Transient Receptor Potential Ankyrin 1 (TRPA1) Modulation by 4-Hydroxynonenal (4-HNE) in Pancreatic Adenocarcinoma Cell Lines: Putative Roles for Therapies
Abstract
1. Introduction
2. Results
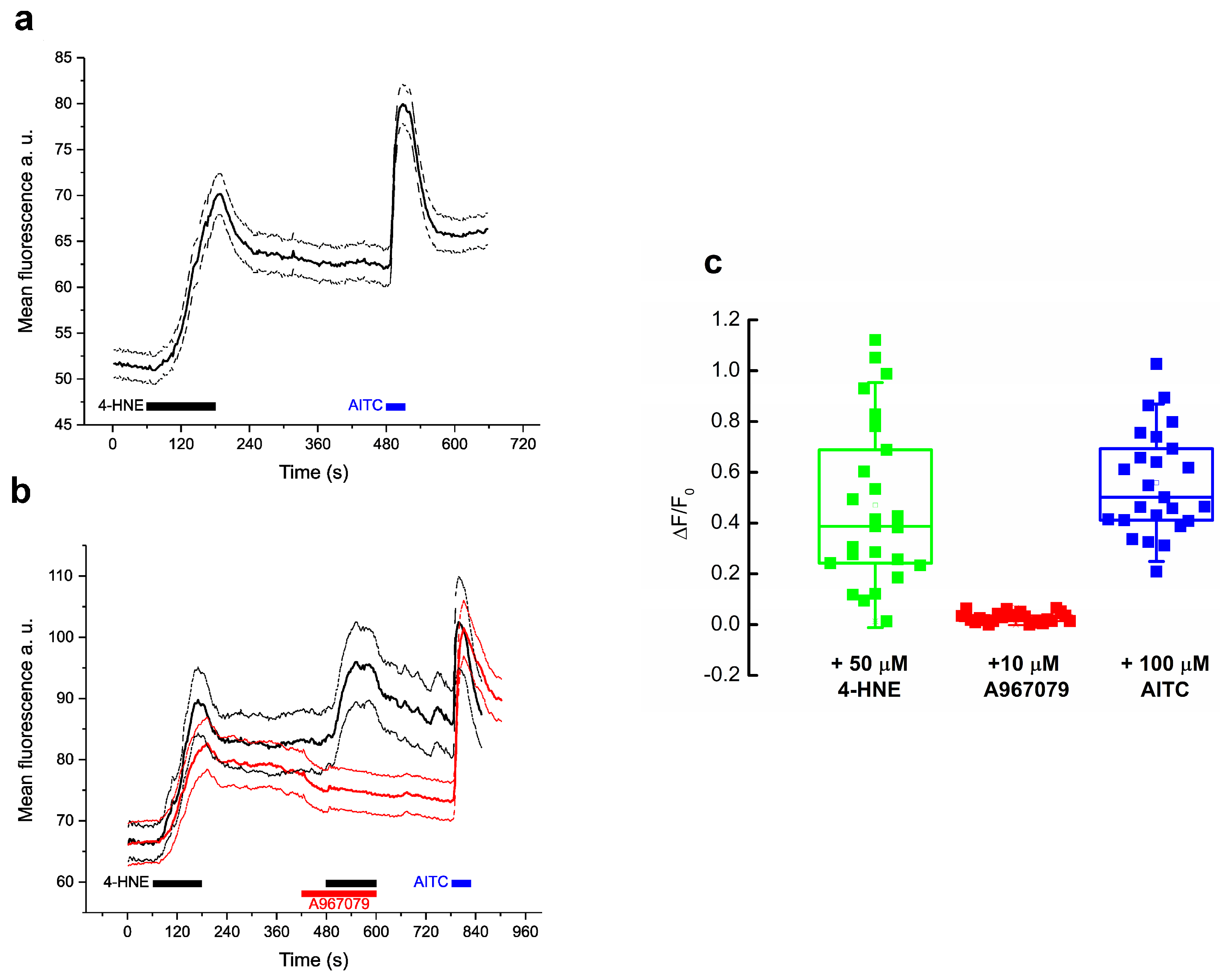
2.1. 4-HNE Directly Induces Ca2+ Uptake via TRPA1 Channel Activation
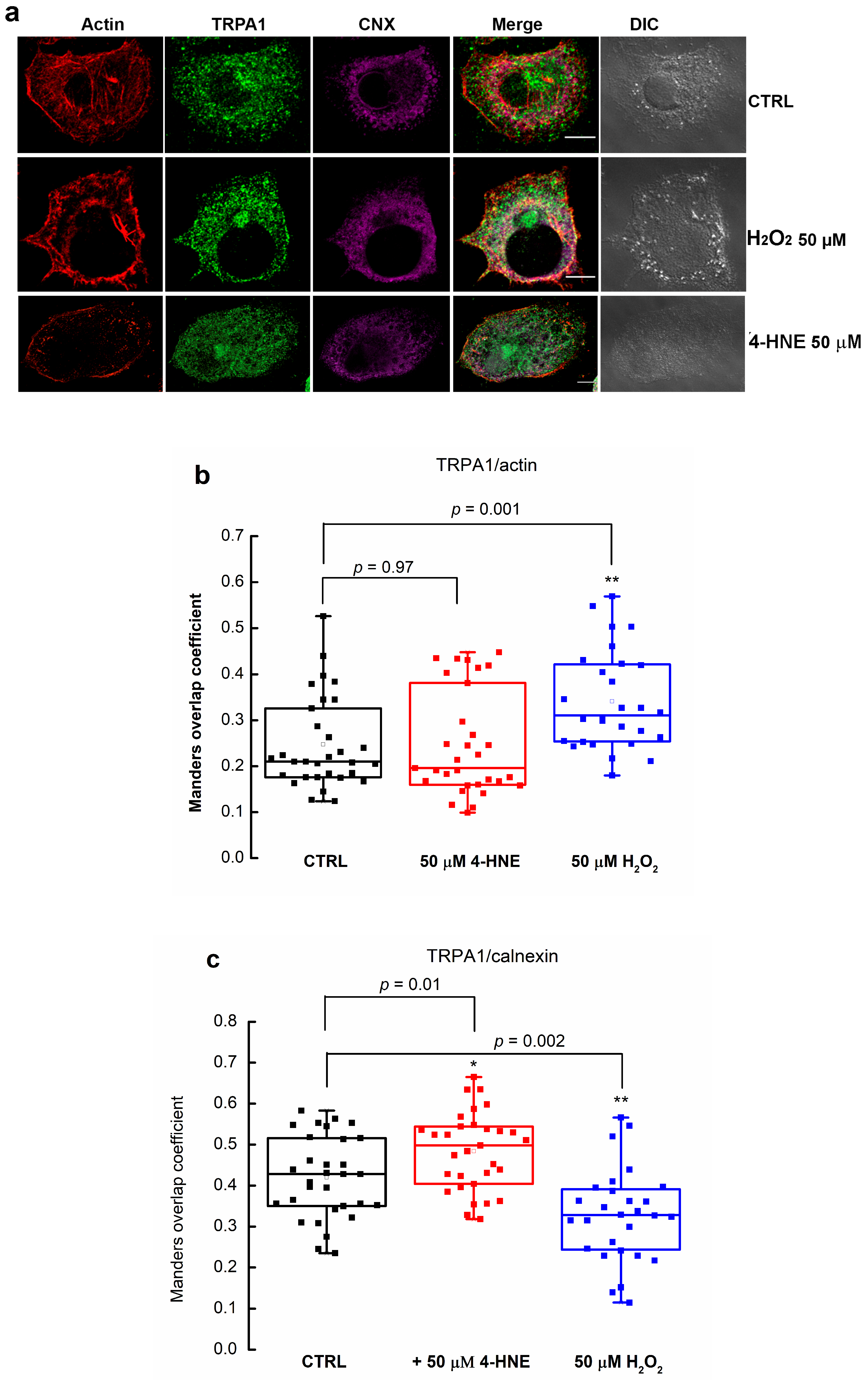
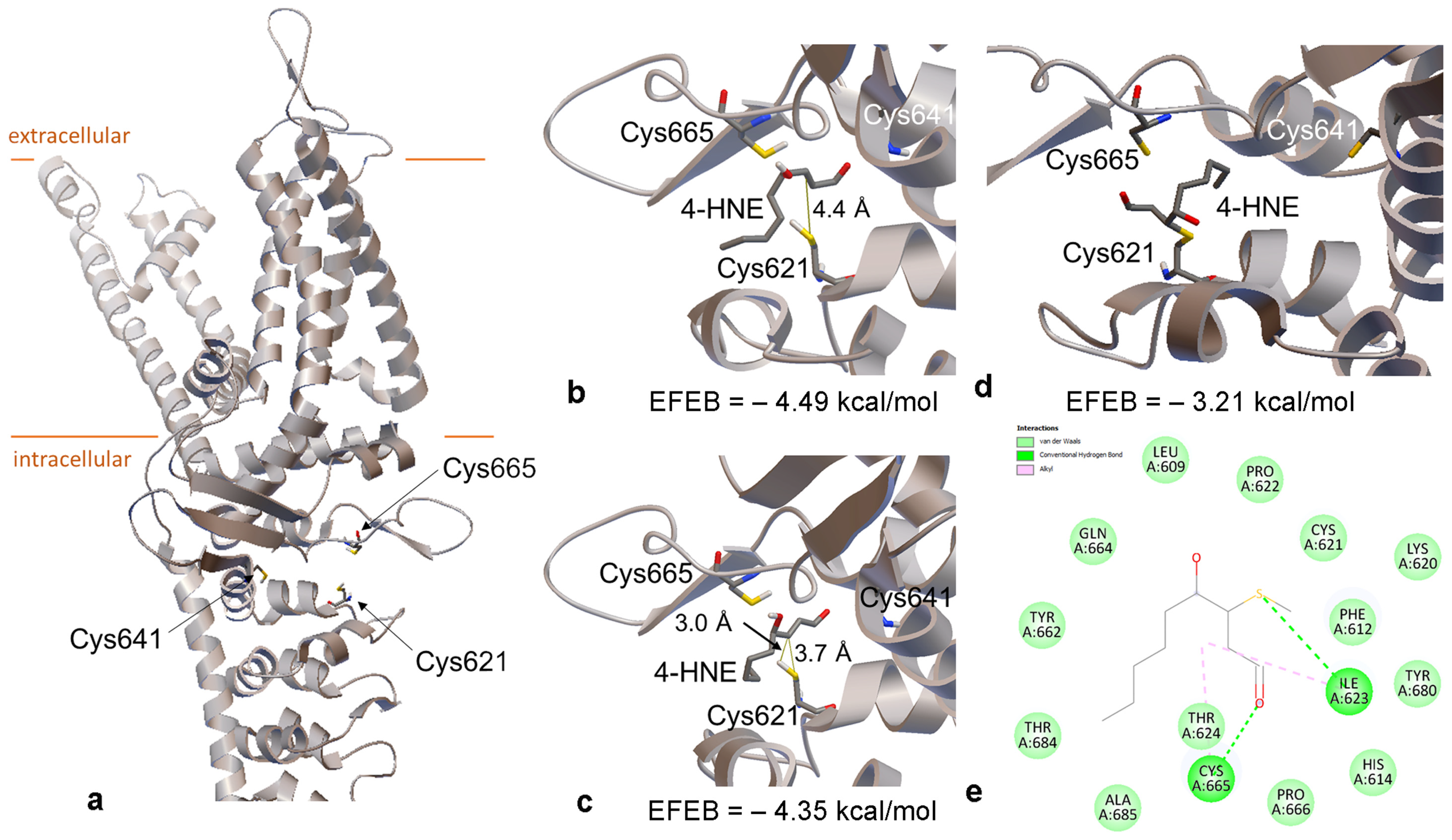
2.2. 4-HNE Stabilizes the Open Structure of TRPA1
2.3. Exogenous 4-HNE Passively Diffuses across the Cell Membrane
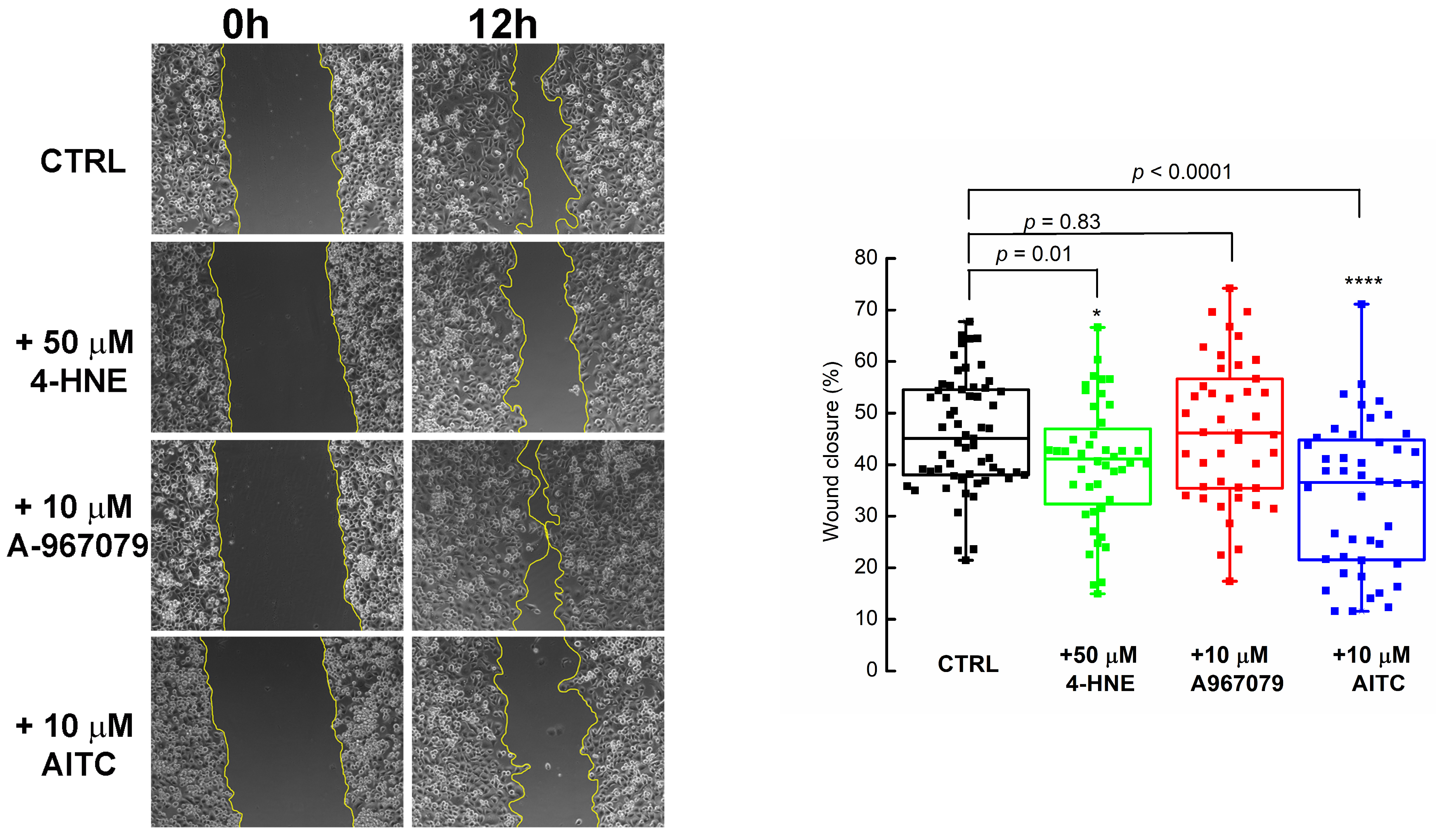
2.4. PDAC Cell Migration Is Impeded by 4-HNE and Recovered in the Presence of the TRPA1 Antagonist
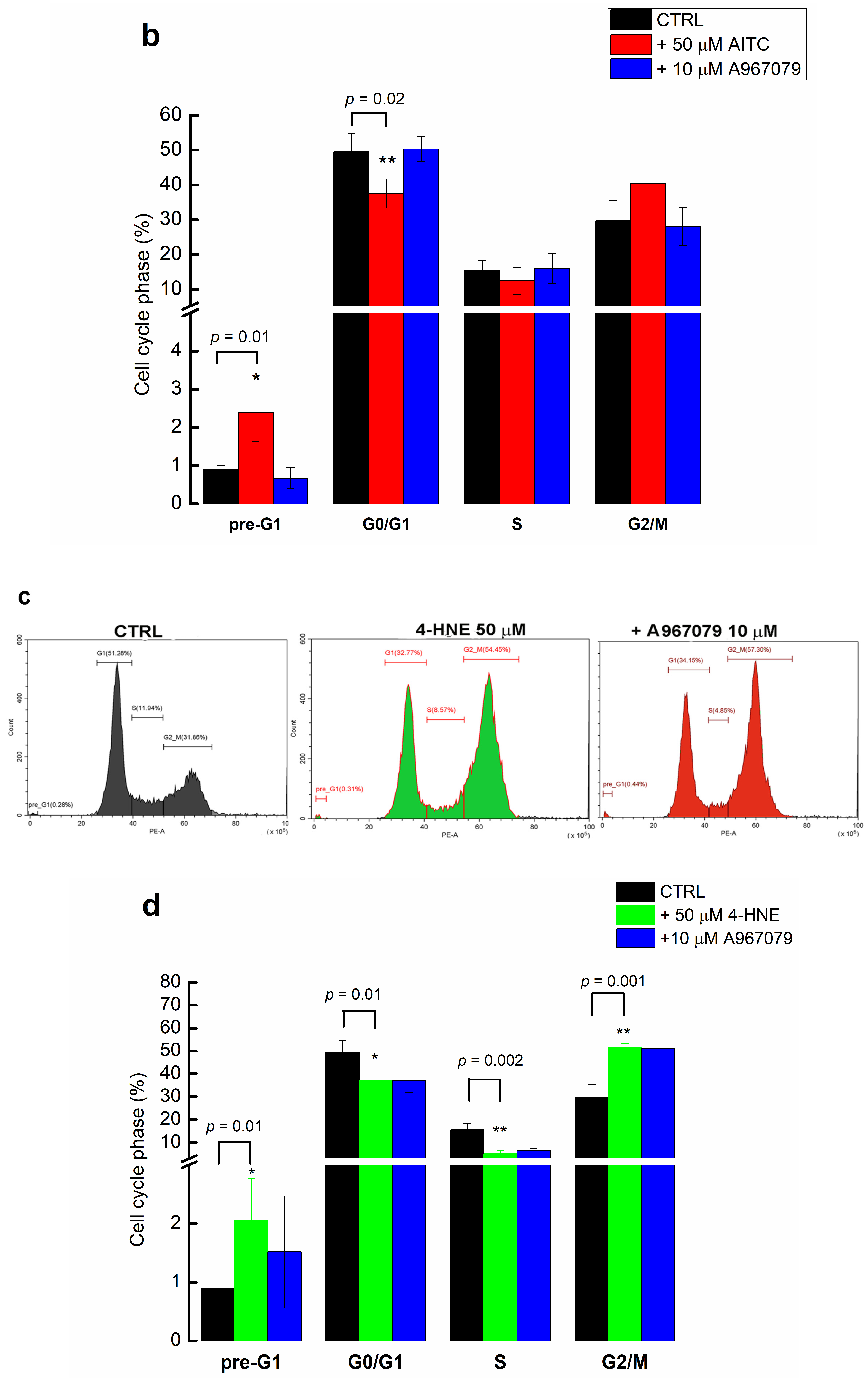
2.5. 4-HNE Stimulates Pre-G1 Phase and Arrests the Cells in the G2/M Phase
3. Discussion
4. Materials and Methods
4.1. In Vitro Cell Culture Model
4.2. Intracellular Nonratiometric Calcium Microfluorimetry
4.3. Immunofluorescence
4.4. Molecular Docking
4.5. Prediction of Membrane Permeability
4.6. Wound Healing Assay
4.7. Cell Cycle
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Partyka, O.; Pajewska, M.; Kwasniewska, D.; Czerw, A.; Deptala, A.; Budzik, M.; Cipora, E.; Gaska, I.; Gazdowicz, L.; Mielnik, A.; et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers 2023, 15, 3634. [Google Scholar] [CrossRef]
- Giuliani, J.; Bonetti, A. FOLFIRINOX is a cost-effective combination chemotherapy in first-line for advanced pancreatic Cancer. Pancreatology 2019, 19, 325–330. [Google Scholar] [CrossRef]
- Marini, M.; Titiz, M.; de Araújo, D.S.M.; Geppetti, P.; Nassini, R.; De Logu, F. TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches. Biomolecules 2023, 13, 1557. [Google Scholar] [CrossRef]
- Piciu, F.; Balas, M.; Badea, M.A.; Cucu, D. TRP Channels in Tumoral Processes Mediated by Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1327. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.; Saimanen, I.; Koskela, R.; Holopainen, A.; Selander, T. Plasma Concentration of the Lipid Peroxidation (LP) Biomarker 4-Etaydroxynonenal (4-HNE) in Benign and Cancer Patients. Vivo 2022, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Santin, Y.; Fazal, L.; Sainte-Marie, Y.; Sicard, P.; Maggiorani, D.; Tortosa, F.; Yucel, Y.Y.; Teyssedre, L.; Rouquette, J.; Marcellin, M.; et al. Mitochondrial 4-HNE derived from MAO-A promotes mitoCa2+ overload in chronic postischemic cardiac remodeling. Cell Death Differ. 2020, 27, 1907–1923. [Google Scholar] [CrossRef] [PubMed]
- Chelaru, N.R.; Chiosa, A.; Sorop, A.; Spiridon, A.; Cojocaru, F.; Domocos, D.; Cucu, D.; Popescu, I.; Dima, S.O. The Association between TRP Channels Expression and Clinicopathological Characteristics of Patients with Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 9045. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.; Selescu, T.; Domocos, D.; Marutescu, L.; Chiritoiu, G.; Chelaru, N.R.; Dima, S.; Mihailescu, D.; Babes, A.; Cucu, D. Functional expression of the transient receptor potential ankyrin type 1 channel in pancreatic adenocarcinoma cells. Sci. Rep. 2021, 11, 2018, Erratum in Sci. Rep. 2021, 11, 8853. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef]
- Milkovic, L.; Zarkovic, N.; Marusic, Z.; Zarkovic, K.; Jaganjac, M. The 4-Hydroxynonenal-Protein Adducts and Their Biological Relevance: Are Some Proteins Preferred Targets? Antioxidants 2023, 12, 856. [Google Scholar] [CrossRef]
- Habgood, M.; Seiferth, D.; Zaki, A.M.; Alibay, I.; Biggin, P.C. Atomistic mechanisms of human TRPA1 activation by electrophile irritants through molecular dynamics simulation and mutual information analysis. Sci. Rep. 2022, 12, 4929. [Google Scholar] [CrossRef]
- Kong, N.; Li, W.; Zhang, J.; Wang, X.; Hu, L.; Xu, Q. TRPM8 as a Potential Biomarker and Therapeutic Target for Gastric Cancer Identified by a Combination of Text Mining and RNA Sequencing. Curr. Gene Ther. 2023, 23, 391–399. [Google Scholar] [CrossRef]
- Haustrate, A.; Mihalache, A.; Cordier, C.; Gosset, P.; Prevarskaya, N.; Lehen’kyi, V. A Novel Anti-TRPV6 Antibody and Its Application in Cancer Diagnosis In Vitro. Int. J. Mol. Sci. 2022, 24, 419. [Google Scholar] [CrossRef]
- Li, C.; Yin, X.; Liu, Z.; Wang, J. Emerging Potential Mechanism and Therapeutic Target of Ferroptosis in PDAC: A Promising Future. Int. J. Mol. Sci. 2022, 23, 15031. [Google Scholar] [CrossRef]
- Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr. Med. Chem. 2014, 21, 230–237. [Google Scholar] [CrossRef]
- Oehler, B.; Kloka, J.; Mohammadi, M.; Ben-Kraiem, A.; Rittner, H.L. D-4F, an ApoA-I mimetic peptide ameliorating TRPA1-mediated nocifensive behaviour in a model of neurogenic inflammation. Mol. Pain. 2020, 16, 1744806920903848. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miura, D.; Kusano, K.F.; Fujimoto, Y.; Sumita-Yoshikawa, W.; Fuke, S.; Nishii, N.; Nagase, S.; Hata, Y.; Morita, H.; et al. 4-Hydroxy-2-nonenal induces calcium overload via the generation of reactive oxygen species in isolated rat cardiac myocytes. J. Card. Fail. 2009, 15, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, J.; Cho, I.; Sheen, Y.Y. Radiotherapy-induced oxidative stress and fibrosis in breast cancer are suppressed by vactosertib, a novel, orally bioavailable TGF-beta/ALK5 inhibitor. Sci. Rep. 2022, 12, 16104. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Nakamura, S.; Zhao, M.; So, K.; Inoue, K.; Numata, T.; Takahashi, N.; Shirakawa, H.; Mori, Y.; Nakagawa, T.; et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat. Commun. 2016, 7, 12840. [Google Scholar] [CrossRef]
- Suo, Y.; Wang, Z.; Zubcevic, L.; Hsu, A.L.; He, Q.; Borgnia, M.J.; Ji, R.R.; Lee, S.Y. Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 2020, 105, 882–894.e885. [Google Scholar] [CrossRef]
- Zhao, J.; Lin King, J.V.; Paulsen, C.E.; Cheng, Y.; Julius, D. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 2020, 585, 141–145. [Google Scholar] [CrossRef]
- Balestrini, A.; Joseph, V.; Dourado, M.; Reese, R.M.; Shields, S.D.; Rouge, L.; Bravo, D.D.; Chernov-Rogan, T.; Austin, C.D.; Chen, H.; et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 2021, 218, e20201637. [Google Scholar] [CrossRef]
- Moccia, F.; Montagna, D. Transient Receptor Potential Ankyrin 1 (TRPA1) Channel as a Sensor of Oxidative Stress in Cancer Cells. Cells 2023, 12, 1261. [Google Scholar] [CrossRef]
- Perkovic, M.N.; Jaganjac, M.; Milkovic, L.; Horvat, T.; Rojo, D.; Zarkovic, K.; Coric, M.; Hudolin, T.; Waeg, G.; Orehovec, B.; et al. Relationship between 4-Hydroxynonenal (4-HNE) as Systemic Biomarker of Lipid Peroxidation and Metabolomic Profiling of Patients with Prostate Cancer. Biomolecules 2023, 13, 145. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, T.; Li, J.; Xia, M.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Kan, D.; Xie, Y.; et al. Oxidative Stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the Pathogenesis and Treatment of Aging-related Diseases. J. Immunol. Res. 2022, 2022, 2233906. [Google Scholar] [CrossRef]
- Watabe, S.; Aruga, Y.; Kato, R.; Kawade, G.; Kubo, Y.; Tatsuzawa, A.; Onishi, I.; Kinowaki, Y.; Ishibashi, S.; Ikeda, M.; et al. Regulation of 4-HNE via SMARCA4 Is Associated with Worse Clinical Outcomes in Hepatocellular Carcinoma. Biomedicines 2023, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- Noe, R.; Inglese, N.; Romani, P.; Serafini, T.; Paoli, C.; Calciolari, B.; Fantuz, M.; Zamborlin, A.; Surdo, N.C.; Spada, V.; et al. Organic Selenium induces ferroptosis in pancreatic cancer cells. Redox Biol. 2023, 68, 102962. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Sharma, R.; Sahu, M.; Vishwanatha, J.K.; Awasthi, S.; Awasthi, Y.C. 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J. Biol. Chem. 2013, 288, 20532–20546. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003.e1007. [Google Scholar] [CrossRef]
- Liu, Q.; Ge, W.; Martinez-Jarquin, S.; He, Y.; Wu, R.; Stoffel, M.; Zenobi, R. Mass Spectrometry Reveals High Levels of Hydrogen Peroxide in Pancreatic Cancer Cells. Angew. Chem. Int. Ed. 2023, 62, e202213703. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Perluigi, M.; Reed, T.; Muharib, T.; Hughes, C.P.; Robinson, R.A.; Sultana, R. Redox proteomics in selected neurodegenerative disorders: From its infancy to future applications. Antioxid. Redox Signal. 2012, 17, 1610–1655. [Google Scholar] [CrossRef]
- Bianco, G.; Forli, S.; Goodsell, D.S.; Olson, A.J. Covalent docking using autodock: Two-point attractor and flexible side chain methods. Protein Sci. 2016, 25, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lomize, A.L.; Hage, J.M.; Schnitzer, K.; Golobokov, K.; LaFaive, M.B.; Forsyth, A.C.; Pogozheva, I.D. PerMM: A Web Tool and Database for Analysis of Passive Membrane Permeability and Translocation Pathways of Bioactive Molecules. J. Chem. Inf. Model. 2019, 59, 3094–3099. [Google Scholar] [CrossRef]
- Sun, J.; Aluvila, S.; Kotaria, R.; Mayor, J.A.; Walters, D.E.; Kaplan, R.S. Mitochondrial and Plasma Membrane Citrate Transporters: Discovery of Selective Inhibitors and Application to Structure/Function Analysis. Mol. Cell. Pharmacol. 2010, 2, 101–110. [Google Scholar] [PubMed]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef]
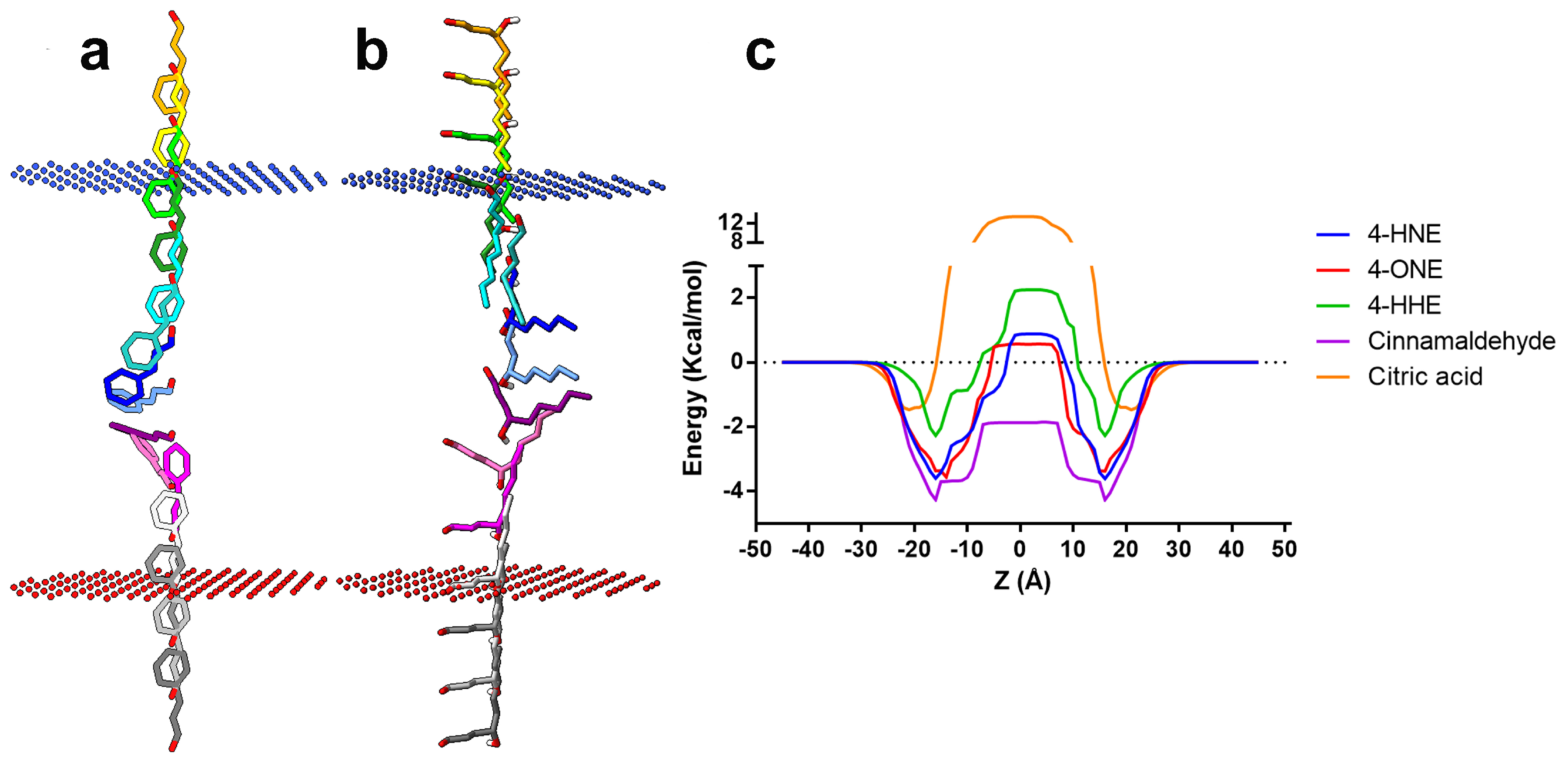

| Compound | ΔG (kcal/mol) | logPerm |
|---|---|---|
| 4-HNE | −3.62 | −0.81 |
| 4-ONE | −3.65 | −0.67 |
| 4-HHE | −2.27 | −1.77 |
| Cinnamaldehyde | −4.29 | 1.21 |
| Citric acid | −1.40 | −10.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piciu, F.; Domocos, D.; Chiritoiu, G.; Chiritoiu-Butnaru, M.; Mernea, M.; Popescu, C.G.; Mihai, D.P.; Galateanu, B.; Hudita, A.; Babes, A.; et al. Transient Receptor Potential Ankyrin 1 (TRPA1) Modulation by 4-Hydroxynonenal (4-HNE) in Pancreatic Adenocarcinoma Cell Lines: Putative Roles for Therapies. Pharmaceuticals 2024, 17, 344. https://doi.org/10.3390/ph17030344
Piciu F, Domocos D, Chiritoiu G, Chiritoiu-Butnaru M, Mernea M, Popescu CG, Mihai DP, Galateanu B, Hudita A, Babes A, et al. Transient Receptor Potential Ankyrin 1 (TRPA1) Modulation by 4-Hydroxynonenal (4-HNE) in Pancreatic Adenocarcinoma Cell Lines: Putative Roles for Therapies. Pharmaceuticals. 2024; 17(3):344. https://doi.org/10.3390/ph17030344
Chicago/Turabian StylePiciu, Florentina, Dan Domocos, Gabriela Chiritoiu, Marioara Chiritoiu-Butnaru, Maria Mernea, Cezar Gabriel Popescu, Dragos Paul Mihai, Bianca Galateanu, Ariana Hudita, Alexandru Babes, and et al. 2024. "Transient Receptor Potential Ankyrin 1 (TRPA1) Modulation by 4-Hydroxynonenal (4-HNE) in Pancreatic Adenocarcinoma Cell Lines: Putative Roles for Therapies" Pharmaceuticals 17, no. 3: 344. https://doi.org/10.3390/ph17030344
APA StylePiciu, F., Domocos, D., Chiritoiu, G., Chiritoiu-Butnaru, M., Mernea, M., Popescu, C. G., Mihai, D. P., Galateanu, B., Hudita, A., Babes, A., & Cucu, D. (2024). Transient Receptor Potential Ankyrin 1 (TRPA1) Modulation by 4-Hydroxynonenal (4-HNE) in Pancreatic Adenocarcinoma Cell Lines: Putative Roles for Therapies. Pharmaceuticals, 17(3), 344. https://doi.org/10.3390/ph17030344








