RETRACTED: Structure–Activity Relationship of Synthetic Linear KTS-Peptides Containing Meta-Aminobenzoic Acid as Antagonists of α1β1 Integrin with Anti-Angiogenic and Melanoma Anti-Tumor Activities
Abstract
1. Introduction
2. Results
2.1. Design and Synthesis of MABA-Peptides
2.2. MABA-Peptides Inhibit Integrin Binding and Cell Adhesion
2.3. In Vitro Metabolic Stability of MABA-Peptides
2.4. In Vitro Anti-Angiogenic Effect of the MABA-Peptides
2.5. Anti-Tumor Effect of the MABA-Peptides in a Murine Melanoma Model
2.6. Safety of MABA-Peptide 4
2.7. Molecular Modeling
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines
4.3. Peptide Synthesis Reagents and General Procedure for Peptides Preparation and Characterization
4.3.1. General
4.3.2. Loading
4.3.3. Fmoc Deprotection
4.3.4. Elongation with the FmocAa
4.3.5. Cleavage from Resin
4.3.6. Mass Spectroscopy
4.4. ELISA to Test GST-α1A Binding to Collagen IV Fragment CB3
4.5. Cell Adhesion Assay
4.6. Stability of the Peptides in Rat Serum
4.7. Tube Formation-Angiogenesis Assay
4.8. In Vivo Characterization of Anti-Tumor Activity
4.9. Cell Death
4.10. Toxicity to Mice
4.11. Molecular Dynamic Simulations
4.12. Molecular Docking
4.13. MM-GBSA Calculations
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moreno-layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J.; Europe PMC Funders Group. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.L.; Picard, M. Integrins as therapeutic targets. Trends Pharmacol. Sci. 2012, 33, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Cox, D. How not to discover a drug—Integrins. Expert. Opin. Drug Discov. 2021, 16, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Kadry, Y.A.; Calderwood, D.A. Chapter 22: Structural and signaling functions of integrins. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183206. [Google Scholar] [CrossRef] [PubMed]
- Topple, A.; Fifkova, E.; Baumgardner, D.; Cullen-Dockstader, K. Effect of age on blood vessels and neurovascular appositions in the CA1 region of the rat hippocampus. Neurobiol. Aging 1991, 12, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pain, B.; Woods, C.M.; Saez, J.; Flickinger, T.; Raines, M.; Peyroll, S.; Moscovici, C.; Moscovici, M.G.; Kung, H.J.; Jurdic, P.; et al. EGF-R as a hemopoietic growth factor receptor: The c-erbB product is present in chicken erythrocytic progenitors and controls their self-renewal. Cell 1991, 65, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Moberg, P.E.; Miles, L.A.; Wagner, S.; Soloway, P.; Gardner, H.A. Elevated matrix metalloprotease and angiostatin levels in integrin α1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, I.; Walsh, E.M.; Rothman, V.L.; Gaathon, A.; Tuszynski, G.P.; Calvete, J.J.; Lazarovici, P.; Marcinkiewicz, C. Effect of VP12 and viperistatin on inhibition of collagen receptors-dependent melanoma metastasis. Cancer Biol. Ther. 2009, 8, 1507–1516. [Google Scholar] [CrossRef]
- Chen, X.; Su, Y.; Fingleton, B.; Acuff, H.; Matrisian, L.M.; Zent, R.; Pozzi, A. Increased plasma MMP9 in integrin α1-null mice enhances lung metastasis of colon carcinoma cells. Int. J. Cancer 2005, 116, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Perruzzi, C.A.; Streit, M.; Koteliansky, V.E.; De Fougerolles, A.R.; Detmar, M. The α1β1 and α2β1 integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am. J. Pathol. 2002, 160, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Niland, S.; Schulz, J.N.; Wang, F.; Eble, J.A.; Leitges, M.; Mauch, C.; Krieg, T.; Zigrino, P.; Eckes, B. Role of Integrins α1β1 and α2β1 in Wound and Tumor Angiogenesis in Mice. Am. J. Pathol. 2016, 186, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Momic, T.; Katzehendler, J.; Benny, O.; Lahiani, A.; Cohen, G.; Noy, E.; Senderowitz, H.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vimocin and vidapin, cyclic KTS peptides, are dual antagonists of α1β1/α2β1 integrins with antiangiogenic activity. J. Pharmacol. Exp. Ther. 2014, 350, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Murciano, M.P.; Monleón, D.; Calvete, J.J.; Celda, B.; Marcinkiewicz, C. Amino acid sequence and homology modeling of obtustatin, a novel non-RGD-containing short disintegrin isolated from the venom of Vipera lebetina obtusa. Protein Sci. 2003, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Murciano, M.P.; Monleón, D.; Marcinkiewicz, C.; Calvete, J.J.; Celda, B. NMR solution structure of the non-RGD disintegrin obtustatin. J. Mol. Biol. 2003, 329, 135–145. [Google Scholar] [CrossRef]
- Kisiel, D.G.; Calvete, J.J.; Katzhendler, J.; Fertala, A.; Lazarovici, P.; Marcinkiewicz, C. Structural determinants of the selectivity of KTS-disintegrins for the α1β1 integrin. FEBS Lett. 2004, 577, 478–482. [Google Scholar] [CrossRef]
- Momic, T.; Arlinghaus, F.T.; Arien-Zakay, H.; Katzhendler, J.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Pharmacological aspects of Vipera xantina palestinae venom. Toxins 2011, 3, 1420–1432. [Google Scholar] [CrossRef]
- Walsh, E.M.; Marcinkiewicz, C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon 2011, 58, 355–362. [Google Scholar] [CrossRef]
- Brown, M.C.; Eble, J.A.; Calvete, J.J.; Marcinkiewicz, C. Structural requirements of KTS-disintegrins for inhibition of α 1β1 integrin. Biochem. J. 2009, 417, 95–101. [Google Scholar] [CrossRef]
- Momic, T.; Katzhendler, J.; Marcinkiewicz, C.; Eble, A.J.; Lazarovici, P. Medicinal Chemistry Approach for Solid Phase Synthesis of Peptide Mimetics of Viperistatin Disintegrin as Lead Compounds for α1/α2 Integrin Receptors. In Proceedings of the 32nd European Peptide Symposium, Athens, Greece, 2–7 September 2012; pp. 304–305. [Google Scholar]
- Zhang, D.W.; Zhao, X.; Hou, J.L.; Li, Z.T. Aromatic amide foldamers: Structures, properties, and functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef] [PubMed]
- Kubik, S. Synthetic Receptors Based on Abiotic Cyclo(pseudo)peptides. Molecules 2022, 27, 2821. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Chen, S.G.; Zhao, X.; Jiang, X.K.; Li, Z.T. Meta-Substituted benzamide oligomers that complex mono-, di- and tricarboxylates: Folding-induced selectivity and chirality. Org. Biomol. Chem. 2011, 9, 8122–8129. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Sakane, T.; Shibukawa, M.; Hashida, M.; Sezaki, H. Absorption and Metabolic Characteristics of p-Aminobenzoic Acid and Its Isomer, m-Aminobenzoic Acid, from the Rat Small Intestine. J. Pharm. Sci. 1991, 80, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Benke, B.P.; Madhavan, N. Active ion transporters from readily accessible acyclic octapeptides containing 3-aminobenzoic acid and alanine. Chem. Commun. 2013, 49, 7340–7342. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, N.; Poojary, B.; Rai, V.; Vasantha, S.P. A Preliminary Study on Quinazolinylaminobenzoyl Monopeptide Esters as Effective Gram-Positive Bacteriostatic Agents. Future Med. Chem. 2019, 11, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, W.; Bae, J.S.; Ma, E. Synthesis and in Vitro and in Vivo anticoagulant and antiplatelet activities of amidino- and non-amidinobenzamides. Molecules 2016, 21, 676. [Google Scholar] [CrossRef] [PubMed]
- Weerapana, E.; Imperiali, B. Peptides to peptidomimetics: Towards the design and synthesis of bioavailable inhibitors of oligosaccharyl transferase. Org. Biomol. Chem. 2003, 1, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. Cell-based adhesion assays for isolation of snake venom’s integrin antagonists. In Snake and Spider Toxins; Methods in Molecular Biology Series; Humana Press Inc.: New York, NY, USA, 2020; pp. 205–223. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef]
- Lazarovici, P.; Lahiani, A.; Gincberg, G.; Haham, D.; Fluksman, A.; Benny, O.; Marcinkiewicz, C.; Lelkes, P.I. Nerve growth factor-induced angiogenesis: 1. Endothelial cell tube formation assay. Methods Mol. Biol. 2018, 1727, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A. Collagen-binding integrins as pharmaceutical targets. Curr. Pharm. Des. 2005, 11, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Dickeson, S.K.; Mathis, N.L.; Rahman, M.; Bergelson, J.M.; Santoro, S.A. Determinants of ligand binding specificity of the α1β1 and α2β1 integrins. J. Biol. Chem. 1999, 274, 32182–32191. [Google Scholar] [CrossRef] [PubMed]
- Monleon, D.; Moreno-Murciano, M.P.; Kovacs, H.; Marcinkiewicz, C.; Calvete, J.J.; Celda, B. Concerted motions of the integrin-binding loop and the C-terminal tail of the non-RGD disintegrin obtustatin. J. Biol. Chem. 2003, 278, 45570–45576. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.G.; Lovdahl, C.M.; Miles, A.J.; Hageini, V.L.M.; Fields, G.B. Solid-Phase synthesis and stability of triple-helical peptides incorporating native collagen sequences. Biopolymers 1993, 33, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Tuckwell, D.S. The α2β1 integrin inhibitor rhodocetin binds to the A-domain of the integrin α2 subunit proximal to the collagen-binding site. Biochem. J. 2003, 376, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A. Titration ELISA as a method to determine the dissociation constant of receptor ligand interaction. J. Vis. Exp. 2018, 132, 57334. [Google Scholar] [CrossRef] [PubMed]
- Schumacher-Klinger, A.; Fanous, J.; Merzbach, S.; Weinmüller, M.; Reichart, F.; Räder, A.F.B.; Gitlin-Domagalska, A.; Gilon, C.; Kessler, H.; Hoffman, A. Enhancing Oral Bioavailability of Cyclic RGD Hexa-peptides by the Lipophilic Prodrug Charge Masking Approach: Redirection of Peptide Intestinal Permeability from a Paracellular to Transcellular Pathway. Mol. Pharm. 2018, 15, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Fluksman, A.; Steinberg, E.; Orehov, N.; Shai, E.; Lahiani, A.; Katzhendler, J.; Marcinkiewicz, C.; Lazarovici, P.; Benny, O. Integrin α2β1-Targeted Self-Assembled Nanocarriers for Tumor Bioimaging. ACS Appl. Bio Mater. 2020, 3, 6059–6070. [Google Scholar] [CrossRef]
- Maatuf, Y.; Priel, A.; Lazarovici, P. Measurements of Cell Death Induced by Snake and Spider’s Venoms and Derived Toxins. Methods Mol. Biol. 2020, 2068, 239–268. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.J.; Chappelle, R. Restrained electrostatic potential atomic partial charges for condensed-phase simulations of carbohydrates. J. Mol. Struct. Theochem. 2000, 527, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, C.; Páll, S.; Fechner, M.; Esztermann, A.; De Groot, B.L.; Grubmüller, H. Best bang for your buck: GPU nodes for GROMACS biomolecular simulations. J. Comput. Chem. 2015, 36, 1990–2008. [Google Scholar] [CrossRef] [PubMed]
- Hess, B. P-LINCS: A parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Bussi, G.; Zykova-Timan, T.; Parrinello, M. Isothermal-isobaric molecular dynamics using stochastic velocity rescaling. J. Chem. Phys. 2009, 130, 074101. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Páll, S.; Hess, B. A flexible algorithm for calculating pair interactions on SIMD architectures. Comput. Phys. Commun. 2013, 184, 2641–2650. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Hockney, R.W.; Goel, S.P.; Eastwood, J.W. Quiet high-resolution computer models of a plasma. J. Comput. Phys. 1974, 14, 148–158. [Google Scholar] [CrossRef]
- Daura, X.; Gademann, K.; Jaun, B.; Seebach, D.; Van Gunsteren, W.F.; Mark, A.E. Peptide folding: When simulation meets experiment. Angew. Chemie Int. Ed. 1999, 38, 236–240. [Google Scholar] [CrossRef]
- Chin, Y.K.Y.; Headey, S.J.; Mohanty, B.; Patil, R.; McEwan, P.A.; Swarbrick, J.D.; Mulhern, T.D.; Emsley, J.; Simpson, J.S.; Scanlon, M.J. The structure of integrin α1I domain in complex with a collagen-mimetic peptide. J. Biol. Chem. 2013, 288, 36796–36809. [Google Scholar] [CrossRef][Green Version]
- Chuang, G.Y.; Kozakov, D.; Brenke, R.; Comeau, S.R.; Vajda, S. DARS (Decoys As the Reference State) potentials for protein-protein docking. Biophys. J. 2008, 95, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Brenke, R.; Comeau, S.R.; Vajda, S. PIPER: An FFT-based protein docking program with pairwise potentials. Proteins 2006, 65, 392–406. [Google Scholar] [CrossRef] [PubMed]
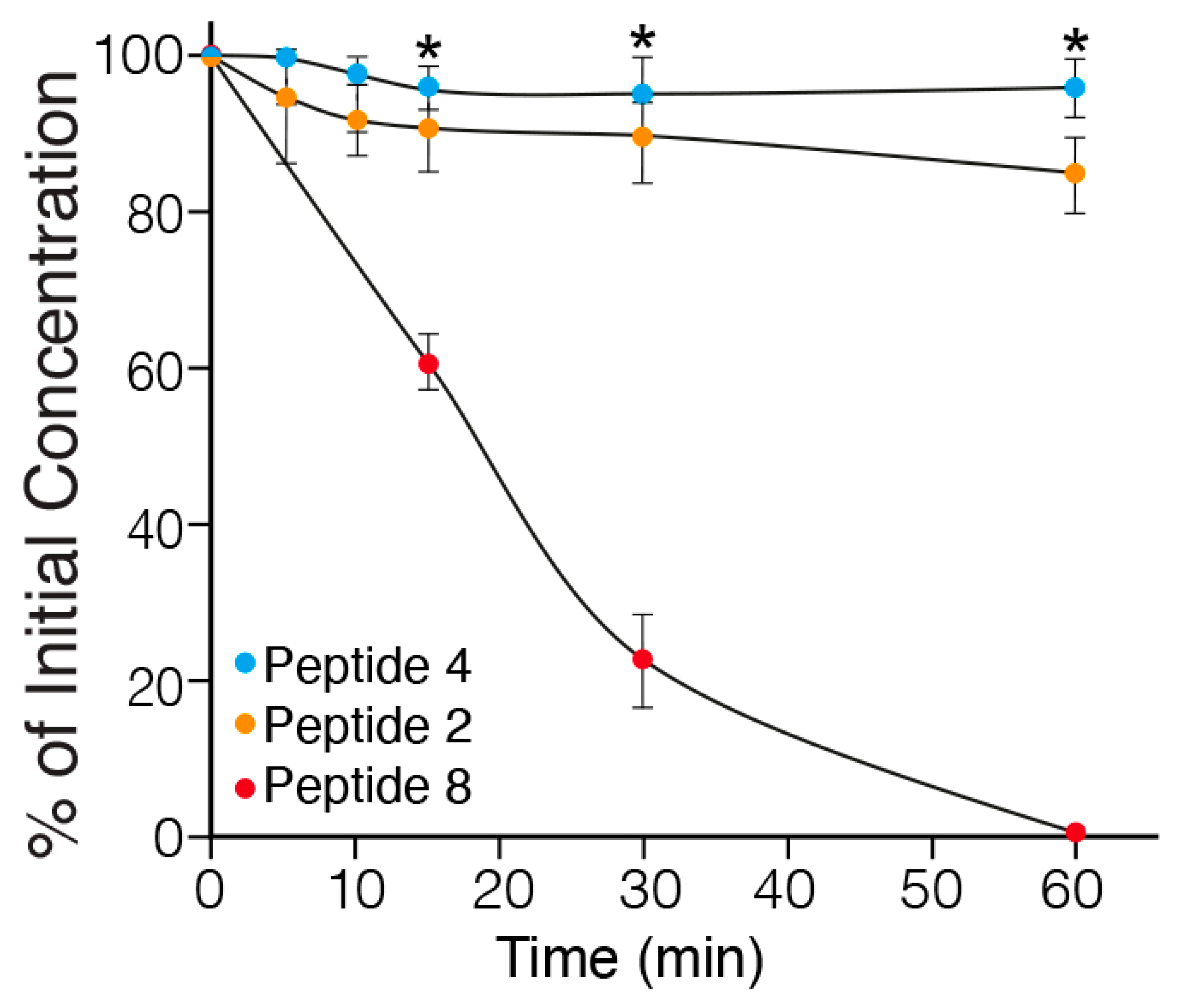

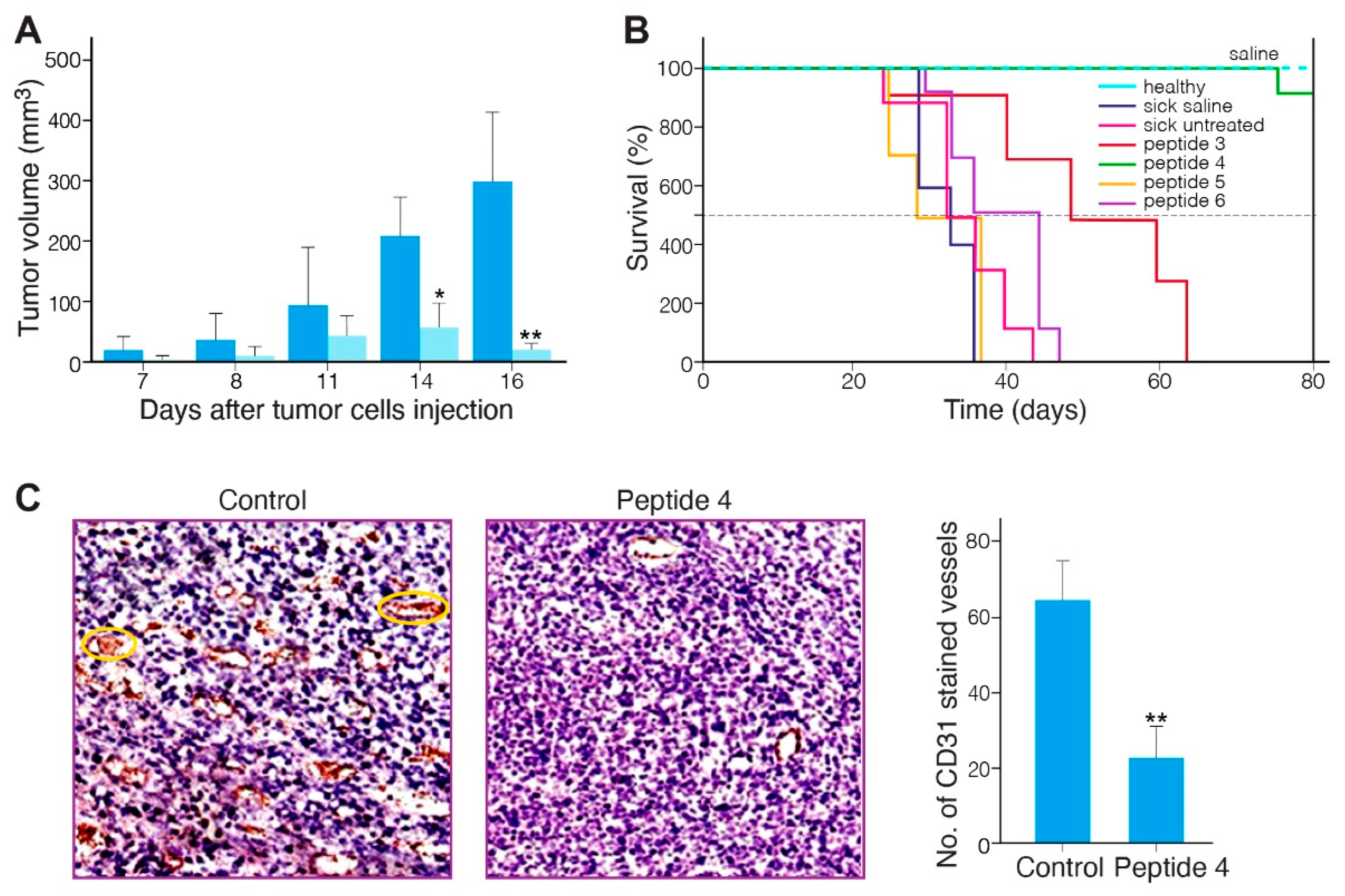
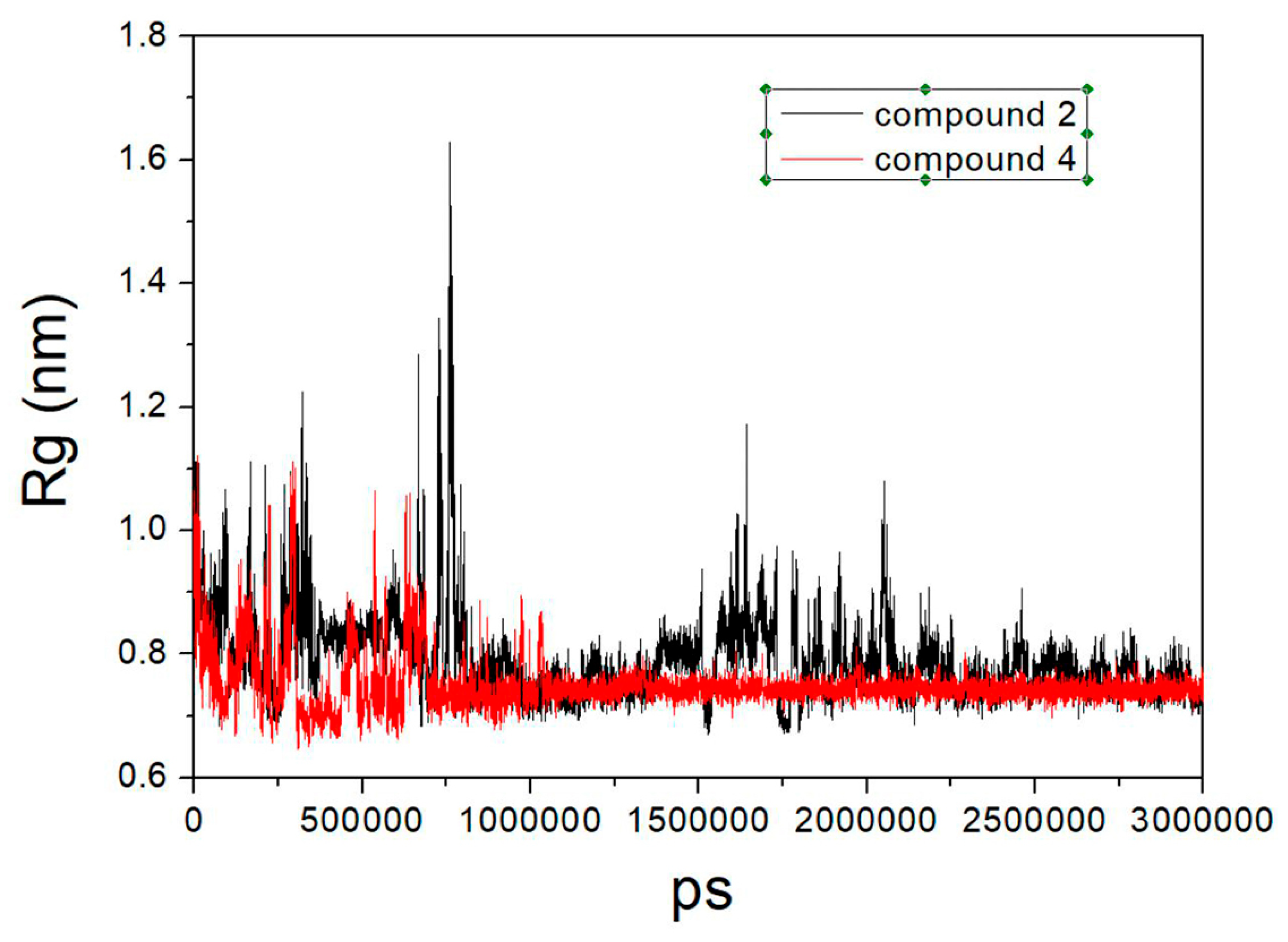
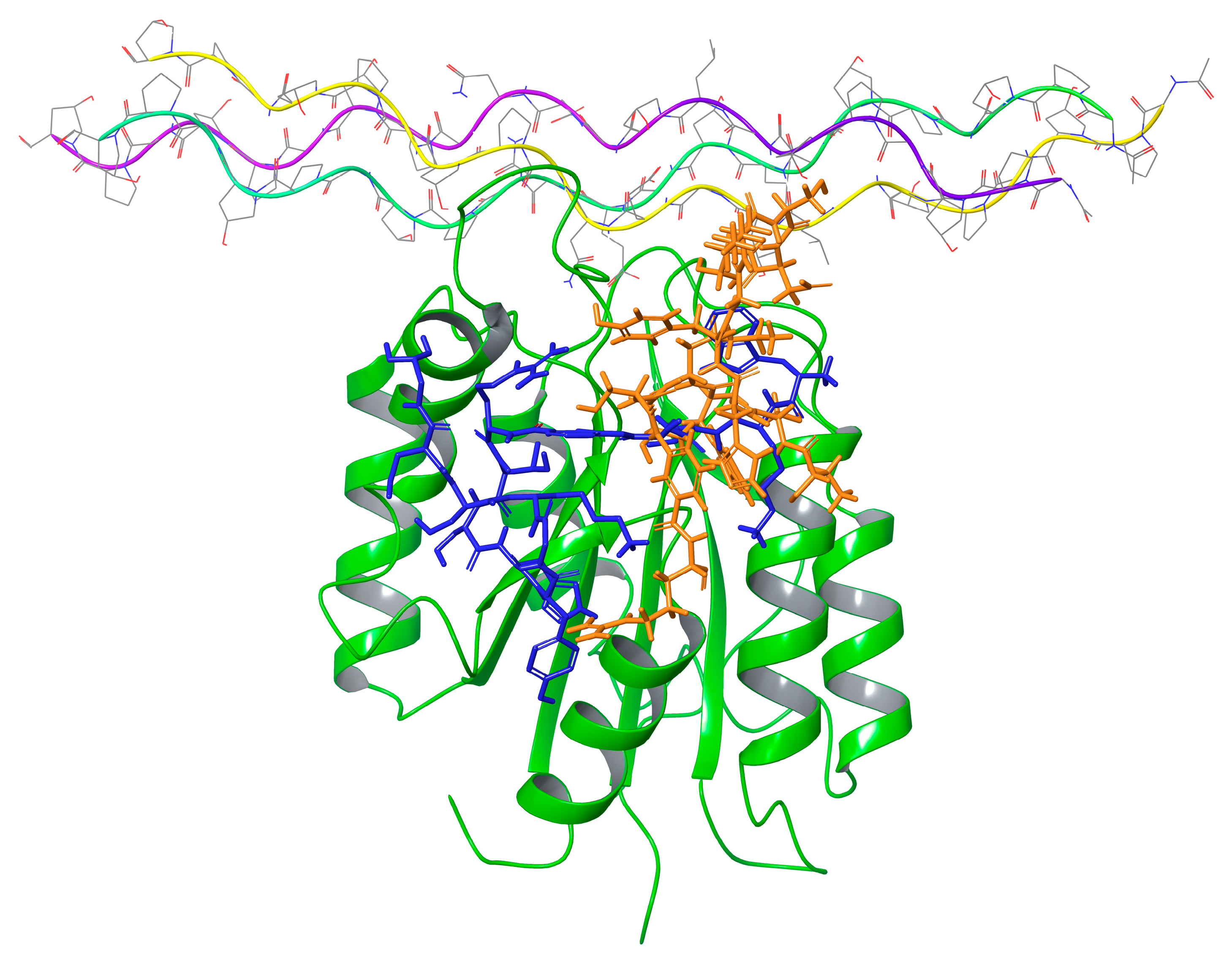
| Peptide Number | Sequence | α1β1 Binding In Vitro Assay IC50 (µM) | α1β1 Adhesion In Cell Assay IC50 (µM) |
|---|---|---|---|
| 1 | H2N-W-KTS-R-TSHY-MABA-TGKSDG-COOH | 828 ± 8 | ≥3000 |
| 2 | H2N-W-KTS-MABA-R-TSHY-GKSDG-COOH | 600 ± 10 | ≥3000 |
| 3 | H2N-KTS-W-KTS-MABA-R-TSHY-GKSDG-COOH | 442 ± 12 | ≥900 ± 75 |
| 4 | H2N-W-KTS-MABA-R-MABA-TSHY-GKSDG-COOH | 324 ± 8 | 550 ± 45 |
| 5 | H2N-W-KTS-MABA-RTSHY-MABA-TGKSDG-COOH | 1000 ± 9 | ≥3000 |
| 6 | H2N-W-KTR-MABA-R-MABA-TSHY-TGKSDG-COOH | 2142 ± 12 | 2006 ± 96 |
| 7 | H2N-W-KTR-MABA-R-MABA-TSHY-MABA-TGKSDG-COOH | 2481 ± 21 | ≥5000 |
| 8 | H2N-20W-KTS-R-TSHY-TGKSDG36-COOH | 970 ± 7 | ≥3000 |
| Peptide 2 | Peptide 4 | |
|---|---|---|
| Cluster No. | MM-GBSA Energy | MM-GBSA Energy |
| 1 | −35.8 | −55.7 |
| 2 | −88.1 | −47.2 |
| 3 | −39.7 | −91.1 |
| 4 | −70.0 | −80.3 |
| 5 | −68.9 | −73.8 |
| 6 | −41.5 | −77.3 |
| 7 | −52.8 |
| Peptide 4 | Interactions between Peptide and Protein | Peptide 2 | Interactions between Peptide and Protein |
|---|---|---|---|
| Trp1 | HB with BB of Tyr 17 | Trp1 | |
| Lys2 | Lys2 | BB HB with Val175 and HB with Asp28 | |
| Thr3 | HB with Ser176 | Thr3 | HB with BB of Leu143 |
| Ser4 | Ser4 | HB BB of Ala174 | |
| MABA 5 | MABA 5 | ||
| Arg6 | 2 HB with Asp177 | Arg6 | HB with Thr153 |
| MABA 7 | Thr7 | HB with Asn174 | |
| Thr8 | Ser8 | HB with Thr168 | |
| Ser9 | HB with Ser21 | His9 | |
| His10 | Tyr10 | HB with Thr183 | |
| Tyr11 | HB with BB of Tyr146 | Gly11 | |
| Gly12 | BB HB with Tyr146 | Lys12 | HB with Asp177 |
| Lys13 | Ser13 | ||
| Ser14 | Asp14 | ||
| Asp15 | Gly15 | BB HB with Glu154 | |
| Gly16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naamneh, M.S.; Momic, T.; Klazas, M.; Grosche, J.; Eble, J.A.; Marcinkiewicz, C.; Khazanov, N.; Senderowitz, H.; Hoffman, A.; Gilon, C.; et al. RETRACTED: Structure–Activity Relationship of Synthetic Linear KTS-Peptides Containing Meta-Aminobenzoic Acid as Antagonists of α1β1 Integrin with Anti-Angiogenic and Melanoma Anti-Tumor Activities. Pharmaceuticals 2024, 17, 549. https://doi.org/10.3390/ph17050549
Naamneh MS, Momic T, Klazas M, Grosche J, Eble JA, Marcinkiewicz C, Khazanov N, Senderowitz H, Hoffman A, Gilon C, et al. RETRACTED: Structure–Activity Relationship of Synthetic Linear KTS-Peptides Containing Meta-Aminobenzoic Acid as Antagonists of α1β1 Integrin with Anti-Angiogenic and Melanoma Anti-Tumor Activities. Pharmaceuticals. 2024; 17(5):549. https://doi.org/10.3390/ph17050549
Chicago/Turabian StyleNaamneh, Majdi Saleem, Tatjana Momic, Michal Klazas, Julius Grosche, Johannes A. Eble, Cezary Marcinkiewicz, Netaly Khazanov, Hanoch Senderowitz, Amnon Hoffman, Chaim Gilon, and et al. 2024. "RETRACTED: Structure–Activity Relationship of Synthetic Linear KTS-Peptides Containing Meta-Aminobenzoic Acid as Antagonists of α1β1 Integrin with Anti-Angiogenic and Melanoma Anti-Tumor Activities" Pharmaceuticals 17, no. 5: 549. https://doi.org/10.3390/ph17050549
APA StyleNaamneh, M. S., Momic, T., Klazas, M., Grosche, J., Eble, J. A., Marcinkiewicz, C., Khazanov, N., Senderowitz, H., Hoffman, A., Gilon, C., Katzhendler, J., & Lazarovici, P. (2024). RETRACTED: Structure–Activity Relationship of Synthetic Linear KTS-Peptides Containing Meta-Aminobenzoic Acid as Antagonists of α1β1 Integrin with Anti-Angiogenic and Melanoma Anti-Tumor Activities. Pharmaceuticals, 17(5), 549. https://doi.org/10.3390/ph17050549










