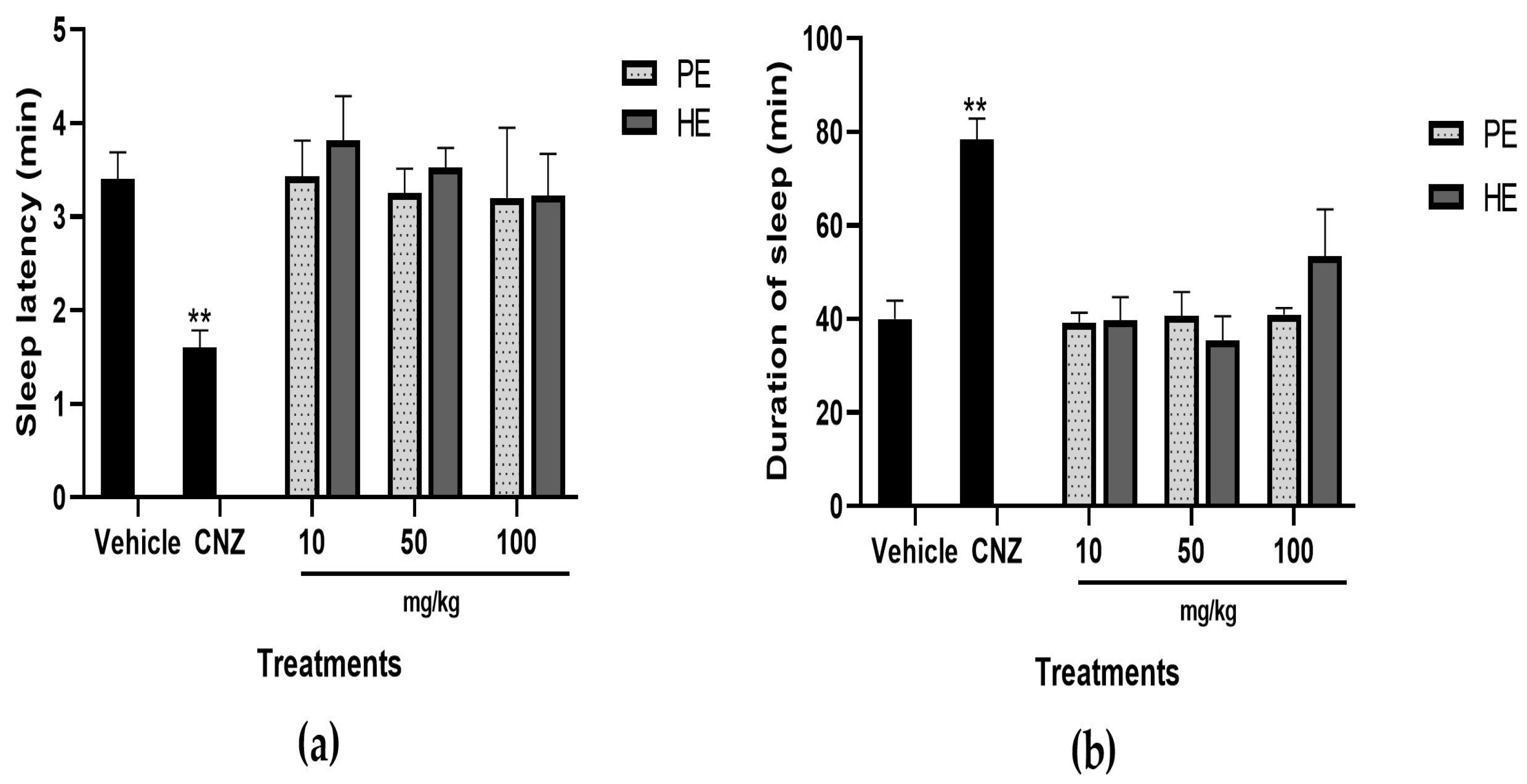
2.5.9. Pentobarbital-Induced Sleep Test
2.5.10. Anticonvulsant Activity
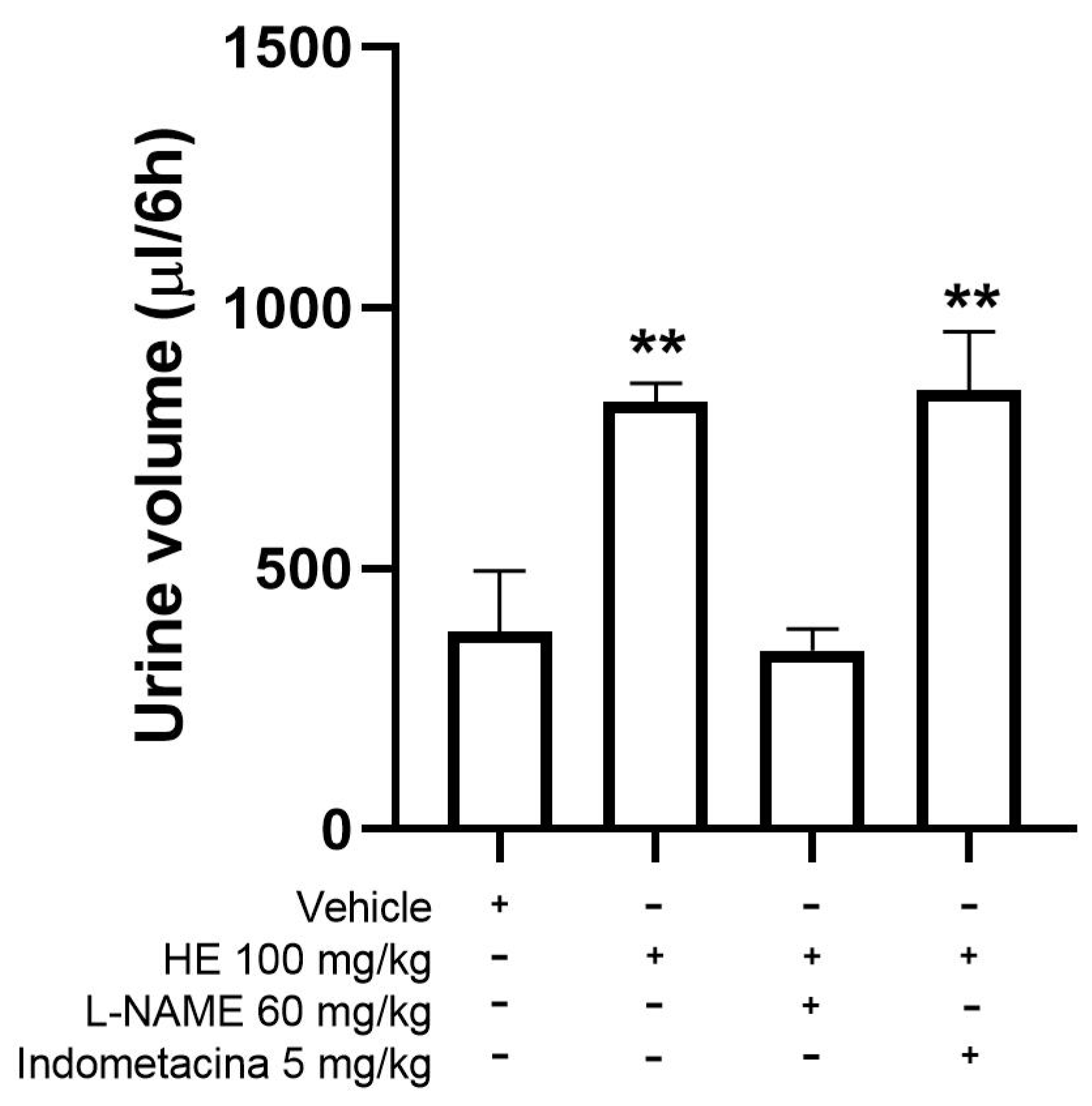
2.6. Diuretic Effect
Mechanism of Action for the Diuretic Effect
3. Discussion
4. Materials and Methods
4.1. Ethnomedicinal Study
4.2. Plant Material
4.3. Reagents
4.4. Plant Extracts
4.5. Chemical Characterization
4.5.1. Sample Derivatization
4.5.2. GC-MS Analysis
4.5.3. Analysis of GC-MS Data
4.6. Determination of the In Vitro Antioxidant Capacity Using ABTS and DPPH
4.7. Experimental Animals
4.8. Acute Toxicity Assessment
4.9. Experimental Model
4.10. Exploratory Cylinder Test
4.11. Hole Board Test
4.12. Elevated plus Maze
4.13. Light–Dark Box Test
4.14. Open-Field Test
4.15. Possible Mechanism of Anxiolytic Action
4.16. Rotarod Test
4.17. Hole Cross Test
4.18. Pentobarbital-Induced Sleep Test
4.19. Anticonvulsant Activity
4.20. Diuretic Activity
4.21. Evaluation of the Possible Mechanism of the Diuretic Activity
4.22. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). World Mental Health Report. Transforming Mental Health for All; World Health Organization (WHO): Geneva, Switzerland, 2022; Volume 50, p. 51. ISBN 978-92-4-004933-8.
- Shahrajabian, M.H. Powerful Stress Relieving Medicinal Plants for Anger, Anxiety, Depression, and Stress During Global Pandemic. Recent Pat. Biotechnol. 2022, 16, 284–310. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Ruiz-Padilla, A.J.; Ramírez-Morales, M.A.; Alcocer-García, S.G.; Ruiz-Noa, Y.; Ibarra-Reynoso, L.D.R.; Solorio-Alvarado, C.R.; Zapata-Morales, J.R.; Mendoza-Macías, C.L.; Deveze-Álvarez, M.A.; et al. Self-treatment with herbal products for weight-loss among overweight and obese subjects from central Mexico. J. Ethnopharmacol. 2019, 234, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sena, S.; Jha, P.; Lekhak, M.M.; Singh, S.K.; Goutam, U.; Arencibia, A.D.; Kumar, V. Arundina graminifolia (D.Don) Hochr. (Orchidaceae): A review of its medicinal importance, phytochemistry and pharmacology activities. S. Afr. J. Bot. 2022, 150, 956–964. [Google Scholar] [CrossRef]
- Fonmboh, D.J.; Fokunang, T.E.; Ndasi, N.P.; Ngangmou, N.T.; Herve, B.; Tita, B.L.; Nubia, K.C.; Awah, T.M.; Aba, E.R.; Fokunang, C.N. An Overview of the Ethnobotanic, Ethnopharmacological and Medicinal Importance of Edible Wild Root Tuber Orchids in Cameroon. Asian J. Biotechnol. Bioresour. Technol. 2021, 7, 11–24. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Khatun, F.; Nasrin, N.; Monira, S.; Asaduzzaman, M.; Apu, A.S. Assessment of neuropharmacological and analgesic potentials of Geodorum densiflorum (Lam.) schltr root extracts in experimental animals. Pharmacologyonline 2013, 3, 16–22. [Google Scholar]
- Watanabe, K.; Tanaka, R.; Sakurai, H.; Iguchi, K.; Yamada, Y.; Hsu, C.S.; Sakuma, C.; Kikuchi, H.; Shibayama, H.; Kawai, T. Structure of cymbidine A, a monomeric peptidoglycan-related compound with hypotensive and diuretic activities, isolated from a higher plant, Cymbidium goeringii (Orchidaceae). Chem. Pharm. Bull. 2007, 55, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Galicia, J.; Ortiz-Andrade, R.; Rivera-Leyva, J.; Castillo-España, P.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Gallardo-Ortiz, I.; Estrada-Soto, S. Vasorelaxant and antihypertensive effects of methanolic extract from roots of Laelia anceps are mediated by calcium-channel antagonism. Fitoterapia 2010, 81, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Dorado-Martinez, C. Ethnopharmacology, Mexico’s therapeutic prolificacy for the sustainable social development. Ecocience Int. J. 2020, 2, 54–65. [Google Scholar] [CrossRef]
- Gonzalez-Rivera, M.L.; Barragan-Galvez, J.C.; Gasca-Martínez, D.; Hidalgo-Figueroa, S.; Isiordia-Espinoza, M.; Alonso-Castro, A.J. In Vivo Neuropharmacological Effects of Neophytadiene. Molecules 2023, 28, 3457. [Google Scholar] [CrossRef]
- Gerlach, G.; Stanhopeinae Mesoamericanae, V. El Aroma Floral de las Stanhopeas de Mexico. Lankesteriana Int. J. Orchid. 2009, 9, 431–442. [Google Scholar]
- Cano-Asseleih, L.M.; Menchaca-García, R.A.; Ruiz-Cruz, J.Y.S. Ethnobotany, Pharmacology and Chemistry of Medicinal Orchids from Veracruz. J. Agric. Sci. Technol. A 2015, 5, 745–754. [Google Scholar] [CrossRef][Green Version]
- Martínez, M.; García, M.; Rebeca, A. Efecto de los compuestos orgánicos en la propagación in vitro de Stanhopea Tigrina Bateman (Orchidaceae). For. Veracruzana 2007, 9, 27–32. [Google Scholar]
- Salazar-Cerezo, S.; Martinez-Montiel, N.; Cruz-Lopez, M.D.C.; Martinez-Contreras, R.D. Fungal diversity and community composition of culturable fungi in Stanhopea trigrina cast gibberellin producers. Front. Microbiol. 2018, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Romero, E.; Moreno-Vera, A.; Villacis-Calderon, A.; Rosado-Sabando, J.; Morales-Moreira, D.; Bravo-Bravo, A. Estudio etnobotánico y comercialización de plantas medicinales del bosque protector Murocomba y su área de influencia del cantón Valencia, Ecuador. Cienc. Y Tecnol. Agropecu. 2019, 20, 491–506. [Google Scholar] [CrossRef]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P.D.Q.; Li, M.S.; Florio, D.; Broersen, K.; De Pandis, M.F.; Roviello, G.N. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo[c]phenanthridine and berberine-based compounds on β-amyloid aggregation. Chem. Biol. Interact. 2021, 334, 109–300. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-L.; Wang, Q.; Wang, J.-X.; Dong, H.-Y.; Xu, X.-K.; Shen, Y.-H.; Li, H.-L.; Zhang, W.-D. Vlasoulamine A, a Neuroprotective [3.2.2] Cyclazine Sesquiterpene Lactone Dimer from the Roots of Vladimiria souliei. Org. Lett. 2018, 20, 7567–7570. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. Caffeine in Kidney Stone Disease: Risk or Benefit? Adv. Nutr. 2018, 9, 419–424. [Google Scholar] [CrossRef]
- Jee, H.J.; Lee, S.G.; Bormate, K.J.; Jung, Y.-S. Effect of Caffeine Consumption on the Risk for Neurological and Psychiatric Disorders: Sex Differences in Human. Nutrients 2020, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, V.; Rivero-Cruz, I.; Laguna-Hernández, G.; Salazar-Chávez, G.; Mata, R. Chemical composition, potential toxicity, and quality control procedures of the crude drug of Cyrtopodium macrobulbon. J. Ethnopharmacol. 2014, 154, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-T.; Tsai, H.-Y.; Hsieh, M.-T.; Chen, C.-F. Analgesic and anti-inflammatory effects of Nervilia purpurea and its active components. J. Tradit. Chin. Med. 1993, 4, 89–107. [Google Scholar] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M. Animal models for screening anxiolytic-like drugs: A perspective. Dialogues Clin. Neurosci. 2015, 17, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Morikawa, K.; Ishida, H.; Chimoto, J.; Maruyama-Fumoto, K.; Yamada, S.; Shinozuka, K. Vasorelaxant effects of benzodiazepines, non-benzodiazepine sedative-hypnotics, and tandospirone on isolated rat arteries. Eur. J. Pharmacol. 2021, 892, 173744. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. Methods Mol. Biol. 2019, 1916, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-anxiety Effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Farzamfard, P.; Rezayof, A.; Alijanpour, S. Ventral hippocampal NMDA receptors mediate the effects of nicotine on stress-induced anxiety/exploratory behaviors in rats. Neurosci. Lett. 2022, 780, 136–649. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, S.; Mori, M.; Harada, H.; Murata, Y.; Ohe, K.; Enjoji, M. Upregulations of α(1) adrenergic receptors and noradrenaline synthases in the medial prefrontal cortex are associated with emotional and cognitive dysregulation induced by post-weaning social isolation in male rats. Neurosci. Lett. 2023, 797, 137071. [Google Scholar] [CrossRef] [PubMed]
- Wahis, J.; Holt, M.G. Astrocytes, Noradrenaline, α1-Adrenoreceptors, and Neuromodulation: Evidence and Unanswered Questions. Front. Cell. Neurosci. 2021, 15, 645691. [Google Scholar] [CrossRef] [PubMed]
- Pędzich, B.D.; Rubens, S.; Sekssaoui, M.; Pierre, A.; Van Schuerbeek, A.; Marin, P.; Bockaert, J.; Valjent, E.; Bécamel, C.; De Bundel, D. Effects of a psychedelic 5-HT2A receptor agonist on anxiety-related behavior and fear processing in mice. Neuropsychopharmacology 2022, 47, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, L.-M.; Dooley, D.; Jahanshahi, A.; Temel, Y.; Hendrix, S. Evaluating rodent motor functions: Which tests to choose? Neurosci. Biobehav. Rev. 2017, 83, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bhat, Z.A.; Kumar, D. Animal models of anxiety: A comprehensive review. J. Pharmacol. Toxicol. Methods 2013, 68, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, M.C.A.; Bachmann, H.S. Diuretics: A contemporary pharmacological classification? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 619–627. [Google Scholar] [CrossRef]
- Schlickmann, F.; Boeing, T.; Mariano, L.N.B.; da Silva, R.C.M.V.A.F.; da Silva, L.M.; de Andrade, S.F.; de Souza, P.; Cechinel-Filho, V. Gallic acid, a phenolic compound isolated from Mimosa bimucronata (DC.) Kuntze leaves, induces diuresis and saluresis in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Merenzon, M.A.; Hincapie Arias, E.; Bhatia, S.; Shah, A.H.; Higgins, D.M.O.; Villaverde, M.; Belgorosky, D.; Eijan, A.M. Nitric oxide synthase inhibitors as potential therapeutic agents for gliomas: A systematic review. Nitric Oxide Biol. Chem. 2023, 138-139, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Nakamura, M.; Suzuki, A.; Tsukada, H.; Horita, S.; Suzuki, M.; Moriya, K.; Seki, G. Effects of Nitric Oxide on Renal Proximal Tubular Na+ Transport. BioMed Res. Int. 2017, 2017, 6871081. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Norma Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio; Diario Oficial de la Federación: Mexico, Mexico, 2001. [Google Scholar]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure, OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Park, C.W.; Hong, K.B.; Suh, H.J.; Ahn, Y. Sleep-promoting activity of amylase-treated Ashwagandha (Withania somnifera L. Dunal) root extract via GABA receptors. J. Food Drug Anal. 2023, 31, 278–288. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.C.; de Oliveira, C.V.; Grigoletto, J.; Ribeiro, L.R.; Funck, V.R.; Grauncke, A.C.B.; de Souza, T.L.; Souto, N.S.; Furian, A.F.; Menezes, I.R.A.; et al. Anticonvulsant activity of β-caryophyllene against pentylenetetrazol-induced seizures. Epilepsy Behav. 2016, 56, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Arana-Argáez, V.; Alonso-Castro, A.J.; Yáñez-Barrientos, E.; Euan-Canto, A.; Torres-Romero, J.C.; Isiordia-Espinoza, M.A.; Brennan-Bourdon, L.M.; Juárez-Vázquez, M.D.C.; González-Ibarra, A.A. In vitro and in vivo anti-inflammatory effects of an ethanol extract from the aerial parts of Eryngium carlinae F. Delaroche (Apiaceae). J. Ethnopharmacol. 2021, 266, 113406. [Google Scholar] [CrossRef]

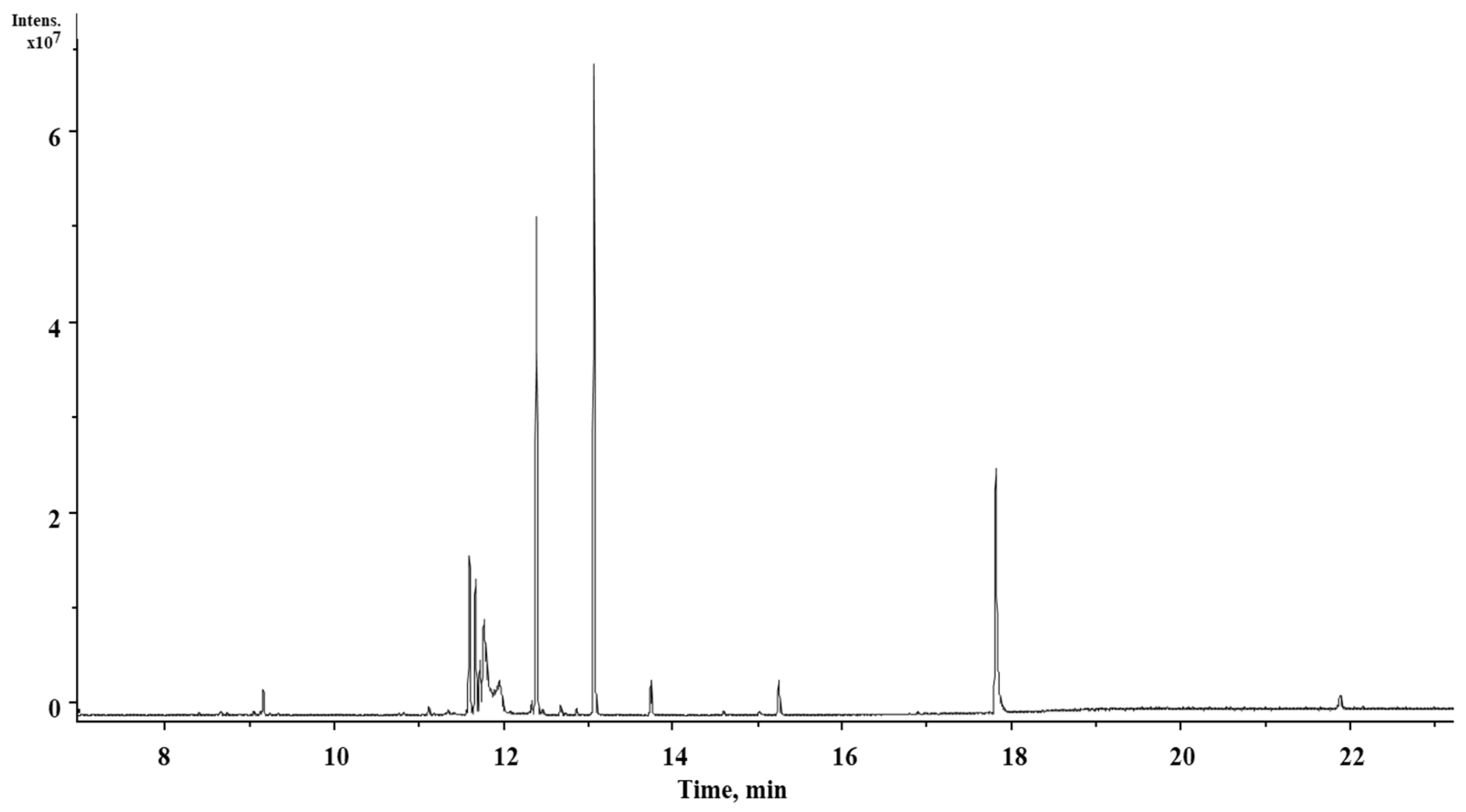


| Peak No. | RT 1 (min) | Name of the Compound | Molecular Formula | Molecular Weight (g/mol) | Peak Area (%) | Compound Nature |
|---|---|---|---|---|---|---|
| 1 | 7.980 | Acetic acid, [(tert-butyldimethylsilyl)oxy]-, tert-butyldimethylsilyl ester | C14H32O3Si2 | 304.57 | 1.11 | Ester |
| 2 | 8.621 | Methyl-2-iodobenzoate | C8H7IO2 | 262.04 | 1.30 | Ester |
| 3 | 8.75 | 2,3,4-Tris[(trimethylsilyl)oxy]butanal | C13H32O4Si3 | 336.65 | 2.40 | Alkane |
| 4 | 8.840 | (4S,5R)-4-trimethylsilyloxy-5-(trimethylsilyloxymethyl)oxolan-2-one | C11H24O4Si2 | 276.48 | 0.80 | Ester |
| 5 | 8.920 | 5-methylsulfanyl-3-phenyl-1,2-oxazole | C10 H9 NOS | 191.25 | 0.85 | Alkaloid |
| 6 | 9.302 | trimethylsilyl 2,3,4-tris(trimethylsilyloxy)butanoate | C16H40O5Si4 | 424.8 | 0.65 | Phenolic acids |
| 7 | 10.041 | Triethyl(1,2,3,4-tetrahydronaphthyl)silane | C16H26Si | 246.47 | 0.92 | Alkene |
| 8 | 10.062 | Neophytadiene | C20H38 | 278.5 | 0.27 | Diterpene |
| 9 | 10.098 | 2,3,4,5-Tetrahydroxypentanoic acid-1,4-lactone, tris(trimethylsilyl)- | C14H32O5Si3 | 364.66 | 0.97 | lactones, coumarin |
| 10 | 10.135 | 2,3-Dimethoxy-1-phenyl-5,5-dimethylcyclopentene | C16H22O2 | 246.35 | 1.33 | Alkene |
| 11 | 10.208 | methyl 3-(4-trimethylsilyloxyphenyl)propanoate | C13H20O3Si | 252.38 | 1.15 | Fatty acid |
| 12 | 10.270 | 2-Methyl-5-(2′,4′,6′-trimethylphenyl)tetrahydrofuran | C14H20O | 204.313 | 1.23 | Ether |
| 13 | 10.315 | Trimethylsilyl laurate | C15H32O2Si | 272.50 | 0.12 | Ester |
| 14 | 10.741 | (Z)-3-(1-Butylidene)phthalide | C12H12O2 | 188.22 | 2.36 | Cumarin |
| 15 | 10.799 | n-Heptadecane | C17H36 | 240.5 | 0.00 | Alkane |
| 16 | 10.845 | Benzopyrido(2,1-a)isoindole | C12H9N | 167.21 | 1.73 | Alkaloid |
| 17 | 11.286 | 3-(methylthio)benzofuro [3,2-e]-1,2,4-triazine | C10H7N3OS | 217.25 | 1.39 | Alkaloid |
| 18 | 11.34 | Hydrocinnamic acid, p-(trimethylsiloxy)-, trimethylsilyl ester | C15H26O3Si2 | 310.54 | 0.46 | Phenolic acids |
| 19 | 11.39 | Trimethylsilyl 2,3,4,5-tetrakis-O-(trimethylsilyl)pentonate | C20H50O6Si5 | 527.00 | 0.63 | Trimethylsilyl esters |
| 20 | 11.439 | 5-Trichloromethyl-3-[1-(cyanothio)ethyl]-4,5-dihydroisoxazol-5-ol | C7H7Cl3N2O2S | 288.00 | 1.20 | Alkaloid |
| 21 | 12.122 | Tetradecanoic acid, trimethylsilyl ester | C17H36O2Si | 300.6 | 0.22 | Organic acid |
| 22 | 12.16 | 1,2,3,4,6-Pentakis-O-(trimethylsilyl)hexopyranose | C21H52O6Si5 | 541.1 | 2.21 | Carbohydrate |
| 23 | 12.35 | D-(-)-Tagatose, pentakis(trimethylsilyl) ether | C21H52O6Si5 | 541.1 | 0.27 | Carbohydrate |
| 24 | 12.40 | β-D-(+)-Mannopyranose, pentakis(trimethylsilyl) ether | C21H52O6Si5 | 541.1 | 0.53 | Carbohydrate |
| 25 | 12.909 | Cinnamic acid, p-(trimethylsiloxy)-, trimethylsilyl ester | C15H24O3Si2 | 308.52 | 0.73 | Phenolic acids |
| 26 | 12.959 | n-Pentanoic acid, trimethylsilyl ester | C18H38O2Si | 314.6 | 0.10 | Fatty acid |
| 27 | 12.981 | Gallic acid—tetrakis(trimethylsilyl) derivative | C31H62O5Si4 | 627.2 | 1.47 | Flavonoids |
| 28 | 13.087 | D-Glucose, pentakis-O-(trimethylsilyl)- | C21H52O6Si5 | 541.1 | 0.51 | Carbohydrate |
| 29 | 13.767 | Trimethylsilyl palmitate | C19H40O2Si | 328.6 | 0.64 | Fatty acid |
| 30 | 14.739 | (2E)-3,7,11,15-Tetramethyl-2-hexadecenyl trimethylsilyl ether | C23H48OSi | 368.7 | 1.72 | Alkane |
| 31 | 15.042 | Linoleic acid trimethylsilyl ester | C21H40O2Si- | 352.6 | 0.82 | Fatty acid |
| 32 | 15.085 | trans-9-Octadecenoic acid, trimethylsilyl ester | C21H42O2Si | 354.6 | 0.86 | Fatty acid |
| 33 | 15.26 | Trimethylsilyl stearate | C21H44O2Si | 356.7 | 2.728 | Fatty acid |
| 34 | 16.207 | β-benzyl-D-glucopyranoside-tetrakis(trimethylsilyl)-ether | C25H50O6Si4 | 559.0 | 0.73 | Carbohydrate |
| 35 | 17.51 | 5,12-Dimethoxy-2,3,8,9-tetramethoxybenzo[c]phenanthridin-6(5H)-one | C22H23NO6 | 397.427 | 1.98 | Alkaloid |
| 36 | 17.812 | Sucrose, octakis(trimethylsilyl) ether | C36H86O11Si8 | 919.745 | 2.49 | Carbohydrate |
| 37 | 18.030 | 3-(2′,2′-Diphenylethenyl)-2,3-dihydro-1H-benzo[e]isoindol-1-one | C26H19NO | 361.444 | 0.18 | Alkaloid |
| 38 | 19.590 | 1,4-Diphenyl-2-[N-(methylcarbazol-2′-yl)amino]-2-butene-1,4-dione | C29H23N2O2 | 431.515 | 0.45 | Alkaloid |
| Peak No. | RT 1 (min) | Name of the Compound | Molecular Formula | Molecular Weight (g/mol) | Peak Área (%) | Compound Nature |
|---|---|---|---|---|---|---|
| 1 | 7.18 | Melibiose | C12H22O11 | 342.30 | 0.21 | Carbohydrate |
| 2 | 8.98 | 4-Acetylpyrazole | C5H6N2O | 110.11 | 0.90 | Alkaloid |
| 3 | 9.08 | (R)-2-(2′-hydroxyethoxy)-2-hydroxymethyl-1,4-dioxane | C7H14O3 | 146.186 | 0.19 | Eter |
| 4 | 9.36 | Tetracosane | C24H50 | 338.7 | 0.05 | Alkane |
| 5 | 10.73 | Per(trimethylsilyl)-D-arabinose | C17H42O5Si4 | 438.9 | 0.13 | Carbohydrate |
| 6 | 10.84 | 5-(2-Oxobutyl)-3-phenyl-2-isoxazoline | C13H17N1O2 | 219.284 | 0.46 | Alakaloid |
| 7 | 11.13 | (3SR,4SR)-4-[(RS)-1-Hydroxy-3-bentenyl]-1-(p-methoxyphenyl)-3-(propenyl)-2-azetidinone methanesulfonate | C17H21NO6S | 367.71 | 0.24 | Alkene |
| 8 | 11.57 | p-(N,N-Dimethylamino)phenylethynyl]dimesitylborane | C28H32BN | 393.4 | 0.04 | Alkene |
| 9 | 11.61 | Undecanoic acid isopropyl ester, 10-hydroxy-11-morpholin-4-yl- | C18H35NO4 | 329.5 | 0.08 | Fatty acid |
| 10 | 11.63 | D-(-)-Fructofuranose, pentakis(trimethylsilyl) ether (isomer 1) | C21H52O6Si5 | 541.061 | 0.12 | Carbohydrate |
| 11 | 11.72 | D-(-)-Fructofuranose, pentakis(trimethylsilyl) ether (isomer 2) | C21H52O6Si5 | 541.061 | 0.20 | Carbohydrate |
| 12 | 11.73 | D-(-)-Tagatofuranose, pentakis(trimethylsilyl) ether (isomer 1) | C21H52O6Si5 | 541.061 | 0.04 | Carbohydrate |
| 13 | 11.78 | 1-Phenylpyrrolo [2,1,5-cd]indolizine | C16H12N | 218.27 | 0.08 | Alkaloid |
| 14 | 11.97 | 1-(p-Acetylbenzoyl)pyrrolidine | C8H10N4O2 | 189.25 | 0.39 | Alkaloid |
| 15 | 12.09 | Caffeine | C8H10N4O2 | 194.19 | 0.31 | Alkaloid |
| 16 | 12.34 | D-Psicose, pentakis(trimethylsilyl) ether | C22H55NO6Si5 | 570.102 | 0.41 | Carbohydrate |
| 17 | 12.40 | β-D-(+)-Mannopyranose, pentakis(trimethylsilyl) ether | C21H52O6Si5 | 541.061 | 0.37 | Carbohydrate |
| 18 | 12.47 | Ethyl 4-(2-Hydroxymethylphenyl)butanoate | C13H18O3 | 222.277 | 0.19 | Alkane |
| 19 | 12.87 | Per(trimethylsilyl)-D-mannose | C21H52O6Si5 | 541.061 | 1.22 | Carbohydrate |
| 20 | 13.08 | β-D-Glucopyranose, 1,2,3,4,6-pentakis-O-(trimethylsilyl)- | C21H52O6Si5 | 541.061 | 0.25 | Carbohydrate |
| 21 | 13.09 | 3,4-Dihydro-2-methylnaphthalene-3,4-diol | C11H10O2 | 174.20 | 0.43 | Phenolic |
| 22 | 13.75 | Trimethylsilyl palmitate | C19H40O2Si | 328.6 | 0.14 | Fatty acid |
| 23 | 15.032 | Linoleic acid trimethylsilyl ester | C21H38O2Si | 350.610 | 0.22 | Fatty acid |
| 24 | 15.25 | Trimethylsilyl stearate | C21H44O2Si | 356.7 | 0.13 | Fatty acid |
| 25 | 17.82 | Sucrose, octakis(trimethylsilyl) ether | C36H86O11Si8 | 919.745 | 0.16 | Carbohydrate |
| Treatment | Total Distance (cm) | Resting Time (s) | Time in Central Squares (s) | Distance in Central Squares (cm) |
|---|---|---|---|---|
| Vehicle | 2095.82 ± 157.64 | 58.04 ± 6.13 | 19.88 ± 1.57 | 252.80 ± 26.36 |
| CNZ 1.5 mg/kg | 764.89 ± 35.69 * | 154.51 ± 4.43 * | 48.04 ± 5.64 * | 516.28 ± 28.34 * |
| PE 10 mg/kg | 2087.44 ± 90.47 | 63.35 ± 4.76 | 17.93 ± 1.48 | 255.06 ± 32.84 |
| PE 50 mg/kg | 2132.38 ± 95.71 | 62.20 ± 2.20 | 19.26 ± 0.90 | 303.17 ± 24.67 |
| PE 100 mg/kg | 2120.15 ± 147.06 | 60.41 ± 3.50 | 28.24 ± 3.41 | 369.92 ± 19.09 * |
| HE 10 mg/kg | 1923.22 ± 105.43 | 60.52 ± 8.90 | 24.70 ± 3.69 | 290.22 ± 44.15 |
| HE 50 mg/kg | 2212.34 ± 121.45 | 64.24 ± 7.31 | 29.20 ± 4.38 | 351.89 ± 31.01 |
| HE 100 mg/kg | 2064.02 ± 109.21 | 63.24 ± 8.02 | 37.30 ± 3.11 * | 432.22 ± 15.17 * |
| Treatments | Onset of Convulsion (s) | Duration of Convulsion (s) | Mortality (%) |
|---|---|---|---|
| Vehicle | 58 ± 3.87 | 155 ± 15.86 | 100 |
| CNZ 1.5 mg/kg | 0 * | 0 * | 0 |
| PE 10 mg/kg | 63.5 ± 8.09 | 160.66 ± 18.62 | 100 |
| PE 50 mg/kg | 59.16 ± 6.98 | 166.83 | 100 |
| PE 100 mg/kg | 72.66 ± 5.38 | 130.66 ± 19.42 | 100 |
| HE 10 mg/kg | 59 ± 5.64 | 160.50 ± 24.47 | 100 |
| HE 50 mg/kg | 76.66 ± 15.21 | 130.50 ± 23.14 | 100 |
| HE 100 mg/kg | 112.83 ± 20.89 * | 41 ± 1.74 * | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Carmen Díaz-Torres, R.; Yáñez-Barrientos, E.; Montes-Rocha, J.Á.; Morales-Tirado, D.J.; Alba-Betancourt, C.; Gasca-Martínez, D.; Gonzalez-Rivera, M.L.; del Carmen Juárez-Vázquez, M.; Deveze-Álvarez, M.A.; Isiordia-Espinoza, M.A.; et al. Ethnomedicinal Study and Evaluation of the Anxiolytic-like and Diuretic Effects of the Orchid Stanhopea tigrina Bateman ex Lindl—(Orchidaceae). Pharmaceuticals 2024, 17, 588. https://doi.org/10.3390/ph17050588
del Carmen Díaz-Torres R, Yáñez-Barrientos E, Montes-Rocha JÁ, Morales-Tirado DJ, Alba-Betancourt C, Gasca-Martínez D, Gonzalez-Rivera ML, del Carmen Juárez-Vázquez M, Deveze-Álvarez MA, Isiordia-Espinoza MA, et al. Ethnomedicinal Study and Evaluation of the Anxiolytic-like and Diuretic Effects of the Orchid Stanhopea tigrina Bateman ex Lindl—(Orchidaceae). Pharmaceuticals. 2024; 17(5):588. https://doi.org/10.3390/ph17050588
Chicago/Turabian Styledel Carmen Díaz-Torres, Rocío, Eunice Yáñez-Barrientos, José Ángel Montes-Rocha, David Jeremías Morales-Tirado, Clara Alba-Betancourt, Deisy Gasca-Martínez, Maria L. Gonzalez-Rivera, María del Carmen Juárez-Vázquez, Martha Alicia Deveze-Álvarez, Mario Alberto Isiordia-Espinoza, and et al. 2024. "Ethnomedicinal Study and Evaluation of the Anxiolytic-like and Diuretic Effects of the Orchid Stanhopea tigrina Bateman ex Lindl—(Orchidaceae)" Pharmaceuticals 17, no. 5: 588. https://doi.org/10.3390/ph17050588
APA Styledel Carmen Díaz-Torres, R., Yáñez-Barrientos, E., Montes-Rocha, J. Á., Morales-Tirado, D. J., Alba-Betancourt, C., Gasca-Martínez, D., Gonzalez-Rivera, M. L., del Carmen Juárez-Vázquez, M., Deveze-Álvarez, M. A., Isiordia-Espinoza, M. A., Carranza-Álvarez, C., & Alonso-Castro, A. J. (2024). Ethnomedicinal Study and Evaluation of the Anxiolytic-like and Diuretic Effects of the Orchid Stanhopea tigrina Bateman ex Lindl—(Orchidaceae). Pharmaceuticals, 17(5), 588. https://doi.org/10.3390/ph17050588








