In Vivo Pharmacokinetic Study of Polygonatum cyrtonema Polysaccharide DPC1 after Oral and Intraperitoneal Administration
Abstract
1. Introduction
2. Results
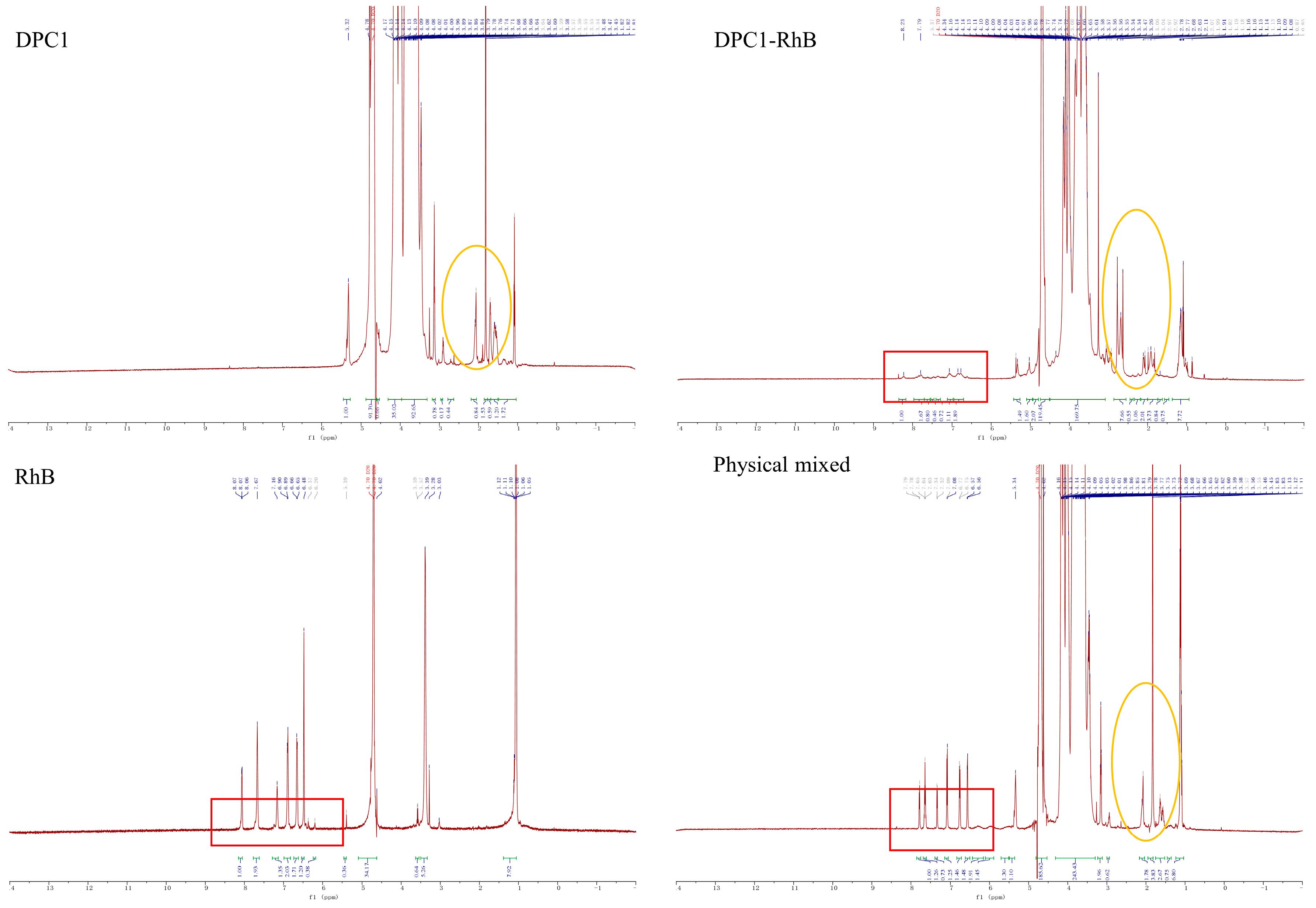
2.1. Characterization of DPC1-RhB
2.2. Determination of the Degree of Fluorescence Substitution
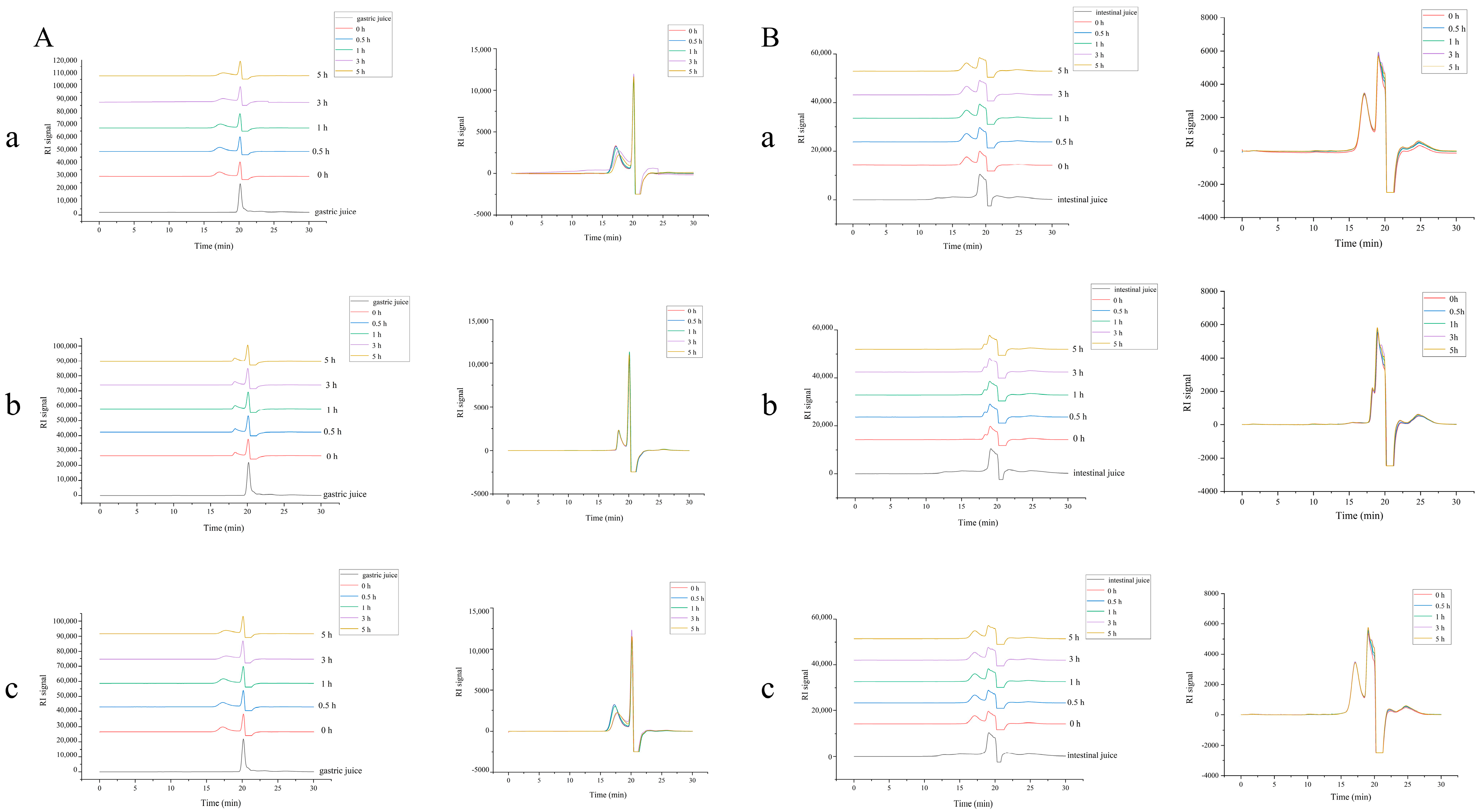
2.3. Stability of DPC1-RhB In Vitro
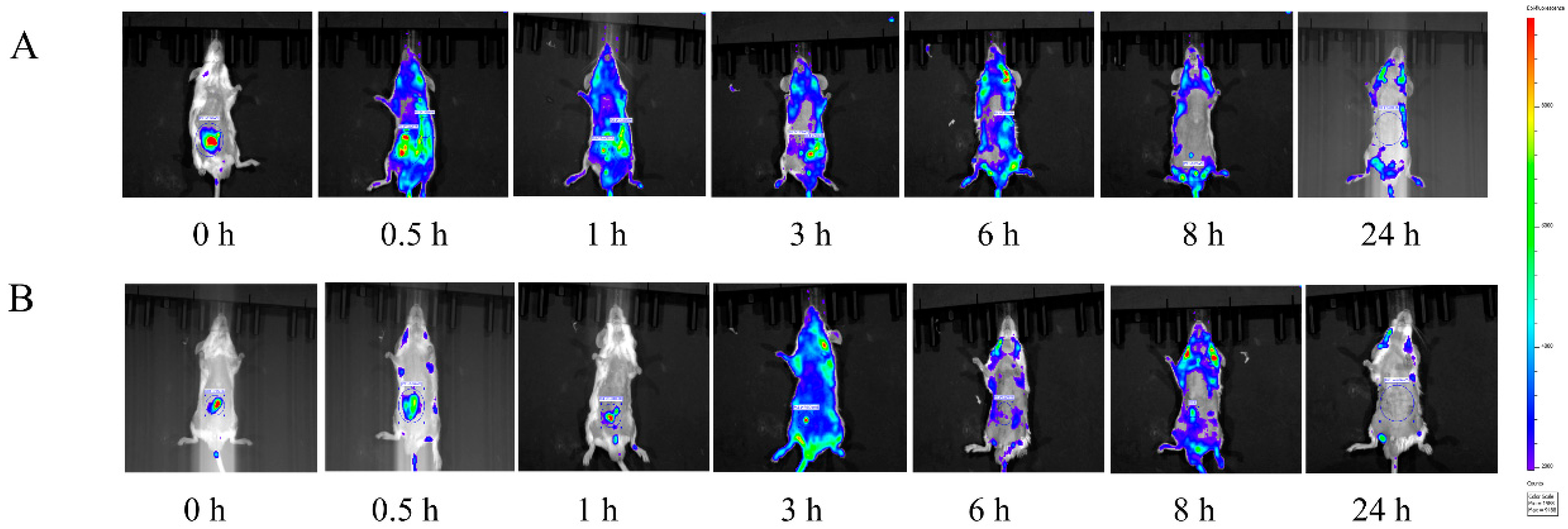
2.4. In Vivo Imaging Studies
2.5. Verification of Quantitative Analysis Method of DPC1-RhB in Plasma and Tissues
2.5.1. Linear Relationship
2.5.2. Precision and Accuracy Test
2.5.3. Stability Test
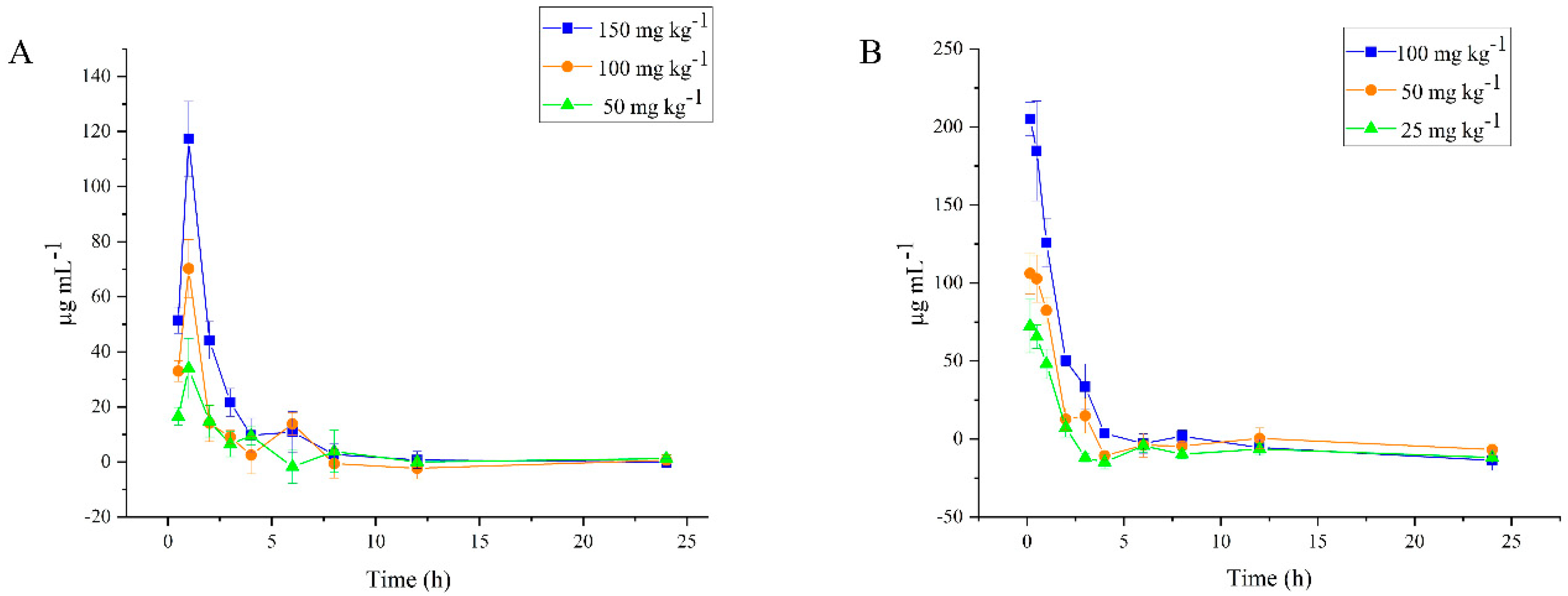
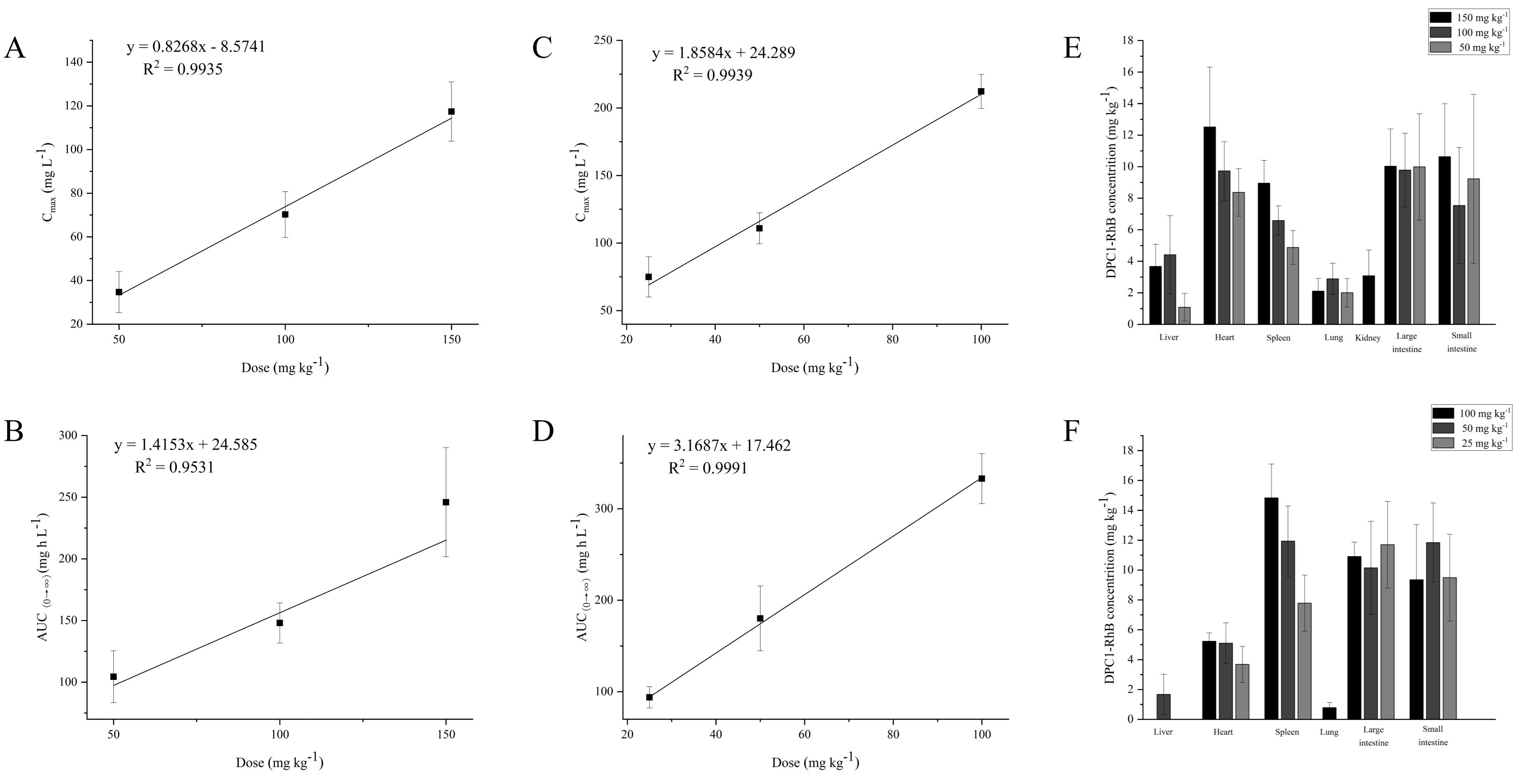
2.6. Plasma Level of DPC1-RhB
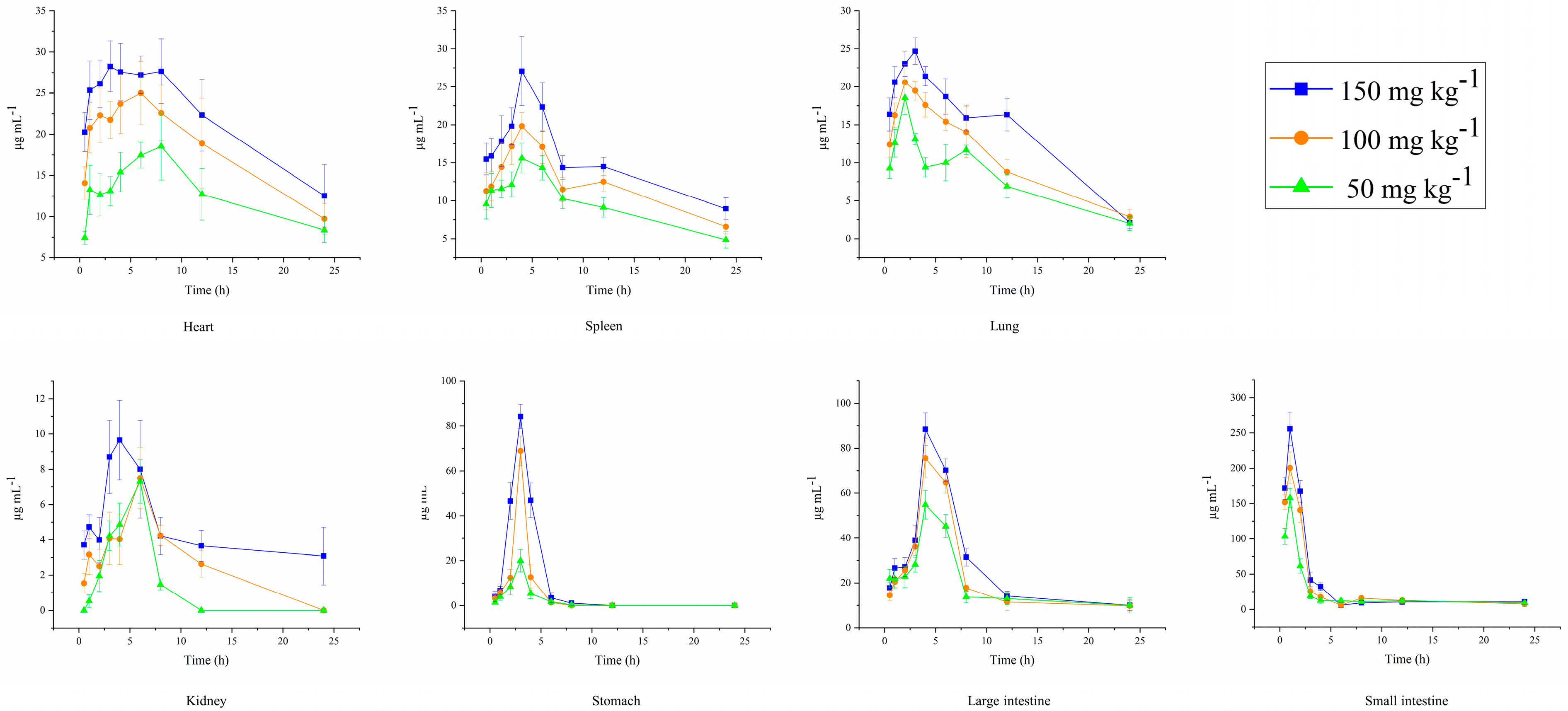
2.7. Tissue Distribution of DPC1-RhB
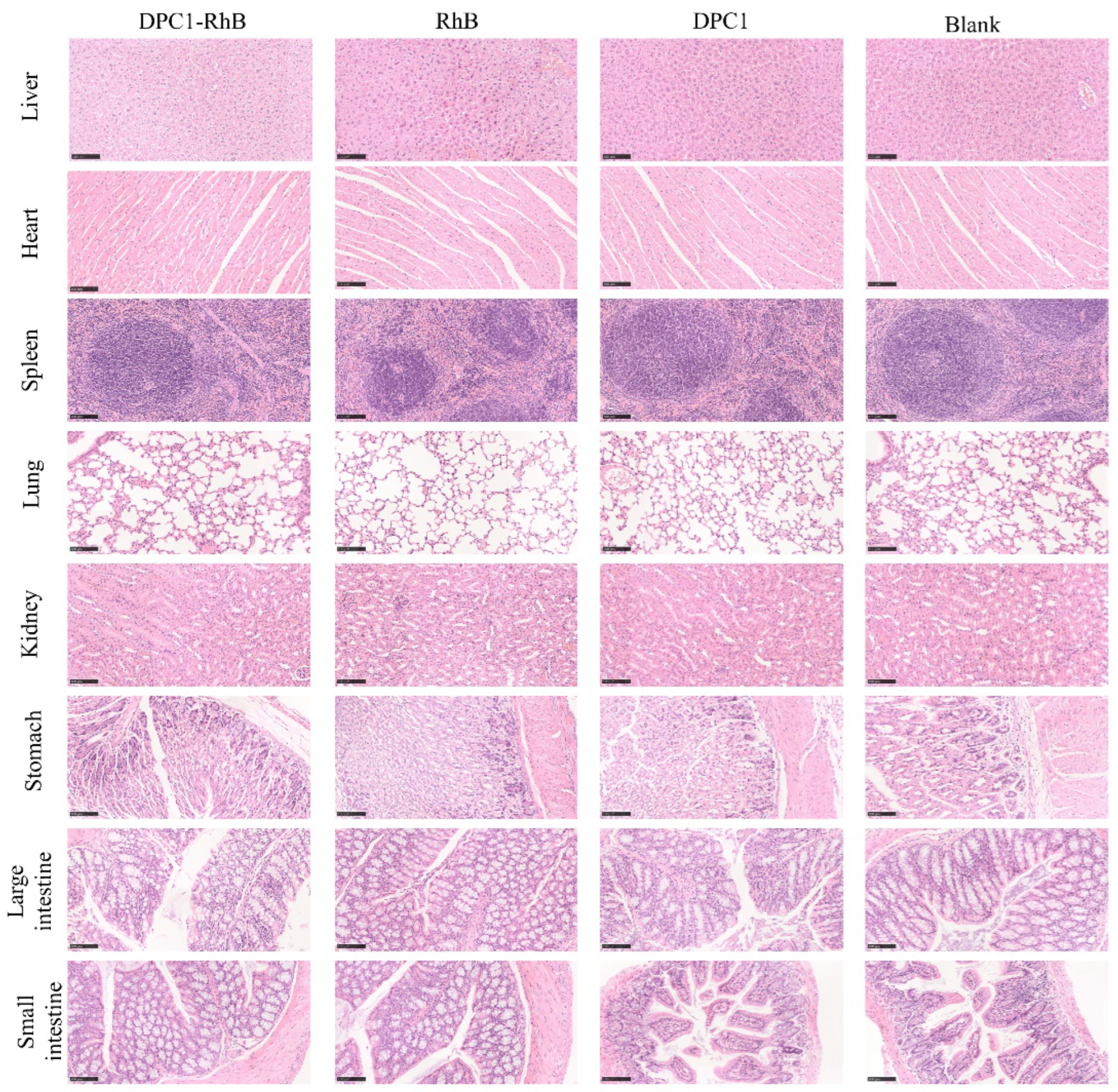
2.8. Toxicity Studies
3. Discussion
4. Materials and Methods
4.1. Materials and Animals
4.2. Extraction and Purification of DPC1
4.3. Preparation of Fluorescently Labeled DPC1
4.4. Characterization of DPC1 and DPC1-RhB
4.5. Fluorescence Labeling Rate of DPC1
4.6. Determination of DPC1-RhB Stability In Vitro
4.7. In Vivo Imaging Studies
4.8. Establishment of Quantitative Analysis Methods for DPC1-RhB in Plasma and Tissues
4.8.1. Linear Relationship
4.8.2. Method Validation
Precision Test
Stability Test
Recovery Rates of Samples
4.9. Pharmacokinetics and Tissue Distribution Studies
4.10. HE Toxicity Studies
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.S. Six features and applications of food therapy for health and wellness in the Supplementary Records of Famous Physicians (Ming Yi Bie Lu). New Chin. Med. 2011, 43, 118–119. [Google Scholar] [CrossRef]
- Chen, X.J.; Duan, J.F.; Liu, K.Q.; Guo, Y.Y.; Wang, D.P.; Liu, M.; Zhao, D.; Li, B.; Li, H.L.; Wang, X.B. Botany, Traditional Uses, and Pharmacology of Polygonati rhizoma. Chin. Med. Cult. 2021, 4, 251–259. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Wang, Y.; Yan, L.; Guo, L.; Huang, L.; Gao, W. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr. Polym. 2020, 233, 115836. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Qiu, Y.; Gong, L.; Wang, W.; Wen, R. A Review of Polygonatum Mill. Genus: Its Taxonomy, Chemical Constituents, and Pharmacological Effect Due to Processing Changes. Molecules 2022, 27, 4821. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, S.; Cao, H.; Guo, H.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Han, C. A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum polysaccharides. Molecules 2018, 23, 1170. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Shang, Y.; Wang, Y.; Gao, J.; Xue, N.; Huang, C.; Li, F.; Li, J. Hypoglycemic and Hypolipidemic Activity of Polygonatum sibiricum Fermented with Lactobacillus brevis YM 1301 in Diabetic C57BL/6 Mice. J. Med. Food 2021, 24, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Wu, S.; Huang, X.-L.; Hu, X.-Q.; Zhang, Y. Hypolipidemic Activity and Antiatherosclerotic Effect of Polysaccharide of Polygonatum sibiricum in Rabbit Model and Related Cellular Mechanisms. Evid.-Based Complement. Altern. Med. 2015, 2015, 391065. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Fu, R.; Ou, J.; Wang, B. Structural characterization and anti-inflammatory activity of a novel polysaccharide PKP2-1 from Polygonatum kingianum. Front. Nutr. 2023, 10, 1156798. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Yuan, L.; Ruan, H.; Zhu, Z.; Fan, X.; Zhu, L.; Peng, X. Blood-Enriching Effects and Immune-Regulation Mechanism of Steam-Processed Polygonatum sibiricum Polysaccharide in Blood Deficiency Syndrome Mice. Front. Immunol. 2022, 13, 813676. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, B.; Huang, J.; Li, W.; Yi, P.; Yi, M.; Peng, W. Identification of the protective effect of Polygonatum sibiricum polysaccharide ond-galactose-induced brain ageing in mice by the systematic characterization of a circular RNA-associated ceRNA network. Pharm. Biol. 2021, 59, 345–364. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Nie, G.; Liu, J.; Mei, H.; He, Z.; Dou, P.; Wang, K. Tracking the gastrointestinal digestive and metabolic behaviour of Dendrobium officinale polysaccharides by fluorescent labelling. Food Funct. 2022, 13, 7274–7286. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, X.; Xu, J.; Wu, Z.; Zhang, Y.; Wang, K. Advances in tracking of polysaccharides in vivo: Labeling strategies, potential factors and applications based on pharmacokinetic characteristics. Int. J. Biol. Macromol. 2020, 163, 1403–1420. [Google Scholar] [CrossRef]
- He, Y.; Xing, Y.; Jiang, T.; Wang, J.; Sang, S.; Rong, H.; Yu, F. Fluorescence labeling of extracellular vesicles for diverse bio-applications in vitro and in vivo. Chem. Commun. 2023, 59, 6609–6626. [Google Scholar] [CrossRef]
- Song, S.; Wei, Q.; Wang, K.; Yang, Q.; Wang, Y.; Ji, A.; Chen, G. Fluorescent Labeling of Polymannuronic Acid and Its Distribution in Mice by Tail Vein Injection. Mar. Drugs 2022, 20, 289. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, H.; Kamiya, M.; Kawatani, M.; Kojima, R.; Yamasoba, T.; Urano, Y. Fluorescence Probes for Imaging Basic Carboxypeptidase Activity in Living Cells with High Intracellular Retention. Anal. Chem. 2021, 93, 3470–3476. [Google Scholar] [CrossRef]
- Ma, X.; Shi, L.; Zhang, B.; Liu, L.; Fu, Y.; Zhang, X. Recent advances in bioprobes and biolabels based on cyanine dyes. Anal. Bioanal. Chem. 2022, 414, 4551–4573. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Zhu, M.; Liu, D.; Liu, D.; Zhao, J. Fluorescence properties of fluorescein and rhodamine supported on alumina nanowire films. Ceram. Int. 2021, 48, 11181–11191. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.B.; Du, L.B.; Lv, Y.G. Synthesis and Study of Fluorescent Probe Molecules Based on Rhodamine Class B Derivatives. Key Eng. Mater. 2021, 881, 117–122. [Google Scholar] [CrossRef]
- Xue, Y.; Lee, J.; Kim, H.-J.; Cho, H.-J.; Zhou, X.; Liu, Y.; Tebon, P.; Hoffman, T.; Qu, M.; Ling, H.; et al. Rhodamine Conjugated Gelatin Methacryloyl Nanoparticles for Stable Cell Imaging. ACS Appl. Bio Mater. 2020, 3, 6908–6918. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.-H.; Zhang, X.-W.; Xu, K.-P.; Jiang, J.-G. Application of fluorescently labeled tracer technique for detection of natural active macromolecules in Chinese medicine. Drug Metab. Rev. 2013, 46, 57–71. [Google Scholar] [CrossRef]
- Sarkar, S.; Chatterjee, A.; Biswas, K. A Recent Update on Rhodamine Dye Based Sensor Molecules: A Review. Crit. Rev. Anal. Chem. 2023, 1–27. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Luo, L.; Zhou, Z.; Wang, Y.; Gao, T.; Yang, L.; Peng, T.; Wu, M. Structures of fructan and galactan from Polygonatum cyrtonema and their utilization by probiotic bacteria. Carbohydr. Polym. 2021, 267, 118219. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Barón-Sevilla, B.; Guerrero-Rentería, Y.; Mata-Sotres, J.A.; Viana, M.T.; Pohlenz, C.; Lazo, J.P. The prebiotic effect of agavin inclusion levels in low fishmeal diets for Totoaba macdonaldi juveniles. Anim. Feed. Sci. Technol. 2023, 303, 115695. [Google Scholar] [CrossRef]
- Flores-Méndez, L.C.; Lizárraga-Velázquez, C.E.; Sánchez-Gutiérrez, E.Y.; Arrizon, J.; Leyva-López, N.; Hernández, C. Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions. Fishes 2022, 7, 340. [Google Scholar] [CrossRef]
- Ochoa-Romo, J.P.; Cornejo-Granados, F.; Lopez-Zavala, A.A.; Viana, M.T.; Sánchez, F.; Gallardo-Becerra, L.; Luque-Villegas, M.; Valdez-López, Y.; Sotelo-Mundo, R.R.; Cota-Huízar, A.; et al. Agavin induces beneficial microbes in the shrimp microbiota under farming conditions. Sci. Rep. 2022, 12, 6392. [Google Scholar] [CrossRef]
- Kilua, A.; Pelpolage, S.; Goto, A.; Nakayama, Y.; Kitazono, E.; Toyohara, K.; Nagata, R.; Fukuma, N.; Han, K.-H.; Fukushima, M. Deciphering the colonic fermentation characteristics of agavin and digestion-resistant maltodextrin in a simulated batch fermentation system. Int. J. Biol. Macromol. 2021, 189, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Mazzocco, E.; Saldaña-Robles, A.; Franco-Robles, E.; Mireles-Arriaga, A.I.; Mares-Mares, E.; Ozuna, C. Optimization of gluten-free muffin formulation with agavin-type fructans as fat and sucrose replacer using response surface methodology. Future Foods 2022, 5, 100112. [Google Scholar] [CrossRef]
- Madia, V.N.; De Vita, D.; Messore, A.; Toniolo, C.; Tudino, V.; De Leo, A.; Pindinello, I.; Ialongo, D.; Saccoliti, F.; D’ursi, A.M.; et al. Analytical Characterization of an Inulin-Type Fructooligosaccharide from Root-Tubers of Asphodelus ramosus L. Pharmaceuticals 2021, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhao, C.; Jin, W.; Chen, Q.; Fan, B.; Qian, C. Study on pharmacokinetics and tissue distribution of Polygonatum sibiricum polysaccharide in rats by fluorescence labeling. Int. J. Biol. Macromol. 2022, 215, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, L.; Wu, J.; Liu, Y.; Zhang, Z.; Guan, Q. Doxorubicin-loaded folate-mediated pH-responsive micelle based on Bletilla striata polysaccharide: Release mechanism, cellular uptake mechanism, distribution, pharmacokinetics, and antitumor effects. Int. J. Biol. Macromol. 2020, 164, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wu, S.; Yan, Y.; Chen, X.; Guan, J.; Bao, Y.; Xiong, X.; Liu, L. Rice bran polysaccharide-metal complexes showed safe antioxidant activity in vitro. Int. J. Biol. Macromol. 2018, 126, 934–940. [Google Scholar] [CrossRef]
- Wu, J.; Guo, X.X.; Li, R.Y.; Jia, M.Z.; LI, Y.Y.; Chen, Y. Fluorescent labeling of Acanthopanax giraldii Harms polysaccharide and its pharmacokinetics in mice. Cent. South Pharm. 2020, 18, 1328–1333. [Google Scholar]
- Li, F.; Wei, Y.; Zhao, J.; Zhang, L.; Li, Q. In vivo pharmacokinetic study of a Cucurbita moschata polysaccharide after oral administration. Int. J. Biol. Macromol. 2022, 203, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shao, Y.-Y.; Zhao, Y.-N.; Zhang, X.; Chang, Z.-P.; Sun, Y.-F.; Liu, J.-J.; Gao, J.; Hou, R.-G. Pharmacokinetics, distribution and excretion of inulin-type fructan CPA after oral or intravenous administration to mice. Food Funct. 2022, 13, 4130–4141. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xiao, B.; Hao, X.; Tan, J.; Gu, J.; Wang, G.; Wang, W.; Zhang, Y. Pumpkin polysaccharide preparation, simulated gastrointestinal digestion, and in vivo biodistribution. Int. J. Biol. Macromol. 2019, 141, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Yang, C.; Zhou, B.; Tang, H.; Yang, L.; Liao, W.; Sun, G. Pharmacokinetics and Excretion Study of Lycium barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling. Foods 2021, 10, 2851. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Z.; Yang, X.; Pan, X.; Yin, L.; Huang, X.; Li, Q.; Shu, Y.; Zhang, Q.; Wang, K. A sensitive and rapid radiolabelling method for the in vivo pharmacokinetic study of lentinan. Food Funct. 2018, 9, 3114–3125. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, C.; Wu, C.; Du, K.; Zhang, J.; Qin, H.; Hou, J.; Du, G. HPLC–MS and HPLC–MS/MS analysis of seven active constituents of Xiao-Xu-Ming decoction and application to a pharmacokinetic study after oral administration to rat. Acta Pharm. Sin. B 2012, 2, 188–197. [Google Scholar] [CrossRef]
- Gidal, B.E.; Majid, O.; Ferry, J.; Hussein, Z.; Yang, H.; Zhu, J.; Fain, R.; Laurenza, A. The practical impact of altered dosing on perampanel plasma concentrations: Pharmacokinetic modeling from clinical studies. Epilepsy Behav. 2014, 35, 6–12. [Google Scholar] [CrossRef]
- Metsugi, Y.; Miyaji, Y.; Ogawara, K.-I.; Higaki, K.; Kimura, T. Appearance of Double Peaks in Plasma Concentration–time Profile after Oral Administration Depends on Gastric Emptying Profile and Weight Function. Pharm. Res. 2007, 25, 886–895. [Google Scholar] [CrossRef]
- Toshimoto, K.; Tomoda, Y.; Chiba, K.; Sugiyama, Y. Analysis of the Change in the Blood Concentration-Time Profile Caused by Complex Drug–Drug Interactions in the Liver Considering the Enterohepatic Circulation: Examining Whether the Inhibition Constants for Uptake, Metabolism, and Biliary Excretion Can be Recovered by the Analyses Using Physiologically Based Pharmacokinetic Modeling. J. Pharm. Sci. 2017, 106, 2727–2738. [Google Scholar] [CrossRef] [PubMed]
- Van der Heyden, S.; Croubels, S.; Gadeyne, C.; Ducatelle, R.; Daminet, S.; Escobar, H.M.; Sterenczak, K.; Polis, I.; Schauvliege, S.; Hesta, M.; et al. Influence of P-glycoprotein modulation on plasma concentrations and pharmacokinetics of orally administered prednisolone in dogs. Am. J. Vet. Res. 2012, 73, 900–907. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Huang, J.; Li, X.; Fan, X.; Li, W.; Wu, G.; Xie, C.; Fan, X.-X.; Zhang, J.; et al. Pharmacokinetics, absorption and transport mechanism for ginseng polysaccharides. Biomed. Pharmacother. 2023, 162, 114610. [Google Scholar] [CrossRef] [PubMed]
- Yu-Hao, D.; Chun, C.; Xiong, F.; Rui-Hai, L. Study on the pharmacokinetics of mulberry fruit polysaccharides through fluorescence labeling. Int. J. Biol. Macromol. 2021, 186, 462–471. [Google Scholar] [CrossRef]
- Tanaka, T.; Fujishima, Y.; Hamano, S.; Kaneo, Y. Cellular disposition of arabinogalactan in primary cultured rat hepatocytes. Eur. J. Pharm. Sci. 2004, 22, 435–444. [Google Scholar] [CrossRef]
- Tanaka, T.; Fujishima, Y.; Hanano, S.; Kaneo, Y. Intracellular disposition of polysaccharides in rat liver parenchymal and nonparenchymal cells. Int. J. Pharm. 2004, 286, 9–17. [Google Scholar] [CrossRef]
- Wenzel, C.; Lapczuk-Romanska, J.; Malinowski, D.; Ostrowski, M.; Drozdzik, M.; Oswald, S. Comparative Intra-Subject Analysis of Gene Expression and Protein Abundance of Major and Minor Drug Metabolizing Enzymes in Healthy Human Jejunum and Liver. Clin. Pharmacol. Ther. 2023, 115, 221–230. [Google Scholar] [CrossRef]
- Bie, B.-J.; Zhao, X.-R.; Yan, J.-R.; Ke, X.-J.; Liu, F.; Yan, G.-P. Dextran Fluorescent Probes Containing Sulfadiazine and Rhodamine B Groups. Molecules 2022, 27, 6747. [Google Scholar] [CrossRef]
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Part Ⅳ; China Pharmaceutical Science and Technology Press: Beijing, China, 2020; Volume 129, p. 424. [Google Scholar]
- Usta, D.Y.; Olgac, S.; Timur, B.; Teksin, Z.S. Development and pharmacokinetic evaluation of Neusilin® US2-based S-SNEDDS tablets for bosentan: Fasted and fed states bioavailability, IVIS® real-time biodistribution, and ex-vivo imaging. Int. J. Pharm. 2023, 643, 123219. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, Y.; Zhou, Z.; Xu, J.; Wang, H.; Chen, D.; Wang, K. Dynamic evaluation of the protective effect of Dendrobium officinale polysaccharide on acute alcoholic liver injury mice in vitro and in vivo by NIR fluorescence imaging. Anal. Bioanal. Chem. 2021, 413, 5715–5724. [Google Scholar] [CrossRef]
- Zhang, Q. Fingerprint and Preliminary Pharmacokinetic Study of Sulfatedhetero-Polysaccharide (UF) Extracted from Saccharina japonica. Master’s thesis, University of Chinese Academy of Sciences, Beijing, China, 2018. [Google Scholar]
- Maiques, O.; Sanz-Moreno, V. Multiplex chromogenic immunohistochemistry to stain and analyze paraffin tissue sections from the mouse or human. STAR Protoc. 2022, 3, 101879. [Google Scholar] [CrossRef] [PubMed]
- Abramova, T.V.; Merkulova, I.B.; Kulbachevskaya, N.Y.; Konyaeva, O.I.; Ermakova, N.P.; Chaley, V.A.; Bukhman, V.M. The comparative pathomorphologic research of the inner organs of rats on the preclinical study of vincristine-ROnC and vincristine-teva. Russ. J. Biother. 2018, 17, 76–82. [Google Scholar] [CrossRef]
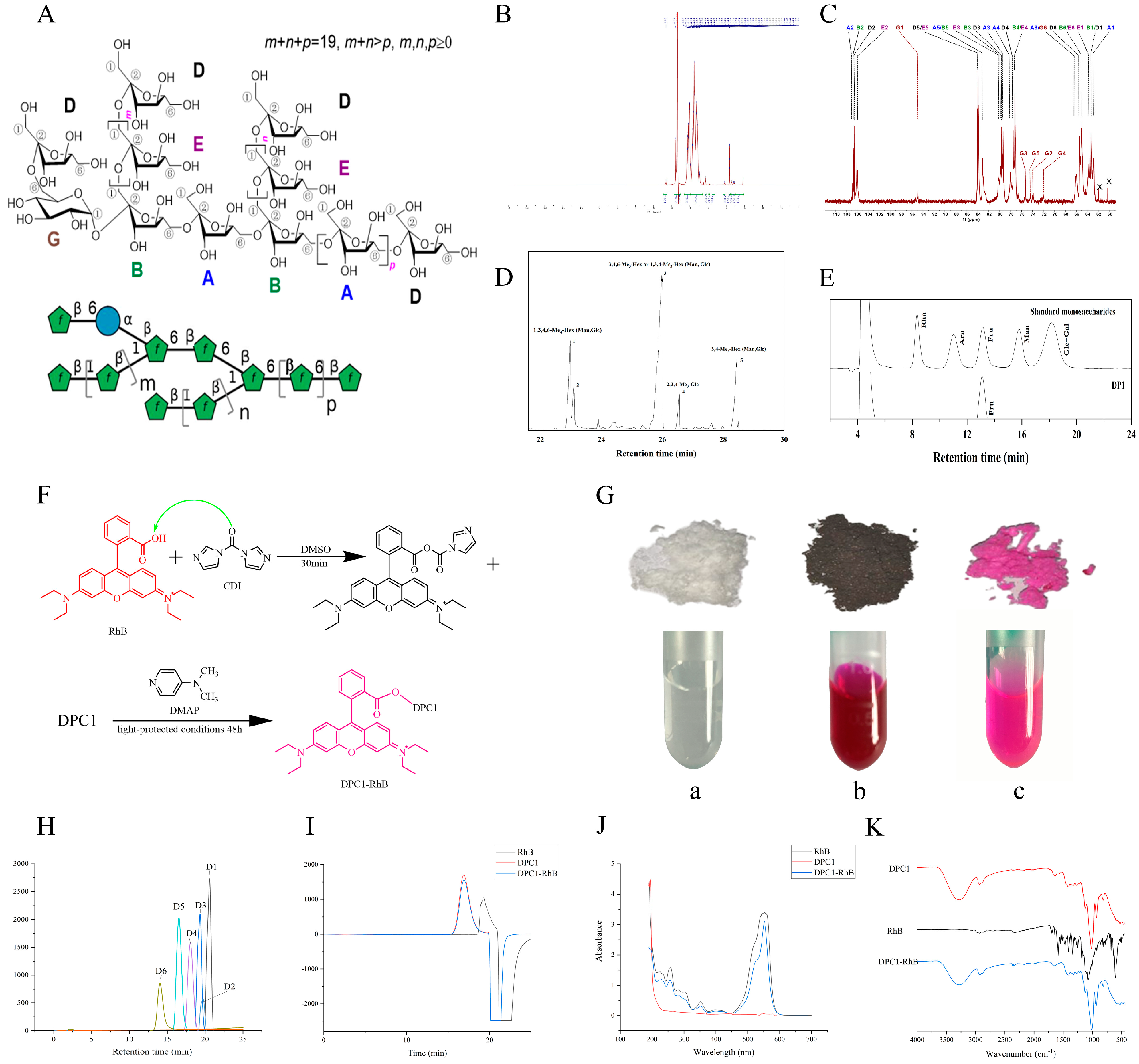

| Sample | Mw (Da) | Mw/Mn | Mw Average | Mw/Mn Average | S | RSD | |
|---|---|---|---|---|---|---|---|
| DPC1 | 1 | 3217 | 1.2230 | 3185 | 1.23 | 58.02 | 1.82% |
| 2 | 3280 | 1.2216 | |||||
| 3 | 3161 | 1.2185 | |||||
| 4 | 3127 | 1.2332 | |||||
| 5 | 3191 | 1.2246 | |||||
| 6 | 3132 | 1.2343 | |||||
| DPC1-RhB | 1 | 3097 | 1.2429 | 3134 | 1.24 | 27.14 | 0.87% |
| 2 | 3124 | 1.2371 | |||||
| 3 | 3137 | 1.226 | |||||
| 4 | 3125 | 1.2447 | |||||
| 5 | 3144 | 1.2354 | |||||
| 6 | 3179 | 1.2242 |
| Sample | Linear Equation | R2 |
|---|---|---|
| Plasma | y = 0.0292x − 0.4713 | 0.9986 |
| Liver | y = 0.0477x + 0.1519 | 0.9994 |
| Heart | y = 0.0627x − 0.2516 | 0.9989 |
| Spleen | y = 0.0315x − 0.0675 | 0.9997 |
| Lung | y = 0.0225x − 0.0446 | 0.9975 |
| Kidney | y = 0.0397x + 0.2638 | 0.9932 |
| Stomach | y = 0.0391x + 0.2153 | 0.9992 |
| Large intestine | y = 0.0294x − 0.2787 | 0.9998 |
| Small intestine | y = 0.0163x − 0.2572 | 0.9978 |
| Sample | Concentration (µg mL−1) | Measured Concentration (µg mL−1) | Accuracy (RE%) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) |
|---|---|---|---|---|---|
| Plasma | 100 | 95.120 ± 10.037 | −4.88 | 4.21 | 4.09 |
| 50 | 54.360 ± 3.715 | 8.72 | 5.39 | 3.52 | |
| 1 | 1.045 ± 0.190 | 4.45 | 9.04 | 6.33 | |
| Liver | 100 | 104.547 ± 8.171 | 4.55 | 5.21 | 6.34 |
| 50 | 50.853 ± 6.037 | 1.71 | 3.84 | 2.11 | |
| 1 | 1.159 ± 0.222 | 15.93 | 9.73 | 7.18 | |
| Heart | 100 | 97.008 ± 4.988 | −2.99 | 10.56 | 8.38 |
| 50 | 52.861 ± 6.728 | 5.72 | 7.42 | 4.33 | |
| 1 | 1.113 ± 0.139 | 11.32 | 4.26 | 6.17 | |
| Spleen | 100 | 103.908 ± 14.948 | 3.91 | 8.29 | 6.78 |
| 50 | 47.737 ± 3.439 | −4.53 | 7.37 | 4.52 | |
| 1 | 0.905 ± 0.147 | −9.52 | 10.42 | 4.39 | |
| Lung | 100 | 105.076 ± 11.102 | 5.08 | 2.62 | 1.17 |
| 50 | 49.049 ± 9.059 | −1.90 | 8.36 | 11.28 | |
| 1 | 0.978 ± 0.195 | −2.22 | 13.99 | 17.52 | |
| Kidney | 100 | 95.718 ± 5.601 | −4.28 | 7.49 | 3.77 |
| 50 | 55.552 ± 6.178 | 11.10 | 2.59 | 6.61 | |
| 1 | 0.907 ± 0.213 | −9.32 | 8.90 | 4.85 | |
| Stomach | 100 | 93.824 ± 5.191 | −6.18 | 4.43 | 2.21 |
| 50 | 53.558 ± 5.068 | 7.12 | 2.62 | 4.94 | |
| 1 | 0.913 ± 0.306 | −8.70 | 15.84 | 9.23 | |
| Large intestine | 100 | 98.010 ± 9.509 | −1.99 | 3.64 | 7.80 |
| 50 | 47.670 ± 3.013 | −4.66 | 1.72 | 4.63 | |
| 1 | 1.051 ± 0.179 | 5.10 | 2.06 | 10.37 | |
| Small intestine | 100 | 103.840 ± 6.209 | 3.84 | 2.07 | 8.58 |
| 50 | 49.865 ± 6.086 | −0.27 | 7.70 | 2.91 | |
| 1 | 0.933 ± 0.371 | −6.75 | 6.36 | 11.84 |
| Sample | Concentration (µg mL−1) | Room Temperature for 6 h | −4 °C for 24 h | −20 °C for 7 d | |||
|---|---|---|---|---|---|---|---|
| Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | ||
| Plasma | 100 | 96.53 | 8.60 | 97.11 | 8.42 | 99.27 | 7.61 |
| 50 | 101.25 | 9.22 | 98.98 | 7.29 | 102.20 | 4.99 | |
| 1 | 113.36 | 17.01 | 96.92 | 16.57 | 106.51 | 10.01 | |
| Liver | 100 | 97.34 | 11.66 | 107.80 | 14.11 | 97.97 | 9.24 |
| 50 | 108.80 | 8.53 | 112.16 | 5.86 | 98.82 | 17.75 | |
| 1 | 100.84 | 18.48 | 113.42 | 9.95 | 94.13 | 15.14 | |
| Heart | 100 | 98.16 | 7.65 | 101.56 | 12.69 | 103.10 | 8.92 |
| 50 | 102.55 | 9.09 | 96.87 | 13.42 | 101.87 | 3.43 | |
| 1 | 94.42 | 16.79 | 98.25 | 14.29 | 97.93 | 15.72 | |
| Spleen | 100 | 101.03 | 18.28 | 90.51 | 8.48 | 104.63 | 9.94 |
| 50 | 102.71 | 13.03 | 95.94 | 7.57 | 113.37 | 11.25 | |
| 1 | 98.73 | 18.56 | 102.54 | 13.91 | 93.02 | 12.40 | |
| Lung | 100 | 105.72 | 9.72 | 88.11 | 7.53 | 100.98 | 8.57 |
| 50 | 104.36 | 10.40 | 95.31 | 9.51 | 94.79 | 8.44 | |
| 1 | 87.11 | 18.04 | 99.56 | 11.56 | 96.00 | 15.35 | |
| Kidney | 100 | 101.00 | 12.95 | 98.84 | 5.70 | 100.63 | 6.09 |
| 50 | 110.61 | 13.99 | 107.19 | 16.95 | 99.76 | 14.87 | |
| 1 | 87.66 | 15.01 | 91.69 | 8.77 | 88.16 | 9.98 | |
| Stomach | 100 | 96.83 | 10.73 | 99.39 | 15.44 | 94.01 | 8.79 |
| 50 | 94.62 | 8.80 | 109.56 | 6.50 | 102.89 | 10.61 | |
| 1 | 106.14 | 17.24 | 97.44 | 15.37 | 95.91 | 11.53 | |
| Large intestine | 100 | 95.02 | 7.62 | 100.60 | 9.73 | 97.23 | 5.38 |
| 50 | 100.96 | 10.28 | 96.48 | 8.07 | 101.03 | 9.91 | |
| 1 | 98.30 | 16.12 | 105.78 | 13.94 | 98.98 | 11.55 | |
| Small intestine | 100 | 95.75 | 4.85 | 91.39 | 12.10 | 98.76 | 8.25 |
| 50 | 107.83 | 10.31 | 103.71 | 12.26 | 96.79 | 6.43 | |
| 1 | 107.98 | 17.33 | 96.93 | 15.24 | 99.39 | 9.76 | |
| Parameter | Dose | ||
|---|---|---|---|
| 50 mg kg−1 | 100 mg kg−1 | 150 mg kg−1 | |
| T1/2 (h) | 2.517 ± 0.867 | 3.091 ± 1.468 | 3.419 ± 1.853 |
| AUC(0→∞) (mg h L−1) | 104.411 ± 21.070 | 147.986 ± 16.240 * | 245.938 ± 44.124 **# |
| CLZ/F (L h−1 kg−1) | 0.495 ± 0.094 | 0.683 ± 0.076 ** | 0.625 ± 0.097 * |
| MRT(0→∞) (h) | 5.129 ± 1.972 | 4.664 ± 1.607 | 3.494 ± 1.037 |
| Cmax (mg L−1) | 34.697 ± 9.425 | 70.236 ± 10.519 ** | 117.374 ± 13.582 **,# |
| Tmax (h) | 0.917 ± 0.204 | 1.000 ± 0.000 | 1.000 ± 0.000 |
| Vz/F (L kg−1) | 1.823 ± 0.821 | 3.079 ± 1.651 | 2.897 ± 1.152 |
| Parameter | Dose | ||
|---|---|---|---|
| 25 mg kg−1 | 50 mg kg−1 | 100 mg kg−1 | |
| T1/2 (h) | 0.478 ± 0.211 | 0.950 ± 0.400 | 1.826 ± 0.823 |
| AUC(0→∞) (mg h L−1) | 93.869 ± 11.636 | 180.113 ± 35.384 ** | 332.926 ± 27.341 **,# |
| CLZ/F (L h−1 kg−1) | 0.270 ± 0.037 | 0.286 ± 0.051 | 0.302 ± 0.025 |
| MRT(0→∞) (h) | 0.855 ± 0.112 | 1.514 ± 0.378 | 1.613 ± 0.493 |
| Cmax (mg L−1) | 74.944 ± 14.915 | 110.921 ± 11.453 ** | 212.231 ± 12.600 **,# |
| Tmax (h) | 0.330 ± 0.186 | 0.330 ± 0.186 | 0.273 ± 0.176 |
| Vz/F (L kg−1) | 0.183 ± 0.068 | 0.370 ± 0.110 | 0.795 ± 0.374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, J.; Zhang, C.; Cao, Y.; Tang, S.; Long, F.; Cao, Z.; Lu, J.; Peng, T. In Vivo Pharmacokinetic Study of Polygonatum cyrtonema Polysaccharide DPC1 after Oral and Intraperitoneal Administration. Pharmaceuticals 2024, 17, 343. https://doi.org/10.3390/ph17030343
Yong J, Zhang C, Cao Y, Tang S, Long F, Cao Z, Lu J, Peng T. In Vivo Pharmacokinetic Study of Polygonatum cyrtonema Polysaccharide DPC1 after Oral and Intraperitoneal Administration. Pharmaceuticals. 2024; 17(3):343. https://doi.org/10.3390/ph17030343
Chicago/Turabian StyleYong, Jin, Chaozheng Zhang, Yuening Cao, Shuang Tang, Fei Long, Zhixing Cao, Jun Lu, and Teng Peng. 2024. "In Vivo Pharmacokinetic Study of Polygonatum cyrtonema Polysaccharide DPC1 after Oral and Intraperitoneal Administration" Pharmaceuticals 17, no. 3: 343. https://doi.org/10.3390/ph17030343
APA StyleYong, J., Zhang, C., Cao, Y., Tang, S., Long, F., Cao, Z., Lu, J., & Peng, T. (2024). In Vivo Pharmacokinetic Study of Polygonatum cyrtonema Polysaccharide DPC1 after Oral and Intraperitoneal Administration. Pharmaceuticals, 17(3), 343. https://doi.org/10.3390/ph17030343






