Fabrication of Nanocrystals for Enhanced Distribution of a Fatty Acid Synthase Inhibitor (Orlistat) as a Promising Method to Relieve Solid Ehrlich Carcinoma-Induced Hepatic Damage in Mice
Abstract
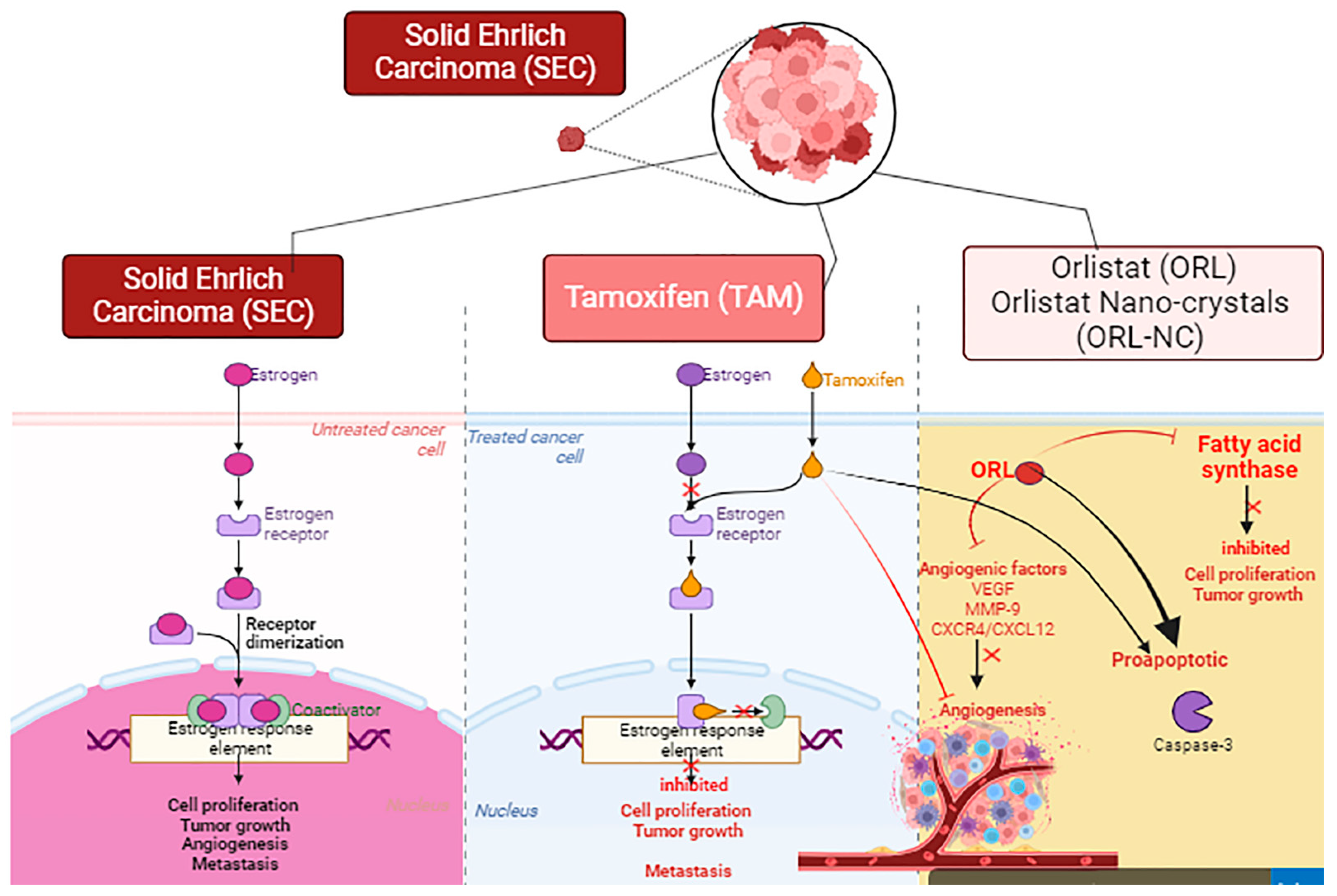
1. Introduction
2. Results
2.1. Saturated Aqueous Solubility
2.2. Particle Size and Zeta Potential Analysis
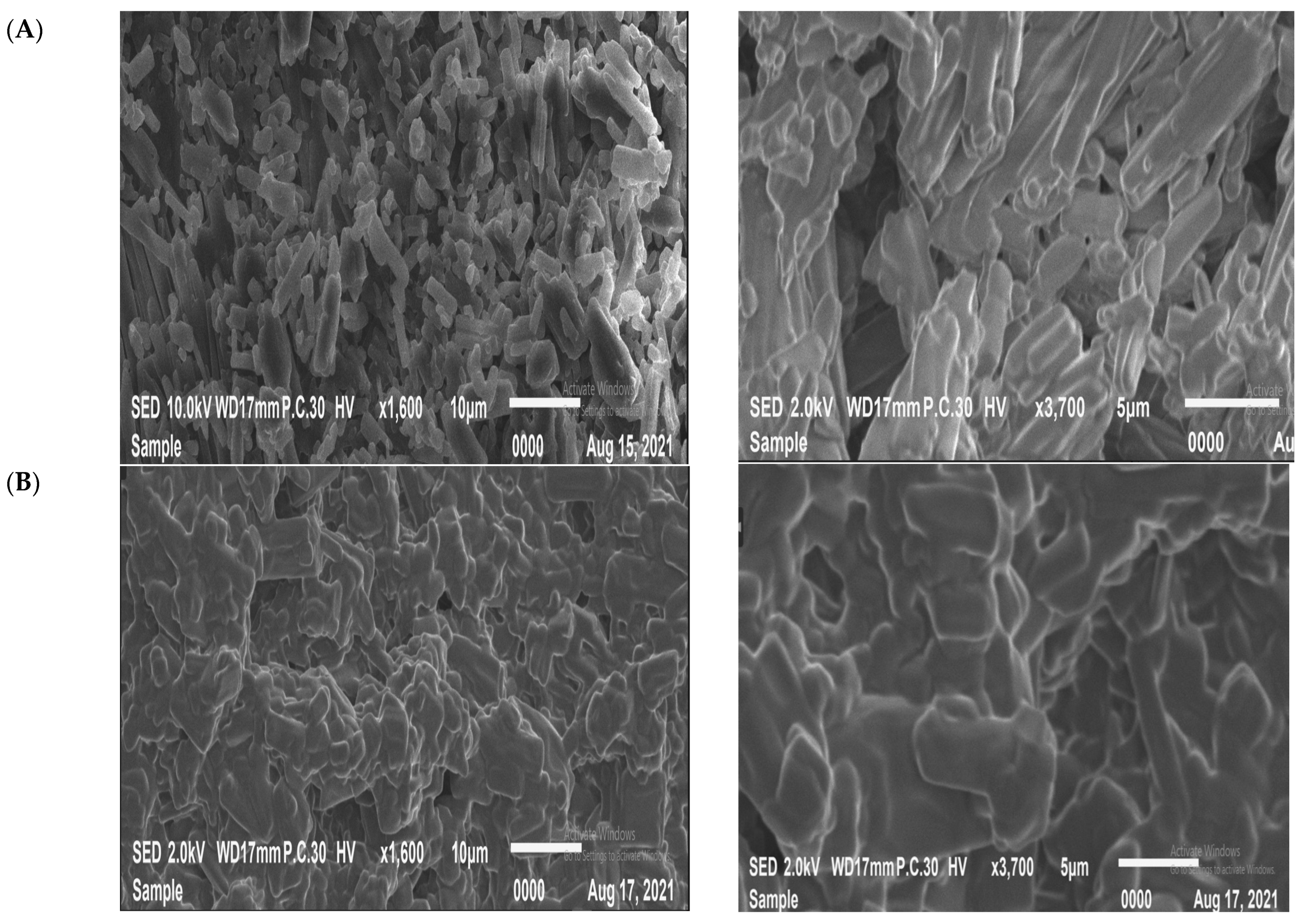
2.3. Scanning Electron Microscope (SEM)
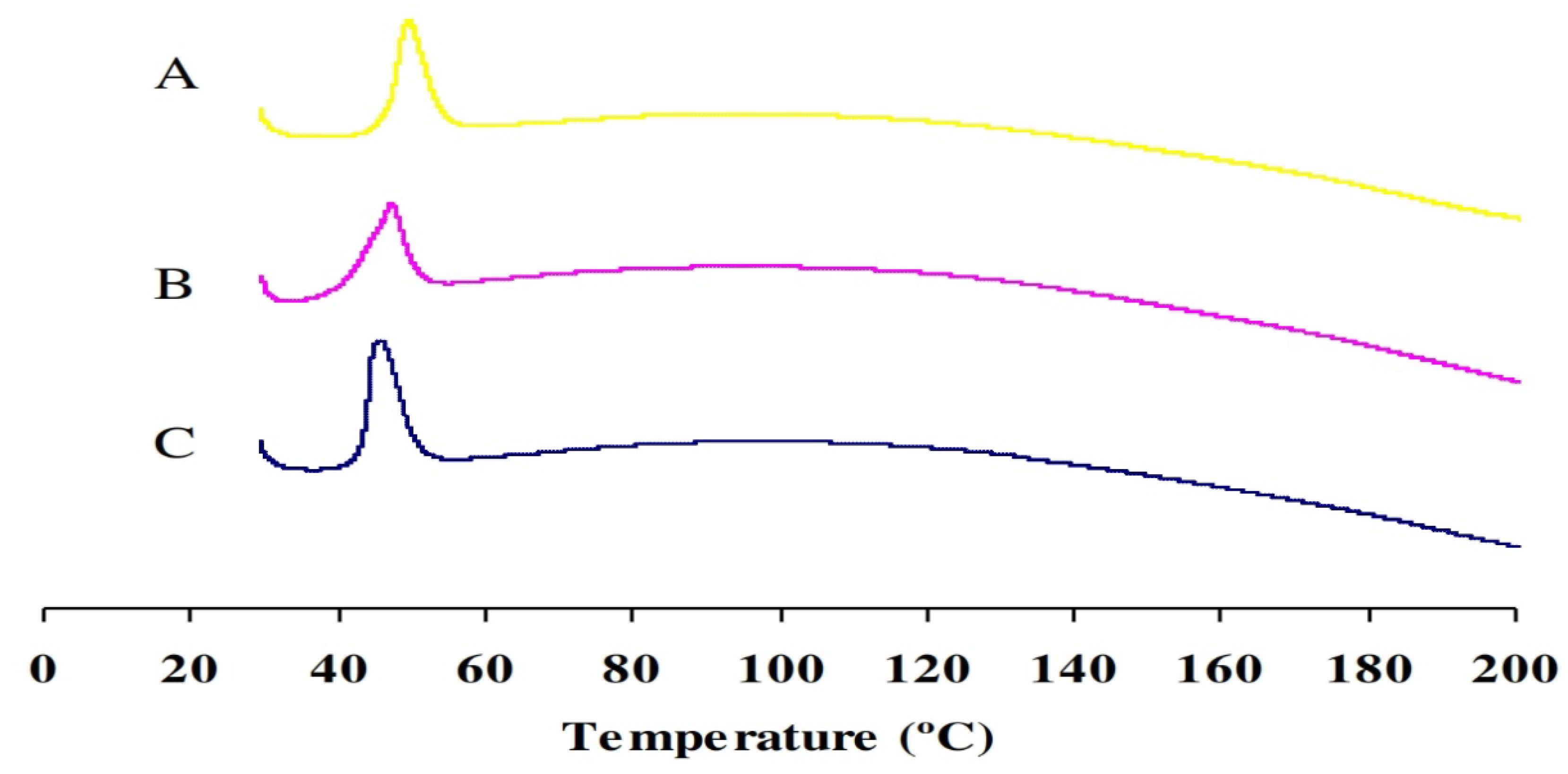
2.4. Differential Scanning Calorimetry (DSC)
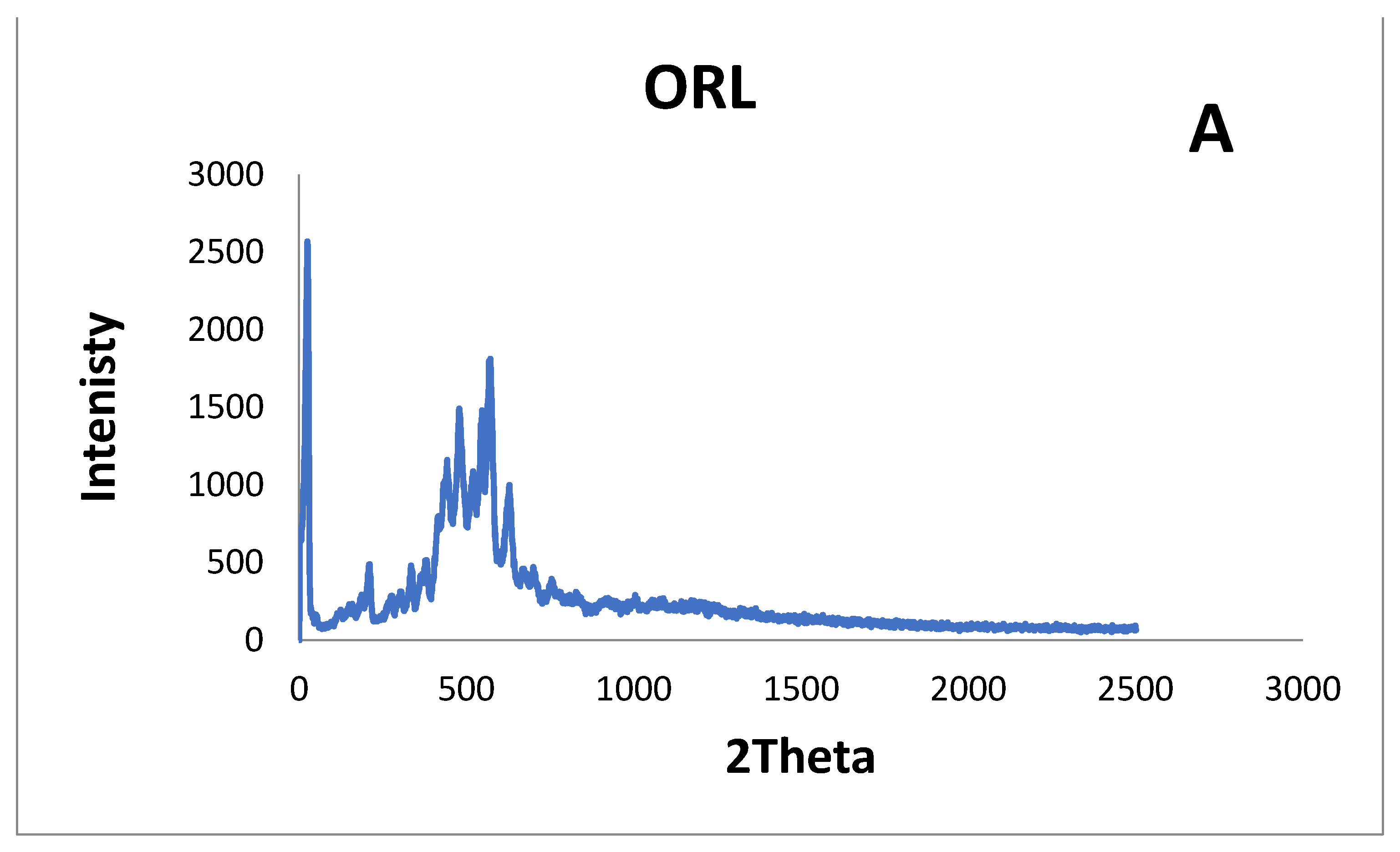
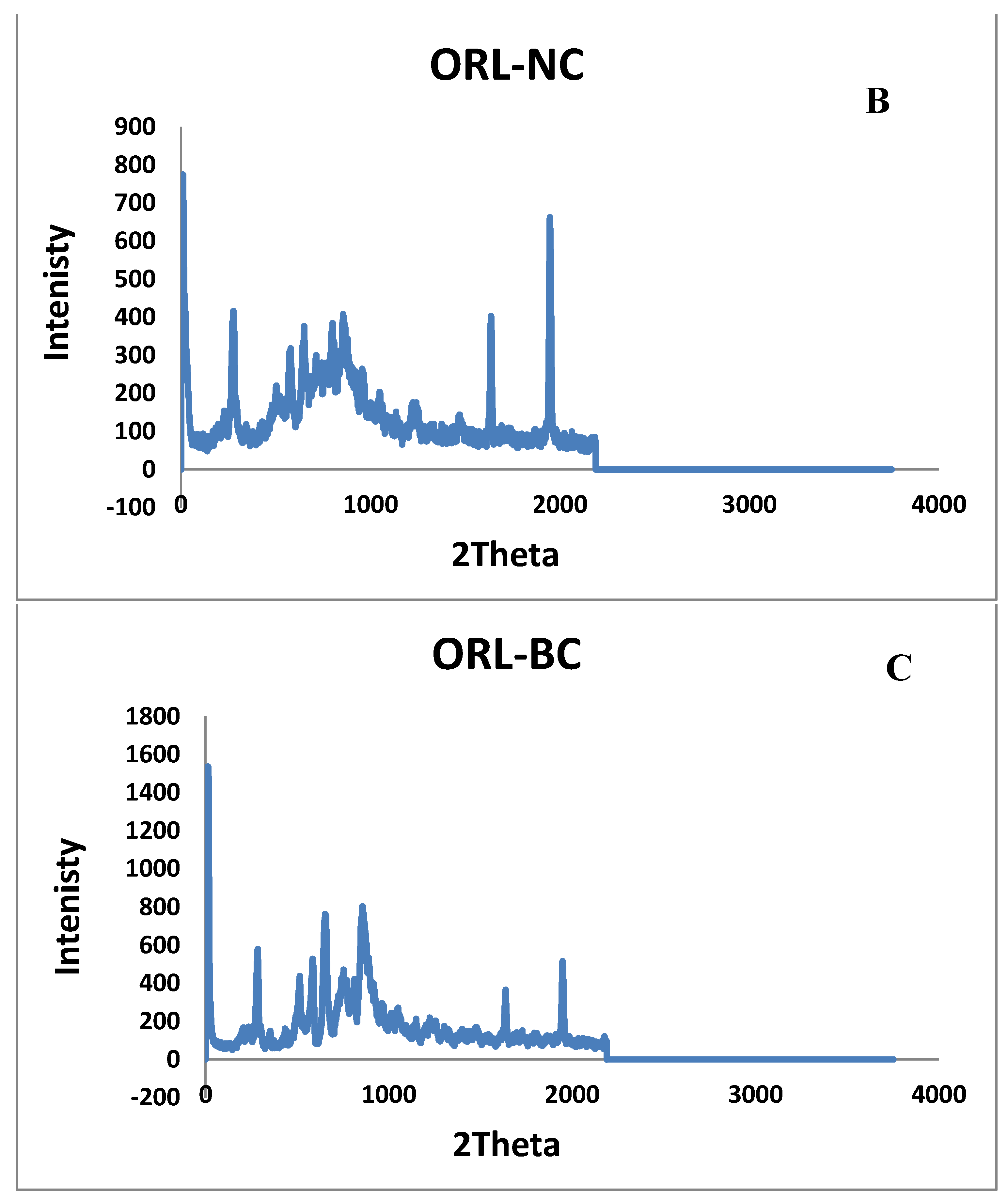
2.5. X-ray Diffraction
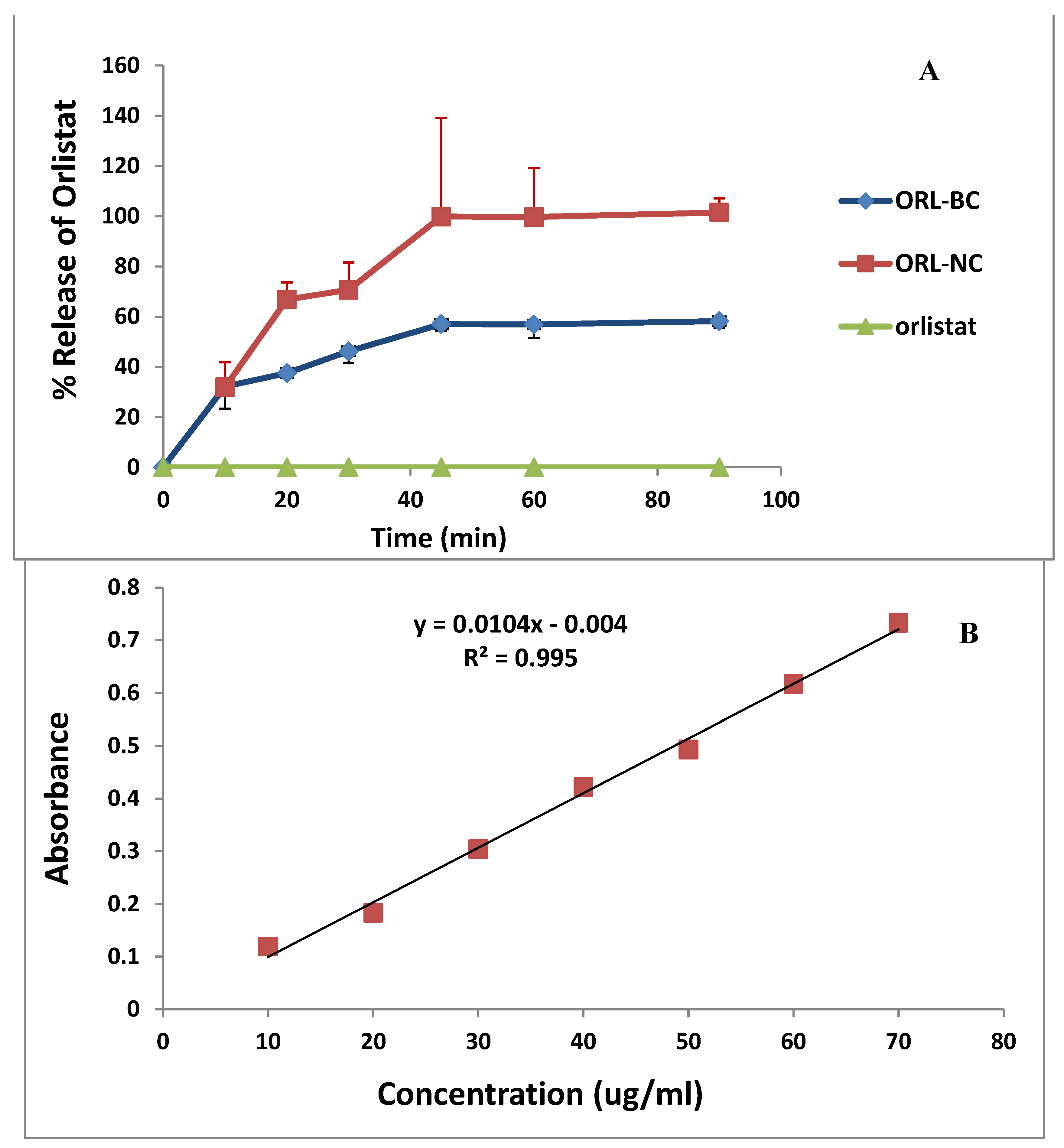
2.6. Dissolution
2.7. Preparation of the 99mTc-Orlistat Nanocrystals Complex
2.8. Radiochemical Yield Optimization
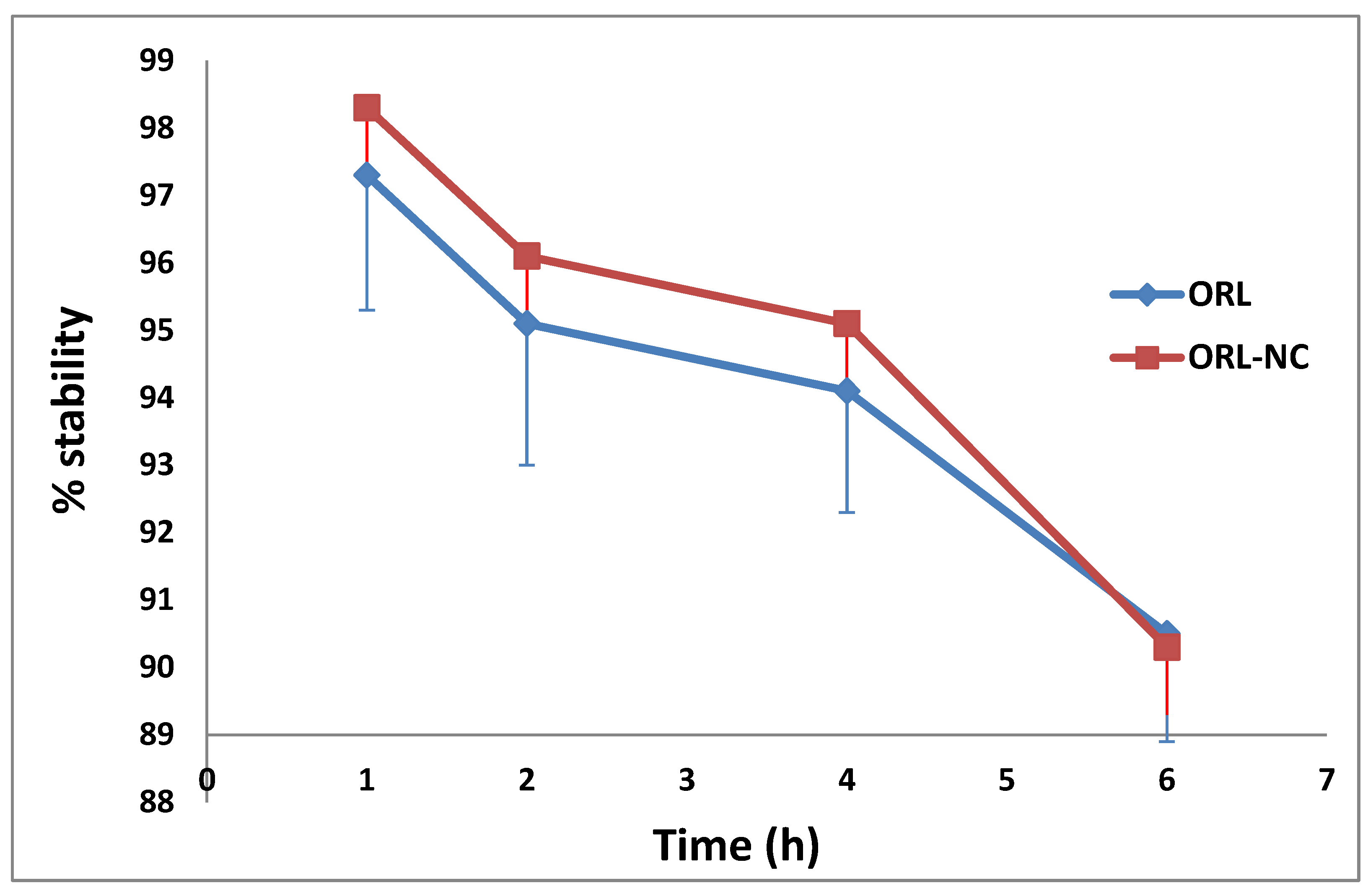
2.9. Serum Stability of the 99mTc-Complex
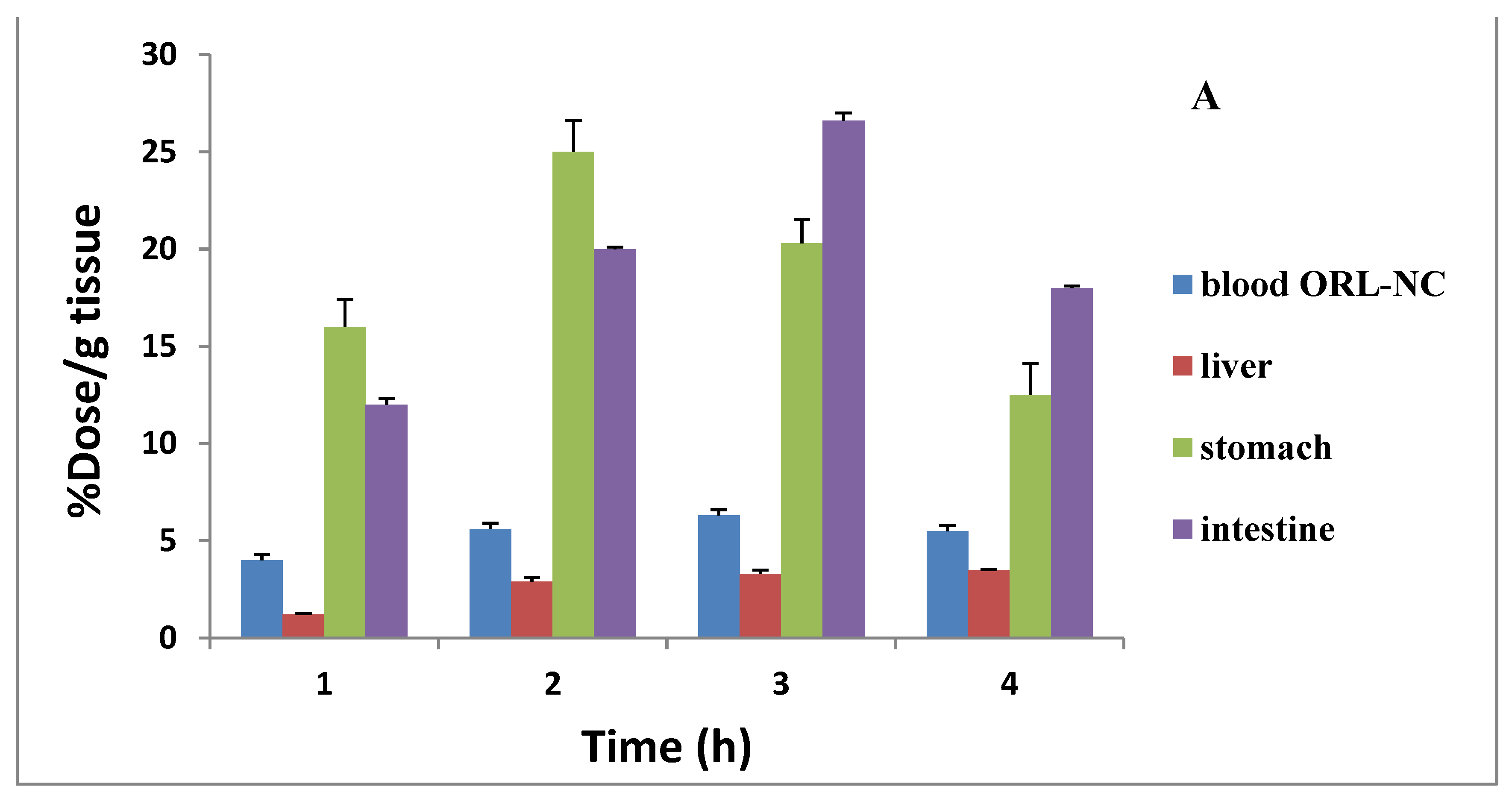
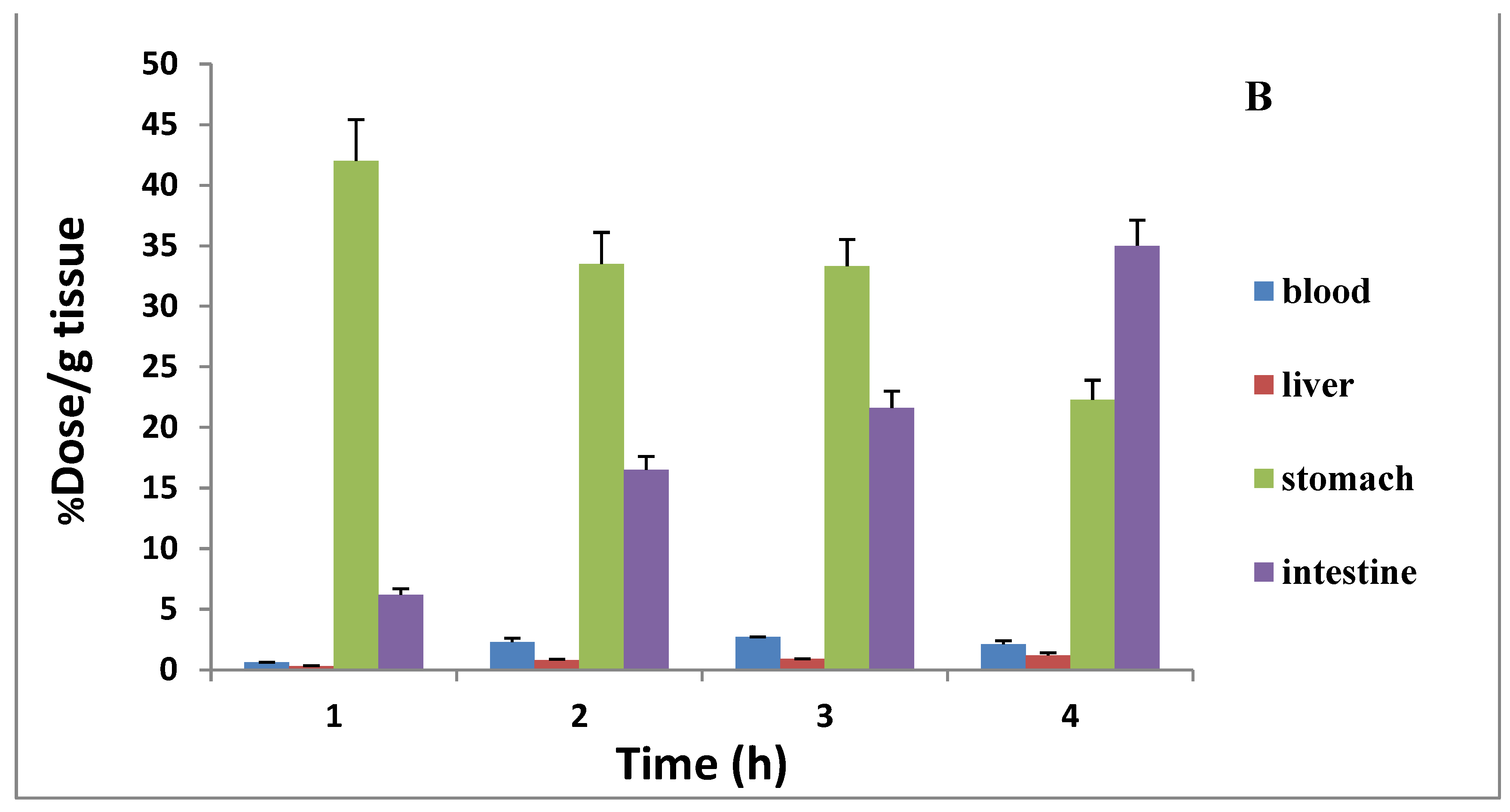
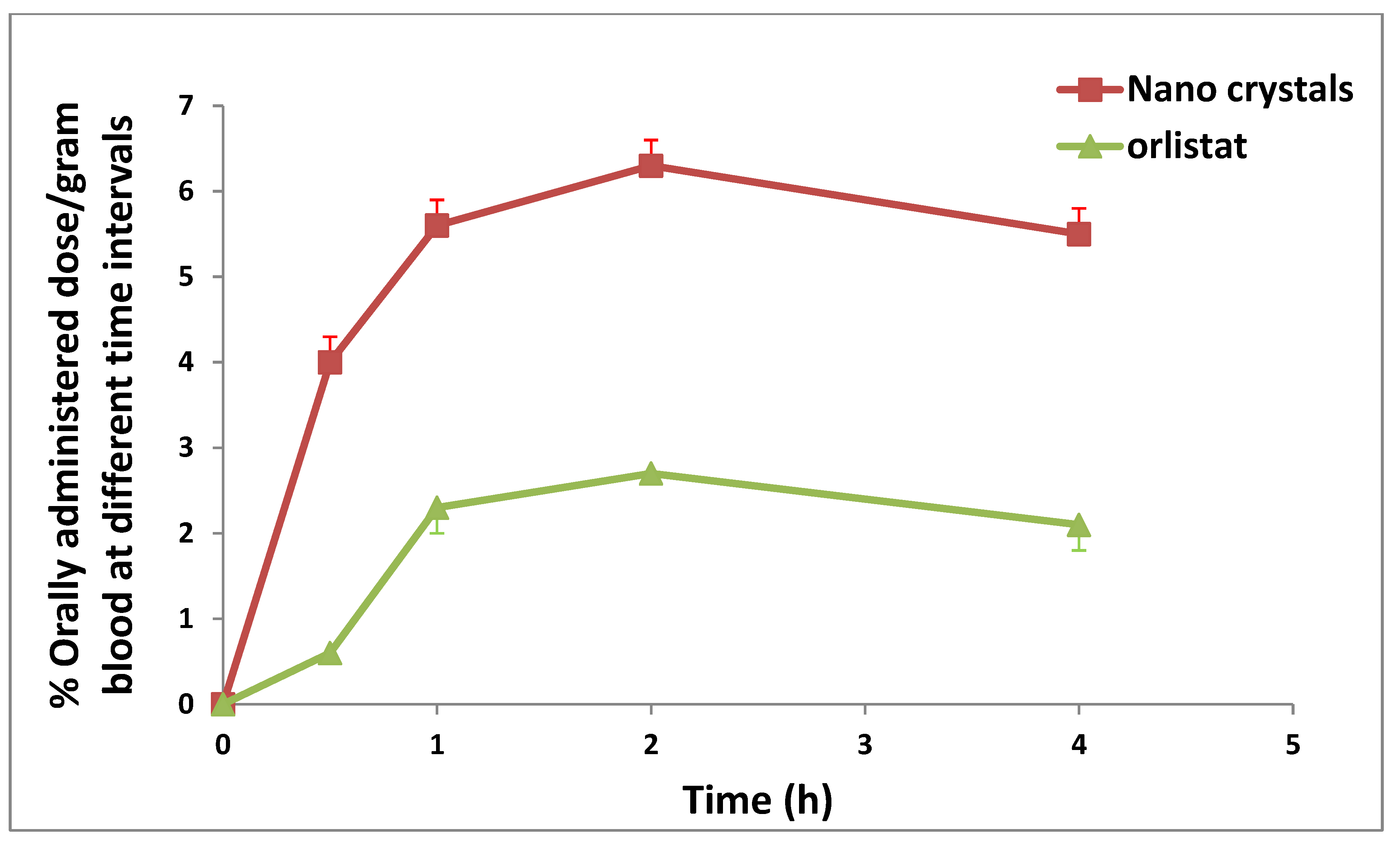
2.10. Assessment of the Distribution and Bioavailability (Radiography)
2.11. In Vivo Experiments
2.11.1. Effect of ORL and ORL-NC on Serum Liver Function Biomarkers
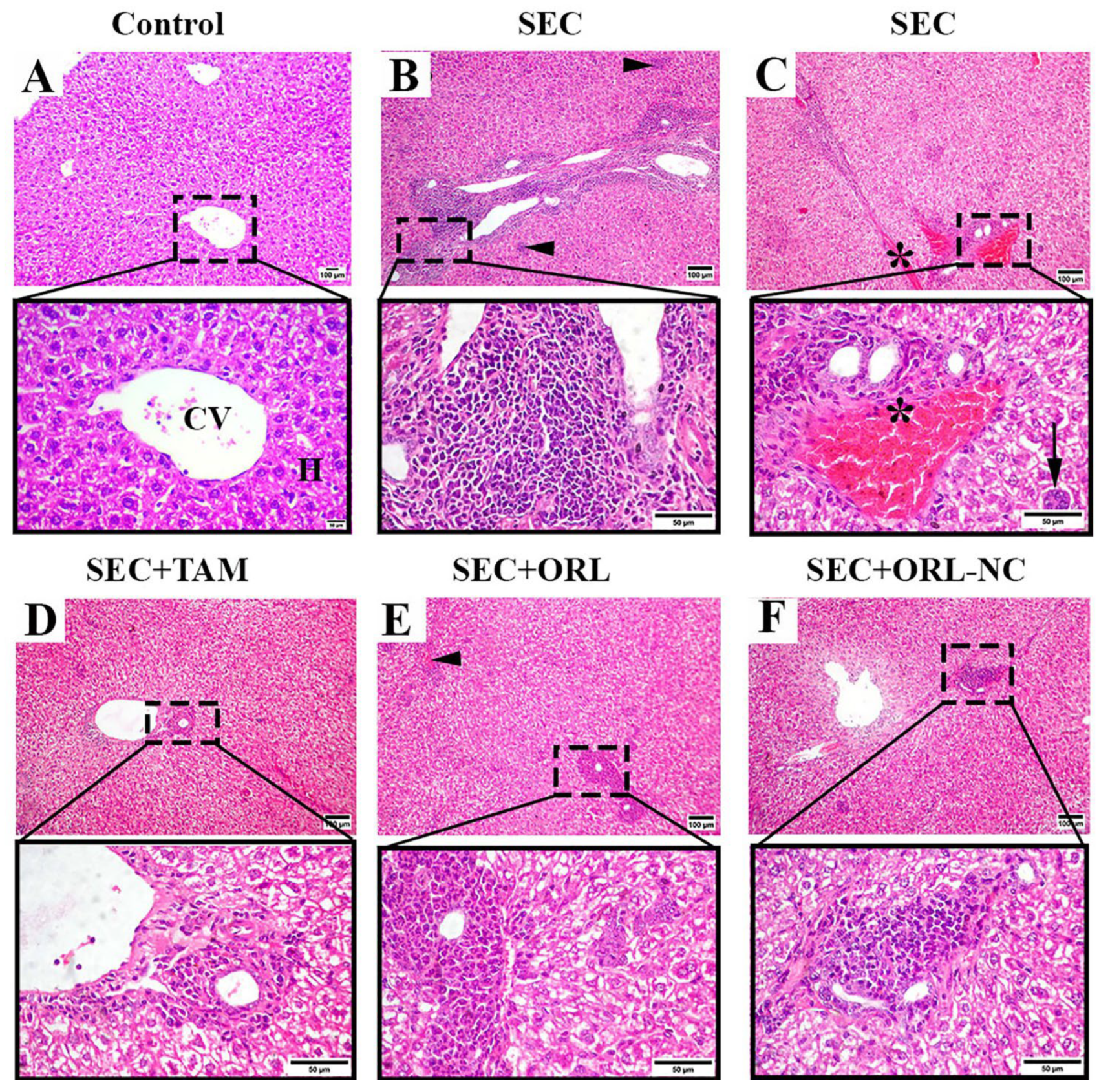
2.11.2. Effect of ORL and ORL-NC Protected the Hepatic Tissue of Mice from SEC-Induced Histopathological Changes
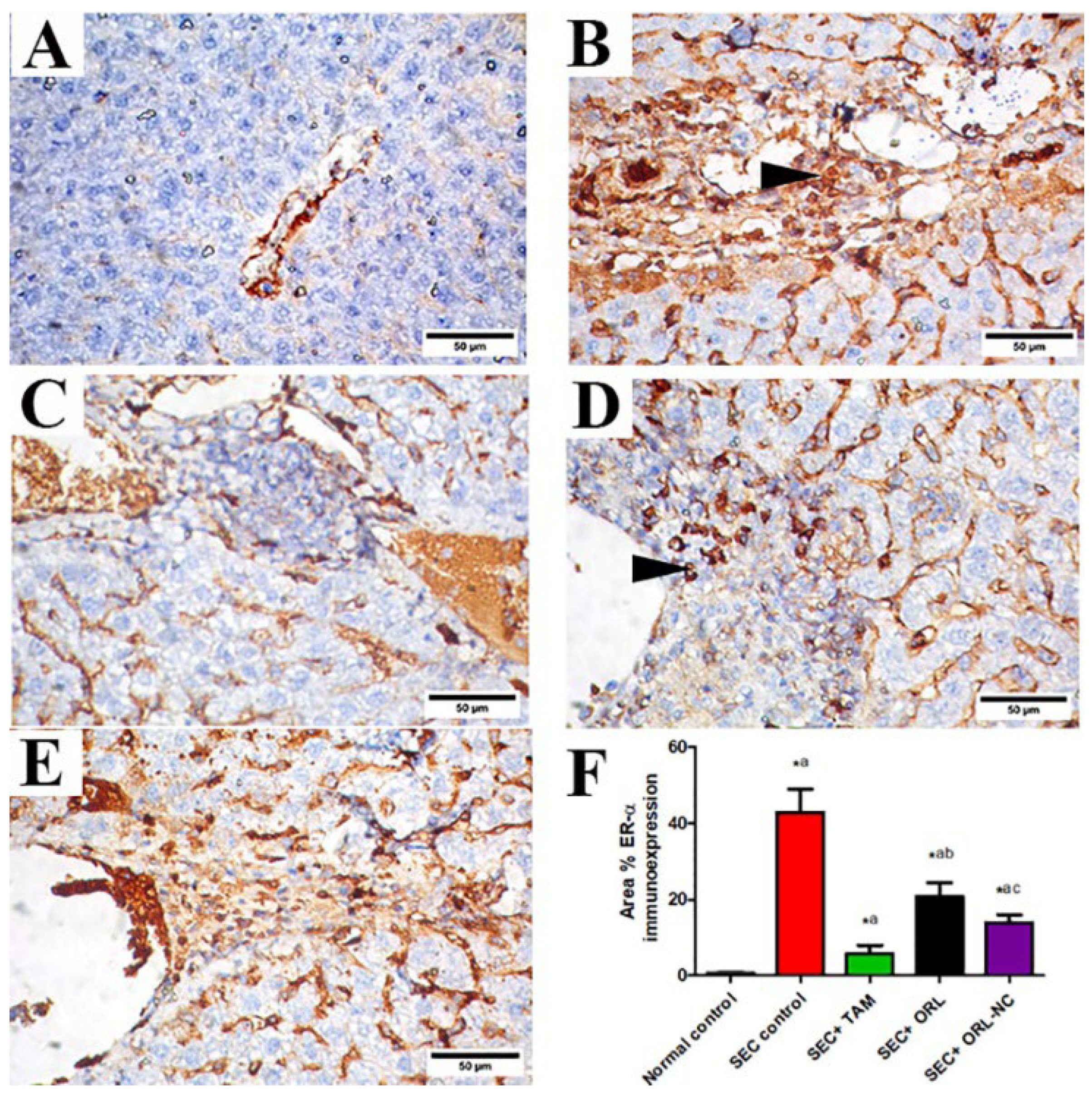
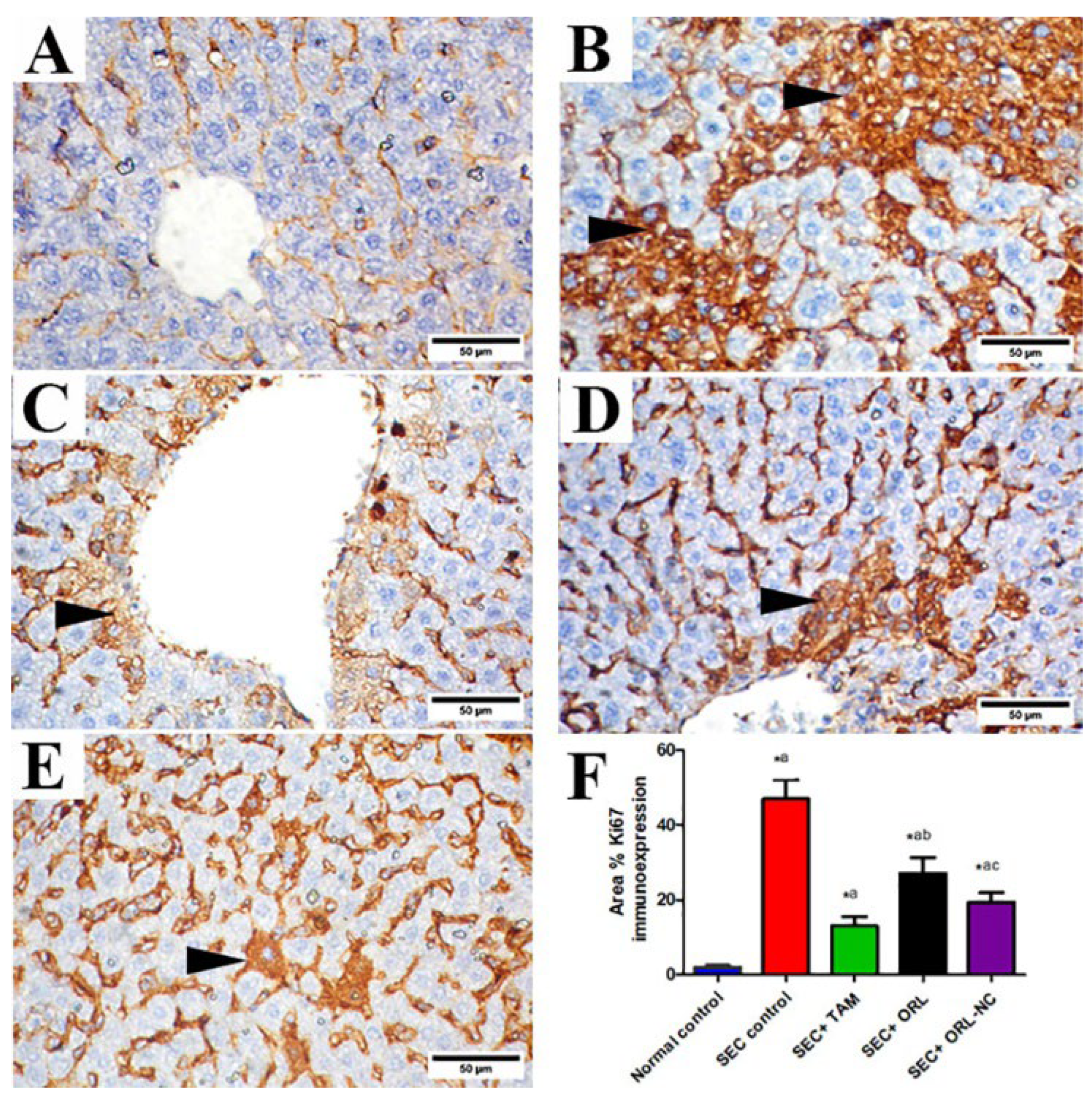
2.11.3. Effect of ORL and ORL-NC Reduced SEC-Induced Increased ER-α and Ki-67
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Preparation of ORL Nanocrystals
4.3. Characterization of ORL Crystals
4.3.1. Saturation Solubility
4.3.2. Particle Size Analysis
4.3.3. Scanning Electron Microscopy (SEM)
4.3.4. Differential Scanning Calorimetry (DSC)
4.3.5. X-ray Powder Diffraction
4.3.6. In Vitro Dissolution Study
4.4. Bioavailability and Tissue Distribution Study
4.4.1. Radiolabeling of ORL Using Technetium-99m
4.4.2. Assessment of Radiochemical Yield
4.4.3. Serum Stability
4.4.4. Assay for Biodistribution
4.5. In Vivo Experiments
4.5.1. Animals
4.5.2. Induction of the Solid Ehrlich Tumor
4.5.3. Experimental Design
Blood and Tissue Collection
Liver Enzyme Measurement
Histopathological Assessments
Immunohistochemical Protein Assay
4.6. Analysis of the Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Liang, J.F. Anticancer Activity of Nano-formulated Orlistat-Dopamine Conjugates Through Self-Assembly. Biocon. Chem. 2023, 34, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Jovankić, J.V.; Nikodijević, D.D.; Milutinović, M.G.; Nikezić, A.G.; Kojić, V.V.; Cvetković, A.M.; Cvetković, D.M. Potential of Orlistat to induce apoptotic and antiangiogenic effects as well as inhibition of fatty acid synthesis in breast cancer cells. Eur. J. Pharmacol. 2023, 939, 175456. [Google Scholar] [CrossRef] [PubMed]
- Qi, X. Review of the Clinical Effect of Orlistat. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012063. [Google Scholar] [CrossRef]
- Vanauberg, D.; Schulz, C.; Lefebvre, T. Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: A promising target in anti-cancer therapies. Oncogenesis 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020, 2, 27–59. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.Y.; Kim, R.M.; Maharjan, P.; Ji, Y.G.; Jang, D.J.; Min, K.A.; Koo, T.S.; Cho, K.H. Orlistat-Loaded Solid SNEDDS for Enhanced Solubility, Dissolution, and In Vivo Performance. Int. J. Nanomed. 2018, 13, 7095–7106. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Jamali-Gojani, Z.; Heidari-Beni, M. Anti-obesity drug orlistat (xenical) is a novel antitumor medication. Shiraz E-Med. J. 2015, 16, e26242. [Google Scholar] [CrossRef]
- Saleh, A.; Elfayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef]
- Shrivastava, N.; Parikh, A.; Dewangan, R.P.; Biswas, L.; Verma, A.K.; Mittal, S.; Ali, J.; Garg, S.; Baboota, S. Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer. Pharmaceutics 2022, 14, 1486. [Google Scholar] [CrossRef]
- Hao, X.M.; Zhu, X.M.; Tian, H.M.; Lai, G.M.; Zhang, W.; Zhou, H.M.; Liu, S. Pharmacological effect and mechanism of orlistat in anti-tumor therapy: A review. Medicine 2023, 102, e34671. [Google Scholar] [CrossRef]
- Hitakarun, A.; Khongwichit, S.; Wikan, N.; Roytrakul, S.; Yoksan, S.; Rajakam, S.; Davidson, A.D.; Smith, D.R. Evaluation of the antiviral activity of orlistat (tetrahydrolipstatin) against dengue virus, Japanese encephalitis virus, Zika virus and chikungunya virus. Sci. Rep. 2020, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Bhargava-Shah, A.; Foygel, K.; Devulapally, R.; Paulmurugan, R. Orlistat and Antisense-miRNA-Loaded PLGA-PEG Nanoparticles for Enhanced Triple-Negative Breast Cancer Therapy. Nanomedicine 2016, 11, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cha, K.H.; Hong, S.H.; Abuzar, S.M.; Ha, E.S.; Kim, J.S.; Kim, M.S.; Hwang, S.J. Melt Amorphisation of Orlistat with Mesoporous Silica Using a Supercritical Carbon Dioxide: Effects of Pressure, Temperature, and Drug Loading Ratio and Comparison with Other Conventional Amorphisation Methods. Pharmaceutics 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An Approach to Enhance the Solubility of Drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Sheokand, S.; Reddy, K.; Bansal, A. Pharmaceutical Nanocrystals: From Fundamentals to Advances. Pharma Times 2018, 50, 20-5. [Google Scholar]
- Dibaei, M.; Rouini, M.-R.; Sheikholeslami, B.; Gholami, M.; Dinarvand, R. The Effect of Surface Treatment on the Brain Delivery of Curcumin Nanosuspension: In Vitro and In Vivo Studies. Int. J. Nanomed. 2019, 14, 5477–5490. [Google Scholar] [CrossRef]
- Patel, J.J.; Alzahrani, T. Myocardial Perfusion Scan; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Radioisotopes, I. Technetium-99m Radiopharmaceuticals: Status and Trends; No, R.S. 1.; IAEA: Vienna, Austria, 2009. [Google Scholar]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R.J.S.R. Subcutaneous Ehrlich Ascites Carcinoma Mice Model for Studying Cancer-Induced Cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef]
- Aldubayan, M.A.; Elgharabawy, R.M.; Ahmed, A.S.; Tousson, E.J. Antineoplastic activity and curative role of avenanthramides against the growth of Ehrlich solid tumors in mice. Oxid. Med. Cell Longev. 2019, 2019, 5162687. [Google Scholar] [CrossRef]
- Filho, A.L.; Lopes, J.M.; Schmitt, F.C. Angiogenesis and Breast Cancer. J. Oncol. 2010, 2010, 576384. [Google Scholar] [CrossRef]
- Ziyad, S.; Iruela-Arispe, M.L.J.G. Molecular Mechanisms of Tumor Angiogenesis. Genes Cancer 2011, 2, 1085–1096. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk. Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Rosmorduc, O.; Housset, C. Hypoxia: A Link between Fibrogenesis, Angiogenesis, and Carcinogenesis in Liver Disease. Semin. Liver Dis. 2010, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H.J.N. Proliferation, Cell Cycle and Apoptosis in Cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Gabri, A.O.; Mm, E.-S.; Somaia, A.N. Prophylactic effect of tamoxifen against induction of mammary carcinoma. Egypt. J. Hosp. Med. 2004, 14, 104–114. [Google Scholar]
- Chaudhary, M. COVID-19 Susceptibility: Potential of ACE2 Polymorphisms. Egypt. J. Med. Hum. Genet. 2020, 21, 54. [Google Scholar] [CrossRef]
- Geskovski, N.; Kuzmanovska, S.; Simonoska Crcarevska, M.; Calis, S.; Dimchevska, S.; Petrusevska, M.; Zdravkovski, P.; Goracinova, K. Comparative Biodistribution Studies of Technetium-99m Radiolabeled Amphiphilic Nanoparticles Using Three Different Reducing Agents During the Labeling Procedure. J. Labelled Comp. Radiopharm. 2013, 56, 689–695. [Google Scholar] [CrossRef]
- Qi, P.; Muddukrishna, S.N.; Torok-Both, R.; Rahn, J.; Chen, A. Direct 99mTc-Labeling of Antibodies by Sodium Dithionite Reduction, and Role of Ascorbate as a Stabilizer in Cysteine Challenge. Nucl. Med. Biol. 1996, 23, 827–835. [Google Scholar] [CrossRef]
- Sanad, M.H. Labeling and Biological Evaluation of 99mTc-azithromycin for Infective Inflammation Diagnosis. Radiochemistry 2013, 55, 539–544. [Google Scholar] [CrossRef]
- Rahman, A.; Haider, M.D.F.; Naseem, N.; Rahman, N. Solubility of Drugs, Their Enhancement, Factors Affecting and Their Limitations: A Review. Int. J. Pharm. Sci. Rev. Res. 2023, 79, 78–94. [Google Scholar] [CrossRef]
- Kridel, S.J.; Axelrod, F.; Rozenkrantz, N.; Smith, J.W. Orlistat Is a Novel Inhibitor of Fatty Acid Synthase with Antitumor Activity. Cancer Res. 2004, 64, 2070–2075. [Google Scholar] [CrossRef]
- Zhong, J.; Shen, Z.; Yang, Y.; Chen, J. Preparation and Characterization of Uniform Nanosized Cephradine by a Combination of Reactive Precipitation and Liquid Anti-solvent Precipitation Under a High Gravity Environment. Int. J. Pharm. 2005, 301, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.; El-Azony, K.M.; Ibrahim, I.T. Application of 99Mo/99mTc Alumina Generator in the Labeling of Metoprolol for Diagnostic Purposes. J. Label. Compd. Radiopharm. 2009, 52, 467–472. [Google Scholar] [CrossRef]
- Singh, A. Evaluation of Orlistat Solid Dispersion Using Poloxomer 188 as a Hydrophilic Carrier. Sch. Res. J. 2011, 1, 48. [Google Scholar] [CrossRef]
- Fernández, L.; Terán, M. Development and Evaluation of 99mTc-Amphotericin Complexes as Potential Diagnostic Agents in Nuclear Medicine. Int. J. Infect. 2017, 4, e62150. [Google Scholar] [CrossRef]
- Zhang, X.; You, L.; Chen, S.; Gao, M.; Guo, Z.; Du, J.; Lu, J.; Zhang, X. Development of a Novel (99m) Tc-Labeled Small Molecular Antagonist for CXCR4 Positive Tumor Imaging. J. Labelled Comp. Radiopharm. 2018, 61, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A Comprehensive Resource for In Silico Drug Discovery and Exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Kabel, A.M.; Abdel-Rahman, M.N.; El-Sisi, A.E.-D.E.; Haleem, M.S.; Ezzat, N.M.; El Rashidy, M.A.J.E. Effect of Atorvastatin and Methotrexate on Solid Ehrlich Tumor. Eur. J. Pharmacol. 2013, 713, 47–53. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Tousson, E.; El Sayed, I.E.T.; Awd, W.M.J.E.T. Ameliorative Effects of Saussurea lappa Root Aqueous Extract Against Ethephon Induced Reproductive Toxicity in Male Rats. Environ. Toxicol. 2019, 34, 150–159. [Google Scholar] [CrossRef]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Sivakumar, T.; Vamsi, M.L.M.J. Antitumor Activity and Antioxidant Status of Caesalpinia Bonducella Against Ehrlich Ascites Carcinoma in Swiss Albino Mice. J. Pharmacol. Sci. 2004, 94, 177–184. [Google Scholar] [CrossRef]
- Sakr, S.; Badr, O.; Abd-Eltawab, H.J.I. Ameliorative Effect of Saffron Extract on Mice Bearing Solid Tumors. J. Sci. Technol. 2011, 12, 60–70. [Google Scholar]
- Ali, D.A.; Badr El-din, N.K.; Abou-El-Magd, R.F. Antioxidant and Hepatoprotective Activities of Grape Seeds and Skin Against Ehrlich Solid Tumor-Induced Oxidative Stress in Mice. Egypt. J. Basic Appl. Sci. 2015, 2, 98–109. [Google Scholar] [CrossRef]
- Hashimoto, S.; Koji, T.; Niu, J.; Kanematsu, T.; Nakane, P.K. Differential Staining of DNA Strand Breaks in Dying Cells by Non-radioactive In Situ Nick Translation. Arch. Histol. Cytol. 1995, 58, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sannappa Gowda, N.G.; Shiragannavar, V.D.; Prabhuswamimath, S.; Tuladhar, S.; Chidambaram, S.B.; Santhekadur, P.K. Ehrlich Ascites Carcinoma Mice Model for Studying Liver Inflammation and Fibrosis. Adv. Cancer Biol. Metastasis 2022, 4, 100029. [Google Scholar] [CrossRef]
- Nofal, A.E.; Elmongy, E.I.; Hassan, E.A.; Tousson, E.; Ahmed, A.A.S.; El Sayed, I.E.T.; Binsuwaidan, R.; Sakr, M. Impact of Synthesized Indoloquinoline Analog to Isolates from Cryptolepis sanguinolenta on Tumor Growth Inhibition and Hepatotoxicity in Ehrlich Solid Tumor-Bearing Female Mice. Cells 2023, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F.; Chatterjee, N.; Ershler, W.B.; Brawley, O.W. Estrogen Receptor Breast Cancer Phenotypes in the Surveillance, Epidemiology, and End Results Database. Breast Cancer Res. Treat. 2002, 76, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.E.; Elsisi, A.E.; Sokkar, S.S.; Abdelrazik, N.M. Enhanced In Vivo Targeting of Estrogen Receptor Alpha Signaling in Murine Mammary Adenocarcinoma by Nilotinib/Rosuvastatin Novel Combination. Toxicol. Appl. Pharmacol. 2020, 404, 115185. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Abdollah, M.R.A.; Elsesy, M.E.; Abou El Ella, D.A.; Zada, S.K.; Tolba, M.F. The Natural Isoflavone Biochanin-A Synergizes 5-Fluorouracil Anticancer Activity In Vitro and In Vivo in the Ehrlich Solid-Phase Carcinoma Model. Phytother. Res. 2022, 36, 1310–1325. [Google Scholar] [CrossRef]
- Guzman, G.; Alagiozian-Angelova, V.; Layden-Almer, J.E.; Layden, T.J.; Testa, G.; Benedetti, E.; Kajdacsy-Balla, A.; Cotler, S.J. p53, Ki-67, and Serum Alpha-Fetoprotein as Predictors of Hepatocellular Carcinoma Recurrence in Liver Transplant Patients. Mod. Pathol. 2005, 18, 1498–1503. [Google Scholar] [CrossRef]
- El-Aarag, B.; El-Saied, F.; Salem, T.; Khedr, N.; Khalifa, S.A.M.; El-Seedi, H.R. New Metal Complexes Derived from Diacetylmonoxime-n(4)antipyrinylthiosemicarbazone: Synthesis, Characterization, and Evaluation of Antitumor Activity Against Ehrlich Solid Tumors Induced in Mice. Arab. J. Chem. 2021, 14, 102993. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a Proliferation Marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Hashem, F.; Teiama, M.; Tantawy, N.; Abdelmoniem, R. Folic Acid Grafted Mixed Polymeric Micelles as a Targeted Delivery Strategy for Tamoxifen Citrate in the Treatment of Breast Cancer; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Özcan Arican, G.; Özalpan, A. Evaluation of the Effect of Paclitaxel, Epirubicin, and Tamoxifen by Cell Kinetics Parameters in Estrogen-Receptor-Positive Ehrlich Ascites Tumor (Eat) Cells Growing In Vitro. Acta Biol. Hung. 2007, 58, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vellon, L.A.; Lupu, R. Antitumoral Actions of the Antiobesity Drug Orlistat (Xenical™) in Breast Cancer Cells: Blockade of Cell Cycle Progression, Promotion of Apoptotic Cell Death and PEA3-Mediated Transcriptional Repression of Her2/Neu (erbB-2) Oncogene. Ann. Oncol. 2005, 16, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, A.J.E. Orlistat in the Treatment of Obesity. Expert Opin. Pharmacother. 2000, 1, 841–847. [Google Scholar] [CrossRef]
- Menendez, J.A.; Colomer, R.; Lupu, R. Why Does Tumor-Associated Fatty Acid Synthase (Oncogenic antigen-519) Ignore Dietary Fatty Acids? Med. Hypotheses 2005, 64, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.S.; Dixon, A.F.; Dixon, J.B. Obesity management: Update on orlistat. Vasc. Health Risk Manag. 2007, 3, 817–821. [Google Scholar] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.; Frenkel, E. Nanoparticles for Drug Delivery in Cancer Treatment. Urol. Oncol. 2008, 26, 57–64. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA Nanoparticles Containing Various Anticancer Agents and Tumor Delivery by EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Devulapally, R.; Paulmurugan, R. Polymer Nanoparticles for Drug and Small Silencing RNA Delivery to Treat Cancers of Different Phenotypes. Nanobiotechnology 2014, 6, 40–60. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A.J.C. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Cunha-Filho, M.S.S.; Martínez-Pacheco, R.; Landín, M. Dissolution Rate Enhancement of the Novel Antitumoral β-Apache by Solvent Change Precipitation of Microparticles. Eur. J. Pharm. Biopharm. 2008, 69, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M. Influence of Microcrystal Formulation on In Vivo Absorption of Celecoxib in Rats. AAPS Pharm. Sci. Technol. 2013, 14, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, P.; Dhanalakshmi, K.; Nagarjuna, G.; Sreenivasa, R.S. Differential Derivative Method Development and Validation of Orlistat by UV: A Spectrophotometric Technique. J. Adv. Pharm. Educ. Res. 2013, 3, 234–237. [Google Scholar]
- Mukund, G.M.; Subhash, B.A.; Jaydevappa, H.P. Stability Indicating Stress Degradation Study of Orlistat by UV Spectrophotometry Method. J. Young Pharm. 2020, 12, 125–128. [Google Scholar] [CrossRef]
- Shamsel-Din, H.A.; Gizawy, M.A.; Zaki, E.G.; Elgendy, A. A Novel (99m) Tc-Diester Complex as Tumor Targeting Agent: Synthesis, Radiolabeling, and Biological Distribution Study. J. Label. Comp. Radiopharm. 2020, 63, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.; Alani, B.G.; Salim, K.S.; Abou-Zeid, L.A.; Aboumanie, M.H.; Motaleb, M.A.; Attallah, K.M.; Ibrahim, I.T.; Hassan, Y.A. Technetium-99m Labeling of Antineoplaston A10 and Its Bioevaluation as a Potential Tumor Imaging Agent. Radiochemistry 2022, 64, 219–227. [Google Scholar] [CrossRef]
- Khedr, N.F.; Khalil, R.M. Effect of Hesperidin on Mice Bearing Ehrlich Solid Carcinoma Maintained on Doxorubicin. Tumour Biol. 2015, 36, 9267–9275. [Google Scholar] [CrossRef]
- Rosenberg, I.; Russell, C.W.; Giles, G.R. Cell viability studies on the exfoliated colonic cancer cell. Brit. J. Surg. 1978, 65, 188–190. [Google Scholar] [CrossRef]
- Osman, A.E.-M.M.; Ahmed, M.M.S.; Khayyal, M.T.E.-D.; El-Merzabani, M.M. Hyperthermic Potentiation of Cisplatin Cytotoxicity on Solid Ehrlich Carcinoma. Tumori 1993, 79, 268–272. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, D.; Yao, A.; Mai, L.; Zhang, Z.; Zhou, Q.J.P.O. Design, Synthesis, and In Vitro and In Vivo Biological Studies of a 3′-Deoxythymidine Conjugate That Potentially Kills Cancer Cells Selectively. PLoS ONE 2012, 7, e52199. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mansour, H.; Shouman, S.; Eissa, A.; Abu El Nour, S.J.H. Modulatory effects of L-carnitine on tamoxifen toxicity and oncolytic activity: In vivo study. Hum. Exp. Toxicol. 2014, 33, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Vis, A.N.; Kranse, R.; Nigg, A.L.; Van Der Kwast, T.H. Quantitative Analysis of the Decay of Immunoreactivity in Stored Prostate Needle Biopsy Sections. Am. J. Clin. Pathol. 2000, 113, 369–373. [Google Scholar] [CrossRef] [PubMed]

| S.S (Mean ± SD, n = 3, µg/mL) | |||||
|---|---|---|---|---|---|
| Particle Size (nm) | Zeta Potential (mV) | PDI | Water | 0.1 N HCl | |
| ORL−NC | 329.59 ± 120.75 | −27.04 | 0.277 | 363.5 ± 43.37 | 457.57 ± 90.35 |
| ORL−BC | 557.63 ± 189.63 | −26.19 | 0.310 | 60.2 ± 11.24 | 36.77 ± 5.29 |
| Melting Point °C | Heat Flow (mV) | |
|---|---|---|
| ORL | 45.67 | 32.204 |
| ORL-NC | 47.22 | 28.595 |
| ORL-BC | 49.48 | 30.62 |
| %Q30 (Mean ± SD, n = 3) | %Q60 (Mean ± SD, n = 3) | %DE 30 min | %DE 60 min | |
|---|---|---|---|---|
| ORL | 00.0 | 00.0 | - | - |
| ORL-NC | 70.67 ± 10.89 | 99.68.0 ± 19.43 | 39.70 | 66.18 |
| ORL-BC | 46.21 ± 4.59 | 56.88 ± 4.59 | 23.93 | 37.95 |
| Factor | Optimum for Plain 99mTc-ORL (98.1%) | Optimum for Nano-99mTc-ORL-NC (84.8%) |
|---|---|---|
| Substrate (ORL or ORL-NC) | 3 mg plain ORL | 1 mg |
| Reducing agent Na2S2O4 | 2 mg | 5 mg |
| Reaction time | 90 min | 60 min |
| pH of the reaction medium | 7 | 7 |
| Organs and Body Fluids | % Orally Administered Dose/Gram Tissue at Different Time Intervals | |||
|---|---|---|---|---|
| 1/2 h | 1 h | 2 h | 4 h | |
| Blood | 0.60 ± 0.03 | 2.30 ± 0.30 * | 2.70 ± 0.03 * | 2.10 ± 0.30 * |
| Bone | 0.40 ± 0.20 | 1.60 ± 0.20 * | 1.90 ± 0.01 * | 1.50 ± 0.10 * |
| Muscle | 0.40 ± 0.04 | 1.40 ± 0.10 | 1.60 ± 0.20 * | 1.50 ± 0.10 |
| Liver | 0.30 ± 0.05 | 0.80 ± 0.07 * | 0.90 ± 0.02 | 1.20 ± 0.20 |
| Stomach | 42.0 ± 3.40 | 33.50 ± 2.60 * | 33.30 ±2.20 * | 22.3 ± 1.60 * |
| Intestine | 6.20 ± 0.50 | 16.50 ± 1.10 * | 21.60 ± 1.40 * | 35.0 ± 2.10 * |
| Lung | 1.30 ± 0.12 | 4.70 ± 0.20 * | 1.90 ± 0.01 * | 1.10 ± 0.02 * |
| Heart | 3.00 ± 0.30 | 3.50 ± 0.30 * | 2.10 ± 0.20 * | 1.30 ± 0.04 * |
| Spleen | 2.50 ± 0.20 | 3.20 ± 0.40 | 2.80 ± 0.05 * | 2.40 ± 0.40 |
| Kidney | 1.00 ± 0.06 | 3.00 ± 0.20 * | 4.50 ± 0.20 * | 3.20 ± 0.30 * |
| Urine | 3.40 ± 0.20 | 7.50 ± 0.30 * | 10.30 ± 1.00 * | 14.10 ± 1.30 * |
| Brain | 3.00 ± 0.60 | 4.10 ± 0.40 * | 3.90 ± 0.02 * | 1.70 ± 0.03 * |
| Thyroid | 3.00 ± 0.20 | 3.10 ± 0.02 | 2.20 ± 0.05 * | 1.50 ± 0.05 |
| Organs and Body Fluids | %Dose/g Tissue at Different Time Intervals | |||
|---|---|---|---|---|
| 1/2 h | 1 h | 2 h | 4 h | |
| Blood | 4.00 ± 0.30 | 5.60 ± 0.30 * | 6.30 ± 0.30 * | 5.50 ± 0.30 * |
| Bone | 1.10 ± 0.20 | 1.50 ± 0.20 * | 1.60 ± 0.01 | 1.50 ± 0.01 |
| Muscle | 1.60 ± 0.04 | 1.80 ± 0.10 | 3.30 ± 0.20 * | 2.20 ± 0.10 |
| Liver | 1.20 ± 0.05 | 2.90± 0.20 * | 3.30 ± 0.20 | 3.50 ± 0.02 |
| Stomach | 16.00 ± 1.40 | 25.0 ± 1.60 * | 20.3 ±1.20 * | 12.5 ± 1.60 * |
| Intestine | 12.0 ± 0.30 | 20.0 ± 0.10 * | 26.60 ± 0.4 * | 18.0 ± 0.10 * |
| Lung | 3.90 ± 0.12 | 3.50 ± 0.20 * | 2.10 ± 0.01 * | 2.10 ± 0.20 * |
| Heart | 5.60± 0.30 | 4.10± 0.30 * | 3.30 ± 0.20 * | 3.00 ± 0.40 * |
| Spleen | 0.80 ± 0.10 | 2.0 ± 0.1 | 3.10 ± 0.30 | 4.1 ± 0.3 |
| Kidney | 1.4 ± 0.6 * | 3.0 ± 0.3 * | 4.1 ± 0.2 * | 4.2 ± 0.3 * |
| Urine | 3.4 ± 0.6 * | 7.5 ± 0.3 * | 11.3 ± 1.0 | 14.1 ± 1.3 * |
| Brain | 3.6 ± 0.6 * | 5.0 ± 0.3 * | 3.0 ± 0.2 * | 2.2 ± 0.2 * |
| Thyroid | 2.7 ± 0.01 * | 4.0 ± 0.02 | 3.3 ± 0.05 * | 2.5 ± 0.02 |
| Organ | 99mTc-ORL | 99mTc-ORL-NC | ||||
|---|---|---|---|---|---|---|
| Cmax (% Dose/g Tissue) | (h) | (% Dose/g TISSUE * h) | Cmax (% Dose/g Tissue) | (h) | (% Dose/g Tissue * h) | |
| Blood | 2.7 | 2 | 8.175 | 6.3 * | 2 | 21.15 * |
| Bone | 1.9 | 2 | 5.75 | 1.6 * | 2 | 5.575 * |
| Muscle | 1.6 | 2 | 5.15 | 3.3 * | 2 | 9.3 * |
| Liver | 1.2 | 4 | 3.3 | 3.5 * | 4 | 11.225 * |
| Stomach | 42 | 0.5 | 118.375 | 25 * | 2 | 69.7 * |
| Intestine | 35 | 4 | 82.875 | 26.6 * | 2 | 78.9 * |
| Lung | 4.7 | 1 | 8.125 | 3.9 * | 0.5 | 9.825 * |
| Heart | 3.5 | 1 | 8.575 | 5.6 * | 0.5 | 13.825 * |
| Spleen | 3.2 | 1 | 10.25 | 4.1 * | 4 | 10.65 * |
| Kidney | 4.5 | 2 | 12.7 | 4.2 * | 4 | 13.3 * |
| Urine | 14.1 | 4 | 36.875 | 14.1 | 4 | 38.375 * |
| Brain | 4.1 | 1 | 12.125 | 5 * | 1 | 17.47 * |
| Thyroid | 3.1 | 1 | 8.625 | 4 * | 1 | 11.8 * |
| Group | SGPT (U/L) | SGOT (U/L) | ALP (U/L) | Total Bilirubin (mg/dL) | Direct Bilirubin (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 41.83 ± 2.31 | 40.17 ± 2.92 | 119.2 ± 4.021 | 0.68 ± 0.11 | 0.36 ± 0.052 |
| SEC control | 129 ± 5.06 * | 130 ± 4.85 * | 408.2 ± 7.44 * | 1.68 ± 0.15 * | 1.8 ± 0.14 * |
| SEC+TAM | 70.67 ± 3.55 *,a | 71.5 ± 7.17 *,a | 183.2 ± 9.51 *,a | 0.96 ± 0.052 *,a | 0.9 ± 0.059 *,a |
| SEC+ORL | 102.3 ± 2.16 *,a,b | 109 ± 3.74 *,a,b | 275.8 ± 5.11 *,a,b | 1.34 ± 0.083 *,a,b | 1.3 ± 0.059 *,a,b |
| SEC+ORL-NC | 81.17 ± 4.62 *,a,c | 85.33 ± 5.78 *,a,c | 202.8 ± 6.01 *,a,c | 1.13 ± 0.095 *,a,c | 1.1 ± 0.072 *,a,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamoudi, J.A.; El-Masry, T.A.; Nasr, M.; Ibrahim, I.T.; Ibrahim, H.A.; Saad, H.M.; El-Nagar, M.M.F.; Alshawwa, S.Z.; Alrashidi, A.; El Zahaby, E.I. Fabrication of Nanocrystals for Enhanced Distribution of a Fatty Acid Synthase Inhibitor (Orlistat) as a Promising Method to Relieve Solid Ehrlich Carcinoma-Induced Hepatic Damage in Mice. Pharmaceuticals 2024, 17, 96. https://doi.org/10.3390/ph17010096
Alamoudi JA, El-Masry TA, Nasr M, Ibrahim IT, Ibrahim HA, Saad HM, El-Nagar MMF, Alshawwa SZ, Alrashidi A, El Zahaby EI. Fabrication of Nanocrystals for Enhanced Distribution of a Fatty Acid Synthase Inhibitor (Orlistat) as a Promising Method to Relieve Solid Ehrlich Carcinoma-Induced Hepatic Damage in Mice. Pharmaceuticals. 2024; 17(1):96. https://doi.org/10.3390/ph17010096
Chicago/Turabian StyleAlamoudi, Jawaher Abdullah, Thanaa A. El-Masry, Mohamed Nasr, Ismail T. Ibrahim, Hanaa A. Ibrahim, Hebatallah M. Saad, Maysa M. F. El-Nagar, Samar Zuhair Alshawwa, Amal Alrashidi, and Enas I. El Zahaby. 2024. "Fabrication of Nanocrystals for Enhanced Distribution of a Fatty Acid Synthase Inhibitor (Orlistat) as a Promising Method to Relieve Solid Ehrlich Carcinoma-Induced Hepatic Damage in Mice" Pharmaceuticals 17, no. 1: 96. https://doi.org/10.3390/ph17010096
APA StyleAlamoudi, J. A., El-Masry, T. A., Nasr, M., Ibrahim, I. T., Ibrahim, H. A., Saad, H. M., El-Nagar, M. M. F., Alshawwa, S. Z., Alrashidi, A., & El Zahaby, E. I. (2024). Fabrication of Nanocrystals for Enhanced Distribution of a Fatty Acid Synthase Inhibitor (Orlistat) as a Promising Method to Relieve Solid Ehrlich Carcinoma-Induced Hepatic Damage in Mice. Pharmaceuticals, 17(1), 96. https://doi.org/10.3390/ph17010096







