The Lipid-Lowering Efficacy of a Nutraceutical Combination Including Leucoselect Phytosome, Red Yeast Rice, Policosanol and Folic Acid in Dyslipidaemia Patients: Real-World Insights
Abstract
1. Introduction
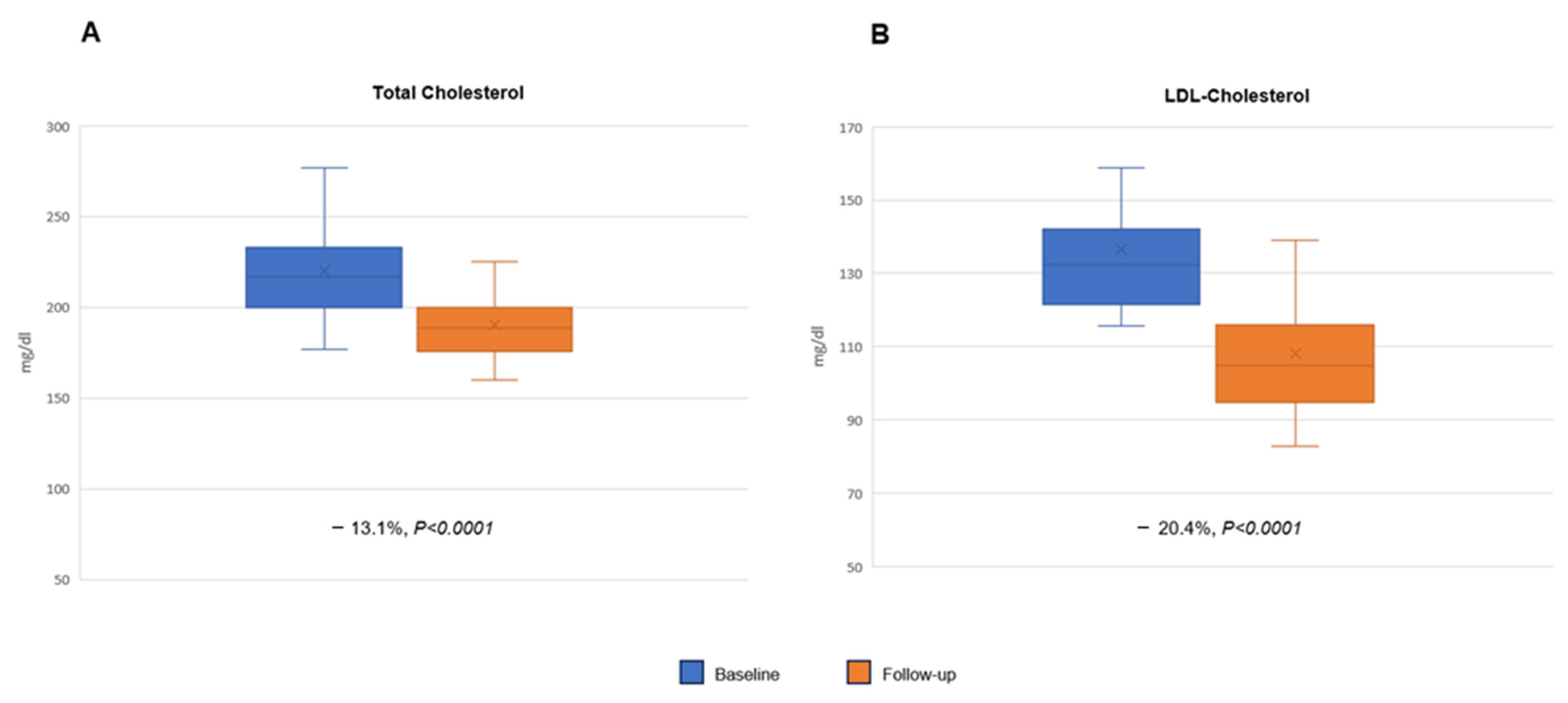
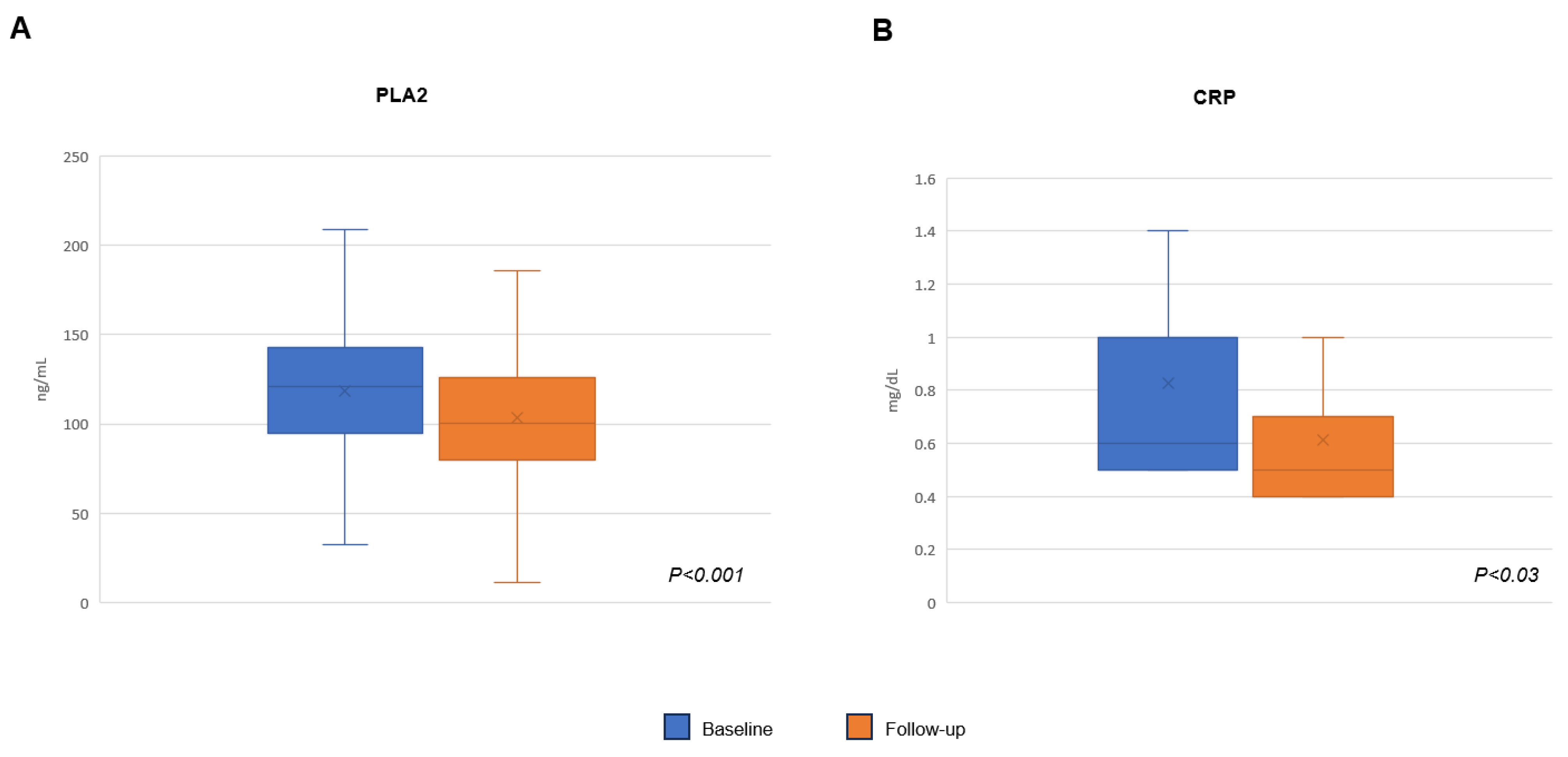
2. Results
3. Discussion
3.1. Nutritional Intervention
3.2. Lipid Lowering Nutraceuticals
3.3. Clinical Studies
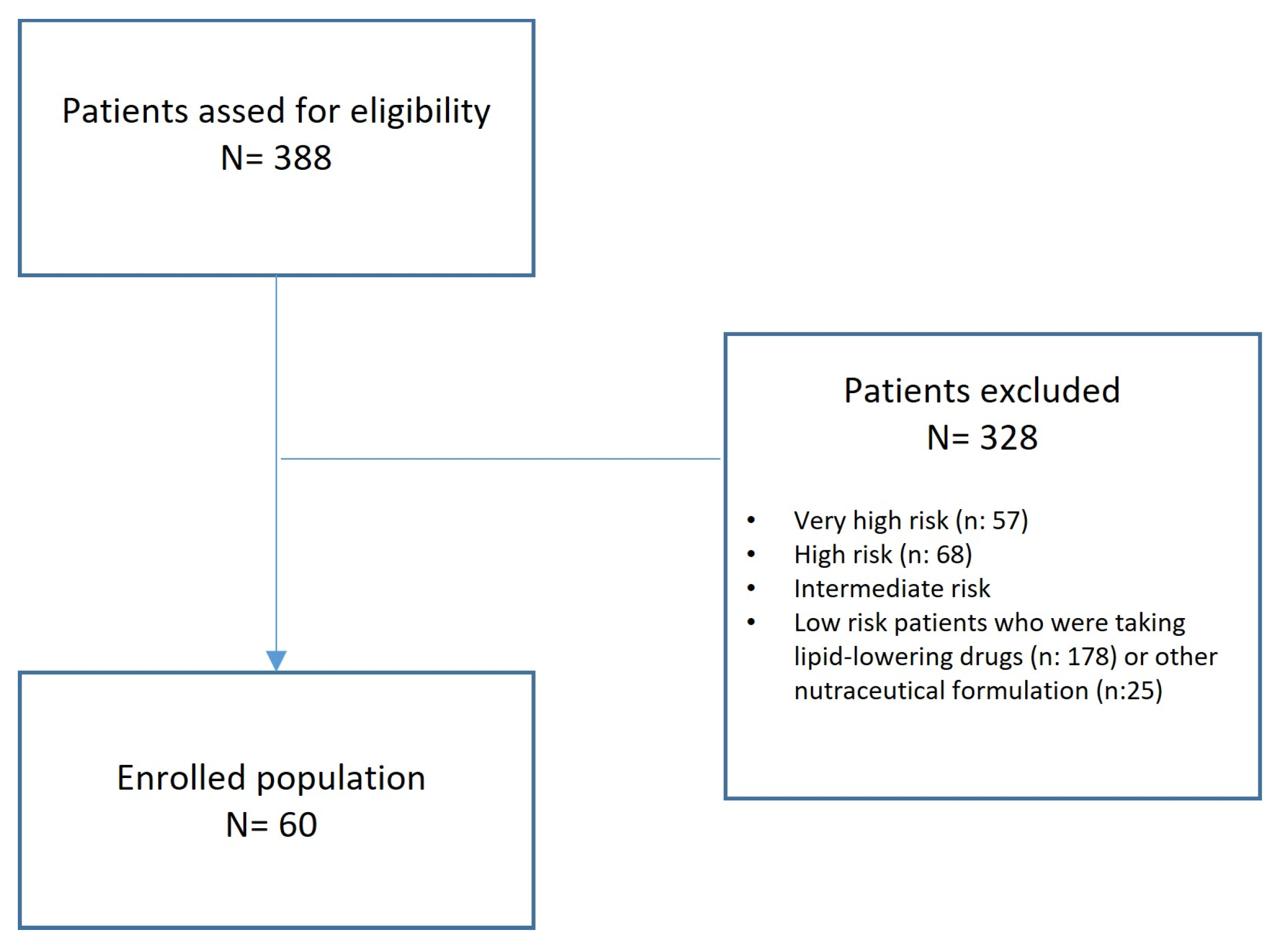
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Reddy, S.; Ôunpuu, S.; Anand, S. Global Burden of Cardiovascular Diseases. Circulation 2001, 104, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Cana-kinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Falco, L.; Tessitore, V.; Ciccarelli, G.; Malvezzi, M.; D’Andrea, A.; Imbalzano, E.; Golino, P.; Russo, V. Antioxidant Properties of Oral Antithrombotic Therapies in Atherosclerotic Disease and Atrial Fibrillation. Antioxidants 2023, 12, 1185. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar]
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [PubMed]
- Ference, B.A.; Kastelein, J.J.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; et al. Association of Triglyceride-Lowering LPL Variants and LDL-C–Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364. [Google Scholar] [CrossRef] [PubMed]
- Raygor, V.; Khera, A. New Recommendations and Revised Concepts in Recent Guidelines on the Management of Dyslipidemias to Prevent Cardiovascular Disease: The 2018 ACC/AHA and 2019 ESC/EAS Guidelines. Curr. Cardiol. Rep. 2020, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Kris-Etherton, P.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B.; et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J. Clin. Lipidol. 2015, 9, S1–S122.e1. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Sialvera, T.E.; Papadopoulou, A.; Efstathiou, S.P.; Trautwein, E.A.; Ras, R.T.; Kollia, N.; Farajian, P.; Goumas, G.; Dimakopoulos, I.; Papavasiliou, K.; et al. Structured advice provided by a dietitian increases adherence of consumers to diet and lifestyle changes and lowers blood low-density lipoprotein (LDL)-cholesterol: The Increasing Adherence of Consumers to Diet & Lifestyle Changes to Lower (LDL) Cholesterol (ACT) randomised controlled trial. J. Hum. Nutr. Diet. 2018, 31, 197–208. [Google Scholar] [PubMed]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Combinations of phytomedicines with different lipid lowering activity for dyslipidemia management: The available clinical data. Phytomedicine 2016, 23, 1113–1118. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Ferroni, A.; Ertek, S. Tolerability and safety of commonly used dietary supplements and nutraceuticals with lipid-lowering effects. Expert Opin. Drug. Saf. 2012, 11, 753–766. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Parini, A.; Rosticci, M. Nutraceuticals and cholesterol-lowering action. IJC Metab. Endocr. 2015, 6, 1–4. [Google Scholar] [CrossRef][Green Version]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of Red Yeast Rice, a Traditional Chinese Food and Medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercholesterolemia. Methodist Debakey Cardiovasc. J. 2019, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the Management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-optimal Lipid Levels? Curr. Atheroscler. Rep. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Catapano, A.L.; Cicero, A.F.G.; Escobar, C.; Foger, B.; Katsiki, N.; Latkovskis, G.; Rakowski, M.; Reiner, Z.; Sahebkar, A.; et al. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: A position paper of the International Lipid Expert Panel. Pharmacol. Res. 2022, 183, 106370. [Google Scholar] [CrossRef]
- Alberts, A.W. Discovery, biochemistry and biology of lovastatin. Am. J. Cardiol. 1988, 62, 10J–15J. [Google Scholar] [CrossRef]
- Li, Y.G.; Zhang, F.; Wang, Z.T.; Hu, Z.B. Identification and chemical profiling of monacolins in red yeast rice using high-performance liquid chromatography with photodiode array detector and mass spectrometry. J. Pharm. Biomed. Anal. 2004, 35, 1101–1112. [Google Scholar] [CrossRef]
- Beltrán, D.; Frutos-Lisón, M.D.; Espín, J.C.; GarcíaVillalba, R. Re-examining the role of the gut microbiota in the conversion of the lipid-lowering statin monacolin K (lovastatin) into its active bhydroxy acid metabolite. Food Funct. 2019, 10, 1787–1791. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.W.; Gerdes, V.E.A. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- Irmak, S.; Dunford, N.T. Policosanol Contents and Compositions of Wheat Varieties. J. Agric. Food Chem. 2005, 53, 5583–5586. [Google Scholar] [CrossRef]
- Nam, D.E.; Yun, J.M.; Kim, D.; Kim, O.K. Policosanol Attenuates Cholesterol Synthesis via AMPK Activation in Hypercholesterolemic Rats. J. Med. Food 2019, 22, 1110–1117. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; Jones, P.J.H.; Kassis, A.N.; Eskin, M.N.A. Policosanols as Nutraceuticals: Fact or Fiction. Crit. Rev. Food Sci. Nutr. 2010, 50, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ishaka, A.; Umar Imam, M.; Mahamud, R.; Zuki, A.B.; Maznah, I. Characterization of rice bran wax policosanol and its nanoemulsion formulation. Int. J. Nanomed. 2014, 9, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Haim, D.; Valenzuela, A.; Brañes, M.C.; Fuenzalida, M.; Videla, L.A. The oleic acid esterification of policosanol increases its bioavailability and hypocholesterolemic action in rats. Grasas Aceites 2012, 63, 345–354. [Google Scholar] [CrossRef]
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; Hatch, G.M.; Chen, L. Berberine Pretreatment Confers Cardioprotection Against Ischemia-Reperfusion Injury in a Rat Model of Type 2 Diabetes. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Ruderman, N.B. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Skarydova, L.; Hofman, J.; Chlebek, J.; Havrankova, J.; Kosanova, K.; Skarka, A.; Hostalkova, A.; Plucha, T.; Cahlikova, L.; Wsol, V. Isoquinoline alkaloids as a novel type of AKR1C3 inhibitors. J. Steroid. Biochem. Mol. Biol. 2014, 143, 250–258. [Google Scholar] [CrossRef]
- Cicero, A.; Ertek, S. Metabolic and cardiovascular effects of berberine: From preclinical evidences to clinical trial results. Clin. Lipidol. 2009, 4, 553–563. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug. Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Caliceti, C.; Franco, P.; Spinozzi, S.; Roda, A.; Cicero, A.F. Berberine: New Insights from Pharmacological Aspects to Clinical Evidences in the Management of Metabolic Disorders. Curr. Med. Chem. 2016, 23, 1460–1476. [Google Scholar] [CrossRef]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug. Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.; Cruickshank, A.; Thorpe, G. Red wine and antioxidant activity in serum. Lancet 1994, 344, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.J.; Nuttall, S.L.; Martin, U. Antioxidant therapy—A new therapeutic option for reducing mortality from coronary artery disease. J. Clin. Pharm. Ther. 1998, 23, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Lavy, A.; Aviram, M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 1995, 61, 549–554. [Google Scholar] [CrossRef]
- Abu-Amsha, R.; Croft, K.D.; Puddey, I.B.; Proudfoot, J.M.; Beilin, L.J. Phenolic Content of Various Beverages Determines the Extent of Inhibition of Human Serum and Low-Density Lipoprotein Oxidation In Vitro: Identification and Mechanism of Action of Some Cinnamic Acid Derivatives from Red Wine. Clin. Sci. 1996, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Facino, R.; Carini, M.; Aldini, G.; Calloni, M.; Bombardelli, E.; Morazzoni, P. Sparing Effect of Procyanidins from Vitis vinifera on Vitamin E: In vitro Studies. Planta Med. 1998, 64, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, S.L.; Kendall, M.J.; Bombardelli, E.; Morazzoni, P. An evaluation of the antioxidant activity of a standardized grape seed extract, Leucoselect®. J. Clin. Pharm. Ther. 1998, 23, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Mao, J.T.; Xue, B.; Fan, S.; Neis, P.; Qualls, C.; Massie, L.; Fiehn, O. Leucoselect Phytosome Modulates Serum Eicosapentaenoic Acid, Docosahexaenoic Acid, and Prostaglandin E3 in a Phase I Lung Cancer Chemoprevention Study. Cancer Prev. Res. 2021, 14, 619–626. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; D’Addato, S.; Borghi, C. A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study). Nutrients 2020, 12, 3127. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Macchi, C.; Botta, M.; Dall’Orto, D.; Del Puppo, M.; Bertolotti, M.; Bosisio, R.; Mombelli, G.; et al. Nutraceutical approach for the management of cardiovascular risk—A combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: Results from a randomized, double-blind, placebo-controlled study. Nutr. J. 2019, 18, 13. [Google Scholar] [CrossRef]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Saccà, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and polico-sanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef]
- Marazzi, G.; Cacciotti, L.; Pelliccia, F.; Iaia, L.; Volterrani, M.; Caminiti, G.; Sposato, B.; Massaro, R.; Grieco, F.; Rosano, G. Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv. Ther. 2011, 28, 1105–1113. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; De Sando, V.; Benedetto, D.; Cevenini, M.; Grandi, E.; Borghi, C. Long-term efficacy and tolerability of a multicomponent lipid-lowering nutraceutical in overweight and normoweight patients. Nutrafoods 2012, 11, 55–61. [Google Scholar] [CrossRef]
- Gonnelli, S.; Caffarelli, C.; Stolakis, K.; Cuda, C.; Giordano, N.; Nuti, R. Efficacy and Tolerability of a Nutraceutical Combination (Red Yeast Rice, Policosanols, and Berberine) in Patients with Low-Moderate Risk Hypercholesterolemia: A Double-Blind, Place-bo-Controlled Study. Curr. Ther. Res. Clin. Exp. 2015, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate car-diometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef]
- Sola, R.; Valls, R.M.; Puzo, J.; Calabuig, J.R.; Brea, A.; Pedret, A.; Morina, D.; Villar, J.; Millan, J.; Anguera, A. Effects of poly-bioactive compounds on lipid profile and body weight in a moderately hypercholesterolemic population with low cardiovascular disease risk: A multicenter randomized trial. PLoS ONE 2014, 9, e101978. [Google Scholar] [CrossRef]
- Barrios, V.; Escobar, C.; Cicero, A.F.G.; Burke, D.; Fasching, P.; Banach, M.; Bruckert, E. A nutraceutical approach (Armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: Review of the clinical evidence. Atheroscler. Suppl. 2017, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Fuchs, D.; Koppenol, W.P.; Schalkwijk, C.G.; Otten-Hofman, A.; Garczarek, U.; Greyling, A.; Wagner, F.; Trautwein, E.A. Effect of a plant sterol-enriched spread on biomarkers of endothelial dysfunction and low-grade inflammation in hypercholesterolaemic subjects. J. Nutr. Sci. 2016, 5, e44. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Ras, R.T.; Gagliardi, A.C.; Mangili, L.C.; Trautwein, E.A.; Santos, R.D. Effects of phytosterols on markers of inflammation: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 76–83. [Google Scholar] [CrossRef]
- Ho, X.L.; Liu, J.J.H.; Loke, W.M. Plant sterol-enriched soy milk consumption modulates 5-lipoxygenase, 12-lipoxygenase, and myeloperoxidase activities in healthy adults—A randomized-controlled trial. Free Radic. Res. 2016, 50, 1396–1407. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padró, T. Phytosterols and Inflammation. Curr. Med. Chem. 2018, 26, 6724–6734. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; Casas, R.; Ruiz-León, A.M.; Sobrino, J.; Ros, E.; Estruch, R. Effects of a Novel Nutraceutical Combination (Aquilea Colesterol®) on the Lipid Profile and Inflammatory Biomarkers: A Randomized Control Trial. Nutrients 2019, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M.; PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef]
- Cicero, A.F.; Morbini, M.; Parini, A.; Urso, R.; Rosticci, M.; Grandi, E.; Borghi, C. Effect of red yeast rice combined with antioxidants on lipid pattern, hs-CRP level, and endothelial function in moderately hypercholesterolemic subjects. Ther. Clin. Risk Manag. 2016, 12, 281–286. [Google Scholar] [CrossRef]
- Badimon, L.; Peña, E.; Arderiu, G.; Padró, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-reactive protein in atherothrombosis and angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Russo, V.; Fabiani, D.; Leonardi, S.; Attena, E.; D’Alterio, G.; Cotticelli, C.; Rago, A.; Sarpa, S.; Maione, B.; D’Onofrio, A.; et al. Dual Pathway Inhibition with Rivaroxaban and Aspirin Reduces Inflammatory Biomarkers in Atherosclerosis. J. Cardiovasc. Pharmacol. 2023, 81, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Viggiano, G.V.; Russo, V.; Mangiacapra, S.; Cavalli, A.; Castaldo, G.; Agrusta, F.; Bellizzi, A.; Amitrano, M.; Iannuzzo, M.; et al. Antithrombotic and Anti-Inflammatory Effects of Fondaparinux and Enoxaparin in Hospitalized COVID-19 Patients: The FONDENOXAVID Study. J. Blood Med. 2021, 12, 69–75. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Campana, P.; Gerundo, G.; Caruso, A.; Grimaldi, M.G.; Russo, V.; Attena, E.; Leosco, D.; Parisi, V. The role of inflammation and metabolic risk factors in the pathogenesis of calcific aortic valve stenosis. Aging Clin. Exp. Res. 2021, 33, 1765–1770. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Poggio, P.; Valerio, V.; Cabaro, S.; Campana, P.; Comentale, G.; Attena, E.; Russo, V.; Pilato, E.; et al. Inflammation and Cardiovascular Diseases in the Elderly: The Role of Epicardial Adipose Tissue. Front. Med. 2022, 9, 844266. [Google Scholar] [CrossRef] [PubMed]
- Caso, V.M.; Manzo, V.; Pecchillo Cimmino, T.; Conti, V.; Caso, P.; Esposito, G.; Russo, V.; Filippelli, A.; Ammendola, R.; Cattaneo, F. Regulation of Inflammation and Oxidative Stress by Formyl Peptide Receptors in Cardiovascular Disease Progression. Life 2021, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Fabiani, D. Put out the fire: The pleiotropic anti-inflammatory action of non-vitamin K oral anticoagulants. Pharmacol. Res. 2022, 182, 106335. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Falco, L.; Tessitore, V.; Mauriello, A.; Catapano, D.; Napolitano, N.; Tariq, M.; Caturano, A.; Ciccarelli, G.; D’Andrea, A.; et al. Anti-Inflammatory and Anticancer Effects of Anticoagulant Therapy in Patients with Malignancy. Life 2023, 13, 1888. [Google Scholar] [CrossRef] [PubMed]
- Vigna, G.B.; Costantini, F.; Aldini, G.; Carini, M.; Catapano, A.; Schena, F.; Tangerini, A.; Zanca, R.; Bombardelli, E.; Morazzoni, P.; et al. Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metabolism 2003, 52, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Wilson, D.; Guthrie, N. A randomized, double-blind, placebo-controlled, pilot study to evaluate the effect of whole grape extract on antioxidant status and lipid profile. J. Funct. Foods 2014, 7, 680–691. [Google Scholar] [CrossRef]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet. Med. 2009, 26, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Tubaro, F.; Rong, J.; Sevanian, A. Optimization of nutrition: Polyphenols and vascular protection. Nutr. Rev. 1999, 57, 241–249. [Google Scholar] [CrossRef]
- Gabetta, B.; Fuzzati, N.; Griffini, A.; Lolla, E.; Pace, R.; Ruffilli, T.; Peterlongo, F. Characterization of proanthocyanidins from grape seeds. Fitoterapia 2000, 71, 162–175. [Google Scholar] [CrossRef]
- Mao, J.T.; Smoake, J.; Park, H.K.; Lu, Q.Y.; Xue, B. Grape Seed Procyanidin Extract Mediates Antineoplastic Effects against Lung Cancer via Modulations of Prostacyclin and 15-HETE Eicosanoid Pathways. Cancer Prev. Res. 2016, 9, 925–932. [Google Scholar] [CrossRef]
- Niki, E. Do free radicals play causal role in atherosclerosis? Low density lipoprotein oxidation and vitamin E revisited. J. Clin. Biochem. Nutr. 2011, 48, 3. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Foglietto Illustrativo.net. Available online: https://www.fogliettoillustrativo.net/bugiardino/ostacol-30cps-921581803 (accessed on 21 March 2024).

| Characteristics | n = 60 |
|---|---|
| Age—yr | 48.02 ± 10 |
| BMI—Kg/m2 | 25.7 ± 3.1 |
| Heart rate—beats/min | 77 ± 18 |
| Systolic blood pressure—mmHg | 116 ± 15 |
| Diastolic blood pressure—mmHg | 67 ± 11 |
| Total Cholesterol, mg/dL | 219 ± 24.6 |
| LDL-C, mg/dL | 137.1 ± 19.3 |
| PLA2, ng/mL | 118.48 ± 42.1 |
| CRP, mg/dL | 0.82 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, V.; Napolitano, N.; Ascrizzi, A.; Leonardi, S.; Pisacane, F.; Di Micco, P.; Imbalzano, E.; Sasso, F.C.; D’Andrea, A.; Caturano, A.; et al. The Lipid-Lowering Efficacy of a Nutraceutical Combination Including Leucoselect Phytosome, Red Yeast Rice, Policosanol and Folic Acid in Dyslipidaemia Patients: Real-World Insights. Pharmaceuticals 2024, 17, 447. https://doi.org/10.3390/ph17040447
Russo V, Napolitano N, Ascrizzi A, Leonardi S, Pisacane F, Di Micco P, Imbalzano E, Sasso FC, D’Andrea A, Caturano A, et al. The Lipid-Lowering Efficacy of a Nutraceutical Combination Including Leucoselect Phytosome, Red Yeast Rice, Policosanol and Folic Acid in Dyslipidaemia Patients: Real-World Insights. Pharmaceuticals. 2024; 17(4):447. https://doi.org/10.3390/ph17040447
Chicago/Turabian StyleRusso, Vincenzo, Nicola Napolitano, Antonia Ascrizzi, Silvia Leonardi, Filomena Pisacane, Pierpaolo Di Micco, Egidio Imbalzano, Ferdinando Carlo Sasso, Antonello D’Andrea, Alfredo Caturano, and et al. 2024. "The Lipid-Lowering Efficacy of a Nutraceutical Combination Including Leucoselect Phytosome, Red Yeast Rice, Policosanol and Folic Acid in Dyslipidaemia Patients: Real-World Insights" Pharmaceuticals 17, no. 4: 447. https://doi.org/10.3390/ph17040447
APA StyleRusso, V., Napolitano, N., Ascrizzi, A., Leonardi, S., Pisacane, F., Di Micco, P., Imbalzano, E., Sasso, F. C., D’Andrea, A., Caturano, A., & Mauriello, A. (2024). The Lipid-Lowering Efficacy of a Nutraceutical Combination Including Leucoselect Phytosome, Red Yeast Rice, Policosanol and Folic Acid in Dyslipidaemia Patients: Real-World Insights. Pharmaceuticals, 17(4), 447. https://doi.org/10.3390/ph17040447












