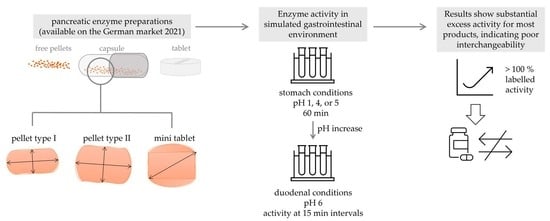
Comparative Investigation of pH–Dependent Availability of Pancreatic Enzyme Preparations In Vitro
Abstract
1. Introduction
2. Results
2.1. Physical Characterization
2.1.1. Particle Imaging
2.1.2. Particle Size
2.1.3. Particle Counting
2.2. Enzymatic Analysis
2.2.1. Enzyme Activities
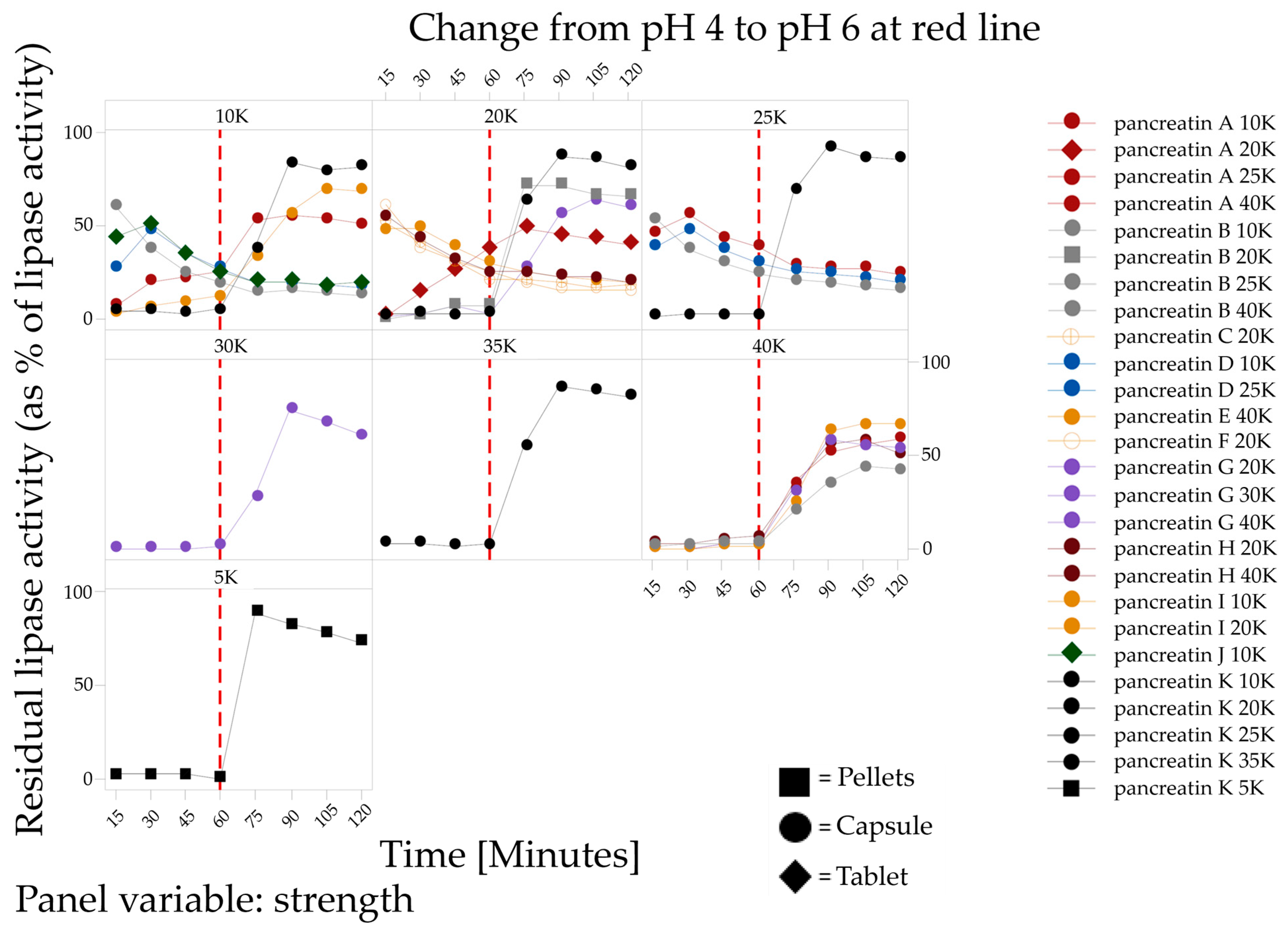
2.2.2. Enzyme Release

3. Discussion
4. Materials and Methods
4.1. Physical Properties
4.1.1. Particle Imaging
4.1.2. Particle Size Distribution
4.1.3. Particle Counting
4.2. Enzyme Activity and Enzyme Release Kinetics
4.2.1. Enzyme Activities
4.2.2. Lipase Activity after Enzyme Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Product | Marketed Name | Supplier | Batch Number |
|---|---|---|---|
| pancreatin A 10,000 | Pangrol | Berlin-Chemie | Batch 1: 83147D Batch 2: 94166E |
| pancreatin A 20,000 | Pangrol | Berlin-Chemie | 92027A |
| pancreatin A 25,000 | Pangrol | Berlin-Chemie | Batch 1: 84231E Batch 2: 93255H |
| pancreatin A 40,000 | Pangrol | Berlin-Chemie | 92019 |
| pancreatin B 10,000 | Panzytrat | Allergan | 337801 |
| pancreatin B 20,000 | Panzytrat | Allergan | 358001 |
| pancreatin B 25,000 | Panzytrat | Allergan | Batch 1: 412801 Batch 2: 413201 |
| pancreatin B 40,000 | Panzytrat | Allergan | 670501 |
| pancreatin C 20,000 | Pankreatin Stada | Nordmark Pharma | 92238 |
| pancreatin D 10,000 | Pankreatan | Nordmark Pharma | 321301 |
| pancreatin D 20,000 | Pankreatan | Nordmark Pharma | 501201 |
| pancreatin D 25,000 | Pankreatan | Nordmark Pharma | 323101 |
| pancreatin E 40,000 | Pankreatin | Nordmark Pharma | 672401 |
| pancreatin F 20,000 | Pankreatin Mikro | Ratiopharm | 321401 |
| pancreatin G 20,000 | Cotazym | UCB o. Cheplapharm | 507401 |
| pancreatin G 30,000 | Cotazym | UCB o. Cheplapharm | 507701 |
| pancreatin G 40,000 | Cotazym | UCB o. Cheplapharm | 659101 |
| pancreatin H 20,000 | Ozym | Trommsdorff | N001 |
| pancreatin H 40,000 | Ozym | Trommsdorff | N002 |
| pancreatin I 10,000 | Pankreatin Laves Mikro | Nordmark Pharma | 012501 |
| pancreatin I 20,000 | Pankreatin Laves Mikro | Nordmark Pharma | 319301 |
| pancreatin J 10,000 | Mezym | Berlin-Chemie | 98013 |
| pancreatin K 5000 | Kreon | Abbott Laboratories | 59042 |
| pancreatin K 10,000 | Kreon | Abbott Laboratories | Batch 1: 57797 Batch 2: 58519 Batch 3: 57234 |
| pancreatin K 20,000 | Kreon | Abbott Laboratories | 58845 |
| pancreatin K 25,000 | Kreon | Abbott Laboratories | Batch 1: 58259 Batch 2: 58888 Batch 3: 57467 |
| pancreatin K 35,000 | Kreon | Abbott Laboratories | 59016 |
References
- Chaudhary, A.; Domínguez-Muñoz, J.E.; Layer, P.; Lerch, M.M. Pancreatic exocrine insufficiency as a complication of gastrointestinal surgery and the impact of pancreatic enzyme replacement therapy. Dig. Dis. 2020, 38, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Radlinger, B.; Ramoser, G.; Kaser, S. Exocrine pancreatic insufficiency in type 1 and type 2 diabetes. Curr. Diabetes Rep. 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Diéguez-Castillo, C.; Jiménez-Luna, C.; Prados, J.; Martín-Ruiz, J.L.; Caba, O. State of the art in exocrine pancreatic insufficiency. Medicina 2020, 56, 523. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Iovanna, J.; Santofimia-Castaño, P. Targeting fibrosis: The bridge that connects pancreatitis and pancreatic cancer. Int. J. Mol. Sci. 2021, 22, 4970. [Google Scholar] [CrossRef] [PubMed]
- Piciucchi, M.; Capurso, G.; Archibugi, L.; Fave, M.M.D.; Capasso, M.; Fave, G.D. Exocrine Pancreatic Insufficiency in Diabetic Patients: Prevalence, Mechanisms, and Treatment. Int. J. Endocrinol. 2015, 2015, 595649. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Del Chiaro, M.; Permert, J.; Löhr, J.M. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, A.; Zelisko, A.; Schauer, P.; Lopez, R.; Kroh, M.; Stevens, T. Acute pancreatitis in patients after bariatric surgery: Incidence, outcomes, and risk factors. Obes. Surg. 2014, 24, 2025–2030. [Google Scholar] [CrossRef]
- Kuhn, R.J.; Eyting, S.; Henniges, F.; Potthoff, A. In vitro comparison of physical parameters, enzyme activity, acid resistance, and pH dissolution characteristics of enteric-coated pancreatic enzyme preparations: Implications for clinical variability and pharmacy substitution. J. Pediatr. Pharmacol. Ther. 2007, 12, 115–128. [Google Scholar] [CrossRef]
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef]
- Hackert, T.; Schütte, K.; Malfertheiner, P. The pancreas: Causes for malabsorption. Viszeralmedizin 2014, 30, 190–197. [Google Scholar] [CrossRef]
- Somaraju, U.R.R.; Solis-Moya, A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Löhr, J.M.; Hummel, F.M.; Pirilis, K.T.; Steinkamp, G.; Körner, A.; Henniges, F. Properties of different pancreatin preparations used in pancreatic exocrine insufficiency. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1024–1031. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Guideline on the Clinical Development of Medicinal Products for the Treatment of Cystic Fibrosis; European Medicines Agency: London, UK, 2009.
- Aloulou, A.; Puccinelli, D.; Sarles, J.; Laugier, R.; Leblond, Y.; Carrière, F. In vitro comparative study of three pancreatic enzyme preparations: Dissolution profiles, active enzyme release and acid stability. Aliment. Pharmacol. Ther. 2008, 27, 283–292. [Google Scholar] [CrossRef]
- Shrikhande, S.V.; Prasad, V.G.; Domínguez-Muñoz, J.E.; Weigl, K.E.; Sarda, K.D. In vitro Comparison of Pancreatic Enzyme Preparations Available in the Indian Market. Drug Des. Dev. Ther. 2021, 15, 3835–3843. [Google Scholar] [CrossRef]
- Maev, I.V.; Kucheryavyy, Y.A.; Gubergrits, N.B.; Bonnacker, I.; Shelest, E.A.; Janssen-van Solingen, G.P.; Domínguez-Muñoz, J.E. Differences in in vitro properties of pancreatin preparations for pancreatic exocrine insufficiency as marketed in Russia and CIS. Drugs RD 2020, 20, 369–376. [Google Scholar] [CrossRef]
- Meyer, J.H.; Elashoff, J.; Porter-Fink, V.; Dressman, J.; Amidon, G.L. Human postprandial gastric emptying of 1–3-mm spheres. Gastroenterology 1988, 94, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Kühnelt, P.; Mundlos, S.; Adler, G. Effect of pellet size of a pancreas enzyme preparation on duodenal lipolytic activity. Z. Gastroenterol. 1991, 29, 417–421. [Google Scholar] [PubMed]
- Lederer, P.C.H. Response to: Properties of different pancreatin preparations used in pancreatic exocrine insufficiency, Löhr JM et al. Eur J Gastroenterol Hepatol 2009; 21(9):1024–1031. Eur. J. Gastroenterol. Hepatol. 2010, 22, 635. [Google Scholar] [CrossRef]
- Piehl, S.; Brinkmann, R.; Windeck, T. Response to: Properties of different pancreatin preparations used in pancreatic exocrine insufficiency. Eur. J. Gastroenterol. Hepatol. 2010, 22, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Mundlos, S.; Kühnelt, P.; Adler, G. Monitoring enzyme replacement treatment in exocrine pancreatic insufficiency using the cholesteryl octanoate breath test. Gut 1990, 31, 1324. [Google Scholar] [CrossRef]
- FitzSimmons, S.C.; Burkhart, G.A.; Borowitz, D.; Grand, R.J.; Hammerstrom, T.; Durie, P.R.; Lloyd-Still, J.D.; Lowenfels, A.B. High-Dose Pancreatic-Enzyme Supplements and Fibrosing Colonopathy in Children with Cystic Fibrosis. N. Engl. J. Med. 1997, 336, 1283–1289. [Google Scholar] [CrossRef]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.P.; Barnett, J.L.; Jarvenpaa, K.M. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.P.; Littlewood, J.M. Pancreatin preparations used in the treatment of cystic fibrosis-lipase content and in vitro release. Aliment. Pharmacol. Ther. 1996, 10, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Borgström, B.; Donnér, J. Binding of bile salts to pancreatic colipase and lipase. J. Lipid Res. 1975, 16, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Brock, A.; Aldag, I.; Edskes, S.; Hartmann, M.; Herzog, T.; Uhl, W.; Schnekenburger, J. Novel ciliate lipases for enzyme replacement during exocrine pancreatic insufficiency. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.U.; Knoll-Ruzicka, M.L.; Domschke, S.; Heptner, G.; Domschke, W. Pancreatic enzyme replacement therapy: Comparative effects of conventional and enteric-coated microspheric pancreatin and acid-stable fungal enzyme preparations on steatorrhoea in chronic pancreatitis. Hepato-Gastroenterology 1985, 32, 97–102. [Google Scholar]
- Xenoulis, P.G.; Zoran, D.L.; Fosgate, G.T.; Suchodolski, J.S.; Steiner, J.M. Feline exocrine pancreatic insufficiency: A retrospective study of 150 cases. J. Vet. Intern. Med. 2016, 30, 1790–1797. [Google Scholar] [CrossRef]
- Taylor, C.J.; Thieroff-Ekerdt, R.; Shiff, S.; Magnus, L.; Fleming, R.; Gommoll, C. Comparison of two pancreatic enzyme products for exocrine insufficiency in patients with cystic fibrosis. J. Cyst. Fibros. 2016, 15, 675–680. [Google Scholar] [CrossRef]
- Peschke, G.J. Pancreatic Enzymes in Health and Disease; Lankisch, P.G., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 55–64. [Google Scholar]
- Hartmann, S.; Rydzewska, G.; Domínguez-Muñoz, J.E. Kreon®(Creon®) vs. Lipancrea®: In vitro comparison of two encapsulated pancreatin preparations. Pharmaceuticals 2022, 15, 1570. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; Chapter 2.9.1.
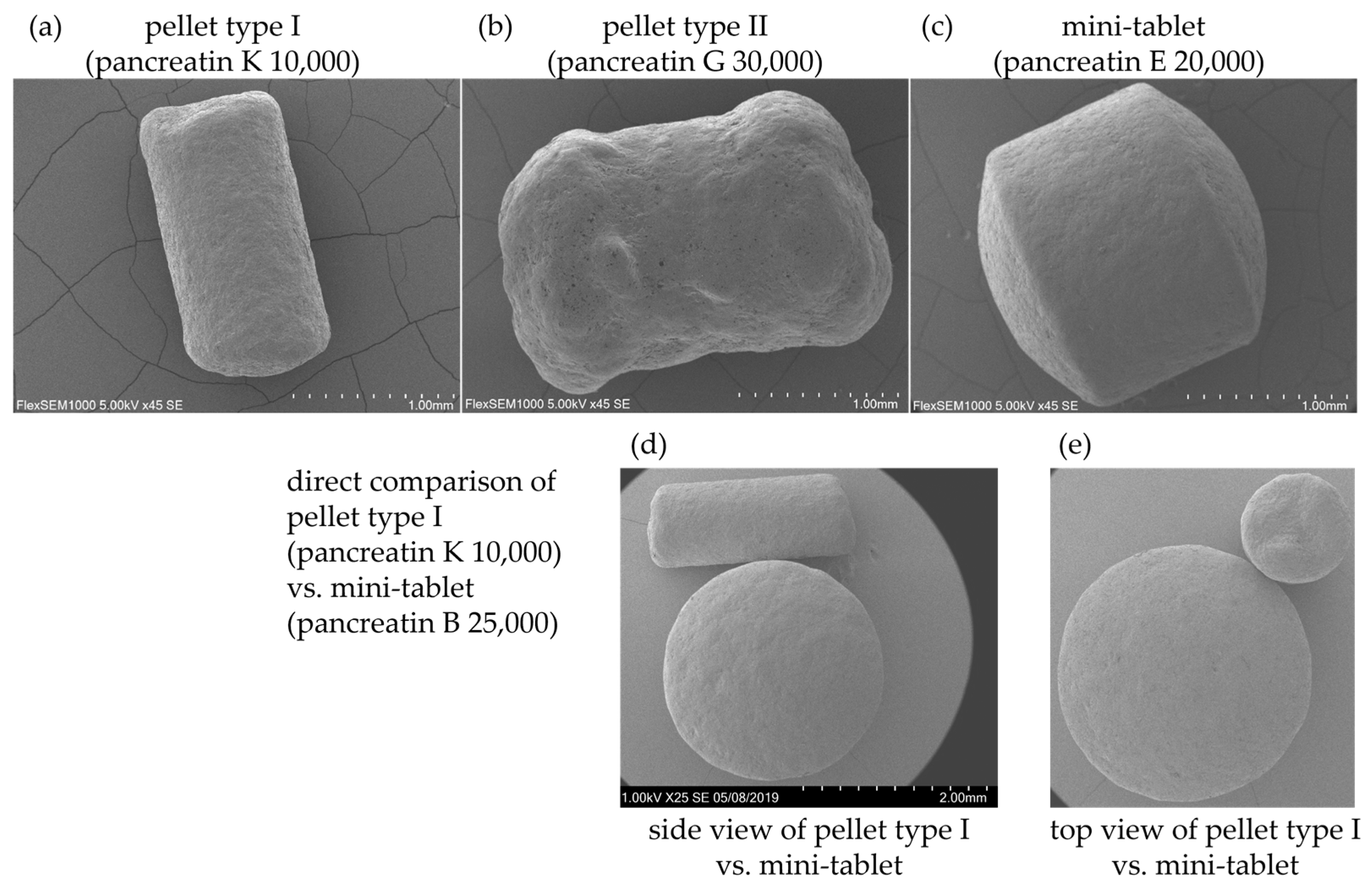
| Presentation | Products |
|---|---|
| pellet type I | pancreatin K 5000, pancreatin K 10,000, pancreatin K 20,000, pancreatin K 25,000, pancreatin K 35,000 |
| pellet type II | pancreatin A 40,000, pancreatin B 40,000, pancreatin E 40,000, pancreatin G 20,000, pancreatin G 30,000, pancreatin G 40,000, pancreatin H 40,000, pancreatin I 10,000 |
| mini-tablets | pancreatin A 10,000, pancreatin A 25,000, pancreatin B 10,000, pancreatin B 20,000, pancreatin B 25,000, pancreatin C 20,000, pancreatin D 10,000, pancreatin D 20,000, pancreatin D 25,000, pancreatin F 20,000, pancreatin H 20,000, pancreatin I 20,000 |
| tablet | pancreatin A 20,000, pancreatin J 10,000 |
| Product | D[v, 0.5]/µm | Presentation |
|---|---|---|
| pancreatin A 10,000 batch 1 | 2632 | Mini-Tablet |
| pancreatin A 10,000 batch 2 | 2616 | Mini-Tablet |
| pancreatin A 20,000 | N/A * | Tablet |
| pancreatin A 25,000 batch 1 | 2615 | Mini-Tablet |
| pancreatin A 25,000 batch 2 | 2617 | Mini-Tablet |
| pancreatin A 40,000 | 2529 | Pellet (type II) |
| pancreatin B 10,000 | 2615 | Mini-Tablet |
| pancreatin B 20,000 | 2615 | Mini-Tablet |
| pancreatin B 25,000 batch 1 | 2614 | Mini-Tablet |
| pancreatin B 25,000 batch 2 | 2614 | Mini-Tablet |
| pancreatin B 40,000 | 2579 | Pellet (type II) |
| pancreatin C 20,000 | 2612 | Mini-Tablet |
| pancreatin D 10,000 | 2612 | Mini-Tablet |
| pancreatin D 20,000 | 2614 | Mini-Tablet |
| pancreatin D 25,000 | 2612 | Mini-Tablet |
| pancreatin E 40,000 | 2519 | Pellet (type II) |
| pancreatin F 20,000 | 2618 | Mini-Tablet |
| pancreatin G 20,000 | 2601 | Pellet (type II) |
| pancreatin G 30,000 | 2547 | Pellet (type II) |
| pancreatin G 40,000 | 2468 | Pellet (type II) |
| pancreatin H 20,000 | 2616 | Mini-Tablet |
| pancreatin H 40,000 | 2528 | Pellet (type II) |
| pancreatin I 10,000 | 1979 | Pellet (type II) |
| pancreatin I 20,000 | 2611 | Mini-Tablet |
| pancreatin J 10,000 | N/A ** | Tablet |
| pancreatin K 5000 | 1449 | Pellet (type I) |
| pancreatin K 10,000 batch 1 | 1541 | Pellet (type I) |
| pancreatin K 10,000 batch 2 | 1566 | Pellet (type I) |
| pancreatin K 10,000 batch 3 | 1537 | Pellet (type I) |
| pancreatin K 20,000 | 1608 | Pellet (type I) |
| pancreatin K 25,000 batch 1 | 1588 | Pellet (type I) |
| pancreatin K 25,000 batch 2 | 1608 | Pellet (type I) |
| pancreatin K 25,000 batch 3 | 1531 | Pellet (type I) |
| pancreatin K 35,000 | 1542 | Pellet (type I) |
| Strength | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5K | 10K | 20K | 25K | 30K | 35K | 40K | ||
| Quantity | Type I pellets | 124–132 | 257–314 | 387–414 | 384–608 | - | 726–765 | - |
| Type II pellets | - | 74–81 | 60–62 | - | 84–93 | - | 92–145 | |
| Mini-tablets | - | 19–30 | 42–50 | 43–61 | - | - | - | |
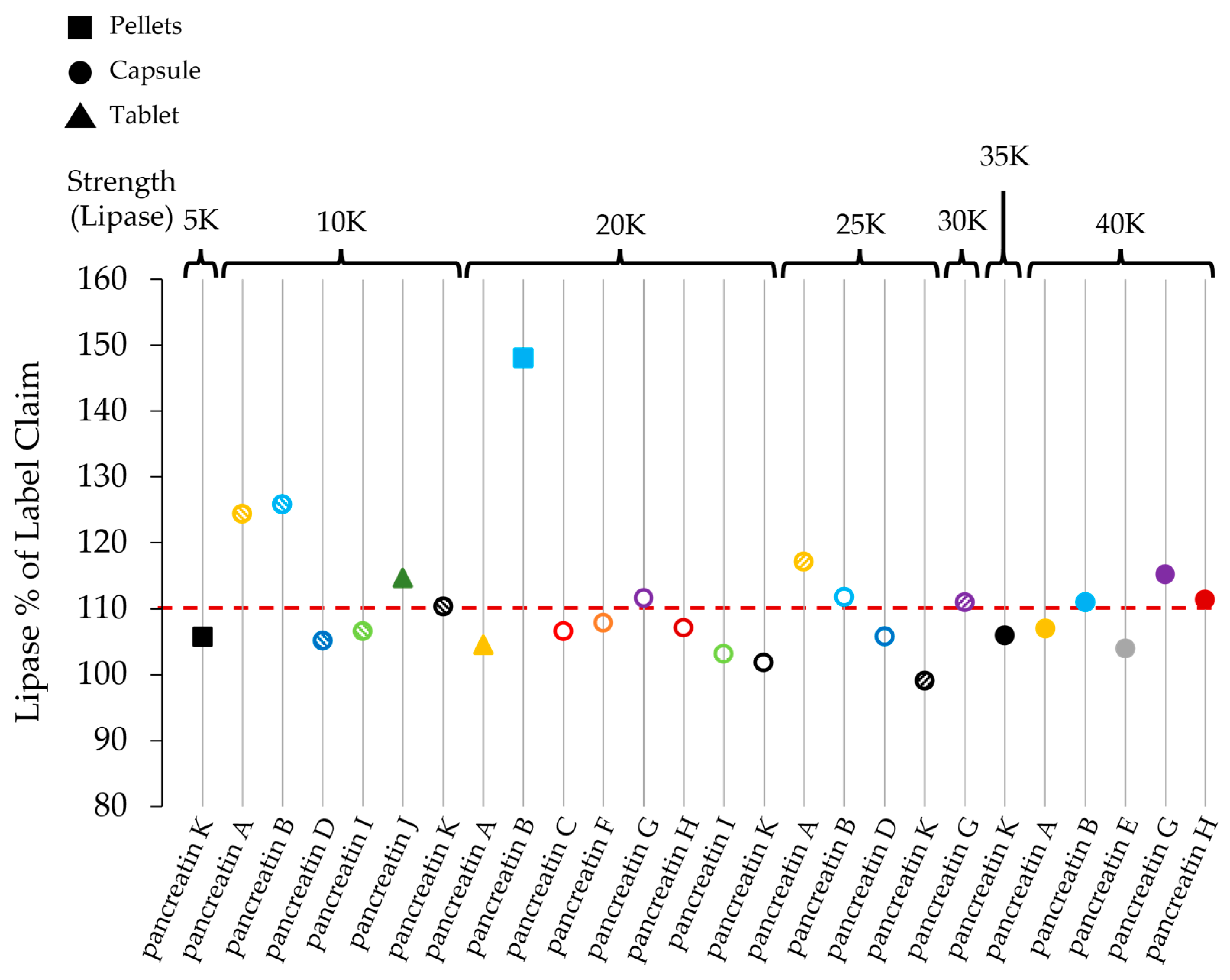
| Lipase Activity | Amylase Activity | Protease Activity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Product | Labelled Lipase Activity/Dose Unit | Lipase Assay/Unit Dose | Lipase % of Label Claim | Labelled Amylase Activity/Dose Unit | Amylase Assay/Unit Dose | Amylase % of Label Claim | Labelled Protease Activity/Dose Unit | Protease Assay/Unit Dose | Protease % of Label Claim |
| pancreatin A 10,000 | 10,000 | 12,444 | 124.4 | 9000 | 11,728 | 130.3 | 500 | 633 | 126.6 |
| pancreatin A 20,000 | 20,000 | 20,900 | 104.5 | 12,000 | 19,244 | 160.4 | 900 | 1290 | 143.3 |
| pancreatin A 25,000 | 25,000 | 29,268 | 117.1 | 22,500 | 28,363 | 126.1 | 1250 | 1529 | 122.3 |
| pancreatin A 40,000 | 40,000 | 42,784 | 107.0 | 25,000 | 33,285 | 133.1 | 1500 | 1735 | 115.7 |
| pancreatin B 10,000 | 10,000 | 12,583 | 125.8 | 9000 | 11,016 | 122.4 | 500 | 599 | 119.7 |
| pancreatin B 20,000 | 20,000 | 29,615 | 148.1 | 18,000 | 21,172 | 117.6 | 1000 | 1109 | 110.9 |
| pancreatin B 25,000 | 25,000 | 27,929 | 111.7 | 15,000 | 20,922 | 139.5 | 800 | 1012 | 126.5 |
| pancreatin B 40,000 | 40,000 | 44,413 | 111.0 | 25,000 | 30,131 | 120.5 | 1500 | 1761 | 117.4 |
| pancreatin C 20,000 | 20,000 | 21,299 | 106.5 | 15,000 | 18,362 | 122.4 | 900 | 1057 | 117.5 |
| pancreatin D 10,000 | 10,000 | 10,516 | 105.2 | 7500 | 10,030 | 133.7 | 450 | 504 | 112.0 |
| pancreatin D 25,000 | 25,000 | 26,442 | 105.8 | 18,750 | 22,667 | 120.9 | 1125 | 1207 | 107.3 |
| pancreatin E 40,000 | 40,000 | 41,555 | 103.9 | 25,000 | 28,979 | 115.9 | 1500 | 1742 | 116.1 |
| pancreatin F 20,000 | 20,000 | 21,572 | 107.9 | 15,000 | 20,545 | 137.0 | 900 | 997 | 110.8 |
| pancreatin G 20,000 | 20,000 | 22,327 | 111.6 | 14,500 | 20,292 | 139.9 | 850 | 1041 | 122.5 |
| pancreatin G 30,000 | 30,000 | 33,283 | 110.9 | 21,750 | 30,033 | 138.1 | 1275 | 1717 | 134.6 |
| pancreatin G 40,000 | 40,000 | 46,081 | 115.2 | 25,000 | 29,655 | 118.6 | 1500 | 1601 | 106.7 |
| pancreatin H 20,000 | 20,000 | 21,428 | 107.1 | 15,000 | 20,609 | 137.4 | 900 | 1060 | 117.8 |
| pancreatin H 40,000 | 40,000 | 44,575 | 111.4 | 25,000 | 33,539 | 134.2 | 1500 | 1667 | 111.1 |
| pancreatin I 10,000 | 10,000 | 10,648 | 106.5 | 7250 | 9665 | 133.3 | 425 | 567 | 133.5 |
| pancreatin I 20,000 | 20,000 | 20,634 | 103.2 | 15,000 | 19,216 | 128.1 | 900 | 987 | 109.7 |
| pancreatin J 10,000 | 10,000 | 11,466 | 114.7 | 7500 | 9213 | 122.8 | 375 | 506 | 135.0 |
| pancreatin K 5000 | 5000 | 5287 | 105.7 | 3600 | 5649 | 156.9 | 200 | 330 | 165.0 |
| pancreatin K 10,000 | 10,000 | 11,037 | 110.4 | 8000 | 12,137 | 151.7 | 600 | 790 | 131.7 |
| pancreatin K 20,000 | 20,000 | 20,368 | 101.8 | 16,000 | 23,967 | 149.8 | 1200 | 1556 | 129.7 |
| pancreatin K 25,000 | 25,000 | 24,785 | 99.1 | 18,000 | 25,975 | 144.3 | 1000 | 1477 | 147.7 |
| pancreatin K 35,000 | 35,000 | 37,101 | 106.0 | 25,200 | 35,906 | 142.5 | 1400 | 2090 | 149.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todd, A.; Bennett-Huntley, E.; Rosendahl, J.; Schnekenburger, J.; Uhl, W. Comparative Investigation of pH–Dependent Availability of Pancreatic Enzyme Preparations In Vitro. Pharmaceuticals 2024, 17, 552. https://doi.org/10.3390/ph17050552
Todd A, Bennett-Huntley E, Rosendahl J, Schnekenburger J, Uhl W. Comparative Investigation of pH–Dependent Availability of Pancreatic Enzyme Preparations In Vitro. Pharmaceuticals. 2024; 17(5):552. https://doi.org/10.3390/ph17050552
Chicago/Turabian StyleTodd, Amy, Emma Bennett-Huntley, Jonas Rosendahl, Jürgen Schnekenburger, and Waldemar Uhl. 2024. "Comparative Investigation of pH–Dependent Availability of Pancreatic Enzyme Preparations In Vitro" Pharmaceuticals 17, no. 5: 552. https://doi.org/10.3390/ph17050552
APA StyleTodd, A., Bennett-Huntley, E., Rosendahl, J., Schnekenburger, J., & Uhl, W. (2024). Comparative Investigation of pH–Dependent Availability of Pancreatic Enzyme Preparations In Vitro. Pharmaceuticals, 17(5), 552. https://doi.org/10.3390/ph17050552