NMI-SO2Cl2-Mediated Amide Bond Formation: Facile Synthesis of Some Dihydrotriazolopyrimidine Amide Derivatives as Potential Anti-Inflammatory and Anti-Tubercular Agents
Abstract
1. Introduction
2. Results and Discussion
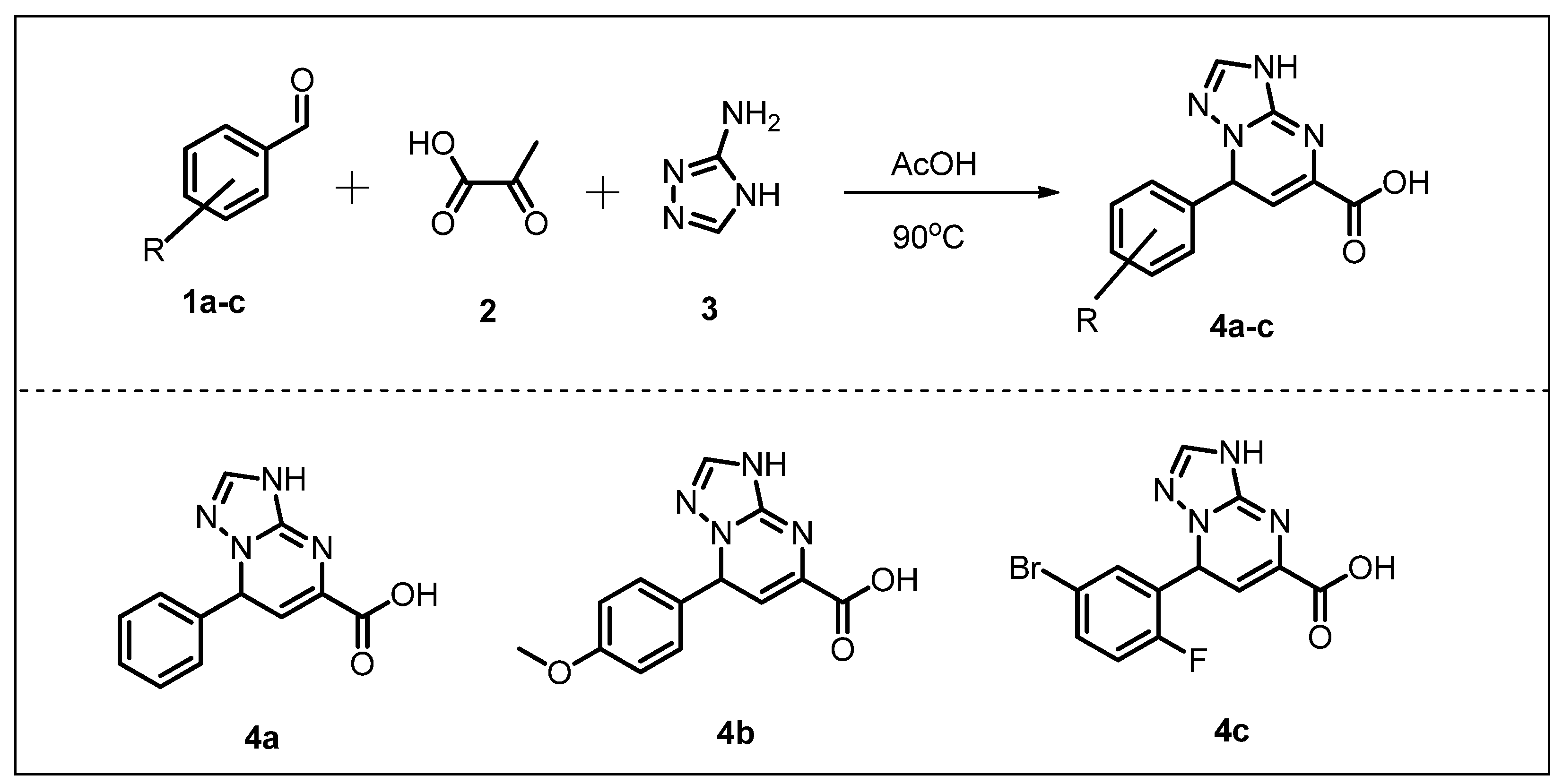
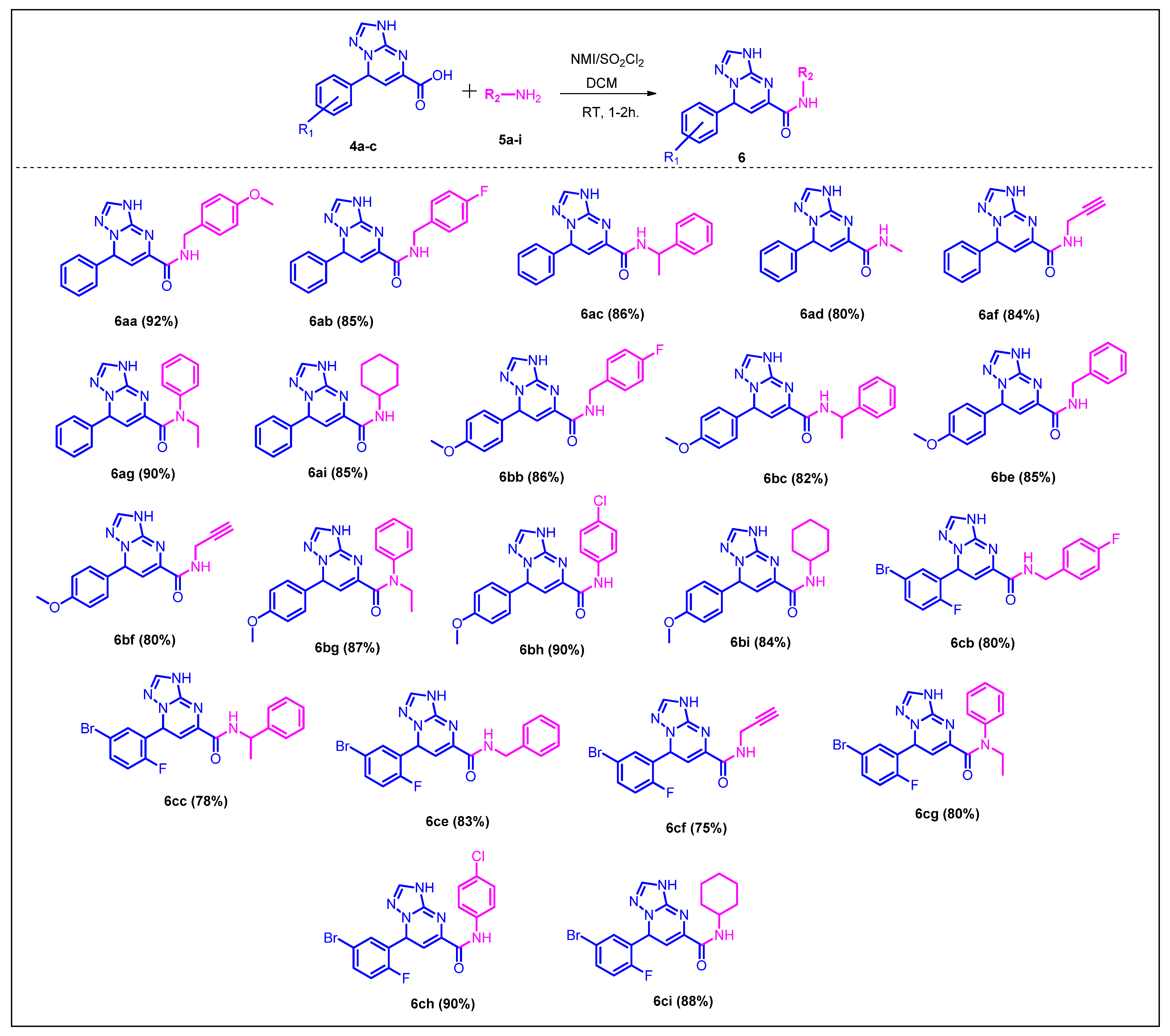
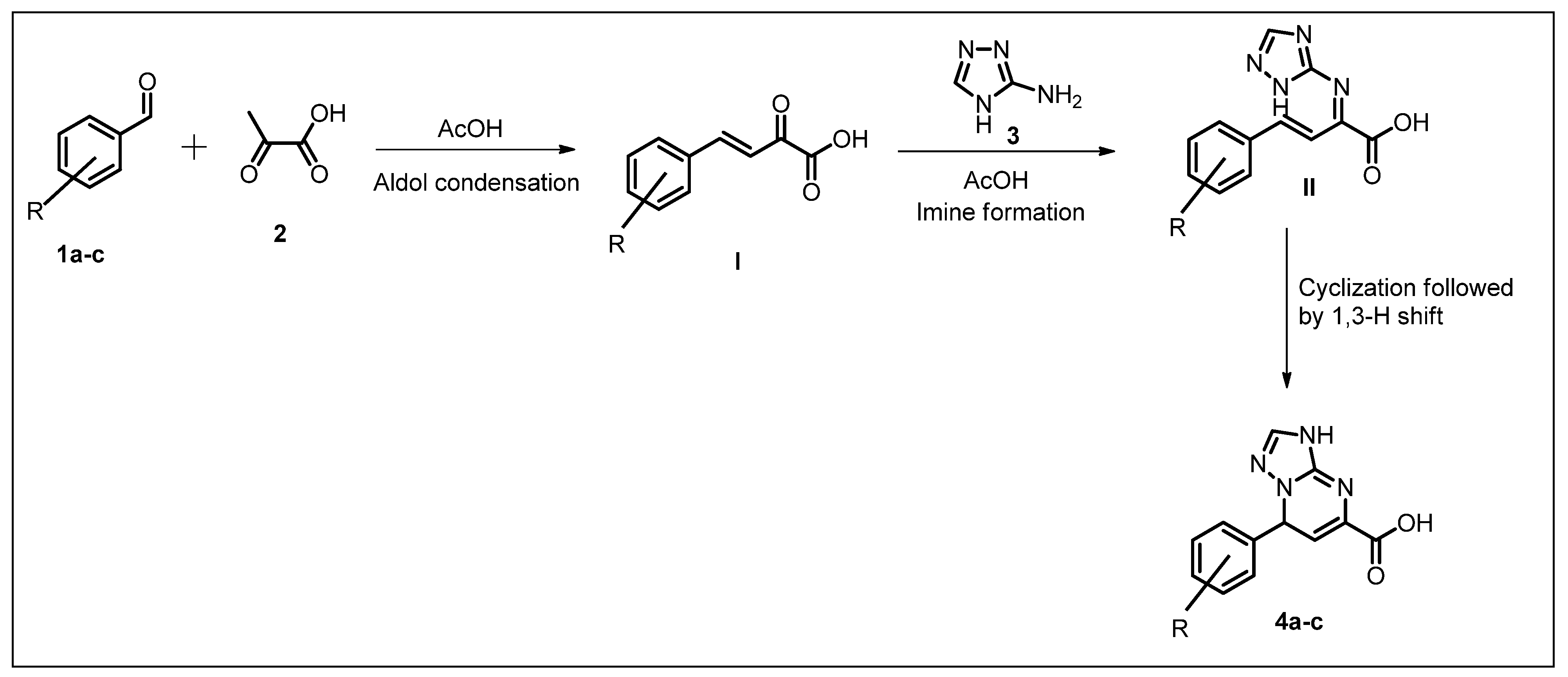
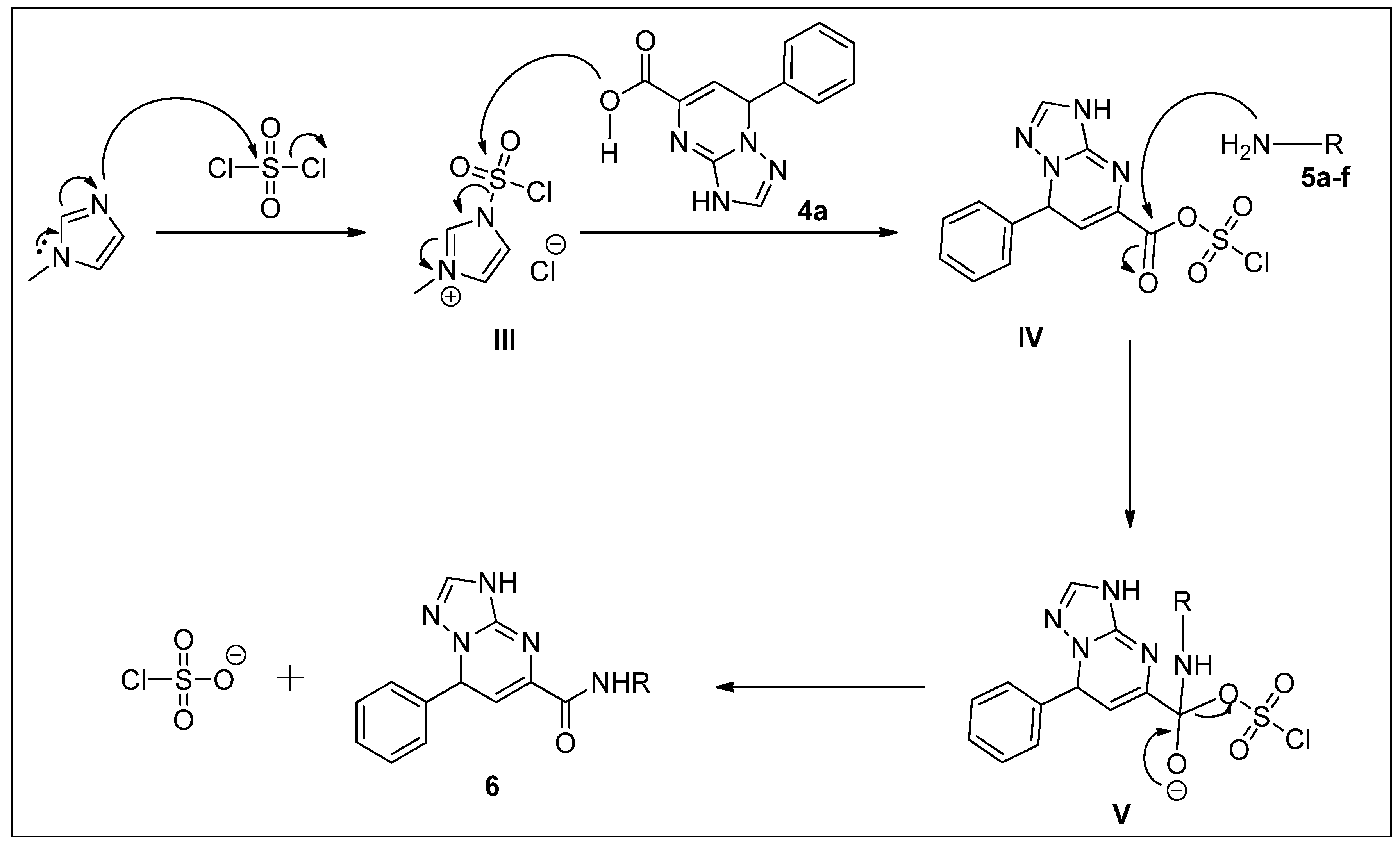
2.1. Chemistry
2.2. Biological Activity of Synthesized Compounds
2.2.1. Anti-Inflammatory Activity
2.2.2. Anti-Tubercular Activity Studies
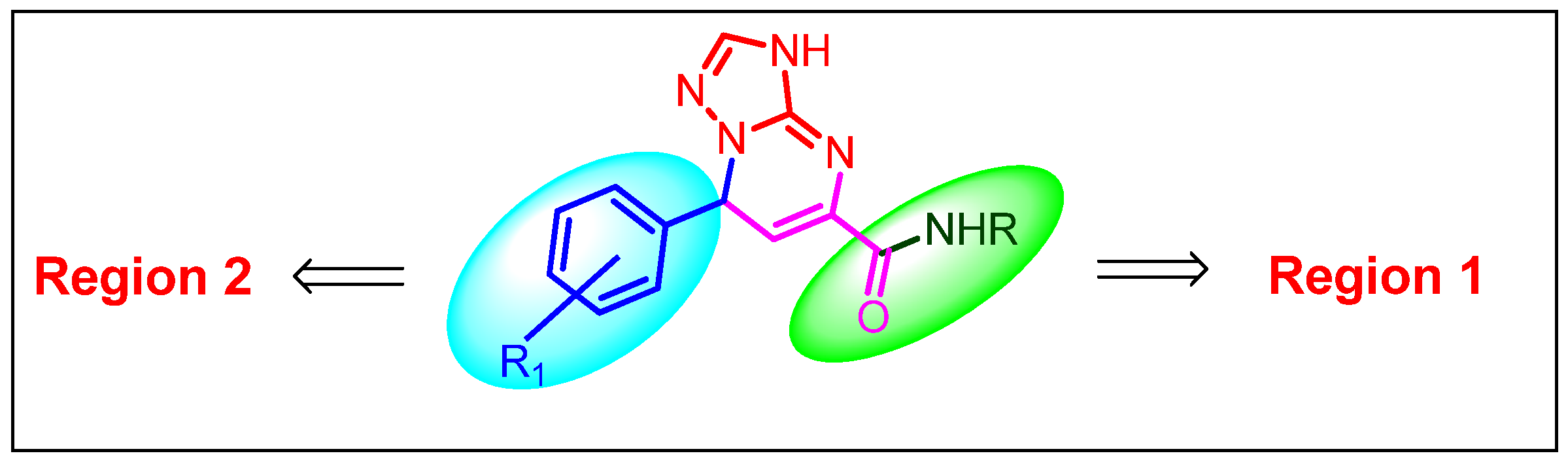
2.2.3. SAR Studies
3. Materials and Methods
3.1. General Considerations
3.2. Experimental Section
3.2.1. General Procedure for the Synthesis of Acid Intermediates 4a–c
3.2.2. General Procedure for the Synthesis of the Final Compounds
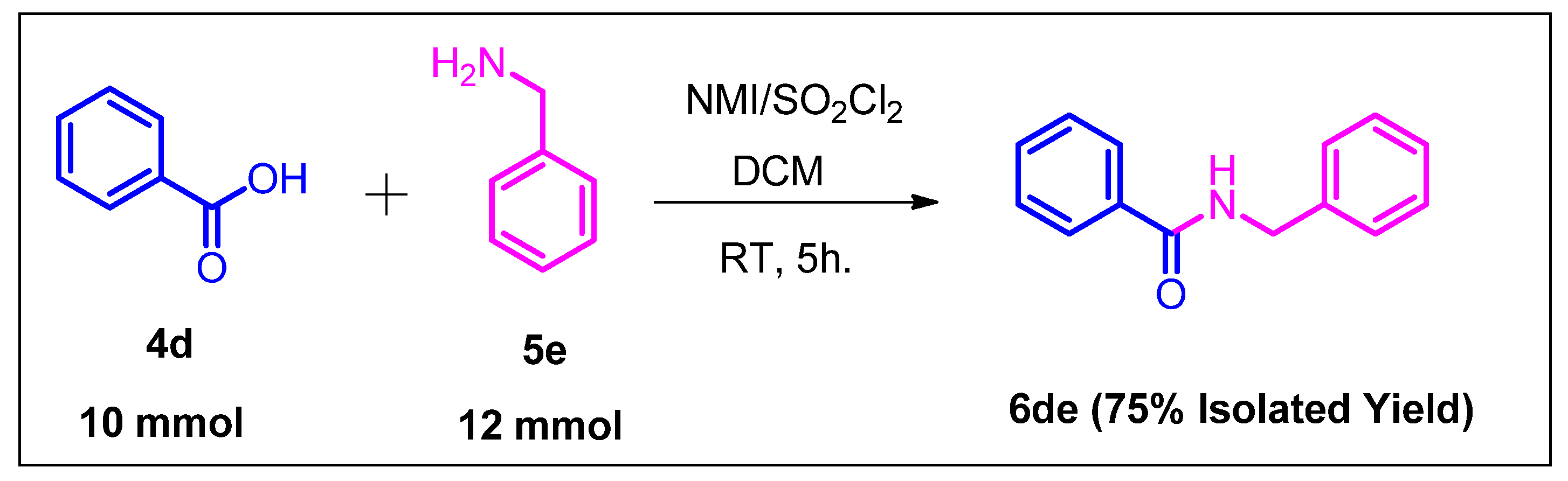
3.2.3. General Procedure for the Gram-Scale Reaction
3.2.4. Procedure for Determining In Vitro Anti-Inflammatory Activity: Anti-Denaturation Assay
3.2.5. Procedure for Determining Anti-Tubercular Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romdhane, A.; Said, A.B.; Cherif, M.; Jannet, H.B. Design, synthesis and anti-acetylcholinesterase evaluation of some new pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives. Med. Chem. Res. 2016, 25, 1358–1368. [Google Scholar] [CrossRef]
- Singh, P.K.; Silakari, O. Molecular dynamics guided development of indole based dual inhibitors of EGFR (T790M) and c-MET. Bioorg. Chem. 2018, 79, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Silakari, O. In silico guided development of imine-based inhibitors for resistance-deriving kinases. J. Biomol. Struct. Dyn. 2019, 37, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Bakulev, V.A.; Shafran, Y.M.; Beliaev, N.A.; Beryozkina, T.V.; Volkova, N.N.; Joy, M.N.; Fan, Z. Heterocyclic azides: Advances in their chemistry. Russ. Chem. Rev. 2022, 91, RCR5042. [Google Scholar] [CrossRef]
- Singh, P.K.; Choudhary, S.; Kashyap, A.; Verma, H.; Kapil, S.; Kumar, M.; Arora, M.; Silakari, O. An exhaustive compilation on chemistry of triazolopyrimidine: A journey through decades. Bioorg. Chem. 2019, 88, 102919. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, W.A.; Ali, O.M.; Faheem, M.S.; Zied, I.F.; Abdel-Rahman, A.A.H. Synthesis and antimicrobial activity of new 1,2,3-triazolopyrimidine derivatives and their glycoside and acyclic nucleoside analogs. J. Heterocycl. Chem. 2012, 49, 607–612. [Google Scholar] [CrossRef]
- Nettekoven, M.; Adam, J.M.; Bendels, S.; Bissantz, C.; Fingerle, J.; Grether, U.; Grüner, S.; Guba, W.; Kimbara, A.; Ottaviani, G.; et al. Novel triazolopyrimidine-derived cannabinoid receptor 2 agonists as potential treatment for inflammatory kidney diseases. ChemMedChem 2016, 11, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Coteron, J.M.; Marco, M.; Esquivias, J.; Deng, X.; White, K.L.; White, J.; Koltun, M.; Mazouni, F.E.; Kokkonda, S.; Katneni, K.; et al. Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J. Med. Chem. 2011, 54, 5540–5561. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; El-Sherbeny, M.A.; El-Ashmawy, M.B.; Bayomi, S.M.; Maarouf, A.R.; Badria, F.A. Synthesis and antitumor testing of certain new fused triazolopyrimidine and triazoloquinazoline derivatives. Arab. J. Chem. 2017, 10, S1345–S1355. [Google Scholar] [CrossRef]
- Omar, A.M.; Razik, H.A.A.E.; Hazzaa, A.A.; El-Attar, M.A.; Demellawy, M.A.E.; Wahab, A.E.A.; Hawash, S.A.M.E. New pyrimidines and triazolopyrimidines as antiproliferative and antioxidants with cyclooxygenase-1/2 inhibitory potential. Future Med. Chem. 2019, 11, 1583–1603. [Google Scholar] [CrossRef]
- Kumar, J.; Meena, P.; Singh, A.; Jameel, E.; Maqbool, M.; Mobashir, M.; Shandilya, A.; Tiwari, M.; Hoda, N.; Jayaram, B. Synthesis and screening of triazolopyrimidine scaffold as multi-functional agents for Alzheimer′s disease therapies. Eur. J. Med. Chem. 2016, 119, 260–277. [Google Scholar] [CrossRef]
- Kumar, J.; Gill, A.; Shaikh, M.; Singh, A.; Shandilya, A.; Jameel, E.; Sharma, N.; Mrinal, N.; Hoda, N.; Jayaram, B. Pyrimidine-triazolopyrimidine and pyrimidine-pyridine hybrids as potential acetylcholinesterase Inhibitors for Alzheimer′s disease. Chemistryselect 2018, 3, 736–747. [Google Scholar] [CrossRef]
- Jameel, E.; Meena, P.; Maqbool, M.; Kumar, J.; Ahmed, W.; Mumtazuddin, S.; Tiwari, M.; Hoda, N.; Jayaram, B. Rational design, synthesis and biological screening of triazine-triazolopyrimidine hybrids as multitarget anti-Alzheimer agents. Eur. J. Med. Chem. 2017, 136, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Rishikesan, R.; Karuvalam, R.P.; Joy, M.N.; Sajith, A.M.; Reddy, E.K.; Pakkath, R.; Haridas, K.R.; Bhaskar, V.; Narasimhamurthy, K.H.; Muralidharan, A. Synthesis of some novel piperidine fused 5-thioxo-1H-1,2,4-triazoles as potential antimicrobial and antitubercular agents. J. Chem. Sci. 2017, 133, 3. [Google Scholar] [CrossRef]
- Gupta, O.; Pradhan, T.; Chawla, G. An updated review on diverse range of biological activities of 1,2,4-triazole derivatives: Insight into structure activity relationship. J. Mol. Struct. 2023, 1274, 134487. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Koksal, M.; Ozkan-Dagliyan, I.; Ozyazici, T.; Kadioglu, B.; Sipahi, H.; Bozkurt, A.; Bilge, S.S. Some novel Mannich bases of 5-(3,4-dichlorophenyl)-1,3,4-oxadiazole-2(3H)-one and their anti-inflammatory activity. Arch. Pharm. 2017, 350, e1700153. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Ambreen, N.; Mughal, U.R.; Jalil, S.; Perveen, S.; Choudhary, M.I. 3-Formylchromones: Potential antiinflammatory agents. Eur. J. Med. Chem. 2010, 45, 4058–4064. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, M.; Jia, X.; Liu, N.; Li, X.; Li, X.; Song, L.; Wang, X.; Qiu, L.; Yu, Y. Combined effects of electrical current and nonsteroidal antiinflammatory drugs (NSAIDs) on microbial community in a three-dimensional electrode biological aerated filter (3DE-BAF). Bioresour. Technol. 2020, 309, 123346. [Google Scholar] [CrossRef]
- Glomb, T.; Wiatrak, B.; Gebczak, K.; Gebarowski, T.; Bodetko, D.; Czyznikowska, Z.; Swiatek, P. New 1,3,4-oxadiazole derivatives of pyridothiazine-1,1-dioxide with anti-inflammatory activity. Int. J. Mol. Sci. 2020, 21, 9122. [Google Scholar] [CrossRef] [PubMed]
- Matteo, Z.; Anna, S.D.; Dennis, F.; Wayne van, G.; Abigail, W.; Armand van, D.; Françoise, P.; Adalbert, L.; Marcos, A.E.; Ariel, P.M.; et al. Twenty years of global surveillance of antituberculosis-drug resistance. N. Engl. J. Med. 2016, 375, 1081–1089. [Google Scholar]
- Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Norton, P.P. Drug resistance: A growing problem. Drug Discov. Today 2010, 15, 583–586. [Google Scholar]
- Sanna, P.; Carta, A.; Nikookar, M.E. Synthesis and antitubercular activity of 3-aryl substituted-2-(1H(2H)benzotriazol-1(2)-yl)acrylonitriles. Eur. J. Med. Chem. 2000, 35, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.N.; Bakulev, V.A.; Bodke, Y.D.; Telkar, S. Synthesis of coumarins coupled with benzamides as potent antimicrobial agents. Pharm. Chem. J. 2020, 54, 604–621. [Google Scholar] [CrossRef]
- Joy, M.N.; Guda, M.R.; Zyryanov, G.V. Evaluation of anti-inflammatory and anti-tubercular activity of 4-methyl-7-substituted coumarin hybrids and their structure activity relationships. Pharmaceuticals 2023, 16, 1326. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, M.A.; Naseem, M.; Joy, M.N.; Sunil, K.; Sajith, A.M.; Howari, F.; Nazzal, Y.; Xavier, C.; Alshammari, M.B.; Haridas, K.R. Application of NMI-TfCl mediated amide bond formation in the synthesis of biologically relevant oxadiazole derivatives employing less basic (hetero)aryl amines. Mol. Divers. 2022, 26, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.N.; Bodke, Y.D.; Telkar, S.; Bakulev, V.A. Synthesis of coumarins linked with 1,2,3-triazoles under microwave irradiation and evaluation of their antimicrobial and antioxidant activity. J. Mex. Chem. Soc. 2020, 64, 53–73. [Google Scholar]
- Reddy, E.K.; Remya, C.; Sajith, A.M.; Dileep, K.V.; Sadasivan, C.; Anwar, S. Functionalised dihydroazo pyrimidine derivatives from Morita–Baylis–Hillman acetates: Synthesis and studies against acetylcholinesterase as its inhibitors. RSC Adv. 2016, 6, 77431–77439. [Google Scholar] [CrossRef]
- Priya, M.G.R.; Girija, K.; Ravichandran, N. Invitro study of anti-inflammatory and antioxidant activity of 4-(3H)-quinazolinone derivatives. Rasayan J. Chem. 2011, 4, 418–424. [Google Scholar]
- Neetu, K.T.; Jaya, S.T. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2007, 60, 288–293. [Google Scholar]
- Parker, M.A.; Kurrasch, D.M.; Nichols, D.E. The role of lipophilicity in determining binding affinity and functional activity for 5-HT2A receptor ligands. Bioorg. Med. Chem. 2008, 16, 4661–4669. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Metro, T.S.; Bonnamour, J.; Reidon, T.; Duprez, A.; Sarpoulet, J.; Martinez, J.; Lamaty, F. Comprehensive study of the organic-solvent-free CDI-mediated acylation of various nucleophiles by mechanochemistry. Chem. Eur. J. 2015, 21, 12787–12796. [Google Scholar] [CrossRef]
- Kulkarni, B.; Manjunatha, K.; Joy, M.N.; Sajith, A.M.; Prashantha, C.N.; Pakkath, R.; Alshammari, M.B. Design, synthesis and molecular docking studies of some 1-(5-(2-fluoro-5-(trifluoromethoxy)phenyl)-1,2,4-oxadiazol-3-yl)piperazine derivatives as potential anti-inflammatory agents. Mol. Divers. 2022, 26, 2893–2905. [Google Scholar] [CrossRef]
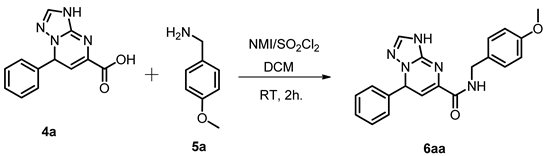 | |||||
|---|---|---|---|---|---|
| Entry | Acid (Equiv.) | Amine (Equiv.) | NMI (Equiv.) | SO2Cl2 (Equiv.) | Yield b 6aa (%) |
| 1 | 1 | 1 | 2 | 2 | 80 |
| 2 c | 1 | 1.2 | 2 | 2 | 92 |
| 3 | 1.2 | 1 | 2 | 2 | 80 |
| 4 | 1 | 1.2 | 1 | 1 | 75 |
| Deviation from the above standard conditions (entry 2) | |||||
| 5 | EDC HCl and HOBT instead of NMI-SO2Cl2 | 65 | |||
| 6 | DIPEA and HATU instead of NMI- SO2Cl2 | 70 | |||
| 7 | TEA instead of NMI | 40 | |||
| 8 | T3P instead of SO2Cl2 | 50 | |||
| 9 | TCFH instead of SO2Cl2 | 75 | |||
| 10 | TfCl instead of SO2Cl2 | 82 | |||
| 11 | MsCl instead of SO2Cl2 | 80 | |||
| 12 | DMF instead of DCM | 70 | |||
| 13 | THF instead of DCM | 60 | |||
| Compounds | % Inhibition of Denaturation at Different Concentrations | ||||
|---|---|---|---|---|---|
| 100 μg/mL | 200 μg/mL | 400 μg/mL | 800 μg/mL | 1600 μg/mL | |
| 6aa | 40 ± 0.65 | 52 ± 0.79 | 62 ± 1.45 | 74 ± 0.59 | 88 ± 0.38 |
| 6ab | 20 ± 0.19 | 32 ± 0.23 | 45 ± 0.36 | 58 ± 0.85 | 70 ± 1.14 |
| 6ac | 37 ± 0.49 | 49 ± 0.54 | 60 ± 1.10 | 76 ± 1.04 | 84 ± 0.85 |
| 6ad | 22 ± 0.49 | 33 ± 1.23 | 44 ± 0.47 | 60 ± 0.67 | 72 ± 0.84 |
| 6af | 25 ± 0.27 | 38 ± 0.28 | 49 ± 1.01 | 62 ± 0.08 | 75 ± 0.66 |
| 6ag | 18 ± 0.21 | 29 ± 0.68 | 40 ± 0.35 | 52 ± 0.58 | 65 ± 0.93 |
| 6ai | 8 ± 0.45 | 20 ± 0.45 | 33 ± 1.12 | 46 ± 0.16 | 57 ± 0.17 |
| 6bb | 21 ± 0.47 | 32 ± 0.54 | 44 ± 0.44 | 59 ± 0.55 | 72 ± 1.06 |
| 6bc | 35 ± 1.22 | 48 ± 1.06 | 60 ± 1.04 | 71 ± 0.63 | 82 ± 1.08 |
| 6be | 44 ± 0.49 | 55 ± 0.18 | 67 ± 0.76 | 80 ± 0.34 | 94 ± 0.48 |
| 6bf | 24 ± 1.22 | 39 ± 0.75 | 50 ± 0.85 | 61 ± 1.12 | 70 ± 1.42 |
| 6bg | 36 ± 0.76 | 47 ± 1.12 | 59 ± 0.45 | 70 ± 0.26 | 84 ± 1.14 |
| 6bh | 23 ± 0.74 | 32 ± 0.58 | 40 ± 0.77 | 53 ± 1.02 | 69 ± 0.92 |
| 6bi | 17 ± 0.18 | 27 ± 0.85 | 39 ± 0.64 | 50 ± 1.15 | 61 ± 1.36 |
| 6cb | 8 ± 0.46 | 19 ± 0.18 | 30 ± 0.05 | 43 ± 1.03 | 55 ± 0.05 |
| 6cc | 6 ± 1.05 | 17 ± 1.16 | 26 ± 0.65 | 38 ± 0.12 | 50 ± 0.45 |
| 6ce | 10 ± 1.11 | 25 ± 1.12 | 35 ± 0.14 | 47 ± 0.04 | 59 ± 0.12 |
| 6cf | 11 ± 1.05 | 23 ± 0.17 | 36 ± 0.65 | 48 ± 0.02 | 56 ± 1.01 |
| 6cg | 20 ± 0.67 | 33 ± 0.69 | 45 ± 0.88 | 58 ± 0.47 | 70 ± 0.30 |
| 6ch | 14 ± 0.14 | 24 ± 0.44 | 36 ± 1.09 | 50 ± 0.51 | 63± 0.14 |
| 6ci | 12 ± 0.56 | 25 ± 0.15 | 38 ± 1.54 | 51 ± 1.14 | 60 ± 0.17 |
| Diclofenac | 41 ± 0.16 | 57 ± 0.48 | 80 ± 0.84 | 86 ± 1.02 | 89 ± 1.06 |
| Preliminary In Vitro Screening Results, MIC (μg/mL) | Second-Level Screening Results, MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | MTB a | MS b | MF c | % d | MTB | MS | MF | MDR-TB |
| 6aa | >100 | >100 | >100 | 0 | - | - | - | - |
| 6ab | 1 ± 0.20 | 10 ± 0.14 | 10 ± 0.35 | 90 | 1.25 ± 0.38 | 2.5 ± 0.16 | >5 | 12.5 ± 0.28 |
| 6ac | >100 | >100 | >100 | 0 | - | - | - | - |
| 6ad | 10 ± 0.14 | 10 ± 0.23 | >100 | <90 | >5 | >5 | >5 | >50 |
| 6af | 10 ± 0.28 | 10 ± 0.22 | 10 ± 0.12 | <90 | >5 | >5 | >5 | >50 |
| 6ag | >100 | >100 | >100 | 0 | - | - | - | - |
| 6ai | >100 | >100 | >100 | 0 | - | - | - | - |
| 6bb | 10 ± 0.45 | 10 ± 0.80 | >100 | <90 | >5 | - | >5 | >50 |
| 6bc | >100 | >100 | >100 | 0 | - | - | - | - |
| 6be | >100 | >100 | >100 | 0 | - | - | - | - |
| 6bf | >100 | >100 | >100 | 0 | - | - | - | - |
| 6bg | >100 | >100 | >100 | 0 | - | - | - | - |
| 6bh | 10 ± 0.32 | 10 ± 0.04 | 10 ± 0.28 | 10± 0.30 | 10 ± 0.45 | 10 ± 0.36 | 10 ± 0.20 | 10 ± 0.55 |
| 6bi | >100 | >100 | >100 | 0 | - | - | - | - |
| 6cb | 1 ± 0.18 | 1 ± 0.29 | 10 ± 0.45 | 95 | 0.625 ± 0.11 | 1.25 ± 0.20 | 5 ± 0.37 | 6.25 ± 0.18 |
| 6cc | 1 ± 0.40 | 10 ± 0.14 | 1 ± 0.35 | 90 | 1.25 ± 0.46 | 1.25 ± 0.20 | >5 | 12.5 ± 0.28 |
| 6ce | 1 ± 0.40 | 1 ± 0.36 | 10 ± 0.55 | 90 | >5 | 1.25 ± 0.20 | >5 | 25 ± 0.26 |
| 6cf | 1 ± 0.38 | 10 ± 0.14 | 1 ± 0.35 | 90 | 1.25 ± 0.14 | 1.25 ± 0.40 | >5 | 12.5 ± 0.62 |
| 6cg | 1 ± 0.20 | 10 ± 0.31 | 1 ± 0.44 | 90 | 1.25 ± 0.24 | 1.25 ± 0.25 | >5 | 12.5 ± 0.29 |
| 6ch | 1 ± 0.15 | 1 ± 0.25 | 10 ± 0.35 | 95 | 0.625 ± 0.07 | 1.25 ± 0.11 | 5 ± 0.27 | 6.25 ± 0.07 |
| 6ci | 1 ± 0.39 | 1 ± 0.24 | 10 ± 0.39 | 90 | >5 | 1.25 ± 0.31 | >5 | 25 ± 0.42 |
| Isoniazid | 0.7 ± 0.05 | 50 ± 0.25 | 12.5 ± 0.34 | 95 | 0.7 ± 0.08 | 50 ± 0.21 | 12.5 ± 0.18 | 12.5 ± 0.20 |
| Rifampicin | 0.5 ± 0.35 | 1.5 ± 0.38 | 1.5 ± 0.34 | 95 | 0.5 ± 0.41 | 1.5 ± 0.16 | 1.5 ± 0.22 | 25 ± 0.21 |
| Compounds | LogP | CLogP |
|---|---|---|
| 6aa | 2.30 | 1.7159 |
| 6ab | 2.58 | 1.9399 |
| 6ac | 2.74 | 2.1059 |
| 6ad | 0.69 | 0.9292 |
| 6af | 0.91 | 0.6929 |
| 6ag | 2.93 | 3.1377 |
| 6ai | 2.24 | 2.1239 |
| 6bb | 2.45 | 1.9840 |
| 6bc | 2.61 | 2.1500 |
| 6be | 2.30 | 1.8410 |
| 6bf | 0.78 | 0.7370 |
| 6bg | 2.80 | 3.0567 |
| 6bh | 2.78 | 2.7994 |
| 6bi | 2.11 | 2.1680 |
| 6cb | 3.57 | 2.9459 |
| 6cc | 3.73 | 3.1119 |
| 6ce | 3.41 | 2.9280 |
| 6cf | 1.89 | 1.8240 |
| 6cg | 3.91 | 4.1437 |
| 6ch | 3.90 | 3.8864 |
| 6ci | 3.22 | 3.2550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babu, A.; Sunil, K.; Sajith, A.M.; Reddy, E.K.; Santra, S.; Zyryanov, G.V.; Venkatesh, T.; Bhadrachari, S.; Nibin Joy, M. NMI-SO2Cl2-Mediated Amide Bond Formation: Facile Synthesis of Some Dihydrotriazolopyrimidine Amide Derivatives as Potential Anti-Inflammatory and Anti-Tubercular Agents. Pharmaceuticals 2024, 17, 548. https://doi.org/10.3390/ph17050548
Babu A, Sunil K, Sajith AM, Reddy EK, Santra S, Zyryanov GV, Venkatesh T, Bhadrachari S, Nibin Joy M. NMI-SO2Cl2-Mediated Amide Bond Formation: Facile Synthesis of Some Dihydrotriazolopyrimidine Amide Derivatives as Potential Anti-Inflammatory and Anti-Tubercular Agents. Pharmaceuticals. 2024; 17(5):548. https://doi.org/10.3390/ph17050548
Chicago/Turabian StyleBabu, Aravinda, Kenchaiah Sunil, Ayyiliath Meleveetil Sajith, Eeda Koti Reddy, Sougata Santra, Grigory V. Zyryanov, Talavara Venkatesh, Somashekara Bhadrachari, and Muthipeedika Nibin Joy. 2024. "NMI-SO2Cl2-Mediated Amide Bond Formation: Facile Synthesis of Some Dihydrotriazolopyrimidine Amide Derivatives as Potential Anti-Inflammatory and Anti-Tubercular Agents" Pharmaceuticals 17, no. 5: 548. https://doi.org/10.3390/ph17050548
APA StyleBabu, A., Sunil, K., Sajith, A. M., Reddy, E. K., Santra, S., Zyryanov, G. V., Venkatesh, T., Bhadrachari, S., & Nibin Joy, M. (2024). NMI-SO2Cl2-Mediated Amide Bond Formation: Facile Synthesis of Some Dihydrotriazolopyrimidine Amide Derivatives as Potential Anti-Inflammatory and Anti-Tubercular Agents. Pharmaceuticals, 17(5), 548. https://doi.org/10.3390/ph17050548





