Potential Anti-Tumorigenic Properties of Diverse Medicinal Plants against the Majority of Common Types of Cancer
Abstract
1. Introduction
2. Methods
2.1. Annona muricata
2.2. Arctium lappa L.
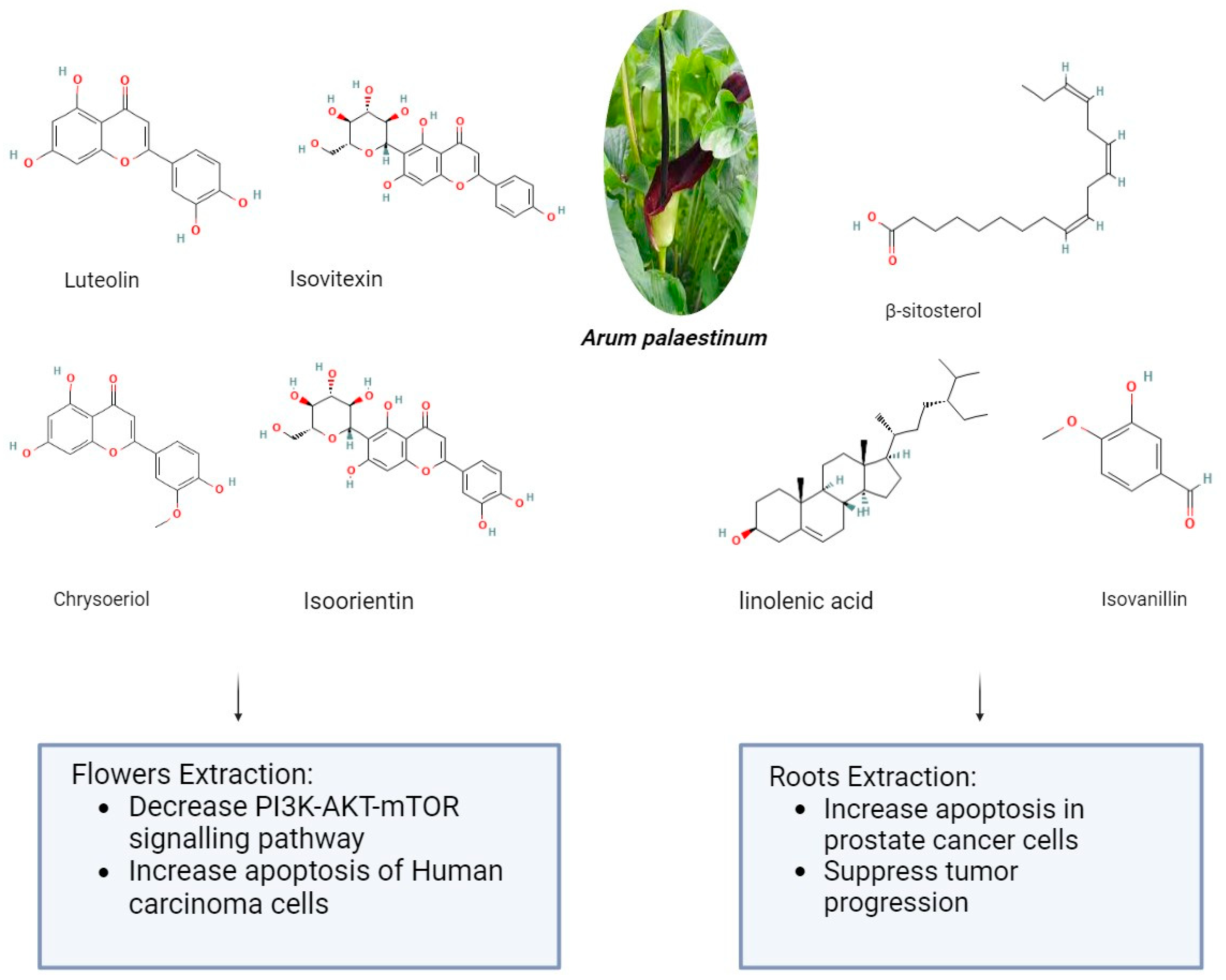
2.3. Arum palaestinum
2.4. Cannabis sativa
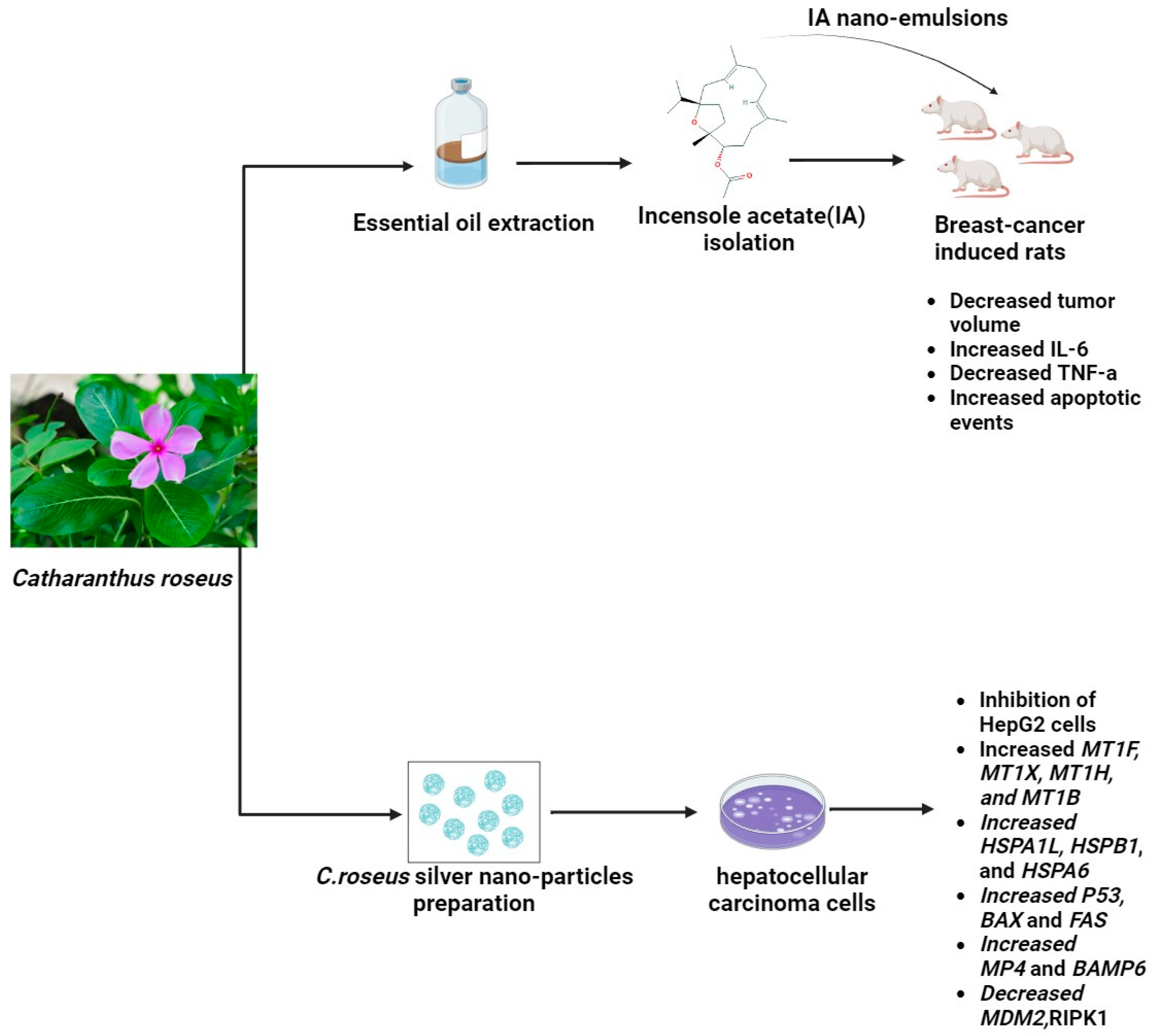
2.5. Catharanthus roseus
2.6. Curcuma longa
2.7. Glycyrrhiza glabra
2.8. Hibiscus
2.9. Kalanchoe blossfeldiana
2.10. Moringa oleifera
2.11. Nerium oleander
2.12. Silybum marianum L.
2.13. Taraxacum officinale
2.14. Urtica dioica
2.15. Withania somnifera
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt Signaling in Colorectal Cancer: Pathogenic Role and Therapeutic Target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services#:~:text=In%202022%2C%20there%20were%20an,women%20die%20from%20the%20disease (accessed on 20 April 2024).
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Intl J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma Lucidum: A Rational Pharmacological Approach to Surmount Cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Hani, U.; Wahab, S.; Siddiqua, A.; Osmani, R.A.M.; Rahamathulla, M. A Comprehensive Review of Current Perspectives on Novel Drug Delivery Systems and Approaches for Lung Cancer Management. J. Pharm. Innov. 2022, 17, 1530–1553. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The Global Burden of Cancer: Priorities for Prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Noorolahi, S.M.; Sadeghi, S.; Mohammadi, M.; Azadi, M.; Rahimi, N.A.; Vahabi, F.; Arjmand, M.; Hosseini, H.; Mosallatpur, S.; Zamani, Z. Metabolomic Profiling of Cancer Cells to Aloe Vera Extract by 1HNMR Spectroscopy. J. Metabolomics 2016, 2, 1. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer Chemoprevention: A Rapidly Evolving Field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O. New Opportunities in Chemoprevention Research. Cancer Investig. 2002, 20, 237–245. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Wang, Q.; Wang, L.; Yu, S. Antitumor Potential of Hedyotis diffusa Willd: A Systematic Review of Bioactive Constituents and Underlying Molecular Mechanisms. Biomed. Pharmacother. 2020, 130, 110735. [Google Scholar] [CrossRef]
- Dutt, R.; Sharma, A.K.; Keservani, R.K.; Garg, V. (Eds.) Promising Drug Molecules of Natural Origin, 1st ed.; Includes bibliographical references and index; Apple Academic Press: Palm Bay, FL, USA, 2020; ISBN 978-1-00-301039-5. [Google Scholar] [CrossRef]
- Pineda-Ramírez, N.; Calzada, F.; Alquisiras-Burgos, I.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Ortiz-Plata, A.; Pinzón Estrada, E.; Torres, I.; Aguilera, P. Antioxidant Properties and Protective Effects of Some Species of the Annonaceae, Lamiaceae, and Geraniaceae Families against Neuronal Damage Induced by Excitotoxicity and Cerebral Ischemia. Antioxidants 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Hogan, S.; Schmelz, E.M.; Ju, Y.H.; Canning, C.; Zhou, K. Selective Growth Inhibition of Human Breast Cancer Cells by Graviola Fruit Extract In Vitro and In Vivo Involving Downregulation of EGFR Expression. Nutr. Cancer 2011, 63, 795–801. [Google Scholar] [CrossRef]
- Sun, B.; Cai, E.; Zhao, Y.; Wang, Y.; Yang, L.; Wang, J.-Y. Arctigenin Triggers Apoptosis and Autophagy via PI3K/Akt/mTOR Inhibition in PC-3M Cells. Chem. Pharm. Bull. 2021, 69, 472–480. [Google Scholar] [CrossRef] [PubMed]
- JianFeng, C.; PengYing, Z.; ChengWei, X.; TaoTao, H.; YunGui, B.; KaoShan, C. Effect of Aqueous Extract of Arctium lappa L. (Burdock) Roots on the Sexual Behavior of Male Rats. BMC Complement. Altern. Med. 2012, 12, 8. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Slaughter, R.J.; Beasley, D.M.G.; Lambie, B.S.; Wilkins, G.T.; Schep, L.J. Poisonous Plants in New Zealand: A Review of Those That Are Most Commonly Enquired about to the National Poisons Centre. N. Z. Med. J. 2012, 125, 87–118. [Google Scholar]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive Effect of the Non-Psychotropic Phytocannabinoid Cannabidiol on Experimental Colon Cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef]
- Azhar, N.A.; Ghozali, S.Z.; Abu Bakar, S.A.; Lim, V.; Ahmad, N.H. Suppressing Growth, Migration, and Invasion of Human Hepatocellular Carcinoma HepG2 Cells by Catharanthus Roseus-silver Nanoparticles. Toxicol. Vitr. 2020, 67, 104910. [Google Scholar] [CrossRef]
- Nair, K.P.P. The Ornamental Curcuma. In The Agronomy and Economy of Turmeric and Ginger; Elsevier: Amsterdam, The Netherlands, 2013; pp. 205–215. ISBN 978-0-12-394801-4. [Google Scholar]
- Zhu, J.; Zhao, B.; Xiong, P.; Wang, C.; Zhang, J.; Tian, X.; Huang, Y. Curcumin Induces Autophagy via Inhibition of Yes-Associated Protein (YAP) in Human Colon Cancer Cells. Med. Sci. Monit. 2018, 24, 7035–7042. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Song, N.-Y.; Suh, J.; Kim, D.-H.; Kim, W.; Ann, J.; Lee, J.; Baek, J.-H.; Na, H.-K.; Surh, Y.-J. Curcumin Suppresses Oncogenicity of Human Colon Cancer Cells by Covalently Modifying the Cysteine 67 Residue of SIRT1. Cancer Lett. 2018, 431, 219–229. [Google Scholar] [CrossRef]
- Tong, W.; Wang, Q.; Sun, D.; Suo, J. Curcumin Suppresses Colon Cancer Cell Invasion via AMPK-Induced Inhibition of NF–κB, uPA Activator and MMP9. Oncol. Lett. 2016, 12, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Zhu, D.-J.; Chen, X.-W.; Chen, Q.-K.; Luo, Z.-T.; Liu, C.-C.; Wang, G.-X.; Zhang, W.-J.; Liao, N.-Z. Curcumin Enhances the Effects of Irinotecan on Colorectal Cancer Cells through the Generation of Reactive Oxygen Species and Activation of the Endoplasmic Reticulum Stress Pathway. Oncotarget 2017, 8, 40264–40275. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, S.; Shao, J.; Qu, X. Primary Research on Chinese Medicine Treatment of Androgen-Independent Prostate Cancer. Chin. J. Integr. Med. 2009, 15, 168–169. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A History of the Therapeutic Use of Liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Bortolotto, L.F.B.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of Trans-Chalcone and Licochalcone A against Breast Cancer Cells Is Due to Apoptosis Induction and Cell Cycle Arrest. Biomed. Pharmacother. 2017, 85, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhang, T.; Zhang, W.; Zhou, L.; Yu, B.; Wang, W.; Yang, Z.; Liu, Z.; Zou, P.; Liang, G. Licochalcone A Inhibits the Proliferation of Human Lung Cancer Cell Lines A549 and H460 by Inducing G2/M Cell Cycle Arrest and ER Stress. Int. J. Mol. Sci. 2017, 18, 1761. [Google Scholar] [CrossRef]
- Lin, X.; Tian, L.; Wang, L.; Li, W.; Xu, Q.; Xiao, X. Antitumor Effects and the Underlying Mechanism of Licochalcone A Combined with 5-Fluorouracil in Gastric Cancer Cells. Oncol. Lett. 2017, 13, 1695–1701. [Google Scholar] [CrossRef]
- Braglia, L.; Bruna, S.; Lanteri, S.; Mercuri, A.; Portis, E. An AFLP-Based Assessment of the Genetic Diversity within Hibiscus Rosa-Sinensis and Its Place within the Hibiscus Genus Complex. Sci. Hortic. 2010, 123, 372–378. [Google Scholar] [CrossRef]
- Kao, E.-S.; Hsu, J.-D.; Wang, C.-J.; Yang, S.-H.; Cheng, S.-Y.; Lee, H.-J. Polyphenols Extracted from Hibiscus sabdariffa L. Inhibited Lipopolysaccharide-Induced Inflammation by Improving Antioxidative Conditions and Regulating Cyclooxygenase-2 Expression. Biosci. Biotechnol. Biochem. 2009, 73, 385–390. [Google Scholar] [CrossRef]
- Hernández-Caballero, M.E.; Sierra-Ramírez, J.A.; Villalobos-Valencia, R.; Seseña-Méndez, E. Potential of Kalanchoe Pinnata as a Cancer Treatment Adjuvant and an Epigenetic Regulator. Molecules 2022, 27, 6425. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa Oleifera: A Review on Nutritive Importance and Its Medicinal Application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Berkovich, L.; Earon, G.; Ron, I.; Rimmon, A.; Vexler, A.; Lev-Ari, S. Moringa Oleifera Aqueous Leaf Extract Down-Regulates Nuclear Factor-kappaB and Increases Cytotoxic Effect of Chemotherapy in Pancreatic Cancer Cells. BMC Complement. Altern. Med. 2013, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Bopape, M.; Tiloke, C.; Ntsapi, C. Moringa oleifera and Autophagy: Evidence from In Vitro Studies on Chaperone-Mediated Autophagy in HepG 2 Cancer Cells. Nutr. Cancer 2023, 75, 1822–1847. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Biswal, A.K.; Dandapat, J.; Debata, P.R. Leaf Extract of Nerium oleander L. Inhibits Cell Proliferation, Migration and Arrest of Cell Cycle at G2/M Phase in HeLa Cervical Cancer Cell. ACAMC Anti-Cancer Agents Med. Chem. 2021, 21, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Dik, B.; Coskun, D.; Er, A. Protective Effect of Nerium oleander Distillate and Tarantula cubensis Alcoholic Extract on Cancer Biomarkers in Colon and Liver Tissues of Rats with Experimental Colon Cancer. ACAMC Anti-Cancer Agents Med. Chem. 2022, 22, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer Chemoprevention Through Dietary Antioxidants: Progress and Promise. Antioxid. Redox Signal. 2008, 10, 475–510. [Google Scholar] [CrossRef]
- Rezaie, H.; Alipanah-Moghadam, R.; Jeddi, F.; Clark, C.C.T.; Aghamohammadi, V.; Nemati, A. Combined Dandelion Extract and All-Trans Retinoic Acid Induces Cytotoxicity in Human Breast Cancer Cells. Sci. Rep. 2023, 13, 15074. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Janda, K.; Szkyrpan, S.; Gutowska, I.; Wolska, J. Stinging nettle (Urtica dioica L.)--botanical characteristics, biochemical composition and health benefits. Pomeranian J. Life Sci. 2015, 61, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Kolluri, S.K.; Lin, F.; Liu, W.; Han, Y.-H.; Cao, X.; Dawson, M.I.; Reed, J.C.; Zhang, X. Conversion of Bcl-2 from Protector to Killer by Interaction with Nuclear Orphan Receptor Nur77/TR3. Cell 2004, 116, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mansoori, B.; Aghapour, M.; Shirjang, S.; Nami, S.; Baradaran, B. The Urtica Dioica Extract Enhances Sensitivity of Paclitaxel Drug to MDA-MB-468 Breast Cancer Cells. Biomed. Pharmacother. 2016, 83, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dahab, R. Antiproliferative Activity of Selected Medicinal Plants of Jordan against a Breast Adenocarcinoma Cell Line (MCF7). Sci. Pharm. 2007, 75, 121–136. [Google Scholar] [CrossRef]
- Cavaleri, F.; Chattopadhyay, S.; Palsule, V.; Kar, P.K.; Chatterjee, R. Study of Drug Targets Associated With Oncogenesis and Cancer Cell Survival and the Therapeutic Activity of Engineered Ashwagandha Extract Having Differential Withanolide Constitutions. Integr. Cancer Ther. 2024, 23, 15347354231223499. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.H.; Al-Saleem, M.S.M.; Abdel-Mageed, W.M.; Al-Attar, A.-S.R.; Shehata, Y.M.; Abdel-Fattah, D.M.; Atta, R.M. LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata. Molecules 2023, 28, 5744. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cai, Y.-Z.; Wong, R.N.S.; Lee, C.K.-F.; Tang, S.C.W.; Sze, S.C.W.; Tong, Y.; Zhang, Y. Comparative Analysis of Caffeoylquinic Acids and Lignans in Roots and Seeds among Various Burdock (Arctium Lappa) Genotypes with High Antioxidant Activity. J. Agric. Food Chem. 2012, 60, 4067–4075. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, Y.; Xu, L.; Hao, N.; Zhao, R.; Wang, J.; Li, S.; Zhang, D.; Zhang, T.; Kang, T. The Diversity of Associated Microorganisms in Different Organs and Rhizospheric Soil of Arctium lappa L. Curr. Microbiol. 2020, 77, 746–754. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. Comprehensive Metabolite Profiling of Arum palaestinum (Araceae) Leaves by Using Liquid Chromatography–Tandem Mass Spectrometry. Food Res. Int. 2015, 70, 74–86. [Google Scholar] [CrossRef]
- Cottone, E.; Pomatto, V.; Cerri, F.; Campantico, E.; Mackie, K.; Delpero, M.; Guastalla, A.; Dati, C.; Bovolin, P.; Franzoni, M.F. Cannabinoid Receptors Are Widely Expressed in Goldfish: Molecular Cloning of a CB2-like Receptor and Evaluation of CB1 and CB2 mRNA Expression Profiles in Different Organs. Fish. Physiol. Biochem. 2013, 39, 1287–1296. [Google Scholar] [CrossRef]
- Mohammad, T.; Ghogare, R.; Morton, L.B.; Dhingra, A.; Potlakayala, S.; Rudrabhatla, S.; Dhir, S.K. Evaluation of Parameters Affecting Agrobacterium-Mediated Transient Gene Expression in Industrial Hemp (Cannabis sativa L.). Plants 2024, 13, 664. [Google Scholar] [CrossRef]
- Malviya, S.; Malviya, N. Medicinal Plants and Cancer Chemoprevention, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-325171-2. Available online: https://www.routledge.com/Medicinal-Plants-and-Cancer-Chemoprevention/Malviya-Malviya/p/book/9781032170763 (accessed on 20 April 2024).
- Chapter 13 Turmeric, the Golden Spice. Available online: https://pubmed.ncbi.nlm.nih.gov/22593922/ (accessed on 20 April 2024).
- Obolentseva, G.V.; Litvinenko, V.I.; Ammosov, A.S.; Popova, T.P.; Sampiev, A.M. Pharmacological and Therapeutic Properties of Licorice Preparations (A Review). Pharm. Chem. J. 1999, 33, 427–434. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Milad, R. Genus Kalanchoe (Crassulaceae): A Review of Its Ethnomedicinal, Botanical, Chemical and Pharmacological Properties. EJMP 2014, 4, 86–104. [Google Scholar] [CrossRef]
- Dogra, P. Kalanchoe Pinnata Is a Miraculous Plant: A Review. J. Biomed. Allied Res. 2023. [Google Scholar] [CrossRef]
- Mbikay, M. Therapeutic Potential of Moringa Oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front. Pharmacol. 2012, 3, 17024. [Google Scholar] [CrossRef]
- Popoola, J.O.; Obembe, O.O. Local Knowledge, Use Pattern and Geographical Distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef]
- Ayouaz, S.; Oliveira-Alves, S.C.; Lefsih, K.; Serra, A.T.; Bento Da Silva, A.; Samah, M.; Karczewski, J.; Madani, K.; Bronze, M.R. Phenolic Compounds from Nerium oleander Leaves: Microwave Assisted Extraction, Characterization, Antiproliferative and Cytotoxic Activities. Food Funct. 2020, 11, 6319–6331. [Google Scholar] [CrossRef]
- Nawaz, A.; Zaib, S.; Khan, I.; Ahmed, A.; Shahzadi, K.; Riaz, H. Silybum Marianum: An Overview of Its Phytochemistry and Pharmacological Activitieswith Emphasis on Potential Anticancer Properties. ACAMC Anti-Cancer Agents Med. Chem. 2023, 23, 1519–1534. [Google Scholar] [CrossRef]
- Vural, A. Trace Element Accumulation Behavior, Ability, and Propensity of Taraxacum officinale F.H. Wigg (Dandelion). Environ. Sci. Pollut. Res. 2024, 31, 16667–16684. [Google Scholar] [CrossRef]
- Huber, M.; Triebwasser-Freese, D.; Reichelt, M.; Heiling, S.; Paetz, C.; Chandran, J.N.; Bartram, S.; Schneider, B.; Gershenzon, J.; Erb, M. Identification, Quantification, Spatiotemporal Distribution and Genetic Variation of Major Latex Secondary Metabolites in the Common Dandelion (Taraxacum officinale Agg.). Phytochemistry 2015, 115, 89–98. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. Food Meas. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Shinde, S.; Balasubramaniam, A.K.; Mulay, V.; Saste, G.; Girme, A.; Hingorani, L. Recent Advancements in Extraction Techniques of Ashwagandha (Withania somnifera) with Insights on Phytochemicals, Structural Significance, Pharmacology, and Current Trends in Food Applications. ACS Omega 2023, 8, 40982–41003. [Google Scholar] [CrossRef]
- Patil, H.V.; Dhankani, M.A.; Dhankani, A.R. A Review on Marvel Fruit: Annona muricata. Med. Sci. Forum 2023, 21, 26. [Google Scholar] [CrossRef]
- Mary, S.J.; Veeravarmal, V.; Thankappan, P.; Angelin, D.; Franklin, R.; Girish, K.L. Evaluation of the Cytotoxic, Anti-Proliferative, Anti-Metastatic and pro-Apoptotic Effect of Aqueous Leaf Extract of Annona muricata on Oral Tongue Squamous Cell Carcinoma Cell Line (SCC-15): An in Vitro Study. J. Oral. Maxillofac. Pathol. 2023, 27, 469–475. [Google Scholar] [CrossRef]
- Silihe, K.K.; Mbou, W.D.; Ngo Pambe, J.C.; Kenmogne, L.V.; Maptouom, L.F.; Kemegne Sipping, M.T.; Zingue, S.; Njamen, D. Comparative Anticancer Effects of Annona muricata Linn (Annonaceae) Leaves and Fruits on DMBA-Induced Breast Cancer in Female Rats. BMC Complement. Med. Ther. 2023, 23, 234. [Google Scholar] [CrossRef]
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Jabir, M.S.; Mohammed, M.K.A.; Nayef, U.M.; AlMalki, F.A.; et al. Biosynthesis of Copper Oxide Nanoparticles Mediated Annona muricata as Cytotoxic and Apoptosis Inducer Factor in Breast Cancer Cell Lines. Sci. Rep. 2022, 12, 16165. [Google Scholar] [CrossRef]
- Salac, E.L.O.; Alvarez, M.R.; Gaurana, R.S.; Grijaldo, S.J.B.; Serrano, L.M.; Juan, F.D.; Abogado, R.; Padolina, I., Jr.; Deniega, F.M.; Delica, K.; et al. Biological Assay-Guided Fractionation and Mass Spectrometry-Based Metabolite Profiling of Annona muricata L. Cytotoxic Compounds against Lung Cancer A549 Cell Line. Plants 2022, 11, 2380. [Google Scholar] [CrossRef]
- Wang, Z.; Song, W.; Song, H.; Huang, W.; Li, Y.; Feng, J. Effects of Extraction Methods on the Physicochemical Properties and Functionalities of Pectic Polysaccharides from Burdock (Arctium lappa L.). Int. J. Biol. Macromol. 2024, 257, 128684. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Tan, J.-Y.; Liu, Z.; Shen, X.-L.; Hu, Y.-J. Lappaol F Regulates the Cell Cycle by Activating CDKN1C/P57 in Human Colorectal Cancer Cells. Pharm. Biol. 2023, 61, 337–344. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Hsin, M.-C.; Chen, P.-N.; Lin, C.-W.; Wang, P.-H.; Yang, S.-F.; Hsiao, Y.-H. Arctiin Inhibits Cervical Cancer Cell Migration and Invasion through Suppression of S100A4 Expression via PI3K/Akt Pathway. Pharmaceutics 2022, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Taleb Agha, M.; Baharetha, H.M.; Al-Mansoub, M.A.; Tabana, Y.M.; Kaz Abdul Aziz, N.H.; Yam, M.F.; Abdul Majid, A.M.S. Proapoptotic and Antiangiogenic Activities of Arctium lappa L. on Breast Cancer Cell Lines. Scientifica 2020, 2020, 7286053. [Google Scholar] [CrossRef]
- Maree, A.; Hashavya, S.; Gross, I.; Asaf, Y.; Bentur, Y. Arum palaestinum Poisoning: Revenge of the Witch. Eur. J. Pediatr. 2020, 179, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Gene, H.; ZaidBeth Ann WolfLarry, D. Crow Nutritional Supplement. Available online: https://patents.justia.com/inventor/gene-h-zaid?page=2 (accessed on 20 April 2024).
- Farid, M.M.; Hussein, S.R.; Ibrahim, L.F.; El Desouky, M.A.; Elsayed, A.M.; El Oqlah, A.A.; Saker, M.M. Cytotoxic Activity and Phytochemical Analysis of Arum palaestinum Boiss. Asian Pac. J. Trop. Biomed. 2015, 5, 944–947. [Google Scholar] [CrossRef]
- Dwikat, M.; Amer, J.; Jaradat, N.; Salhab, A.; Rahim, A.A.; Qadi, M.; Aref, A.; Ghanim, M.; Murad, H.; Modallal, A.; et al. Arum palaestinum Delays Hepatocellular Carcinoma Proliferation through the PI3K-AKT-mTOR Signaling Pathway and Exhibits Anticoagulant Effects with Antimicrobial Properties. Front. Pharmacol. 2023, 14, 1180262. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.; Burgoyne, T.; Lee, A.; Stehno-Bittel, L.; Zaid, G. Erratum to: Arum palaestinum with Isovanillin, Linolenic Acid and β-Sitosterol Inhibits Prostate Cancer Spheroids and Reduces the Growth Rate of Prostate Tumors in Mice. BMC Complement. Altern. Med. 2015, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Crocq, M.-A. History of Cannabis and the Endocannabinoid System. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Worster, B.; Hajjar, E.R.; Handley, N. Cannabis Use in Patients with Cancer: A Clinical Review. JCO Oncol. Pract. 2022, 18, 743–749. [Google Scholar] [CrossRef]
- Lal, S.; Shekher, A.; Puneet; Narula, A.S.; Abrahamse, H.; Gupta, S.C. Cannabis and Its Constituents for Cancer: History, Biogenesis, Chemistry and Pharmacological Activities. Pharmacol. Res. 2021, 163, 105302. [Google Scholar] [CrossRef]
- Javid, F.A.; Phillips, R.M.; Afshinjavid, S.; Verde, R.; Ligresti, A. Cannabinoid Pharmacology in Cancer Research: A New Hope for Cancer Patients? Eur. J. Pharmacol. 2016, 775, 1–14. [Google Scholar] [CrossRef]
- Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer. Cancers 2021, 13, 4353. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon Carcinogenesis Is Inhibited by the TRPM8 Antagonist Cannabigerol, a Cannabis-Derived Non-Psychotropic Cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-Induced Apoptosis Is Mediated by Activation of Noxa in Human Colorectal Cancer Cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef]
- Donati, L.; Casagrande Pierantoni, D.; Conti, A.; Calzoni, E.; Corte, L.; Santi, C.; Rosati, O.; Cardinali, G.; Emiliani, C. Water Extracts from Industrial Hemp Waste Inhibit the Adhesion and Development of Candida Biofilm and Showed Antioxidant Activity on HT-29 Colon Cancer Cells. Int. J. Mol. Sci. 2024, 25, 3979. [Google Scholar] [CrossRef]
- Wang, T.; Collet, J.-P.; Shapiro, S.; Ware, M.A. Adverse Effects of Medical Cannabinoids: A Systematic Review. Can. Med. Assoc. J. 2008, 178, 1669–1678. [Google Scholar] [CrossRef]
- Pratt, M.; Stevens, A.; Thuku, M.; Butler, C.; Skidmore, B.; Wieland, L.S.; Clemons, M.; Kanji, S.; Hutton, B. Benefits and Harms of Medical Cannabis: A Scoping Review of Systematic Reviews. Syst. Rev. 2019, 8, 320. [Google Scholar] [CrossRef]
- Nagarajaiah, S.; Shivanna Giresha, A.; Gopala Krishna, P.; Manikrao Gadewar, M.; Praveen, M.; Nanda, N.; Urs, D.; Krishnappa Dharmappa, K.; Mutta Nagabhushana, B.; Rao, S.; et al. Anti-oncogenic Potential and Inflammation Modulatory Response of Green Synthesized Biocompatible Silver Nanoparticles. Chem. Biodivers. 2024, 21, e202301533. [Google Scholar] [CrossRef]
- Azhar, N.A.; Abu Bakar, S.A.; Citartan, M.; Ahmad, N.H. mRNA Transcriptome Profiling of Human Hepatocellular Carcinoma Cells HepG2 Treated with Catharanthus roseus-Silver Nanoparticles. World J. Hepatol. 2023, 15, 393–409. [Google Scholar] [CrossRef]
- Nayila, I.; Sharif, S.; Lodhi, M.S.; Rehman, M.F.U.; Aman, F. Synthesis, Characterization and Anti-Breast Cancer Potential of an Incensole Acetate Nanoemulsion from Catharanthus roseus Essential Oil; in Silico, in Vitro, and in Vivo Study. RSC Adv. 2023, 13, 32335–32362. [Google Scholar] [CrossRef]
- Chuah, Y.Y.; Lee, Y.Y.; Chou, C.-K.; Chang, L.-J. Catharanthus Roseus Intoxication Mimicking Acute Cholangitis. BMC Complement. Med. Ther. 2024, 24, 139. [Google Scholar] [CrossRef]
- Moshe, Y.; Bentur, O.S.; Lishner, M.; Avivi, I. The Management of Hodgkin Lymphomas in Pregnancies. Eur. J. Haematol. 2017, 99, 385–391. [Google Scholar] [CrossRef]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Curcumin: Historical Background, Chemistry, Pharmacological Action, and Potential Therapeutic Value. In Curcumin for Neurological and Psychiatric Disorders; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–44. ISBN 978-0-12-815461-8. Available online: https://www.researchgate.net/publication/332443753_Curcumin_Historical_Background_Chemistry_Pharmacological_Action_and_Potential_Therapeutic_Value (accessed on 20 April 2024).
- Lüer, S.C.; Goette, J.; Troller, R.; Aebi, C. Synthetic versus Natural Curcumin: Bioequivalence in an in Vitro Oral Mucositis Model. BMC Complement. Altern. Med. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D. Farmer to Pharmacist: Curcumin as an Anti-Invasive and Antimetastatic Agent for the Treatment of Cancer. Front. Chem. 2014, 2, 113. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Salehi, E.; Mirtavoos-mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of Apoptosis Modulation by Curcumin: Implications for Cancer Therapy. J. Cell. Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Loo, W.T.Y.; Sze, S.C.W.; Tong, Y. Curcumin Inhibits Cell Proliferation of MDA-MB-231 and BT-483 Breast Cancer Cells Mediated by down-Regulation of NFκB, cyclinD and MMP-1 Transcription. Phytomedicine 2009, 16, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Noh, E.-M.; Kwon, K.-B.; Kim, J.-S.; You, Y.-O.; Hwang, J.-K.; Hwang, B.-M.; Kim, B.-S.; Lee, S.-H.; Lee, S.J.; et al. Curcumin Suppresses the TPA-Induced Invasion through Inhibition of PKCα-Dependent MMP-Expression in MCF-7 Human Breast Cancer Cells. Phytomedicine 2012, 19, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Yim-im, W.; Sawatdichaikul, O.; Semsri, S.; Horata, N.; Mokmak, W.; Tongsima, S.; Suksamrarn, A.; Choowongkomon, K. Computational Analyses of Curcuminoid Analogs against Kinase Domain of HER2. BMC Bioinform. 2014, 15, 261. [Google Scholar] [CrossRef]
- Catania, A.; Barrajón-Catalán, E.; Nicolosi, S.; Cicirata, F.; Micol, V. Immunoliposome Encapsulation Increases Cytotoxic Activity and Selectivity of Curcumin and Resveratrol against HER2 Overexpressing Human Breast Cancer Cells. Breast Cancer Res. Treat. 2013, 141, 55–65. [Google Scholar] [CrossRef]
- Starok, M.; Preira, P.; Vayssade, M.; Haupt, K.; Salomé, L.; Rossi, C. EGFR Inhibition by Curcumin in Cancer Cells: A Dual Mode of Action. Biomacromolecules 2015, 16, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-D.; Liu, X.-E.; Huang, D.-S. Curcumin Induces Apoptosis of Triple-Negative Breast Cancer Cells by Inhibition of EGFR Expression. Mol. Med. Rep. 2012, 6, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The Combination of Epigallocatechin Gallate and Curcumin Suppresses ERα-breast Cancer Cell Growth in Vitro and in Vivo. Int. J. Cancer 2008, 122, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, Y.; Chen, X.; Dai, J.; Jiang, Z.; Li, N.; Zhang, Z. Protective Effect of Curcumin against Formaldehyde-induced Genotoxicity in A549 Cell Lines. J. Appl. Toxicol. 2013, 33, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin Inhibits Cell Proliferation and Induces Apoptosis of Human Non-Small Cell Lung Cancer Cells through the Upregulation of miR-192-5p and Suppression of PI3K/Akt Signaling Pathway. Oncol. Rep. 2015, 34, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Lee, J.; Huh, T.-L.; Lee, Y.S. Curcumin Induces Apoptosis in Human Colorectal Carcinoma (HCT-15) Cells by Regulating Expression of Prp4 and P53. Mol. Cells 2013, 35, 526–532. [Google Scholar] [CrossRef]
- Rajitha, B.; Belalcazar, A.; Nagaraju, G.P.; Shaib, W.L.; Snyder, J.P.; Shoji, M.; Pattnaik, S.; Alam, A.; El-Rayes, B.F. Inhibition of NF-κB Translocation by Curcumin Analogs Induces G0/G1 Arrest and Downregulates Thymidylate Synthase in Colorectal Cancer. Cancer Lett. 2016, 373, 227–233. [Google Scholar] [CrossRef]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin Suppresses the Colon Cancer Proliferation by Inhibiting Wnt/β-Catenin Pathways via miR-130a. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Li, B.; Shi, C.; Li, B.; Zhao, J.; Wang, L. The Effects of Curcumin on HCT-116 Cells Proliferation and Apoptosis via the miR-491/PEG10 Pathway. J. Cell. Biochem. 2018, 119, 3091–3098. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The Antiviral and Antimicrobial Activities of Licorice, a Widely-Used Chinese Herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The Therapeutic Wound Healing Bioactivities of Various Medicinal Plants. Life 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Chiang, Y.-F.; Shieh, T.-M.; Chen, H.-Y.; Shih, C.-K.; Wang, T.-H.; Wang, K.-L.; Huang, T.-C.; Hong, Y.-H.; Li, S.-C.; et al. Dietary Compound Isoliquiritigenin, an Antioxidant from Licorice, Suppresses Triple-Negative Breast Tumor Growth via Apoptotic Death Program Activation in Cell and Xenograft Animal Models. Antioxidants 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-O.; Joo, S.H.; Lee, J.-Y.; Kwak, A.-W.; Kim, K.-T.; Cho, S.-S.; Yoon, G.; Choi, Y.H.; Park, J.W.; Shim, J.-H. Licochalcone C Inhibits the Growth of Human Colorectal Cancer HCT116 Cells Resistant to Oxaliplatin. Biomol. Ther. 2024, 32, 104–114. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Hsieh, W.-C.; Chen, S.-H.; Li, Y.-Z.; Liao, H.-F.; Lin, M.-Y.; Sheu, S.-M. 18β-Glycyrrhetinic Acid Modulated Autophagy Is Cytotoxic to Breast Cancer Cells. Int. J. Med. Sci. 2023, 20, 444–454. [Google Scholar] [CrossRef]
- Wu, M.; Hsieh, Y.; Lin, C.; Ying, T.; Hsia, S.; Hsieh, S.; Lee, C.; Lin, C. Licochalcone A Induces Endoplasmic Reticulum Stress-mediated Apoptosis of Endometrial Cancer Cells via Upregulation of GRP78 Expression. Environ. Toxicol. 2024, 39, 2961–2969. [Google Scholar] [CrossRef]
- Magdalita, P.M.; San Pascual, A.O. Hibiscus (Hibiscus rosa-sinensis): Importance and Classification. In Floriculture and Ornamental Plants; Datta, S.K., Gupta, Y.C., Eds.; Springer Nature: Singapore, 2022; pp. 483–522. ISBN 9789811535178. Available online: https://www.researchgate.net/publication/360455514_Hibiscus_Rosa_Sinensis_Linn_A_phytochemical_and_pharmacological_review (accessed on 20 April 2024).
- Bedi, P.S.; Bekele, M.; Gure, G. Phyto-Chemistry and Pharmacological Activities of Hibiscus sabdariffa Linn.—A Review. IRJPAC 2020, 21, 41–54. [Google Scholar] [CrossRef]
- Yasmin, R.; Gogoi, S.; Bora, J.; Chakraborty, A.; Dey, S.; Ghaziri, G.; Bhattacharjee, S.; Singh, L.H. Novel Insight into the Cellular and Molecular Signalling Pathways on Cancer Preventing Effects of Hibiscus sabdariffa: A Review. J. Cancer Prev. 2023, 28, 77–92. [Google Scholar] [CrossRef]
- Ajiboye, T.; Ajala-Lawal, R.; Adeyiga, A. Caffeic Acid Abrogates 1,3-Dichloro-2-Propanol-Induced Hepatotoxicity by Upregulating Nuclear Erythroid-Related Factor 2 and Downregulating Nuclear Factor-Kappa B. Hum. Exp. Toxicol. 2019, 38, 1092–1101. [Google Scholar] [CrossRef]
- Chen, C.; Yi, L.; Jin, X.; Zhang, T.; Fu, Y.; Zhu, J.; Mi, M.; Zhang, Q.; Ling, W.; Yu, B. Inhibitory Effect of Delphinidin on Monocyte–Endothelial Cell Adhesion Induced by Oxidized Low-Density Lipoprotein via ROS/p38MAPK/NF-κB Pathway. Cell Biochem. Biophys. 2011, 61, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Khaghani, S.; Razi, F.; Yajloo, M.M.; Paknejad, M.; Shariftabrizi, A.; Pasalar, P. Selective Cytotoxicity and Apoptogenic Activity of Hibiscus sabdariffa Aqueous Extract Against MCF-7 Human Breast Cancer Cell Line. JCT 2011, 2, 394–400. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells—Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Chen, C.-C.; Tseng, T.-H.; Chang, Y.-C.; Lin, Y.-J.; Tsai, I.-N.; Wang, C.-C.; Wang, C.-J. Hibiscus Anthocyanins Extracts Induce Apoptosis by Activating AMP-Activated Protein Kinase in Human Colorectal Cancer Cells. Nutrients 2023, 15, 3972. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Muzitano, M.F.; Camargo, L.M.M.; Coutinho, M.A.S. Therapeutic Potential of Kalanchoe Species: Flavonoids and Other Secondary Metabolites. Nat. Product. Commun. 2008, 3, 1934578X0800301. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Sztormowska-Achranowicz, K.; Kowalczyk, M.; Soluch, A.; Ochocka, J.R. An In Vitro Anticancer, Antioxidant, and Phytochemical Study on Water Extract of Kalanchoe daigremontiana Raym.-Hamet and H. Perrier. Molecules 2022, 27, 2280. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Gucwa, M.; Moniuszko-Szajwaj, B.; Stochmal, A.; Kawiak, A.; Ochocka, J.R. Bersaldegenin-1,3,5-Orthoacetate Induces Caspase-Independent Cell Death, DNA Damage and Cell Cycle Arrest in Human Cervical Cancer HeLa Cells. Pharm. Biol. 2021, 59, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Chang, W.-T.; Lee, M.-S.; Chen, H.-Y.; Chen, Y.-H.; Lin, C.-C.; Lin, M.-K. Three Bufadienolides Induce Cell Death in the Human Lung Cancer Cell Line CL1-5 Mainly through Autophagy. Bioorganic Med. Chem. Lett. 2021, 31, 127715. [Google Scholar] [CrossRef]
- Hip Kam, A.; Neergheen, V.S. Nutraceutical and Phytopharmaceuticals in Immune Health. In Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 445–475. ISBN 978-0-12-821232-5. [Google Scholar]
- Umar, H.; Rabiu Aliyu, M. Moringa Oleifera-Mediated Iron Oxide Nanoparticles, Characterization and Their Anti-Proliferative Potential on MDA-MB 231 Human Breast Cancer Cells. Pak. J. Pharm. Sci. 2023, 36, 1875–1883. [Google Scholar]
- Sultan, R.; Ahmed, A.; Wei, L.; Saeed, H.; Islam, M.; Ishaq, M. The Anticancer Potential of Chemical Constituents of Moringa Oleifera Targeting CDK-2 Inhibition in Estrogen Receptor Positive Breast Cancer Using in-Silico and in Vitro Approches. BMC Complement. Med. Ther. 2023, 23, 396. [Google Scholar] [CrossRef]
- Singhal, K.G.; Gupta, G.D. Hepatoprotective and Antioxidant Activity of Methanolic Extract of Flowers of Nerium Oleander against CCl4–Induced Liver Injury in Rats. Asian Pac. J. Trop. Med. 2012, 5, 677–685. [Google Scholar] [CrossRef]
- Bakir Çilesizoğlu, N.; Yalçin, E.; Çavuşoğlu, K.; Sipahi Kuloğlu, S. Qualitative and Quantitative Phytochemical Screening of Nerium Oleander L. Extracts Associated with Toxicity Profile. Sci. Rep. 2022, 12, 21421. [Google Scholar] [CrossRef]
- Crandell Addington Particles from Processing of Oleander Leaves. Available online: https://patents.google.com/patent/US20060188536A1/en (accessed on 20 April 2024).
- Calderón-Montaño, J.; Burgos-Morón, E.; Orta, M.; Mateos, S.; López-Lázaro, M. A Hydroalcoholic Extract from the Leaves of Nerium Oleander Inhibits Glycolysis and Induces Selective Killing of Lung Cancer Cells. Planta Med. 2013, 79, 1017–1023. [Google Scholar] [CrossRef]
- Rashan, L.J.; Özenver, N.; Boulos, J.C.; Dawood, M.; Roos, W.P.; Franke, K.; Papasotiriou, I.; Wessjohann, L.A.; Fiebig, H.-H.; Efferth, T. Molecular Modes of Action of an Aqueous Nerium Oleander Extract in Cancer Cells In Vitro and In Vivo. Molecules 2023, 28, 1871. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Kianmehr, M.; Kazemi, T.; Samarghandian, S.; Khazdair, M. Toxicity Effects of Nerium oleander, Basic and Clinical Evidence: A Comprehensive Review. Hum. Exp. Toxicol. 2020, 39, 773–784. [Google Scholar] [CrossRef]
- Ayyappan, S.; N, A.; Toi, P.C. Accidental Fatal Poisoning in a Child Due to Ingestion of Nerium oleander Leaf. Forensic Sci. Med. Pathol. 2023. [Google Scholar] [CrossRef]
- Bhatia, N.; Agarwal, C.; Agarwal, R. Differential Responses of Skin Cancer-Chemopreventive Agents Silibinin, Quercetin, and Epigallocatechin 3-Gallate on Mitogenic Signaling and Cell Cycle Regulators in Human Epidermoid Carcinoma A431 Cells. Nutr. Cancer 2001, 39, 292–299. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The Silymarin Composition… and Why Does It Matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Nathalie Chevreau Topical Compositions and Methods for Reducing Oxidative Stress. Available online: https://patents.google.com/patent/US9889171B2/en (accessed on 20 April 2024).
- Zhang, X.; Jiang, Y.; Guo, N.; Ding, Y.; Feng, J.; Miao, C.; Lv, Y. Application of SNAP-Tag-EGFR Cell Membrane Chromatography Model in Screening Antitumor Active Components of Silybum marianum (L.) Gaertn. J. Pharm. Biomed. Anal. 2024, 238, 115816. [Google Scholar] [CrossRef]
- Gjörloff Wingren, A.; Ziyad Faik, R.; Holefors, A.; Filecovic, E.; Gustafsson, A. In Vitro Effects of Undifferentiated Callus Extracts from Plantago major L., Rhodiola rosea L. and Silybum marianum L. in Normal and Malignant Human Skin Cells. Heliyon 2023, 9, e16480. [Google Scholar] [CrossRef]
- Mi, X.; Choi, H.S.; Perumalsamy, H.; Shanmugam, R.; Thangavelu, L.; Balusamy, S.R.; Kim, Y.-J. Biosynthesis and Cytotoxic Effect of Silymarin-Functionalized Selenium Nanoparticles Induced Autophagy Mediated Cellular Apoptosis via Downregulation of PI3K/Akt/mTOR Pathway in Gastric Cancer. Phytomedicine 2022, 99, 154014. [Google Scholar] [CrossRef]
- Kacar, S.; Bektur Aykanat, N.E.; Sahinturk, V. Silymarin Inhibited DU145 Cells by Activating SLIT2 Protein and Suppressing Expression of CXCR4. Med. Oncol. 2020, 37, 18. [Google Scholar] [CrossRef]
- Zecca, E.; Zuppa, A.A.; D’Antuono, A.; Tiberi, E.; Giordano, L.; Pianini, T.; Romagnoli, C. Efficacy of a Galactogogue Containing Silymarin-Phosphatidylserine and Galega in Mothers of Preterm Infants: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2016, 70, 1151–1154. [Google Scholar] [CrossRef]
- McBride, G.M.; Stevenson, R.; Zizzo, G.; Rumbold, A.R.; Amir, L.H.; Keir, A.K.; Grzeskowiak, L.E. Use and Experiences of Galactagogues While Breastfeeding among Australian Women. PLoS ONE 2021, 16, e0254049. [Google Scholar] [CrossRef]
- Bokelmann, J.M. Dandelion (Taraxacum Officinale). In Medicinal Herbs in Primary Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 303–307. ISBN 978-0-323-84676-9. Available online: https://shop.elsevier.com/books/medicinal-herbs-in-primary-care/bokelmann/978-0-323-84676-9 (accessed on 20 April 2024).
- Richards, A.J. The Origin of Taraxacum Agamospecies. Bot. J. Linn. Soc. 1973, 66, 189–211. [Google Scholar] [CrossRef]
- Qu, J.; Ke, F.; Liu, Z.; Yang, X.; Li, X.; Xu, H.; Li, Q.; Bi, K. Uncovering the Mechanisms of Dandelion against Triple-Negative Breast Cancer Using a Combined Network Pharmacology, Molecular Pharmacology and Metabolomics Approach. Phytomedicine 2022, 99, 153986. [Google Scholar] [CrossRef]
- Ovadje, P.; Chochkeh, M.; Akbari-Asl, P.; Hamm, C.; Pandey, S. Selective Induction of Apoptosis and Autophagy Through Treatment With Dandelion Root Extract in Human Pancreatic Cancer Cells. Pancreas 2012, 41, 1039–1047. [Google Scholar] [CrossRef]
- El-Emam, S.Z.; Abo El-Ella, D.M.; Fayez, S.M.; Asker, M.; Nazeam, J.A. Novel Dandelion Mannan-Lipid Nanoparticle: Exploring the Molecular Mechanism Underlying the Potent Anticancer Effect against Non-Small Lung Carcinoma. J. Funct. Foods 2021, 87, 104781. [Google Scholar] [CrossRef]
- Ahmadi, S.; Saberivand, A.; Jalili, C.; Asadpour, R.; Khordadmehr, M.; Saberivand, M. Hydroalcoholic Extract of Taraxacum officinale Induces Apoptosis and Autophagy in 4T1 Breast Cancer Cells. Vet. Res. Forum 2023. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Y.; Chen, L.; Min, L.; Huang, D.; Zhang, Y.; Li, C.; Li, Z. Suppression of Migration and Invasion by Taraxerol in the Triple-Negative Breast Cancer Cell Line MDA-MB-231 via the ERK/Slug Axis. PLoS ONE 2023, 18, e0291693. [Google Scholar] [CrossRef]
- De Vico, G.; Guida, V.; Carella, F. Urtica Dioica (Stinging nettle): A Neglected Plant With Emerging Growth Promoter/Immunostimulant Properties for Farmed Fish. Front. Physiol. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of Pharmacological Uses of Urtica Dioica and Others Benefits. Progress. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P. Therapeutic Perspectives of Molecules from Urtica Dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Ardekani, A.M.; Zabihi, E.; Abedian, Z.; Mostafazadeh, A.; Pourbagher, R.; Akhavan-Niaki, H. Antioxidant and Apoptotic Effects of an Aqueous Extract of Urtica Dioica on the MCF-7 Human Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2013, 14, 5317–5323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Jin, L.; Casero, R.A.; Davidson, N.E.; Huang, Y. Role of Ornithine Decarboxylase in Regulation of Estrogen Receptor Alpha Expression and Growth in Human Breast Cancer Cells. Breast Cancer Res. Treat. 2012, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Telo, S.; Halifeoglu, I.; Ozercan, I.H. Effects of Stinging Nettle (Urtica dioica L.) on Antioxidant Enzyme Activities in Rat Model of Mammary Gland Cancer. Iran. J. Pharm. Res. 2017, 16, 164–170. [Google Scholar] [PubMed][Green Version]
- Tekin, V.; Kozgus Guldu, O.; Medine, E.I.; Biber Muftuler, F.Z. Examination of the Association Between 3,4-Divanillyltetrahydrofuran Lignan (Urtica dioica Origin) and Prostate Cancer Cells by 131 I Radiolabeling. Cancer Biother. Radiopharm. 2021, 36, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kardan, M.; Rafiei, A.; Golpour, M.; Ebrahimzadeh, M.A.; Akhavan-Niaki, H.; Fattahi, S. Urtica Dioica Extract Inhibits Cell Proliferation and Induces Apoptosis in HepG2 and HTC116 as Gastrointestinal Cancer Cell Lines. ACAMC Anti-Cancer Agents Med. Chem. 2020, 20, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Srinivasan, M.; Gangurde, A.; Chandane, A.Y.; Tagalpallewar, A.; Pawar, A.; Baheti, A.M. Integrating Network Pharmacology and in Silico Analysis Deciphers Withaferin-A’s Anti-Breast Cancer Potential via Hedgehog Pathway and Target Network Interplay. Brief. Bioinform. 2024, 25, bbae032. [Google Scholar] [CrossRef]
- Kulavi, S.; Dhar, D.; Kamal, I.M.; Chakrabarti, S.; Bandyopadhyay, J. EIF4A3 Targeted Therapeutic Intervention in Glioblastoma Multiforme Using Phytochemicals from Indian Medicinal Plants—An Integration of Phytotherapy into Precision Onco-Medicine. J. Biomol. Struct. Dyn. 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Al Awadh, A.A.; Sakagami, H.; Amano, S.; Sayed, A.M.; Abouelela, M.E.; Alhasaniah, A.H.; Aldabaan, N.; Refaey, M.S.; Abdelhamid, R.A.; Khalil, H.M.A.; et al. In Vitro Cytotoxicity of Withania somnifera (L.) Roots and Fruits on Oral Squamous Cell Carcinoma Cell Lines: A Study Supported by Flow Cytometry, Spectral, and Computational Investigations. Front. Pharmacol. 2024, 15, 1325272. [Google Scholar] [CrossRef] [PubMed]


| Medicinal Plant | Biological Outcome | Effective Pathway | Type of Cancer | Regional Source | Reference |
|---|---|---|---|---|---|
| Annona muricata | ↓cell migration ↑apoptosis | ↓Bcl-2 ↑P53 ↓EGFR | Tongue cancer Breast cancer | America Asia | [16,17] |
| Arctium lappa | ↓cell migration ↑autophagy ↑apoptosis | ↓S100A4 protein ↓PI3K ↓Bcl-2 ↑LC3B-II ↑Beclin-1 | Cervical cancer Colon cancer Prostate cancer Breast cancer | Asia Europe | [18,19,20] |
| Arum palaestinum | ↓proliferation ↑apoptosis | ↓PI3K/AKT/mTOR | Liver cancer Prostate canceer | Asia Europe Africa Europe | [21] |
| Cannabis sativa | ↑apoptosis ↓proliferation | ↑caspase 3 ↓phosphoprotein kinase B | Colorectal cancer | Asia | [22] |
| Catharanthus roseus | ↑apoptosis ↓proliferation | ↑p53 ↑MTs ↑HS ↑HMOX | Lung cancer Breast cancer | Asia | [23] |
| Curcuma longa | ↑apoptosis ↑autophagy ↓proliferation | ↓YAP ↓SIRT1 ↑ROS ↑AMPK | Colorectal cancer Breast cancer Lung cancer | Asia | [24,25,26,27,28] |
| Glycyrrhiza glabra | ↑apoptosis ↑autophagy | ↑LC3-II ↓PI3K/RAC-α ↓Cyclin B1 ↓CDK1 | Breast cancer, Prostate cancer Lung cancer Gastric cancer | Asia Europe | [29,30,31,32,33] |
| Hibiscus | ↑apoptosis ↓proliferation | ↑p53 ↓cyclin E/cdk2 | Prostate cancer Cervical cancer Breast cancer | Asia America Europe Australia Africa | [34,35] |
| Kalanchoe blossfeldiana | ↑apoptosis ↑autophagy | ↑p53 ↓p-mTOR | Lung cancer Breast cancer Cervical cancer | Arica Asia America | [36] |
| Moringa oleifera | ↑apoptosis ↑autophagy | ↓NF-kB ↓Vimentin mRNA | Pancreatic cancer Breast cancer | Africa Asia America | [37,38,39] |
| Nerium oleander | ↓proliferation ↑apoptosis | ↓EGFR ↓TGF-β, VEGF, AFP, COX-2 | Cervical cancer Lung cancer Liver cancer Colon cancer | Asia Europe | [40,41] |
| Silybum marianum | ↑apoptosis ↑autophagy ↓proliferation | ↑Bax/Bcl-2 ↓PI3K/AKT/mTOR ↓COX-2 | skin cancer gastric cancer liver cancer | Asia America Australia | [42] |
| Taraxacum officinale | ↑apoptosis ↓proliferation | ↓MMP-9 ↓IL-1β ↑p53 ↑KAI1 | Breast cancer | Europe America Asia Australia | [43] |
| Urtica dioica | ↑apoptosis ↓proliferation | ↑OCD1 ↓miR-21 ↓MMP1 ↓MMP9 ↓MMP13 ↓Bcl-2 | Breast cancer Prostate cancer Gastrointestinal cancer | Asia North Africa North America Europe | [44,45,46,47] |
| Withania somnifera L. | ↓proliferation ↑apoptosis | ↑caspase 3 ↓RSK1, Akt1, and mTOR | Prostate cancer Breast cancer Ovarian | Asia | [48] |
| Medicinal Plant | Main Active Component/s | Effective Plant Parts | Reference |
|---|---|---|---|
| Annona muricata | Acetogenins Alkaloids Phenolic compounds Flavonoids | Roots Bark Seeds Leaves | [49] |
| Arctium lappa | Lignans Caffeoylquinic acids Phenolic compounds Flavonoids Polysaccharides | Roots Seeds Leaves Fruits Stem | [50,51] |
| Arum palaestinum | Piperazirum Isoorientin Diketopiperazine Phenolic compounds Flavonoids | Flowers Leaves Roots | [52] |
| Cannabis sativa | Cannabidiol Tetrahydrocannabinol Cannabinol β-caryophyllene Cannabigerol | Fibers Oil Seeds | [53,54] |
| Catharanthus roseus | Vinblastine Vincristine Carbohydrates Alkaloids Flavonoids Saponins | Roots Flowers Basal stem | [55] |
| Curcuma longa | Curcumin | Rhizome | [56] |
| Glycyrrhiza glabra | Glycyrrhizin Glycyrrhetinic acid Isoliquiritigenin | Roots | [57] |
| Hibiscus | Anthocyanins Polysaccharides Flavonoids Organic acids | Flowers Leaves Seeds | [58] |
| Kalanchoe blossfeldiana | Flavonoids Anthocyanins Coumarins Phenolic acids Sterols | Roots Stem Leaves | [59,60] |
| Moringa oleifera | Flavonoids Tannins Alkaloids Glucosinolates Isothiocyanates Oleic acids | Leaves Flowers Seeds Pods Bark | [61,62] |
| Nerium oleander | Chlorogenic acid Rutin Quinic acid esters Oleandrin Flavonoids Carbohydrates Alkaloids Polysaccharides Tannins | Leaves Roots Bark Flowers | [63] |
| Silybum marianum L. | Silandrin Silybin silychristin Silydianin Silymarin Silymonin | Flowers Leaves Roots Achene | [64] |
| Taraxacum officinale | Sesquiterpene lactones Triterpene Chicoric acid Flavonoids Phenolic acids 4-hydroxyphenylacetate inositol esters | Roots Stem Leaves Flowers | [65,66] |
| Urtica dioica | Phenolic compounds Ferulic acid Lignans Phytosterols Isolectins Coumarins | Roots Stalk Leaves | [67] |
| Withania somnifera | Withamolides Sitoindosides Alkaloids | Roots Leaves Fruits | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albahri, G.; Badran, A.; Abdel Baki, Z.; Alame, M.; Hijazi, A.; Daou, A.; Baydoun, E. Potential Anti-Tumorigenic Properties of Diverse Medicinal Plants against the Majority of Common Types of Cancer. Pharmaceuticals 2024, 17, 574. https://doi.org/10.3390/ph17050574
Albahri G, Badran A, Abdel Baki Z, Alame M, Hijazi A, Daou A, Baydoun E. Potential Anti-Tumorigenic Properties of Diverse Medicinal Plants against the Majority of Common Types of Cancer. Pharmaceuticals. 2024; 17(5):574. https://doi.org/10.3390/ph17050574
Chicago/Turabian StyleAlbahri, Ghosoon, Adnan Badran, Zaher Abdel Baki, Mohamad Alame, Akram Hijazi, Anis Daou, and Elias Baydoun. 2024. "Potential Anti-Tumorigenic Properties of Diverse Medicinal Plants against the Majority of Common Types of Cancer" Pharmaceuticals 17, no. 5: 574. https://doi.org/10.3390/ph17050574
APA StyleAlbahri, G., Badran, A., Abdel Baki, Z., Alame, M., Hijazi, A., Daou, A., & Baydoun, E. (2024). Potential Anti-Tumorigenic Properties of Diverse Medicinal Plants against the Majority of Common Types of Cancer. Pharmaceuticals, 17(5), 574. https://doi.org/10.3390/ph17050574







