Effects of CYP3A5 Genotype on Tacrolimus Pharmacokinetics and Graft-versus-Host Disease Incidence in Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Patients
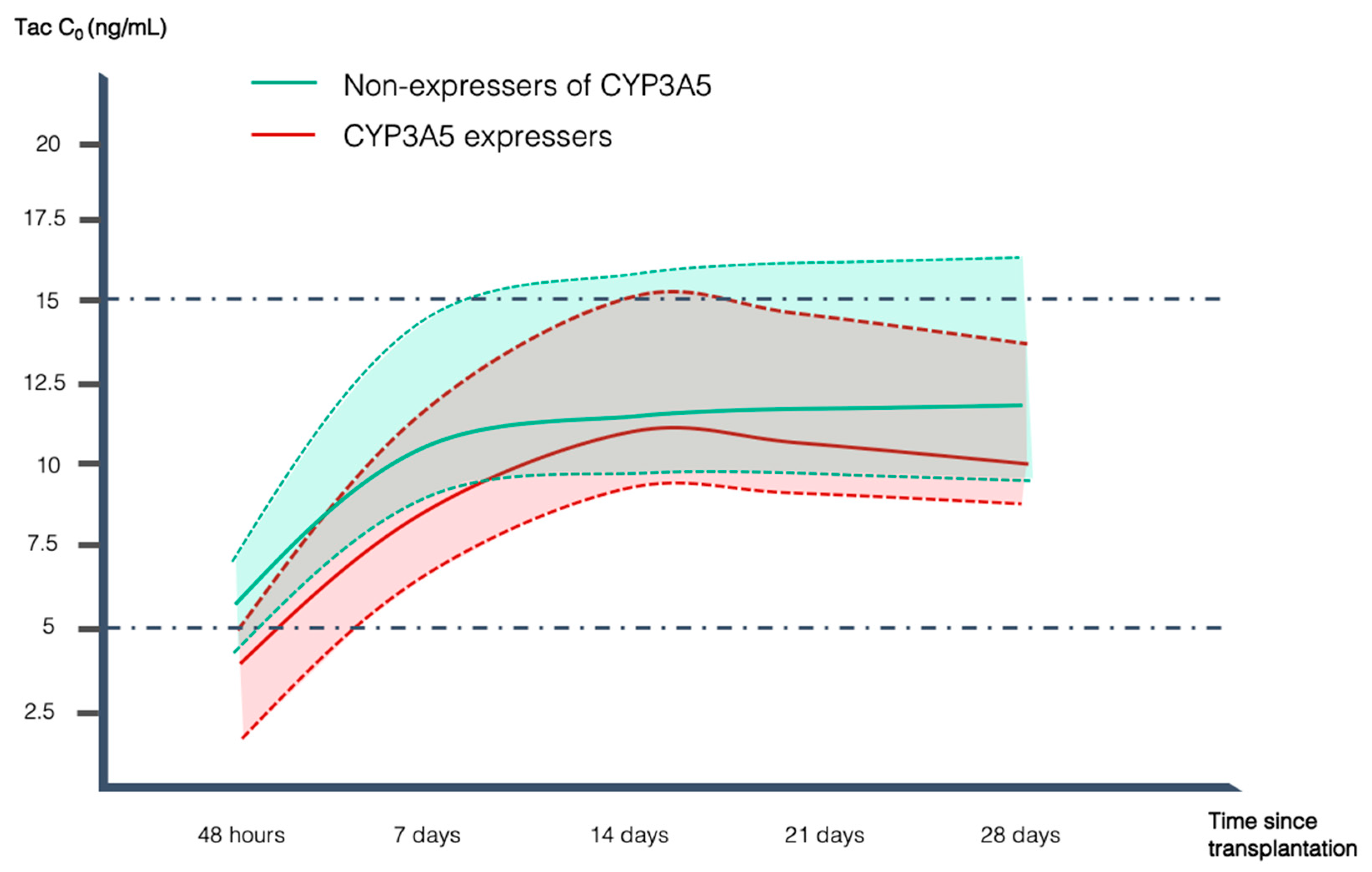
2.2. CYP3A5 and CYP3A4 Genotypes and Tac Pharmacokinetics
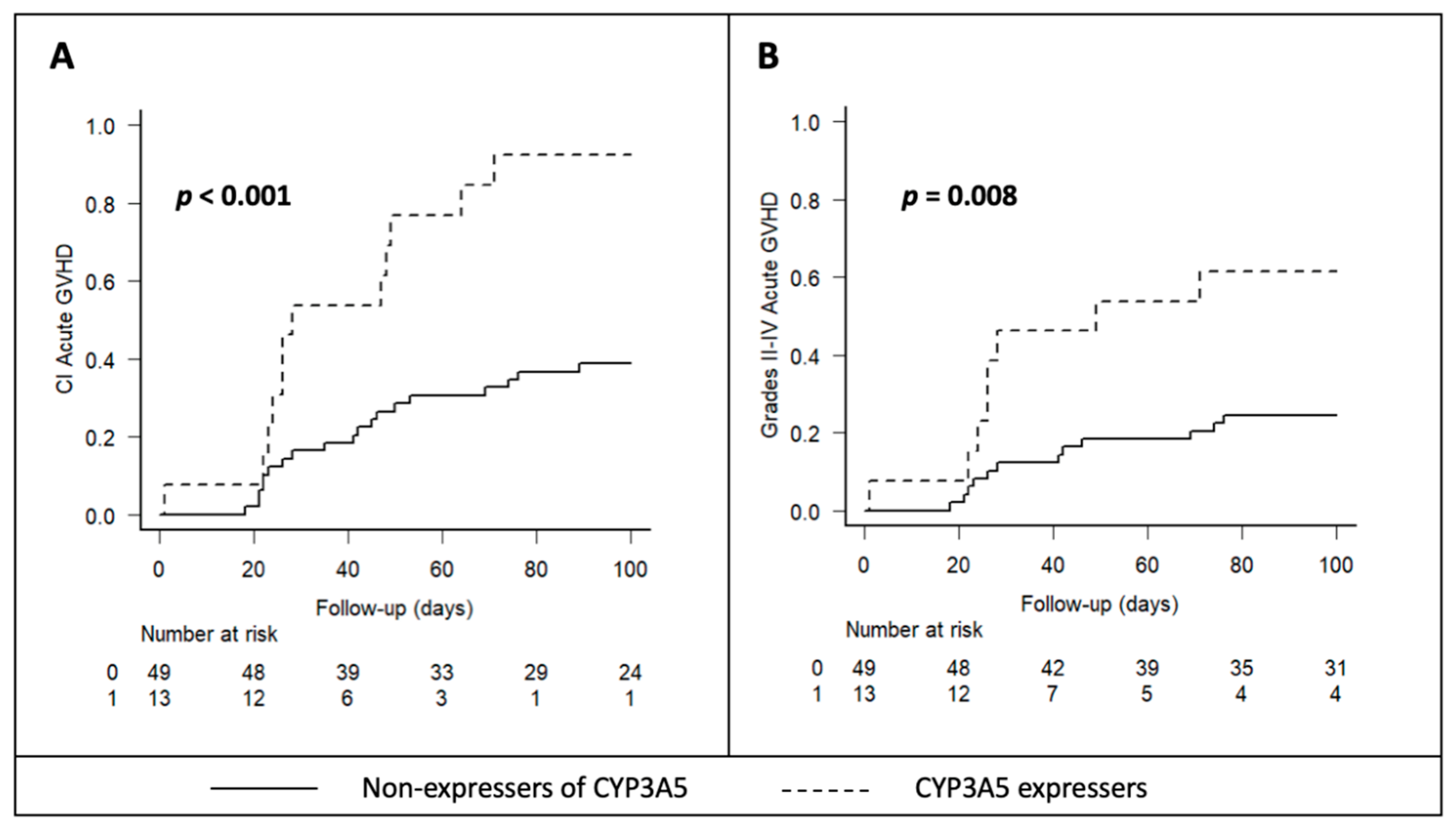
2.3. CYP3A5 and CYP3A4 Genotypes and Acute GVHD Incidence
2.4. CYP3A5 and CYP3A4 Genotypes and Tac-Related Adverse Events
3. Discussion
4. Materials and Methods
4.1. Patients and Donors
4.2. Treatment Protocol and Supportive Care
4.3. Tac Pharmacokinetic Parameters
4.4. CYP3A5 and CYP3A4 Genetic Polymorphism Analysis
4.5. Endpoints of the Study
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Penack, O.; Marchetti, M.; Aljurf, M.; Arat, M.; Bonifazi, F.; Duarte, R.F.; Giebel, S.; Greinix, H.; Hazenberg, M.D.; Kröger, N.; et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024, 11, e147–e159. [Google Scholar] [CrossRef] [PubMed]
- De Jong, C.N.; Meijer, E.; Bakunina, K.; Nur, E.; Kooij, M.V.M.; De Groot, M.R.; Van Gelder, M.; Maertens, J.A.; Kuball, J.H.; Deeren, D.; et al. Post-Transplantation Cyclophosphamide after Allogeneic Hematopoietic Stem Cell Transplantation: Results of the Prospective Randomized HOVON-96 Trial in Recipients of Matched Related and Unrelated Donors. Blood 2019, 134 (Suppl. S1), 1. [Google Scholar] [CrossRef]
- Wingard, J.R.; Nash, R.A.; Przepiorka, D.; Klein, J.L.; Weisdorf, D.J.; Fay, J.W.; Zhu, J.; Maher, R.M.; Fitzsimmons, W.E.; Ratanatharathorn, V. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol. Blood Marrow Transplant. 1998, 4, 157–163. [Google Scholar] [CrossRef]
- Przepiorka, D.; Devine, S.M.; Fay, J.W.; Uberti, J.P.; Wingard, J.R. Practical considerations in the use of tacrolimus for allogeneic marrow transplantation. Bone Marrow Transplant. 1999, 24, 1053–1056. [Google Scholar] [CrossRef]
- Przepiorka, D.; Nash, R.A.; Wingard, J.R.; Zhu, J.; Maher, R.M.; Fitzsimmons, W.E.; Fay, J.W. Relationship of Tacrolimus Whole Blood Levels to Efficacy and Safety Outcomes after Unrelated Donor Marrow Transplantation. Biol. Blood Marrow Transplant. 1999, 5, 94–97. [Google Scholar] [CrossRef]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef]
- Mori, T.; Kato, J.; Shimizu, T.; Aisa, Y.; Nakazato, T.; Yamane, A.; Ono, Y.; Kunimoto, H.; Okamoto, S. Effect of Early Posttransplantation Tacrolimus Concentration on the Development of Acute Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation fromUnrelated Donors. Biol. Blood Marrow Transplant. 2012, 18, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ganetsky, A.; Shah, A.; Miano, T.A.; Hwang, W.-T.; He, J.; Loren, A.W.; Hexner, E.O.; Frey, N.V.; Porter, D.L.; Reshef, R. Higher tacrolimus concentrations early after transplant reduce the risk of acute GvHD in reduced-intensity allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Marco, D.N.; Salas, M.Q.; Gutiérrez-García, G.; Monge, I.; Riu, G.; Carcelero, E.; Roma, J.R.; Llobet, N.; Arcarons, J.; Suárez-Lledó, M.; et al. Impact of Early Intrapatient Variability of Tacrolimus Concentrations on the Risk of Graft-Versus-Host Disease after Allogeneic Stem Cell Transplantation Using High-Dose Post-Transplant Cyclophosphamide. Pharmaceuticals 2022, 15, 1529. [Google Scholar] [CrossRef]
- Yao, J.M.; Yang, D.; Clark, M.C.; Otoukesh, S.; Cao, T.; Ali, H.; Arslan, S.; Aldoss, I.; Artz, A.; Amanam, I.; et al. Tacrolimus initial steady state level in post-transplant cyclophosphamide-based GvHD prophylaxis regimens. Bone Marrow Transplant. 2022, 57, 232–242. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.; Stein, C.; Sadee, W.; Wang, D.; Vinks, A.; He, Y.; Swen, J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sissung, T.M.; Baum, C.E.; Kirkland, C.T.; Gao, R.; Gardner, E.R.; Figg, W.D. Pharmacogenetics of membrane transporters: An update on current approaches. Mol Biotechnol. 2010, 44, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; Van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Degraeve, A.L.; Moudio, S.; Haufroid, V.; Eddour, D.C.; Mourad, M.; Bindels, L.B.; Elens, L. Predictors of tacrolimus pharmacokinetic variability: Current evidences and future perspectives. Expert Opin. Drug Metab. Toxicol. 2020, 16, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, D.A.; Bouamar, R.; Elens, L.; Van Schaik, R.H.N.; Van Gelder, T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin. Pharmacokinet. 2014, 53, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Wrighton, S.A.; Cooke, G.E.; Sadee, W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharm. J. 2011, 11, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Goodman, L.K.; Tett, S.E. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin. Pharmacokinet. 2010, 49, 141–175. [Google Scholar] [CrossRef]
- De Jonge, H.; De Loor, H.; Verbeke, K.; Vanrenterghem, Y.; Kuypers, D.R. In Vivo CYP3A4 Activity, CYP3A5 Genotype, and Hematocrit Predict Tacrolimus Dose Requirements and Clearance in Renal Transplant Patients. Clin. Pharmacol. Ther. 2012, 92, 366–375. [Google Scholar] [CrossRef]
- Thölking, G.; Gerth, H.U.; Schuette-Nuetgen, K.; Reuter, S. Influence of tacrolimus metabolism rate on renal function after solid organ transplantation. World J. Transplant. 2017, 7, 26–33. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, T.; Meziyerh, S.; Swen, J.J.; de Vries, A.P.J.; Moes, D.J.A.R. The Clinical Impact of the C0/D Ratio and the CYP3A5 Genotype on Outcome in Tacrolimus Treated Kidney Transplant Recipients. Front. Pharmacol. 2020, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; Pastor-Anglada, M. Insights into the Pharmacogenetics of Tacrolimus Pharmacokinetics and Pharmacodynamics. Pharmaceutics 2022, 14, 1755. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.K.; Palmer, J.M.; Herzog, J.; Stiller, T.; Tsai, N.-C.; Senitzer, D.; Liu, X.; Thomas, S.H.; Shayani, S.; Weitzel, J.; et al. Influence of Absorption, Distribution, Metabolism, and Excretion Genomic Variants on Tacrolimus/Sirolimus Blood Levels and Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, M.; Kunii, N.; Toyosaki, M.; Machida, S.; Ohgiya, D.; Ogawa, Y.; Kawada, H.; Inoko, H.; Ando, K. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011, 46, 1113–1117. [Google Scholar] [CrossRef]
- Yamashita, T.; Fujishima, N.; Miura, M.; Niioka, T.; Abumiya, M.; Shinohara, Y.; Ubukawa, K.; Nara, M.; Fujishima, M.; Kameoka, Y.; et al. Effects of CYP3A5 polymorphism on the pharmacokinetics of a once-daily modified-release tacrolimus formulation and acute kidney injury in hematopoietic stem cell transplantation. Cancer Chemother. Pharmacol. 2016, 78, 111–118. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Zhang, Q.; Steuerwald, N.; Hamilton, A.; Druhan, L.J.; McSwain, M.; Diez, Y.; Rusin, S.; Han, Y.; Symanowski, J.; et al. Effect of CYP3A4, CYP3A5, and ABCB1 Polymorphisms on Intravenous Tacrolimus Exposure and Adverse Events in Adult Allogeneic Stem Cell Transplant Patients. Biol. Blood Marrow Transplant. 2019, 25, 656–663. [Google Scholar] [CrossRef]
- Zhang, S.; Pillai, V.C.; Mada, S.R.; Strom, S.; Venkataramanan, R. Effect of voriconazole and other azole antifungal agents on CYP3A activity and metabolism of tacrolimus in human liver microsomes. Xenobiotica 2012, 42, 409–416. [Google Scholar] [CrossRef]
- Anglicheau, D.; Flamant, M.; Schlageter, M.H.; Martinez, F.; Cassinat, B.; Beaune, P.; Legendre, C.; Thervet, E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrology Dialysis Transplantation. 2003, 18, 2409–2414. [Google Scholar] [CrossRef]
- Limsrichamrern, S.; Chanapul, C.; Mahawithitwong, P.; Sirivatanauksorn, Y.; Kositamongkol, P.; Asavakarn, S.; Tovikkai, C.; Dumronggittigule, W. Correlation of Hematocrit and Tacrolimus Level in Liver Transplant Recipients. Transplant. Proc. 2016, 48, 1176–1178. [Google Scholar] [CrossRef]
- Sikma, M.A.; Hunault, C.C.; Huitema, A.D.R.; De Lange, D.W.; Van Maarseveen, E.M. Clinical Pharmacokinetics and Impact of Hematocrit on Monitoring and Dosing of Tacrolimus Early after Heart and Lung Transplantation. Clin. Pharmacokinet. 2020, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Schütte-Nütgen, K.; Thölking, G.; Steinke, J.; Pavenstädt, H.; Schmidt, R.; Suwelack, B.; Reuter, S. Fast Tac Metabolizers at Risk—It is Time for a C/D Ratio Calculation. J. Clin. Med. 2019, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Hustert, E.; Haberl, M.; Burk, O.; Wolbold, R.; He, Y.-Q.; Klein, K.; Nuessler, A.C.; Neuhaus, P.; Klattig, J.; Eiselt, R.; et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001, 11, 773–779. [Google Scholar] [CrossRef]
- Lee, S.J.; Usmani, K.A.; Chanas, B.; Ghanayem, B.; Xi, T.; Hodgson, E.; Mohrenweiser, H.W.; Goldstein, J.A. Genetic findings and functional studies of human CYP3A5 single nucleotide polymorphisms in different ethnic groups. Pharmacogenetics 2003, 13, 461–472. [Google Scholar] [CrossRef]
- Pascual, J.; Del Castillo, D.; Cabello, M.; Pallardó, L.; Grinyó, J.M.; Fernández, A.M.; Brunet, M. Interaction between everolimus and tacrolimus in renal transplant recipients: A pharmacokinetic controlled trial. Transplantation 2010, 89, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- ICH M10 on Bioanalytical Method Validation—Scientific Guideline|European Medicines Agency. Available online: https://www.ema.europa.eu/en/ich-m10-bioanalytical-method-validation-scientific-guideline (accessed on 16 April 2024).
- Levine, J.E.; Hogan, W.J.; Harris, A.C.; Litzow, M.R.; Efebera, Y.A.; Devine, S.M.; Reshef, R.; Ferrara, J.L. Improved accuracy of acute graft-versus-host disease staging among multiple centers. Best Pract. Res. Clin. Haematol. 2014, 27, 283–287. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. Available online: https://experts.umn.edu/en/publications/kidney-disease-improving-global-outcomes-kdigo-acute-kidney-inju (accessed on 16 April 2024).
- Jodele, S.; Laskin, B.L.; Dandoy, C.E.; Myers, K.C.; El-Bietar, J.; Davies, S.M.; Goebel, J.; Dixon, B.P. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015, 29, 191–204. [Google Scholar] [CrossRef]
- Cho, B.S.; Yahng, S.A.; Lee, S.E.; Eom, K.-S.; Kim, Y.-J.; Kim, H.-J.; Lee, S.; Min, C.-K.; Cho, S.-G.; Kim, D.-W.; et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 2010, 90, 918–926. [Google Scholar] [CrossRef]
| Characteristics | All Patients N = 62 | CYP3A5*3/*3 N = 49 | CYP3A5*1 † N = 13 | p Value †† |
|---|---|---|---|---|
| Mean age, years (SD) | 49 (15) | 49 (14) | 50 (17) | 0.7 |
| Female sex | 30 (48) | 24 (49) | 6 (46) | 1 |
| Mean Weight, kg (SD) | 68.6 (12) | 69.1 (12) | 67.6 (14) | 0.7 |
| Primary diagnosis | ||||
| Acute leukemia | 26 (42) | 17 (35) | 9 (69) | 0.16 |
| Myelodysplastic syndromes | 14 (22) | 12 (25) | 2 (15) | 0.3 |
| Chronic myeloproliferative syndromes | 11 (18) | 10 (20) | 1 (8) | - |
| Lymphoma/multiple myeloma | 11 (18) | 10 (20) | 1 (8) | - |
| Disease status at transplant | ||||
| Complete response | 36 (58) | 27 (55) | 9 (69) | 0.6 |
| Partial response | 10 (16) | 8 (16) | 2 (15) | 0.9 |
| Stable disease/progression | 16 (26) | 14 (29) | 2 (15) | 0.4 |
| Conditioning regimen | ||||
| Myeloablative | 27 (44) | 21 (43) | 6 (46) | 1 |
| Reduced intensity | 35 (56) | 28 (57) | 7 (54) | - |
| Donor type | ||||
| HLA match 10/10 | 35 (56) | 28 (57) | 7 (54) | 1 |
| Identical sibling | 7 (11) | 4 (8) | 3 (23) | 0.23 |
| Haploidentical sibling | 11 (16) | 15 (31) | 1 (8) | - |
| Discordant sex ††† | 12 (19) | 9 (18) | 3 (23) | 0.7 |
| GVHD prophylaxis regimen | ||||
| PTCy-based | 48 (77) | 35 (71) | 13 (100) | 0.03 |
| Tac IV | 2 (3) | 2 (4) | 0 (0) | - |
| Tac BID | 15 (24) | 11 (22) | 4 (31) | 0.65 |
| Tac QD | 45 (73) | 36 (74) | 9 (69) | 0.8 |
| Hematocrit (SD) | ||||
| Day 0 | 0.29 (0.06) | 0.29 (0.06) | 0.29 (0.06) | 0.9 |
| At 48 h | 0.29 (0.05) | 0.29 (0.06) | 0.29 (0.06) | 0.9 |
| Mean of first month | 0.27 (0.03) | 0.27 (0.03) | 0.28 (0.03) | 0.6 |
| Change in azole regimen | 15 (24) | 11 (22) | 4 (31) | 0.72 |
| Corticosteroids treatment | 31 (50) | 22 (45) | 9 (69) | 0.21 |
| Characteristics | Univariate Analysis | p-Value | Multivariate Analysis, HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Patient age | Continuous variable (years) | 48.5 vs. 49.7 | 0.75 | 1.01 (0.97–1.03) | 0.7 |
| Conditioning regimen | RIC (vs. myeloablative) | 25.7 vs. 40.7 | 0.28 | 0.47 (0.2–1.09) | 0.08 |
| Disease status at transplant | Complete remission (vs. other) | 31.4 vs. 33.3 | 1 | ||
| Donor sex | Female donor to male (vs. other) | 41.7 vs. 30 | 0.5 | 0.81 (0.33–1.93) | 0.64 |
| Donor type | Any mismatch (vs. HLA matched) | 33.3 vs. 31.3 | 1 | 1.78 (0.9–3.51) | 0.1 |
| Tac TISS | <5 ng/mL (vs. ≥5) | 37.9 vs. 27.3 | 0.42 | ||
| Tac C0 at 7 days | <5 ng/mL (vs. ≥5) | 75 vs. 29.3 | 0.09 | 7.3 (0.7–17.1) | 0.1 |
| Tac formulation | QD (vs. other) | 31.1 vs. 35.3 | 0.7 | ||
| Hematocrit at 48 h | <25th percentile (vs. ≥25th) | 56.3 vs. 23.9 | 0.029 | 5.3 (1.4–19.7) | 0.01 |
| <50th percentile (vs. ≥50th) | 39.4 vs. 24.1 | 0.28 | |||
| <75th percentile (vs. ≥75th) | 37.8 vs. 17.6 | 0.22 | |||
| CMV status | High risk (vs. other) | 38.9 vs. 29.5 | 0.55 | 2.5 (0.3–13.1) | 0.62 |
| CYP3A5 | *1/*3 or *1/*1 (vs. *3/*3) | 61.5 vs. 24.5 | 0.019 | 4.51 (2.37–8.6) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco, D.N.; Molina, M.; Guio, A.-M.; Julian, J.; Fortuna, V.; Fabregat-Zaragoza, V.-L.; Salas, M.-Q.; Monge-Escartín, I.; Riu-Viladoms, G.; Carcelero, E.; et al. Effects of CYP3A5 Genotype on Tacrolimus Pharmacokinetics and Graft-versus-Host Disease Incidence in Allogeneic Hematopoietic Stem Cell Transplantation. Pharmaceuticals 2024, 17, 553. https://doi.org/10.3390/ph17050553
Marco DN, Molina M, Guio A-M, Julian J, Fortuna V, Fabregat-Zaragoza V-L, Salas M-Q, Monge-Escartín I, Riu-Viladoms G, Carcelero E, et al. Effects of CYP3A5 Genotype on Tacrolimus Pharmacokinetics and Graft-versus-Host Disease Incidence in Allogeneic Hematopoietic Stem Cell Transplantation. Pharmaceuticals. 2024; 17(5):553. https://doi.org/10.3390/ph17050553
Chicago/Turabian StyleMarco, Daniel N., Mònica Molina, Ana-María Guio, Judit Julian, Virginia Fortuna, Virginia-Lucila Fabregat-Zaragoza, María-Queralt Salas, Inés Monge-Escartín, Gisela Riu-Viladoms, Esther Carcelero, and et al. 2024. "Effects of CYP3A5 Genotype on Tacrolimus Pharmacokinetics and Graft-versus-Host Disease Incidence in Allogeneic Hematopoietic Stem Cell Transplantation" Pharmaceuticals 17, no. 5: 553. https://doi.org/10.3390/ph17050553
APA StyleMarco, D. N., Molina, M., Guio, A.-M., Julian, J., Fortuna, V., Fabregat-Zaragoza, V.-L., Salas, M.-Q., Monge-Escartín, I., Riu-Viladoms, G., Carcelero, E., Roma, J. R., Llobet, N., Arcarons, J., Suárez-Lledó, M., Rosiñol, L., Fernández-Avilés, F., Rovira, M., Brunet, M., & Martínez, C. (2024). Effects of CYP3A5 Genotype on Tacrolimus Pharmacokinetics and Graft-versus-Host Disease Incidence in Allogeneic Hematopoietic Stem Cell Transplantation. Pharmaceuticals, 17(5), 553. https://doi.org/10.3390/ph17050553






