Design, Synthesis and Biological Assessment of N′-(2-Oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazides as Potential Anti-Proliferative Agents toward MCF-7 Breast Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anticancer Activity
2.2.1. Cytotoxicity of the Designed Compounds 6a–l and 8a–g on MCF-7 and MCF-10A
2.2.2. SARs of Compounds 6a–l and 8a–g on MCF-7 Cell Line
2.2.3. VGEFR-2 Inhibitory Activity (IC50) for Compounds 6b, 6i and 6j
2.2.4. PCR Assessment for Compound 6i
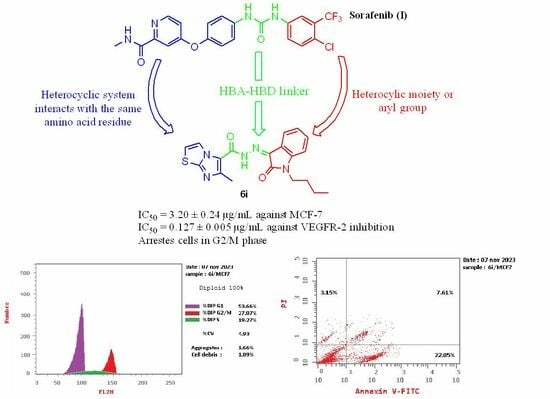
2.2.5. Cell Cycle Analysis of Compound 6i on MCF-7
2.2.6. Apoptotic Assay for Compound 6i
2.3. Molecular Modeling Study
2.4. Computational ADME Study
3. Materials and Methods
3.1. Chemistry
3.1.1. Preparation of Carbohydrazide 4
3.1.2. Synthesis of Hybrids 6a–l
6-Methyl-N′-(2-oxoindolin-3-ylidene)imidazo[2,1-b]thiazole-5-carbohydrazide (6a)
N′-(5-Fluoro-2-oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6b)
N′-(5-Chloro-2-oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6c)
N′-(5-Bromo-2-oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6d)
6-Methyl-N′-(2-oxo-1-propylindolin-3-ylidene)imidazo[2,1-b]thiazole-5-carbohydrazide (6e)
N′-(5-Fluoro-2-oxo-1-propylindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6f)
N′-(5-Chloro-2-oxo-1-propylindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6g)
N′-(5-Bromo-2-oxo-1-propylindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6h)
N′-(1-Butyl-2-oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6i)
N′-(1-Butyl-5-fluoro-2-oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6j)
N′-(1-Butyl-5-chloro-2-oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6k)
N′-(5-Romo-1-butyl-2-oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazide (6l)
3.1.3. Synthesis of Urea Derivatives 8a–g
1-(6-Methylimidazo[2,1-b]thiazol-5-yl)-3-phenylurea (8a)
1-(6-Methylimidazo[2,1-b]thiazol-5-yl)-3-(p-tolyl)urea (8b)
1-(4-Methoxyphenyl)-3-(6-methylimidazo[2,1-b]thiazol-5-yl)urea (8c)
1-(4-Fluorophenyl)-3-(6-methylimidazo[2,1-b]thiazol-5-yl)urea (8d)
1-(4-Chlorophenyl)-3-(6-methylimidazo[2,1-b]thiazol-5-yl)urea (8e)
1-(4-Bromophenyl)-3-(6-methylimidazo[2,1-b]thiazol-5-yl)urea (8f)
4-(3-(6-Methylimidazo[2,1-b]thiazol-5-yl)ureido)benzenesulfonamide (8g)
3.2. Anticancer Activity
3.2.1. MTT Assay
3.2.2. VEGFR-2 Kinase Activity
3.2.3. PCR Assay
3.2.4. Cell Cycle Analysis and Apoptosis
3.3. Docking Protocol
3.4. In Silico Predictive ADME Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Pincheira, J. Invited Review Cell proliferation and cancer. Histol. Histopathol. 1998, 13, 1197–1214. [Google Scholar]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Karamysheva, A.F. Mechanisms of angiogenesis. Biochemistry 2008, 73, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Tumor angiogenesis: Past, present and the near future. Carcinogenesis 2000, 21, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Traxler, P. Tyrosine kinases as targets in cancer therapy—Successes and failures. Expert Opin. Ther. Targets 2003, 7, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Weich, H.A.; Brokelmann, M.; Horiguchi, S.; Funata, N.; Ogawa, T.; Toi, M. Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br. J. Cancer 2005, 92, 553–561. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, W. The emerging regulation of VEGFR-2 in triple-negative breast cancer. Front. Endocrinol. 2015, 6, 159. [Google Scholar] [CrossRef]
- Shiau, J.P.; Wu, C.C.; Chang, S.J.; Pan, M.R.; Liu, W.; Ou-Yang, F.; Chen, F.M.; Hou, M.F.; Shih, S.L.; Luo, C.W. FAK regulates VEGFR2 expression and promotes angiogenesis in triple-negative breast cancer. Biomedicines 2021, 9, 1789. [Google Scholar] [CrossRef]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 2007, 19, 2003–2012. [Google Scholar] [CrossRef]
- Narayanan, J.; Tamilanban, T.; Kumar, P.S.; Guru, A.; Muthupandian, S.; Kathiravan, M.K.; Arockiaraj, J. Role and mechanistic actions of protein kinase inhibitors as an effective drug target for cancer and COVID. Arch. Microbiol. 2023, 205, 238. [Google Scholar] [CrossRef]
- Musumeci, F.; Radi, M.; Brullo, C.; Schenone, S. Vascular endothelial growth factor (VEGF) receptors: Drugs and new inhibitors. J. Med. Chem. 2012, 55, 10797–10822. [Google Scholar] [CrossRef]
- El Hadi, S.R.A.; Lasheen, D.S.; Soliman, D.H.; Elrazaz, E.Z.; Abouzid, K.A.M. Scaffold hopping and redesign approaches for quinazoline based urea derivatives as potent VEGFR-2 inhibitors. Bioorg. Chem. 2020, 101, 103961. [Google Scholar] [CrossRef]
- Fodor, D.; Jung, I.; Turdean, S.; Satala, C.; Gurzu, S. Angiogenesis of hepatocellular carcinoma: An immunohistochemistry study. World J. Hepatol. 2019, 11, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.W.; Liu, D.K.; Zhang, Q.W.; Xu, Y.G.; Shi, L. VEGFR-2 inhibitors and the therapeutic applications thereof: A patent review (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- Oguro, Y.; Miyamoto, N.; Okada, K.; Takagi, T.; Iwata, H.; Awazu, Y.; Miki, H.; Hori, A.; Kamiyama, K.; Imamura, S. Design, synthesis, and evaluation of 5-methyl-4-phenoxy-5H-pyrrolo[3,2-d] pyrimidine derivatives: Novel VEGFR2 kinase inhibitors binding to inactive kinase conformation. Bioorg. Med. Chem. 2010, 18, 7260–7273. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Serya, R.A.T.; Lasheen, D.S.; Abdel-Aziz, A.K.; Esmat, A.; Mansour, A.M.; Singab, A.N.; Abouzid, K. Discovery of Potent VEGFR-2 Inhibitors based on Furopyrimidine and Thienopyrimidne Scaffolds as Cancer Targeting Agents. Sci. Rep. 2016, 6, 24460. [Google Scholar] [CrossRef]
- Dweedar, H.E.; Mahrous, H.; Ibrahim, H.S.; Abdel-Aziz, H.A. Analogue-based design, synthesis and biological evaluation of 3-substituted-(methylenehydrazono)indolin-2-ones as anticancer agents. Eur. J. Med. Chem. 2014, 78, 275–280. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Altoukhy, A.; Mahrous, H.; Abdel-Aziz, H.A. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur. J. Med. Chem. 2015, 90, 684–694. [Google Scholar] [CrossRef]
- Fares, M.; Eldehna, W.M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Aly, M.H.; Tolba, M.F. Design, synthesis and in Vitro antiproliferative activity of novel isatin-quinazoline hybrids. Arch. Pharm. 2015, 348, 144–154. [Google Scholar] [CrossRef]
- Kamal, A.; Dastagiri, D.; Ramaiah, M.J.; Reddy, J.S.; Bharathi, E.V.; Srinivas, C.; Pushpavalli, S.N.; Pal, D.; Pal-Bhadra, M. Synthesis of imidazothiazole-chalcone derivatives as anticancer and apoptosis inducing agents. ChemMedChem 2010, 5, 1937–1947. [Google Scholar] [CrossRef]
- Elsawi, A.E.; Elbadawi, M.M.; Nocentini, A.; Almahli, H.; Giovannuzzi, S.; Shaldam, M.; Salem, R.; Ibrahim, T.M.; Abdel-Aziz, H.A.; Supuran, C.T.; et al. 1,5-diaryl-1,2,4-triazole ureas as new SLC-0111 analogues endowed with dual carbonic anhydrase and VEGFR-2 inhibitory activities. J. Med. Chem. 2023, 66, 10. [Google Scholar] [CrossRef]
- Fakhry, M.M.; Mattar, A.A.; Alsulaimany, M.; Al-Olayan, E.M.; Al-Rashood, S.T.; Abdel-Aziz, H.A. New Thiazolyl-Pyrazoline Derivatives as Potential Dual EGFR/HER2 Inhibitors: Design, Synthesis, Anticancer Activity Evaluation and In Silico Study. Molecules 2023, 28, 7455. [Google Scholar] [CrossRef]
- Abdelsalam, E.A.; Abd El-Hafeez, A.A.; Eldehna, W.M.; El Hassab, M.A.; Marzouk, H.M.M.; Elaasser, M.M.; Abou Taleb, N.A.; Amin, K.M.; Abdel-Aziz, H.A.; Ghosh, P.; et al. Discovery of novel thiazolyl-pyrazolines as dual EGFR and VEGFR-2 inhibitors endowed with in vitro antitumor activity towards non-small lung cancer. J. Enzyme Inhib. Med. Chem. 2022, 37, 2265–2282. [Google Scholar] [CrossRef] [PubMed]
- Elewa, M.A.F.; Eldehna, W.M.; Hamdan, A.M.E.; Abd El-kawi, S.H.; El-Kalaawy, A.M.; Majrashi, T.A.; Barghash, R.F.; Abdel-Aziz, H.A.; Hashem, K.S.; Al-Gayyar, M.M.H. WRH-2412 alleviates the progression of hepatocellular carcinoma through regulation of TGF-β/β-catenin/α-SMA pathway. J. Enzyme Inhib. Med. Chem. 2023, 38, 2185761. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Mohammed, E.E.; Al-Ansary, G.H.; Berrino, E.; Elbadawi, M.M.; Ibrahim, T.M.; Jaballah, M.Y.; Al-Rashood, S.T.; Binjubair, F.A.; Celik, M.; et al. Design and synthesis of 6-arylpyridine-tethered sulfonamides as novel selective inhibitors of carbonic anhydrase IX with promising antitumor features toward the human colorectal cancer. Eur. J. Med. Chem. 2023, 258, 115538. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Alshaye, N.A.; Elrubi, N.; Hamed, E.A.; Omar, A.Z. Pyrimidines-Based Heterocyclic Compounds: Synthesis, Cytoxicity Evaluation and Molecular Docking. Molecules 2022, 27, 4912. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Hulme, C.; Hurley, L.H. The design, synthesis, and evaluation of 8 hybrid DFG-out allosteric kinase inhibitors: A structural analysis of the binding interactions of Gleevec ®, Nexavar®, and BIRB-796. Bioorg. Med. Chem. 2010, 18, 5738–5748. [Google Scholar] [CrossRef]
- Xie, Q.Q.; Xie, H.Z.; Ren, J.X.; Li, L.L.; Yang, S.Y. Pharmacophore modeling studies of type I and type II kinase inhibitors of Tie2. J. Mol. Graph. Model. 2009, 27, 751–758. [Google Scholar] [CrossRef]
- Sobhy, M.K.; Mowafy, S.; Lasheen, D.S.; Farag, N.A.; Abouzid, K.A.M. 3D-QSAR pharmacophore modelling, virtual screening and docking studies for lead discovery of a novel scaffold for VEGFR 2 inhibitors: Design, synthesis and biological evaluation. Bioorg. Chem. 2019, 89, 102988. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.T.; Abdullaziz, M.A.; El Kerdawy, A.M.; Ragab, F.A.F.; Flanagan, K.J.; Mahmoud, A.E.E.; Ali, M.M.; Diwani, H.I.E.; Senge, M.O. Targeting receptor tyrosine kinase VEGFR-2 in hepatocellular cancer: Rational design, synthesis and biological evaluation of 1,2-disubstituted benzimidazoles. Molecules 2020, 25, 770. [Google Scholar] [CrossRef] [PubMed]
- Hyde, C.A.C.; Giese, A.; Stuttfeld, E.; Abram Saliba, J.; Villemagne, D.; Schleier, T.; Binz, H.K.; Ballmer-Hofer, K. Targeting Extracellular Domains D4 and D7 of Vascular Endothelial Growth Factor Receptor 2 Reveals Allosteric Receptor Regulatory Sites. Mol. Cell Biol. 2012, 32, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.J.; Kulkarni, V.M. Exploration of structural requirements for the inhibition of VEGFR-2 tyrosine kinase: Binding site analysis of type II, ‘DFG-out’ inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 5712–5727. [Google Scholar] [CrossRef] [PubMed]
- El-Adl, K.; Ibrahim, M.K.; Khedr, F.; Abulkhair, H.S.; Eissa, I.H. Design, synthesis, docking, and anticancer evaluations of phthalazines as VEGFR-2 inhibitors. Arch. Pharm. 2022, 355, e2100278. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, Y.C. Transformation of MCF-10A human breast epithelial cells by zeranol and estradiol-17β. Breast J. 2004, 10, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, A.; Zhang, L. An Overview of Scoring Functions Used for Protein–Ligand Interactions in Molecular Docking. Interdiscip. Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Duchowicz, P.R.; Talevi, A.; Bellera, C.; Bruno-Blanch, L.E.; Castro, E.A. Application of descriptors based on Lipinski’s rules in the QSPR study of aqueous solubilities. Bioorg. Med. Chem. 2007, 15, 3711–3719. [Google Scholar] [CrossRef]
- Samala, G.; Devi, P.B.; Saxena, S.; Meda, N.; Yogeeswari, P.; Sriram, D. Design, synthesis and biological evaluation of imidazo[2,1-b]thiazole and benzo[d]imidazo[2,1-b]thiazole derivatives as Mycobacterium tuberculosis pantothenate synthetase inhibitors. Bioorganic Med. Chem. 2016, 24, 1298–1307. [Google Scholar] [CrossRef]
- Cesur, N.; Cesur, Z.; Guner, H. Fused Heterocycles: Synthesis of Some New Imidazothiazoles. Heterocycl. Commun. 2002, 8, 433–438. [Google Scholar] [CrossRef]
- Slater, T.F.; Sawyer, B.; Sträuli, U.; van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J. Studies on succinate-tetrazolium reductase systems. III. Points of coupling of four different tetrazolium salts III. Points of coupling of four different tetrazolium salts. J. Immunol. Methods 1994, 77, 311–320. [Google Scholar]
- van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M.A.C. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.; Ongaro, E.; Bolzonello, S.; Guardascione, M.; Fasola, G.; Aprile, G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann. Transl. Med. 2014, 2, 123. [Google Scholar] [PubMed]
- Sharma, K.; Suresh, P.S.; Mullangi, R.; Srinivas, N.R. Quantitation of VEGFR2 (vascular endothelial growth factor receptor) inhibitors—Review of assay methodologies and perspectives. Biomed. Chromatogr. 2015, 29, 803–834. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z. Critical aspects in analysis of cellular DNA content. Curr. Protoc. Cytom. 2011, 7, 7.2.1–7.2.8. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]








 | |||
| Compound | R1 | R2 | IC50 (µM) |
| 6a | H | H | 23.97 ± 0.67 |
| 6b | H | F | 11.50 ± 0.52 |
| 6c | H | Cl | 43.10 ± 3.69 |
| 6d | H | Br | 22.80 ± 1.38 |
| 6e | n-propyl | H | 61.39 ± 3.15 |
| 6f | n-propyl | F | 82.55 ± 2.75 |
| 6g | n-propyl | Cl | 52.47 ± 1.26 |
| 6h | n-propyl | Br | 74.34 ± 2.19 |
| 6i | n-butyl | H | 8.38 ± 0.62 |
| 6j | n-butyl | F | 11.67 ± 0.52 |
| 6k | n-butyl | Cl | 42.72 ± 2.01 |
| 6l | n-butyl | Br | 52.55 ± 2.36 |
| 8a | H | – | 109.68 ± 4.62 |
| 8b | CH3 | – | 114.61 ± 4.99 |
| 8c | OCH3 | – | 106.43 ± 5.32 |
| 8d | F | – | 72.33 ± 4.71 |
| 8e | Cl | – | 35.72 ± 3.32 |
| 8f | Br | – | 31.12 ± 2.47 |
| 8g | SO2NH2 | – | 41.57 ± 1.59 |
| Sorafenib (I) | – | – | 7.55 ± 0.40 |
| Compound | IC50 (µM) |
|---|---|
| 6b | 125.26 ± 2.7 |
| 6i | 54.63 ± 1.29 |
| 6j | 47.73 ± 0.53 |
| Sorafenib (I) | 22.35 ± 1.29 |
| Compounds | R1 | R2 | IC50 (µM) |
|---|---|---|---|
| 6b | H | F | 3.85 ± 0.15 |
| 6i | n-butyl | H | 0.33 ± 0.01 |
| 6j | n-butyl | F | 1.51 ± 0.06 |
| Sorafenib (I) | – | – | 0.09 ± 0.004 |
| RT-PCR Fold Change | |||||
|---|---|---|---|---|---|
| Bax | Caspase-8 | Caspase-9 | Cytochrome C | Bcl-2 | |
| 6i/MCF-7 | 4.337 | 2.727 | 4.947 | 2.420 | 0.359 |
| Control MCF-7 | 1 | 1 | 1 | 1 | 1 |
| %G0–G1 | %S | %G2/M | |
|---|---|---|---|
| 6i/MCF-7 | 53.66 | 19.27 | 27.07 |
| Cont. MCF-7 | 64.73 | 23.96 | 11.31 |
| Total | Early | Late | Necrosis | |
|---|---|---|---|---|
| 6i/MCF-7 | 32.81 | 22.05 | 7.61 | 3.15 |
| Cont. MCF-7 | 1.91 | 0.43 | 0.11 | 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaye, N.A.; Elgohary, M.K.; Elkotamy, M.S.; Abdel-Aziz, H.A. Design, Synthesis and Biological Assessment of N′-(2-Oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazides as Potential Anti-Proliferative Agents toward MCF-7 Breast Cancer. Pharmaceuticals 2024, 17, 216. https://doi.org/10.3390/ph17020216
Alshaye NA, Elgohary MK, Elkotamy MS, Abdel-Aziz HA. Design, Synthesis and Biological Assessment of N′-(2-Oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazides as Potential Anti-Proliferative Agents toward MCF-7 Breast Cancer. Pharmaceuticals. 2024; 17(2):216. https://doi.org/10.3390/ph17020216
Chicago/Turabian StyleAlshaye, Najla A., Mohamed K. Elgohary, Mahmoud S. Elkotamy, and Hatem A. Abdel-Aziz. 2024. "Design, Synthesis and Biological Assessment of N′-(2-Oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazides as Potential Anti-Proliferative Agents toward MCF-7 Breast Cancer" Pharmaceuticals 17, no. 2: 216. https://doi.org/10.3390/ph17020216
APA StyleAlshaye, N. A., Elgohary, M. K., Elkotamy, M. S., & Abdel-Aziz, H. A. (2024). Design, Synthesis and Biological Assessment of N′-(2-Oxoindolin-3-ylidene)-6-methylimidazo[2,1-b]thiazole-5-carbohydrazides as Potential Anti-Proliferative Agents toward MCF-7 Breast Cancer. Pharmaceuticals, 17(2), 216. https://doi.org/10.3390/ph17020216