Intestinal Fibrogenesis in Inflammatory Bowel Diseases: Exploring the Potential Role of Gut Microbiota Metabolites as Modulators
Abstract
1. Overview of Inflammatory Bowel Diseases (IBDs)
1.1. Epidemiology and Global Prevalence
1.2. Clinical Manifestations and Disease Course
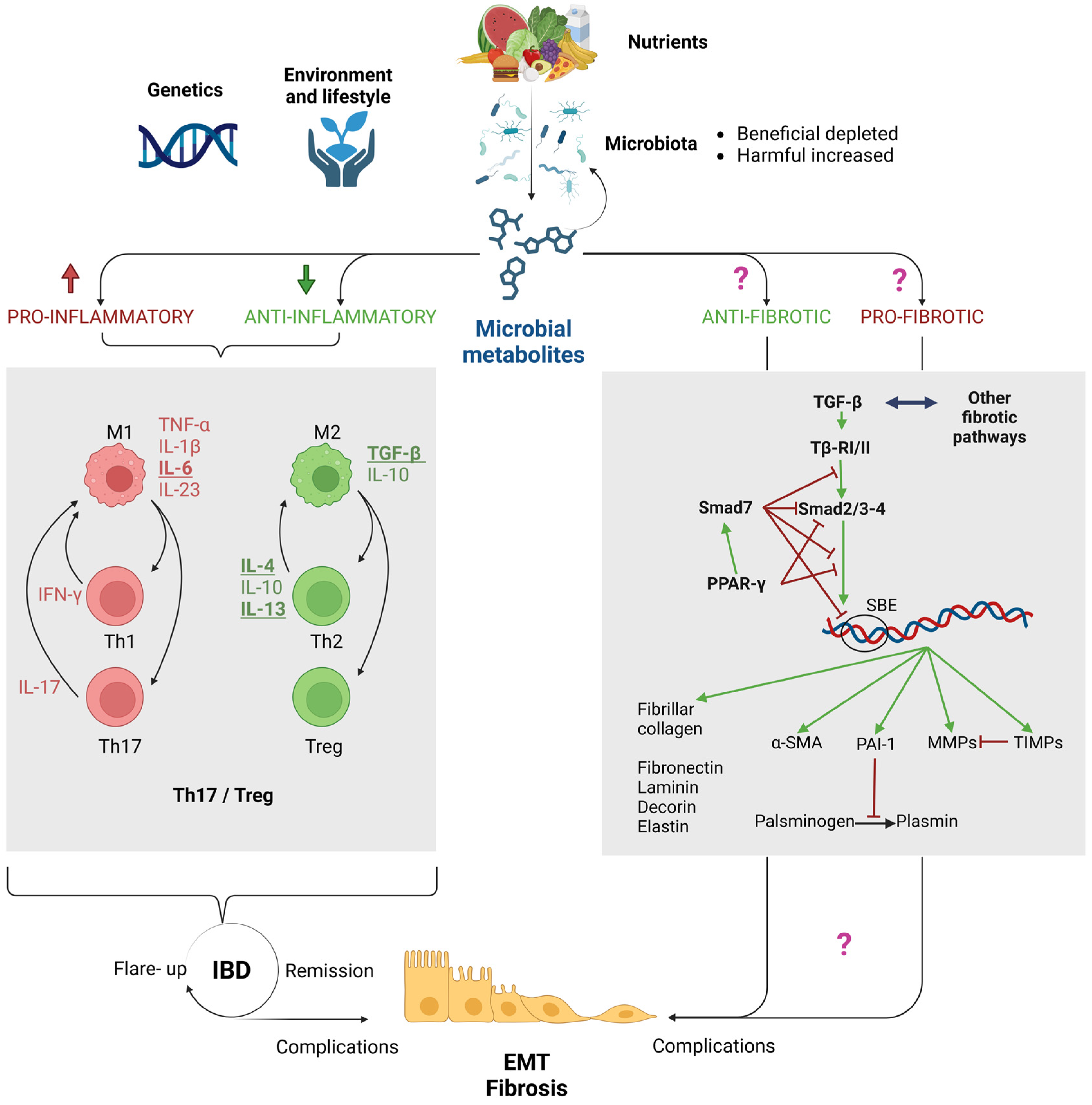
2. Purpose of this Review: Are the Microbiota and Its Metabolites Key Players in Fibrogenesis in IBDs?
3. The Intestinal Barrier in Health and in IBDs
3.1. Dysbiosis: Alterations in the Intestinal Microbiota
3.2. Impairment of the Mucus Layer
3.3. Epithelial Dysfunction and Increased Permeability: The “Leaky Gut”
3.4. Dysregulation of Mucosal Immunity
4. The Role of Immune Dysregulation in Driving Epithelial-to-Mesenchymal Transition (EMT) and Fibrosis in IBDs
5. Microbial Metabolites: Established Players in Intestinal Homeostasis and IBD Inflammation, with Potential Implications in Fibrosis
5.1. Short-Chain Fatty Acids (SCFAs)
5.2. Lactic Acid (LA)
5.3. Indoles
| IAA | IPA | ILA | IS | IC | |
|---|---|---|---|---|---|
| AhR | Kidney: IPA suppresses the IS effect on the receptor [147]. | Gut: Supplementation with L. acidophilus, or its metabolite ILA, attenuates inflammation and restores IL-22 levels through AhR signaling in mice [142]. Similar results were observed in a mice model of DSS-induced colitis supplemented with two strains of ILA-producing B. bifidum [143]. | Liver: IS is an agonist of the AhR receptor [147]. | Gut: depletion of dietary IC is fatal in AhR IEC-deficient mice and worsens chronic colitis in C57BL/6 mice; in contrast, its administration reduces the Th17/Treg ratio in the same model [145,148]. | |
| TGF-β | Peritoneum: the novel IAA analogue MA-35 reduces TGF-β-positive cells in a murine model of peritoneal fibrosis [149]. | Kidney: IPA suppresses the IS effect on the receptor [147]. Liver: IPA aggravates CCl4-induced fibrosis by activating TGF-β1/Smads signaling in HSCs [150]. | Kidney: IS induces fibrosis through the stimulation of TGF-β1 [147]. | ||
| Smads | Kidney: the IAA novel analogue, MA-35, inhibits the phosphorylation of Smad3, thus reducing TGF-β1 signaling and related renal fibrosis [151]. | Liver: IPA aggravates CCl4-induced fibrosis by activating TGF-β1/Smads signaling in HSCs [150]. | |||
| PPAR-γ | Adipocytes: the administration of I3C restores the levels of PPAR-γ, which were deregulated in mice fed with a high-fat diet [146]. | ||||
| ECM | Peritoneum: the treatment with the novel IAA analogue MA-35 reduces α-SMA-positive myofibroblasts in a murine model of peritoneal fibrosis [149]. | Liver: IPA reduces α-SMA and collagen deposition and MMP expression while inducing TIMPs in TGF-β1-stimulated hepatic stellate cells [152]. Liver: IPA aggravates CCl4-induced fibrosis by activating TGF-β1/Smads signaling in HSCs [150]. | Kidney: IS enhances α-SMA expression [147]. | ||
| PXR | Gut: IPA reduces PXR-induced fibrosis in a mice model of colitis; IBD patients showed lower levels of PXR and fecal IPA [68]. |
| QUERY | (“IBD” OR “Gut”) AND (“TGF-Beta” OR “Smad” OR “PPAR-Gamma” OR “Fibrosis” OR “EMT” OR “Alpha-SMA” OR “MMP” OR “PAI-1” OR “TIMP”) | Title and Abstract Check | |
|---|---|---|---|
| AND | |||
| “butyrate” OR “butyric acid” | 83 | 16 | |
| “acetate” OR “acetic acid” | 58 | 4 | |
| “propionate” OR “propionic acid” | 41 | 5 | |
| “lactic acid” | 27 | 1 | |
| “indole-3-acetic acid” | 5 | 1 | |
| “indole-3-carbinol” | 2 | 0 | |
| “indole-3-lactic acid” | 0 | 0 | |
| “indole-3-propionic acid” | 5 | 2 | |
| “indoxyl sulfate” | 1 | 0 | |
| “urolithin” | 5 | 1 | |
| “hydrogen sulfide” | 1 | 0 | |
| “trimethylamine” OR “TMAO” OR “trimethylamine-N-oxide” | 52 | 8 | |
| Total | 280 | 38 | |
5.4. Urolithins (Uros)
5.5. Hydrogen Sulfide (H2S)
5.6. Trimethylamine (TMA) and Trimethylamine-N-Oxide (TMAO)
6. Discussion: Current Knowledge and Therapeutic Perspectives of Microbiota Metabolite Modulation in Intestinal Fibrogenesis
7. Methods
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, Z.; Beattie, R.M.; Ashton, J.J. Recent Developments in the Assessment and Management of Inflammatory Bowel Disease in Childhood: A Narrative Review. Transl. Pediatr. 2023, 12, 1853–1874. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Khera, R.; Dulai, P.S.; Boland, B.S.; Ohno-Machado, L.; Sandborn, W.J.; Singh, S. National Estimates of Financial Hardship From Medical Bills and Cost-Related Medication Nonadherence in Patients With Inflammatory Bowel Diseases in the United States. Inflamm. Bowel Dis. 2021, 27, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Götz, M.; Herrlinger, K.; Steurer, W.; Stange, E.F. Inflammatory Bowel Disease: Crohn’s Disease and Ulcerative Colitis. Dtsch. Ärztebl. Int. 2016, 113, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia hominis and Faecalibacterium prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut Microbiota Composition and Functional Changes in Inflammatory Bowel Disease and Irritable Bowel Syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Kang, S.; Denman, S.E.; Morrison, M.; Yu, Z.; Dore, J.; Leclerc, M.; McSweeney, C.S. Dysbiosis of Fecal Microbiota in Crohn’s Disease Patients as Revealed by a Custom Phylogenetic Microarray. Inflamm. Bowel Dis. 2010, 16, 2034–2042. [Google Scholar] [CrossRef]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the Faecal Microbiota in Patients with Crohn’s Disease and Their Unaffected Relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low Counts of Faecalibacterium Prausnitzii in Colitis Microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Seksik, P.; Rigottier-Gois, L.; Lay, C.; Lepage, P.; Podglajen, I.; Marteau, P.; Doré, J. Specificities of the Fecal Microbiota in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2006, 12, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Russell, R.K.; Reiff, C.; Louis, P.; McIntosh, F.; Berry, S.H.; Mukhopadhya, I.; Bisset, M.W.; Barclay, A.R.; Bishop, J.; et al. Microbiota of De-Novo Pediatric IBD: Increased Faecalibacterium Prausnitzii and Reduced Bacterial Diversity in Crohn’s But Not in Ulcerative Colitis. Am. J. Gastroenterol. 2012, 107, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased Proportions of Bifidobacterium and the Lactobacillus Group and Loss of Butyrate-Producing Bacteria in Inflammatory Bowel Disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- Fyderek, K.; Strus, M.; Kowalska-Duplaga, K.; Gosiewski, T.; Wędrychowicz, A.; Jedynak-Wąsowicz, U.; Sładek, M.; Pieczarkowski, S.; Adamski, P.; Kochan, P.; et al. Mucosal Bacterial Microflora and Mucus Layer Thickness Inadolescents with Inflammatory Bowel Disease. World J. Gastroenterol. 2009, 15, 5287. [Google Scholar] [CrossRef]
- Zhong, W.; Lu, X.; Shi, H.; Zhao, G.; Song, Y.; Wang, Y.; Zhang, J.; Jin, Y.; Wang, S. Distinct Microbial Populations Exist in the Mucosa-Associated Microbiota of Diarrhea Predominant Irritable Bowel Syndrome and Ulcerative Colitis. J. Clin. Gastroenterol. 2019, 53, 660–672. [Google Scholar] [CrossRef]
- Gophna, U.; Sommerfeld, K.; Gophna, S.; Doolittle, W.F.; Veldhuyzen Van Zanten, S.J.O. Differences between Tissue-Associated Intestinal Microfloras of Patients with Crohn’s Disease and Ulcerative Colitis. J. Clin. Microbiol. 2006, 44, 4136–4141. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Gaiani, F.; Mancabelli, L.; Milani, C.; Grandi, A.; Bolchi, A.; Santoni, A.; Lugli, G.A.; Ferrario, C.; Mangifesta, M.; et al. Elucidating the Gut Microbiome of Ulcerative Colitis: Bifidobacteria as Novel Microbial Biomarkers. FEMS Microbiol. Ecol. 2016, 92, fiw191. [Google Scholar] [CrossRef]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T. Growth and Activities of Sulphate-Reducing Bacteria in Gut Contents of Healthy Subjects and Patients with Ulcerative Colitis. FEMS Microbiol. Ecol. 1991, 9, 103–111. [Google Scholar] [CrossRef]
- Zheng, M.; Han, R.; Yuan, Y.; Xing, Y.; Zhang, W.; Sun, Z.; Liu, Y.; Li, J.; Mao, T. The Role of Akkermansia Muciniphila in Inflammatory Bowel Disease: Current Knowledge and Perspectives. Front. Immunol. 2023, 13, 1089600. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Valid Publication of the Names of Forty-Two Phyla of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Ortega Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 10224. [Google Scholar] [CrossRef]
- Bruewer, M.; Samarin, S.; Nusrat, A. Inflammatory Bowel Disease and the Apical Junctional Complex. Ann. N. Y. Acad. Sci. 2006, 1072, 242–252. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Pedicord, V.A. Intestinal Epithelial Cells: At the Interface of the Microbiota and Mucosal Immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Strober, W.; Asano, N.; Fuss, I.; Kitani, A.; Watanabe, T. Cellular and Molecular Mechanisms Underlying NOD 2 Risk-associated Polymorphisms in C Rohn’s Disease. Immunol. Rev. 2014, 260, 249–260. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.; Liang, Y.; Xu, J.; Xu, H.; Nie, Y.; Wang, L.; Yao, J.; Li, D. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. JIR 2022, 15, 1825–1844. [Google Scholar] [CrossRef]
- Tindemans, I.; Joosse, M.E.; Samsom, J.N. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells 2020, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Zhang, Q.; Yang, J.; Liu, G. Roles of Macrophages on Ulcerative Colitis and Colitis-Associated Colorectal Cancer. Front. Immunol. 2023, 14, 1103617. [Google Scholar] [CrossRef] [PubMed]
- Kałużna, A.; Olczyk, P.; Komosińska-Vassev, K. The Role of Innate and Adaptive Immune Cells in the Pathogenesis and Development of the Inflammatory Response in Ulcerative Colitis. J. Clin. Med. 2022, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The Influence of Cytokines on the Complex Pathology of Ulcerative Colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Heller, F.; Fromm, A.; Gitter, A.H.; Mankertz, J.; Schulzke, J.-D. Epithelial Apoptosis Is a Prominent Feature of the Epithelial Barrier Disturbance in Intestinal Inflammation: Effect of pro-Inflammatory Interleukin-13 on Epithelial Cell Function. Mucosal Immunol. 2008, 1, S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Sewell, G.W.; Levine, A.P.; Chew, T.S.; Dunne, J.; O’Shea, N.R.; Smith, P.J.; Harrison, P.J.; Macdonald, C.M.; Bloom, S.L.; et al. Disruption of Macrophage Pro-inflammatory Cytokine Release in C Rohn’s Disease Is Associated with Reduced Optineurin Expression in a Subset of Patients. Immunology 2015, 144, 45–55. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s Disease Inflammation and Recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Q.; Liu, Y.; Shi, Z.; Hu, L.; Zeng, Z.; Tu, Y.; Xiao, Z.; Xu, Q. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J. Immunol. Res. 2021, 2021, 8816041. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Luo, M.; Chen, Z.; He, B. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef]
- Giuffrida, P.; Cococcia, S.; Delliponti, M.; Lenti, M.V.; Di Sabatino, A. Controlling Gut Inflammation by Restoring Anti-Inflammatory Pathways in Inflammatory Bowel Disease. Cells 2019, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Goebel, E.J.; Hart, K.N.; McCoy, J.C.; Thompson, T.B. Structural Biology of the TGFβ Family. Exp. Biol. Med. 2019, 244, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Moustakas, A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Z.; Chen, Y. Regulation of TGF-β Signaling by Smad7. Acta Biochim. Biophys. Sin. 2009, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad Pathways in TGF-β Signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Coskun, M.; Olsen, J.; Seidelin, J.B.; Nielsen, O.H. MAP Kinases in Inflammatory Bowel Disease. Clin. Chim. Acta 2011, 412, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.T.; Hassan, N.F.; Abdulameer, S.J.; Farhan, Z.M.; Suleiman, A.A.; Al-Azzawi, A.K.; Zabibah, R.; Fadhil, A. Phosphatidylinositol 3-kinase Signaling Pathway and Inflammatory Bowel Disease: Current Status and Future Prospects. Fundam. Clin. Pharmacol. 2023, 37, 910–917. [Google Scholar] [CrossRef]
- Lijnen, P.; Petrov, V. Transforming Growth Factor-Beta 1-Induced Collagen Production Incultures of Cardiac Fibroblasts Is the Result of the Appearance Ofmyofibroblasts. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 333. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming Growth Factor-β Signaling Through the Smad Pathway: Role in Extracellular Matrix Gene Expression and Regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef]
- Branton, M.H.; Kopp, J.B. TGF-β and Fibrosis. Microbes Infect. 1999, 1, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W.; Pinchuk, I.V.; Saada, J.I.; Chen, X.; Mifflin, R.C. Mesenchymal Cells of the Intestinal Lamina Propria. Annu. Rev. Physiol. 2011, 73, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J. From Shape-Shifting Embryonic Cells to Oncology: The Fascinating History of Epithelial Mesenchymal Transition. Semin. Cancer Biol. 2023, 96, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Kardooni, A.; Bahrampour, A.; Golmohammadi, S.; Jalili, A.; Alishahi, M.M. The Role of Epithelial Mesenchymal Transition (EMT) in Pathogenesis of Cardiotoxicity: Diagnostic & Prognostic Approach. Mol. Biotechnol. 2023, 65, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Hadpech, S.; Thongboonkerd, V. Epithelial–Mesenchymal Plasticity in Kidney Fibrosis. Genesis 2023, 62, e23529. [Google Scholar] [CrossRef]
- Mottais, A.; Riberi, L.; Falco, A.; Soccal, S.; Gohy, S.; De Rose, V. Epithelial–Mesenchymal Transition Mechanisms in Chronic Airway Diseases: A Common Process to Target? Int. J. Mol. Sci. 2023, 24, 12412. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A. Molecular Basis of Intestinal Fibrosis in Inflammatory Bowel Disease. Inflamm. Intest. Dis. 2022, 7, 119–127. [Google Scholar] [CrossRef]
- Akhmetkaliyev, A.; Alibrahim, N.; Shafiee, D.; Tulchinsky, E. EMT/MET Plasticity in Cancer and Go-or-Grow Decisions in Quiescence: The Two Sides of the Same Coin? Mol. Cancer 2023, 22, 90. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, C.; Chen, Y. TGF-β in Inflammatory Bowel Diseases: A Tale of the Janus-Like Cytokine. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 335–347. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Sferra, R.; Speca, S.; Vetuschi, A.; Dubuquoy, C.; Desreumaux, P.; Pompili, S.; Cristiano, L.; Gaudio, E.; Flati, V.; et al. Role of Glycogen Synthase Kinase-3β and PPAR-γ on Epithelial-to-Mesenchymal Transition in DSS-Induced Colorectal Fibrosis. PLoS ONE 2017, 12, e0171093. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Interactions between TGF-Β1, Canonical WNT/β-Catenin Pathway and PPAR γ in Radiation-Induced Fibrosis. Oncotarget 2017, 8, 90579–90604. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Xue, Z.; Cheng, Y.; Zhang, X. PPARγ: The Central Mucus Barrier Coordinator in Ulcerative Colitis. Inflamm. Bowel Dis. 2021, 27, 732–741. [Google Scholar] [CrossRef]
- McCormack, N.; O’Dea, S. Regulation of Epithelial to Mesenchymal Transition by Bone Morphogenetic Proteins. Cell. Signal. 2013, 25, 2856–2862. [Google Scholar] [CrossRef]
- Hatamzade Esfahani, N.; Day, A.S. The Role of TGF-β, Activin and Follistatin in Inflammatory Bowel Disease. Gastrointest. Disord. 2023, 5, 167–186. [Google Scholar] [CrossRef]
- Cicchinelli, S.; Pignataro, G.; Gemma, S.; Piccioni, A.; Picozzi, D.; Ojetti, V.; Franceschi, F.; Candelli, M. PAMPs and DAMPs in Sepsis: A Review of Their Molecular Features and Potential Clinical Implications. Int. J. Mol. Sci. 2024, 25, 962. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karmakar, S.; Babu, S.P.S. TLR2 and TLR4 Mediated Host Immune Responses in Major Infectious Diseases: A Review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kelley, K.; Melichian, D.S.; Tamaki, Z.; Fang, F.; Su, Y.; Feng, G.; Pope, R.M.; Budinger, G.R.S.; Mutlu, G.M.; et al. Toll-Like Receptor 4 Signaling Augments Transforming Growth Factor-β Responses. Am. J. Pathol. 2013, 182, 192–205. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Nieves, K.M.; Szczepanski, H.E.; Serra, A.; Lee, J.W.; Alston, L.A.; Ramay, H.; Mani, S.; Hirota, S.A. The Pregnane X Receptor and Indole-3-Propionic Acid Shape the Intestinal Mesenchyme to Restrain Inflammation and Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 765–795. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Comalada, M.; Bailón, E.; De Haro, O.; Lara-Villoslada, F.; Xaus, J.; Zarzuelo, A.; Gálvez, J. The Effects of Short-Chain Fatty Acids on Colon Epithelial Proliferation and Survival Depend on the Cellular Phenotype. J. Cancer Res. Clin. Oncol. 2006, 132, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut Microbiota-Derived Butyrate Regulates Gut Mucus Barrier Repair by Activating the Macrophage/WNT/ERK Signaling Pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate Metabolism in Human Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.L.; Abraham, A.G.; Rumbo, M. Is Lactate an Undervalued Functional Component of Fermented Food Products? Front. Microbiol. 2015, 6, 629. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Pan, C.; Chen, X.; Jing, M.; Xie, J.; Gao, Y.; Huang, J.; Chen, X.; Gao, Y.; Xu, C.; et al. Probiotic Lactic Acid Bacteria Alleviate Pediatric IBD and Remodel Gut Microbiota by Modulating Macrophage Polarization and Suppressing Epithelial Apoptosis. Front. Microbiol. 2023, 14, 1168924. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Dokter-Fokkens, J.; Figueroa Lozano, S.; Zhang, Q.; De Haan, B.J.; Zhang, H.; Faas, M.M.; De Vos, P. Lactic Acid Bacteria May Impact Intestinal Barrier Function by Modulating Goblet Cells. Mol. Nutr. Food Res. 2018, 62, 1700572. [Google Scholar] [CrossRef] [PubMed]
- Tennoune, N.; Andriamihaja, M.; Blachier, F. Production of Indole and Indole-Related Compounds by the Intestinal Microbiota and Consequences for the Host: The Good, the Bad, and the Ugly. Microorganisms 2022, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Cho, M.H.; Lee, J. Indole and 3-Indolylacetonitrile Inhibit Spore Maturation in Paenibacillus Alvei. BMC Microbiol. 2011, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, H.; Anjum, K.; Zhong, X.; Miao, S.; Zheng, G.; Liu, W.; Li, L. Dual Role of Indoles Derived From Intestinal Microbiota on Human Health. Front. Immunol. 2022, 13, 903526. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on Their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, K.N.M.; Kalo, M.Z.; Beer-Hammer, S.; Lang, F. The Gut Microbiota Metabolite Urolithin A Inhibits NF-κB Activation in LPS Stimulated BMDMs. Sci. Rep. 2021, 11, 7117. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Andriamihaja, M.; Larraufie, P.; Ahn, E.; Lan, A.; Kim, E. Production of Hydrogen Sulfide by the Intestinal Microbiota and Epithelial Cells and Consequences for the Colonic and Rectal Mucosa. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G125–G135. [Google Scholar] [CrossRef] [PubMed]
- Rabus, R.; Venceslau, S.S.; Wöhlbrand, L.; Voordouw, G.; Wall, J.D.; Pereira, I.A.C. A Post-Genomic View of the Ecophysiology, Catabolism and Biotechnological Relevance of Sulphate-Reducing Prokaryotes. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 66, pp. 55–321. ISBN 978-0-12-803299-2. [Google Scholar]
- Singh, S.; Lin, H. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef]
- Mironov, A.; Seregina, T.; Nagornykh, M.; Luhachack, L.G.; Korolkova, N.; Lopes, L.E.; Kotova, V.; Zavilgelsky, G.; Shakulov, R.; Shatalin, K.; et al. Mechanism of H 2 S-Mediated Protection against Oxidative Stress in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 6022–6027. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; Van Mil, S.W.C.; Müller, M.; Kleerebezem, M.; Van Der Meer, R. Gut Microbiota Facilitates Dietary Heme-Induced Epithelial Hyperproliferation by Opening the Mucus Barrier in Colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E.W.; Moore, J.; Babidge, W. Colonic Sulfide in Pathogenesis and Treatment of Ulcerative Colitis. Dig. Dis. Sci. 1997, 42, 1571–1579. [Google Scholar] [CrossRef]
- Christl, S.U.; Eisner, H.-D.; Dusel, G.; Kasper, H.; Scheppach, W. Antagonistic Effects of Sulfide and Butyrate on Proliferation of Colonic Mucosa: A Potential Role for These Agents in the Pathogenesis of Ulcerative Colitis. Dig. Dis. Sci. 1996, 41, 2477–2481. [Google Scholar] [CrossRef]
- Hoyles, L.; Jiménez-Pranteda, M.L.; Chilloux, J.; Brial, F.; Myridakis, A.; Aranias, T.; Magnan, C.; Gibson, G.R.; Sanderson, J.D.; Nicholson, J.K.; et al. Metabolic Retroconversion of Trimethylamine N-Oxide and the Gut Microbiota. Microbiome 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the Trimethylamine-Producing Bacteria of the Human Gut Microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Xu, H.-M.; Zhao, H.-L.; Guo, G.-J.; Xu, J.; Zhou, Y.-L.; Huang, H.-L.; Nie, Y.-Q. Characterization of Short-Chain Fatty Acids in Patients with Ulcerative Colitis: A Meta-Analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.-M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of Butyrate Enemas on the Colonic Mucosa in Distal Ulcerative Colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef]
- Vieira, E.L.M.; Leonel, A.J.; Sad, A.P.; Beltrão, N.R.M.; Costa, T.F.; Ferreira, T.M.R.; Gomes-Santos, A.C.; Faria, A.M.C.; Peluzio, M.C.G.; Cara, D.C.; et al. Oral Administration of Sodium Butyrate Attenuates Inflammation and Mucosal Lesion in Experimental Acute Ulcerative Colitis. J. Nutr. Biochem. 2012, 23, 430–436. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Cazzola, P.; Ciccocioppo, R.; Morera, R.; Biancheri, P.; Rovedatti, L.; Cantoro, L.; Vanoli, A.; Tinozzi, F.P.; Tinozzi, S.; et al. Efficacy of Butyrate in the Treatment of Mild to Moderate Crohn’s Disease. Dig. Liver Dis. Suppl. 2007, 1, 31–35. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van De Wiele, T. Butyrate-Producing Bacteria Supplemented in Vitro to Crohn’s Disease Patient Microbiota Increased Butyrate Production and Enhanced Intestinal Epithelial Barrier Integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Butyrate in Inflammatory Bowel Disease Therapy. Gastroenterology 2020, 158, 1511. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate Producers, “The Sentinel of Gut”: Their Intestinal Significance with and beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Q.; Tang, C.; Li, Y.; Zhang, K.; Li, F.; Zhang, J. Butyrate Mitigates Weanling Piglets From Lipopolysaccharide-Induced Colitis by Regulating Microbiota and Energy Metabolism of the Gut–Liver Axis. Front. Microbiol. 2020, 11, 588666. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.L.; Aristizabal-Henao, J.J.; Patuel, S.J.; Bowden, J.A.; Zubcevic, J.; Martyniuk, C.J. Interaction between Butyrate and Tumor Necrosis Factor α in Primary Rat Colonocytes. Biomolecules 2023, 13, 258. [Google Scholar] [CrossRef]
- Matsumoto, N.; Riley, S.; Fraser, D.; Al-Assaf, S.; Ishimura, E.; Wolever, T.; Phillips, G.O.; Phillips, A.O. Butyrate Modulates TGF-Β1 Generation and Function: Potential Renal Benefit for Acacia(Sen) SUPERGUMTM (Gum Arabic)? Kidney Int. 2006, 69, 257–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsiao, Y.-P.; Chen, H.-L.; Tsai, J.-N.; Lin, M.-Y.; Liao, J.-W.; Wei, M.-S.; Ko, J.-L.; Ou, C.-C. Administration of Lactobacillus Reuteri Combined with Clostridium Butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation. Nutrients 2021, 13, 2792. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, T.; He, Y.; Xie, Y.; Xu, Y.; Huang, W. The Role and Mechanism of Butyrate in the Prevention and Treatment of Diabetic Kidney Disease. Front. Microbiol. 2022, 13, 961536. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; You, N.; Zhao, X.; Li, J.; Jiang, W. Butyric Acid Ameliorates Myocardial Fibrosis by Regulating M1/M2 Polarization of Macrophages and Promoting Recovery of Mitochondrial Function. Front. Nutr. 2022, 9, 875473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Li, L.; Zhang, H.; Pang, D.; Ouyang, H.; Jin, X.; Tang, X. Clostridium Butyricum and Bifidobacterium Pseudolongum Attenuate the Development of Cardiac Fibrosis in Mice. Microbiol. Spectr. 2022, 10, e02524-22. [Google Scholar] [CrossRef]
- Alhusaini, A.M.; Alsoghayer, R.; Alhushan, L.; Alanazi, A.M.; Hasan, I.H. Acetyl-L-Carnitine and Liposomal Co-Enzyme Q10 Attenuate Hepatic Inflammation, Apoptosis, and Fibrosis Induced by Propionic Acid. Int. J. Mol. Sci. 2023, 24, 11519. [Google Scholar] [CrossRef] [PubMed]
- Gart, E.; Van Duyvenvoorde, W.; Toet, K.; Caspers, M.P.M.; Verschuren, L.; Nielsen, M.J.; Leeming, D.J.; Souto Lima, E.; Menke, A.; Hanemaaijer, R.; et al. Butyrate Protects against Diet-Induced NASH and Liver Fibrosis and Suppresses Specific Non-Canonical TGF-β Signaling Pathways in Human Hepatic Stellate Cells. Biomedicines 2021, 9, 1954. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, M.; Gong, J.; Zhang, X.; Ge, S.; Zhao, L. Sodium Acetate Inhibit TGF-Β1-Induced Activation of Hepatic Stellate Cells by Restoring AMPK or c-Jun Signaling. Front. Nutr. 2021, 8, 729583. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct Signatures of Gut Microbiome and Metabolites Associated with Significant Fibrosis in Non-Obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]
- Thing, M.; Werge, M.P.; Kimer, N.; Hetland, L.E.; Rashu, E.B.; Nabilou, P.; Junker, A.E.; Galsgaard, E.D.; Bendtsen, F.; Laupsa-Borge, J.; et al. Targeted Metabolomics Reveals Plasma Short-Chain Fatty Acids Are Associated with Metabolic Dysfunction-Associated Steatotic Liver Disease. BMC Gastroenterol. 2024, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Qiu, Y.; Gao, Z.; Wu, Y.-X.; Wan, B.; Liu, G.; Chen, J.; Zhou, Q.; Yu, R.; Pang, Q. Sodium Propionate Attenuates the Lipopolysaccharide-Induced Epithelial–Mesenchymal Transition via the PI3K/Akt/mTOR Signaling Pathway. J. Agric. Food Chem. 2020, 68, 6554–6563. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Kamada, N. Contribution of the Gut Microbiota to Intestinal Fibrosis in Crohn’s Disease. Front. Med. 2022, 9, 826240. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, H.; Cheng, X.; He, Y.; Ren, Q.; Li, D.; Xie, Y.; Gao, C.; Zhang, Y.; Sun, X.; et al. Sodium Butyrate Attenuates Diabetic Kidney Disease Partially via Histone Butyrylation Modification. Mediat. Inflamm. 2022, 2022, 7643322. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Guan, X.; Wang, X.; Qu, S.; Zhang, S.; Hui, W.; Men, L.; Chen, X. Dynamic Modulation of Gut Microbiota Improves Post-Myocardial Infarct Tissue Repair in Rats via Butyric Acid-Mediated Histone Deacetylase Inhibition. FASEB J. 2021, 35, e21385. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Li, Y.J.; Ma, J.; Loh, Y.W.; Chadban, S.J.; Wu, H. Short-Chain Fatty Acids Directly Exert Anti-Inflammatory Responses in Podocytes and Tubular Epithelial Cells Exposed to High Glucose. Front. Cell Dev. Biol. 2023, 11, 1182570. [Google Scholar] [CrossRef]
- Lin, C.-J.; Cheng, Y.-C.; Chen, H.-C.; Chao, Y.-K.; Nicholson, M.W.; Yen, E.C.L.; Kamp, T.J.; Hsieh, P.C.H. Commensal Gut Microbiota-Derived Acetate and Propionate Enhance Heart Adaptation in Response to Cardiac Pressure Overload in Mice. Theranostics 2022, 12, 7319–7334. [Google Scholar] [CrossRef]
- Aoki, R.; Onuki, M.; Hattori, K.; Ito, M.; Yamada, T.; Kamikado, K.; Kim, Y.-G.; Nakamoto, N.; Kimura, I.; Clarke, J.M.; et al. Commensal Microbe-Derived Acetate Suppresses NAFLD/NASH Development via Hepatic FFAR2 Signalling in Mice. Microbiome 2021, 9, 188. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; Ledue, F.; Blottière, H.M.; Lapaque, N. Butyrate Produced by Gut Commensal Bacteria Activates TGF-Beta1 Expression through the Transcription Factor SP1 in Human Intestinal Epithelial Cells. Sci. Rep. 2018, 8, 9742. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-Activated PPAR-γ Signaling Inhibits Dysbiotic Enterobacteriaceae Expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Q.; Zhu, Y.; Zhang, Y.; Xia, Y.; Wei, Z.; Dai, Y. Rectal Administration of Butyrate Ameliorates Pulmonary Fibrosis in Mice through Induction of Hepatocyte Growth Factor in the Colon via the HDAC-PPARγ Pathway. Life Sci. 2022, 309, 120972. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; He, L.; Zhong, Z.; Zhao, R.; Weng, S.; Mi, H.; Liu, F. Stigmasterol Restores the Balance of Treg/Th17 Cells by Activating the Butyrate-PPARγ Axis in Colitis. Front. Immunol. 2021, 12, 741934. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M.; et al. An Orally Administered Butyrate-releasing Derivative Reduces Neutrophil Recruitment and Inflammation in Dextran Sulphate Sodium-induced Murine Colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Chen, M.; Chen, L.; Xu, F.; Xiao, Z.; Yi, A.; Tian, Y.; Ping, Y.; Lv, L.; Cheng, Y.; et al. Roseburia Intestinalis and Its Metabolite Butyrate Inhibit Colitis and Upregulate TLR5 through the SP3 Signaling Pathway. Nutrients 2022, 14, 3041. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, X.; Zhang, W.; Zhang, G.; Nguyen, A.K.; Liu, X.; Jimenez, F.; Cox, C.S.; Townsend, C.M.; Ko, T.C. Dietary Fiber Enhances TGF-β Signaling and Growth Inhibition in the Gut. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G156–G164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dong, Y.; Li, M.; Zhang, W.; Ding, Y.; Wang, X.; Chen, D.; Liu, T.; Wang, B.; Cao, H.; et al. Clostridium butyricum Inhibits Epithelial–Mesenchymal Transition of Intestinal Carcinogenesis through Downregulating METTL3. Cancer Sci. 2023, 114, 3114–3127. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces Cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Jiang, L.; Han, D.; Si, D.; Sun, Z.; Wu, Z.; Dai, Z. Limosilactobacillus Mucosae and Lactobacillus Amylovorus Protect Against Experimental Colitis via Upregulation of Colonic 5-Hydroxytryptamine Receptor 4 and Transforming Growth Factor-Β2. J. Nutr. 2023, 153, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Kottmann, R.M.; Kulkarni, A.A.; Smolnycki, K.A.; Lyda, E.; Dahanayake, T.; Salibi, R.; Honnons, S.; Jones, C.; Isern, N.G.; Hu, J.Z.; et al. Lactic Acid Is Elevated in Idiopathic Pulmonary Fibrosis and Induces Myofibroblast Differentiation via pH-Dependent Activation of Transforming Growth Factor-β. Am. J. Respir. Crit. Care Med. 2012, 186, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-García, D.; Jiménez, M.; Rivas-Santiago, C.E.; Gallegos-Alcalá, P.; Hernández-Mercado, A.; Santoyo-Payán, L.S.; Loera-Arias, M.D.J.; Saucedo-Cardenas, O.; Montes De Oca-Luna, R.; Salinas, E. Lactococcus lactis NZ9000 Prevents Asthmatic Airway Inflammation and Remodelling in Rats through the Improvement of Intestinal Barrier Function and Systemic TGF-β Production. Int. Arch. Allergy Immunol. 2021, 182, 277–291. [Google Scholar] [CrossRef]
- Jantararussamee, C.; Rodniem, S.; Taweechotipatr, M.; Showpittapornchai, U.; Pradidarcheep, W. Hepatoprotective Effect of Probiotic Lactic Acid Bacteria on Thioacetamide-Induced Liver Fibrosis in Rats. Probiotics Antimicrob. Proteins 2021, 13, 40–50. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Lactic Acid Bacteria Improve the Photoprotective Effect via MAPK/AP-1/MMP Signaling Pathway on Skin Fibroblasts. Microorganisms 2022, 10, 2481. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Xie, X.; Chen, M.; Xue, L.; Wang, J.; Ye, Q.; Wu, S.; Yang, R.; Zhao, H.; et al. Exploration of the Molecular Mechanisms Underlying the Anti-Photoaging Effect of Limosilactobacillus Fermentum XJC60. Front. Cell. Infect. Microbiol. 2022, 12, 838060. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Hosoya, T.; Ono-Ohmachi, A.; Ukibe, K.; Ogawa, A.; Moriya, T.; Kadooka, Y.; Shiozaki, T.; Nakagawa, H.; Nakayama, Y.; et al. Lactobacillus Gasseri SBT2055 Induces TGF-β Expression in Dendritic Cells and Activates TLR2 Signal to Produce IgA in the Small Intestine. PLoS ONE 2014, 9, e105370. [Google Scholar] [CrossRef]
- Madjirebaye, P.; Peng, F.; Mueed, A.; Huang, T.; Mahamat, B.; Pahane, M.M.; Xi, Q.; Chen, X.; Moussa, K.; Kadebe, Z.T.; et al. Exploring Impact of Probiotic-Fermented Soymilk on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Modulating Inflammation and Gut Microbiota Profile. Mol. Nutr. Food Res. 2024, 68, 2300586. [Google Scholar] [CrossRef] [PubMed]
- Remund, B.; Yilmaz, B.; Sokollik, C. D-Lactate: Implications for Gastrointestinal Diseases. Children 2023, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, C.; Li, R.; Zheng, M.; Feng, B.; Gao, J.; Long, X.; Li, L.; Li, S.; Zuo, X.; et al. Lactobacillus-Derived Indole-3-Lactic Acid Ameliorates Colitis in Cesarean-Born Offspring via Activation of Aryl Hydrocarbon Receptor. iScience 2023, 26, 108279. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Z.; Tian, X.; Liang, X.; Lu, Y.; Shi, Y.; Kuerman, M.; Wang, R.; Zhou, Y.; Gong, P.; et al. Bifidobacterium bifidum Ameliorates DSS-Induced Colitis in Mice by Regulating AHR/NRF2/NLRP3 Inflammasome Pathways through Indole-3-Lactic Acid Production. J. Agric. Food Chem. 2023, 71, 1970–1981. [Google Scholar] [CrossRef]
- Wang, S.; Van Schooten, F.-J.; Jin, H.; Jonkers, D.; Godschalk, R. The Involvement of Intestinal Tryptophan Metabolism in Inflammatory Bowel Disease Identified by a Meta-Analysis of the Transcriptome and a Systematic Review of the Metabolome. Nutrients 2023, 15, 2886. [Google Scholar] [CrossRef] [PubMed]
- Qazi, A.; Comiskey, S.; Calzadilla, N.; Amin, F.; Sharma, A.; Khin, E.; Holton, N.; Weber, C.R.; Saksena, S.; Kumar, A.; et al. Potential Dietary and Therapeutic Strategies Involving Indole-3-Carbinole in Preclinical Models of Intestinal Inflammation. Nutrients 2023, 15, 4980. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Park, S.; Lee, K.W.; Park, T. Indole-3-Carbinol Prevents Diet-Induced Obesity through Modulation of Multiple Genes Related to Adipogenesis, Thermogenesis or Inflammation in the Visceral Adipose Tissue of Mice. J. Nutr. Biochem. 2012, 23, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Yisireyili, M.; Takeshita, K.; Saito, S.; Murohara, T.; Niwa, T. Indole-3-Propionic Acid Suppresses Indoxyl Sulfate-Induced Expression of Fibrotic and Inflammatory Genes in Proximal Tubular Cells. Nagoya J. Med. Sci. 2017, 79, 477–486. [Google Scholar] [PubMed]
- Singh, N.P.; Singh, U.P.; Rouse, M.; Zhang, J.; Chatterjee, S.; Nagarkatti, P.S.; Nagarkatti, M. Dietary Indoles Suppress Delayed-Type Hypersensitivity by Inducing a Switch from Proinflammatory Th17 Cells to Anti-Inflammatory Regulatory T Cells through Regulation of MicroRNA. J. Immunol. 2016, 196, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Torigoe, K.; Torigoe, M.; Muta, K.; Obata, Y.; Suzuki, T.; Suzuki, C.; Abe, T.; Koji, T.; Mukae, H.; et al. Mitochonic Acid-5 Ameliorates Chlorhexidine Gluconate-Induced Peritoneal Fibrosis in Mice. Med. Mol. Morphol. 2022, 55, 27–40. [Google Scholar] [CrossRef]
- Liu, F.; Sun, C.; Chen, Y.; Du, F.; Yang, Y.; Wu, G. Indole-3-Propionic Acid-Aggravated CCl4-Induced Liver Fibrosis via the TGF-Β1/Smads Signaling Pathway. J. Clin. Transl. Hepatol. 2021, 9, 917–930. [Google Scholar] [CrossRef]
- Shima, H.; Sasaki, K.; Suzuki, T.; Mukawa, C.; Obara, T.; Oba, Y.; Matsuo, A.; Kobayashi, T.; Mishima, E.; Watanabe, S.; et al. A Novel Indole Compound MA-35 Attenuates Renal Fibrosis by Inhibiting Both TNF-α and TGF-Β1 Pathways. Sci. Rep. 2017, 7, 1884. [Google Scholar] [CrossRef]
- Sehgal, R.; Ilha, M.; Vaittinen, M.; Kaminska, D.; Männistö, V.; Kärjä, V.; Tuomainen, M.; Hanhineva, K.; Romeo, S.; Pajukanta, P.; et al. Indole-3-Propionic Acid, a Gut-Derived Tryptophan Metabolite, Associates with Hepatic Fibrosis. Nutrients 2021, 13, 3509. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pei, J.; Wang, X.; Tai, S.; Tang, L.; Hu, X. Gut Bacterial Metabolite Urolithin A Inhibits Myocardial Fibrosis through Activation of Nrf2 Pathway In Vitro and In Vivo. Mol. Med. 2022, 28, 19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Tu, J.; Zhang, H.; Zhang, Y.; Zhou, B. Urolithin A Attenuates Renal Fibrosis by Inhibiting TGF-Β1/Smad and MAPK Signaling Pathways. J. Funct. Foods 2021, 83, 104547. [Google Scholar] [CrossRef]
- Duan, J.; Pan, J.; Sun, M.; Fang, Y. Comparative Multiomics Study of the Effects of Ellagic Acid on the Gut Environment in Young and Adult Mice. Food Res. Int. 2022, 161, 111819. [Google Scholar] [CrossRef]
- Cheng, F.; Dou, J.; Zhang, Y.; Wang, X.; Wei, H.; Zhang, Z.; Cao, Y.; Wu, Z. Urolithin A Inhibits Epithelial–Mesenchymal Transition in Lung Cancer Cells via P53-Mdm2-Snail Pathway. OncoTargets Ther. 2021, 14, 3199–3208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, R.; Zhou, X.; Ji, F.; Zhang, B. Hydrogen Sulfide Improves Colonic Barrier Integrity in DSS-Induced Inflammation in Caco-2 Cells and Mice. Int. Immunopharmacol. 2016, 39, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mottawea, W.; Chiang, C.-K.; Mühlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S.; et al. Altered Intestinal Microbiota–Host Mitochondria Crosstalk in New Onset Crohn’s Disease. Nat. Commun. 2016, 7, 13419. [Google Scholar] [CrossRef] [PubMed]
- Chirindoth, S.S.; Cancarevic, I. Role of Hydrogen Sulfide in the Treatment of Fibrosis. Cureus 2021, 13, e18088. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Shi, X.; Wang, H.; Fan, J.; Feng, Y.; Lin, X.; Yang, J.; Cui, Q.; Tang, C.; Xu, G.; et al. Cystathionine γ Lyase–Hydrogen Sulfide Increases Peroxisome Proliferator-Activated Receptor γ Activity by Sulfhydration at C139 Site Thereby Promoting Glucose Uptake and Lipid Storage in Adipocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, J.; Wang, C.; Yang, C.; Wang, Y.; Li, Y.; Li, Y. Hydrogen Sulfide Alleviates Cigarette Smoke-Induced COPD through Inhibition of the TGF-β1/Smad Pathway. Exp. Biol. Med. 2020, 245, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, J.; Zhao, J.; Ni, Q.; Zhao, C.; Zheng, Y.; Chen, X.; Wang, L. Trimethylamine N-Oxide Exacerbates Cardiac Fibrosis via Activating the NLRP3 Inflammasome. Front. Physiol. 2019, 10, 866. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, S.; Zhu, J.; Jiang, H.; Jia, D.; Ou, T.; Qi, Z.; Zou, Y.; Qian, J.; Sun, A.; et al. Gut Microbe-Derived Metabolite Trimethylamine N-Oxide Accelerates Fibroblast-Myofibroblast Differentiation and Induces Cardiac Fibrosis. J. Mol. Cell. Cardiol. 2019, 134, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kong, B.; Shuai, W.; Fu, H.; Jiang, X.; Huang, H. 3,3-Dimethyl-1-Butanol Attenuates Cardiac Remodeling in Pressure-Overload-Induced Heart Failure Mice. J. Nutr. Biochem. 2020, 78, 108341. [Google Scholar] [CrossRef]
- Moludi, J.; Saiedi, S.; Ebrahimi, B.; Alizadeh, M.; Khajebishak, Y.; Ghadimi, S.S. Probiotics Supplementation on Cardiac Remodeling Following Myocardial Infarction: A Single-Center Double-Blind Clinical Study. J. Cardiovasc. Trans. Res. 2021, 14, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Wu, H.; Shi, H.; Bai, J.; Zhao, W.; Jiang, D.; Jiang, X. Lactobacillus Rhamnosus GG Strain Mitigated the Development of Obstructive Sleep Apnea-Induced Hypertension in a High Salt Diet via Regulating TMAO Level and CD4+ T Cell Induced-Type I Inflammation. Biomed. Pharmacother. 2019, 112, 108580. [Google Scholar] [CrossRef]
- Kapetanaki, S.; Kumawat, A.K.; Persson, K.; Demirel, I. The Fibrotic Effects of TMAO on Human Renal Fibroblasts Is Mediated by NLRP3, Caspase-1 and the PERK/Akt/mTOR Pathway. Int. J. Mol. Sci. 2021, 22, 11864. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zheng, B.; Liu, N.; Liu, J.; Liu, W.; Huang, X.; Zeng, X.; Chen, L.; Li, Z.; Ouyang, D. Trimethylamine N-Oxide Exacerbates Renal Inflammation and Fibrosis in Rats With Diabetic Kidney Disease. Front. Physiol. 2021, 12, 682482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Miikeda, A.; Zuckerman, J.; Jia, X.; Charugundla, S.; Zhou, Z.; Kaczor-Urbanowicz, K.E.; Magyar, C.; Guo, F.; Wang, Z.; et al. Inhibition of Microbiota-Dependent TMAO Production Attenuates Chronic Kidney Disease in Mice. Sci. Rep. 2021, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Bale, S.; Verma, P.; Wan, Q.; Ma, F.; Gudjonsson, J.E.; Hazen, S.L.; Harms, P.W.; Tsou, P.-S.; Khanna, D.; et al. Gut Microbe-Derived Metabolite Trimethylamine N-Oxide Activates PERK to Drive Fibrogenic Mesenchymal Differentiation. iScience 2022, 25, 104669. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Zhou, T.; Jiang, C.; A, L.; Xu, W. Gut Microbiota-Dependent Trimethylamine n-Oxide Pathway Contributes to the Bidirectional Relationship between Intestinal Inflammation and Periodontitis. Front. Cell. Infect. Microbiol. 2023, 12, 1125463. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Buffa, J.A.; Roberts, A.B.; Sangwan, N.; Skye, S.M.; Li, L.; Ho, K.J.; Varga, J.; DiDonato, J.A.; Tang, W.H.W.; et al. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1239–1255. [Google Scholar] [CrossRef]
- Organ, C.L.; Li, Z.; Sharp, T.E.; Polhemus, D.J.; Gupta, N.; Goodchild, T.T.; Tang, W.H.W.; Hazen, S.L.; Lefer, D.J. Nonlethal Inhibition of Gut Microbial Trimethylamine N-oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef]
- Massey, W.; Mukherjee, P.; Nguyen, Q.T.; Mrdjen, M.; Wang, Z.; Brown, J.M.; Rieder, F. THE PATHOGENIC ROLE OF MICROBIAL TRIMETHYLAMINE IN IBD ASSOCIATED INTESTINAL FIBROSIS. Inflamm. Bowel Dis. 2024, 30, S64. [Google Scholar] [CrossRef]
- Jalandra, R.; Makharia, G.K.; Sharma, M.; Kumar, A. Inflammatory and Deleterious Role of Gut Microbiota-Derived Trimethylamine on Colon Cells. Front. Immunol. 2023, 13, 1101429. [Google Scholar] [CrossRef]
- Banno, Y.; Nomura, M.; Hara, R.; Asami, M.; Tanaka, K.; Mukai, Y.; Tomata, Y. Trimethylamine N-Oxide and Risk of Inflammatory Bowel Disease: A Mendelian Randomization Study. Medicine 2023, 102, e34758. [Google Scholar] [CrossRef]
- Wilson, A.; Teft, W.A.; Morse, B.L.; Choi, Y.-H.; Woolsey, S.; DeGorter, M.K.; Hegele, R.A.; Tirona, R.G.; Kim, R.B. Trimethylamine-N-Oxide: A Novel Biomarker for the Identification of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 3620–3630. [Google Scholar] [CrossRef] [PubMed]
- Bunt, D.; Minnaard, A.; El Aidy, S. Potential Modulatory Microbiome Therapies for Prevention or Treatment of Inflammatory Bowel Diseases. Pharmaceuticals 2021, 14, 506. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, N.; Lee, C. Mysteries of TGF-Î2 Paradox in Benign and Malignant Cells. Front. Oncol. 2014, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Stenke, E.; Bourke, B.; Knaus, U. Crohn’s Strictures—Moving Away from the Knife. Front. Pediatr. 2017, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Golob, J.; Rao, K.; Berinstein, J.; Chey, W.; Owyang, C.; Kamada, N.; Higgins, P.; Young, V.; Bishu, S.; Lee, A. The Fecal Microbiome in Quiescent Crohn’s Disease with Persistent Gastrointestinal Symptoms Show Enrichment of Oral Microbes But Depletion of Butyrate and Indole Producers. medRxiv 2023. [Google Scholar] [CrossRef]
- Shashni, B.; Tajika, Y.; Ikeda, Y.; Nishikawa, Y.; Nagasaki, Y. Self-Assembling Polymer-Based Short Chain Fatty Acid Prodrugs Ameliorate Non-Alcoholic Steatohepatitis and Liver Fibrosis. Biomaterials 2023, 295, 122047. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hao, W.; Wang, X.; Zhou, R.; Lin, Q. Probiotics for the Treatment of Ulcerative Colitis: A Review of Experimental Research from 2018 to 2022. Front. Microbiol. 2023, 14, 1211271. [Google Scholar] [CrossRef] [PubMed]
| Phylum | Class | Order | Family | Genus | Species | CD | Ref. | UC | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Firmicutes | Clostridia | ↓ | [9] | ↓ | [9] | ||||
| Clostridiales | Lachnospiraceae | Roseburia | R. hominis | ↓ | [10] | ||||
| R. intestinalis | ↓ | [11] | ↓ | [11] | |||||
| Ruminococcus | R. albus | ↓ | [12] | ||||||
| R. callidus | ↓ | [12] | |||||||
| R. bromii | ↓ | [12] | |||||||
| R. gnavus | ↑ | [13] | ↑ | [13] | |||||
| R. torques | ↑ | [13] | ↑ | [13] | |||||
| Acidaminococcaceae | Dialister | D. invisus | ↓ | [14] | |||||
| Eubacteriaceae | Eubacterium | E. rectale | ↓ | [12] | |||||
| Clostridiaceae | Clostridium | C. difficile | ↑ | [12] | |||||
| C. coccoides | ↓ | [15] | ↓ | [15,16] | |||||
| C. leptum | ↓ | [12,15,16] | ↓ | [15] | |||||
| Faecalibacterium | F. prausnitzii | ↑ | [17] | ↓ | [10,11,15] | ||||
| ↓ | [11,12,14,15] | ||||||||
| Bacilli | ↑ | [9] | ↑ | [9] | |||||
| Bacillales | Listeriaceae | Listeria | ↑ | [12] | |||||
| Lactobacillales | Enterococcaceae | Enterococcus | ↑ | [12] | |||||
| Lactobacillaceae | Lactobacillus | ↑ ↓ | [12,18] [19] | ↓ | [20] | ||||
| Bacteroidetes | Bacteroidetes | ↓ | [9] | ↓ | [9] | ||||
| Bacteroidales | Bacteroidaceae | Bacteroides | B. fragilis | ↓ | [11,12] | ↓ | [11] | ||
| ↑ | [21] | ||||||||
| B. vulgatus | ↓ | [11,12] | ↓ | [11] | |||||
| ↑ | [21] | ||||||||
| Actinobacteria | Actinobacteria | ↑ | [9] | ↓ | [22] | ||||
| Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | B. longum | ↑ | [11] | ||||
| B. bifidum | ↓ | [22] | |||||||
| Proteobacteria | ↑ | [9] | ↑ | [9] | |||||
| δ | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | ↑ | [23] | ||||
| γ | Enterobacteriales | Enterobacteriaceae | Escherichia | ↑ | [11,21] | ||||
| Shigella | ↑ | [11] | |||||||
| S. flexneri | ↑ | [12] | |||||||
| Pseudomonadales | Moraxellaceae | Acinetobacter | A. junii | ↑ | [21] | ||||
| Verrucomicrobia | Verrucomicrobiae | ↓ | [13,24] | ↓ | [13,24] | ||||
| Verrucomicrobiales | Verrucomicrobiaceae | Akkermansia | A. muciniphila | ↓ | [13,24] | ↓ | [13,24] |
| Main Effects on the Parts of the Gut Barrier | |||||||
|---|---|---|---|---|---|---|---|
| Microbial Metabolites | Precursor | Species Involved in the Metabolism | Microbiota | Mucus | Epithelium | IIS | Ref. |
Short-chain fatty acids (SCFAs)
| Non-digestible dietary fibers, amino acids, and lactate. |
| SCFAs interact with other bacteria such as Lactobacilli and Bifidobacteria, enhancing their growth. | SCFAs stimulate goblet cells and induce the MUC2 gene. | SCFAs are the principal energetic source for colonocytes and contribute to the integrity of the APC. | SCFAs regulate TLR and FFAR activation, the differentiation of Tregs, and IL-10 secretion. | [70,71,72,73] |
| Lactic acid (LA) | Fermented foods: carbohydrate fermentation. | “LAB”, Gram-positive catalase-negative bacteria resistant to low pH, mainly belonging to the Lactobacillus genus. | LAB produce bacteriocins, peptides involved in the mucosal defense. | Various strains of LAB differently affect goblet cell functions and the expression of mucus-related genes, MUC2 included. | LA promotes the TCA for energy production, maintains the cellular redox state, stimulates the ACC for fatty acid synthesis, and contributes to normal epithelial proliferation. | LAB administration promotes macrophage M2 polarization and a reduction in pro-inflammatory cytokines (e.g., IL-1β and IL-6) | [74,75,76,77] |
| Indoles | Tryptophan, the essential amino acid found in meat, fish, dairy, eggs, nuts, seeds, legumes, and whole grains. | Tryptophanase-expressing bacteria, such as Clostridium, Bacteroides, Lactobacillus, and Bifidobacterium spp. | Indoles influence bacterial communication, limiting virulence gene expression and bacterial invasiveness, in a dose-dependent manner. | Indoles boost MUC2 and MUC4 expression and goblet cell activity. | Indoles reduce the epithelial permeability by enhancing tight junctions. | [78,79,80,81,82] | |
| Urolithin A (UA) | Polyphenolic compounds (ellagitannins) in fruits, nuts, and tea. | In the small intestine, ellagitannins are hydrolyzed to ellagic and gallic acid intermediates, and further metabolized by Gordonibacter urolithinfaciens and Ellagibactrer into UA. Only about 40% of elderly humans possess a suitable gut microbiota able to produce UA. | UA and its synthetic analogue, UAS03, have been reported to upregulate tight junction proteins. | UA reduces the production of ROS and suppresses the TLR4, MAPK, and PI3K pathways, with decrease in the expression of pro-inflammatory mi-RNA and cytokines (IL-1β, IL-6, and TNF-α). | [83,84,85] | ||
| Hydrogen sulfide (H2S) | Sulfate (SO42−) derived from amino acids (mainly cysteine and methionine), additives, preservatives, and IEC production (CBS activity). | Sulfate-reducing bacteria (SRB), like colonic Desulfovibrio, Desulfotomaculum, and Bilophila, utilize SO42− as a terminal electron acceptor in their metabolic pathways, reducing it to H2S. | Exogenous H2S confers to the bacteria’s high resistance to oxidative stress. | High concentrations of H2S destabilize the disulfide bonds of the mucin-2 network, resulting in increased contact between bacteria and the epithelium. | H2S is the primary mineral energy substrate for colonocytes, but in high concentrations, it inhibits the mitochondrial respiratory chain. Also, it negatively interferes with butyrate metabolism. | [86,87,88,89,90,91,92] | |
| Trimethylamine (TMA) | Choline, carnitine, and betaine, contained in red meat, eggs, fish, and dairy. | Several bacterial species (e.g., E. coli, Enterococcus, Clostridium, Proteus, Shigella, Klebsiella, and Providentia spp.) transform the precursors in TMA, which is further oxidized in the liver to form TMAO. | TMA and TMAO modulate the composition of the microbiota. | [93,94] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicchinelli, S.; Gemma, S.; Pignataro, G.; Piccioni, A.; Ojetti, V.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Intestinal Fibrogenesis in Inflammatory Bowel Diseases: Exploring the Potential Role of Gut Microbiota Metabolites as Modulators. Pharmaceuticals 2024, 17, 490. https://doi.org/10.3390/ph17040490
Cicchinelli S, Gemma S, Pignataro G, Piccioni A, Ojetti V, Gasbarrini A, Franceschi F, Candelli M. Intestinal Fibrogenesis in Inflammatory Bowel Diseases: Exploring the Potential Role of Gut Microbiota Metabolites as Modulators. Pharmaceuticals. 2024; 17(4):490. https://doi.org/10.3390/ph17040490
Chicago/Turabian StyleCicchinelli, Sara, Stefania Gemma, Giulia Pignataro, Andrea Piccioni, Veronica Ojetti, Antonio Gasbarrini, Francesco Franceschi, and Marcello Candelli. 2024. "Intestinal Fibrogenesis in Inflammatory Bowel Diseases: Exploring the Potential Role of Gut Microbiota Metabolites as Modulators" Pharmaceuticals 17, no. 4: 490. https://doi.org/10.3390/ph17040490
APA StyleCicchinelli, S., Gemma, S., Pignataro, G., Piccioni, A., Ojetti, V., Gasbarrini, A., Franceschi, F., & Candelli, M. (2024). Intestinal Fibrogenesis in Inflammatory Bowel Diseases: Exploring the Potential Role of Gut Microbiota Metabolites as Modulators. Pharmaceuticals, 17(4), 490. https://doi.org/10.3390/ph17040490









