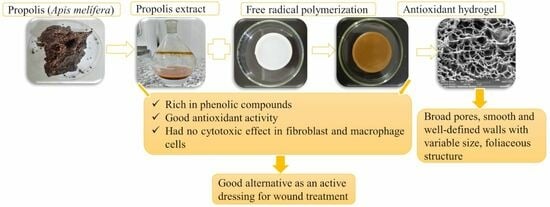
Characterization, Biocompatibility and Antioxidant Activity of Hydrogels Containing Propolis Extract as an Alternative Treatment in Wound Healing
Abstract
1. Introduction
2. Results
2.1. Gas Chromatography-Mass Spectrometry Assay (GC-MS) of Propolis Extract
2.2. Nuclear Magnetic Resonance Spectrometry (NMR) of Propolis Extract
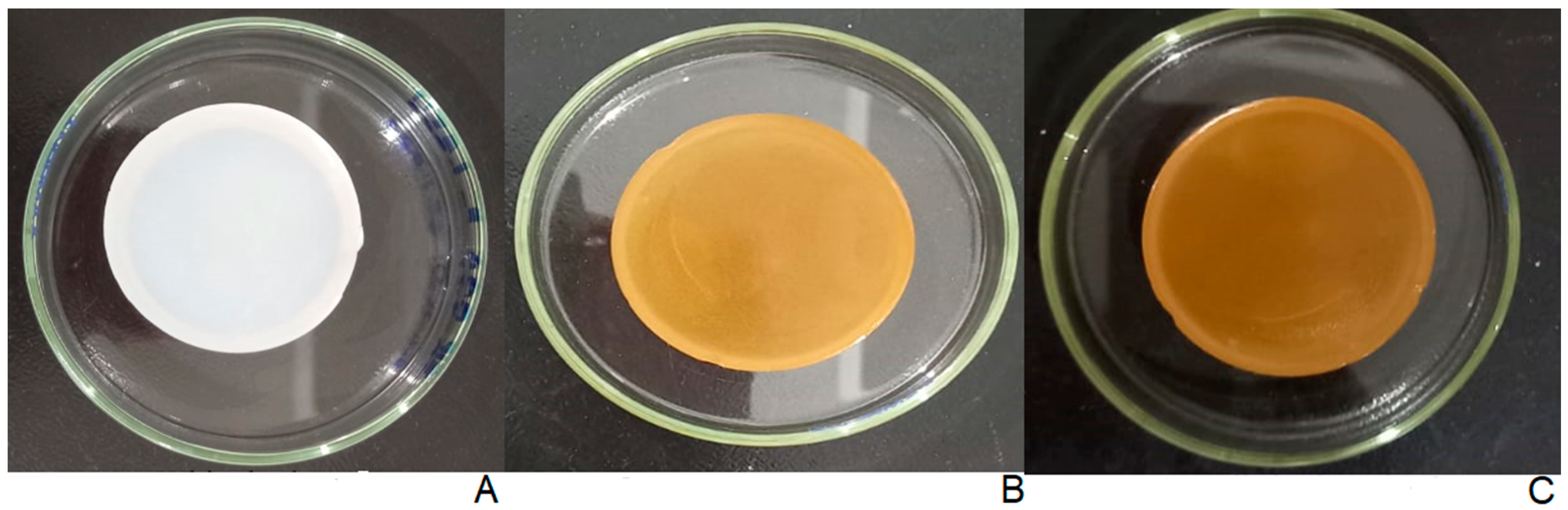
2.3. Physical Appearance of Hydrogels
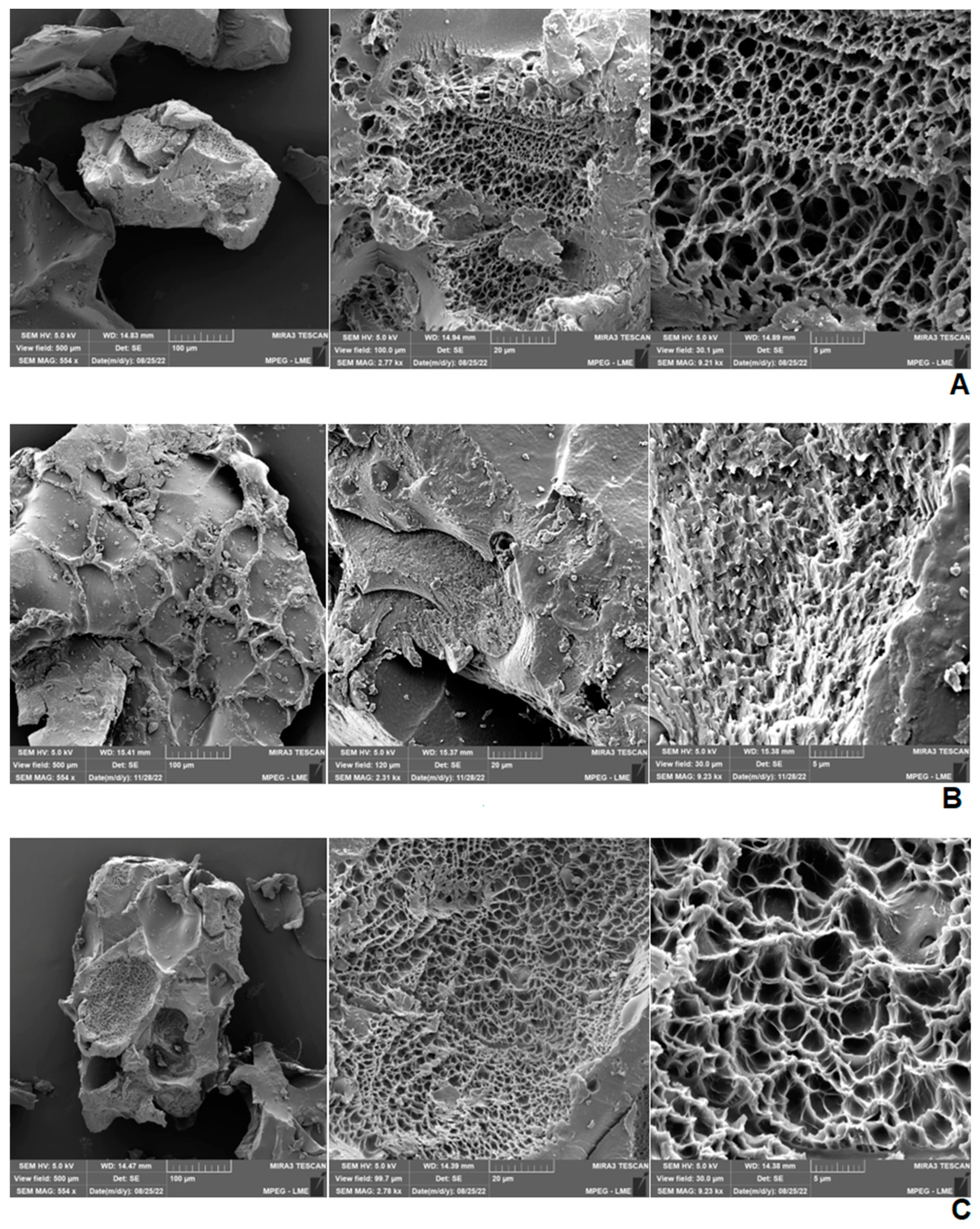
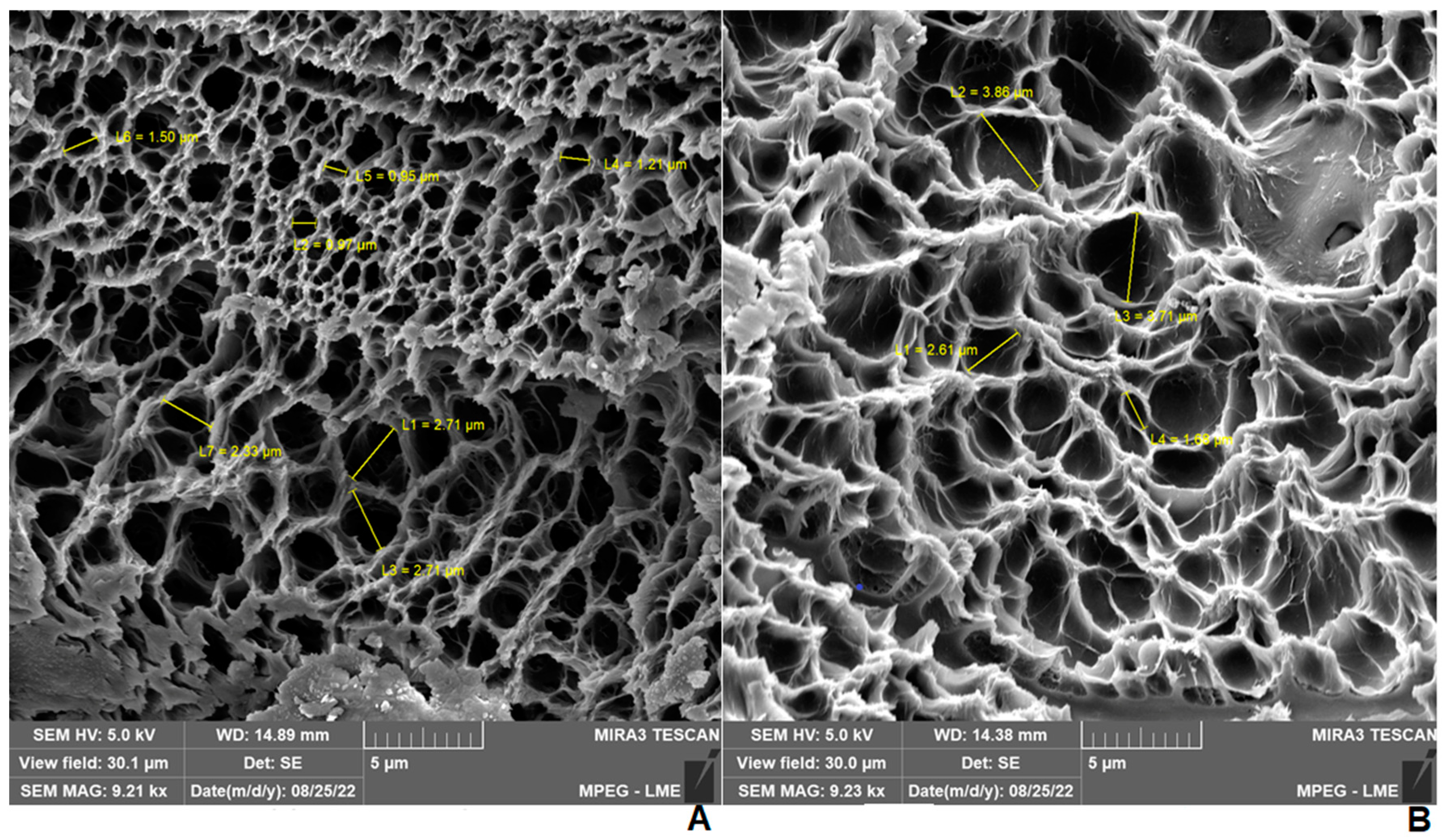
2.4. Scanning Electron Microscopy (SEM) of Hydrogels
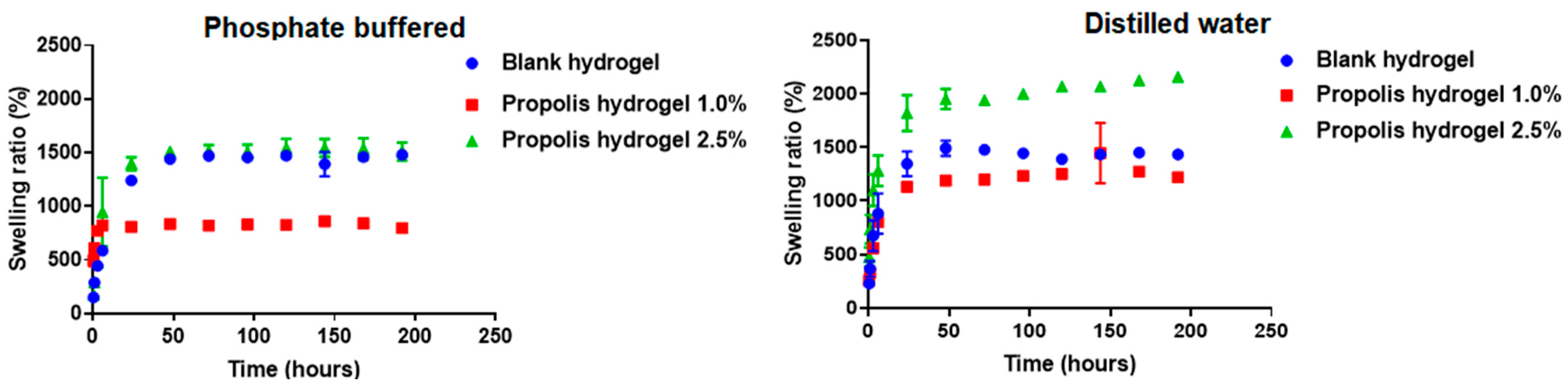
2.5. Swelling Capacity of Hydrogels
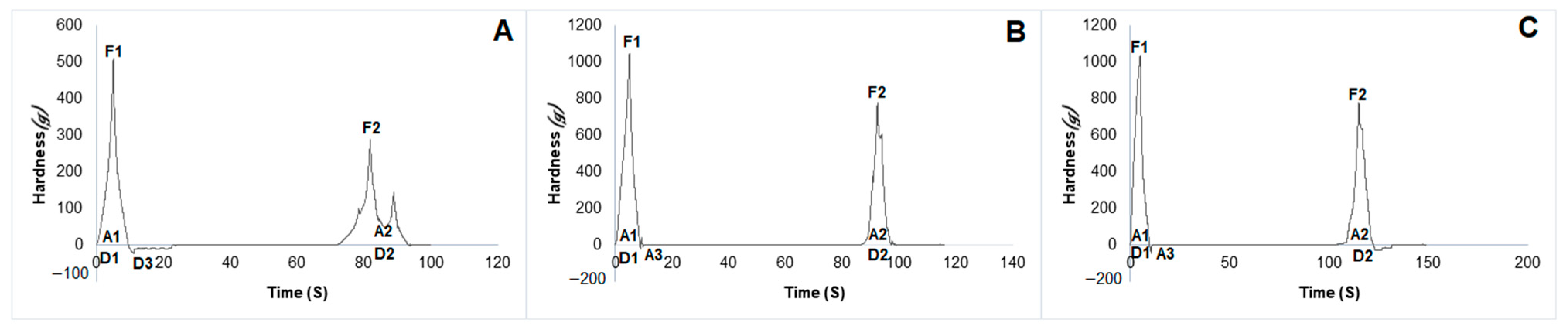
2.6. Mechanical Properties of Hydrogels
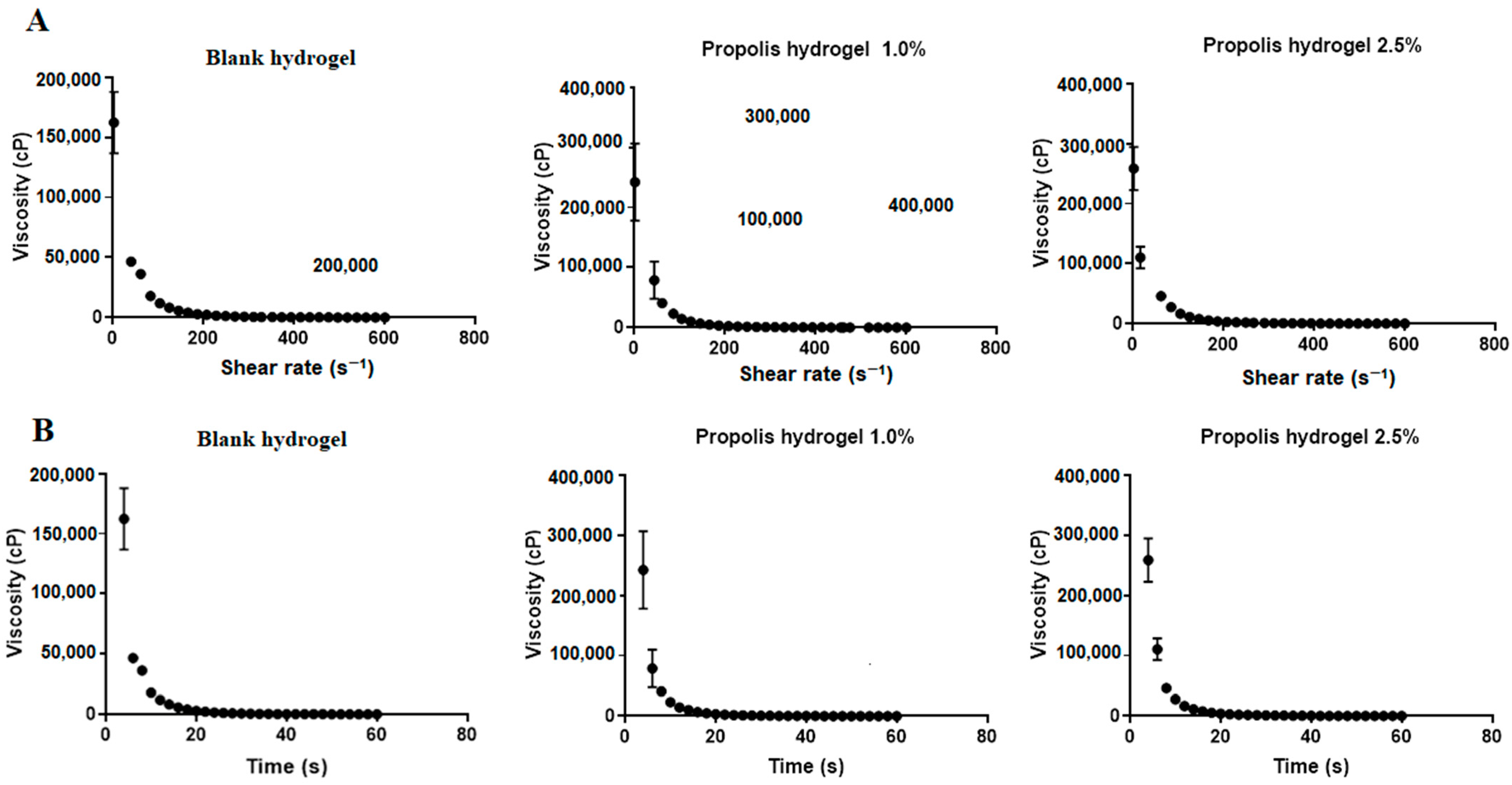
2.7. Rheological Behavior of Hydrogels
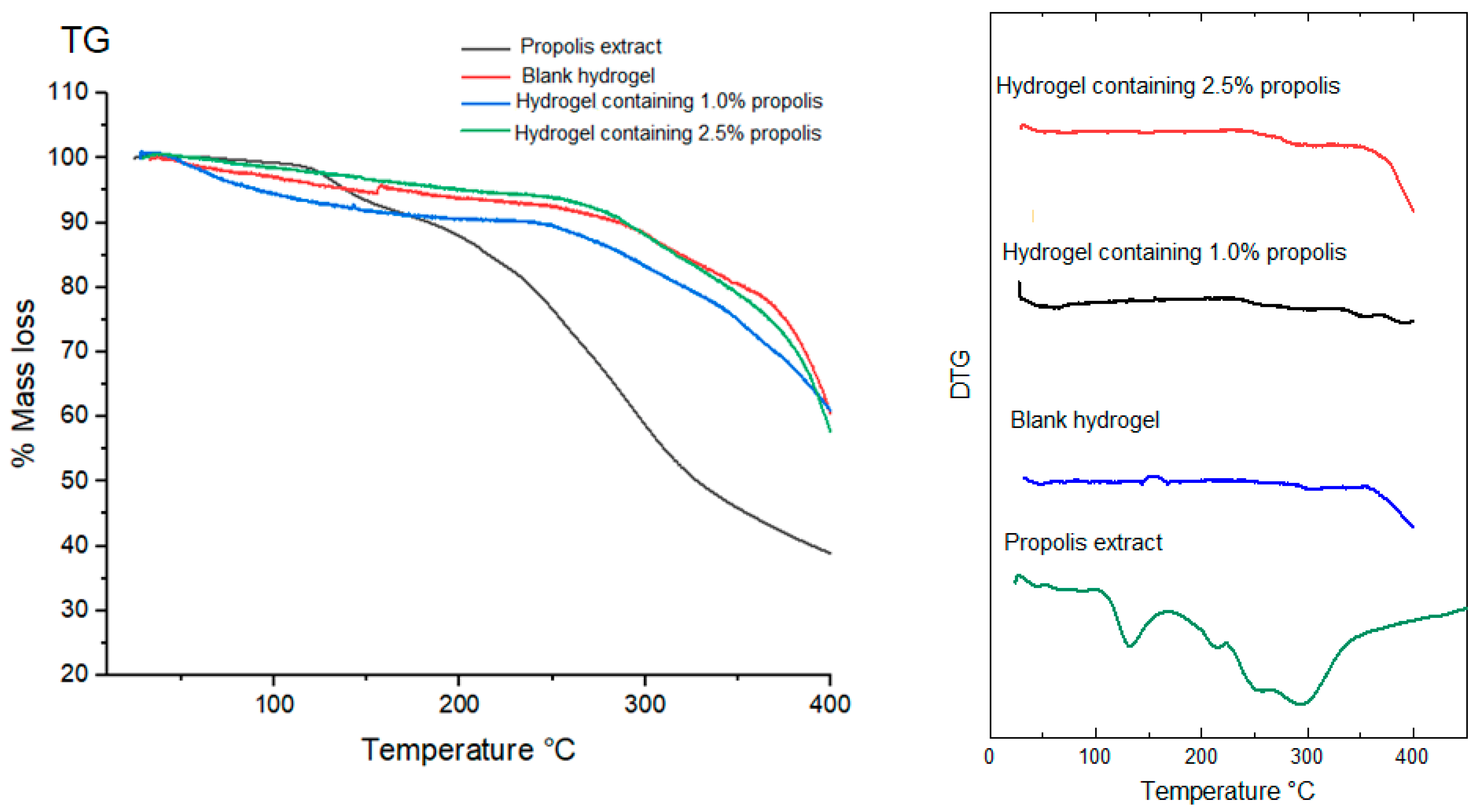
2.8. Thermal Behavior of Propolis Extract and Hydrogels by Thermogravimetry (TG/DTG)
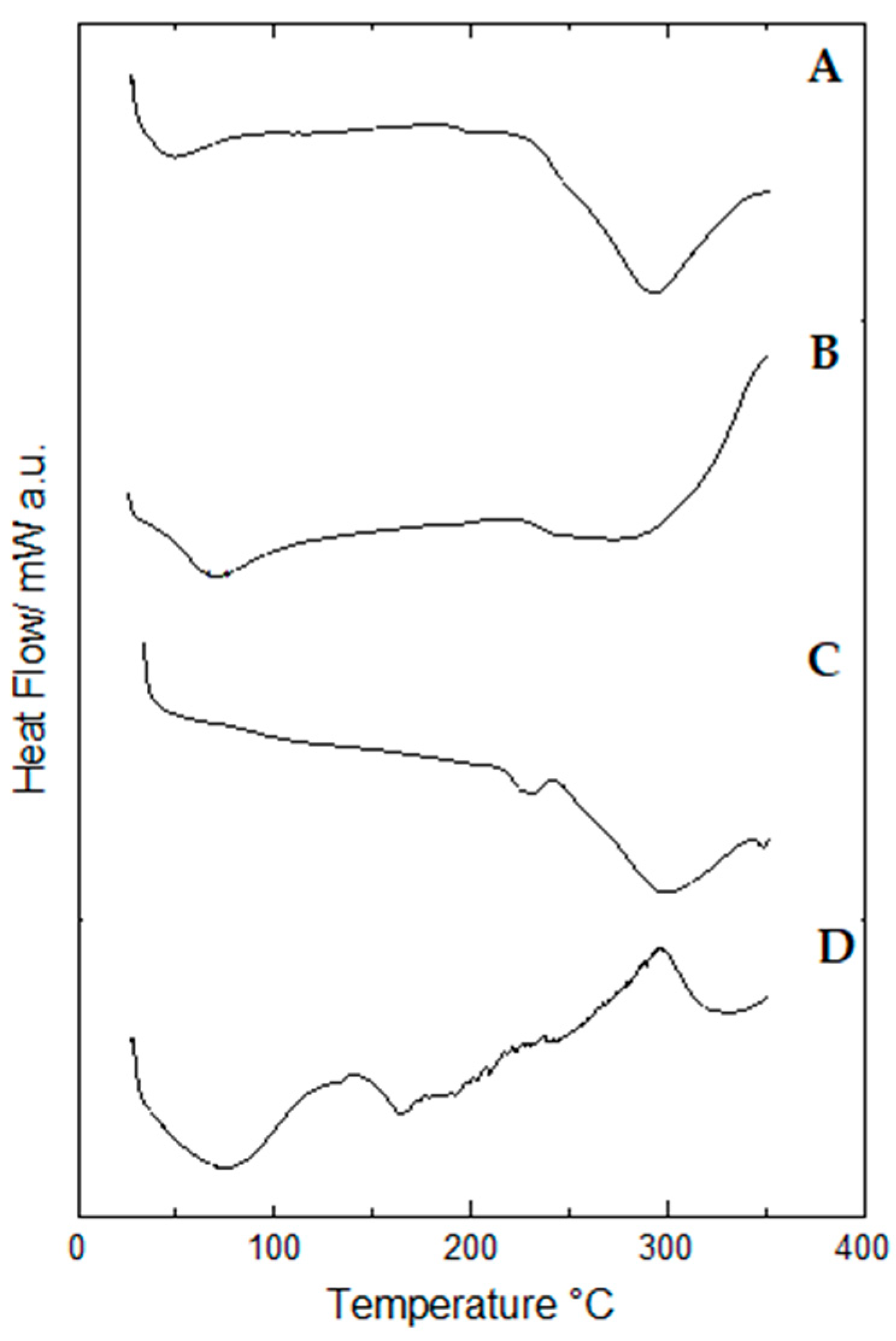
2.9. Thermal Behavior of Propolis Extract and Hydrogels by Differential Scanning Calorimetry (DSC)
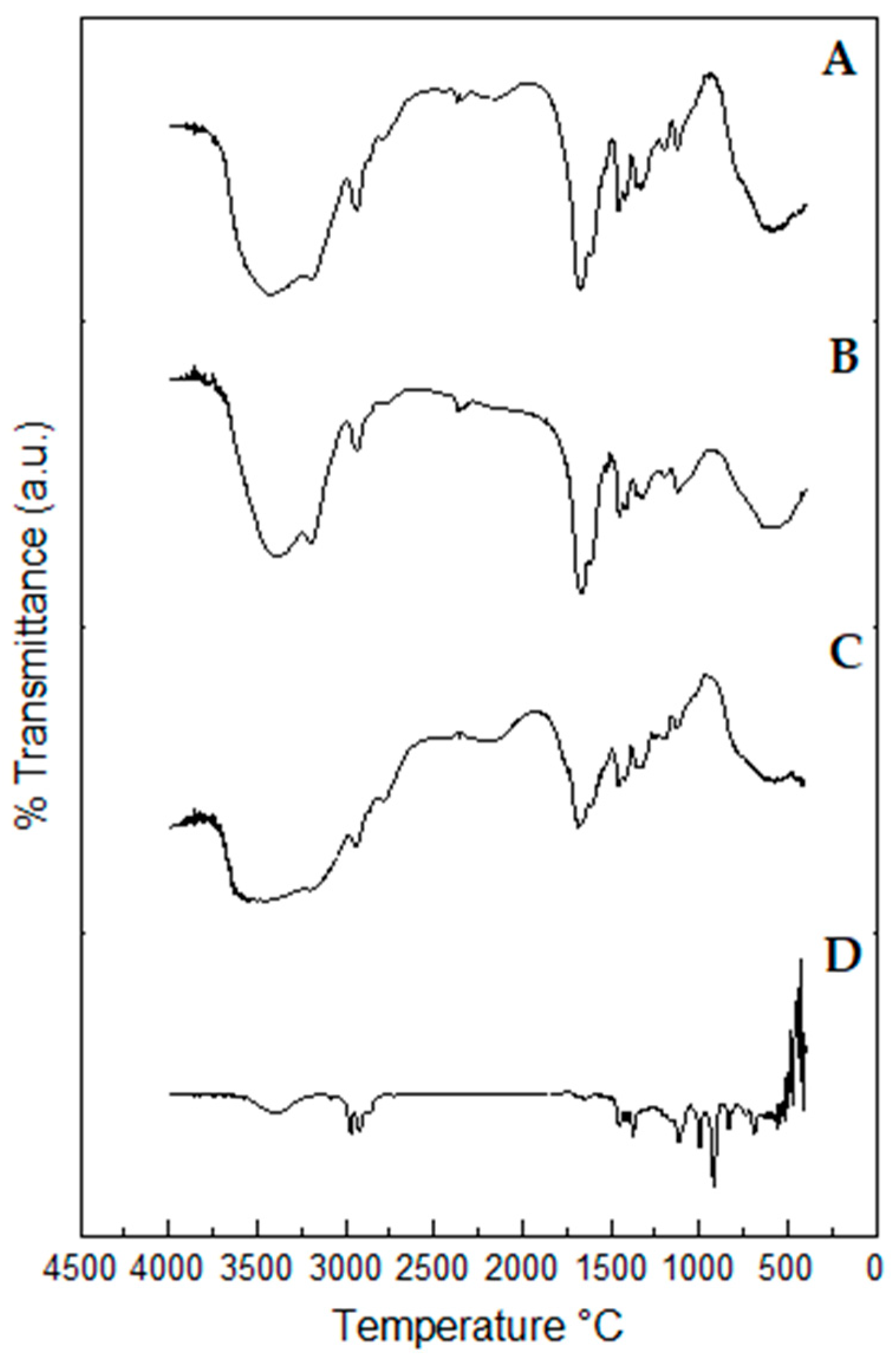
2.10. Fourier-Transform Infrared (FTIR) Spectroscopy Profiles of Propolis Extract and Hydrogels
2.11. Total Polyphenol and Flavonoid Contents of Propolis Extract and Hydrogels
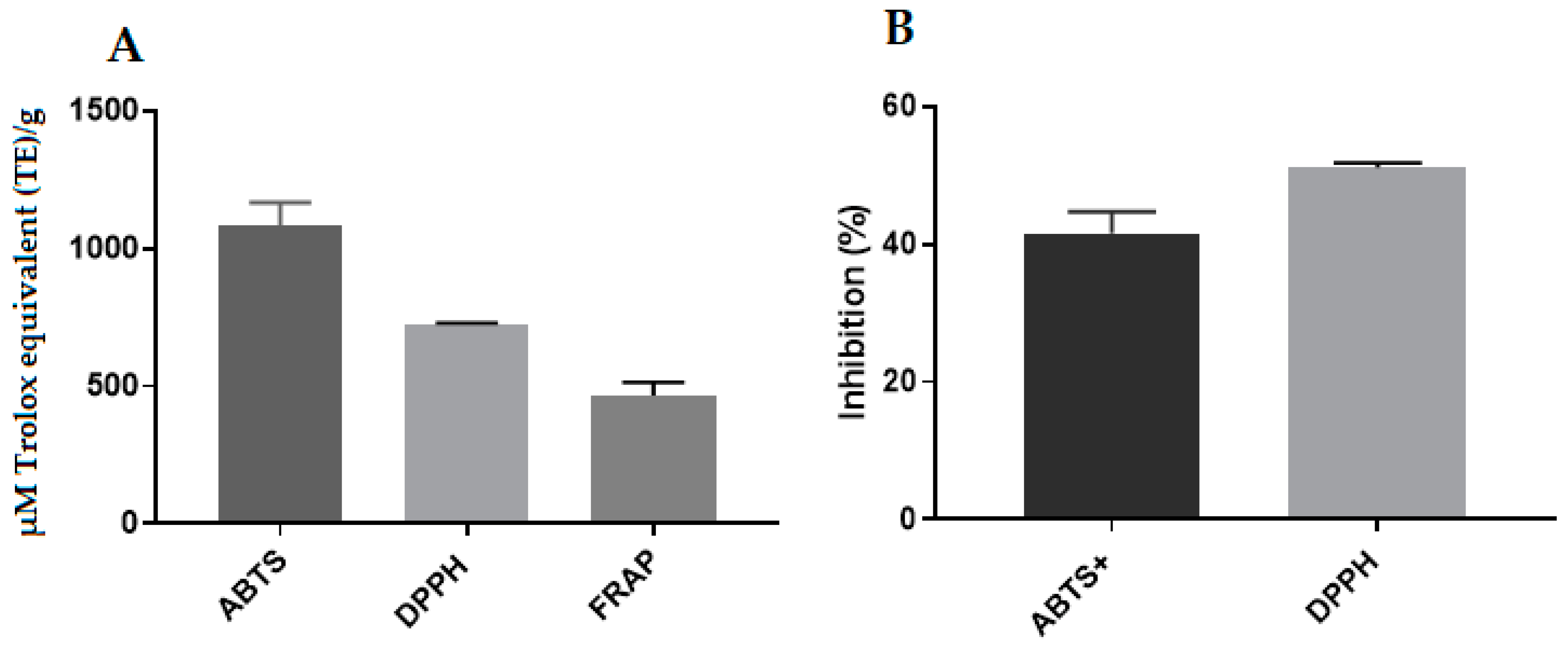
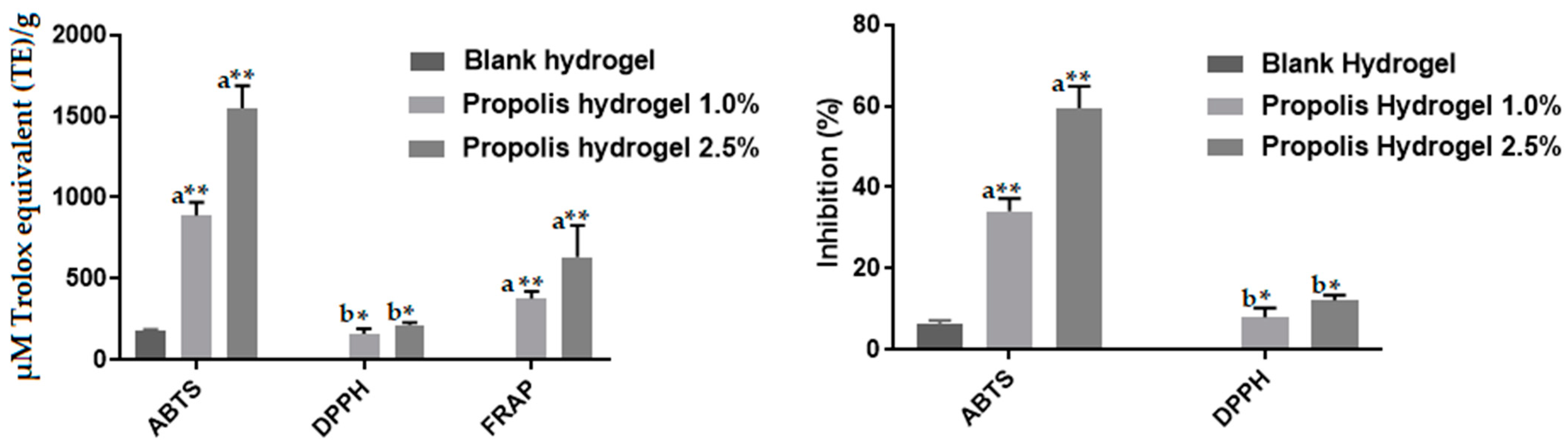
2.12. Antioxidant Activity of Propolis Extract and Hydrogels
2.13. Biocompatibility of Propolis Extract and Hydrogels
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Propolis Samples
4.3. Preparation of the Propolis Extract
4.4. Gas Chromatography-Mass Spectrometry Assay (GC-MS) of Propolis Extract
4.5. Nuclear Magnetic Resonance Spectrometry (NMR) of Propolis Extract
4.6. Preparation of Propolis-Containing Hydrogels
4.7. Scanning Electron Microscopy (SEM) of Hydrogels
4.8. Swelling Capacity of Hydrogels
4.9. Mechanical Properties of Hydrogels
4.10. Rheological Properties of Hydrogels
4.11. Thermal Behavior by Thermogravimetry (TG/DTG) of Propolis Extract and Hydrogels
4.12. Thermal Behavior by Differential Scanning Calorimetry (DSC) of Propolis Extract and Hydrogels
4.13. Fourier-Transform Infrared (FTIR) Spectroscopy Profile of Propolis Extract and Hydrogels
4.14. Extraction of Phenolic Compounds from the Hydrogel Matrix
4.14.1. Determination of Total Polyphenol Content of Propolis Extract and Hydrogels
4.14.2. Determination of Total Flavonoid Content of Propolis Extract and Hydrogels
4.15. Antioxidant Activity Determination of Propolis Extract and Hydrogels
4.15.1. ABTS Assay
4.15.2. DPPH Assay
4.15.3. FRAP Assay
4.16. In Vitro Hydrogel and Extract Biocompatibility
4.16.1. Cultivation of Fibroblast and Macrophage Cells
4.16.2. Cell Viability Analysis
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díez-García, I.; de Costa Lemma, M.R.; Barud, H.S.; Eceiza, A.; Tercjak, A. Hydrogels based on waterborne poly(urethane-urea)s by physically cross-linking with sodium alginate and calcium chloride. Carbohydr. Polym. 2020, 250, 116940. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Heitz, K.; Strømme, M.; Welch, K.; Ferraz, N. Ion-crosslinked wood-derived nanocellulose hydrogels with tunable antibacterial properties: Candidate materials for advanced wound care applications. Carbohydr. Polym. 2018, 181, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, N.; Abbas, A.; Afshan, R.; Irfan, M.; Khalid, S.H.; Asghar, S.; Munir, M.U.; Rizg, W.Y.; Majrashi, K.A.; Alshehri, S.; et al. In vitro and in vivo evaluation of hydroxypropyl-β-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) semi-interpenetrating matrices of dexamethasone sodium phosphate. Pharmaceuticals 2022, 15, 1399. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.Y.; Park, W.H. Injectable Methylcellulose Hydrogel Containing Silver Oxide Nanoparticles for Burn Wound Healing. Carbohydr. Polym. 2018, 181, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, X.; Wu, Y.; Liu, C.; Xia, H.; Cheng, X. In vitro evaluation of kaempferol-loaded hydrogel as ph-sensitive drug delivery systems. Polymers 2022, 14, 3205. [Google Scholar] [CrossRef] [PubMed]
- Alesa Gyles, D.; Pereira Júnior, A.D.; Diniz Castro, L.; Santa Brigida, A.; Nobre Lamarão, M.L.; Ramos Barbosa, W.L.; Carréra Silva Júnior, J.O.; Ribeiro-Costa, R.M. Polyacrylamide-metilcellulose hydrogels containing aloe barbadensis extract as dressing for treatment of chronic cutaneous skin lesions. Polymers 2020, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A Review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Catanzano, O.; Gomez d’Ayala, G.; D’Agostino, A.; Di Lorenzo, F.; Schiraldi, C.; Malinconico, M.; Lanzetta, R.; Bonina, F.; Laurienzo, P. PEG-crosslinked-chitosan hydrogel films for in situ delivery of Opuntia ficus-indica extract. Carbohydr. Polym. 2021, 264, 117987. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.; Macocinschi, D.; Zaltariov, M.-F.; Ciubotaru, B.-I.; Bargan, A.; Varganici, C.-D.; Vasiliu, A.-L.; Peptanariu, D.; Balan-Porcarasu, M.; Timofte-Zorila, M.-M. Hydroxypropyl cellulose/pluronic-based composite hydrogels as biodegradable mucoadhesive scaffolds for tissue engineering. Gels 2022, 8, 519. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Bandeira, E.d.S.; Gomes, M.F.; Lynch, D.G.; Bastos, G.N.T.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide hydrogel containing calendula extract as a wound healing bandage: In vivo test. Int. J. Mol. Sci. 2023, 24, 3806. [Google Scholar] [CrossRef]
- Morozova, S. Methylcellulose Fibrils: A mini review. Polym. Int. 2020, 69, 125–130. [Google Scholar] [CrossRef]
- Coughlin, M.L.; Liberman, L.; Ertem, S.P.; Edmund, J.; Bates, F.S.; Lodge, T.P. Methyl cellulose solutions and gels: Fibril formation and gelation properties. Prog. Polym. Sci. 2021, 112, 101324. [Google Scholar] [CrossRef]
- Hynninen, V.; Patrakka, J.; Nonappa. Methylcellulose–cellulose nanocrystal composites for optomechanically tunable hydrogels and fibers. Materials 2021, 14, 5137. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.V.G.; Tavares, E.J.M.; Aouada, F.A.; Negrão, C.A.B.; Oliveira, M.E.C.; Duarte Júnior, A.P.; Ferreira Da Costa, C.E.; Silva Júnior, J.O.C.; Ribeiro Costa, R.M. Thermal analysis characterization of PAAm-Co-MC hydrogels. J. Therm. Anal. Calorim. 2011, 106, 717–724. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Longo, E.; Mattoso, L.H.C. Investigação do processo de absorção de água de hidrogéis de polissacarídeo: Efeito da carga iônica, presença de sais, concentrações de monômero e polissacarídeo. Polímeros 2012, 22, 311–317. [Google Scholar] [CrossRef][Green Version]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.C. Thermo-sensitive poly (N-isopropylacrylamide-co-polyacrylamide) hydrogel for pH-responsive therapeutic delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef]
- Andriukonis, E.; Butkevicius, M.; Simonis, P.; Ramanavicius, A. Development of a disposable polyacrylamide hydrogel-based semipermeable membrane for micro ag/agcl reference electrode. Sensors 2023, 23, 2510. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, S.; Blais, A.S.; Morin, F.; Moore, K.; Cloutier, J.; Bolduc, S. Polyacrylamide hydrogel as a bulking agent for the endoscopic treatment of vesicoureteral reflux: Long-term results and safety. J. Urol. 2017, 197, 963–967. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Chavda, V.P.; Chaudhari, A.Z.; Teli, D.; Balar, P.; Vora, L. Propolis and their active constituents for chronic diseases. Biomedicines 2023, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Assis, D.S.; Ramos, L.D.P.; Hasna, A.A.; Queiroz, T.S.D.; Lima, N.D.; Berretta, A.A. Antimicrobial and antibiofilm effect of Brazilian green propolis aqueous extract against dental anaerobic bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic constituents, antioxidant and antimicrobial activity and clustering analysis of propolis samples based on PCA from different regions of anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.d.M.C.; Souza, P.D.Q.d.; Pereira, R.R.; da Silva, E.O.; Barbosa, W.L.R.; Silva-Júnior, J.O.C.; Converti, A.; Ribeiro-Costa, R.M. Preliminary study on the chemical and biological properties of propolis extract from stingless bees from the Northern region of Brazil. Processes 2024, 12, 700. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Elakkad, Y.E.; Elwakeel, A.A.; Allam, R.M.; Mousa, M.R. Formulation and characterization of propolis and tea tree oil nanoemulsion loaded with clindamycin hydrochloride for wound healing: In-vitro and in-vivo wound healing assessment. Saudi Pharm. J. 2021, 29, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.d.A.; Cerqueira, J.C.; Reis, J.H.d.O.; Hodel, K.V.S.; Gama, L.A.; Anjos, J.P.; Minafra-Rezende, C.S.; Andrade, L.N.; Amaral, R.G.; Pessoa, C.d.Ó.; et al. Evaluation of the potential of Brazilian red propolis extracts: An analysis of the chemical composition and biological properties. App. Sci. 2022, 12, 11741. [Google Scholar] [CrossRef]
- Ahmed, E.T.; Abo-Salem, O.M.; Osman, A. The influence of Egyptian propolis on induced burn wound healing in diabetic rats; antibacterial mechanism. Sci. J. Med. Clin. Trials 2012, 2011–2317. [Google Scholar]
- Jeffreys, M.F.; Nunez, C.V. Triterpenes of leaves from Piranhea trifoliata (Picrodendraceae). Acta Amaz. 2016, 46, 189–194. [Google Scholar] [CrossRef][Green Version]
- Menezes-de-Oliveira, D.; Aguilar, M.-I.; King-Díaz, B.; Vieira-Filho, S.A.; Pains-Duarte, L.; Silva, G.-D.d.F.; Lotina-Hennsen, B. The Triterpenes 3β-Lup-20(29)-En-3-Ol and 3β-Lup-20(29)-En-3-Yl acetate and the carbohydrate 1,2,3,4,5,6-hexa-O-acetyl-dulcitol as photosynthesis light reactions inhibitors. Molecules 2011, 16, 9939–9956. [Google Scholar] [CrossRef]
- Khedr, A.I.M.; Ibrahim, S.R.M.; Mohamed, G.A.; Ahmed, H.E.A.; Ahmad, A.S.; Ramadan, M.A.; El-Baky, A.E.A.; Yamada, K.; Ross, S.A. New ursane triterpenoids from Ficus pandurata and their binding affinity for human cannabinoid and opioid receptors. Arch. Pharm. Res. 2016, 39, 897–911. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. α-Amyrin and β-Amyrin isolated from Celastrus hindsii leaves and their antioxidant, anti-xanthine oxidase, and anti-tyrosinase potentials. Molecules 2021, 26, 7248. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introdução à Espectroscopia, 4th ed.; Editora Cengage: São Paulo, Brasil, 2010; 716p, ISBN 978-85-221-0708-7. [Google Scholar]
- Fan, X.; Wang, S.; Fang, Y.; Li, P.; Zhou, W.; Wang, Z.; Chen, M.; Liu, H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C 2020, 109, 110649. [Google Scholar] [CrossRef] [PubMed]
- Balçık Tamer, Y. A new design of poly(N-isopropylacrylamide) hydrogels using biodegradable poly(beta-aminoester) crosslinkers as fertilizer reservoirs for agricultural applications. Gels 2023, 9, 127. [Google Scholar] [CrossRef]
- Barak, A.; Goel, Y.; Kumar, R.; Shukla, S.K. Removal of methyl orange over TiO2/polyacrylamide hydrogel. Mater. Today Proc. 2019, 12, 529–535. [Google Scholar] [CrossRef]
- Said dos Santos, R.; Bassi da Silva, J.; Rosseto, H.C.; Vecchi, C.F.; Campanholi, K.d.S.S.; Caetano, W.; Bruschi, M.L. Emulgels containing propolis and curcumin: The effect of type of vegetable oil, poly(acrylic acid) and bioactive agent on physicochemical stability, mechanical and rheological properties. Gels 2021, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, I.; Gębski, J.; Stangierska, D.; Szymańska, A. Determinants of honey and other bee products use for culinary, cosmetic, and medical purposes. Nutrients 2023, 15, 737. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different types of gel carriers as metronidazole delivery systems to the oral mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and antifungal activity of pullulan edible films enriched with propolis extract for active packaging. Foods 2022, 11, 2319. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Krzemińska-Fiedorowicz, L.; Lenart-Boroń, A.; Białecka, A.; Krupka, M.; Krzan, M.; Blaszyńska, K.; Hanula, M.; et al. Synthesis and investigation of physicochemical and biological properties of films containing encapsulated propolis in hyaluronic matrix. Polymers 2023, 15, 1271. [Google Scholar] [CrossRef]
- Sotirova, Y.; Gugleva, V.; Stoeva, S.; Kolev, I.; Nikolova, R.; Marudova, M.; Nikolova, K.; Kiselova-Kaneva, Y.; Hristova, M.; Andonova, V. Bigel formulations of nanoencapsulated St. John’s wort extract-an approach for enhanced wound healing. Gels 2023, 9, 360. [Google Scholar] [CrossRef]
- De Cássia Almeida Sampaio, R.; Da Costa, R.S.; De Souza, C.R.F.; Duarte Júnior, A.P.; Ribeiro-Costa, R.M.; Da Costa, C.E.F.; De Oliveira, W.P.; Converti, A.; Silva Júnior, J.O.C. Thermal characterization of Arrabidaea chica (Humb. & Bonpl.) B. Verl. dry extracts obtained by spray dryer. J. Therm. Anal. Calorim. 2016, 123, 2469–2475. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; David, L.B., Ed.; Wiley: Sao Paolo, Brazil, 2007; pp. 71–107. ISBN 978-85-216-3637-3. [Google Scholar]
- Gabbay Alves, T.V.; Silva da Costa, R.; Aguiar Gomes, A.T.; Ferreira da Costa, C.E.; Perego, P.; Carréra Silva Júnior, J.O.; Converti, A.; Ribeiro Costa, R.M. Quality control of Amazonian cocoa (Theobroma cacao L.) by-products and microencapsulated extract by thermal analysis. J. Therm. Anal. Calorim. 2018, 134, 993–1000. [Google Scholar] [CrossRef]
- do Nascimento, T.G.; de Almeida, C.P.; da Conceição, M.M.; dos Santos Silva, A.; de Almeida, L.M.; de Freitas, J.M.D.; Grillo, L.A.M.; Dornelas, C.B.; Ribeiro, A.S.; da Silva, J.F.; et al. Caseinates loaded with Brazilian Red propolis extract: Preparation, protein-flavonoids interaction, antioxidant and antibacterial activities. J. Therm. Anal. Calorim. 2022, 147, 1329–1343. [Google Scholar] [CrossRef]
- Rodrigues Sousa, H.; Lima, I.S.; Neris, L.M.L.; Silva, A.S.; Santos Nascimento, A.M.S.; Araújo, F.P.; Ratke, R.F.; Silva, D.A.; Osajima, J.A.; Bezerra, L.R.; et al. Superabsorbent hydrogels based to polyacrylamide/cashew tree gum for the controlled release of water and plant nutrients. Molecules 2021, 26, 2680. [Google Scholar] [CrossRef] [PubMed]
- Okińczyc, P.; Widelski, J.; Szperlik, J.; Żuk, M.; Mroczek, T.; Skalicka-Woźniak, K.; Sakipova, Z.; Widelska, G.; Kuś, P.M. Article impact of plant origin on Eurasian propolis on phenolic profile and classical antioxidant activity. Biomolecules 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.M.D.M.; Silva, H.R.E.; Vieira, G.M.; Ayres, M.C.C.; Da Costa, C.L.S.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.D.M.; Brandão, M.S.; et al. Fenóis Totais e atividade antioxidante de cinco plantas medicinais. Química Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Wang, R.; Zhang, M.; Zhang, Q.; Zhang, Y.; Wang, S.; Cheng, Y. Bioactive skin-mimicking hydrogel band-aids for diabetic wound healing and infectious skin incision treatment. Bioact. Mater. 2021, 6, 3962–3975. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Alkadhem, N.; Progri, H.; Nikfarjam, S.; Jeon, J.; Kotturi, H.; Vaughan, M.B. Glutathione immobilized polycaprolactone nanofiber mesh as a dermal drug delivery mechanism for wound healing in a diabetic patient. Processes 2022, 10, 512. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Mohammed, S.N.F.; Syed Abd Halim, S.A.; Ghafar, N.A.; Abdul Jalil, N.A. Therapeutic potential of honey and propolis on ocular disease. Pharmaceuticals 2022, 15, 1419. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Thanyacharoen, T.; Techasakul, S.; Svasti, J.; Nooeaid, P. Turmeric herb extract-incorporated biopolymer dressings with beneficial antibacterial, antioxidant and anti-inflammatory properties for wound healing. Polymers 2023, 15, 1090. [Google Scholar] [CrossRef]
- Caracterização de Polímeros Multifásicos. Parte 1: Processamento e Morfologia. Available online: http://revistapolimeros.org.br/article/588371397f8c9d0a0c8b47bc (accessed on 27 October 2023).
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on polymeric hydrogel membranes for wound dressing applications: Pva-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Umar, A.; Wang, Y. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Murali Mohan, Y.; Keshava Murthy, P.S.; Mohana Raju, K. Preparation and swelling behavior of macroporous poly(acrylamide-co-sodium methacrylate) superabsorbent hydrogels. J. Appl. Polym. Sci. 2006, 101, 3202–3214. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and characterization of collagen/PVA dual-layer membranes for periodontal bone regeneration. Front. Bioeng. Biotechnol. 2021, 9, 630977. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty tough hydrogels and their biomedical applications. Adv. Healthc. Mater. 2020, 9, 1901396. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Martinović, M.; Ćirić, A.; Fraj, J. Composite chitosan hydrogels as advanced wound dressings with sustained ibuprofen release and suitable application characteristics. Pharm. Dev. Technol. 2020, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Solanki, D.; Vinchhi, P.; Patel, M.M. Design considerations, formulation approaches, and strategic advances of hydrogel dressings for chronic wound management. ACS Omega 2023, 8, 8172–8189. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, M.V.; Borghi-Pangoni, F.B.; Ferreira, S.B.d.S.; Bruschi, M.L. Evaluation of the methylene blue addition in binary polymeric systems composed by poloxamer 407 and carbopol 934p using quality by design: Rheological, textural, and mucoadhesive analysis. Drug Dev. Ind. Pharm. 2016, 42, 2009–2019. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-incorporated crosslinked sodium alginate-g-poly (N-isopropyl acrylamide) thermo-responsive hydrogel as an in-situ forming injectable dressing for wound healing: In vitro characterization and in vivo evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; da Silva Almeida, J.R.G. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Svirskis, D.; Waterhouse, G.I.N.; Wu, Z. Hydroxypropyl methylcellulose bioadhesive hydrogels for topical application and sustained drug release: The effect of polyvinylpyrrolidone on the physicomechanical properties of hydrogel. Pharmaceutics 2023, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Skin Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, M.; Leyk, E. Coupled and simultaneous thermal analysis techniques in the study of pharmaceuticals. Pharmaceutics 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical composition and its applications in endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Angelo, P.M.; Jorge, N. Compostos fenólicos em alimentos—Uma breve revisão. Rev. Inst. Adolfo Lutz 2007, 66, 1–9. [Google Scholar] [CrossRef]
- David, J.M.; David, J.P.; Lima, L.S. Avaliação da atividade antioxidante de flavonóides. Dialogos Ciência 2007, 12, 1–8. [Google Scholar]
- Freitas, C.P.; Bilibio, D. Atividade antioxidante da própolis de abelhas jataí antioxidant activity of propolis of Jataí bees. Braz. J. Anim. Environ. Res. 2021, 4, 989–996. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Preocugutierrez-Gonçalves, M.E.J. Activities, a. atividades antimicrobiana e antioxidante da própolis do estado do Ceará antimicrobial and antioxidant activities of própolis from ceará state. Rev. Fitos 2009, 4, 81–86. [Google Scholar] [CrossRef]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Tamer, T.M.; Augustyniak, M.; El Wakil, A. Carboxymethyl cellulose/sericin-based hydrogels with intrinsic antibacterial, antioxidant, and anti-inflammatory properties promote re-epithelization of diabetic wounds in rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Fucoidan-loaded hydrogels facilitates wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydr. Polym. 2020, 247, 116624. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef] [PubMed]
- Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and mechanical properties of carboxymethyl chitosan hydrogels. Polymers 2023, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.F.; Dias, A.M.A.; Leal, E.C.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Chitosan-based dressings loaded with neurotensin--an efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Ecke, B.; Zigrino, P.; Kessler, D.; Holtkötter, O.; Shepard, P.; Mauch, C.; Krieg, T. Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol. 2000, 19, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Leibovich, S.J. Inflammatory Cells during Wound Repair: The Good, the Bad and the Ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef]
- de Carvalho, F.M.d.A.; Schneider, J.K.; de Jesus, C.V.F.; de Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Caramão Bastos, E.; et al. Brazilian red propolis: Extracts production, physicochemical characterization, and cytotoxicity profile for antitumor activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef]
- Schleiff, M.; Sommers, C.; Yang, J.; Shen, X.; Rodriguez, J.D.; Shu, Q. Development and validation of a quantitative proton nmr method for the analysis of pregnenolone. J. Pharm. Biomed. Anal. 2023, 222, 115073. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Funari, C.S.; Ferro, V.O. Análise de Própolis. Food Sci. Technol. 2006, 26, 171–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Trolox assay. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.M.; Moysés, D.A.; Nascimento, H.F.S.; Mota, T.C.; Bonfim, L.T.; Cardoso, P.C.S.; Burbano, R.M.R.; Bahia, M.O. Genotoxic and cytotoxic effects of the drug dipyrone sodium in african green monkey kidney (Vero) cell line exposed in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1529–1535. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]



| Identified Compounds | Retention Time | Area % |
|---|---|---|
| Pentan-2-ol, 4-allyloxy-2-methyl- | 2.070 | 4.77 |
| Hexane, 1-(3-butenyloxy) | 2.170 | 2.49 |
| 2-Pentene, 4,4-dimethyl-, (Z)- | 2.284 | 2.71 |
| Cyclopropane, 1,1,2,3-tetrameth | 2.480 | 0.94 |
| 2-Cyclopenten-1-one, 3-methyl- | 2.535 | 1.99 |
| 2-Methyl-5-octyn-4-ol | 2.538 | 0.72 |
| 2-Hexanone, 6-hydroxy- | 2.640 | 0.82 |
| 1,4-Hexadiene, 4-methyl- | 2.962 | 1.32 |
| Hexadecanoic acid, methyl ester | 29.305 | 11.25 |
| 9,12-Octadecadienoic acid (Z,Z) | 34.683 | 1.48 |
| 9-Octadecenoic acid, methyl ester | 34.879 | 32.20 |
| Methyl stearate | 35.696 | 1.92 |
| Docosanoic acid, methyl ester | 47.031 | 1.98 |
| Tetracosanoic acid, methyl ester | 52.084 | 1.79 |
| Terpenes | ||
| Lanosterol | 48.073 | 6.00 |
| β-Amyrin | 49.608 | 9.83 |
| 24-Norursa-3,12-diene | 50.459 | 17.13 |
| Lup-20(29)-en-3-ol, acetate(3. beta.)- | 52.400 | 7.33 |
| Thunbergol | 66.174 | 2.29 |
| RMN 1H | |||
|---|---|---|---|
| Chemical Shift δ (ppm) | Multiplicity | Type of Proton | Class |
| 0.85 | Singlet | CH3 | Methyl |
| 1.23 | Singlet | CH3 | Methyl |
| 1.55 | Singlet | CH2 | Methylene |
| 1.63 | Singlet | CH2 | Methylene |
| 1.99 | Singlet | (C=C–C–H) | Methylene |
| 2.17 | Singlet | (C=C–C–H) | Methylene |
| 2.50 | Singlet | (C=C–H) sp2 | DMSO |
| 5.05 | Duplet | (C=C–H) sp2 | Alkenes |
| 5.32 | Singlet | (C=C–H) sp2 | Alkenes |
| 6.58 | Singlet | (C=C–H) sp2 | Alkenes |
| 6.82 | Duplet | (C=C–H) sp2 | Alkenes/aromatics |
| 7.83 | Duplet | (C=C–H) sp2 | Alkenes/aromatics |
| Cycle 1 | Cycle 2 | ||||
|---|---|---|---|---|---|
| Formulation | Hardness (g) | Adhesiveness (mJ) | Stiffness (mm) | Cohesiveness (Dimensionless) | Elasticity (mm) |
| Blank hydrogel | 415.57 ± 131.79 a** | 0.52 ± 0.37 b* | 2.03 ± 0.37 a** | 0.98 ± 0.01 a** | 31.55 ± 18.88 a** |
| Hydrogel containing 1.0% propolis | 1430.70 ± 185.83 a** | 0.83 ± 0.12 b* | 0.62 ± 0.14 a** | 0.82 ± 0.15 a** | 9.96 ± 0.13 a** |
| Hydrogel containing 2.5% propolis | 1337.13 ± 377.13 a** | 1.44 ± 0.51 b* | 0.80 ± 0.17 a** | 0.95 ± 0.24 a** | 10.41 ± 0.60 a** |
| Formulation | Viscosity (cP) | Shear Rate (s−1) | Shear Stress (Pa) |
|---|---|---|---|
| Blank hydrogel | 108.44 ± 10.15 a** | 601.84 ± 0.031 | 63.10 ± 6.97 b** |
| Hydrogel containing 1.0% propolis | 149.50 ± 21.92 a** | 601.92 ± 0.012 | 90.01 ± 13.19 b** |
| Hydrogel containing 2.5% propolis | 445.67 ± 5.18 a** | 601.68 ± 0.106 | 268.36 ± 2.99 b** |
| Sample | 1st Stage | 2nd Stage | |||
|---|---|---|---|---|---|
| Temperature Range (°C) | Mass Loss (%) | Temperature Range (°C) | Mass Loss (%) | ||
| Propolis extract | 124.57–146.59 | 7.35 | 268.52–341.47 | 44.92 | |
| Non-lyophilized samples | Blank hydrogel | 32.02–170.22 | 46.55 | 359.57–485.09 | 25.95 |
| Hydrogel containing 1.0% propolis | 41.01–170.92 | 62.42 | 365.05–472.71 | 14.46 | |
| Hydrogel containing 2.5% propolis | 44.22–173.00 | 4.02 | 195.27–570.81 | 38.37 | |
| Lyophilized samples | Blank hydrogel | 379.68–391.45 | 17.65 | – | – |
| Hydrogel containing 1.0% propolis | 352.56–392.12 | 25.37 | – | – | |
| Hydrogel containing 2.5% propolis | 287.10–393.11 | 27.06 | – | – | |
| 1st Event | 2nd Event | 3rd Event | |||||
|---|---|---|---|---|---|---|---|
| Sample | Peak Temperature (°C) | Enthalpy Variation (J/g) | Peak Temperature (°C) | Enthalpy Variation (J/g) | Peak Temperature (°C) | Enthalpy Variation (J/g) | |
| Propolis extract | 74.8 | −43.76 | 327.4 | −1.02 | 215.53 | −2.48 | |
| Non-lyophilized samples | Blank hydrogel | 54.71 | −222.66 | - | - | ||
| Hydrogel containing 1.0% propolis | 67.92 | −502.27 | - | - | |||
| Hydrogel containing 2.5% propolis | 97.13 | −1.52 | - | - | |||
| Lyophilized samples | Blank hydrogel | 299.66 | −152.45 | ||||
| Hydrogel containing 1.0% propolis | 70.71 | −119.52 | 273.37 | −397.26 | |||
| Hydrogel containing 2.5% propolis | 43.31 | −33.24 | 293.28 | −311.88 | |||
| Sample | Total Polyphenols (mg GAE/g) | Total Flavonoids (mg QE/g) |
|---|---|---|
| Propolis extract | 72.80 ± 1.20 | 35.19 ± 0.24 |
| Blank hydrogel | - | - |
| Hydrogel containing 1.0% propolis | 24.74 ± 1.71 a** | 8.01 ± 0.99 a** |
| Hydrogel containing 2.5% propolis | 32.10 ± 1.01 a** | 13.81 ± 0.71 a** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.M.d.M.C.; Modesto, Y.Y.; Souza, P.D.Q.d.; Nascimento, F.C.d.A.; Pereira, R.R.; Converti, A.; Lynch, D.G.; Brasil, D.d.S.B.; da Silva, E.O.; Silva-Júnior, J.O.C.; et al. Characterization, Biocompatibility and Antioxidant Activity of Hydrogels Containing Propolis Extract as an Alternative Treatment in Wound Healing. Pharmaceuticals 2024, 17, 575. https://doi.org/10.3390/ph17050575
Ferreira LMdMC, Modesto YY, Souza PDQd, Nascimento FCdA, Pereira RR, Converti A, Lynch DG, Brasil DdSB, da Silva EO, Silva-Júnior JOC, et al. Characterization, Biocompatibility and Antioxidant Activity of Hydrogels Containing Propolis Extract as an Alternative Treatment in Wound Healing. Pharmaceuticals. 2024; 17(5):575. https://doi.org/10.3390/ph17050575
Chicago/Turabian StyleFerreira, Lindalva Maria de Meneses Costa, Yuri Yoshioka Modesto, Poliana Dimsan Queiroz de Souza, Fabiana Cristina de Araújo Nascimento, Rayanne Rocha Pereira, Attilio Converti, Desireé Gyles Lynch, Davi do Socorro Barros Brasil, Edilene Oliveira da Silva, José Otávio Carréra Silva-Júnior, and et al. 2024. "Characterization, Biocompatibility and Antioxidant Activity of Hydrogels Containing Propolis Extract as an Alternative Treatment in Wound Healing" Pharmaceuticals 17, no. 5: 575. https://doi.org/10.3390/ph17050575
APA StyleFerreira, L. M. d. M. C., Modesto, Y. Y., Souza, P. D. Q. d., Nascimento, F. C. d. A., Pereira, R. R., Converti, A., Lynch, D. G., Brasil, D. d. S. B., da Silva, E. O., Silva-Júnior, J. O. C., & Ribeiro-Costa, R. M. (2024). Characterization, Biocompatibility and Antioxidant Activity of Hydrogels Containing Propolis Extract as an Alternative Treatment in Wound Healing. Pharmaceuticals, 17(5), 575. https://doi.org/10.3390/ph17050575