Nanomedicines: Emerging Platforms in Smart Chemotherapy Treatment—A Recent Review
Abstract
1. Introduction
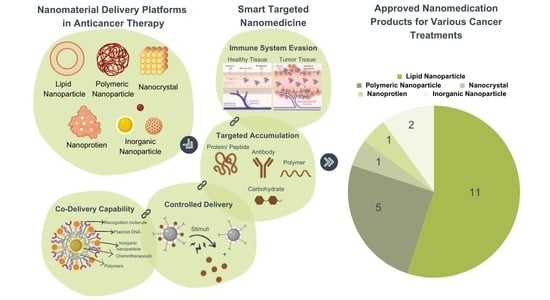
2. Types of Nanomaterial
2.1. Lipid-Based Nanoparticle
2.1.1. Liposomes
2.1.2. Solid Lipid Nanoparticles (SLNs)
2.1.3. Nanostructured Lipid Carriers (NLCs)
2.2. Polymers
2.2.1. Polymer–Drug Conjugates
2.2.2. Polymeric Micelles
2.2.3. Dendrimers
2.3. Nanocrystal
2.4. Nanoprotein
2.5. Inorganic Nanoparticle
2.6. Stealth Nanocarriers
2.7. Comparison of Nanomaterial Platforms for Drug Delivery in Cancer Therapy
3. Nanoparticle Properties and Characteristics
3.1. Physiochemical Properties
3.1.1. Nanoparticle Size
3.1.2. The Shape of Nanoparticles
3.1.3. Surface Charge of the Nanoparticle
3.2. Nanoparticle Lipophilicity
3.3. Nanoparticle Drug Release
4. Nanoparticle-Based Cancer Drug Delivery Systems
4.1. Smart Targeted Therapy
4.2. Passive Drug Delivery
4.3. Active Drug Delivery
4.4. Tumor Microenvironment (TME) Responsive Drug Delivery
5. Contemporary Landscape in the Field of Cancer Nanomedicine
6. Challenges Encountered in Developing Nanomedication
6.1. Overcoming EPR-Based Limitations
6.2. Ensuring Nanomaterial Safety and Effectiveness
6.3. Scaling up Nanoparticle Production
6.4. Regulatory Hurdles in Nanotherapeutics Development
6.5. Ethical Considerations
7. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Brand Name (Active Ingredient) | Developer | Indication(s) [Approval Year and Region] |
|---|---|---|
| Polymer Nanoparticle | ||
| Adagen® (Pegademase bovine) | Sigma-Tau Pharmaceuticals | Adensine deaminase (ADA) deficiency in patients with severe combined immunodeficiency disease (SCID) [1990 US] Disc. * 2019 |
| Adynovate® (Antihemophilic factor) (recombinant), PEGylated | Shire | Hemophilia [2015 US] |
| Cimzia® (Certolizumab pegol) | UCB | Crohn’s disease [2008 US] [2009 EU] |
| Copaxone®; Glatopa ® (Glatiramer acetate) | Teva Neuroscience | Multiple sclerosis (MS) [1996 US] |
| Apealea® (paclitaxel) | Oasmia Pharmaceutical AB | Ovarian cancer, peritoneal cancer, fallopian tube cancer [2018 EU] |
| Genexol-PM® (paclitaxel) | Lupin Ltd. | Breast cancer [2007 US] |
| Eligard® (Leuprolide acetate) | Tolmar Pharmaceuticals | Prostate cancer [2002 US] |
| Krystexxa® (Pegloticase) | Crealta Pharmaceuticals | Chronic gout [2010 US] [2013 EU] |
| Macugen® (Pegaptanib) | Bausch & Lomb | Neovascular age-related macular degeneration [2004 US] [2006 EU] Disc. * |
| Mircera® (mPEG-epoetin beta) | Roche | Anemia associated with chronic kidney disease [2007 US/EU] |
| Neulasta® (Pegfilgrastim) | Amgen | Chemotherapy induced neutropenia [2002 US/EU] |
| Oncaspar® (Pegaspargase) | Shire | Acute lymphoblastic leukemia [1994 US] [2016 EU] |
| PegIntron® (alpha interferon (INF) molecule) | Merk & Co. Inc. | Hepatitis C [2000 EU] [2001 US] |
| Pegasys® (Peginterferon alpha-2a) | Roche | Hepatitis B and C [2002 US/EU] |
| Plegridy® (Peginterferon beta-1a) | Biogen | Multiple sclerosis (MS) [2014 US/EU] |
| Renvela®/Renagel® (Sevelamer hydrochloride) | Genzyme | Chronic kidney disease [2000 US/EU] |
| Mircera (epoetin β (EPO) | Vifor | Anemia [2007 EU] [2018 US] |
| Macugen® (pegatinib sodium) | Pfizer | Choroidal neovascularization caused by wet age-related macular degeneration [2004 US] |
| Somavert® (Pegvisomant) | Pfizer | Acromegaly [2003 US] [2002 EU] |
| Rebinyn® (recombinant DNA-derived coagulation FIX) | NovoNordisk | Hemophilia B [2017 US] |
| Restasis® (cyclosporine) | Allergan | Chronic dry eye [2003 US] |
| Zilretta® (Triamcinolone acetonide) | Flexion Therapeutics | Osteoarthritis knee pain [2017 US] |
| Sublocade® (Buprenorphine) | Indivior | Opioid use disorder [2017 US] |
| Liposome Nanoparticle | ||
| Abelcet® (Amphotericin B) | Sigma-Tau Pharmaceuticals | Fungal infections [1995 US] |
| AmBisome® (Amphotericin B) | Gilead Sciences | Fungal/protozoal infections [1997 US] |
| Curosurf® (Poractant alpha) | Chiesei | Respiratory distress syndrome [1999 US] |
| DaunoXome® (Daunorubicin) | Galen | Kaposi’s sarcoma [1996 US] Disc. * 2016 |
| DepoCyt© (Cytarabine) | Pacira Pharmaceuticals | Lymphomatous meningitis [1999 US] [2001 EU] Disc. * 2017 |
| Zevalin® (90Y-ibritumomab tiuxetan) | Bayer Pharma | lymphoma [2002 US] [2004 EU] |
| DepoDur® (Morphine sulfate) | Pacira Pharmaceuticals | Analgesia (post-operative) [2004 US] Disc. * |
| Doxil®/CaelyxTM (Doxorubicin) | Janssen | Karposi’s sarcoma [1995 US] [1996 EU] Ovarian cancer [2005 US] [1996 EU] Multiple myeloma [2008 US] [1996 EU] Breast cancer [1996 EU] |
| Lipodox® (doxorubicin) | doxorubicinSun Pharma Global FZE | Metastatic ovarian cancer, HIV-associated KS [2013 US] |
| Marqibo® (Vincristine sulfate) | Spectrum Pharmaceuticals | Acute lymphoblastic leukemia [2012 US] Disc. * 2022 |
| Mepact® (Mifamurtide) | IDM Pharma | Bone cancer [2009 EU] |
| Myocet® (Doxorubicin) | Teva UK | Bone cancer [2000 EU] |
| Onivyde® (Irinotecan hydrochloride) | Ipsen Biopharmaceuticals | Pancreatic cancer [2015 US] [2016 EU] |
| Vyxeos® (daunorubicin) | Jazz Pharmaceuticals | AML, AML with myelodysplasia related changes [2017 US] |
| Onpattro® (patisiran) | Alnylam | Hereditary transthyretin (TTR) mediated amyloidosis [2018 FDA and EMA] |
| Visudyne® (Verteporfin) | Novartis | Age-related macular degeneration [2000 US/EU] Pathologic myopia [2000 US/EU] Ocular histoplasmosis [2000 US/EU] |
| Micellar Nanoparticle | ||
| EstrasorbTM® (Estradiol hemihydrate) | Novavax/Graceway | Menopausal therapy [2003 US] Disc. * |
| Taxol® (Paclitaxel) | Bristol Myres Squibb | Ovarian cancer [1992 US] Breast cancer [1994 US] Disc. * |
| Taxotere® (Docetaxel) | Sanofi-Aventis | Head and neck cancer [2006 US] [1995 EU] |
| Protein Nanoparticle | ||
| Abraxane® (ABI-007 Protein-bound paclitaxel) | Celgene | Breast cancer [2005 US] [2008 EU] |
| Ontak® (Denileukin diftitox) | Eisai | Cutaneous T-cell lymphoma [1999 US] |
| Nanocrystals | ||
| Avinza® (Morphine sulfate) | Pfizer | Pain management [2002 US] Disc. * |
| Emend® (Aprepitant) | Merck | Antiemetic [2003 US/EU] |
| Ivemend® (fosaprepitant dimeglumine) | Merck | Antiemetic [2008 US/EU] |
| Focalin XR® (Dexmethylphenidate hydrochloride) | Novartis | Attention deficit hyperactivity disorder [2005 US] |
| Invega Sustenna®/Xeplion® (Paliperidone palmitate) | Janssen Pharms | Schizophrenia [2009 US] [2011 EU] |
| Zyprexa® (Olanzapine) | Lilly Pharma | Schizophrenia [1996] |
| Megace ES® (Megestrol acetate) | Endo Pharms | Anti-anorexic [2005 US] |
| Rapamune® (Sirolimus) | Pfizer | Immunosuppresent [1999 US] [2001 EU] |
| Ritalin LA® (Methylphenidate hydrochloride) | Novartis | Attention deficit hyperactivity disorder [2002 US] |
| Ryanodex® (Dantrolene sodium) | Eagle Pharmaceuticals | Malignant hypothermia [2014 US] |
| Tricor® (Fenofibrate) | AbbVie | High cholesterol and high triglyceride levels [2004 US] |
| Triglide® (Fenofibrate) | SkyePharma AG | High cholesterol and triglycerides [2005 US] |
| Zanaflex® (Tizanidine hydrochloride) | Acorda | Muscle relaxant [1996 US] |
| Inorganic and Metallic Nanoparticles | ||
| Dexferrum®/ DexIron® (iron dextran) | Luitpold Pharmaceuticals | Iron deficiency [1996 US] Disc. * 2014 |
| FerahemeTM/Rienso® Ferumoxytol (ferumoxytol) | AMAG pharmaceuticals | Iron deficiency anemia in chronic kidney disease [2009 US] [2012 EU] |
| Ferrlecit® (iron carboxymaltose colloid) | Sanofi Avertis | Iron deficiency anemia in chronic kidney disease [1999 US] |
| INFeD® (iron dextran) | Allergan Pharma | Iron deficiency anemia in chronic kidney disease [1974 US] |
| Injectafer®/Ferinject® (iron carboxymaltose colloid) | Luitpold Pharmaceuticals | Iron deficiency anemia in chronic kidney disease [2013 US] |
| Hensify® (hafnium oxide nanoparticles) | Nanobiotix | Locally advanced squamous cell carcinoma [2019 EU] |
| Venofer® (iron sucrose) | Luitpold Pharmaceuticals | Iron deficiency anemia in chronic kidney disease [2000 US] |
| Nano-therm | MagForce | Recurrent glioblastoma, Prostate Cancer [2010 EU, 2018 US] |
| Dendrimer based Nanoparticles | ||
| VivaGel® BV (astodrimer sodium) | Starpharma | Anti-infective for prevention of recurrent bacterial vaginosis (BV) [2015 US] |
References
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 29 January 2024).
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 205031212110343. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, Y.I. A Comparative Review of Haute Autorité de Santé and National Institute for Health and Care Excellence Health Technology Assessments of Ikervis® to Treat Severe Keratitis in Adult Patients with Dry Eye Disease Which Has Not Improved despite Treatment With. J. Mark. Access Health Policy 2017, 5, 1336043. [Google Scholar] [CrossRef]
- Chaniotakis, N.; Thermos, K.; Kalomiraki, M. Dendrimers as Tunable Vectors of Drug Delivery Systems and Biomedical and Ocular Applications. Int. J. Nanomed. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; de la Fuente, M. Nanotherapies for the Treatment of Ocular Diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef]
- Bondì, M.L.; Craparo, E.F.; Giammona, G.; Drago, F. Brain-Targeted Solid Lipid Nanoparticles Containing Riluzole: Preparation, Characterization and Biodistribution. Nanomedicine 2010, 5, 25–32. [Google Scholar] [CrossRef]
- Bell, C.; Anderson, J.; Ganguly, T.; Prescott, J.; Capila, I.; Lansing, J.C.; Sachleben, R.; Iyer, M.; Fier, I.; Roach, J.; et al. Development of Glatopa® (Glatiramer Acetate): The First FDA-Approved Generic Disease-Modifying Therapy for Relapsing Forms of Multiple Sclerosis. J. Pharm. Pract. 2018, 31, 481–488. [Google Scholar] [CrossRef]
- Pasero, C.; McCaffery, M. Extended-Release Epidural Morphine (DepoDurTM). J. PeriAnesthesia Nurs. 2005, 20, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Armstead-Williams, C.; Hyatali, F.; Cox, K.S.; Kaye, R.J.; Eng, L.K.; Farooq Anwar, M.A.; Patel, P.V.; Patil, S.; Cornett, E.M. Exparel for Postoperative Pain Management: A Comprehensive Review. Curr. Pain Headache Rep. 2020, 24, 73. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Geetha, K.M. Lipid Nanoparticles in the Development of MRNA Vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on Responsive Drug Delivery Based on Liposome Vehicles for Cancer Treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef] [PubMed]
- Bostanudin, M.F.; Arafat, M.; Tan, S.F.; Sarker, M.Z.I. Investigations of Pectin Nanostructures for Enhanced Percutaneous Delivery of Fusidic Acid. J. Appl. Polym. Sci. 2022, 139, e52760. [Google Scholar] [CrossRef]
- Minhas, M.U.; Khan, K.U.; Sarfraz, M.; Badshah, S.F.; Munir, A.; Barkat, K.; Basit, A.; Arafat, M. Polyvinylpyrrolidone K-30-Based Crosslinked Fast Swelling Nanogels: An Impeccable Approach for Drug’s Solubility Improvement. BioMed Res. Int. 2022, 2022, 5883239. [Google Scholar] [CrossRef]
- Sharifi, M.; Cho, W.C.; Ansariesfahani, A.; Tarharoudi, R.; Malekisarvar, H.; Sari, S.; Bloukh, S.H.; Edis, Z.; Amin, M.; Gleghorn, J.P.; et al. An Updated Review on EPR-Based Solid Tumor Targeting Nanocarriers for Cancer Treatment. Cancers 2022, 14, 2868. [Google Scholar] [CrossRef]
- Batool, S.; Sohail, S.; ud Din, F.; Alamri, A.H.; Alqahtani, A.S.; Alshahrani, M.A.; Alshehri, M.A.; Choi, H.G. A Detailed Insight of the Tumor Targeting Using Nanocarrier Drug Delivery System. Drug Deliv. 2023, 30, 2183815. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Sarfraz, M.; Arafat, M.; Zaidi, S.H.H.; Eltaib, L.; Siddique, M.I.; Kamal, M.; Ali, A.; Asdaq, S.M.B.; Khan, A.; Aaghaz, S.; et al. Resveratrol-Laden Nano-Systems in the Cancer Environment: Views and Reviews. Cancers 2023, 15, 4499. [Google Scholar] [CrossRef]
- Fornaguera, C.; García-Celma, M. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Thapa, R.K.; Kim, J.O. Nanomedicine-Based Commercial Formulations: Current Developments and Future Prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer Nanomedicine: A Review of Recent Success in Drug Delivery. Clin. Transl. Med. 2017, 6, e44. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose-Response 2020, 18, 155932582093616. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration Drug Approvals and Databases. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed on 29 January 2024).
- Yuan, Z.; Zhang, Y.; Cao, D.; Shen, K.; Li, Q.; Zhang, G.; Wu, X.; Cui, M.; Yue, Y.; Cheng, W.; et al. Pegylated Liposomal Doxorubicin in Patients with Epithelial Ovarian Cancer. J. Ovarian Res. 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Lang, T.; Yin, Q.; Li, Y. Nano Drug Delivery Systems Improve Metastatic Breast Cancer Therapy. Med. Rev. 2021, 1, 244–274. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yao, W. Albumin-Bound Paclitaxel: Worthy of Further Study in Sarcomas. Front. Oncol. 2022, 12, 815900. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A. Toxicity of Nanoparticles_ Challenges and Opportunities. Appl. Microsc. 2019, 49, 2. [Google Scholar] [CrossRef]
- Layek, B.; Gidwani, B.; Tiwari, S.; Joshi, V.; Jain, V.; Vyas, A. Recent Advances in Lipid-Based Nanodrug Delivery Systems in Cancer Therapy. Curr. Pharm. Des. 2020, 26, 3218–3233. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y.; et al. Nanoparticle-Based Medicines in Clinical Cancer Therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Arafat, M.; Kirchhoefer, C.; Mikov, M.; Sarfraz, M.; Löbenberg, R. Nanosized Liposomes Containing Bile Salt: A Vesicular Nanocarrier for Enhancing Oral Bioavailability of BCS Class III Drug. J. Pharm. Pharm. Sci. 2017, 20, 305. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Mehrdadi, S. Drug Delivery of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) to Target Brain Tumors. Adv. Pharm. Bull. 2023, 13, 512–520. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for Tumor Targeted Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Mellinger, A.; Lubitz, L.J.; Gazaille, C.; Leneweit, G.; Bastiat, G.; Lépinoux-Chambaud, C.; Eyer, J. The Use of Liposomes Functionalized with the NFL-TBS.40-63 Peptide as a Targeting Agent to Cross the in Vitro Blood-Brain Barrier and Target Glioblastoma Cells. Int. J. Pharm. 2023, 646, 123421. [Google Scholar] [CrossRef] [PubMed]
- Hattab, D.; Bakhtiar, A. Bioengineered SiRNA-Based Nanoplatforms Targeting Molecular Signaling Pathways for the Treatment of Triple Negative Breast Cancer: Preclinical and Clinical Advancements. Pharmaceutics 2020, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Zardavas, D.; Lemort, M.; Wilke, C.; Vanderbeeken, M.-C.; D’Hondt, V.; De Azambuja, E.; Gombos, A.; Lebrun, F.; Dal Lago, L.; et al. Feasibility Study of EndoTAG-1, a Tumor Endothelial Targeting Agent, in Combination with Paclitaxel Followed by FEC as Induction Therapy in HER2-Negative Breast Cancer. PLoS ONE 2016, 11, e0154009. [Google Scholar] [CrossRef]
- Espelin, C.W.; Leonard, S.C.; Geretti, E.; Wickham, T.J.; Hendriks, B.S. Dual HER2 Targeting with Trastuzumab and Liposomal-Encapsulated Doxorubicin (MM-302) Demonstrates Synergistic Antitumor Activity in Breast and Gastric Cancer. Cancer Res. 2016, 76, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Affram, K.O.; Smith, T.; Ofori, E.; Krishnan, S.; Underwood, P.; Trevino, J.G.; Agyare, E. Cytotoxic Effects of Gemcitabine-Loaded Solid Lipid Nanoparticles in Pancreatic Cancer Cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101374. [Google Scholar] [CrossRef]
- Bhagwat, G.S.; Athawale, R.B.; Gude, R.P.; Md, S.; Alhakamy, N.A.; Fahmy, U.A.; Kesharwani, P. Formulation and Development of Transferrin Targeted Solid Lipid Nanoparticles for Breast Cancer Therapy. Front. Pharmacol. 2020, 11, 614290. [Google Scholar] [CrossRef]
- Zheng, G.; Zheng, M.; Yang, B.; Fu, H.; Li, Y. Improving Breast Cancer Therapy Using Doxorubicin Loaded Solid Lipid Nanoparticles: Synthesis of a Novel Arginine-Glycine-Aspartic Tripeptide Conjugated, PH Sensitive Lipid and Evaluation of the Nanomedicine in Vitro and in Vivo. Biomed. Pharmacother. 2019, 116, 109006. [Google Scholar] [CrossRef]
- Larian, Z.; Homayouni Tabrizi, M.; Karimi, E.; Khatamian, N.; Hosseini Torshizi, G.; Pourmohammadi, H. The Folate-Chitosan-Decorated Harmaline Nanostructured Lipid Carrier (FCH-NLC), the Efficient Selective Anticancer Nano Drug Delivery System. J. Drug Deliv. Sci. Technol. 2023, 88, 104864. [Google Scholar] [CrossRef]
- Kumari, P.; Dang, S. Dual Drug Loaded Nanostructured Lipid Carrier for Cytotoxic Effect against Breast Cancer—A Drug Repurposing Approach. Surf. Interfaces 2023, 40, 103138. [Google Scholar] [CrossRef]
- Huang, L.; Teng, W.; Cao, J.; Wang, J. Liposomes as Delivery System for Applications in Meat Products. Foods 2022, 11, 3017. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef] [PubMed]
- Moosavian, S.A.; Bianconi, V.; Pirro, M.; Sahebkar, A. Challenges and Pitfalls in the Development of Liposomal Delivery Systems for Cancer Therapy. Semin. Cancer Biol. 2021, 69, 337–348. [Google Scholar] [CrossRef]
- Yang, S.; Shim, M.K.; Song, S.; Cho, H.; Choi, J.; Jeon, S.I.; Kim, W.J.; Um, W.; Park, J.H.; Yoon, H.Y.; et al. Liposome-Mediated PD-L1 Multivalent Binding Promotes the Lysosomal Degradation of PD-L1 for T Cell-Mediated Antitumor Immunity. Biomaterials 2022, 290, 121841. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Sakkal, M.; Yuvaraju, P.; Esmaeil, A.; Poulose, V.; Aburuz, S. Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets. Pharmaceuticals 2023, 16, 539. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor Microenvironment-Activated Cancer Cell Membrane-Liposome Hybrid Nanoparticle-Mediated Synergistic Metabolic Therapy and Chemotherapy for Non-Small Cell Lung Cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Sarpietro, M.G.; Castelli, F.; Lauro, M.R.; Torrisi, C.; Russo, S.; Puglia, C. Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations. Molecules 2023, 28, 1545. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.; Mithu, M.S.H.; Douroumis, D. Solid Lipid Nanoparticles: An Effective Lipid-Based Technology for Cancer Treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Shamsuddin, N.A.M.; Zulfakar, M.H. Nanostructured Lipid Carriers for the Delivery of Natural Bioactive Compounds. Curr. Drug Deliv. 2023, 20, 127–143. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, M.Z.; Garg, A.; Ahmad, J. Advancement in Design of Nanostructured Lipid Carriers for Cancer Targeting and Theranostic Application. Biochim. Biophys. Acta—Gen. Subj. 2021, 1865, 129936. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Arafat, M.; Sarfraz, M.; Bostanudin, M.F.; Esmaeil, A.; Salam, A.; AbuRuz, S. In Vitro and In Vivo Evaluation of Oral Controlled Release Formulation of BCS Class I Drug Using Polymer Matrix System. Pharmaceuticals 2021, 14, 929. [Google Scholar] [CrossRef]
- Li, X.; Duan, Z.; Li, Z.; Gu, L.; Li, Y.; Gong, Q.; Gu, Z.; Luo, K. Dendritic Polymer-Functionalized Nanomedicine Potentiates Immunotherapy via Lethal Energy Crisis-Induced PD-L1 Degradation. Biomaterials 2023, 302, 122294. [Google Scholar] [CrossRef]
- Al-Hanbali, O.A.; Hamed, R.; Arafat, M.; Bakkour, Y.; Al-Matubsi, H.; Mansour, R.; Al-Bataineh, Y.; Aldhoun, M.; Sarfraz, M.; Dardas, A.K.Y. Formulation and Evaluation of Diclofenac Controlled Release Matrix Tablets Made of HPMC and Poloxamer 188 Polymer: An Assessment on Mechanism of Drug Release. Pak. J. Pharm. Sci. 2018, 31, 345–351. [Google Scholar]
- Arafat, M.; Sarfraz, M.; AbuRuz, S. Development and In Vitro Evaluation of Controlled Release Viagra® Containing Poloxamer-188 Using GastroplusTM PBPK Modeling Software for In Vivo Predictions and Pharmacokinetic Assessments. Pharmaceuticals 2021, 14, 479. [Google Scholar] [CrossRef]
- Soni, V.; Pandey, V.; Asati, S.; Gour, V.; Tekade, R.K. Biodegradable Block Copolymers and Their Applications for Drug Delivery. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 401–447. [Google Scholar]
- Heo, Y.-A.; Syed, Y.Y.; Keam, S.J. Pegaspargase: A Review in Acute Lymphoblastic Leukaemia. Drugs 2019, 79, 767–777. [Google Scholar] [CrossRef]
- Chen, B.-M.; Cheng, T.-L.; Roffler, S.R. Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-Polyethylene Glycol Antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar]
- Arafat, M.; Kirchhoefer, C.; Mikov, M. Mixed Micelles Loaded with Bile Salt: An Approach to Enhance Intestinal Transport of the BCS Class III Drug Cefotaxime in Rats. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-W.; Rejinold, N.S.; Choy, J.-H. Multifunctional Polymeric Micelles for Cancer Therapy. Polymers 2022, 14, 4839. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Emami, J.; Maghzi, P.; Hasanzadeh, F.; Sadeghi, H.; Mirian, M.; Rostami, M. PLGA-PEG-RA-Based Polymeric Micelles for Tumor Targeted Delivery of Irinotecan. Pharm. Dev. Technol. 2018, 23, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shamul, J.G.; Shah, S.R.; Shin, A.; Lee, B.J.; Quinones-Hinojosa, A.; Green, J.J. Verteporfin-Loaded Poly(Ethylene Glycol)-Poly(Beta-Amino Ester)-Poly(Ethylene Glycol) Triblock Micelles for Cancer Therapy. Biomacromolecules 2018, 19, 3361–3370. [Google Scholar] [CrossRef]
- Panagi, M.; Mpekris, F.; Chen, P.; Voutouri, C.; Nakagawa, Y.; Martin, J.D.; Hiroi, T.; Hashimoto, H.; Demetriou, P.; Pierides, C.; et al. Polymeric Micelles Effectively Reprogram the Tumor Microenvironment to Potentiate Nano-Immunotherapy in Mouse Breast Cancer Models. Nat. Commun. 2022, 13, 7165. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics 2020, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shen, M.; Rodrigues, J.; Mignani, S.; Majoral, J.-P.; Shi, X. Superstructured Poly(Amidoamine) Dendrimer-Based Nanoconstructs as Platforms for Cancer Nanomedicine: A Concise Review. Coord. Chem. Rev. 2020, 421, 213463. [Google Scholar] [CrossRef]
- Gigliobianco, M.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Chandra, A.; Jain, K.; Talegaonkar, S. Nanocrystalization: An Emerging Technology to Enhance the Bioavailability of Poorly Soluble Drugs. Pharm. Nanotechnol. 2019, 7, 259–278. [Google Scholar] [CrossRef]
- Jeon, H.J.; Lee, H.-E.; Yang, J. Safety and Efficacy of Rapamune® (Sirolimus) in Kidney Transplant Recipients: Results of a Prospective Post-Marketing Surveillance Study in Korea. BMC Nephrol. 2018, 19, 201. [Google Scholar] [CrossRef]
- Stater, E.P.; Sonay, A.Y.; Hart, C.; Grimm, J. The Ancillary Effects of Nanoparticles and Their Implications for Nanomedicine. Nat. Nanotechnol. 2021, 16, 1180–1194. [Google Scholar] [CrossRef]
- Yenurkar, D.; Nayak, M.; Mukherjee, S. Recent Advances of Nanocrystals in Cancer Theranostics. Nanoscale Adv. 2023, 5, 4018–4040. [Google Scholar] [CrossRef]
- Miao, Y.; Yang, T.; Yang, S.; Yang, M.; Mao, C. Protein Nanoparticles Directed Cancer Imaging and Therapy. Nano Converg. 2022, 9, 2. [Google Scholar] [CrossRef]
- Zhao, M.; Lei, C.; Yang, Y.; Bu, X.; Ma, H.; Gong, H.; Liu, J.; Fang, X.; Hu, Z.; Fang, Q. Abraxane, the Nanoparticle Formulation of Paclitaxel Can Induce Drug Resistance by Up-Regulation of P-Gp. PLoS ONE 2015, 10, e0131429. [Google Scholar] [CrossRef]
- Fuentes, A.C.; Szwed, E.; Spears, C.D.; Thaper, S.; Dang, L.H.; Dang, N.H. Denileukin Diftitox (Ontak) as Maintenance Therapy for Peripheral T-Cell Lymphomas: Three Cases with Sustained Remission. Case Rep. Oncol. Med. 2015, 2015, 123756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ilhan-Ayisigi, E.; Yesil-Celiktas, O. Silica-based Organic-inorganic Hybrid Nanoparticles and Nanoconjugates for Improved Anticancer Drug Delivery. Eng. Life Sci. 2018, 18, 882–892. [Google Scholar] [CrossRef]
- Kopeckova, K.; Eckschlager, T.; Sirc, J.; Hobzova, R.; Plch, J.; Hrabeta, J.; Michalek, J. Nanodrugs Used in Cancer Therapy. Biomed. Pap. 2019, 163, 122–131. [Google Scholar] [CrossRef]
- Kumar, L.; Verma, S.; Utreja, P.; Kumar, D. Overview of Inorganic Nanoparticles: An Expanding Horizon in Tumor Therapeutics. Recent Pat. Anticancer. Drug Discov. 2023, 18, 343–363. [Google Scholar] [CrossRef]
- Gupta, A.; Kushwaha, S.S.; Mishra, A. A Review on Recent Technologies and Patents on Silica Nanoparticles for Cancer Treatment and Diagnosis. Recent Pat. Drug Deliv. Formul. 2020, 14, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y.; Lu, H.; Zhao, D. Silver Nanoparticles Coupled to Anti-EGFR Antibodies Sensitize Nasopharyngeal Carcinoma Cells to Irradiation. Mol. Med. Rep. 2017, 16, 9005–9010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- ELhabal, S.F.; Elwy, H.M.; Hassanin, S.; El-Rashedy, A.A.; Hamza, A.A.; Khasawneh, M.A. Biosynthesis and Characterization of Gold and Copper Nanoparticles from Salvadora Persica Fruit Extracts and Their Biological Properties. Int. J. Nanomed. 2022, 17, 6095–6112. [Google Scholar] [CrossRef]
- Xu, P.; Wang, R.; Ouyang, J.; Chen, B. A New Strategy for TiO2 Whiskers Mediated Multi-Mode Cancer Treatment. Nanoscale Res. Lett. 2015, 10, 94. [Google Scholar] [CrossRef]
- Salve, R.; Kumar, P.; Ngamcherdtrakul, W.; Gajbhiye, V.; Yantasee, W. Stimuli-Responsive Mesoporous Silica Nanoparticles: A Custom-Tailored next Generation Approach in Cargo Delivery. Mater. Sci. Eng. C 2021, 124, 112084. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Sun, M.; Wang, T.; Li, L.; Li, X.; Zhai, Y.; Zhang, J.; Li, W. The Application of Inorganic Nanoparticles in Molecular Targeted Cancer Therapy: EGFR Targeting. Front. Pharmacol. 2021, 12, 702445. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in Cancer: Reviewing the Combination of Hyperthermia and Triggered Chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Dehghanian, A.R. The Application of Titanium Dioxide (TiO2) Nanoparticles in the Photo-Thermal Therapy of Melanoma Cancer Model. Iran. J. Basic Med. Sci. 2018, 21, 1133–1139. [Google Scholar] [CrossRef]
- Giri, P.M.; Banerjee, A.; Layek, B. A Recent Review on Cancer Nanomedicine. Cancers 2023, 15, 2256. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and Pseudo-Stealth Nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nagasaki, Y.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Long-Circulating Poly(Ethylene Glycol)–Poly(d,l-Lactide) Block Copolymer Micelles with Modulated Surface Charge. J. Control. Release 2001, 77, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Caliceti, P. Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Ghandehari, H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Thummarati, P. Innovative Design of Targeted Nanoparticles: Polymer–Drug Conjugates for Enhanced Cancer Therapy. Pharmaceutics 2023, 15, 2216. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Rani, R.; Malik, P.; Dhania, S.; Mukherjee, T.K. Recent Advances in Mesoporous Silica Nanoparticle-Mediated Drug Delivery for Breast Cancer Treatment. Pharmaceutics 2023, 15, 227. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Abbasi, M.; Moaddeli, M.R.; Vaez, A.; Shafiee, M.; Kasaee, S.R.; Amani, A.M.; Hatam, S. Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview. Nanotheranostics 2022, 6, 400–423. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding Cellular Interactions with Nanomaterials: Towards a Rational Design of Medical Nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping Cancer Nanomedicine: The Effect of Particle Shape on the in Vivo Journey of Nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Bostanudin, M.F.; Arafat, M.; Sarfraz, M.; Górecki, D.C.; Barbu, E. Butylglyceryl Pectin Nanoparticles: Synthesis, Formulation and Characterization. Polymers 2019, 11, 789. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Sitia, L.; Sevieri, M.; Signati, L.; Bonizzi, A.; Chesi, A.; Mainini, F.; Corsi, F.; Mazzucchelli, S. HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment. Cancers 2022, 14, 2424. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ge, Z.; Toh, K.; Liu, X.; Dirisala, A.; Ke, W.; Wen, P.; Zhou, H.; Wang, Z.; Xiao, S.; et al. Enzymatically Transformable Polymersome-Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv. Mater. 2021, 33, 2105254. [Google Scholar] [CrossRef] [PubMed]
- Kayani, A.; Raza, A.; Si, J.; Dutta, D.; Zhou, Q.; Ge, Z. Polymersome Membrane Engineering with Active Targeting or Controlled Permeability for Responsive Drug Delivery. Biomacromolecules 2023, 24, 4622–4645. [Google Scholar] [CrossRef]
- Najer, A.; Rifaie-Graham, O.; Yeow, J.; Adrianus, C.; Chami, M.; Stevens, M.M. Differences in Human Plasma Protein Interactions between Various Polymersomes and Stealth Liposomes as Observed by Fluorescence Correlation Spectroscopy. Macromol. Biosci. 2023, 23, 2200424. [Google Scholar] [CrossRef]
- Kataoka, K.; Anraku, Y.; Sueyoshi, D. Nanoreactor Using Polyion Complex Polymersomes, and Method for Producing Same. U.S. Patent No 11,096,991, 24 August 2021. [Google Scholar]
- Japir, A.A.-W.M.M.; Ke, W.; Li, J.; Mukerabigwi, J.F.; Ibrahim, A.; Wang, Y.; Li, X.; Zhou, Q.; Mohammed, F.; Ge, Z. Tumor-Dilated Polymersome Nanofactories for Enhanced Enzyme Prodrug Chemo-Immunotherapy. J. Control. Release 2021, 339, 418–429. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Li, X.; Lu, N.; Ge, Z. Polymersome Nanoreactor-Mediated Combination Chemodynamic-Immunotherapy via ROS Production and Enhanced STING Activation. Adv. Ther. 2021, 4, 2100130. [Google Scholar] [CrossRef]
- Khalid, N.; Sarfraz, M.; Arafat, M.; Akhtar, M.; Löbenberg, R.; Ur Rehman, N. Nano-Sized Droplets of Self-Emulsifying System for Enhancing Oral Bioavailability of Chemotherapeutic Agent VP-16 in Rats: A Nano Lipid Carrier for BCS Class IV Drugs. J. Pharm. Pharm. Sci. 2018, 21, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(Ethylene Glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.-M.; Kim, D.-H.; Kim, G.-L.; Seo, S.-H.; Park, J.-W.; Jeong, S.H.; et al. Functional Ligands for Improving Anticancer Drug Therapy: Current Status and Applications to Drug Delivery Systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Salam, A.; Mahmood, A.; Arafat, M.; Kaharudin, A.K.; Sahudin, S.; Lazim, A.M.; Azfaralariff, A. Formulation and In-Vitro Characterisation of Cross-Linked Amphiphilic Guar Gum Nanocarriers for Percutaneous Delivery of Arbutin. J. Pharm. Sci. 2021, 12, 3918. [Google Scholar] [CrossRef]
- Kamal, T.; Sarfraz, M.; Arafat, M.; Mikov, M.; Rahman, N. Cross-Linked Guar Gum and Sodium Borate Based Microspheres as Colon-Targeted Anticancer Drug Delivery Systems for 5-Fluorouracil. Pak. J. Pharm. Sci. 2017, 30, 2329–2336. [Google Scholar]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Feitosa, R.C.; Geraldes, D.C.; Beraldo-de-Araújo, V.L.; Costa, J.S.R.; Oliveira-Nascimento, L. Pharmacokinetic Aspects of Nanoparticle-in-Matrix Drug Delivery Systems for Oral/Buccal Delivery. Front. Pharmacol. 2019, 10, 1057. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart Drug Delivery Systems for Precise Cancer Therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xin, X.; Wu, R.; Guan, W.; Zhou, W. Advances in Intelligent-Responsive Nanocarriers for Cancer Therapy. Pharmacol. Res. 2022, 178, 106184. [Google Scholar] [CrossRef] [PubMed]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; et al. Smart Nanocarriers as an Emerging Platform for Cancer Therapy: A Review. Molecules 2021, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Vanbilloen, W.J.F.; Rechberger, J.S.; Anderson, J.B.; Nonnenbroich, L.F.; Zhang, L.; Daniels, D.J. Nanoparticle Strategies to Improve the Delivery of Anticancer Drugs across the Blood–Brain Barrier to Treat Brain Tumors. Pharmaceutics 2023, 15, 1804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kataoka, K. Chemo-Physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huo, Y.; Yao, L.; Xu, Y.; Meng, F.; Li, H.; Sun, K.; Zhou, G.; Kohane, D.S.; Tao, K. Transcytosis of Nanomedicine for Tumor Penetration. Nano Lett. 2019, 19, 8010–8020. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, C.; Lu, L.; Jiang, H.; Wang, F.; Zhang, X. Transcytosable Peptide-Paclitaxel Prodrug Nanoparticle for Targeted Treatment of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 4646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zou, M.; Xu, Y.; Lin, P.; Lei, C.; Xia, X. Nano Drug Delivery System for Tumor Immunotherapy: Next-Generation Therapeutics. Front. Oncol. 2022, 12, 864301. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.P.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, H.; Lim, D.-K.; Joo, M.K.; Kim, K. Perspectives for Improving the Tumor Targeting of Nanomedicine via the EPR Effect in Clinical Tumors. Int. J. Mol. Sci. 2023, 24, 10082. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Colino, C.I.; Lanao, J.M.; Gutierrez-Millan, C. Targeting of Hepatic Macrophages by Therapeutic Nanoparticles. Front. Immunol. 2020, 11, 218. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-Based Drug Delivery Systems Targeting Cancer Cell Surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin Receptor 1 in Cancer: A New Sight for Cancer Therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a Tumor Biomarker and Therapeutic Target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Han, J.; Gong, Y.; Liu, C.; Yu, H.; Xie, N. Nanoparticle-Based Drug Delivery Systems Targeting Tumor Microenvironment for Cancer Immunotherapy Resistance: Current Advances and Applications. Pharmaceutics 2022, 14, 1990. [Google Scholar] [CrossRef] [PubMed]
- Sikder, A.; Vambhurkar, G.; Amulya, E.; Bagasariya, D.; Famta, P.; Shah, S.; Khatri, D.K.; Singh, S.B.; Sinha, V.R.; Srivastava, S. Advancements in Redox-Sensitive Micelles as Nanotheranostics: A New Horizon in Cancer Management. J. Control. Release 2022, 349, 1009–1030. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Yang, Y.; Shen, P.H.; Gao, X.J.; He, S.Q.; Liu, H.; Zhu, C.S. Facile and Simple Preparation of PH-Sensitive Chitosan-Mesoporous Silica Nanoparticles for Future Breast Cancer Treatment. Express Polym. Lett. 2015, 9, 1068–1075. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Du, X.; Wang, T. Redox-Responsive Micelles for Triggered Drug Delivery and Effective Laryngopharyngeal Cancer Therapy. Int. J. Biol. Macromol. 2018, 112, 65–73. [Google Scholar] [CrossRef]
- Lee, S.; Song, S.J.; Lee, J.; Ha, T.H.; Choi, J.S. Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells. Pharmaceutics 2020, 12, 876. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Xu, F.; Han, Z.; Wei, D.; Jia, D.; Zhou, Y. Tough Magnetic Chitosan Hydrogel Nanocomposites for Remotely Stimulated Drug Release. Biomacromolecules 2018, 19, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox Dual-Stimuli Responsive Drug Delivery Systems for Improving Tumor-Targeting Ability and Reducing Adverse Side Effects. Asian J. Pharm. Sci. 2020, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Fatfat, Z.; Fatfat, M.; Gali-Muhtasib, H. Micelles as Potential Drug Delivery Systems for Colorectal Cancer Treatment. World J. Gastroenterol. 2022, 28, 2867–2880. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T.; et al. Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery. Nano Today 2020, 34, 100914. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nejadnik, H.; Daldrup-Link, H.E. Next-Generation Superparamagnetic Iron Oxide Nanoparticles for Cancer Theranostics. Drug Discov. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Awan, U.A.; Raza, A.; Ali, S.; Saeed, R.F.; Akhtar, N. Doxorubicin-Loaded Gold Nanorods: A Multifunctional Chemo-Photothermal Nanoplatform for Cancer Management. Beilstein J. Nanotechnol. 2021, 12, 295–303. [Google Scholar] [CrossRef]
- Su, C.; Ren, X.; Yang, F.; Li, B.; Wu, H.; Li, H.; Nie, F. Ultrasound-Sensitive SiRNA-Loaded Nanobubbles Fabrication and Antagonism in Drug Resistance for NSCLC. Drug Deliv. 2022, 29, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Margel, S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Wang, T.; Chang, T.M.S. Superparamagnetic Artificial Cells PLGA-Fe3O4 Micro/Nanocapsules for Cancer Targeted Delivery. Cancers 2023, 15, 5807. [Google Scholar] [CrossRef]
- Su, C.; Ren, X.; Nie, F.; Li, T.; Lv, W.; Li, H.; Zhang, Y. Current Advances in Ultrasound-Combined Nanobubbles for Cancer-Targeted Therapy: A Review of the Current Status and Future Perspectives. RSC Adv. 2021, 11, 12915–12928. [Google Scholar] [CrossRef]
- Kemp, J.A.; Kwon, Y.J. Cancer Nanotechnology: Current Status and Perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, Challenges, and Future of Nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Approves DaunoXome as First-Line Therapy for Kaposi’s Sarcoma. J. Int. Assoc. Physicians AIDS Care 1996, 2, 50–51. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, J.F.; Tagawa, S.T.; Atkinson, S.N.; Boldt-Houle, D.M.; Moul, J.W. Subcutaneous in Situ Gel Delivered Leuprolide Acetate’s Consistent and Prolonged Drug Delivery Maintains Effective Testosterone Suppression Independent of Age and Weight in Men with Prostate Cancer. BJUI Compass 2020, 1, 64–73. [Google Scholar] [CrossRef]
- Grillo-López, A.J. Zevalin: The First Radioimmunotherapy Approved for the Treatment of Lymphoma. Expert Rev. Anticancer Ther. 2002, 2, 485–493. [Google Scholar] [CrossRef]
- Ando, K.; Mori, K.; Corradini, N.; Redini, F.; Heymann, D. Mifamurtide for the Treatment of Nonmetastatic Osteosarcoma. Expert Opin. Pharmacother. 2011, 12, 285–292. [Google Scholar] [CrossRef]
- Egea-Benavente, D.; Ovejero, J.G.; Morales, M.d.P.; Barber, D.F. Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation. Cancers 2021, 13, 4583. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (Vincristine Sulfate Liposome Injection) Improves the Pharmacokinetics and Pharmacodynamics of Vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Tkaczuk, K.; Yared, J. Update on Taxane Development: New Analogs and New Formulations. Drug Des. Dev. Ther. 2012, 371, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Choi, J.M.; In, J.; Sung, T.; Kim, Y.B.; Sultana, S. Current Clinical Application of Dantrolene Sodium. Anesth. Pain Med. 2023, 18, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.E.; Levien, T.L. Irinotecan Liposome Injection. Hosp. Pharm. 2017, 52, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Seo, J.H.; Ahn, J.-H.; Kim, T.-Y.; Kang, S.Y.; Sohn, J.; Yang, Y.; Park, K.H.; Moon, Y.W.; Lim, S.; et al. Phase II Study of DHP107 (Oral Paclitaxel) in the First-Line Treatment of HER2-Negative Recurrent or Metastatic Breast Cancer (OPTIMAL Study). Ther. Adv. Med. Oncol. 2021, 13, 175883592110619. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Daunorubicin/Cytarabine Liposome: A Review in Acute Myeloid Leukaemia. Drugs 2018, 78, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a First-in-Class Radioenhancer Hafnium Oxide Nanoparticle, plus Radiotherapy versus Radiotherapy Alone in Patients with Locally Advanced Soft-Tissue Sarcoma (Act.In.Sarc): A Multicentre, Phase 2–3, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Albalawi, F.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J. Engineered Nanomaterials: The Challenges and Opportunities for Nanomedicines. Int. J. Nanomed. 2021, 16, 161–184. [Google Scholar] [CrossRef]
- El-Kady, M.M.; Ansari, I.; Arora, C.; Rai, N.; Soni, S.; Verma, D.K.; Singh, P.; Mahmoud, A.E.D. Nanomaterials: A Comprehensive Review of Applications, Toxicity, Impact, and Fate to Environment. J. Mol. Liq. 2023, 370, 121046. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega 2023, 8, 14290–14320. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Lam, A.K.; Sykes, E.A.; Rocheleau, J.V.; Chan, W.C.W. Tumour-on-a-Chip Provides an Optical Window into Nanoparticle Tissue Transport. Nat. Commun. 2013, 4, 2718. [Google Scholar] [CrossRef] [PubMed]
- Bleijs, M.; van de Wetering, M.; Clevers, H.; Drost, J. Xenograft and Organoid Model Systems in Cancer Research. EMBO J. 2019, 38, e101654. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.-A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Pınar, S.G.; Oktay, A.N.; Karaküçük, A.E.; Çelebi, N. Formulation Strategies of Nanosuspensions for Various Administration Routes. Pharmaceutics 2023, 15, 1520. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, V.M.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhao, P.; Yu, T.; Gu, N. Current Applications and Future Prospects of Nanotechnology in Cancer Immunotherapy. Cancer Biol. Med. 2019, 16, 487–497. [Google Scholar] [CrossRef]
- Dutta Gupta, Y.; Mackeyev, Y.; Krishnan, S.; Bhandary, S. Mesoporous Silica Nanotechnology: Promising Advances in Augmenting Cancer Theranostics. Cancer Nanotechnol. 2024, 15, 9. [Google Scholar] [CrossRef]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in Cancer Diagnosis: Progress, Challenges and Opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alsowinea, A.F. Approved and Marketed Nanoparticles for Disease Targeting and Applications in COVID-19. Nanotechnol. Rev. 2021, 10, 1941–1977. [Google Scholar] [CrossRef]
| Lipid-Based Nanoparticle | Examples of Prominent Applications | Reference |
|---|---|---|
| Liposomes | Attachment of anti-CD22 monoclonal antibodies to PEGylated liposomes loaded with doxorubicin (DOX) for enhanced drug accumulation in non-Hodgkin’s lymphoma tumors. | [44] |
| Evaluation of paclitaxel liposomes targeting the folate receptor (FR) in cancer therapy. | [20] | |
| Evaluation of TfR-targeted liposomes (Tf-PEG-liposomes) in a mouse model of colon cancer. | [45] | |
| Investigation of liposomes functionalized with the neurofilament-derived peptide, NFL-TBS.40–63, for targeted delivery to glioblastoma cells across the blood–brain barrier (BBB). | [46] | |
| Phase I trial of siRNA targeting EPHA2 with liposomes (siRNA-EPHA2-DOPC) for advanced neoplasm. | [47] | |
| Phase III trial of liposomal paclitaxel (EndoTAG-1) for breast cancer. | [48] | |
| Phase III trial of HER2-targeted liposomal doxorubicin hydrochloride (MM-302) for breast cancer. | [49] | |
| Phase III trials of thermally sensitive liposomal doxorubicin (ThermoDox) for breast cancer. | [15] | |
| Solid Lipid Nanoparticles | Evaluation of solid lipid nanoparticles (SLNs) loaded with gemcitabine on patient-derived primary pancreatic cancer cell lines (PPCL-46) and MiaPaCa-2 pancreatic cancer cell lines. | [50] |
| Development of transferrin-conjugated solid lipid nanoparticles (SLNs) for targeted delivery of tamoxifen citrate for breast cancer treatment. | [51] | |
| Development of arginine-glycine-aspartic (RGD) tripeptide-modified solid lipid nanoparticles (SLNs) for targeted delivery of doxorubicin (DOX) for breast cancer treatment. | [52] | |
| Nanostructured Lipid Carriers | Evaluation of folate-chitosan-coated nanostructured lipid carriers (FCH-NLCs) encapsulating harmaline for targeted breast cancer therapy. | [53] |
| Optimization of nanostructured lipid carriers (NLCs) loaded with metformin and thymoquinone for breast cancer therapy. | [54] |
| Type of the Inorganic Nanoparticle | Characteristics | Examples of Prominent Applications | Reference |
|---|---|---|---|
| Gold Nanoparticles (AuNPs) | AuNPs have unique thermal and optical properties, which can be controlled by changing its size, shape, and/or surface chemistry. | Chitosan-folic acid-coated gold nanoparticles are biocompatible and can be used to deliver drugs more selectively to tumor cells. | [106] |
| Silver Nanoparticles (AgNPs) | AgNPs, typically smaller than 100 nm and composed of 20 to 15,000 silver atoms, have unique physicochemical and biological properties that are influenced by their size and shape. | C225-coated Ag NPs (Ag/C225) are effective radiosensitizers for nasopharyngeal carcinoma epithelial cell lines, with an average preserved anti-EGFR antibody activity of about 82%. | [107] |
| Iron Oxide Nanoparticles | Iron oxide nanoparticles are made up of a solid iron oxide core surrounded by a layer of water-soluble polymers such as dextran or sucrose. | A nanotherm is a type of nanoparticle made up of iron oxide coated with aminosilane. It is used to eradicate cancer cells by heating them with an alternating magnetic field. | [108] |
| Copper Nanoparticles (CuNPs) | CuNPs exhibit strong near-infrared light absorption and can generate heat. This characteristic makes them valuable in photothermal therapy. | Gold and copper nanoparticles have the potential to be used in the treatment of breast cancer when applied to MCF-7 and MDA-MB-231 breast cancer cells. | [103] |
| Titanium Dioxide Nanoparticles (TiO2 NPs) | TiO2 NPs can serve as anti-cancer agents due to their significant cellular accumulation, which can induce alterations in metabolic pathways, ultimately resulting in necrosis. | TiO2 has been used to deliver various anti-cancer drugs, including daunorubicin, temozolomide, doxorubicin, and cisplatin, to cancer cells. | [109] |
| Mesoporous Silica Nanoparticles (MSNs) | MSNs can transform crystalline drugs into their amorphous state, facilitating enhanced cellular absorption. | MSNs increased paclitaxel cytotoxicity by 4.3-fold against HepG2 cells and camptothecin cytotoxicity by 86% against Capan-1 human pancreatic adenocarcinoma cells. | [110] |
| Characteristics of Smart Nanoparticles | Strategies for Smart Nanoparticles | Reference |
|---|---|---|
| Immune System Evasion | Achieved through PEGylation to evade immune system clearance. | [152] |
| Targeted Accumulation | Surface modification with ligands matching cancer cell overexpressed proteins for precise targeting. | [153] |
| Controlled Delivery | Delivering therapeutic agents to the desired location at specific concentrations, using external or internal stimuli. Achieving this control often involves grafting various chemical groups onto the surface of the nanocarrier. | [153] |
| Co-Delivery Capability | Capable of delivering multiple substances, such as anti-cancer drugs, genetic materials, and imaging agents. | [154] |
| Receptor | Targeting Ligands | Description | Reference |
|---|---|---|---|
| Folate receptor (FR) | Folic acid (FA) | FA, also known as vitamin B9, is crucial for DNA-related processes. When combined with the FR, it enters cancer cells through endocytosis. FR is highly expressed in various epithelial tumors, including ovarian, lung, breast, endometrial, cervical, renal, bladder, and brain cancers. | [165] |
| Integrin receptor | Arginylglycylaspartic acid peptide (RGD) | The RGD peptide is a common integrin-binding moiety found in the extracellular matrix. It binds most strongly to αvβ3 and αvβ5 integrins, which are not expressed in normal tissues. Mainly in lung cancer and breast cancer. | [166] |
| Epidermal Factor Receptor (EGFR) | Anti-EGFR | EGFR is a transmembrane glycoprotein in the tyrosine kinase receptor family. EGFR plays a huge role in the development of several cancers, such as colon, non-small-cell lung, breast, head, and ovarian cancers. | [47] |
| Transferrin receptor (TfR) | Transferrinreceptor ligand | TfR regulates iron distribution in normal human cells. TfR is more expressed in breast cancer, glioma, lung adenocarcinoma, and chronic lymphocytic leukemia. | [167] |
| Cluster of Differentiation 44 (CD44) | Hyaluronic acid | CD44, a transmembrane adhesion glycoprotein, participates in various physiological and pathological pathways, especially in tumor development, progression, and metastasis. CD44 is overexpressed on the surfaces of many tumors, including liver, breast, colon, and lymphoma. | [169] |
| Endogenous Stimulus Factor | Description | Example | Reference |
|---|---|---|---|
| The pH-responsive stimulus | The variance in pH levels between normal and cancer cells provides a robust basis for developing a stimulus-responsive drug delivery system. | A mesoporous silica nanoparticle-chitosan system was prepared for pH-responsive drug delivery, demonstrating enhanced Ibuprofen release at pH 6.8 over pH 7.4, promising for breast cancer treatment. | [122] |
| Redox sensitive stimulus | Glutathione sulfhydryl (GSH), a potent antioxidant, is abundant in mammalian tissues, especially within tumors, where its concentration is four times higher than in normal cells. GSH can reduce disulfide bonds in nanocarriers, leading to precise drug release, making it a key component in targeted drug delivery. | Stable micelles were developed by coupling heparosan with deoxycholic acid via disulfide bonds to deliver DOX to cancer tissues. These micelles exhibited strong drug-loading capacity and glutathione-triggered drug release. | [177,178] |
| Enzyme stimulus | Extracellular enzymes target tumor sites due to elevated activity but are not suitable for intracellular drug release because enzyme levels in cancer and healthy cells are similar. Proteases are ideal for drug release from liposomes. | Doxorubicin-loaded GLFG liposomes, degraded by overexpressed cathepsin B in cancer cells, effectively inhibited cancer cell proliferation in Hep G2 cells. | [175] |
| Exogenous Stimulus Factor | Description | Example | Reference |
|---|---|---|---|
| Magnetic field responsive stimulus | Magnetic systems attract drug-loaded nanocarriers to tumor sites using an extracorporeal magnetic field. | Implantable magnetic chitosan hydrogel loaded with both rifampicin and adriamycin drugs responds to low-frequency alternating magnetic fields, releasing drugs intermittently without inducing magnetic hyperthermia, enhancing precision and reducing post-surgical infection risk. | [183] |
| Thermo-responsive stimulus | Exceeding the critical solution temperature of the polymer nanoparticle disrupts the hydrophilic–hydrophobic balance, leading to polymer chain dehydration and structural changes, releasing the drug. | Superparamagnetic nanoparticles loaded with camptothecin and formulated to be thermo-responsive. This nanocomposite enhanced cytotoxicity against cancer cells compared to free drugs. | [184] |
| Light triggered stimulus | Light-responsive drug delivery systems achieve precise drug release upon exposure to external light sources, including visible, infrared, or ultraviolet light. | The release of DOX from the gold nanocarrier is enhanced when exposed to 808 nm illumination. | [181] |
| Ultrasound responsive stimulus | It can induce both mechanical and thermal effects within nanocarriers, leading to the release of loaded medications. | Ultrasound-sensitive nanobubbles loaded with paclitaxel and siRNA for hepatocellular carcinoma were developed. When exposed to low-frequency ultrasound, this system induces apoptosis in cancer cells and reduces tumor volume. | [185] |
| Product (Active Ingredient) | Type of Nanomaterial | Indication(s) | Developer | Initial Approved Year and Region | Reference |
|---|---|---|---|---|---|
| Doxil (Doxorubicin) | PEGylated liposome | Kaposi’s sarcoma, breast cancer, ovarian cancer, multiple myeloma | Janssen | FDA (1995) EMA (1996) | [49] |
| DaunoXome (Daunorubicin) | Liposome | Kaposi’s sarcoma | Galen | FDA (1996) | [190] |
| Lipo-Dox (Doxorubicin) | PEGylated liposome | Kaposi’s sarcoma, breast cancer, ovarian cancer | Taiwan Liposome | Taiwan (1998) | [33] |
| DepoCyt (Cytarabine) | Liposome | Lymphomatous meningitis | Pacira Pharmaceuticals | FDA (1999) | [191] |
| Myocet (Doxorubicin) | Liposome | Metastatic breast cancer | Teva UK | EMA (2000) | [192] |
| Eligard (Leuprolide acetate) | Polymer | Prostate cancer | Tolmar Pharmaceuticals | FDA (2002) | [193] |
| Zevalin (90Y-ibritumomab tiuxetan) | Liposome | Lymphoma | Bayer Pharma | FDA (2002) EMA (2004) | [194] |
| Abraxane (Paclitaxel) | Albumin nanoparticle | Advanced NSCLC, metastatic breast cancer, metastatic pancreatic cancer | Abraxis BioScience/Celgene | FDA (2005) EMA (2008) | [127] |
| Oncaspar (L-asparaginase) | Polymer protein conjugate | NSCLC, ovarian cancer, and breast cancer | Les Laboratoires Servier | State Food and Drug Administration of China (2006) | [127] |
| Genexol-PM (Paclitaxel) | PEG-b-PLA polymeric micelle | Breast cancer, ovarian cancer, and NSCLC | Samyang Biopharmaceutical | South Korea (2007) | [80] |
| Mepact (Mifamurtide) | Liposome | Osteosarcoma | Takeda | EMA (2009) | [195] |
| NanoTherm | Iron oxide nanoparticle | Thermal ablation of glioblastoma, prostate cancer | MagForce Nano | EMA (2010) FDA (2018) | [196] |
| Marqibo (Vincristine) | Liposome | Acute lymphoblastic leukemia | Talon Therapeutics Inc. | FDA (2012) | [197] |
| Opaxio (Paclitaxel) | Polymer | Head and neck cancer; Glioblastoma | Cell Therapeutics, Inc. | FDA (2012) | [198] |
| Ryanodex (Dantrolene sodium) | Nanocrystal | Malignant hypothermia | Eagle Pharmaceuticals | FDA (2014) | [199] |
| Onivyde (Irinotecan) | PEGylated liposome | Metastatic pancreatic cancer | Merrimack Pharmaceuticals | FDA (2015) | [200] |
| DHP107 (Paclitaxel) | Lipid nanoparticle | Gastric cancer | Daehwa Pharmaceutical | South Korea (2016) | [201] |
| Vyxeos CPX-351 (Daunorubicin:cytarabine [1:5 molar ratio]) | Liposome | Acute myeloid leukemia | Jazz Pharmaceuticals | FDA (2017) EMA (2018) | [202] |
| Apealea (Paclitaxel) | Micelle | Ovarian, peritoneal, and fallopian tube cancer | Oasmia Pharmaceutical | EMA (2018) | [28] |
| Hensify | Hafnium oxide nanoparticle | Locally advanced soft tissue sarcoma | Nanobiotix | CE mark (2019) | [203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafat, M.; Sakkal, M.; Beiram, R.; AbuRuz, S. Nanomedicines: Emerging Platforms in Smart Chemotherapy Treatment—A Recent Review. Pharmaceuticals 2024, 17, 315. https://doi.org/10.3390/ph17030315
Arafat M, Sakkal M, Beiram R, AbuRuz S. Nanomedicines: Emerging Platforms in Smart Chemotherapy Treatment—A Recent Review. Pharmaceuticals. 2024; 17(3):315. https://doi.org/10.3390/ph17030315
Chicago/Turabian StyleArafat, Mosab, Molham Sakkal, Rami Beiram, and Salahdein AbuRuz. 2024. "Nanomedicines: Emerging Platforms in Smart Chemotherapy Treatment—A Recent Review" Pharmaceuticals 17, no. 3: 315. https://doi.org/10.3390/ph17030315
APA StyleArafat, M., Sakkal, M., Beiram, R., & AbuRuz, S. (2024). Nanomedicines: Emerging Platforms in Smart Chemotherapy Treatment—A Recent Review. Pharmaceuticals, 17(3), 315. https://doi.org/10.3390/ph17030315