A Drug Safety Briefing (II) in Transplantation from Real-World Individual Pharmacotherapy Management to Prevent Patient and Graft from Polypharmacy Risks at the Very Earliest Stage
Abstract
1. Introduction
2. Methods
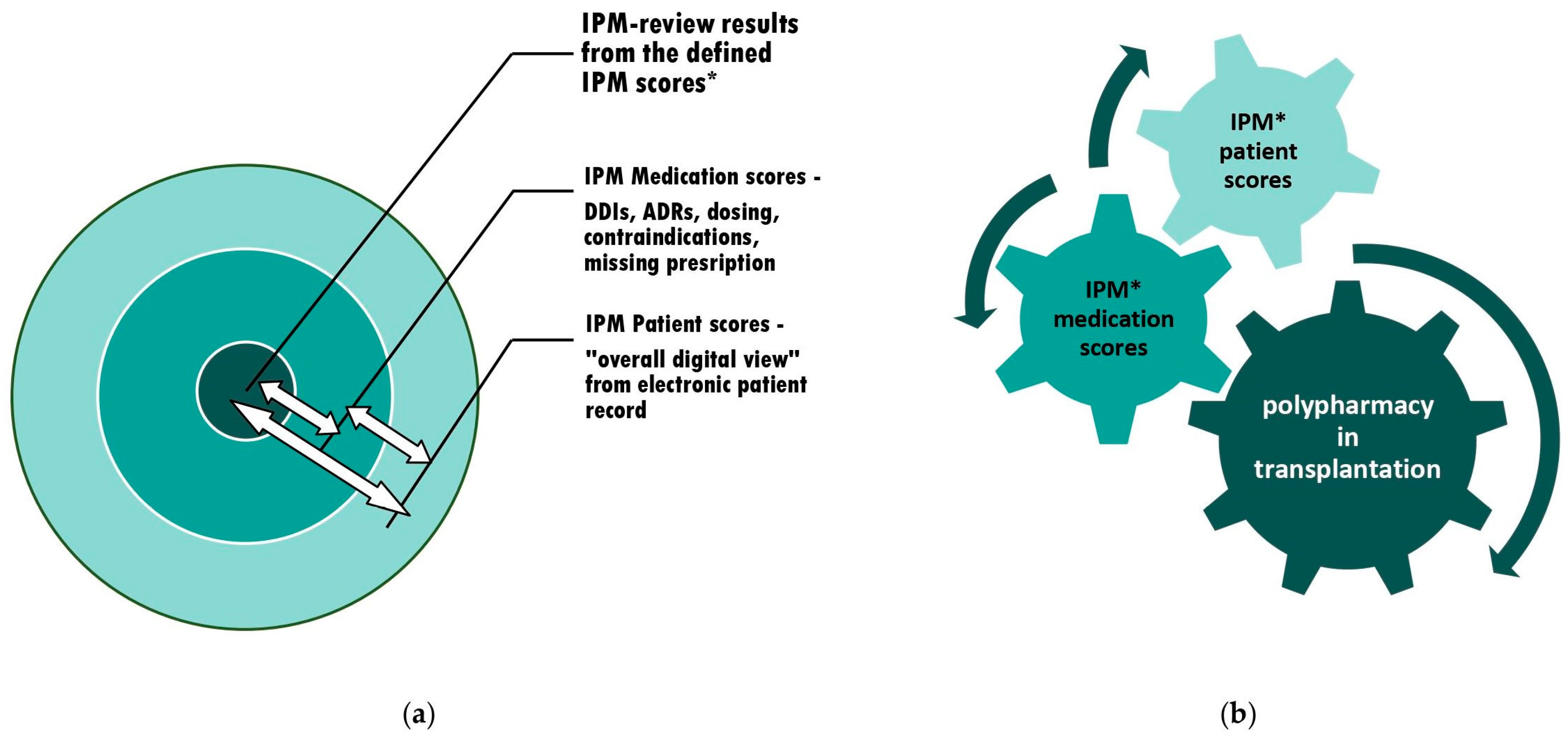
2.1. IPM Concept and Implementation of a Digital Interdisciplinary Networking Strategy [9]
2.2. Briefing Toolset on Relevant IPM Medication Scores and Preventive Countermeasures to Avoid Iatrogenic Patients and Graft Injuries
3. Results
3.1. IPM in Practice
3.2. Tabulated Extracts as the Relevant Medication Scores of Common Critical Coadministered Drugs in Posttransplant Polypharmacy
3.3. Briefing on Awareness and Preventive Countermeasures in Unavoidable Drug-Induced Risk Situations
3.3.1. Kidney Injury
3.3.2. QTc Prolongation
3.3.3. The Differential Diagnosis and Follow-up of Cytomegalovirus Infection (CMV)
3.3.4. Hypogammaglobulinemia
3.3.5. Risks in Analgesics and Sedatives
3.3.6. Life-Threatening Infectious Diseases—Sepsis
3.3.7. Surgical Interventions—Wound Healing
3.3.8. Rhabdomyolysis—Statins
3.3.9. Calcium Channel Blockers—DDI-Grading
3.3.10. Diarrhea
3.3.11. Loperamide-Induced Cardiotoxicity
3.3.12. Acute Liver Dysfunction
3.3.13. Exchanging CSA or TAC within Different Formulations
3.3.14. Attention Letermovir Metabolism; Letermovir with Voriconazole
3.3.15. Monitoring Differentiated ADRs of Immunosuppressants and Integrating Continuous Patient Education
4. Discussion
5. Strengths and Weaknesses
6. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandimmun Concentrate for Solution for Infusion 50 mg/mL, Novartis Pharmaceuticals UK Limited, SmPC, Updated emc 15 September 2023. Available online: https://www.medicines.org.uk/emc/product/1036/smpc#gref (accessed on 23 October 2023).
- Prograf 0.5 mg, 1 mg, 5 mg Hard Capsules, Astellas Pharma Co., Ltd., SmPc 5 December 2022. Available online: https://www.medicines.ie/medicines/prograf-capsules-33450/spc (accessed on 23 October 2023).
- Rapamune 1 mg Coated Tablets. Summary of Product Characteristics, Updated emc 2 August 2021|Pfizer Limited. Rapamune 1 mg Coated Tablets—Summary of Product Characteristics (SmPC)—Print Friendly—(emc). Available online: https://www.medicines.org.uk/emc/product/10398/smpc#gref (accessed on 10 October 2023).
- Rapamune 2 mg Coated Tablets, Pfizer Limited. SmPC, Updated emc July 2021. Available online: https://www.medicines.org.uk/emc/product/10399/smpc#about-medicine (accessed on 23 October 2023).
- Marty, F.M.; Lowry, C.M.; Cutler, C.S.; Campbell, B.J.; Fiumara, K.; Baden, L.R.; Antin, J.H. Voriconazole and sirolimus coadministration after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2006, 12, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.P.; Manivannan, J.; John, G.T.; Jacob, C.K. Sirolimus and ketoconazole co-prescription in renal transplant recipients. Transplantation 2004, 77, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.J. Exposure-response relationships and drug interactions of sirolimus. AAPS J. 2004, 6, e28. [Google Scholar] [CrossRef] [PubMed]
- Afinitor 2.5 mg, 5 mg, 10 mg Tablets, Novartis Pharmaceuticals UK Ltd., SmPC, Updated emc 12 July 2022. Available online: https://www.medicines.org.uk/emc/product/6658/smpc#gref (accessed on 12 October 2023).
- Wolf, U. A Drug Safety Concept (I) to Avoid Polypharmacy Risks in Transplantation by Individual Pharmacotherapy Management in Therapeutic Drug Monitoring of Immunosuppressants. Pharmaceutics 2023, 15, 2300. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.W.; Galanko, J.A.; Shrestha, R.; Fried, M.W.; Watkins, P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transplant. 2004, 10, 1018–1023. [Google Scholar] [CrossRef]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, M.; González-Grande, R.; García-Cortés, M.; Andrade, R.J. Drug-Induced Liver Injury after Liver Transplantation. Liver Transplant. 2020, 26, 1167–1176. [Google Scholar] [CrossRef]
- Bethesda, M.D. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; Updated 23 November 2023; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Yasrebi-de Kom, I.A.R.; Dongelmans, D.A.; Abu-Hanna, A.; Schut, M.C.; de Lange, D.W.; van Roon, E.N.; de Jonge, E.; Bouman, C.S.C.; de Keizer, N.F.; Jager, K.J.; et al. Acute kidney injury associated with nephrotoxic drugs in critically ill patients: A multicenter cohort study using electronic health record data. Clin. Kidney J. 2023, 16, 2549–2558. [Google Scholar] [CrossRef]
- Naughton, C.A. Drug-induced nephrotoxicity. Am. Fam. Physician 2008, 78, 743–750. [Google Scholar]
- Jacobs, U.; Klein, B.; Miersch, W.D.; Molitor, D.; Klehr, H.U. Dilemma: Maintenance therapy enhances sclerogenic risk profile. Transplant. Proc. 1996, 28, 3227–3230. [Google Scholar]
- Jacobs, U.; Ferber, J.; Heimbach, D.; Klehr, H.U. Manifestation of metabolic risk factors after renal transplantation: I: Association with long-term allograft function. Transplant. Proc. 1995, 27, 2048–2049. [Google Scholar]
- Jacobs, U.; Ferber, J.; Heimbach, D.; Klehr, H.U. Manifestation of metabolic risk factors after renal transplantation: II. Impact of maintenance therapy. Transplant. Proc. 1995, 27, 2050–2051. [Google Scholar]
- Jacobs, U.; Ferber, J.; Klehr, H.U. Manifestation of metabolic risk factors after renal transplantation—II. Impact of maintenance therapy. 25. Kongress der Gesellschaft für Nephrologie, Zürich, September 1994. Kidney Int. 1995, 47, 979. [Google Scholar]
- Jacobs, U.; Ferber, J.; Heimbach, D.; Klehr, H.U. Manifestation of metabolic risk factors after renal transplantation: III. Impact on cerebrocardiovascular complications. Transplant. Proc. 1995, 27, 2052–2053. [Google Scholar]
- Jacobs, U.; Brensing, K.A.; Klehr, H.U. Chronic allograft destruction vs. chronic allograft rejection. Transplant. Proc. 1994, 26, 3119–3120. [Google Scholar] [PubMed]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef]
- Dosing. Arzneimitteldosierung bei Niereninsuffizienz. 1998–2023 Abt. Klinische Pharmakologie & Pharmakoepidemiologie, Universitätsklinikum Heidelberg. Available online: https://dosing.de/nierelst.php/ (accessed on 23 July 2023).
- Stanford Health Care Antimicrobial Dosing Reference Guide. ABX Subcommittee Approved: 12/2022. Pharmacy & Therapeutics Committee Approved 1/2023. Available online: https://med.stanford.edu/content/dam/sm/bugsanddrugs/documents/antimicrobial-dosing-protocols/SHC%20Antimicrobial%20Dosing%20Guide.pdfhttps://med.stanford.edu/content/dam/sm/bugsanddrugs/documents/antimicrobial-dosing-protocols/SHC%20Antimicrobial%20Dosing%20Guide.pdf (accessed on 23 July 2023).
- Renal Dose Adjustment Guidelines for Antimicrobials. CRRT Dosing Recommendations. Prepared by Peitz, G.; Rolek, K.; Van Schooneveld, T. Approved by Antimicrobial Stewardship Program: June 2016. Available online: https://www.unmc.edu/intmed/_documents/id/asp/dose-renal-dose-adjustment-guidelines-for-antimicrobial.pdf (accessed on 23 July 2023).
- Drugs.com. Drug Interaction Checker. Check for Multi-Drug Interactions Including Alcohol, Food, Supplements & Diseases. Available online: https://www.drugs.com/drug_interactions.html (accessed on 23 July 2023).
- DrugBank Online. Interaction Checker. Available online: https://go.drugbank.com/drug-interaction-checker#results (accessed on 23 July 2023).
- Hesselink, D.A.; van Schaik, R.H.; van der Heiden, I.P.; van der Werf, M.; Gregoor, P.J.; Lindemans, J.; Weimar, W.; van Gelder, T. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin. Pharmacol. Ther. 2003, 74, 245–254. [Google Scholar] [CrossRef]
- Su, L.; Yin, L.; Yang, J.; Sun, L. Correlation between gene polymorphism and blood concentration of calcineurin inhibitors in renal transplant recipients: An overview of systematic reviews. Medicine 2019, 98, e16113. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; Pastor-Anglada, M. Insights into the Pharmacogenetics of Tacrolimus Pharmacokinetics and Pharmacodynamics. Pharmaceutics 2022, 14, 1755. [Google Scholar] [CrossRef]
- Wolf, U.; Eckert, S.; Walter, G.; Wienke, A.; Bartel, S.; Plontke, S.K.; Naumann, C. Prevalence of oropharyngeal dysphagia in geriatric patients and real-life associations with diseases and drugs. Sci. Rep. 2021, 11, 21955. [Google Scholar] [CrossRef] [PubMed]
- Wolf, U.; Ghadir, H.; Drewas, L.; Neef, R. Underdiagnosed CKD in Geriatric Trauma Patients and Potent Prevention of Renal Impairment from Polypharmacy Risks through Individual Pharmacotherapy Management (IPM-III). J. Clin. Med. 2023, 12, 4545. [Google Scholar] [CrossRef] [PubMed]
- Aciclovir 200 mg Tablets, Wockhardt UK Ltd., SmPC, Updated emc 21 September 2016. Available online: https://www.medicines.org.uk/emc/product/2353/smpc#gref (accessed on 19 October 2023).
- Allopurinol Tablets BP 100 mg Aurobindo Pharma—Milpharm Ltd. SmPC, Updated emc 7 February 2022. Available online: https://www.medicines.org.uk/emc/product/7004/smpc#gref (accessed on 19 October 2023).
- Amiodarone Hydrochloride 200 mg Tablets. Summary of Product Characteristics, Updated emc 20 February 2023|Ennogen Pharma Ltd. Available online: https://www.medicines.org.uk/emc/product/13964/smpc/print (accessed on 12 October 2023).
- Amlovie 10—10 mg Amlodipine, Dafra Pharma GmbH, Updated SmPc January 2019. Available online: https://www.dafrapharma.com/wp-content/uploads/2021/07/AMLOVIE-10-SmPC-2019-revkvh.pdf (accessed on 18 October 2023).
- AmBisome Liposomal 50 mg Powder for Dispersion for Infusion Gilead Sciences Ltd. SmPC, Updated emc October 2019. Available online: https://www.medicines.org.uk/emc/product/1022/smpc#gref (accessed on 19 October 2023).
- Eliquis 2.5 mg Film-Coated Tablets. Summary of Product Characteristics, Updated 23 June 2023—Bristol-Myers Squibb/Pfizer EEIG. Available online: https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf (accessed on 12 October 2023).
- Aprepitant 80 mg and 125 mg Hard Capsules. Summary of Product Characteristics, Updated emc 14 September 2020|Sandoz Limited. Available online: https://www.medicines.org.uk/emc/product/11726/smpc/print (accessed on 12 October 2023).
- EMEND 125 mg Hard Capsules, 80 mg Hard Capsules, Merck Sharp & Dohme B.V. Available online: https://www.ema.europa.eu/en/documents/product-information/emend-epar-product-information_en.pdf (accessed on 18 October 2023).
- Aspirin 75 mg Dispersible Tablets, Actavis UK Ltd., Updated SmPC September 2013. Available online: https://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PA0176-015-003_23092013104042.pdf (accessed on 18 October 2023).
- Acetylsalicylzuur Ratiopharm 500 mg, Tablette, Ratiopharm GmbH, SmPC 15 February 2022. Available online: https://www.geneesmiddeleninformatiebank.nl/smpc/h126642_smpc_en.pdf (accessed on 18 October 2023).
- Atorvastatin Tablets 10 mg, 20 mg, 40 mg, 80 mg. Atorkey (Trade Name) Summary of Product Characteristics Updated March 2021, Aurobindo Pharma Ltd., Unit-XV. Available online: https://www.tmda.go.tz/uploads/1620043518-T20H0069SmPCv1.pdf (accessed on 10 October 2023).
- Azithromycin 500 mg Tablets. Summary of Product Characteristics, Updated emc 9 June 2022|Sandoz Limited. Available online: https://www.medicines.org.uk/emc/product/6541/smpc/print (accessed on 10 October 2023).
- Buprenorphine 8 mg Sublingual Tablets, Ranbaxy (UK) Limited a Sun Pharmaceutical Company, SmPC, Updated emc 2 June 2021. Available online: https://www.medicines.org.uk/emc/product/2050/smpc#gref (accessed on 20 October 2023).
- Tegretol® 100 mg, 200 mg and 400 mg Tablets, Novartis Pharmaceuticals UK Limited, SmPC, Updated emc 25 May 2022. Available online: https://www.medicines.org.uk/emc/product/1040/smpc#gref (accessed on 20 October 2023).
- Vistide—Cidofovir Equivalent to 375 mg/5 mL (75 mg/mL) Cidofovir Anhydrous. Gilead Sciences Limited, UK, SmPC. Available online: https://ec.europa.eu/health/documents/community-register/1997/199704232996/anx_2996_en.pdf (accessed on 12 October 2023).
- Ciprofloxacin 2 mg/mL, Solution for Infusion. SmPC Updated 16 September 2019—Baxter Holding B.V. Available online: https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA2299-034-001_16092019092404.pdf (accessed on 12 October 2023).
- Citalopram 20 mg Tablets. Zentiva Pharma UK Limited. SmPC, Updated emc 23 June 2023. Available online: https://www.medicines.org.uk/emc/product/5160/smpc#gref (accessed on 19 October 2023).
- Clarithromycin 500 mg Powder for Solution for Infusion. Ibigen S.r.l., SmPC, Updated emc 4 October 2023. Available online: https://www.medicines.org.uk/emc/product/8825/smpc#about-medicine (accessed on 23 October 2023).
- Clopidogrel 75 mg Film-Coated Tablets, Aurobindo Pharma—Milpharm Ltd. SmPC, Updated emc 4 August 2022. Available online: https://www.medicines.org.uk/emc/product/5207/smpc#gref (accessed on 19 October 2023).
- Prednisolone 5 mg Tablets, Wockhardt UK Ltd., SmPC Updated emc 13 May 2021. Available online: https://www.medicines.org.uk/emc/product/2427/smpc#gref (accessed on 23 October 2023).
- Jardiance 10 mg and 25 mg Film-Coated Tablets, Empaglifozin. Boehringer Ingelheim International GmbH, SmPC, Updated emc September 2023. Available online: https://www.medicines.org.uk/emc/product/5441/smpc#gref (accessed on 23 October 2023).
- Febuxostat STADA 80 mg Film-Coated Tablets Thornton & Ross Ltd. (Trading as ‘STADA’). SmPC, Updated emc 3 January 2018. Available online: https://www.medicines.org.uk/emc/product/11120/smpc#gref (accessed on 19 October 2023).
- Vascalpha 5 mg Prolonged Release Tablets (Felodipine). Summary of Product Characteristics, Updated 13 August 2023—Accord-UK Ltd. Available online: https://accord-healthcare-products.co.uk/products/f/felodipine-tablets (accessed on 10 October 2023).
- Sandoz Fentanyl Patch, Fentanyl Transdermal System 12 mcg/h, 25 mcg/h, 37 mcg/h, 50 mcg/h, 75 mcg/h, and 100 mcg/h. Health Professional Information, Updated 26 February 2020—Sandoz Canada Inc. Available online: https://www.sandoz.ca/sites/www.sandoz.ca/files/Sandoz%20Fentanyl%20Patch%20Product%20Monograph_0.pdf (accessed on 12 October 2023).
- Fluconazole 150 mg Capsules Gedeon Richter Plc. Updated SmPc April 2018. Available online: http://www.hpra.ie/img/uploaded/swedocuments/2198529.PA1330_014_002.9d608f93-bc7b-4e4c-bd6f-14466eb52367.000001pil.180501.pdf (accessed on 17 October 2023).
- Diflucan 100 mg, 200 mg Hartkapseln Pfizer Pharma GmbH. Fachinformation Updated October 2022. Available online: https://figi.pfizer.de/sites/default/files/FI-7630.pdf (accessed on 17 October 2023).
- Fluvastatin 20 mg Hard Capsules, Sandoz Limited, SmPC, Updated emc 6 September 2019. Available online: https://www.medicines.org.uk/emc/product/4465/smpc#gref (accessed on 9 October 2023).
- Foscarnet Sodium Hexahydrate Tillomed 24 mg/mL Solution for Infusion. SmPC Updated 12 August 2022. Available online: http://www.hpra.ie/img/uploaded/swedocuments/Licence_PA22720-009-001_12082022163443.pdf (accessed on 12 October 2023).
- Cymevene and Associated Names, 500 mg Powder for Concentrate for Solution for Infusion. Available online: https://www.ema.europa.eu/en/documents/referral/cymevene-article-30-referral-annex-iii_en.pdf (accessed on 12 October 2023).
- Valganciclovir 450 mg Film-Coated Tablets. Amarox Limited. Summary of Product Characteristics Updated 9 October 2023. Available online: https://www.medicines.org.uk/emc/product/13566/smpc#about-medicine (accessed on 12 October 2023).
- Ibuprofen 200 mg, 400 mg Film-Coated Tablets, McDermott Laboratories Ltd. SmPC Updated December 2016. Available online: https://www.hpra.ie/img/uploaded/swedocuments/PIL-2184772-20012017111614-636205077751693750.pdf (accessed on 15 October 2023).
- Lercanidipine Clonmel 10 mg, 20 mg Film-Coated Tablet, Clonmel Healthcare Ltd., Updated SmPC March 2011. Available online: http://clonmedica.ie/wp-content/uploads/spc/Lercanidipine_Clonmel_20_and_40_mg_Tabs_(Mar_2011).pdf (accessed on 28 October 2023).
- Prevymis 240 mg and 480 mg Film-Coated Tablets. Summary of Product Characteristics Updated 20 June 2023—Merck Sharp & Dohme B.V. Available online: https://www.ema.europa.eu/en/documents/product-information/prevymis-epar-product-information_en.pdf (accessed on 12 October 2023).
- Linezolid 600 mg Film-Coated Tablets. Summary of Product Characteristics, Updated emc 29 September 2020|Sandoz Limited. Available online: https://www.medicines.org.uk/emc/product/2006/smpc#gref (accessed on 10 October 2023).
- Imodium Classic 2 mg Capsules. Summary of Product Characteristics, Updated emc 24 January 2023|McNeil Products Ltd. Available online: https://www.medicines.org.uk/emc/product/522/smpc#gref (accessed on 10 October 2023).
- Metamizol AbZ 500 mg Tabletten AbZ-Pharma GmbH, Fachinformation Updated July 2022. Available online: https://www.abz.de/assets/products/de/label/Metamizol%20AbZ%20500%20mg%20Tabletten%20-%207.pdf?pzn=9436064 (accessed on 17 October 2023).
- Optalgin® Teva 1 g/2 mL, Solution for I.V. or I.M. Injection, Dipyrone (Metamizole Sodium); SmPC, Updated June 2022. Available online: https://www.pharmaline.co.il/wp-content/uploads/2022/08/Optalgin-Teva-Solution-for-I.V.-or-I.M.-Injection-2.8D.pdf (accessed on 17 October 2023).
- Metformin 500 mg Film Coated Tablets, Brown & Burk UK Ltd. SmPC, Updated emc 11 May 2022. Available online: https://www.medicines.org.uk/emc/product/10759/smpc#gref (accessed on 20 October 2023).
- Methotrexate 2.5 mg Tablets, Mercury Pharmaceuticals Ltd., SmPC Updated emc 14 September 2023. Available online: https://www.medicines.org.uk/emc/product/511/smpc#gref (accessed on 12 October 2023).
- Metoclopramide 5 mg/mL Solution for Injection. SmPC Updated September 2022, Mercury Pharmaceuticals (Ireland) Ltd. Available online: https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA0073-084-001_16092022104709.pdf (accessed on 10 October 2023).
- Midazolam 5 mg/mL Solution for Injection/Infusion, Mercury Pharmaceuticals (Ireland) Ltd., SmPC Updated 14 February 2020. Available online: https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA0073-147-001_14022020112213.pdf (accessed on 8 October 2023).
- Mirtazapine 30 mg Tablets. Summary of Product Characteristics, Updated emc 17 December 2021|Aurobindo Pharma—Milpharm Ltd. Available online: https://www.medicines.org.uk/emc/product/535/smpc/print (accessed on 10 October 2023).
- Moxonidine 400 Microgram Film-Coated Tablets, Sandoz Limited, SmPC, Updated emc 29 October 2020. Available online: https://www.medicines.org.uk/emc/product/4138/smpc#gref (accessed on 10 October 2023).
- CellCept 250 mg Capsules (Mycophenolate Mofetil) Roche Registration GmbH. SmPC, Updated 8 September 2023. Available online: https://rss.medsinfo.com.au/ro/pi.cfm?product=ropccept10215 (accessed on 20 October 2023).
- Büchler, M.; Lebranchu, Y.; Bénéton, M.; Lemeur, Y.; Heng, A.E.; Westeel, P.F.; Leguellec, C.; Libert, F.; Hary, L.; Marquet, P.; et al. Higher exposure to mycophenolic acid with sirolimus than with cyclosporine cotreatment. Clin. Pharmacol. Ther. 2005, 78, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gabardi, S.; Olyaei, A. Evaluation of potential interactions between mycophenolic acid derivatives and proton pump inhibitors. Ann. Pharmacother. 2012, 46, 1054–1064. [Google Scholar] [CrossRef]
- Rissling, O.; Glander, P.; Hambach, P.; Mai, M.; Brakemeier, S.; Klonower, D.; Halleck, F.; Singer, E.; Schrezenmeier, E.; Dürr, M.; et al. No relevant pharmacokinetic interaction between pantoprazole and mycophenolate in renal transplant patients: A randomized crossover study. Br. J. Clin. Pharmacol. 2015, 80, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Pantoprazole Mylan 40 mg Gastro-Resistant Tablets McDermott Laboratories Ltd. t/a Gerard Laboratories. SmPC Updated 27 January 2022. Available online: https://www.medicines.ie/medicines/pantoprazole-mylan-40mg-gastro-resistant-tablets-33314/spc (accessed on 15 October 2023).
- Flothow, D.J.G.; Suwelack, B.; Pavenstädt, H.; Schütte-Nütgen, K.; Reuter, S. The Effect of Proton Pump Inhibitor Use on Renal Function in Kidney Transplanted Patients. Br. J. Clin. Pharmacol. 2015, 80, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.L.; Parkin, L.; Paul, C.; Herbison, P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014, 86, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Yan, Y.; Al-Aly, Z. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017, 91, 1482–1494. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Maddukuri, G.; Xie, Y. Proton Pump Inhibitors and the Kidney: Implications of Current Evidence for Clinical Practice and When and How to Deprescribe. Am. J. Kidney Dis. 2020, 75, 497–507. [Google Scholar] [CrossRef]
- Paracetamol 500 mg Tablets. Zentiva, Summary of Product Characteristics Updated 8 August 2023. Available online: https://www.medicines.org.uk/emc/product/5164/smpc/print (accessed on 20 October 2023).
- Paracetamol Ratiopharm, 500 mg Tabletten, SmPC Updated October 2021. Available online: https://www.ratiopharm.de/assets/products/de/label/Paracetamol-ratiopharm%20500%20mg%20Tabletten%20-%207.pdf?pzn=1126111 (accessed on 20 October 2023).
- Wenn Paracetamol die Leber Vergiftet. 10 January 2023, News, Leibniz-Institut für Arbeitsforschung an der TU Dortmund, Lebenswissenschaften, Projekte. Available online: https://www.leibniz-gemeinschaft.de/ueber-uns/neues/forschungsnachrichten/forschungsnachrichten-single/newsdetails/leberschaedigung-auf-der-spur (accessed on 20 October 2023).
- Dynastat 40 mg Powder for Solution for Injection, Parecoxib, Pfizer Europe MA EEIG. Updated SmPC June 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/dynastat-epar-product-information_en.pdf (accessed on 15 October 2023).
- Noxafil 40 mg/mL Oral Suspension Merck Sharp & Dohme B.V. SmPC. Available online: https://www.ema.europa.eu/en/documents/product-information/noxafil-epar-product-information_en.pdf (accessed on 17 October 2023).
- EU-Risk Management Plan for Posaconazole DE-H-5573-001-DC, V1.4. STADA Arzneimittel AG, Part VI: Summary of the Risk Management Plan. Available online: https://laegemiddelstyrelsen.dk/upload/rmp/28106098818%2002-10-2019.pdf (accessed on 17 October 2023).
- Pravastatin Sodium 10 mg Tablets, Milpharm Limited, SmPC, Updated emc 17 July 2023. Available online: https://www.medicines.org.uk/emc/product/5321/smpc#gref (accessed on 12 October 2023).
- Seroquel XR—Quetiapine Fumarate Extended-Release Tablets, 50, 150, 200, 300 and 400 mg, Quetiapin, Oral Use AstraZeneca Canada Inc. SmPc, Updated emc 4 January 2022. Available online: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/seroquel-xr-product-monograph-en.pdf (accessed on 15 October 2023).
- Veklury 100 mg Concentrate for Solution for Infusion, Remdesivir, Gilead Sciences Ireland UC, SmPC Updated 25 June 2020. Available online: https://www.ema.europa.eu/en/documents/other/veklury-product-information-approved-chmp-25-june-2020-pending-endorsement-european-commission_en.pdf (accessed on 12 October 2023).
- Rifampicin 300 mg Capsules, Generics [UK] Limited t/a Mylan, SmPC Updated emc June 2022. Available online: https://www.medicines.org.uk/emc/product/8789/smpc#gref (accessed on 10 October 2023).
- Targaxan 550 mg Film-Coated Tablets, 550 mg Rifaximin, Norgine Pharmaceuticals Limited, SmPC, Updated emc 8 September 2022. Available online: https://www.medicines.org.uk/emc/product/2976/smpc#gref (accessed on 12 October 2023).
- Rosuvastatin 20 mg Film-Coated Tablets, Milpharm Limited, SmPC Updated emc 3 October 2023. Available online: https://www.medicines.org.uk/emc/product/4366/smpc#gref (accessed on 12 October 2023).
- Jakavi 5 mg, 10 mg, 15 mg, 20 mg Tablets, Ruxolitinib, Novartis Pharmaceuticals UK Limited, SmPC Updated emc 24 March 2022. Available online: https://www.medicines.org.uk/emc/product/7786/smpc#gref (accessed on 11 October 2023).
- Sertraline 50 mg Film-Coated Tablets. Summary of Product Characteristics Updated 4 March 2013—Dr. Reddy’s Laboratories (UK) Ltd. Available online: https://www.drreddys.com/media/109893/sertraline-50-spc.pdf (accessed on 12 October 2023).
- Sertralin Basics 50 mg und 100 mg Filmtabletten Fachinformation Updated August 2019 Basics GmbH. Available online: https://www.basics.de/wp-content/uploads/2020/04/de-spc-sertralin50100mgv0800clean.pdf (accessed on 12 October 2023).
- Simvastatin Heumann Filmtabletten. Fachinformation Updated Mar-2023—Heumann Pharma. Available online: https://www.heumann.de/fileadmin/user_upload/produkte/infos/Fachinformation-Simvastatin-Heumann-20-30-40-60-80-mg-Filmtabletten.pdf (accessed on 12 October 2023).
- Zocord 10 mg, 20 mg, 40 mg, 80 mg Film-Coated Tablets. Summary of Product Characteristics. Laboratoires Merck Sharp & Dohme. Available online: https://www.ema.europa.eu/en/documents/referral/zocord-article-30-referral-annex-i-ii-iii_en.pdf (accessed on 12 October 2023).
- Zocor Simvastatin Tablet, Film Coated, Organon LLC, Summary of Product Characteristics Updated August 2023. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=8f55d5de-5a4f-4a39-8c84-c53976dd6af9&type=display (accessed on 12 October 2023).
- Sitagliptin Accord 25 mg, 50 mg, 100 mg Film-Coated Tablets, Accord Healthcare S.L.U, SmPC 5 July 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/sitagliptin-accord-epar-product-information_en.pdf (accessed on 10 October 2023).
- Co-Trimoxazole 800 mg/160 mg Forte Tablets, Aspen Pharma Trading Limited, SmPC, Updated emc 21 November 2022. Available online: https://www.medicines.org.uk/emc/product/6997/smpc#gref (accessed on 9 October 2023).
- Tamsulosin Hydrochloride Zentiva 400 Microgram, Prolonged-Release Hard Capsules, Zentiva Pharma UK Limited, SmPC, Updated emc 9 March 2023. Available online: https://www.medicines.org.uk/emc/product/9245/smpc#gref (accessed on 14 October 2023).
- Brilique 90 mg Film-Coated Tablets, AstraZeneca UK Limitedm SmPC, Updated emc 24 November 2022. Available online: https://www.medicines.org.uk/emc/product/5767/smpc#gref (accessed on 14 October 2023).
- Tygacil 50 mg Powder for Solution for Infusion. Summary of Product Characteristics Updated 11 October 2022—Pfizer Europe MA EEIG. Available online: https://www.ema.europa.eu/en/documents/product-information/tygacil-epar-product-information_en.pdf (accessed on 12 October 2023).
- Drugs.com. Valganciclovir, medically reviewed by Drugs.com, 2023, written by Cerner Multum. Available online: https://www.drugs.com/mtm/valganciclovir.html (accessed on 10 December 2023).
- Vancocin Powder for Solution. Summary of Product Characteristics, Updated emc 13 October 2023|Flynn Pharma Ltd. Available online: https://www.medicines.org.uk/emc/product/6407/smpc#gref (accessed on 20 October 2023).
- Vancomycin Hydrochloride for Injection USP 500 mg & 1 g Mylan Laboratories Limited. SmPc June 2020. Available online: https://fdaghana.gov.gh/img/smpc/Vancomycin%201g%20Injection%20(Vanomycin%20Hydrochloride).pdf (accessed on 16 October 2023).
- Voriconazole Pfizer 50 mg, 200 mg Film-Coated Tablets, Pfizer Limited, SmPC, Updated emc May 2023. Available online: https://www.medicines.org.uk/emc/product/7981/smpc#gref (accessed on 15 October 2023).
- Wohlt, P.D.; Zheng, L.; Gunderson, S.; Balzar, S.A.; Johnson, B.D.; Fish, J.T. Recommendations for the use of medications with continuous enteral nutrition. Am. J. Health Syst. Pharm. 2009, 66, 1438–1467. [Google Scholar] [CrossRef]
- Zolpidemtartraat Ratiopharm 5 mg, 10 mg Filmomhulde Tabletten—Ratiopharm GmbH. Updated SmPc 3 March 2023. Available online: https://www.geneesmiddeleninformatiebank.nl/smpc/h26427_smpc_en.pdf (accessed on 15 October 2023).
- Uchida, M.; Hanada, N.; Yamazaki, S.; Takatsuka, H.; Imai, C.; Utsumi, A.; Shiko, Y.; Kawasaki, Y.; Suzuki, T.; Ishii, I. Analysis of the variable factors affecting changes in the blood concentration of cyclosporine before and after transfusion of red blood cell concentrate. Pharm. Health Care Sci. 2022, 8, 4. [Google Scholar] [CrossRef]
- The American Association of Psychiatric Pharmacists (AAPP). Education ansd Resources. Monoamine Oxidase Inhibitors (MAOI): Significant Drug-Drug/Drug-Food Interactions with MAOIs. Available online: https://aapp.org/guideline/maoi/interactions (accessed on 16 October 2023).
- Danziger-Isakov, L.; Kumar, D.; AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Di Minno, A.; Frigerio, B.; Spadarella, G.; Ravani, A.; Sansaro, D.; Amato, M.; Kitzmiller, J.P.; Pepi, M.; Tremoli, E.; Baldassarre, D. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev. 2017, 31, 193–203. [Google Scholar] [CrossRef]
- Antoniou, T.; Bodkin, J.; Ho, J.M. Drug interactions with cannabinoids. CMAJ 2020, 192, E206. [Google Scholar] [CrossRef]
- Jacobs, U.; Eis-Hübinger, A.; Miersch, W.D.; Klehr, H.U. Immunologic diagnostic and therapeutic aspects of cytomegalovirus, human herpes virus-6, and Epstein-Barr virus disease posttransplantation. Transplant. Proc. 1996, 28, 3238. [Google Scholar]
- Jacobs, U.; Klehr, H.U. Predictive value of immunological parameters in the early diagnosis of cytomegalovirus infection in renal transplantation. Transplant. Proc. 1993, 25, 2670. [Google Scholar]
- Jacobs, U.; Klehr, H.U.; Oehlmann, U. Frühzeitige Erkennung des CMV-Infektes—Verbesserung der Transplantat-Überlebensrate auf 100% in den beiden letzten Jahren. In Proceedings of the 159th Tagung der Rheinisch-Westfälischen Gesellschaft für Innere Medizin, Düsseldorf, Germany, 4–5 December 1992. [Google Scholar]
- Amundsen, R.; Åsberg, A.; Ohm, I.K.; Christensen, H. Cyclosporine A- and tacrolimus-mediated inibition of CYP3A4 and CYP3A5 in vitro. Drug Metab. Dispos. 2012, 40, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Serotonin-Syndrom als Additive Wechselwirkung unter Fentanyl. Arzneimittelbrief 2013, 47, 43. Available online: https://der-arzneimittelbrief.com/artikel/2013/serotonin-syndrom-als-additive-wechselwirkung-unter-fentanyl (accessed on 28 October 2023).
- Dean, P.G.; Lund, W.J.; Larson, T.S.; Prieto, M.; Nyberg, S.L.; Ishitani, M.B.; Kremers, W.K.; Stegall, M.D. Wound-healing complications after kidney transplantation: A prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004, 77, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. Drug Interaction Checker. Interaction Report. Available online: https://www.drugs.com/interactions-check.php?drug_list=763-0,2067-0,2142-0&professional=1 (accessed on 24 October 2023).
- Hammoud, S.H.; Alkhansa, S.; Mahjoub, N.; Omar, A.G.; El-Mas, M.M.; Eid, A.A. Molecular basis of the counteraction by calcium channel blockers of cyclosporine nephrotoxicity. Am. J. Physiol. Renal Physiol. 2018, 315, F572–F582. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. Drug Interaction Checker. Interaction Report. Available online: https://www.drugs.com/interactions-check.php?drug_list=172-0,763-0,2142-0&professional=1 (accessed on 20 October 2023).
- Felodipin Retard Heumann, SmPc Updated January 2020. Available online: https://www.heumann.de/fileadmin/user_upload/produkte/infos/Fachinformation-Felodipin-retard-Heumann.pdf (accessed on 20 October 2023).
- Lercanidipin Heumann 10 mg, 20 mg Filmtabletten. SmPC November 2020. Available online: https://www.heumann.de/fileadmin/user_upload/produkte/infos/Lercanidipin_Heumann_Filmtabletten_012457_2019-07.pdf (accessed on 20 October 2023).
- Drugs.com. Drug Interaction Checker. Interaction Report. Available online: https://www.drugs.com/interactions-check.php?drug_list=763-0,1482-0,2142-0&professional=1 (accessed on 24 October 2023).
- Drugs.com. Drug Interaction Checker. Interaction Report. Available online: https://www.drugs.com/interactions-check.php?drug_list=763-0,3873-0,2309-0&professional=1 (accessed on 24 October 2023).
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Friman, S.; Scheuermann, E.; Rostaing, L.; Jenssen, T.; Campistol, J.M.; Uchida, K.; Pescovitz, M.D.; Marchetti, P.; Tuncer, M.; et al. DIRECT (Diabetes Incidence after Renal Transplantation: Neoral C Monitoring vs. Tacrolimus) Investigators. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine vs. tacrolimus. Am. J. Transplant. 2007, 7, 1506–1514. [Google Scholar] [CrossRef]
- Benotmane, I.; Solis, M.; Velay, A.; Cognard, N.; Olagne, J.; Gautier Vargas, G.; Perrin, P.; Marx, D.; Soulier, E.; Gallais, F.; et al. Intravenous immunoglobulin as a preventive strategy against BK virus viremia and BKV-associated nephropathy in kidney transplant recipients-Results from a proof-of-concept study. Am. J. Transplant. 2021, 21, 329–337. [Google Scholar] [CrossRef]
- Chow, E.J.; Mueller, B.A.; Baker, K.S.; Cushing-Haugen, K.L.; Flowers, M.E.; Martin, P.J.; Friedman, D.L.; Lee, S.J. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann. Intern. Med. 2011, 155, 21–32. [Google Scholar] [CrossRef]
- Halegoua-DeMarzio, D.; Navarro, V.; Ahmad, J.; Avula, B.; Barnhart, H.; Barritt, A.S.; Bonkovsky, H.L.; Fontana, R.J.; Ghabril, M.S.; Hoofnagle, J.H.; et al. Liver Injury Associated with Turmeric-A Growing Problem: Ten Cases from the Drug-Induced Liver Injury Network [DILIN]. Am. J. Med. 2023, 136, 200–206. [Google Scholar] [CrossRef]
- Ikitimur, B.; Cosansu, K.; Karadag, B.; Cakmak, H.A.; Avci, B.K.; Erturk, E.; Seyahi, N.; Ongen, Z. Long-Term Impact of Different Immunosuppressive Drugs on QT and PR Intervals in Renal Transplant Patients. Ann. Noninvasive Electrocardiol. 2015, 20, 426–432. [Google Scholar] [CrossRef]
- Drewas, L.; Ghadir, H.; Neef, R.; Delank, K.S.; Wolf, U. Individual Pharmacotherapy Management (IPM)—I: A group-matched retrospective controlled clinical study on prevention of complicating delirium in the elderly trauma patients and identification of associated factors. BMC Geriatr. 2022, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Wolf, U.; Baust, H.; Neef, R.; Steinke, T. Individual Pharmacotherapy Management (IPM)—IV: Optimized Usage of Approved Antimicrobials Addressing Under-Recognized Adverse Drug Reactions and Drug-Drug Interactions in Polypharmacy. Antibiotics 2022, 11, 1381. [Google Scholar] [CrossRef] [PubMed]
- Coemans, M.; Süsal, C.; Döhler, B.; Anglicheau, D.; Giral, M.; Bestard, O.; Legendre, C.; Emonds, M.P.; Kuypers, D.; Molenberghs, G.; et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int. 2018, 94, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, I.; Kolonko, A.; Chudek, J.; Nowak, Ł.; Farnik, M.; Więcek, A. Influence of Polypharmacy on the Quality of Life in Stable Kidney Transplant Recipients. Transplant. Proc. 2018, 50, 1896–1899. [Google Scholar] [CrossRef]
- Van Osten, M.J.M.; Logtenberg, S.J.J.; Hemmelder, M.H.; Leegte, M.J.H.; Bilo, H.J.G.; Jager, K.J.; Stel, V.S. Polypharmacy and medication use in patients with chronic kidney disease with and without kidney replacement therapy compared to matched controls. Clin. Kidney J. 2021, 14, 2497–2523. [Google Scholar] [CrossRef]
- Sugidono, M.; Lo, M.; Young, R.; Rosario, K.; Jung, Y.; Huang, C.Y.; Sheng, Y.; Huang, L.W.; Olin, R.L. Impact of Polypharmacy Prior to Allogeneic Hematopoietic Stem Cell Transplantation in Older Adults. Transplant. Cell Ther. 2021, 27, 344.e1–344.e5. [Google Scholar] [CrossRef] [PubMed]
- Glotzbecker, B.; Duncan, C.; Alyea, E., 3rd; Campbell, B.; Soiffer, R. Important drug interactions in hematopoietic stem cell transplantation: What every physician should know. Biol. Blood Marrow Transplant. 2012, 18, 989–1006. [Google Scholar] [CrossRef]
- Ahmed, R.; Hassan, Z.; Haseeb, A.; Masood, A.; Ali, I. Multiple Adverse Drug Reactions to Calcineurin Inhibitors in a Renal Transplant Patient. Uro 2021, 1, 180–186. [Google Scholar] [CrossRef]
- Jacobs, U.; Klein, B.; Klehr, H.U. Cumulative side effects of cyclosporine and Ca antagonists: Hypergalactinemia, mastadenoma, and gynecomastia. Transplant. Proc. 1994, 26, 3122. [Google Scholar] [PubMed]
- Reuben, A. Drug-Induced Liver Injury in Liver Transplant Recipients: Informed Insights and Sage Advice from Andalusia. Liver Transplant. 2020, 26, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Moradi, O.; Karimzadeh, I.; Davani-Davari, D.; Shafiekhani, M.; Sagheb, M.M.; Raees-Jalali, G.A. Drug-Drug Interactions among Kidney Transplant Recipients in The Outpatient Setting. Int. J. Organ. Transplant. Med. 2020, 11, 185–195. [Google Scholar]
- Drenth-van Maanen, A.C.; Wilting, I.; Jansen, P.A.F. Prescribing medicines to older people-How to consider the impact of ageing on human organ and body functions. Br. J. Clin. Pharmacol. 2020, 86, 1921–1930. [Google Scholar] [CrossRef]
- Branthwaite, J.P.; Nicholls, A. Cyclosporin and diclofenac interaction in rheumatoid arthritis. Lancet 1991, 337, 252. [Google Scholar] [CrossRef]
- Harris, K.P.; Jenkins, D.; Walls, J. Nonsteroidal antiinflammatory drugs and cyclosporine. Transplantation 1988, 46, 598–599. [Google Scholar] [CrossRef]
- Deray, G.; Le Hoang, P.; Aupetit, B.; Achour, A.; Rottembourg, J.; Baumelou, A. Enhancement of cyclosporine A nephrotoxicity by diclofenac. Clin. Nephrol. 1987, 27, 213–214. [Google Scholar]
- Lapi, F.; Azoulay, L.; Yin, H.; Nessim, S.J.; Suissa, S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: Nested case-control study. BMJ 2013, 346, e8525. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Ren, H.; Chen, X.; Xie, J.; Chen, N. Diuretics associated acute kidney injury: Clinical and pathological analysis. Ren. Fail. 2014, 36, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Fouassier, D.; Blanchard, A.; Fayol, A.; Bobrie, G.; Boutouyrie, P.; Azizi, M.; Hulot, J.S. Sequential nephron blockade with combined diuretics improves diastolic function in patients with resistant hypertension. ESC Heart Fail. 2020, 7, 2561–2571. [Google Scholar] [CrossRef]
- Fliser, D.; Schröter, M.; Neubeck, M.; Ritz, E. Coadministration of thiazides increases the efficacy of loop diuretics even in patients with advanced renal failure. Kidney Int. 1994, 46, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. CJASN 2010, 5, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.S.; Hawkey, C.J.; Ford, I.; Greenlaw, N.; Pigazzani, F.; Rogers, A.; Struthers, A.D.; Begg, A.G.; Wei, L.; Avery, A.J.; et al. Allopurinol versus usual care in patients with ischaemic heart disease (ALL-HEART): A multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 1195–1205. [Google Scholar] [CrossRef]
- Tanaka, A.; Node, K. Xanthine oxidase inhibition for cardiovascular disease prevention. Lancet 2022, 400, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Febuxostat-Ratiopharm 80 mg Filmtabletten. Fachinformation 2019. Available online: https://www.ratiopharm.de/assets/products/de/label/Febuxostat-ratiopharm%2080%20mg%20Filmtabletten%20-%202.pdf?pzn=14168559 (accessed on 23 October 2023).
- Eleftheriadis, T.; Golphinopoulos, S.; Pissas, G.; Stefanidis, I. Asymptomatic hyperuricemia and chronic kidney disease: Narrative review of a treatment controversial. J. Adv. Res. 2017, 8, 555–560. [Google Scholar] [CrossRef]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 5–14. Available online: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf (accessed on 28 October 2023).
- Migliozzi, D.R.; Asal, N.J. Clinical Controversy in Transplantation: Tacrolimus Versus Cyclosporine in Statin Drug Interactions. Ann. Pharmacother. 2020, 54, 171–177. [Google Scholar] [CrossRef]
- Bae, S.; Ahn, J.B.; Joseph, C.; Whisler, R.; Schnitzler, M.A.; Lentine, K.L.; Kadosh, B.S.; Segev, D.L.; McAdams-DeMarco, M.A. Incidence of Statin-Associated Adverse Events in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2023, 18, 626–633. [Google Scholar] [CrossRef]
- Launay-Vacher, V.; Izzedine, H.; Deray, G. Statins’ dosage in patients with renal failure and cyclosporine drug-drug interactions in transplant recipient patients. Int. J. Cardiol. 2005, 101, 9–17. [Google Scholar] [CrossRef] [PubMed]
- El Bardai, G.; Chouhani, B.A.; Haddane, W.; Kabbali, N.; Sqalli Houssaini, T. Beware of Rhabdomyolysis after a Renal Graft. Cureus 2022, 14, e30546. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Corsini, A.; Davidson, M.H.; Holdaas, H.; Jacobson, T.A.; Leitersdorf, E.; März, W.; Reckless, J.P.D.; Stein, E.A. Risk for Myopathy with Statin Therapy in High-Risk Patients. Arch. Intern. Med. 2003, 163, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Breckenridge, A.M.; Kitteringham, N.R.; Park, B.K. Adverse drug reactions. BMJ 1998, 316, 1295–1298. [Google Scholar] [CrossRef]
- Alomar, M.J. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm. J. 2014, 22, 83–94. [Google Scholar] [CrossRef]
- Page, R.L., 2nd; Miller, G.G.; Lindenfeld, J. Drug therapy in the heart transplant recipient: Part IV: Drug-drug interactions. Circulation 2005, 111, 230–239. [Google Scholar] [CrossRef]
- Tatro, D.S. (Ed.) Drug Interaction Facts; Facts and Comparisons/Wolters Kluwer Health Inc.: Saint Louis, MO, USA, 2004; pp. xv–xviii. [Google Scholar]
- Calne, R.Y.; Thiru, S.; Mcmaster, P.; Craddock, G.N.; White, D.J.G.; Evans, D.B.; Dunn, D.C.; Pentlow, B.D.; Rolles, K. Cyclospin A in patients receiving renal allografts from cadaver donors. Lancet 1978, 312, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Klintmalm, G.B.; Iwatsuki, S.; Starzl, T.E. Nephrotoxicity of cyclosporin A in liver and kidney transplant patients. Lancet 1981, 1, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, K.; Takeda, A.; Uchida, K.; Mihatsch, M.J. Cyclosporine nephrotoxicity: How does it affect renal allograft function and transplant morphology? Transplant. Proc. 2004, 36, 251–256. [Google Scholar] [CrossRef]
- He, X.; Johnston, A. Variable cyclosporine exposure: A risk factor for chronic allograft nephropathy and graft loss? Transplant. Proc. 2004, 36, 1321–1326. [Google Scholar] [CrossRef]
- Jacobs, U.; Niese, D.; Miersch, W.D.; Klehr, H.U. Acute rejection relapses posttransplant: Definition of risk group and evaluation of potent therapeutic regimens. Transpl. Int. 1996, 9, 34–37. [Google Scholar] [CrossRef]
- Jacobs, U.; Paar, D.; Buszello, H.; Klehr, H.U. Tumors after transplantation: Are there associated factors? Transplant. Proc. 1996, 28, 3248. [Google Scholar]
- Jacobs, U.; Niese, D.; Brensing, K.A.; Eis-Hübinger, A.; Buszello, H.; Klehr, H.U. Immunologic disorders in posttransplant lymphoma: Therapeutic implications. Transplant. Proc. 1996, 28, 3249–3250. [Google Scholar]
- Jacobs, U.; Ferber, J.; Klehr, H.U. Severe allograft dysfunction after OKT3-induced human herpes virus-6 reactivation. Transplant. Proc. 1994, 26, 3121. [Google Scholar]
- Jacobs, U.; Niese, D.; Klein, B.; Paar, D.; Miersch, W.D.; Klehr, H.U. Cold ischemia, histocompatibility, donor and recipient age: Impact on early lymphocyte subsets and transplant outcome. Transplant. Proc. 1996, 28, 3251–3252. [Google Scholar] [PubMed]
- Jacobs, U.; Niese, D.; Brensing, K.A.; Klein, B.; Buszello, H.; Klehr, H.U. Clinical and immunologic characteristics of transplant recipients with recurrent acute rejection episodes. Transplant. Proc. 1996, 28, 3231–3233. [Google Scholar] [PubMed]
- Wolf, U.; Presek, P. Tailored Pharmacotherapy—Clinical Pharmacological Fine-Tuning to Optimize Acute and Long-Term Patient’s and Transplant’s Outcome. Abstract. Transplantation 2012, 94, 775. Available online: https://journals.lww.com/transplantjournal/fulltext/2012/11271/tailored_pharmacotherapy___clinical.1517.aspx (accessed on 28 October 2023).
- Wolf, U.; Desole, M.; Presek, P. Increasing Patient’s Trend towards “Natural Comedication”—Supportive or Harmful in Transplantation?—A Case Report Documenting Sage to Severely Decrease Cyclosporine Bioavailability. Transplantation 2012, 94, 775. Available online: https://journals.lww.com/transplantjournal/Fulltext/2012/11271/Increasing_Patient__S_Trend_Towards__Natural.1518.aspx (accessed on 28 October 2023).
- Wolf, U. Innovative Consequences for Clinical Pharmacological Approach: HELP—Hierarchical Elements for Long-term Prevaillance. Poster presentation. In Proceedings of the 22nd Annual Meeting of the German Transplantation Society, Frankfurt, Germany, 24–26 October 2013; Volume 26. [Google Scholar]
- Wolf, U.; Klipp, G.; Müller, T.J.; Schneider, I.; Wartenberg, K.E.; Wolf, H.H.; Presek, P. Interdisciplinary effort to diagnose and treat a posttransplant patient with severe cerebral lesions in synopsis with the intraindividually complex pharmacological situation. Oral presentation. In Proceedings of the 15th Congress of the European Society Organ Transplantation (ESOT), Glasgow, Scotland, 7 September 2011; Volume 24, p. 10366. [Google Scholar]
- Wolf, C.P.J.G.; Rachow, T.; Ernst, T.; Hochhaus, A.; Zomorodbakhsch, B.; Foller, S.; Rengsberger, M.; Hartmann, M.; Hübner, J. Interactions in cancer treatment considering cancer therapy, concomitant medications, food, herbal medicine and other supplements. J. Cancer Res. Clin. Oncol. 2022, 148, 461–473. [Google Scholar] [CrossRef]
- Wolf, C.P.J.G.; Rachow, T.; Ernst, T.; Hochhaus, A.; Zomorodbakhsch, B.; Foller, S.; Rengsberger, M.; Hartmann, M.; Hübner, J. Complementary and alternative medicine (CAM) supplements in cancer outpatients: Analyses of usage and of interaction risks with cancer treatment. J. Cancer Res. Clin. Oncol. 2022, 148, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, Q.; Wang, J.; Hu, M.; Zeng, F.; Wang, Z.; Zhang, Y. Genetic polymorphisms affecting tacrolimus metabolism and the relationship to posttransplant outcomes in kidney transplant recipients. Pharmgenom. Pers. Med. 2021, 14, 1463–1474. [Google Scholar] [CrossRef]
- World Health Organization. Medication Safety in Polypharmacy, 2019, Geneva, (WHO/UHC/SDS/2019.11). License: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/docs/default-source/patient-safety/who-uhc-sds-2019-11-eng.pdf (accessed on 25 July 2023).

| Medication Risks to Be Recognized in Solid Organ and Hematopoietic Stem Cell Transplantation | ||||
|---|---|---|---|---|
| Drug | ADR * | DDI | CI ** | Other Aspects |
| Aciclovir [33] | headache, dizziness; nausea, vomiting, diarrhea, abdominal pains; fever, fatigue; symptoms of overdose include agitation, coma, seizures, lethargy, and precipitation in renal tubules, more common in patients given high doses without monitoring of fluid and electrolyte balancing or reduced kidney function | eliminated primarily unchanged via active renal tubular secretion, any concurrent drug competing with this mechanism may increase aciclovir plasma concentrations, probenecid and cimetidine increase the AUC of aciclovir by this mechanism, and reduce aciclovir renal clearance; similarly increases in the plasma AUCs of aciclovir, mycophenolate mofetil, and mycophenolate acid when coadministered; the risk of renal impairment is increased by the concomitant use of other nephrotoxic drugs; may increase serum theophylline levels; risk or severity of nephrotoxicity can be increased with CsA, TAC; excretion of aciclovir can be decreased when combined with methotrexate | with caution in patients with underlying neurological abnormalities, severe hepatic or electrolyte abnormalities or significant hypoxia; maintain adequate hydration; dosage reduction may be required in elderly; renal dose adjustment | |
| Allopurinol [34] | should be withdrawn immediately if a skin rash or other evidence of sensitivity occurs, as this could result in more serious hypersensitivity reactions (chronic renal impairment and concomitant diuretic use, in particular thiazides, may increase the risk of hypersensitivity reactions, including SJS/TEN, HLA-B*5801 allele has been shown to be associated with the risk of developing allopurinol-related hypersensitivity syndrome and SJS/TEN, up to 20% prevalence in the Han Chinese population); xanthine deposition in the urinary track, minimized by adequate hydration; increased TSH values; very rare reports have been received of thrombocytopenia, agranulocytosis, and aplastic anemia, particularly in individuals with impaired renal or hepatic function | CsA level may be increased; 6-mercaptopurine or azathioprine at only one-quarter of the usual dose because inhibition of xanthine oxidase will prolong their activity; vidarabine is increased, enhancing toxic effects; oxipurinol, the major metabolite of allopurinol and itself therapeutically active, is excreted by the kidney in a similar way to urate, hence, drugs with uricosuric activity, such as probenecid or large doses of salicylate, may accelerate the excretion of oxipurinol, which may decrease the therapeutic activity of allopurinol; with chlorpropamide in poor renal function, the increased risk of prolonged hypoglycemic activity because allopurinol and chlorpropamide may compete for excretion in the renal tubule; increased effect of warfarin and other coumarin anticoagulants; hepatic oxidation of phenytoin inhibited; metabolism of theophylline inhibited; increase in the frequency of skin rashes among patients receiving ampicillin or amoxicillin concurrently, it is recommended that in patients receiving allopurinol, an alternative to ampicillin or amoxicillin is used where available; with cyclophosphamide, doxorubicin, bleomycin, procarbazine, mechlorethamine, enhanced bone marrow suppression is likely; didanosine level elevated, combination not recommended; with furosemide, increased serum urate and plasma oxypurinol concentrations; increased risk of hypersensitivity with diuretics, in particular thiazides, and ACE inhibitors, especially in renal impairments; with cytostatics (e.g., cyclophosphamide, doxorubicin, bleomycin, procarbazine, alkyl halogenides), blood dyscrasias more frequently; with aluminum hydroxide concomitantly, allopurinol may have an attenuated effect, there should be an interval of at least 3 h between taking both medicines | asymptomatic hyperuricemia per se is generally not considered an indication for the use of allopurinol; should not be started until an acute attack of gout has completely subsided, as further attacks may be precipitated | reduced doses in patients with renal or hepatic impairment |
| Amiodarone [35] | bradycardia, conduction disturbances; the pharmacological action of amiodarone induces ECG changes: QTc prolongation (related to prolonged repolarization) with the possible development of U-waves and deformed T-waves; these changes do not reflect toxicity; in the elderly, heart rate may decrease markedly; treatment should be discontinued in case of onset of second or third degree AV block, sino-atrial block, or fascicular block; amiodarone has a low pro-arrhythmic effect, despite QTc interval prolongation, amiodarone exhibits a low torsadogenic activity; hypothyroidism, hyperthyroidism; eye disorders, corneal microdeposits; benign gastrointestinal disorders (nausea, vomiting, dysgeusia), usually occurring with loading dosage and resolving with dose reduction; constipation; hepatobiliary disorders with isolated increases in serum transaminases, acute liver disorders with high serum transaminases and/or jaundice, including hepatic failure, which are sometimes fatal; peripheral sensorimotor neuropathy and/or myopathy, may be severe; extrapyramidal tremor, nightmares, sleep disorders; pulmonary toxicity (sometimes fatal); photosensitivity | increase in CsA, TAC, SIR, EVR, simvastatin, atorvastatin, lovastatin, amlodipine, lercanidipine, felodipine, fentanyl, buprenorphine, midazolam, quetiapine, mirtazapine, aprepitant, lidocaine, DOAC; pharmacodynamic DDIs with drugs inducing torsade de pointes or prolonging QTc intervals incl. erythromycin i.v., co-trimoxazole, haloperidol, doxepin, amitriptyline, moxifloxacin; potentially severe complications have been reported in patients undergoing general anesthesia: bradycardia unresponsive to atropine, hypotension, disturbances of conduction, decreased cardiac output; a few cases of adult respiratory distress syndrome, sometimes fatal, most often in the period immediately after surgery, have been observed, a possible interaction with a high oxygen concentration may be implicated; with hepatitis C medicines with or without other medicines that lower the heart rate risk of bradycardia and heart block; concomitant use not recommended with beta-blockers, heart rate lowering calcium channel inhibitors (verapamil, diltiazem), stimulant laxative agents which may cause hypokalemia; increased plasma levels of flecainide, should be reduced accordingly and the patient closely monitored; not to be taken alongside grapefruit juice consumption | thyroid dysfunction; before surgery, the anesthetist should be informed that the patient is taking amiodarone; sinus bradycardia and sino-atrial heart block, in patients with severe conduction disturbances (high grade AV block, bifascicular or trifascicular block) or sinus node disease be used only in conjunction with a pacemaker; before starting, it is recommended to perform an ECG and serum potassium and magnesium measurements; monitoring of ECG is recommended during treatment; may increase the defibrillation threshold and/or pacing threshold in patients with an implantable cardioverter defibrillator or a pacemaker, which may adversely affect the efficacy of the device, regular tests are recommended to ensure the proper function of the device; combination with drugs which may induce torsades de pointes, such as moxifloxacin (cave fluoroquinolones), contraindicated | treatment should be limited; half-life of 50 days (20–100); monitor thyroid function: Primary Graft Dysfunction (PGD) post-cardiac transplant: in retrospective studies, amiodarone use in the transplant recipient prior to heart transplant has been associated with an increased risk of PGD (left, right, or biventricular dysfunction occurring within the first 24 h of transplant surgery, for which there is no identifiable secondary cause, may be irreversible), for patients who are on the heart transplant waiting list, consideration should be given to use an alternative antiarrhythmic drug as early as possible before transplantation |
| Amlodipine [36] DDIs present class effect, also with other dihydropyridine calcium antagonists | oedema; fatigue, asthenia; somnolence, dizziness, headache (especially at the beginning of the treatment); visual disturbance (including diplopia); palpitations; flushing; dyspnea; abdominal pain, nausea, dyspepsia, altered bowel habits (including diarrhea and constipation), ankle swelling, muscle cramp | with strong or moderate CYP3A4 inhibitors (protease inhibitors, azole antifungals, macrolides like erythromycin or clarithromycin, verapamil or diltiazem) may give rise to a significant increase in amlodipine exposure; decreased amlodipine exposure with strong CYP3A4 inducers (e.g., rifampicin, St. John’s wort); increased blood levels of CsA, TAC, SIR, EVR; increased risk of angioedema with mTORIs; limit the dose of simvastatin in patients on amlodipine to 20 mg daily | not in severe hypotension, shock, including cardiogenic shock, obstruction of the outflow-tract of the left ventricle (e.g., high grade aortic stenosis), hemodynamically unstable heart failure after acute myocardial infarction | with hepatic impairment, dose selection should be cautious and should start at the lower end of the dosing range; in the elderly, any increase of the dosage should take place with care |
| Amphotericin B, AmBisome Liposomal [37] | renal toxicity, hypokalemia hypomagnesemia, hypocalcemia notably higher with high dose, regular evaluation of electrolytes, renal, hepatic, and hematopoietic function, appropriate potassium supplementation; high portion of sucrose caution in diabetes mellitus; hyperglycemia; headache; tachycardia; hypotension, vasodilatation, flushing; dyspnea; nausea, vomiting, diarrhea, abdominal pain; abnormal liver function tests, hyperbilirubinemia, increased alkaline phosphatase; renal toxicity, increased creatinine, increased blood urea; rigors, pyrexia; chest pain | with other nephrotoxic agents (for example CsA, TAC, aminoglycosides, polymyxins, and pentamidine) may enhance the potential for drug-induced renal toxicity; corticosteroids, ACTH, and diuretics (loop and thiazide) may potentiate hypokalemia; antineoplastic agents may enhance the potential for renal toxicity, bronchospasm, and hypotension, and should be given concomitantly with caution | acute pulmonary toxicity given amphotericin B (such as sodium deoxycholate complexes) during or shortly after leukocyte transfusions, recommended that these infusions are separated by as long a period as possible, and pulmonary function should be monitored | if clinically significant reduction in renal function, then dose reduction or treatment interruption; no benefit from the use of flucytosine with AmBisome has been observed, whilst synergy between amphotericin and flucytosine reported, concurrent use may increase the toxicity of flucytosine by possibly increasing its cellular uptake and/or impairing its renal excretion; false elevations of serum phosphate in PHOSm assays |
| Apixaban [38] As an example for DOACs | hemorrhage, eye hemorrhage contusion, epistaxis, hematoma; nausea; gastrointestinal hemorrhage; mouth hemorrhage; rectal hemorrhage, gingival bleeding, hematuria, anemia; YGT, ALT increased; abnormal vaginal hemorrhage, urogenital hemorrhage; contusion | closer monitoring of increased anticoagulation effects of apixaban whenever a CYP450 3A4 or P-gp inhibitor is added, incl. CsA, TAC; caution increased bleeding risk with SSRIs/SNRIs, NSAIDs, ASA, and/or P2Y12 inhibitors; following surgery, other platelet aggregation inhibitors are not recommended concomitantly with apixaban; active substances which are not considered strong inhibitors of both CYP3A4 and P-gp, e.g., amiodarone, clarithromycin, diltiazem, fluconazole, naproxen, quinidine, verapamil, are expected to increase the apixaban plasma concentration to a lesser extent | not recommended in concomitant systemic treatment with strong inhibitors of both CYP3A4 and P-gp, such as azole-antimycotics, e.g., ketoconazole, itraconazole, voriconazole, posaconazole, and HIV protease inhibitors, e.g., ritonavir; strong CYP3A4 and P-gp inducers, e.g., rifampicin, phenytoin, carbamazepine, phenobarbital, or St. John’s Wort may lead to a ~50% reduction in apixaban exposure, thus not to be used for the treatment of DVT and treatment of PE; not in active clinically significant bleeding, lesions, or conditions if considered a significant risk factor for major bleeding; not in hepatic diseases associated with coagulopathy and clinically relevant bleeding risk; not in severe hepatic impairment, coagulative disease; caution in elevated ALT/AST > 2 × ULN or total bilirubin ≥ 1.5 × ULN; no concomitant treatment with any other anticoagulant agent; DOACs are not recommended for patients diagnosed with antiphospholipid syndrome; GPIIb/IIIa receptor antagonists, dipyridamole, dextran, sulfinpyrazone, or thrombolytic agents not recommended | caution in conditions with increased risks of hemorrhage, e.g., thrombocytopenia; for the prevention of stroke and systemic embolism in patients with NVAF and serum creatinine ≥ 1.5 mg/dL (133 micromole/L) associated with age ≥ 80 years or body weight ≤ 60 kg, a dose reduction is necessary; in patients with severe renal impairment (creatinine clearance 15–29 mL/min), dose reduction is required (compared to, e.g., rivaroxaban, which already requires dose reduction at creatinine clearance < 50 mL/min); creatinine clearance < 15 mL/min, or in patients undergoing dialysis not recommended; calibrated quantitative anti-Factor Xa assays in acute bleeding risks; antidot adexanet alfa available |
| Aprepitant [39,40] | decreased appetite, headache, hiccups, constipation, dyspepsia, fatigue; increased ALT | several-fold increase in plasma concentrations of aprepitant with active substances that inhibit CYP3A4 activity (e.g., ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin, nefazodone, and protease inhibitors); strong CYP3A4 inducers (e.g., rifampicin, phenytoin, carbamazepine, phenobarbital) reduce aprepitant efficacy; transient moderate increase, followed by a mild decrease in the exposure of CSA, TAC, SIR, EVR; increased exposure of simvastatin, atorvastatin, lovastatin, amlodipine, lercanidipine, felodipine, fentanyl, uprenorphine, midazolam, quetiapine, mirtazapine, ergot alkaloid derivatives, quinidine, and irinotecan; reduced efficacy of hormonal contraceptives during and for 28 days after administration, alternative non-hormonal back-up methods of contraception should be used during treatment with aprepitant and for 2 months following the last dose; oral dexamethasone and methylprednisolone dose should be reduced by approximately 50% when coadministered; during continuous treatment with methylprednisolone, the AUC of methylprednisolone may decrease at later time points within 2 weeks following the initiation of the aprepitant dose, due to the additional inducing effect of aprepitant on CYP3A4; caution potential increase of orally administered chemotherapeutic medicinal products metabolized primarily or partly by CYP3A4 (e.g., etoposide, vinorelbine, ifosfamide); decrease in warfarin, acenocoumarol, tolbutamide, and phenytoin via CYP2C9/CYP3A4 induction, apparent only after the end of a 3-day treatment with aprepitant, the induction is transient with a maximum effect reached 3–5 days after the end of the aprepitant 3-day treatment, the effect is maintained for a few days | no coadministration with pimozide, terfenadine, astemizole, or cisapride, not with St. John’s wort | caution in patients with moderate to severe hepatic impairment |
| ASA [41,42] acetyl salicylic acid | bleeding and hemorrhagic tendency (epistaxis, bleeding gums, purpura, etc.), bleeding risk may persist for 4 to 8 days after discontinuation, increased risk of hemorrhage in the event of surgery, intracranial and gastrointestinal hemorrhage; anaphylactic reactions, asthma, angioedema; rhinitis, dyspnea, bronchospasm; headache, dizziness, sensation of hearing loss, tinnitus, which are usually indicative of an overdose; abdominal pain, dyspepsia, nausea, vomiting; occult or patent gastrointestinal hemorrhage (hematemesis, melaena, etc.), resulting in iron-deficiency anemia, the bleeding risk is dose-dependent, gastric ulcers and perforations; elevation of hepatic enzymes, hepatocellular liver injury; impaired renal function; Reye’s syndrome; reduces the excretion of uric acid, risks of gout attacks in predisposed patients | cardioprotective value of LD-ASA can be compromised in patients who take NSAIDs concomitantly, because some NSAIDs competitively bind to critical amino-acid residues on cyclooxygenase (COX) enzymes and interfere with the mechanism of the antiplatelet activity of LD-ASA, take ASA hrs in advance; not recommended with: oral anticoagulants or other NSAIDs or heparins or clopidogrel (beyond the approved indications for this combination in patients with acute coronary syndrome) or ticlopidine or glucocorticoids (except hydrocortisone replacement therapy) for anti-inflammatory doses or anagrelide increased bleeding risk; reduction in the uricosuric effects of benzbromarone, probenecid; increased risk of pemetrexed toxicity in mild-to-moderate renal impairment (creatinine clearance between 45 mL/min and 80 mL/min); precaution in combinations with diuretics, ACE inhibitors, sartans (acute renal failure may occur in dehydrated patients due to decreased GFR secondary to decreased synthesis of renal prostaglandin); methotrexate at doses ≤15 mg/week, requires regular blood counts, clopidogrel; gastrointestinal topicals, antacids, and charcoal at least 2 h apart; with deferasirox, thrombolytics, SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline), an increased risk of gastrointestinal ulcers and hemorrhage | not with a history of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy, not with an active or a history of recurrent peptic ulcers/hemorrhages (two or more distinct episodes of proven ulceration or bleeding); not with severe heart failure; not be given to children and adolescents with signs of viral infection aged under 16 years for risk of fatal Reye’s syndrome, affecting the brain (vomiting, disturbances of consciousness or abnormal behavior) and liver; not with a history of asthma induced by NSAIDs; esp. 500 mg doses, not in severe renal or hepatic insufficiencies; severe uncontrolled cardiac insufficiency, coadministration with methotrexate > 15 mg/week; coadministration of oral anticoagulants with a history of gastroduodenal ulcers | on long-term (>3 months) with administration every two days or more frequently, medication-overuse headache (MOH) should be suspected, headache induced by overuse of analgesics (MOH); risk of persistent renal lesions and renal insufficiency; in severe G6PD deficiency, ASA may induce hemolysis; monitoring in metrorrhagia or menorrhagia; with alcohol increased bleeding risk |
| Atorvastatin [43] | myalgia, arthralgia; caution in patients with pre-disposing factors for rhabdomyolysis, e.g., renal impairment, hypothyroidism; liver function test abnormal, blood creatine kinase increased; in elderly (age > 70 years), creatine phosphokinase (CPK) measurements should be considered, according to the presence of other predisposing factors for rhabdomyolysis; patients must be asked to promptly report muscle pain, cramps, or weakness; constipation, flatulence, dyspepsia, nausea, diarrhea; insomnia, headache, dizziness, paresthesia, hypoesthesia; asthenia, chest pain, back pain, peripheral oedema; elevated serum transaminases; hyperglycemia; nasopharyngitis, pharyngolaryngeal pain | risk of rhabdomyolysis is increased with CsA, TAC, SIR, EVR, clarithromycin, itraconazole, ketoconazole, posaconazole, voriconazole, nefazodone, niacin, gemfibrozil, other fibric acid derivates, or HIV-protease inhibitors, grapefruit juice, amiodarone; oral contraceptives with an increase in plasma concentrations of norethindrone and ethinyl estradiol; also moderate CYP3A4 inhibitors (e.g., erythromycin, diltiazem, verapamil and fluconazole) increase plasma concentrations of atorvastatin; in general, lovastatin and simvastatin should preferably be avoided in patients treated with CsA, TAC, and SIR, due to the potential rhabdomyolysis; atorvastatin may be used with caution, although the dosage should start low; pravastatin and fluvastatin are the safest alternatives, since they are not metabolized by CYP450 3A4; all patients treated with HMG-CoA reductase inhibitors should be advised to promptly report any unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever; therapy should be discontinued if creatine kinase is markedly elevated in the absence of strenuous exercise or if myopathy is otherwise suspected or diagnosed | with active liver disease or unexplained persistent elevations of serum transaminases exceeding three times the upper limit of normal; in women of childbearing potential not using appropriate contraceptive measures; not with the hepatitis C antivirals gearlever/pibrentasvir; coadministration of potent CYP3A4 inhibitors (e.g., CsA, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole, and HIV protease inhibitors including ritonavir, lopinavir, atazanavir, indinavir, darunavir, etc.) should be avoided | in primary hypercholesterolemia and combined hyperlipidemia, the majority of patients are controlled with atorvastatin 10 mg/d; all patients treated with HMG-CoA reductase inhibitors should be advised to promptly report any unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever, and checked for CK increase |
| Azithromycin [44] | QTc prolongation (keep serum-Mg++ and –K+ high normal); diarrhea; vomiting, abdominal pain, nausea; hepatotoxicity (cases of fulminant hepatitis); headache, anorexia, dizziness, paresthesia, dysgeusia; visual impairment; arthralgia; fatigue; deafness; decreased lymphocyte and eosinophil count; decreased blood bicarbonate | increased exposure of CsA (TAC); increased exposure of P-gp substrates, such as digoxin, colchicines; use antacids with an interval of 2 h; rises in theophylline, astemizole, alfentanil levels not investigated, presumed; post-marketing rhabdomyolysis cases with statins; with cisapride or hydroxychloroquine risks of QTc prolongation | not in severe liver dysfunction; not with ergot derivates; not with QTc prolonging drugs | caution in neurologic or psychiatric disorders; in severe renal impairment (GFR < 10 mL/min) 33% increased systemic exposure |
| Buprenorphine [45] | CNS depression, drowsiness; dependence, discontinue gradually, delayed withdrawal syndrome; respiratory depression and death; liver function tests at regular intervals, transient asymptomatic elevations in hepatic transaminases, case reports of cytolytic hepatitis, hepatic failure, hepatic necrosis, hepatorenal syndrome, hepatic encephalopathy, and death; in many cases, the presence of pre-existing liver enzyme abnormalities, infection with hepatitis B, or hepatitis C virus, concomitant use of other potentially hepatotoxic drugs and ongoing injecting drug use may have a causative or contributory role; orthostatic hypotension; caution in elderly or debilitated patients; caution in patients with head injury, increased intracranial pressure, hypotension, prostatic hypertrophy, or urethral stenosis; sleep-related breathing disorders, including central sleep apnea (CSA) and sleep-related hypoxemia; bronchitis, infection, influenza, pharyngitis, rhinitis; lymphadenopathy; insomnia; agitation, anxiety, depression, hostility, nervousness, paranoia, abnormal thinking, headache, dizziness, hypertonia, migraine, paranesthesia, somnolence, syncope, tremor; mydriasis; palpitations; vasodilatation; cough, dyspnea, yawning; nausea; constipation, abdominal pain, diarrhea, dry mouth, dyspepsia, gastrointestinal disorder, flatulence, tooth disorder, vomiting; hyperhidrosis; arthralgia, back pain, bone pain, muscle spasms, myalgia; dysmenorrhea; drug withdrawal syndrome, pain, asthenia, chest pain, chills, malaise, oedema peripheral, pyrexia | reduce dose with CYP3A4 inhibitors (e.g., protease inhibitors ritonavir, nelfinavir or indinavir, azole antifungals, such as ketoconazole and itraconazole, macrolide antibiotics, amiodarone); CYP3A4 inducers decrease buprenorphine plasma concentrations (e.g., phenobarbital, carbamazepine, phenytoin or rifampicin); CNS depression, drowsiness, sedation, respiratory depression, coma, and death, which may be exacerbated by other centrally acting agents, such as alcohol, tranquilizers, sedatives, hypnotics; buprenorphine and other serotonergic agents, such as MAO inhibitors, SSRIs, SNRIs, or tricyclic antidepressants, may result in serotonin syndrome (mental-status changes, autonomic instability, neuromuscular abnormalities, and/or gastrointestinal symptoms); naltrexone is an opioid antagonist that can block the pharmacological effects of buprenorphine | severe respiratory insufficiency; severe hepatic insufficiency; acute alcoholism or delirium tremens; use with care in chronic obstructive pulmonary disease, asthma, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, pre-existing respiratory depression, or kyphoscoliosis; not in severe hepatic impairment | higher plasma levels in patients with moderate and severe hepatic impairment; caution with dosing in patients with severe renal impairment (creatinine clearance < 30 mL/min); some risks of misuse and abuse include overdose, spread of blood borne viral or localized infections, respiratory depression, and hepatic injury; protect children and non-dependent persons against exposure |
| Carbamazepine [46] | leucopenia, thrombocytopenia, eosinophilia; agranulocytosis and aplastic anemia; oedema, fluid retention, weight increase, hyponatremia and blood osmolarity decreased due to an antidiuretic hormone (ADH)-like effect, leading in rare cases to water intoxication, accompanied by lethargy, vomiting, headache, confusional state, neurological disorders; suicidal ideation and behavior; sometimes fatal cutaneous reactions (higher risk in Asians); hypothyroidism; anticholinergic effects; activation of a latent psychosis and, in elderly patients, of confusion or agitation; ataxia, dizziness, somnolence, hypotension, confusional state, sedation, which may lead to falls, fractures, or other injuries; headache, diplopia; accommodation disorders (e.g., blurred vision), lenticular opacities; vomiting, nausea; dry mouth; urticaria, which may be severe dermatitis allergic; fatigue; YGT increased (due to hepatic enzyme induction), usually not clinically relevant; increased AP | decreased exposure of CSA, TAC, SIR, EVR; increased exposure and ADRs with inhibitors of CYP3A4 or inhibitors of epoxide hydrolase: dextropropoxyphene, danazol, macrolide antibiotics (e.g., erythromycin, clarithromycin), ciprofloxacine; fluoxetine, fluvoxamine, paroxetine, trazodone, vigabatrin; azoles, e.g., itraconazole, ketoconazole, fluconazole, voriconazole, loratadine, olanzapine, isoniazid, protease inhibitors (e.g., ritonavir), acetazolamide, diltiazem, verapamil, amiodarone, cimetidine, omeprazole, grapefruit juice, nicotinamide; dose adjustment and plasma level monitoring with quetiapine, progabide, valproic acid, valnoctamide, valpromide, primidone, brivaracetam; decreased reliability of hormonal contraceptives may be adversely affected and decreased; potent inducer of CYP3A4 and other phase I and phase II enzyme systems, leads to ineffectiveness of hormonal contraceptives; decreased carbamazepine exposure with CYP3A4 inducers oxcarbazepine, phenobarbital, phenytoin, fosphenytoin, primidone, and possibly clonazepam, cisplatin, or doxorubicin, rifampicin, theophylline, aminophylline, isotretinoin, St. John’s wort; carbamazepine may lower the plasma level, diminish, or even abolish, the activity of certain drugs, the dosage of the following drugs may have to be adjusted for clinical requirements of buprenorphine, methadone, paracetamol tramadol, doxycycline, rifabutin, oral anticoagulants (e.g., warfarin, acenocoumarol, rivaroxaban, dabigatran, apixaban and edoxaban), bupropion, citalopram, mianserin, sertraline, trazodone, tricyclic antidepressants (e.g., imipramine, amitriptyline, nortriptyline, clomipramine), aprepitant, clobazam, clonazepam, ethosuximide, lamotrigine, eslicarbazepine, oxcarbazepine, primidone, tiagabine, topiramate, valproic acid, zonisamide, itraconazole, voriconazole, albendazole, imatinib, cyclophosphamide, lapatinib, temsirolimus, clozapine, haloperidol, bromperidol, olanzapine, quetiapine, risperidone, aripiprazole, paliperidone, indinavir, ritonavir, saquinavir, alprazolam, theophylline, calcium channel blockers (dihydropyridine group), e.g., felodipine, digoxin, simvastatin, atorvastatin, lovastatin, cerivastatin, ivabradine, corticosteroids (e.g., prednisolone, dexamethasone), tadalafil, levothyroxine, estrogens and/or progesterones; levetiracetam may increase carbamazepine-induced toxicity; may increase isoniazid-induced hepatotoxicity; lithium may enhance neurotoxicity; metoclopramide or major tranquilizers, e.g., haloperidol, thioridazine, may increase neurological ADRs; with some diuretics (hydrochlorothiazide, furosemide), a risk of symptomatic hyponatremia; may antagonize the effects of non-depolarizing muscle relaxants (e.g., pancuronium); abstain from alcohol for reduced tolerance; reduced plasma concentrations of DOACs, with risks of thrombosis (rivaroxaban, dabigatran, apixaban, and edoxaban), to avoid phenytoin intoxication and subtherapeutic concentrations of carbamazepine, it is recommended to adjust the plasma concentration of phenytoin to 13 micrograms/mL before adding carbamazepine to the treatment | not in patients with atrioventricular block, a history of bone marrow depression, or a history of hepatic porphyrias (e.g., acute intermittent porphyria, variegate porphyria, porphyria cutanea tarda); not with MAOIs, should be discontinued for a minimum of 2 weeks before starting carbamazepine | only after a critical benefit-risk appraisal and under close monitoring in patients with a history of cardiac, hepatic, or renal damage, adverse hematological reactions to other drugs, or interrupted courses of therapy with carbamazepine; withdraw immediately in cases of aggravated liver dysfunction or acute liver disease; carbamazepine withdrawal should be gradual |
| Cidofovir [47] | dose-dependent nephrotoxicity is the major dose-limiting toxicity; neutropenia; potentially carcinogen; increased risk of developing ocular hypotony with diabetes mellitus; embryotoxic; proteinuria, fever, death (AIDS complications), infection, dyspnea, pneumonia, asthenia, and nausea with vomiting; alopecia | interactions of cidofovir, probenecid, and anti-HIV drugs, including anti-HIV protease inhibitors, have not been investigated in clinical trials, may reduce renal clearance | considered contraindicated with CsA, TAC, SIR, EVR, and other nephrotoxic drugs, because of severely enhanced nephrotoxicity; not in patients with renal impairment [serum creatinine > 133 μmol/L (>1.5 mg/dL) or creatinine clearance ≤ 0.92 mL/s (≤55 mL/min) or proteinuria ≥ 100 mg/dL (≥2+ proteinuria)]; not with aminoglycosides, amphotericin B, foscarnet, intravenous pentamidine and vancomycin; direct intraocular injection is contraindicated; safety and efficacy have not been established in patients with hepatic disease | renal function (serum creatinine and urine protein) must be monitored prior to each dose of cidofovir; hydration to minimize the potential for nephrotoxicity; women of childbearing potential should be advised to use effective contraception during and after treatment; men should be advised to practice barrier contraceptive methods during and for 3 months after treatment with cidofovir |
| Ciprofloxacin [48] | triggers seizures up to status epilepticus; vision disorders; depression or psychosis can progress to suicide; nausea, diarrhea, vomiting, transient increase in transaminases, rash; potentially irreversible sensory or sensorimotor polyneuropathy, resulting in paranesthesia, hypoesthesia, dysesthesia, or weakness; QTc prolongation cave Class IA and III anti-arrhythmics, tricyclic antidepressants, macrolides, antipsychotics, hypokalemia, hypomagnesemia, elderly, cardiac disease; hypoglycemia in diabetic patients; crystalluria; very rare cases of prolonged (continuing months or years), disabling, and potentially irreversible serious adverse drug reactions, affecting different body systems (musculoskeletal, nervous, psychiatric, and sensory); tendinitis and tendon rupture (especially but not limited to Achilles tendon), sometimes bilateral, may occur as early as within 48 h of starting treatment with quinolones and fluoroquinolones, and have been reported to occur even up to several months after discontinuation of treatment; the risk of tendinitis and tendon rupture is increased in older patients, patients with renal impairment, patients with solid organ transplants, and those treated concurrently with corticosteroids, concomitant use of corticosteroids should be avoided; photosensitivity reactions, avoid direct exposure to either extensive sunlight or UV irradiation during treatment; increased risk of aortic aneurysm and dissection | transient rise in serum creatinine with CsA; ciprofloxacin inhibits CYP1A2 substrates, e.g., theophylline (determination of serum concentration) caffeine or pentoxifylline (oxpentifylline), clozapine, olanzapine, ropinirole, tizanidine, duloxetine, sildenafil, monitor closely for signs of overdose; probenecid reduces renal ciprofloxacin secretion; reduced or increased phenytoin levels; augments vitamin K antagonist effect; increased risk of nephrotoxicity and neurotoxicity with CsA and TAC; peroral hours apart from Ca++, Mg++, Fe++, Zn++, Se++ | contraindicated with tizanidine; not with agomelatine, zolpidem; discontinue in symptoms of hepatic diseases; inhibits the renal tubular transport of methotrexate with increased risks of methotrexate toxicity; careful risk-benefit assessment in positive family history of aneurysm disease, or in patients diagnosed with pre-existing aortic aneurysm and/or aortic dissection, or in the presence of other risk factors or conditions predisposing for aortic aneurysm and dissection (e.g., Marfan syndrome, vascular Ehlers-Danlos syndrome, Takayasu arteritis, giant cell arteritis, Behcet’s disease, hypertension, known atherosclerosis) | dose adjustment with impaired renal function; monotherapy not suited for the treatment of severe infections and infections that might be due to Gram-positive or anaerobic pathogens; local prevalence of resistance in Escherichia coli to fluoroquinolones; caution in patients with myasthenia gravis |
| Citalopram [49] | paradoxical anxiety; risk of suicide; sleep disorders; agitation, anxiety, nervousness, confusional state, abnormal dreams, anorexia, apathy, somnolence, insomnia, headache, Tremor, paranesthesia, dizziness, disturbance in attention, migraine, amnesia, syncope; asthenia; abnormal accommodation; tinnitus; hemorrhage, e.g., SSRIs/SNRIs may increase the risk of postpartum hemorrhage, caution is advised in patients taking SSRIs, particularly in concomitant use with drugs known to affect platelet function, e.g., atypical antipsychotics and phenothiazines, most tricyclic antidepressants, ASA and NSAIDs, or other active substances that can increase the risk of hemorrhage, as well as in patients with a history of bleeding disorders; hyponatremia (SIADH); akathisia, restlessness (increasing dose may be detrimental); mania; seizures; alters glycemic control in diabetes mellitus; mydriasis, glaucoma; serotonin syndrome; sexual dysfunction with SSRIs and SNRIs; increase of psychotic symptoms; dose-dependent QTc prolongation (predominantly in female gender, with hypokalemia, or with pre-existing QTc prolongation, or other cardiac diseases), caution advised in bradycardia or in patients with recent acute myocardial infarction or uncompensated heart failure, electrolyte disturbances, such as hypokalemia and hypomagnesemia, increase the risk of malignant arrhythmias and should be corrected before treatment with citalopram is started; yawning, rhinitis; myalgia, arthralgia; urinary retention | hemorrhage with anticoagulants, ASA, NDAIDs, DOACs, clopidogrel, prasugrel, ticagrelor, dipyridamole, ticlopidine, atypical antipsychotics, phenothiazines, and tricyclic depressants; not advisable with alcohol; caution with drugs that cause hypokalemia/hypomagnesemia or any other QTc interval prolonging with increased risks of malignant arrhythmias; SSRIs can lower the seizure threshold cumulative with, e.g., antidepressants (tricyclics, SSRIs, SNRIs), neuroleptics (phenothiazines, thioxanthenes, and butyrophenones), mefloquine, bupropion, and tramadol; with CYP2C19 inhibitors omeprazole, esomeprazole, fluconazole, fluvoxamine, lansoprazole, ticlopidine, or cimetidine, moderate increases are likely; escitalopram (the active enantiomer of citalopram) is an inhibitor of the enzyme CYP2D6, caution when citalopram is coadministered with medicinal products that are mainly metabolized by CYP2D6, and that have a narrow therapeutic index, e.g., flecainide, propafenone, and metoprolol (when used in cardiac failure) or some CNS-acting medicinal products that are mainly metabolized by CYP2D6, e.g., antidepressants such as desipramine, clomipramine, and nortriptyline, or antipsychotics like risperidone, thioridazine, and haloperidol, dosage adjustment may be warranted; dose reduction may be needed in cases of increased desipramine | not with DOACs; not with and not within 2 weeks of monoamine oxidase inhibitors (MAOIs, selegeline, linezolid, moclobemide), lithium, risk of serotonin syndrome; not in patients with known QTc intervals or with medicinal products that are known to prolong the QTc interval; not with linezolid; not with pimozide; should be avoided in patients with unstable epilepsy, patients with controlled epilepsy should be carefully monitored; not with medicinal products with serotonergic effects, such as triptans (including sumatriptan and oxitriptan), opioids (including tramadol and buprenorphine) and tryptophan, due to risk of serotonin syndrome; not with St. John’s wort; citalopram with medicinal products that prolong the QTc interval, such as Class IA and III antiarrhythmics, antipsychotics (e.g., phenothiazine derivatives, pimozide, haloperidol), tricyclic antidepressants, certain antimicrobial agents (e.g., sparfloxacin, moxifloxacin, erythromycin IV, pentamidine, anti-malarial treatment particularly halofantrine), and certain antihistamines (astemizole, mizolastine), is contraindicated | risk of suicide may increase in the early stages of recovery; other psychiatric conditions for which citalopram is prescribed can also be associated with an increased risk of suicide-related events; increased risk of suicidal behavior in patients less than 25 years old; caution in the treatment of elderly patients; caution with reduced kidney and liver function; discontinue gradually; ECG monitoring may be advisable in case of overdose or conditions of altered metabolism with increased peak levels, e.g., liver impairment; if signs of cardiac arrhythmia occur during treatment with citalopram, the treatment should be withdrawn, and ECG control is needed |
| Clarithromycin [50] | QTc prolongation; risk of adverse cardiac outcomes; hepatic dysfunction (discontinue in severe course); rhabdomyolysis with statins; statin that is not dependent on CYP3A metabolism (e.g., fluvastatin, pravastatin) can be considered; abdominal pain, diarrhea, nausea, vomiting, and taste perversion; vasodilation; insomnia; dysgeusia, headache | dose reduction required for carbamazepine, alprazolam, cilostazol, CsA, TAC, SIR, EVR (EVR should not be combined) disopyramide, ibrutinib, methadone, methylprednisolone, midazolam, omeprazole, oral anticoagulants, atypical antipsychotics (e.g., quetiapine), quinidine, rifabutin, sildenafil, triazolam and vinblastine, phenytoin, theophylline, and valproate; risk of serious hemorrhage with DOACs and warfarin; caution drugs with QTc prolongation other than the contraindicated, e.g., torsades de pointes with increased quinidine and disopyramide; hypoglycemia with disopyramide; hydroxychloroquine or chloroquine can increase the risk of fatal cardiovascular events; oral hypoglycemics such as nateglinide, repaglinide, and/or insulin can result in significant hypoglycemia; rhabdomyolysis with statins that are not dependent on CYP3A metabolism (e.g., fluvastatin, pravastatin) can be considered; CYP3A4 inducers (rifampicin, phenytoin, carbamazepine, phenobarbital, efavirenz, nevirapine, St. John’s wort) may lead to sub-therapeutic levels of clarithromycin and reduced efficacy, and vice versa, the CYP3A inducers may increase, owing to the inhibition of CYP3A by clarithromycin; exposure decreased by etravirine; only slight increase with fluconazol; ritonavir with concomitant renal impairment require clarithromycin dose reduction; increase in sildenafil, tadalafil, and vardenafil; theophylline, carbamazepine increased; tolterodine increased in poor CYP2D6 metabolizers; zidovudine to 4 h intervals between each medication; bidirectional drug interactions with increases in clarithromycin and calcium channel blockers, atazanavir, saquinavir, itraconazole; possible failure of oral contraceptives | not with ergotamine or dihydroergotamine, midazolam; not with astemizole, cisapride, domperidone, pimozide, terfenadine and ivabradine because QTc prolongation and cardiac arrhythmias, not be given to patients with a history of QTc prolongation; not ticagrelor, ranolazine, lomitapide, lovastatin, or simvastatin; not with colchicine; not with hypokalemia or hypomagnesemia; not with severe hepatic failure in combination with renal impairment; combination with EVR should be avoided | in patients with renal impairment who have creatinine clearance less than 30 mL/min, the dosage of clarithromycin should be reduced to one half of the normal recommended dose; caution with impaired hepatic function and with moderate-to-severe renal impairment; with coronary artery disease, severe cardiac insufficiency, conduction disturbances, or clinically relevant bradycardia; sensitivity testing must be performed when prescribing clarithromycin for community-acquired pneumonia |
| Clopidrogel [51] | risk of bleeding with hematoma, epistaxis, bleeding at puncture side, eye bleeding, hematuria; gastrointestinal hemorrhage; diarrhea, abdominal pain, dyspepsia, and hematological ADRs; Thrombotic Thrombocytopenic Purpura (TTP): thrombocytopenia and microangiopathic hemolytic anemia, associated with either neurological findings, renal dysfunction, or fever, acquired hemophilia; bruising | increased bleeding risk with ASA, heparin, glycoprotein IIb/IIIa inhibitors, or non-steroidal anti-inflammatory drugs (NSAIDs), including Cox-2inhibitors, selective serotonin reuptake inhibitors (SSRIs), strong CYP2C19 inducers, or other medicinal products associated with bleeding risk, such as pentoxifylline; the concomitant administration with DOACS increases the intensity of bleedings; reduced clopidogrel effect and risk of thrombosis with strong or moderate CYP2C19 inhibitors including, e.g., omeprazole, esomeprazole, fluvoxamine, fluoxetine, moclobemide, voriconazole, fluconazole, ticlopidine, carbamazepine, and efavirenz; with inducers of CYP2C19, e.g., rifampicin, potentiates the risk of bleeding; less pronounced reductions of metabolite exposure has been observed with pantoprazole or lansoprazole compared to omeprazole; significantly reduced platelet inhibition in HIV patients treated with ritonavir or cobicistat-boosted anti-retroviral therapy (similar with nirmatrelvir/ritonavir); increase in repaglinide, paclitaxel exposure; increase in rosuvastatin exposure | not with strong or moderate CYP2C19 inhibitors; not in severe hepatic impairment; not in active pathological bleeding, such as peptic ulcer or intracranial hemorrhage; not with omeprazole; 600 mg loading dose is not recommended in patients with non-ST segment elevation acute coronary syndrome and ≥75 years of age, due to increased bleeding risks in this population | in poor CYP2C19 metabolizers (tests available), clopidogrel at recommended doses forms less of the active metabolite of clopidogrel and has a smaller effect on platelet function; increased bleeding risk from trauma, surgery, or other pathological conditions, if possible, discontinue 7 days prior to surgery; caution in patients who have lesions with a propensity to bleed (particularly gastrointestinal and intraocular); there are no data to support the use of dual antiplatelet therapy in patients for whom treatment with carotid endarterectomy or intravascular thrombectomy is indicated, or in patients planned for thrombolysis or anticoagulant therapy, dual antiplatelet therapy is not recommended in these situations |
| Corticosteroids Prednisolone [52] | a wide range of psychiatric reactions, including affective disorders (such as irritable, euphoric, depressed and labile mood, and suicidal thoughts), psychotic reactions (including mania, delusions, hallucinations, and aggravation of schizophrenia), behavioural disturbances, irritability, anxiety, sleep disturbances, and cognitive dysfunction, including confusion and amnesia; precaution in hypertension, congestive heart failure, and hepatic disease; chickenpox, measles; cataract; Cushing’s disease; osteoporosis, diabetes, hypertension, hypokalemia, susceptibility to infection, and thinning of the skin; adrenocortical insufficiency; renal insufficiency; epilepsy, and/or seizure disorders; dyspepsia, nausea, peptic ulceration with perforation and hemorrhage, abdominal distension, abdominal pain, diarrhea, esophageal ulceration, acute pancreatitis; hirsutism, skin atrophy, bruising, striae, telangiectasia, acne, increased sweating, pruritus, rash, urticaria; proximal myopathy, osteoporosis, vertebral and long bone fractures, avascular osteonecrosis, tendon rupture, tendinopathies (particularly of the Achilles and patellar tendons), myalgia, growth suppression in infancy, childhood, and adolescence; caution in patients with myasthenia gravis receiving anticholinesterase therapy; increased blood coagulability, thromboembolism; scleroderma renal crisis; leukocytosis; sodium and water retention, hypokalemic alkalosis, potassium loss, negative nitrogen and calcium balance, protein catabolism; increase in both high and low density lipoprotein cholesterol, increased appetite, weight gain, obesity, hyperglycemia; congestive heart failure in susceptible patients, hypertension, increased risk of heart failure, increased risk of cardiovascular disease, including myocardial infarction, bradycardia; menstrual irregularity, amenorrhea; fatigue, malaise, impaired healing; increased intra-ocular pressure | risk of seizures in patients receiving CsA with high-dose methylprednisolone; altered blood levels of CsA, TAC with prednisolone (we find decreased trough levels); reduced effect with rifampicin; can lower plasma levels of isoniazid; response to anticoagulants may be reduced or, less often, enhanced by corticosteroids; concurrent insulin and/or oral hypoglycemic agents may require increased dosage; carbamazepine, phenobarbital, phenytoin, and primidone reduce corticosteroid effect; risk of hypokalemia may be increased with amphotericin, combination should be avoided; ketoconazole inhibits metabolism of methylprednisolone and possibly other corticosteroids; with another antimuscarinic drug risk of impairment to memory and attention in the elderly; carbimazole and thiamazole decrease the effect; increased with antiviral drugs, such as ritonavir and indinavir; CsA may increase effects; increased risk of hematological toxicity with methotrexate; CYP3A inducers, such as phenobarbital, phenytoin, rifampicin, rifabutin, carbamazepine, primidone, and aminoglutethimide may reduce the therapeutic efficacy of corticosteroids by increasing the rate of metabolism, lack of expected response may be observed and dosage of deltacortril gastro-resistant tablets may need to be increased; CYP3A4 inhibitors (e.g., ketoconazole, troleandomycin) may increase glucocorticoid effect; oral contraceptives increased prednisolone concentrations by 131%; increased exposure of contraceptives containing ethinylestradiol, mestranol, desogestrel, levonorgestrel, norgestrel, or norethisterone; increased risk of GI ulceration with concomitant ulcerogenic drugs, such as indomethacin; salicylates and corticosteroids should be used concurrently with caution, reduced effect of salicylate and increased at withdrawal; estrogens may potentiate the effects of glucocorticoid; ritonavir possibly increases plasma concentrations of prednisolone and other corticosteroids; the desired effects of hypoglycemic agents (including insulin), antihypertensives, and diuretics are antagonized by corticosteroids; and the hypokalemic effect of acetazolamide, loop diuretics, thiazide diuretics, carbenoxolone, and theophylline are enhanced; increased risk of hypokalemia, if high doses of corticosteroids are given with high doses of bambuterol, fenoterol, formoterol, ritodrine, salbutamol, salmeterol, and terbutaline | not with ocular herpes simplex, because of possible perforation; not with systemic infection, unless specific anti-infective therapy is employed; no live vaccines with high dose corticosteroids | incidence of predictable undesirable effects, including hypothalamic-pituitary adrenal suppression correlates with the relative potency of the drug, dosage, timing of administration, and the duration of treatment; enteric coated form should be used with caution in any condition characterized by diarrhea or a rapid transit time; in patients with acute and active hepatitis, protein binding of the glucocorticoids will be reduced and peak concentrations of administered glucocorticoids increased, elimination of prednisolone will also be impaired, enhanced effect of corticosteroids in patients with cirrhosis; effect of corticosteroids may be reduced for 3–4 days after mifepristone; may suppress reactions to skin tests |
| Cyclosporine A (CsA) [1] | due to its nephrotoxic potential, the careful monitoring of renal function is recommended; experience in the elderly is limited, more likely to develop systolic hypertension during therapy, and more likely to show serum creatinine rises ≥ 50% above the baseline after 3 to 4 months of therapy; increases the risk of developing lymphomas and other malignancies, particularly those of the skin, enhanced with treatment regimen containing multiple immunosuppressants, has to avoid excess unprotected sun exposure and should not receive concomitant ultraviolet B irradiation or PUVA photochemotherapy; predisposes patients to the development of a variety of bacterial, fungal, parasitic, and viral infections, often with opportunistic pathogens, the activation of latent polyomavirus infections which may lead to polyomavirus-associated nephropathy (PVAN), especially to BK virus nephropathy (BKVN), or to JC virus-associated progressive multifocal leukoencephalopathy (PML), to be considered in the differential diagnosis in immunosuppressed patients with deteriorating renal function or neurological symptoms, serious and/or fatal outcomes have been reported; frequent and potentially serious increases in serum creatinine and urea, these functional changes are dose-dependent and are initially reversible, usually responding to dose reduction, during long-term treatment, some patients may develop structural changes in the kidney (e.g., interstitial fibrosis), which, in renal transplant patients, must be differentiated from changes due to chronic rejection, the frequent monitoring of renal function, esp. in the elderly, is therefore required; hepatotoxicity and liver injury, including cholestasis, jaundice, hepatitis, and liver failure, necessitates dose reduction; hypertension requires antihypertensives, preferentially those without DDIs; hyperlipidemia; hyperkalemia; serum Mg++ measures for risks of hypomagnesemia; tremor, headache, convulsions, paresthesia; hirsutism; diarrhea, anorexia, nausea, vomiting, abdominal discomfort/pain, diarrhea, gingival hyperplasia, peptic ulcer; leucopenia; hyperlipidemia; hepatic function abnormal; hirsutism, acne, hypertrichosis; myalgia, muscle cramps; pyrexia, fatigue; hearing impairment with high doses; pain of lower extremities has also been noted as part of calcineurin-inhibitor induced pain syndrome (CIPS) | caution when coadministering CsA with drugs that substantially increase or decrease CsA blood concentrations, through the inhibition or induction of CYP3A4 and/or P-gp; all inducers of CYP3A4 and/or P-gp are expected to decrease CsA levels; drugs that decrease CsA levels are metamizole, barbiturates, carbamazepine, oxcarbazepine, phenytoin; nafcillin, intravenous sulfadimidine, probucol, orlistat, St. John’s wort, ticlopidine, sulfinpyrazone, terbinafine, bosentan; rifampicin induces CsA intestinal and liver metabolism, CsA may need to be increased 3- to 5-fold during coadministration; octreotide decreases the oral absorption of CsA and a 50% increase in the CsA dose, or a switch to intravenous administration could be necessary; all inhibitors of CYP3A4 and/or P-gp may lead to increased levels of CsA, such as nicardipine, metoclopramide, oral contraceptives, methylprednisolone (high dose) (we have found decreased CsA through blood levels with steroids), allopurinol, cholic acid and derivatives, protease inhibitors, imatinib, colchicine, nefazodone, macrolide antibiotics, e.g., erythromycin can increase CsA exposure 4- to 7-fold, sometimes resulting in nephrotoxicity, clarithromycin has been reported to double the exposure of CsA, azitromycin increases CsA levels by around 20%, azole antimycotics, e.g., ketoconazole, fluconazole, itraconazole, and voriconazole could more than double CsA exposure, verapamil increases CsA blood concentrations two- to threefold, coadministration with telaprevir resulted in approximately 4.64-fold increase in CsA dose normalized exposure (AUC); amiodarone substantially increases the plasma CsA concentration concurrently with an increase in serum creatinine, this interaction can occur for a long time after withdrawal of amiodarone, due to its very long half-life (about 50 days), danazol has been reported to increase CsA blood concentrations by approximately 50%, diltiazem (at doses of 90 mg/day) can increase CsA plasma concentrations by up to 50%, imatinib could increase CsA exposure by around 20%, cannabidiol (P-gp inhibitor) inhibits intestinal P-gp efflux, leading to the increased bioavailability of calcineurin inhibitor, grapefruit and grapefruit juice increase CsA; exhibit nephrotoxic synergy, such as aminoglycosides (including gentamycin, tobramycin), amphotericin B, ciprofloxacin, vancomycin, trimethoprim (+sulfamethoxazole); fibric acid derivatives (e.g., bezafibrate, fenofibrate); NSAIDs (including diclofenac, naproxen, sulindac), melphalan histamine H2-receptor antagonists (e.g., cimetidine, ranitidine), methotrexate, close monitoring of renal function should be performed, if a significant impairment of renal function occurs, the dosage of the coadministered drug should be reduced, or alternative treatment considered; due to the risk for nephrotoxicity and pharmacokinetic interactions via CYP3A4 and/or P-gp, concomitant use of CsA and TAC should be avoided; CsA is an inhibitor of CYP3A4, the multidrug efflux transporter P-gp and organic anion transporter proteins (OATP); statins increase with risk of rhabdomyolysis; in concomitant administration of CsA and lercanidipine, the AUC of lercanidipine was increased 3-fold and CsA 21%, only administer lercanidipine 3 h before CsA, otherwise avoid combination; pharmacokinetics of CsA may be impacted by changes in liver function during DAA therapy, related to the clearance of HCV virus; CsA may reduce the clearance of digoxin, colchicine, statins (dose reductions), and etoposide; with nifedipine, there is an increased risk of gingival hyperplasia; significant increase in diclofenac bioavailability and risk of renal impairment; CsA significantly increases SIR and EVR exposure; significantly elevated hyperkalemia risk with potassium-sparing diuretics, ACE inhibitors, angiotensin II receptor antagonists, and potassium-containing drugs; increased repaglinide with risks of hypoglycemia; increased ambrisentan exposure; increased anthracycline antibiotics (e.g., doxorubicin, mitoxantrone, daunorubicin); CsA with mycophenolate sodium or mycophenolate mofetil in transplant patients may decrease the mean exposure MPA by 20–50%, when compared with other immunosuppressants, to be considered esp. in the case of the interruption or discontinuation of CsA therapy; decreases eltrombag exposure | decreased CsA with St, John’s Wort; not with substrates for the multidrug efflux transporter P-gp or the organic anion transporter proteins (OATP), and for which elevated plasma concentrations are associated with serious and/or life-threatening events, e.g., bosentan, dabigatran etexilate, and aliskiren; vaccination less effective, the use of live attenuated vaccines should be avoided; in non-transplant patients, the relationship between blood level and clinical effects is less well established, if drugs known to increase CsA levels are given concomitantly, the frequent assessment of renal function and the careful monitoring of CsA-ADRs may be more appropriate than blood level measurement; not with aliskiren; not recommended with dabigatran; not with bosentan, CsA exposure is decreased by 35%, and bosentan exposure increased several-fold; at increased risks of acute or chronic nephrotoxicity, cases of acute nephrotoxicity reported disorders of ion homeostasis, such as hyperkalemia, hypomagnesemia, and hyperuricemia, cases reporting chronic morphological changes included arteriolar hyalinosis, tubular atrophy, and interstitial fibrosis | TDM is an important safety measure; CsA is extensively metabolized by the liver, an approximate 2- to 3-fold increase in CsA exposure may be observed in patients with hepatic impairment, dose reduction may be necessary in patients with severe liver impairment; higher doses of oral CsA or the use of CsA intravenous therapy, may be necessary in the presence of gastrointestinal disturbances, which might decrease absorption; due to its nephrotoxic potential, the careful monitoring of renal function is recommended, adequate follow-up, including regular full physical examination, blood pressure measurements, and control of laboratory safety parameters; transplantation patients on CsA should be managed in facilities with adequate laboratory and supportive medical resources; the physician responsible for maintenance therapy should receive complete information for the follow-up of the patient |
| Empaglifozin [53] | ketoacidosis (nausea, vomiting, anorexia, abdominal pain, excessive thirst, difficulty breathing, confusion, unusual fatigue, or sleepiness); risk for volume depletion by osmotic diureses with a decrease in blood pressure, therefore caution in patients with known cardiovascular disease, patients on antihypertensive therapy with a history of hypotension, or patients aged 75 years and older, in case of conditions that may lead to fluid loss (e.g., gastrointestinal illness), with coadministered drugs, leading to volume depletion (e.g., diuretics, ACE inhibitors); thirst; complicated urinary tract infections, including pyelonephritis and urosepsis; vaginal moniliasis, vulvovaginitis, balanitis, and other genital infection; necrotizing fasciitis of the perineum, (Fournier’s gangrene), advise patients for early symptoms, awareness that either urogenital infection or perineal abscess may precede necrotizing fasciitis; increase in cases of lower limb amputation (primarily of the toe); hepatic injury; positive glucose test in urine according to the drug’s mechanism of action; hypoglycemia when used with sulphonylurea or insulin; constipation; increased serum lipids | adds to the diuretic effect of thiazide and loop diuretics, and may increase the risk of dehydration and hypotension; insulin and insulin secretagogues, such as sulphonylureas, may increase the risk of hypoglycemia, therefore, a lower dose of insulin or an insulin secretagogue may be required; co-treatment with known UTG inducers (e.g., by rifampicin or phenytoin) not recommended, due to a potential risk of decreased efficacy; may increase renal lithium excretion | should not be used in patients with type 1 diabetes, increased ketoacidosis occurrence with empagliflozin 10 mg and 25 mg as an adjunct to insulin; not recommended with eGFR < 20 mL/min/1.73 m2 due to limited experience | monitor renal function, dose reduction with eGFR < 60 mL/min/1.73 m2; interrupt SGLT2 inhibitors in patients who are hospitalized for major surgical procedures or acute serious medical illnesses; higher risk of ketoacidosis with beta-cell function reserve (e.g., type 2 diabetes patients with low C-peptide or latent autoimmune diabetes in adults (LADA) or with a history of pancreatitis), with conditions that lead to restricted food intake or severe dehydration, patients for whom insulin doses are reduced, and patients with increased insulin requirements due to acute medical illness, surgery, or alcohol abuse; the glucose lowering efficacy of empagliflozin is dependent on renal function, reduced in patients with an eGFR < 45 mL/min/1.73 m2 and is likely absent in patients with an eGFR < 30 mL/min/1.73 m2 |
| Everolimus (EVR) [8] | stomatitis, including mouth ulcerations and oral mucositis; hypertriglyceridemia, hypercholesterolemia, hyperglycemia; hypophosphatemia, hypokalemia; hypocalcemia; dehydration; opportunistic infections (e.g., pneumocystis jirovecii (carinii) pneumonia (PJP/PCP), thrombocytopenia, neutropenia, leukopenia, lymphopenia, anemia; non-infectious pneumonitis; localized and systemic infections, including pneumonia, other bacterial infections, invasive fungal infections, such as aspergillosis, candidiasis, or PJP/PCP, and viral infections, including the reactivation of hepatitis B virus with risk of sepsis or respiratory and hepatic failure; proteinuria, renal failure; insomnia, dysgeusia, headache; eyelid oedema; hemorrhage, hypertension, lymphoedema; pneumonitis, epistaxis, cough, dyspnea; diarrhea, nausea, vomiting, dyspepsia, dysphagia; increased AST, ALT; rash, pruritus, dry skin, skin lesions; arthralgia; menstruation irregular; fatigue, asthenia, oedema peripheral; pyrexia; decreased weight; impaired wound healing, caution in the peri-surgical period; severe radiation reactions (such as radiation esophagitis, radiation pneumonitis, and radiation skin injury), including fatal cases, during or shortly after radiation therapy, the potentiation of radiotherapy toxicity in close temporal relationship with radiation therapy and radiation recall syndrome (RRS) | large increased exposure with coadministered strong CYP3A4 and/or Pgp inhibitors and thus not recommended (ketoconazole, itraconazole, posaconazole, voriconazole, telithromycin, clarithromycin, nefazodone, ritonavir, atazanavir, saquinavir, darunavir, indinavir, nelfinavir), caution and decrease dosage with moderate CYP3A4/Pgp inhibitors (erythromycin, imatinib, verapamil, CsA p.o., cannabidiol, fluconazole, diltiazem, dronedarone, amiodarone, amprenavir, fosamprenavir, grapefruit juice); decreased exposure and not recommended with inducers of CYP3A4 and/or Pgp (rifampicin, carbamazepine, phenobarbital, phenytoin, dexamethasone, efavirenz, nevirapine, St. John’s Wort) should be avoided, if unavoidable, increase the everolimus dosage with monitoring, and respect the washout phase of at least 3–5 days of the inducer’s effect; prophylaxis for PJP/PCP to be considered when concomitant use of corticosteroids or other immunosuppressive agents are required; with ACE, increased risk for angioedema; caution with orally administered CYP3A4 substrates with a narrow therapeutic index (e.g., pimozide, terfenadine, astemizole, cisapride, quinidine, or ergot alkaloid derivatives), monitor for ADRs | not in severe hepatic impairment (Child-Pugh C), only recommended if the desired benefit outweighs the risk, a dose of 2.5 mg daily must not be exceeded; dose adjustment due to adverse reactions; in invasive systemic fungal infections, discontinue promptly and permanently; stop in radiation recall syndrome (RRS) | dose reduction with mild and moderate hepatic impairment; ADRs leading to the discontinuation of the medicinal product were higher in patients ≥ 65 years, most common ADRs leading to discontinuation were pneumonitis (including interstitial lung disease), stomatitis, fatigue, and dyspnea; live vaccines (intranasal influenza, measles, mumps, rubella, oral polio, BCG (Bacillus Calmette–Guérin), yellow fever, varicella, and TY21a typhoid vaccines) should be avoided |
| Febuxostat [54] | liver function abnormalities, liver function test is recommended prior to the initiation and periodically thereafter; increased TSH values; gout flares, diarrhea, nausea; headache, rash, oedema; atrial fibrillation, palpitations, ECG abnormal; renal failure | concomitant mercaptopurine or azathioprine not recommended; coadministration with rosiglitazone or other CYP2C8 substrates is not expected to require dose adjustment for those compounds; febuxostat metabolism depends on uridine glucuronosyl transferase (UGT) enzymes, medicinal products that inhibit glucuronidation, such as NSAIDs and probenecid, may increase febuxostat; potent inducers of UGT enzymes might increase metabolism and decrease the efficacy of febuxostat; febuxostat is a weak inhibitor of CYP2D6, a small increase in AUC of desipramine, a CYP2D6 substrate | in patients with ischemic heart disease or congestive heart failure, not recommended; not with mercaptopurine/azathioprine; no experience in organ transplant recipients, the use in such patients is not recommended | |
| Felodipine [55] | significant hypotension with subsequent tachycardia; peripheral oedema; headache; flush; the renal vascular resistance is decreased by felodipine; in CsA-treated renal transplant recipients, felodipine improves both the renal blood flow and the glomerular filtration rate, and may also improve early renal graft function | AUC increased sixfold when coadministered with the strong CYP3A4 inhibitor itraconazole, with erythromycin AUC increasing 2.5-fold; felodipine dose reduction required in coadministered CYP3A4 inhibitors; CsA may significantly increase serum felodipine concentrations and vice versa via the inhibition of CYP3A4 first pass metabolism, amlodipine undergoes less first-pass metabolism, to be considered as an alternative; TAC and SIR expose increased | not in decompensated heart failure, unstable angina pectoris, hemodynamically significant cardiac valvular obstruction, dynamic cardiac outflow obstruction | dose reduction in impaired hepatic function; strong CYP3A4 inhibitors and grapefruit juice should be avoided; plasma protein (albumin) binding is approximately 99% |
| Fentanyl [56] | life-threatening respiratory depression; potential for serious or life-threatening hypoventilation exists, even if the lowest dose is used in initiating therapy in opioid-naïve patients, especially in elderly or patients with hepatic or renal impairment; fentanyl patches may have more severe adverse effects in patients with chronic obstructive or other pulmonary disease, in such patients, opioids may decrease respiratory drive and increase airway resistance; if withdrawal symptoms are experienced, do not discontinue abruptly; tolerance, physical, and psychological dependence; repeated use of fentanyl may lead to opioid use disorder (OUD with drug seeking behavior); increased skin temperature, potential for temperature-dependent increases in fentanyl released from the system, resulting in possible overdoses and death, patients to be advised to avoid exposing the application site of fentanyl patches to direct external heat sources, such as heating pads, electric blankets, heated water beds, heat or tanning lamps, sunbathing, hot water bottles, prolonged hot baths, saunas, and hot whirlpool spa baths; bradycardia; hypotension; constipation (prophylactic laxative be considered); caution in myasthenia gravis; opioid-induced hyperalgesia (OIH); anorexia; insomnia, depression, anxiety, confusional state, hallucination; somnolence, dizziness, headache, tremor, paresthesia; vertigo, tachycardia, palpitations, bradycardia; dry mouth, nausea, vomiting, abdominal pain, hyperhidrosis, muscle spasms, urinary retention, fatigue, peripheral oedema, asthenia, malaise, feeling cool | caution with coadministered drugs that affect the serotonergic neurotransmitter systems (SSRIs, SNRIs, MAOIs), risk of potentially life-threatening serotonin syndrome; with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death; not recommended with CYP3A4 inhibitors (amiodarone, cimetidine, clarithromycin, diltiazem, erythromycin, fluconazole, itraconazole, ketoconazole, nefazodone, ritonavir, verapamil, and voriconazole—this list is not exhaustive, e.g., to a lesser extend also CsA), they increase fentanyl plasma concentrations, generally, a patient should wait 2 days after stopping treatment with a CYP3A4 inhibitor before applying the first fentanyl patch; however, the duration of inhibition varies, and for some CYP3A4 inhibitors with a long elimination half-life, such as amiodarone, or for time-dependent inhibitors, such as erythromycin, idelalisib, nicardipine, and ritonavir, this period may need to be longer, a patient who is treated with fentanyl patches should wait at least 1 week after the removal of the last patch before initiating treatment with a CYP3A4 inhibitor; not with concomitant buprenorphine, nalbuphine, or pentazocine; with CYP3A4 inducers, decrease in fentanyl plasma concentrations and a decreased therapeutic effect (by carbamazepine, phenobarbital, phenytoin and rifampicin—list not exhaustive); effects of the inducer decline gradually and may result in increased fentanyl plasma concentrations | not with MAOIs or within 14 days of such therapy; suspected surgical abdomen; acute or perioperative pain; opioid-I patients; respiratory depression; acute or severe bronchial asthma; mechanical gastrointestinal obstruction, ileus of any type; acute alcoholism, delirium tremens, and convulsive disorders; increased cerebrospinal or intracranial pressure, and head injury; alcohol serious additive injury, potentially death; fentanyl patch should only be prescribed to patients who require continuous opioids for pain management, and who are tolerant to at least the morphine equivalent of the lowest initiating fentanyl patch dose; dose reduction in hepatic impairment because of the risk of toxicity; caution in renal impairment | in elderly, cachectic, or debilitated patients, fentanyl transdermal system may have altered pharmacokinetics due to poor fat stores, muscle wasting, or altered clearance, initiate on a low dose; risks of addiction, abuse, and misuse; only to be used in patients for whom alternative treatment options are ineffective or not tolerated (e.g., non-opioid analgesics), or would be otherwise inadequate to provide sufficient management of pain (e.g., immediate-release opioids); accidental exposure, such as in cases of hugging, sharing a bed, or moving a patient, esp. in children, can lead to serious medical consequences, including death; patients and their carers must be advised to keep fentanyl in a safe and secure place, not accessible by others; elderly patients may have reduced clearance, a prolonged half-life, and they may be more sensitive to the active substance |
| Fluconazole [57,58] | may affect liver; headache; stomach discomfort, feeling sick, vomiting; increases in blood tests of liver function; QTc prolongation, strongly inhibits the HERG channel, potentially proarrhythmic conditions, such as congenital or documented acquired QTc prolongation, cardiomyopathy, particularly when heart failure is present, sinus bradycardia, existing symptomatic arrhythmias, concomitant medication known to prolong QTc interval, hypokalemia, hypomagnesemia, and hypocalcemia should be corrected prior to the initiation of fluconazole treatment; adrenal insufficiency with chronic or long lasting fatigue, muscle weakness, loss of appetite, weight loss, abdominal pain, patients receiving long-term therapy with fluconazole and prednisone should be closely monitored for signs of adrenal insufficiency when fluconazole is withdrawn | increase in CsA, TAC (5-fold if peroral, nephrotoxicity increased risk), SIR, EVR, needs dose reduction; caution with loperamide and QTc prolongation; caution because of inhibited metabolizm and QTc prolongation with amidodarone; rifampicin and rifabutin reduce fluconazole bioavalability and require fluconazole dose elevation; increased abrocitinib, olaparib, ibrutinib, ivacaftor, lurasidone, methadone, naproxen, lornoxicam, meloxicam, diclofenac, phenytoin, rifabutin exposure, dose reduction required; increased alfentanil, fentanyl (can lead to respiratory depression), amitriptyline, nortriptyline, theopfylline, trimetrexate, zidovodine, dose reduction required; amphotericin B in therapy likely antagonizes systemic Aspergillus fumigatus; enhanced anticoagulative effects of coumarin or indandione drugs; elevated midazolam and triazolam exposures require dose reduction; risk of carbamazepin toxicity should lead to extensive monitoring; elevated exposure of nifedipine, isradipine, amlodipine, verapamil, lercanidipine, felodipine, celecoxib; with cyclophosphamide, increased bilirubin and crea tinin serum concentrations; risk of rhabdomyolysis with atorvastatin, simvastatin, fluvastatin; decreased activation of losartan; prolongs the plasma half-life of concomitantly administered sulphonyl urea (chlorpropamide, glibenclamide, glipizide, and tolbutamide) with risk of hypoglycema, voriconazole warfarin, or similar medicines, carbamazepine, phenytoin, nifedipine, isradipine, amlodipine, felodipine, and losartan, CsA, TAC, SIR, EVR, cyclophosphamide, vinca alkaloids (vincristine, vinblastine, or similar medicines), halofantrine, statins (atorvastatin, simvastatin, and fluvastatin), methadone, celecoxib, flurbiprofen, naproxen, ibuprofen, lornoxicam, meloxicam, diclofenac, oral contraceptives, prednisone, zidovudine, saquinavir, chlorpropamide, glibenclamide, glipizide, or tolbutamide, theophylline, vitamin A, ivacaftor, amiodarone, hydrochlorothiazide; risk of adrenal insufficiency in combination with prednisone | caution in liver impairment; not with drugs that prolong QTc interval and are metabolized via CYP3A4, such as astemizole, terfenadine, cisapride, pimozide, quinidine, erythromycin, halofantrine | dose adjustment to renal function; fluconazole is a moderate CYP2C9 and CYP3A4 inhibitor, and a strong inhibitor of CYP2C19 |
| Fluvastatin [59] | blood creatine phosphokinase increased, blood transaminases increased; hepatic failure; increase in AST or ALT three times the upper limit of normal and persisting, therapy should be discontinued; insomnia; headache; nausea, abdominal pain, dyspepsia; pancreatitis; myalgia, muscular weakness, myopathy; sleep disturbances, insomnia, nightmares; memory loss; depression; interstitial lung disease; diabetes mellitus; interstitial lung disease; hyperglycemia; tendinopathy; very rarely immune-mediated necrotizing myopathy (IMNM); caution in patients with predisposing factors for rhabdomyolysis and its complications; creatine kinase level before starting treatment in the following situations (predisposing factors for rhabdomyolysis): renal impairment, hypothyroidism, personal or familial history of hereditary muscular disorders, previous history of muscular toxicity with a statin or fibrate, alcohol abuse, sepsis, hypotension, excessive exercise of muscles, major surgery, severe metabolic, endocrine or electrolyte disorders, elderly (age > 70 years) | increased risk of myopathy/rhabdomyolysis with bezafibrate, gemfibrozil, ciprofibrate, or niacin (nicotinic acid), immunosuppressive agents (including CsA), erythromycin, colchicine; for warfarin or other coumarin derivatives, recommendation to monitor prothrombin times; rifampicin reduces exposure; with glibenclamide, increases of both; with bile acid sequestrants, at least 4 h after the resin (e.g., cholestyramine) to avoid a significant interaction due to the drug binding of the resin; may be used with azole antifungal agents, except for fluconazole and voriconazole, both of which can inhibit fluvastatin metabolism via CYP450 2C9; with the potent cytochrome P450 (CYP) 3A4 inhibitors itraconazole and erythromycin, minimal effects on the bioavailability | not in patients with active liver disease, or unexplained, persistent elevations in serum transaminases; not with systemic formulations of fusidic acid or within 7 days of stopping fusidic acid (rhabdomyolysis) | as with other lipid-lowering agents, recommended liver function tests before initiation of treatment and at 12 weeks following initiation of treatment, or elevation in dose and periodically thereafter; cases of hepatic failures with some statins including fluvastatin; caution in history of liver disease and heavy alcohol ingestion; CK not to be measured following strenuous exercise |
| Foscarnet [60] | renal, myelosuppressive, and neurotoxicity (severe seizures); rate of infusion must be no more than 1 mg/kg/min; hydration is recommended with each infusion to reduce renal toxicity; hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, hypokalemia; potential to chelate divalent metal ions, such as calcium and magnesium; seizures (associated with death) related to mineral and electrolyte abnormalities; QTs prolongation; saline load (caution in cardiomyopathy); perioral tingling, numbness in the extremities or paresthesia during or after infusion as possible symptoms of electrolyte abnormalities; potentially carcinogenesis, mutagenesis, impairment of ncreasty, teratogenic effect; fever; nausea; diarrhea; anemia; renal toxicity; seizures; marrow suppression; fever, fatigue, rigors, asthenia, malaise, pain, infection, sepsis, death; depression, confusion, anxiety; vision abnormalities; urine concentrations genital irritations, ulcers | decreases serum concentrations of ionized calcium, concurrent treatment with other drugs known to influence serum calcium concentrations should be used with particular caution; avoid combination with potentially nephrotoxic drugs, such as aminoglycosides, CsA, TAC, amphotericin B, aciclovir, methotrexat, and intravenous pentamidine; renal impairment and symptomatic hypocalcemia (Trousseau’s and Chvostek’ signs) with i.v. pentamidine; abnormal renal function with ritonavir, saquinavir; when diuretics are indicated, thiazides are recommended; avoid QTc prolonging drugs (e.g., quinidine, amiodarone, sotalol)—antiarrhythmic or neuroleptic agents | not with pentamidine i.v.—enhanced hypocalcemia and nephrotoxicity | initial and maintenance therapy with dose adjustment to renal function; monitor renal function every second day during induction therapy, and once weekly during maintenance therapy, adequate hydration, monitor serum calcium and magnesium |
| Ganciclovir, Valganciclovir [61,62] | neutropenia, anemia, thrombocytopenia; infections; complete blood counts and platelet counts should be monitored regularly during therapy, increased hematological monitoring may be warranted in patients with renal impairment and pediatrics; decreased appetite and weight; depression, confusion, anxiety; headache, dizziness, hypo-paresthesia, seizure, dysgeusia; visual impairment; ear pain; hypotension; cough, dyspnea; diarrhea, nausea; vomiting, dyspepsia, flatulence, constipation, dysphagia, pancreatitis; hepato-biliary disorders; dermatitis; night sweats; back pain, myalgia, arthralgia, muscle spasms; renal impairment; pyrexia; fatigue; pain, asthenia; potential teratogen and carcinogen in humans, caution should be observed in handling broken tablets; retinal detachment reported in AIDS patients treated for CMV retinitis | with CsA and TAC enhanced risk of myelotoxicity, nephrotoxicity, and neurotoxicity; with MMF or trimethoprim, enhanced myelosuppressive toxicity; seizures with imipenem-cilastatin; probenecid competition for renal tubular secretion enhances toxicity; increased didanosine toxicity, pancreatitis; renal impairment enhances risk of myelosuppression; toxicity may be enhanced with other drugs known to be myelosuppressive or associated with renal impairment, such as nucleoside (e.g., zidovudine, didanosine, stavudine) and nucleotide analogues (e.g., tenofovir, adefovir), immunosuppressants (e.g., CsA, TAC, MMF), antineoplastic agents (e.g., doxorubicin, vinblastine, vincristine, hydroxyurea), and anti-infective agents (trimethoprim/sulphonamides, dapsone, amphotericin B, flucytosine, pentamidine)—these drugs should only be considered for concomitant use with valganciclovir if the potential benefits outweigh the potential risks | should not be initiated if the absolute neutrophil count is less than 500 cells/μL, or the platelet count is less than 25,000/μL, or the hemoglobin level is less than 8 g/dL; antiviral drugs that share the tubular secretion pathway enhance exposure, e.g., nucleos(t)ide analog reverse transcriptase inhibitors (NRTIs) (including those used for HBV therapy), e.g., lamivudine, emtricitabine, tenofovir, adefovir, and entecavir; renal clearance inhibited, due to nephrotoxicity caused by drugs, such as cidofovir, foscarnet, NRTIs (e.g., tenofovir, adefovir)—concomitant use of valganciclovir with any of these drugs should be considered only if the potential benefits outweigh the potential risks; toxicity may be enhanced with, or is given immediately before or after, other drugs that inhibit the replication of rapidly dividing cell populations, such as in bone marrow, testes, and germinal layers of the skin, and gastrointestinal mucosa, such as dapsone, pentamidine, flucytosine, vincristine, vinblastine, Adriamycin, amphotericin B, trimethoprim/sulfa combinations, nucleoside analogues and hydroxyurea, and pegylated interferons/ribavirin (with or without boceprevir or telaprevir) | dose adjustment to renal function; not in patients on hemodialysis; safety and efficacy of valganciclovir tablets have not been studied in patients with hepatic impairment, not in elderly patients; if there is a significant deterioration of blood cell counts during therapy with valganciclovir, treatment with hematopoietic growth factors and/or dose interruption should be considered; when extending prophylaxis beyond 100 days, the possible risk of developing leucopenia and neutropenia should be taken into account |
| Ibuprofen [63] | dose-dependent reduction in prostaglandin formation and precipitate renal failure—at increased risk with impaired renal function, cardiac impairment, liver dysfunction, those taking diuretics, and the elderly; lasting severe renal impairment, increased under physical strain associated with loss of salt and dehydration; prolonged use of any type of analgesic for headaches can make them worse; medication-overuse headache (MOH); GI bleeding, ulceration or perforation; precipitate bronchospasm, urticaria, or angioedema; caution heart failure or hypertension | with TAC, CsA increased risk of nephrotoxicity; corticosteroids increased risk of gastrointestinal ulceration or bleeding with NSAIDs; may affect or be affected by anti-coagulants ASA, warfarin, ticlopidine, medicines that reduce high blood pressure (ACE-inhibitors, beta-blockers angiotensin-II receptor antagonists); CYP2C9 inhibitors, e.g., voriconazole and fluconazole, increase the ibuprofen exposure; quinolones increased risk of developing convulsions; anti-platelet agents and SSRIs/SNRIs leads to an increased risk of gastrointestinal bleeding with NSAIDs; NSAIDs may decrease the excretion of aminoglycosides; increased risk of hematological toxicity with zidovudine; may potentiate the effects of sulfonylurea medications with hypoglycemia; cardioprotective value of LD-ASA can be compromised in patients who take NSAIDs concomitantly, because some NSAIDs competitively bind to critical amino-acid residues on cyclooxygenase (COX) enzymes and interfere with the mechanism of antiplatelet activity of LD-ASA, important to take ASA hrs in advance to NSADIs; enhanced effects of methotrexate, lithium, probenecid, and sulphinpyrazones; increased risk of gastrointestinal ulcers with bisphosphonates; quinolone antibiotics increased risk for convulsions; zidovudine increased risk of bleeding | not with TAC, CsA; not with DOACs; previous allergic reaction such as asthma, difficulty in breathing, swelling of the face, tongue, or throat nettle rash, itchy runny nose attributed to acetylsalicylic acid (ASA) or other NSAIDs; stomach ulcer or bleeding; gastrointestinal perforation or bleeding when taking NSAIDs; suffering from cerebrovascular or other active bleeding; suffering from unclarified blood-formation disturbances; severe dehydration (caused by vomiting, diarrhea, or insufficient fluid intake); severe liver, kidney, or heart failure | minimum effective dose for the shortest period of time; the elderly are at increased risk of ADRs; combination therapy with protective agents, such as PPIs; NSAIDs may mask symptoms of infection and fever; Ginkgo biloba may potentiate the risk of bleeding with NSAIDs; monitor renal function |
| Lercanidipine [64] | headache, dizziness, peripheral oedema, tachycardia, palpitations, flushing; care in patients with sick sinus syndrome, with LV dysfunction, ischemic heart disease; overdose might be expected to cause excessive peripheral vasodilatation with marked hypotension and reflex tachycardia | potentiates the effect of vasodilating antihypertensive medicines; reduced exposure by inducers of CYP3A4, like anticonvulsants (e.g., phenytoin, carbamazepine) and rifampicin; increased exposure with CYP3A4 (e.g., ketoconazole (15-fold AUC increase), itraconazole, ritonavir, erythromycin, troleandomycin), avoid combination; caution with CYP3A4 substrates, like terfenadine, astemizole, class III antiarrhythmic medicines, such as amiodarone or quinidine; metoprolol reduced lercanidipine bioavailability by 50%, likely due to reduced hepatic blood flow caused by β-blockers; increased digoxin exposure; with simvastatin 12 h apart recommended | not in patients with left ventricular outflow tract obstruction, untreated congestive cardiac failure, unstable angina pectoris, severe renal, or hepatic impairment, within 1 month of a myocardial infarction; not with strong inhibitors of CYP3A4, not with CsA (3-fold increase of the plasma levels of lercanidipine and a 21% increase of the CsA AUC), grapefruit juice; not in patients with severe hepatic impairment or with severe renal impairments (GFR < 30 mL/min) | gradual dose titration, as it may take about 2 weeks before the maximal antihypertensive effect is apparent; antihypertensive effect may be enhanced in patients with hepatic impairment, dose adjustment required |
| Letermovir [65] | nausea, diarrhea, vomiting common; increase in blood creatinine or alanine aminotransferase, aspartate aminotransferase uncommon | in CYP3A substrates, such as alfentanil, fentanyl, midazolam, quinidine, CsA, TAC, SIR, coadministration increased exposure (close monitoring and dose adjustment); increased OATP1B1/3 transported statins; through inducing effects, reduced voriconazole and dabigatran exposure; combination of CsA and letermovir may lead to more marked or additional effects on concomitant medicinal products as compared to letermovir alone; increase in amiodarone (frequent monitoring of concentrations), SIR, TAC, quinidine are expected, but not yet studied; decrease in omeprazole, pantoprazole, voriconazole, oral contraceptive steroids, warfarin, dabigatran | not with severe hepatic impairment; not with combined moderate hepatic and moderate renal impairment; subtherapeutic letermovir exposure through rifampicin, rifabutin, thioridazine, bosentan, phenytoin, carbamazepine, St. John’s wort, phenobarbital, efavirenz, etravirine, nevirapine, ritonavir, lopinavir, modafinil; in combination with CsA, concomitant use of dabigatran, atorvastatin, simvastatin, rosuvastatin, or pitavastatin is contraindicated; fluvastatin, pravastatin increased exposure; pimozide; ergot alkaloids | CsA (potent OATP1B1/3 inhibitor), requires decreased letermovir dose of 240 mg/d; caution if other OATP1B1/3 inhibitors are added to letermovir combined with CsA, such as gemfibrozil, erythromycin, clarithromycin, and several protease inhibitors (atazanavir, simeprevir); monitoring of CMV DNA; it is still unknown whether letermovir may reduce the exposure of piperacillin/tazobactam, amphotericine B, and micafungin; extensively bound (98.2%) to human plasma proteins |
| Linezolid [66] | myelosuppression; lactic acidosis; increased LDH, lipase, AST, ALT or alkaline phosphatase; diarrhea, nausea, vomiting; headache; peripheral and optic neuropathy; risk of serotonin syndrome | linezolid may enhance the increases in blood pressure caused by pseudoephedrine and phenylpropanolamine hydrochloride | not with and not within two weeks of taking monoamine oxidase A or B inhibitors; not with serotonergic agents; not with tyramine rich foods | caution in patients with severe renal insufficiency, and in patients with severe hepatic insufficiency |
| Loperamide [67] | constipation; risk for toxic megacolon; CNS depression, urinary retention, QTc interval and QRS complex prolongation and torsades de pointes, other serious ventricular arrhythmias, cardiac arrest and syncope in association with overdose, hepatic impairment, or DDI-induced overexposure, some cases had a fatal outcome | increased exposure and risk of cardiotoxicity with drugs that inhibit CYP3A4 (e.g., azole antifungal agents, clarithromycin, cobicistat, conivaptan, delavirdine, erythromycin, idelalisib, nefazodone, protease inhibitors, telithromycin) and CYP2C8 (e.g., gemfibrozil, clopidogrel) with inhibitors of P-glycoprotein transport (e.g., amiodarone, CsA, diltiazem, dronedarone, quinidine, verapamil) and hepatic impairment | not in children < 12 yrs; not in acute severe inflammatory colitis or acute dysentery with blood in stool and fever | caution in hepatic impairment |
| Metamizole (Dipyrone) [68,69] | agranulocytosis; pancytopenia; severe skin reactions; deterioration of renal impairment, interstitial nephritis; parenteral administration is associated with a high risk of anaphylactic/anaphylactoid reactions; risk of a hypotensive reaction; hepatotoxicity | decrease in blood levels of CsA and TAC SIR, EVR requires dose elevation and monitoring; increased methotrexate hematotoxicity, in particular in elderly patients, combination to be avoided; reduced efficacy of ASA on platelet aggregation, take ASA hours in advance or avoid combination; reduction in bupropion exposure and increase of its active metabolite hydroxybupropion; upropion; reduced bioavailability of efavirenz, methadone, valproate, sertraline | previous experience with agranulocytosis; hypotension or unstable hemodynamics, impaired bone marrow function (e.g., after chemotherapy), or impaired hematopoiesis; analgesic asthma or analgesic intolerance of the urticaria/angioedema type, i.e., patients with a known occurrence of bronchospasm or other anaphylactoid reactions (e.g., urticaria, rhinitis, angioedema) after the administration of salicylates, paracetamol, or other on-narcotic analgesics, e.g., diclofenac, ibuprofen, indomethacin, naproxen; elimination rate is reduced when renal or hepatic function is impaired, multiple high doses should be avoided | dose reduction in elderly population (AUC increased by two- to threefold), debilitated patients, and patients with reduced creatinine clearance; acute overdose reactions include nausea, vomiting, abdominal pain, deterioration of renal function/acute renal failure (e.g., due to interstitial nephritis), rare central nervous system symptoms (dizziness, somnolence, coma, convulsions) and a drop in blood pressure (sometimes progressing to shock), as well as heart arrhythmia (tachycardia); excretion of a harmless metabolite (ribonic acid) may cause the red coloration of urine after very high doses |
| Metformin [70] | lactic acidosis, most often occurs at the acute worsening of renal function, cardiorespiratory illness, or sepsis, metformin accumulation with acute reduction in renal function increasing the risk of lactic acidosis; may reduce vitamin B12 serum levels, monitor for appropriate corrective treatment for vitamin B12 deficiency; taste disturbance; nausea, vomiting, diarrhea, abdominal pain, and loss of appetite, most frequently during the initiation of therapy, to prevent them, it is recommended to take metformin in 2 or 3 daily doses and to increase the doses slowly | drugs that can acutely impair renal function (such as antihypertensives, diuretics, and NSAIDs) should be initiated with caution in metformin-treated patients; other risk factors for lactic acidosis are excessive alcohol intake, hepatic insufficiency, inadequately controlled diabetes, ketosis, prolonged fasting, and any conditions associated with hypoxia, as well as concomitant use of medicinal products that may cause lactic acidosis, metformin alone does not cause hypoglycemia, but caution is advised when it is used in combination with insulin or other oral antidiabetics (e.g., sulfonylureas or meglitinides); alcohol intoxication is associated with an increased risk of lactic acidosis, particularly in cases of fasting or malnutrition, hepatic impairment; drugs that can adversely affect renal function may increase the risk of lactic acidosis, e.g., NSAIDs, including selective cyclo-oxygenase (COX) II inhibitors, ACE inhibitors, angiotensin II receptor antagonists, and diuretics, especially loop diuretics; drugs with intrinsic hyperglycemic ncreaity (e.g., glucocorticoids—systemic and local routes—and sympathomimetics) require more frequent blood glucose monitoring and metformin dose adjustment; metformin is a substrate of both transporters OCT1 and OCT2, inhibitors of OCT1 (such as verapamil) may reduce the efficacy of metformin; inducers of OCT1 (such as rifampicin) may increase the gastrointestinal absorption and efficacy of metformin; inhibitors of OCT2 (such as cimetidine, dolutegravir, ranolazine, trimethoprim, vandetanib, isavuconazole) may decrease the renal elimination of metformin and thus lead to an increase in metformin plasma concentration; inhibitors of both OCT1 and OCT2 (such as crizotinib, Olaparib) may alter the efficacy and renal elimination of metformin, caution especially in patients with renal impairment, when these drugs are coadministered with metformin, as the metformin plasma concentration may increase | must be discontinued at the time of surgery under general, spinal, or epidural anesthesia, therapy may be restarted no earlier than 48 h following surgery or resumption of oral nutrition and provided that renal function remained stable; intravascular administration of iodinated contrast agents may lead to contrast induced nephropathy, metformin accumulation, and an increased risk of lactic acidosis, metformin should be discontinued prior to or at the time of the imaging procedure, and should not restarted until at least 48 h after provided stable renal function; diabetic pre-coma; any type of acute metabolic acidosis (such as lactic acidosis, diabetic ketoacidosis); severe renal failure (GFR < 30 mL/min); acute conditions with the potential to alter renal function such as dehydration, severe infection, shock; disease which may cause tissue hypoxia (especially acute disease, or worsening of chronic disease), such as: decompensated heart failure, respiratory failure, recent myocardial infarction, shock; hepatic insufficiency; acute alcohol intoxication, alcoholism | in dehydration (severe diarrhea or vomiting, fever, or reduced fluid intake), metformin should be temporarily discontinued; with overdose lactic acidosis, the most effective method to remove lactate and metformin is hemodialysis |
| Methotrexate [71] | supervise for toxic signs and symptoms; increased risk of raised methotrexate blood levels and ARDs with, e.g., dehydration, impaired renal function, additional, or increased dose drugs, such as NSAIDs, administered concomitantly; hematopoietic suppression; deaths have been reported associated with the use of methotrexate in psoriasis; lung manifestations of RA and other connective tissue disorders; pleural effusions and ascites should be drained before methotrexate is started, can accumulate in these fluids and may be re-excreted into the circulation, prolonging the serum half-life, and resulting in unexpected toxicity; adequate hydration prior to and during treatment is required to limit the risk of renal toxicity; folate deficiency may increase methotrexate toxicity; systemic toxicity may follow intrathecal use; tumor lysis syndrome may occur in patients with rapidly growing tumors; If acute methotrexate toxicity occurs, patients may require folinic acid (to neutralize bone marrow effects); in patients with rheumatoid arthritis or psoriasis, folic acid or folinic acid supplementation may reduce toxicity, such as gastrointestinal symptoms, stomatitis, alopecia, and elevated liver enzymes, plasma methotrexate levels should be monitored in order to calculate the appropriate dose; check levels of vitamin B12 prior to initiating folic acid supplementation, particularly in adults aged over 50 years, as folic acid intake may mask a vitamin B12 deficiency; infections; encephalopathy/leukoencephalopathy; progressive multifocal leukoencephalopathy (PML); monitor hepatotoxicity; acute or chronic interstitial pneumonitis, often associated with blood eosinophilia, treatment resistant interstitial fibrosis, pulmonary alveolar hemorrhage; may cause renal damage that may lead to acute renal failure, monitor; diarrhea and ulcerative stomatitis; inactive chronic infections (e.g., herpes zoster, tuberculosis, hepatitis B or C) potential activation; development of malignant lymphomas; headache, dizziness, fatigue | CsA may potentiate efficacy and toxicity, there is a risk of excessive immunosuppression with risk of lymphoproliferation when the combination is used; concomitant use of proton pump inhibitors (PPIs) and a high dose methotrexate should be avoided, especially in patients with renal impairment; the concomitant administration of hepatotoxic or haematotoxin DMARDs (e.g., leflunomide) is not advisable, due to the possibility of fatal or severe toxic reactions; serious ADRs including deaths have been reported with the concomitant administration of methotrexate (usually in high doses) and nonsteroidal anti-inflammatory drugs (NSAIDs); in the treatment of rheumatoid arthritis, treatment with ASA and NSAIDs as well as small-dose steroids can be continued, but the possible increased risk of toxicity needs to be monitored; the steroid dose can be reduced gradually in patients who exhibit therapeutic response to methotrexate; the concomitant administration of folate antagonists, such as trimethoprim/sulfamethoxazole, has been reported to cause an acute megaloblastic pancytopenia; vitamin preparations containing folic acid (or its derivatives) may alter the response to methotrexate; salicylates and sulphonamides, whether antibacterial, hypoglycemic, or diuretic, should not be given, are extensively protein bound, and may displace, or be displaced by, other acidic drugs, the concurrent administration of agents, such as diphenylhydantoin, acidic anti-inflammatory agents, salicylates, phenylbutazone, phenytoin, barbiturates, tranquilizers, oral contraceptives, amidopyrine derivatives, p-aminobenzoic acid, thiazidediuretics, oral hypoglycaemics, doxorubicin, tetracyclines, probenecid, or sulfinpyrazone or oral hypoglycaemics will decrease the methotrexate transport function of renal tubules, thereby reducing excretion and almost certainly increasing methotrexate toxicity; increased risk of agranulocytosis with olanzapine; plasma concentrations of methotrexate increased by acitretin—also an increased risk of hepatotoxicity; azapropazone reduced the excretion of methotrexate; NSAIDs (aspirin, ibuprofen or indomethacin) increase and prolonged serum methotrexate concentrations with consequent increased gastrointestinal and hematological toxicity; sulfamethoxazole and folate antagonists, such as trimethoprim (as co-trimoxazole)—increased risk of hematological toxicity; probenecid and weak organic acids (e.g., loop diuretics: pyrazoles)—the excretion of methotrexate reduced (increased risk of toxicity) | not in significantly impaired hepatic function, severe/significantly impaired renal function (creatinine clearance less than 30 mL/min) for methotrexate doses <100 mg/m2, and moderate renal impairment (creatinine clearance less than 60 mL/min) for methotrexate doses >100 mg/m2, liver disease including fibrosis, cirrhosis, recent or active hepatitis, active infectious disease, pre-existing blood dyscrasias, such as bone marrow hypoplasia, significant anemia, leucopenia, or thrombocytopenia, alcoholism, severe acute or chronic infections, immunodeficiency syndrome, stomatitis, ulcers of the oral cavity and known active gastrointestinal ulcer disease, and concurrent vaccination with live vaccines; methotrexate tablets should not be used concomitantly with drugs with antifolate properties (e.g., co-trimoxazole); methotrexate is teratogenic and should not be given during pregnancy or to mothers who are breast-feeding; following administration to an adult, conception should be avoided by using an effective contraceptive method for at least 6 months after using methotrexate 2.5 mg tablets | the prescriber should specify the day of intake on the prescription once a week; patients should be instructed on the importance of adhering to the once-weekly intakes; full blood count (including hematocrit), hepatic and renal function tests (including urinalysis), the routine examination of lymph nodes, patients should report any unusual swelling; stop in acute infection; radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis; radiation-induced dermatitis and sun burn can reappear under methotrexate therapy (recall reaction); psoriatic lesions may get worse if methotrexate is combined with ultraviolet radiation/PUVA |
| Metoclopramide [72] | somnolence; depression; extra-pyramidal disorders; tardive dyskinesia, potentially irreversible, especially in the elderly; neuroleptic malignant syndrome; severe bradycardia, cardiac arrest, and QTc prolongation | increases CsA exposure by 22%; serotonergic drugs such as SSRIs may increase the risk of serotonin syndrome | not in patients with epilepsy; Parkinson’s disease; gastrointestinal hemorrhage, mechanical ncreasetion, or gastro-intestinal perforation | dose adjustment in renal impairment and in severe hepatic impairment |
| Midazolam [73] | lower doses in elderly people due to higher levels sensitivity to effects of benzodiazepines; severe cardiorespiratory ADRs incl. respiratory depression, apnea, respiratory arrest, dyspnea, laryngospasm cardiac arrest, and bradycardia; when used as a premedication, adequate observation of the patient after administration is mandatory as interindividual sensitivity varies and symptoms of overdose may occur; high risk patients are as follows: adults over 60 years of age, chronically ill or debilitated patients, e.g., patients with chronic respiratory insufficiency, patients with chronic renal failure, impaired hepatic function or with impaired cardiac function, pediatric patients especially those with cardiovascular instability; caution with myasthenia gravis; tolerance; dependence; withdrawal symptoms; anterograde amnesia; paradoxical reactions; sedation, amnesia, impaired attention, and impaired muscular function may adversely affect the ability to drive or use machines; nausea, vomiting, constipation, dry mouth; fatigue, falls and fracture; hypotension, vasodilating effects, thrombophlebitis, thrombosis; greater likelihood of adverse drug reactions in patients with severe renal impairment and in elderly | elimination may be altered in patients receiving compounds that inhibit or induce CYP3A4 (azoles, etc.), and the dose of midazolam may need to be adjusted accordingly; the concomitant use of midazolam with alcohol or/and CNS depressants should be avoided; concomitant opioids may result in sedation, respiratory depression, coma, and death; avoid use in patients with a medical history of alcohol or drug abuse; pharmacokinetic interactions with CYP3A4 inhibitors or inducers are more pronounced for oral use when compared to IV midazolam, particularly since CYP3A4 also exists in the upper gastro-intestinal tract; the administration of high doses or long-term infusions of midazolam to patients receiving strong CYP3A4inhibitors, e.g., during intensive care, may result in long-lasting hypnotic effects, delayed recovery and respiratory depression, thus requiring dose adjustments; for strong inducers, a relevant induction even after short-term treatment cannot be excluded; increased with verapamil/diltiazem, erythromycin, clarithromycin, telithromycin, IV fentanyl, propofol, protease inhibitors, nefazodone, tyrosine kinase inhibitors, aprepitant; decreased drug exposure by inducers rifampicin, ticagrelor, carbamazepine, phenytoin, mitotane (strong), enzalutamide (strong), clobazam and efavirenz, vemurafenib, St. John’s Wort | not to be used for conscious sedation in patients with severe ncreastory failure or acute respiratory depression; protease inhibitors should not be coadministered with orally administered midazolam | hepatic impairment reduces the clearance of intravenous midazolam, reduce dose and monitor vital signs; after prolonged infusion in intensive care unit patients, the mean duration of the sedative effect in the renal failure population was considerably increased most likely due to accumulation of α-hydroxymidazolam glucuronide; no specific data in patients with severe renal impairment (creatinine clearance below 30 mL/min) receiving midazolam for induction of anesthesia; to be administered only by experienced physicians in a setting fully equipped for the monitoring and support of respiratory and cardiovascular function and by persons specifically trained in the recognition and management of expected adverse events, including respiratory and cardiac resuscitation; elimination delayed in patients with liver dysfunction, low cardiac output |
| Mirtazapine [74] | bone-marrow depression, usually presenting as granulocytopenia or agranulocytosis; jaundice; akathisia, psychomotor restlessness; cases of QTc prolongation, torsade de pointes, ventricular tachycardia, and sudden death have been reported during post-marketing use; hyponatremia, serotonin syndrome; the elderly are often more sensitive, especially with regard to the undesirable effects of antidepressants; increase in appetite and weight; abnormal dreams, confusion, anxiety, insomnia; somnolence, sedation, headache; orthostatic hypotension | the concomitant administration of serotonergic agents, such as MAO inhibitors, selective serotonin re-uptake inhibitors (SSRIs), serotonin norepinephrine re-uptake inhibitors (SNRIs) or tricyclic antidepressants, and buprenorphine-containing medicinal products, may result in serotonin syndrome, a potentially life-threatening condition; serotonergic active substances (L-tryptophan, triptans, tramadol, linezolid, methylene blue, SSRIs, venlafaxine, lithium, and St. John’s Wort) may lead to an incidence of serotonin associated effects; may increase the sedating properties of benzodiazepines and other sedatives (notably, most antipsychotics, antihistamine H1 antagonists, opioids); risk of QTC prolongation and/or ventricular arrhythmias (e.g., torsade de pointes) may be increased with QTc-prolonging antipsychotics and antibiotics; carbamazepine and phenytoin, CYP3A4 inducers, about a 2-fold increase in mirtazapine clearance; potent CYP3A4 inhibitors, such as HIV protease inhibitors, azole antifungals, erythromycin, cimetidine, or nefazodone, increase exposure; cimetidine increases exposure | not with or within two weeks of monoamine oxidase (MAO) inhibitors; caution in cardiac diseases, low blood pressure, diabetes mellitus; avoid alcohol; caution with buprenorphine for risk of serotonin syndrome | careful dosing as well as regular and close monitoring in hepatic and renal impairment; increased suicidal risk with antidepressants compared to placebo in patients less than 25 years old; close supervision of patients, in particular those at high risk, especially those in early treatment and those experiencing dose changes |
| Moxonidine [75] | headache, dizziness, somnolence, vertigo; dry mouth; diarrhea, nausea, vomiting, dyspepsia; asthenia, insomnia; back pain; bradycardia, syncope, hypotension (including orthostatic), varying degrees of AV block | not recommended with tricyclic antidepressants (reduce the effectiveness of centrally acting antihypertensives); can potentiate the sedative effect of tricyclic antidepressants (avoid co-prescribing), tranquilizers, alcohol, and hypnotics; may enhance the sedative effect of benzodiazepines; interaction with other drugs that are excreted through tubular excretion cannot be excluded; in combination with a β-blocker, for discontinuation, the β-blocker should be discontinued first, and then moxonidine after a few days (doses should be reduced gradually over a period of two weeks) | not in sick sinus syndrome, bradycardia (resting heart rate < 50 beats/min), AV-block 2nd and 3rd degree, and cardiac insufficiency | caution with renal impairment, excreted primarily via the kidney, careful titration of the dose is recommended; in 1st degree AV block, special care should be exercised to avoid bradycardia, in severe coronary artery disease or unstable angina pectoris, special care should be exercised because of limited experience; the elderly may be more susceptible to the cardiovascular effects of antihypertensives, start low and increment dose with caution |
| MMF Mycophenolate mofetil [76,77,78,79] | hypogammaglobulinemia; risk of lymphomas and other malignancies, particularly of the skin; infections, latent viral reactivation, progressive multifocal leukoencephalopathy (PML) associated with the JC virus, BK virus-associated nephropathy sometimes leading to renal graft loss; leukopenia, anemia, thrombocytopenia and pancytopenia, pure red cell aplasia (PRCA); increased incidence of digestive system adverse events, including uncommon cases of gastrointestinal tract ulceration, hemorrhage, and perforation (colon, gall bladder); elderly patients > 65yrs may be at an increased risk of ADRs, such as certain infections (includeing CMV tissue invasive disease), possible gastrointestinal hemorrhages, and pulmonary oedema, compared with younger individuals; diarrhea, leucopenia, sepsis, and vomiting, and there is evidence of a higher frequency of certain types of infections, such as tuberculosis and atypical mycobacterial infection; uncommon but serious life-threatening infections such as meningitis and infectious endocarditis have been reported; bronchopulmonary infections; hypercholesterolemia; hypophosphatemia; headache; hypertension; cough, dyspnea; diarrhea (endoscopic investigation of patients with CellCept-related diarrhea have revealed isolated cases of intestinal villous atrophy), dyspepsia, nausea, vomiting; hematuria; oedema; pyrexia | PPIs significantly reduce the overall exposure to MPA after oral administration of mycophenolate mofetil, this interaction does not exist between PPIs and enteric-coated mycophenolic acid (EC-MPA), immunosuppressive effect may probably not be impaired in renal transplants; increase of approximately 20% in TAC AUC when multiple doses of CellCept (1.5 g twice daily) were administered to patients taking TAC; CsA interferes with MPA enterohepatic recirculation, resulting in reduced MPA exposures by 30–50% in renal transplant patients, similar to other patients devoid of this effect, e.g., belatacept, cholestyramine, or vice versa, as this might result in reduced MPA exposure; for drugs which interfere with MPA’s enterohepatic cycle, e.g., cholestyramine, antibiotics should be used with caution due to their potential to reduce the plasma levels and efficacy of MPA; as MPAG plasma concentrations are increased in renal impairment, so are aciclovir concentrations, the potential exists for the MPA and aciclovir or its prodrugs, e.g., valaciclovir, to compete for tubular secretion and thus further increase the concentrations of both drugs; with antacids, such as magnesium and aluminum hydroxides, MMF absorption decreased; antibiotics eliminating glucuronidase-produ-cing bacteria in the intestine (e.g., aminoglycoside, cephalosporin, fluoroquinolone, and penicillin classes of antibiotics) may interfere with MPAG/MPA enterohepatic recirculation, thus leading to reduced systemic MPA exposure; norfloxacin and metronidazole reduced the MPA AUC, drugs inhibiting the glucuronidation of MPA (isavuconazole) may increase MPA exposure; telmisartan enhances UGT1A9 expression and activity and decreases MPA; increased with SIR; as MPAG plasma and ganciclovir concentrations are increased in the presence of renal impairment, the potential exists for the two medicines to compete for tubular secretion, and thus further increases in concentrations of both medicines may occur; iron supplements should be administered at least 3 h following MPA; rifampicin decreases MPA (CellCept) exposure by 70%; sevelamer and other calcium-free phosphate binders should preferentially be given two hours after CellCept intake to minimize the impact on the reduced absorption of MPA; the renal clearance of MPAG occurs by renal tubular secretion, as well as glomerular filtration, the coadministration of probenecid, a known inhibitor of tubular secretion, with MMF raises the plasma AUC of MPAG threefold, thus, other medicines known to undergo renal tubular secretion may compete with MPAG, thereby raising the plasma concentrations of MPAG or the other drug undergoing tubular secretion | contraindicated in women with childbearing potential not using highly effective contraceptive methods, men should not donate semen during therapy and for 90 days following discontinuation; if neutropenia develops (absolute neutrophil count < 1.3 × 103/μL), dosing with CellCept should be interrupted; not with azathioprine; in severe chronic renal impairment (GFR < 25 mL/min/1.73m2), the administration of MPA doses greater than 1g twice daily should be avoided; with delayed graft function posttransplant, mean MPA AUC0-12 was comparable, but MPAG AUC0-12 was two- or threefold higher, monitor carefully; no live vaccines | patients should be switched from IV to oral CellCept as soon as they can tolerate oral medication; patients receiving MPA should be instructed to immediately report any evidence of infection, unexpected bruising, bleeding, or any other manifestation of bone marrow depression; vaccinations may be less effective, and the use of live attenuated vaccines should be avoided, influenza vaccination may be of value; the therapeutic drug monitoring of MPA may be appropriate when switching combination therapy (e.g., from CsA to TAC or vice versa) or to ensure adequate immunosuppression in patients with high immunological risk (e.g., risk of rejection, treatment with antibiotics, addition or removal of an interacting medication) |
| Pantoprazole, Omeprazole other PPIs [80,81,82,83,84] | reduced absorption of vitamin B12 due to hypo- or achlorhydria; increased risk of gastrointestinal infections caused by bacteria such as Salmonella and Campylobacter or C. difficile; hypomagnesemia with serious manifestations, such as fatigue, tetany, delirium, convulsions, dizziness, and ventricular arrhythmia; risk of hip, wrist, and spine fracture, predominantly in the elderly; rare cases of agranulocytosis, very rare thrombocytopenia, leukopenia, pancytopenia; benign fundic gland polyps; headache, dizziness, sleep disorders; diarrhea, nausea, vomiting, abdominal distension, and bloating, constipation, dry mouth, abdominal pain and discomfort; increase in hepatobiliary enzymes; asthenia, fatigue, and malaise | may interfere with the absorption of other medicinal products where gastric pH is an important determinant of oral bioavailability, e.g., some azole antifungals, such as ketoconazole, itraconazole, posaconazole, and other medicines like erlotinib, HIV protease inhibitor may need to be adjusted; with warfarin or phenprocoumon increases in INR and prothrombin time; increased levels of high dose methotrexate (e.g., 300 mg) necessitate consideration of PPI withdrawal; inhibitors of CYP2C19 such as fluvoxamine could increase the systemic exposure of pantoprazole, a dose reduction may be considered for patients treated long-term with high doses of pantoprazole, or those with hepatic impairment; CYP2C19 and CYP3A4 inducers, such as rifampicin and St. John´s wort, may decrease the PPI plasma concentrations | in severe hepatic impairment max. 20 pantoprazole mg/d, in the case of a rise in liver enzymes, discontinue use; not recommended with HIV protease inhibitors for which absorption is dependent on acidic intragastric pH, such as atazanavir | should be swallowed whole 1 h before a meal; tube feeding, PEG: administer omeprazole (pantoprazole not possible); in long-term treatment, especially >1 year, patients should be kept under regular surveillance; differentiate prophylactic versus therapeutic doses (e.g. pantoprazol 20 mg versus ≥40 mg/per day); deprescribing with biweekly dose halving to prevent risk of rebound; lowering the PPI dose (e.g., from twice to once daily, or halving the dose, or taking every second day) or stopping the PPI and using it on-demand are equally recommended strong options according to the algorithm by Farrell et al. |
| Paracetamol, (Acetaminophen) [85,86,87] | overdose: liver damage is possible in adults who have taken 10 g or more of paracetamol, ingestion of 5 g or more of paracetamol may lead to liver damage if the patient has risk factors (see below): risk factors long term treatment with carbamazepine, phenobarbital, phenytoin, primidone, rifampicin, St. John’s Wort, or other drugs that induce liver enzymes, or the regular consumption of ethanol in excess amounts, or likely to be glutathione deplete, e.g., eating disorders, cystic fibrosis, HIV infection, starvation, cachexia; blood dyscrasias, including thrombocytopenia and agranulocytosis | the anticoagulant effect of warfarin and other coumarins may be enhanced by the prolonged regular daily use of paracetamol with an increased risk of bleeding; occasional doses have no significant effect; caution should be taken when paracetamol is used concomitantly with flucloxacillin, as concurrent intake has been associated with high anion gap metabolic acidosis, especially in patients with risks factors | 2 tablets (500 mg) every 4 h (German version: 6 h) as required, not more than eight tablets (4 g) in 24 h, do not take for more than 3 days without consulting; these doses should not be given more frequently than every four hours, nor should more than four doses be given in any 24 h period; symptoms of paracetamol overdosage, in the first 24 h, are pallor, nausea, vomiting, anorexia, and abdominal pain; liver damage may become apparent 12 to 48 h after ingestion; abnormalities of glucose metabolism and metabolic acidosis may occur; in severe poisoning, hepatic failure may progress to encephalopathy, disseminated intravascular coagulation, hemorrhage, hypoglycemia, cerebral oedema, gastrointestinal bleeding, and death; acute renal failure with acute tubular necrosis, strongly suggested by loin pain, hematuria, and proteinuria may develop, even in the absence of severe liver damage; cardiac arrhythmias and pancreatitis have been reported; antidot: N-acetylcysteine may be used up to 24 h after the ingestion of paracetamol; however, the maximum protective effect is obtained up to 8 h post-ingestion | intoxications with paracetamol (APAP) are the second most common causes of liver transplants worldwide [87]—the maximum permitted daily dose over 3 days in >43 kg adolescents (from 12 years) and adults max 4 × 1 g; the minimal time intervals between each dose differs comparing SmPCs: 4 h for the USA version versus 6h for the German version; reduce dose and care in patients with renal or severe hepatic impairment; the hazard of overdose is greater in those with non-cirrhotic alcoholic liver disease; do not exceed the recommended dose; do not take for more than 3 days without consulting; do not take with any other paracetmol-contai-ning products; keep out of the reach of children; caution if administered concomitantly with flucloxacillin, due to the increased risk of high anion gap metabolic acidosis (HAGMA), particularly in patients with severe renal impairment, sepsis, malnutrition, and other sources of glutathione deficiency (e.g., chronic alcoholism), as well as those using maximum daily doses of paracetamol, close monitoring, including measurement of urinary 5-oxoproline, is recommended; immediate medical advice should be sought in the event of an overdose, even if one feels well, because of the risk of delayed, serious liver damage |
| Parecoxib [88] | COX-2 inhibitors have been associated with the increased risk of cardiovascular and thrombotic ADRs when taken long term, caution in patients with significant risk factors for cardiovascular events (e.g., hypertension, hyperlipidemia, diabetes mellitus, smoking); aggravation of soft tissue infections; upper gastrointestinal (GI) complications (perforations, ulcers or bleedings [PUBs]), higher risk in elderly, or patients with a prior history of gastrointestinal disease, such as ulceration and GI bleeding, or patients using ASA concomitantly, anticoagulants, such as warfarin/coumarin-type and DOACs, glucocorticoids, SSRIs/SNPIs, antiplatelet drugs, other NSAIDs, or patients ingesting alcohol; severe skin reactions; fluid retention, oedema, oliguria, renal impairment; pharyngitis, alveolar osteitis; anemia postoperative; hypokalemia; agitation, insomnia; hypoesthesia, dizziness; hypertension, hypotension; respiratory insufficiency, bronchospasm; abdominal pain, vomiting, constipation, dyspepsia, flatulence; hepatitis probably; significant greater incidence of cardiovascular/thromboem-bolic events | increased nephrotoxicity with CsA, TAC; diminishes the antihypertensive effect of ACE inhibitors, angiotensin II antagonists, beta-blockers, and diuretics; in elderly patients, volume-depleted (including those on diuretic therapy), or with compromised renal function, the coadministration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors or angiotensin-II antagonists, may result in the further deterioration of renal function, including possible acute renal failure; fluconazole increases the exposure and requires dose reduction; inhibits CYP2D6 and thus enhances the exposure of, e.g., flecainide, propafenone, metoprolol; inhibits CYP2C19, and thus may enhance the exposure of, e.g., phenytoin, diazepam, imipramine, omeprazole; may result in increased plasma levels of methotrexate; significant decreases in lithium serum clearance; may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women | not in severe hepatic impairment; caution needed for lowest dose and monitoring in severe renal impairment; previous serious allergic drug reaction of any type; active peptic ulceration or GI bleeding; patients who have experienced bronchospasm, acute rhinitis, nasal polyps, angioneurotic oedema, urticaria, or other allergic-type reactions after taking ASA or NSAIDs, including COX-2 inhibitors; inflammatory bowel disease; congestive heart failure (NYHA II-IV); treatment of post-operative pain following coronary artery bypass graft (CABG) surgery; established ischemic heart disease, peripheral arterial disease, and/or cerebrovascular disease | may mask fever and other signs of inflammation; caution in patients with compromised cardiac function, preexisting oedema, or other conditions predisposing to, or worsened by, fluid retention, including those taking diuretic treatment or otherwise at risk of hypovolemia; in healthy elderly subjects, the apparent oral clearance of valdecoxib (activated parecoxib) was reduced, resulting in an approximately 40% higher plasma exposure to valdecoxib |
| Posaconazole [89,90] | hepatic toxicity—ALT, AST, bilirubin alkaline phosphatase, GGT increased; nausea, vomiting, diarrhea (may overlap/mimic symptoms of CMV, GvHD)—patients with severe diarrhea or vomiting should be monitored closely for breakthrough fungal infections; thrombotic thrombocytopenia purpura; hemolytic uremic syndrome; QTc prolongation and torsade de pointes—administer with caution to patients with pro-arrhythmic conditions, such as congenital or acquired QTc prolongation, cardiomyopathy, especially in the presence of cardiac failure, sinus bradycardia, existing symptomatic arrhythmias, concomitant use with medicinal products known to prolong the QTc interval, electrolyte disturbances, especially those involving potassium, magnesium, or calcium levels, should be monitored and corrected as necessary before and during posaconazole therapy; agranulocytosis, aplastic anemia; depression; adrenal insufficiency; convulsion; hypertension; hypokalemia; photopsia, visual brightness, visual disturbances; paresthesia, dizziness, somnolence, headache, dysgeusia; pyrexia, asthenia, fatigue | potent inhibitor of CYP3A4, coadministration with CYP3A4 substrates may result in large increases in exposure, e.g., CsA (nephrotoxicity and fatal cases of leukoencephalopathy), dose of CsA should be reduced (e.g., to about three quarters of the current dose), TAC (dose of TAC should be reduced, e.g., to about one third of the current dose), SIR (not to combine), EVR (not recommended), atazanavir, simvastatin, atorvastatin, lovastatin; venetoclax; prolonged sedation and possible respiratory depression, coadministration with any benzodiazepines metabolized by CYP3A4 (e.g., midazolam, triazolam, alprazolam)—avoid or reduce dose; caution vincristine, vinblastine, and venetoclax toxicity; significantly lowered posaconazole concentrations with rifampicin, rifabutin, phenytoin, carbamazepine, phenobarbital, primidone, efavirenz, fosamprenavir—avoid combination; posaconazole is metabolized via UDP glucuronidation, and is a substrate for P-gp efflux, inhibitors, e.g., verapamil, CsA, quinidine, clarithromycin, erythromycin increase posaconazole plasma concentrations; increased HIV protease inhibitors; diltiazem, verapamil, nifedipine, nisoldipine, felodipine, lercanidipine increase, dose adjustment | no coadministration with ergot alkaloids with CYP3A4 substrates terfenadine, astemizole, cisapride, pimozide, halofantrine, or quinidine with increased plasma concentrations, QTc prolongation, or the risk of torsades de pointes; coadministration with the HMG-CoA reductase inhibitors simvastatin, lovastatin, and atorvastatin; coadministration during the initiation and dose-titration phase of venetoclax in chronic lymphocytic leukemia (CLL) patients; increased ergot alkaloids; simvastatin, lovastatin, and atorvastatin must be discontinued for the risk of rhabdomyolysis; rifabutin increased; not with SIR for 8-fold AUC increase | Caution: with hepatic impairment increased plasma exposure; tablets should be swallowed whole; posaconazole tablets and oral suspension are not interchangeable, plasma concentrations following the administration of posaconazole tablets are generally higher than those obtained with posaconazole oral suspension; posaconazole plasma concentrations following the administration of posaconazole tablets may increase over time in some patients; posaconazole is an inhibitor of CYP3A4 |
| Pravastatin [91] | arthralgia, muscle cramps, myalgia, muscle weakness, and elevated CK levels; elevations of serum transaminases; peripheral polyneuropathy is very rare, particularly if used for long periods of time, as are paresthesia, pancreatitis, jaundice, hepatitis, fulminant hepatic necrosis, rhabdomyolysis, which can be associated with acute renal failure, secondary to myoglobinuria, myopathy, myositis, polymyositis, tendon disorders, which may induce de novo or aggravate pre-existing myasthenia gravis or ocular myasthenia; immune-mediated necrotizing myopathy; photosensitivity reaction; class effects: nightmares, memory loss, depression, exceptional cases of interstitial lung disease, especially with long term therapy, diabetes mellitus; interstitial lung disease have been reported with some statins (presenting features can include dyspnea, a non-productive cough, and a deterioration in general health (fatigue, weight loss, and fever) | CsA leads to an approximately 4-fold increase in pravastatin systemic exposure, in some patients, however, the increase in pravastatin exposure may be larger; the risk and severity of muscular disorders during statin therapy is increased via the coadministration of interacting medicines, such as CsA, clarithromycin, and other macrolides or niacin, an increase in the incidence of myopathy has also been described in patients receiving other statins in combination with inhibitors of cytochrome P450 metabolism, this may result from pharmacokinetic interactions that have not been documented for pravastatin; caution with colchicine (rhabdomyolysis); increased exposure of vitamin K antagonists requires the appropriate monitoring of INR is needed; increased statin exposure, cautiously with macrolide antibiotics (e.g., erythromycin, clarithromycin, roxithromycin); not metabolized to a clinically significant extent by the cytochrome P450 system, in contrast to other statins; the absence of a significant pharmacokinetic interaction with pravastatin has been specifically demonstrated for several products, particularly those that are substrates/inhibitors of CYP3A4, e.g., diltiazem, verapamil, itraconazole, ketoconazole, protease inhibitors, grapefruit juice, and CYP2C9 inhibitors (e.g., fluconazole); rifampicin may decrease the oral bioavailability of pravastatin; but also, with rifampicin, an increase in pravastatin AUC and Cmax was observed, caution if both are given at the same time, no interaction is expected if their dosing is administered at least two hours apart; increased risk of rhabdomyolysis with lenalidomide | not in active liver disease, including unexplained persistent elevations of serum transaminase elevation exceeding 3 × the upper limit of normal (ULN); as for other HMG-CoA reductase inhibitors, the combination of pravastatin with fibrates is not recommended; statins, including pravastatin, must not be coadministered with systemic formulations of fusidic acid or within 7 days of stopping fusidic acid; patients should be advised to report promptly unexplained muscle pain, tenderness, weakness, or cramps, in these cases, the CK levels should be measured | predisposing factors including advanced age (>65), uncontrolled hypothyroidism, and renal impairment may increase the risk of muscular toxicity, and can therefore justify a careful evaluation of the benefit/risk and special clinical monitoring, CK measurement is indicated before starting statin therapy in these patients; predisposing factors such as renal impairment, hypothyroidism, previous history of muscular toxicity with a statin or fibrate, personal or familial history of hereditary muscular disorders, or alcohol abuse, in these cases, CK levels should be measured prior to initiation of therapy; CK measurement should also be considered before starting treatment in persons over 70 years of age, especially in the presence of other predisposing factors in this population |
| Quetiapine [92] | weight gain; hyperlipidemia; hyperglycemia; tardive dyskinesia; and neuroleptic malignant syndrome; mean plasma clearance is reduced by 30% to 50% in the elderly; high α1 adrenergic receptor blocking activity may induce orthostatic hypotension, tachycardia, dizziness, and sometimes syncope, which may lead to falls; cardiomyopathy and myocarditis; myelosuppression; venous thromboembolism; hepatic, biliary, pancreatic risks; rhabdomyolysis; neuroleptic malignant syndrome; EPS, tardive dyskinesia; dysphagia; QTC prolongation; dry mouth; constipation; cognitive and motor impairment; rhabdomyolysis; hepatic failure | CsA, TAC, and SIR enhance exposure (less vice versa); inhibitors of CYP3A4, e.g., ketoconazole, increase the exposure by up to 6.2-fold (cave: azole antifungals, macrolide antibiotics, protease inhibitors, amiodarone, diltiazem, verapamil, nefazodone, and grapefruit juice); QTc prolongation with concomitant neuroleptics (some antibiotics, azoles, etc.), especially for patients with an increased risk of QTc prolongation, i.e., the elderly, patients with congenital long QTc syndrome, congestive heart failure, heart hypertrophy, hypokalemia, or hypomagnesemia; urinary retention with other anticholinergic drugs; CYP3A4 inducers, such as carbamazepine and phenytoin, reduce exposure | use for the shortest time that is clinically indicated; not indicated in elderly patients with dementia—increased risk of death | reduce dose in mild hepatic impairment; no data on renal impairment dosing, caution; drowsiness and sedation, tachycardia, hypotension, and anticholinergic effects if overdosed; caution in patients with known cardiovascular disease, cerebrovascular disease, or other conditions predisposing to hypotension, e.g., dehydration, hypovolemia, and treatment with antihypertensive medications |
| Remdesivir [93] | transaminase elevations; severe renal toxicity was observed (not to be used in patients with eGFR < 30 mL/min); headache, nausea, rash | risk of reduced antiviral activity when coadministered with chloroquine or hydroxychloroquine (antagonistic effect); potential of the interaction of remdesivir with inhibitors/inducers of the hydrolytic pathway (esterase) or CYP2C8, 2D6, or 3A4 has not been studied, the risk of clinically relevant interaction is unknown, strong inhibitors may result in increased remdesivir exposure; the use of strong inducers (e.g., rifampicin) may decrease plasma concentrations of remdesivir and is not recommended; may transiently increase plasma concentrations of substrates of CYP3A or OATP 1B1/1B3, no data is available, however, it can be suggested that medicinal products that are substrates of CYP3A4 or substrates of OATP 1B1/1B3 should be administered at least 2 h after remdesivir; induced CYP1A2 and potentially CYP3A in vitro, coadministration of remdesivir with CYP1A2 or CYP3A4 substrates with narrow therapeutic index may lead to the loss of their efficacy | remdesivir should not be initiated in patients with ALT ≥ five times the upper limit of normal at baseline; contains betadex sulfobutyl ether sodium, which is renally cleared and accumulates in patients with decreased renal function, which may potentially adversely affect renal function and therefore should not be used in patients with eGFR < 30 mL/min | this has not been evaluated in patients with renal impairment, patients with eGFR ≥ 30 mL/min have received remdesivir for the treatment of COVID-19 with no dose adjustment, remdesivir should not be used in patients with eGFR < 30 mL/min; pharmacokinetics of remdesivir have not been evaluated in patients with hepatic impairment, it is not known if a dosage adjustment is appropriate in patients with hepatic impairment |
| Rifampicin [94] | monitor liver function; severe, systemic hypersensitivity reactions; enhanced metabolism of endogenous substrates including adrenal hormones, thyroid hormones and vitamin D; severe skin reactions, preceded by fever, lymphadenopathy, or biological abnormalities (including eosinophilia, liver abnormalities) discoloration (yellow, orange, red, brown) of the teeth, urine, sweat, sputum, and tears; thrombocytopenia, leukopenia, disseminated intravascular coagulation, eosinophilia, agranulocytosis, hemolytic anemia, Vitamin K dependent coagulation disorders; anaphylactic reaction; adrenal insufficiency; decreased appetite; psychotic disorder; headache, dizziness; cerebral hemorrhage and fatalities have been reported when rifampicin administration has been continued or resumed after the appearance of purpura; shock, flushing, vasculitis, bleeding; dyspnea, wheezing, sputum discolored; nausea, vomiting, diarrhea, tooth discoloration (which may be permanent); hepatitis, hyperbilirubinemia; erythema multiforme, including Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, acute generalized exanthematous pustulosis (AGEP), skin reactions, sweat discoloration; muscle weakness, myopathy, bone pain; acute kidney injury, usually due to renal tubular necrosis or tubulointerstitial nephritis; post-partum hemorrhage; menstrual disorder; edema; blood bilirubin increased, aspartate aminotransferase increased, alanine aminotransferase increased; blood pressure decreased; blood creatinine increased | with halothane (should be avoided) or isoniazid, the potential for hepatotoxicity is increased; vitamin K-dependent coagulation and severe bleeding (supplement in vitamin K deficiency, hypoprothrombinemia); with other antibiotics causing vitamin K-dependent coagulopathy, such as cefazolin (or other cephalosporins with N-methylthiotetrazole side chain) should be avoided; rifampicin capsules may accelerate the metabolism and reduce the activity of certain coadministered drugs, and have the potential to perpetuate clinically important drug–drug interactions in order to maintain optimum therapeutic blood levels, dosages of drugs may require adjustment when starting or stopping concomitantly administered rifampicin capsules: antiarrhythmics (e.g., disopyramide, mexiletine, quinidine, propafenone, tocainide), antiepileptics (e.g., phenytoin), hormone antagonist (antiestrogens, e.g., tamoxifen, toremifene, gestinone), antipsychotics (e.g., haloperidol, aripiprazole), anticoagulants (e.g., coumarins, DOACs, antifungals (e.g., fluconazole, itraconazole, ketoconazole, voriconazole), antivirals (e.g., saquinavir, indinavir, efavirenz, amprenavir, nelfinavir, atazanavir, lopinavir, nevirapine), barbiturate, beta-blockers (e.g., bisoprolol, propranolol), anxiolytics and hypnotics (e.g., diazepam, benzodiazepines, zopiclone, zolpidem), calcium channel blockers (e.g., diltiazem, nifedipine, verapamil, nimodipine, isradipine, nicardipine, nisoldipine), antibacterials (e.g., chloramphenicol, clarithromycin, dapsone, doxycycline, fluoroquinolones, telithromycin), corticosteroids, cardiac glycosides (digitoxin, digoxin), clofibrate, systemic hormonal contraceptives, including estrogens and progestogens, antidiabetic (e.g., chlorpropamide, tolbutamide, sulfonylureas, rosiglitazone), immunosuppressive agents CsA, TAC, SIR, EVR, irinotecan, thyroid hormone, losartan, analgesics (e.g., methadone, narcotic analgesics), praziquantel, quinine, riluzole, selective 5-HT3 receptor antagonists (e.g., ondansetron), statins metabolized by CYP 3A4 (e.g., simvastatin), theophylline, tricyclic antidepressants (e.g., amitriptyline, nortriptyline), cytotoxics (e.g., imatinib), diuretics (e.g., eplerenone); enalapril: decrease enalapril active metabolite exposure; dosage adjustments should be made if indicated by the patient’s clinical condition, ncreastis-C antiviral drugs (e.g., daclatasvir, simeprevir, sofosbuvir, telaprevir), concurrent use of treatment of hepatitis-C antiviral drugs and rifampicin should be avoided; morphine: plasma concentration and the analgesic effect of morphine may be reduced by rifampicin; clopidogrel increases active metabolite exposure, rifampicin strongly induces CYP2C19, resulting in both an increased level of clopidogrel active metabolite and platelet inhibition, which in particular might potentiate the risk of bleeding; as a precaution, the concomitant use of clopidogrel and rifampicin should be discouraged; rifampicin treatment reduces the systemic exposure of oral contraceptives; also, diabetes may become more difficult to control; the concurrent use of ketoconazole and rifampicin has resulted in the decreased serum concentrations of both drugs; if p-aminosalicylic acid and rifampicin are both included in the treatment regimen, they should be given not less than eight hours apart to ensure satisfactory blood levels; the concomitant antacid administration may reduce the absorption of rifampicin, daily doses of rifampicin should be given at least 1 h before the antacids; taken concomitantly, decreased concentrations of atovaquone and increased concentrations of rifampicin were observed | not with the combination of saquinavir/ritonavir (hepatotoxicity) | caution in case of renal impairment if dose >600 mg/day; paradoxical drug reaction, after the initial improvement of tuberculosis under therapy with rifampicin capsules, the symptoms may worsen again; therapeutic levels of rifampicin have been shown to inhibit standard microbiological assays for serum folate and Vitamin B12; transient elevation of BSP and serum bilirubin has been reported, rifampicin may impair biliary the excretion of contrast media used for the visualization of the gallbladder, due to the competition for biliary excretion, therefore, these tests should be performed before the morning dose of rifampicin |
| Rifaximin [95] | Clostridium difficile-associated diarrhea (CDAD-reported with the use of nearly all antibacterial agents); reddish discoloration of the urine; anemia; anorexia, hyperkalemia; depression, confusional state, anxiety, hypersomnia, insomnia; dizziness, headache, balance disorders, amnesia, convulsion, attention disorders, hypoesthesia, memory impairment; dyspnea, pleural effusion; upper abdominal pain, abdominal distension, diarrhea, nausea, vomiting, ascites, esophageal varices hemorrhage, dry mouth; rashes, pruritus; muscle spasms, arthralgia, myalgia; dysuria, pollakiuria, proteinuria; oedema; pyrexia; fall | in healthy subjects, clinical drug interaction studies demonstrated that rifaximin did not significantly affect the pharmacokinetics of CYP3A4 substrates; however, in hepatic impaired patients, it cannot be excluded that rifaximin may decrease the exposure of the concomitant CYP3A4 substrates administered (e.g., warfarin—monitor INR, antiepileptics, antiarrhythmics, oral contraceptives), due to the higher systemic exposure with respect to healthy subjects; not to combine with other rifamycin; it is unknown whether concomitant drugs which inhibit P-gp and/or CYP3A4 can increase the systemic exposure of rifaximin, the coadministration of a single dose CsA (600 mg), a potent P-glycoprotein inhibitor, with a single dose of rifaximin (550 mg) resulted in 83-fold and 124-fold increases in rifaximin mean Cmax and AUC∞, clinical significance unknown | not in cases of intestinal obstruction | caution in patients with severe (Child–Pugh C) hepatic impairment and in patients with MELD (Model for End-Stage Liver Disease) score > 25 |
| Rosuvastatin [96] | diabetes mellitus; thrombocytopenia; headache, dizziness; constipation, nausea, abdominal pain, pancreatits; myalgia, myopathy (including myositis), rhabdomyolysis, Lupus-like syndrome, muscle rupture; asthenia; polyneuropathy, memory loss; depression, peripheral neuropathy; sleep disturbances (including insomnia and nightmares), myasthenia gravis; as with other HMG-CoA reductase inhibitors, a dose-related increase in transaminases has been observed; proteinuria; interstitial lung disease | concomitant protease inhibitor use may strongly increase rosuvastatin exposure; gemfibrozil, fenofibrate, other fibrates and lipid-lowering doses (>or equal to 1 g/day) of niacin (nicotinic acid) increase the risk of myopathy; low increase by ezetimibe; decreased with erythromycin (via increase in gut motility); ticagrelor might affect the renal excretion of rosuvastatin, increasing the risk for rosuvastatin accumulation, concomitant use of ticagrelor and rosuvastatin led to renal function decrease, increased CPK level, and rhabdomyolysis; rosuvastatin is neither an inhibitor nor an inducer of cytochrome P450 isoenzymes; therefore, drug interactions resulting from cytochrome P450-mediated metabolism are not expected; rosuvastatin is a poor substrate for these isoenzymes, no clinically relevant interactions have been observed between rosuvastatin and either fluconazole (an inhibitor of CYP2C9 and CYP3A4) or ketoconazole (an inhibitor of CYP2A6 and CYP3A4), febuxo-stat, clopidogrel, gemfibrozil increase exposure (2-fold) | not in patients with active liver disease including unexplained, persistent elevations of serum transaminases and any serum transaminase elevation, exceeding three times the upper limit of normal; not with severe renal impairment (creatinine clearance < 30 mL/min), not with myopathy, the concomitant combination of sofosbuvir/velpatasvir/voxilaprevir, not with concomitant CsA; a 40 mg dose is contraindicated in patients fibrates (start with 5 mg dose) or with pre-disposing factors for myopathy/rhabdomyolysis, such as moderate renal impairment (creatinine clearance < 60 mL/min), hypothyroidism, personal or family history of hereditary muscular disorders, previous history of muscular toxicity with another HMG-CoA reductase inhibitor or fibrate, alcohol abuse, situations where an increase in plasma levels may occur, Asian patients, or the concomitant use of fibrates | |
| Ruxolitinib [97] | myelosuppression, treatment should be discontinued in patients with platelet count less than 50,000/mm3 or absolute neutrophil count less than 500/mm3; serious bacterial, mycobacterial, fungal, viral and other opportunistic infections; sepsis; hepatitis B viral load (HBV-DNA titer) increases; herpes zoster; progressive multifocal leukoencephalopathy (PML); non-melanoma skin cancer; lipid abnormalities/elevations; bleeding (gastrointestinal, intracranial, epistaxis, post-procedural hemorrhage and hematuria); bruising; hyperlipidemia; dizziness, headache; elevated lipase, constipation, flatulence; elevated transaminases; hypertension | when administering ruxolitinib with strong CYP3A4 inhibitors (such as, but not limited to, boceprevir, clarithromycin, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir, ritonavir, mibefradil, nefazodone, nelfinavir, posaconazole, saquinavir, telaprevir, telithromycin, voriconazole) in MF (myelofibrosis) and PV (polycythemia vera) patients or dual inhibitors of CYP3A4 and CYP2C9 enzymes (e.g., fluconazole) in MF, PV, and GvHD patients, the unit dose must be reduced by approximately 50%, administered twice daily; 50% dose reduction with dual inhibitors of CYP2C9 and CYP3A4 enzymes (e.g., fluconazole); avoid the concomitant use of ruxolitinib with fluconazole doses greater than 200 mg daily; with CYP3A4 inducers (such as, but not limited to, avasimibe, carbamazepine, phenobarbital, phenytoin, rifabutin, rifampin (rifampicin), St. John’s wort), an increase of the ruxolitinib dose may be needed (effects not very strong); no dose adjustment is recommended when coadministered with mild or moderate CYP3A4 inhibitors (e.g., erythromycin); however, patients should be closely monitored for cytopenia when initiating therapy with a moderate CYP3A4 inhibitor; inhibits P-glycoprotein and breast cancer resistance protein (BCRP) in the intestine with elevated exposure of dabigatran etexilate, CsA, rosuvastatin, and potentially digoxin, TDM, or the clinical monitoring of the affected substance is advised, possibly the potential inhibition of P-gp and BCRP in the intestine can be minimized if the time between administrations is kept apart as long as possible | starting doses should be reduced in patients with severe renal impairment; for patients with end-stage renal disease on hemodialysis, the starting dose should be based on platelet counts for MF patients; the starting dose should be reduced by approximately 50% in MF and PV patients with hepatic impairment; in GvHD patients with hepatic impairment not related to GvHD, the starting dose should be reduced by approximately 50% | |
| Sertraline [98,99] | hemorrhage—caution in concomitant use with drugs known to affect platelet function incl. atypical antipsychotics and phenothiazines, most tricyclic antidepressants, aspirin, NSAIDs, in thrombocytopenia, patients with a history of bleeding disorders; nausea, diarrhea, anorexia, dyspepsia, tremor, seizures, dizziness, insomnia, somnolence, hallucinations, aggressive reaction, agitation, anxiety, psychosis, depersonalisation, increased sweating, dry mouth, urinary retention, sexual dysfunction; hyponatremia, SIADH; postural hypotension; serotonin syndrome; pharyngitis; mydriasis; palpitation, tachycardia; arthralgia, myalgia; chest pain | increase in plasma levels of the tricyclic antidepressants (less than some other SSRIs); strong and moderately strong CYP3A4 inhibitors, e.g., protease inhibitors, ketoconazole, itraconazole, posaconazole, voriconazole, clarithromycin, telithromycin and nefazodone, aprepitant, erythromycin, fluconazole, verapamil, and diltiazem greater increase following sertraline exposure; increased exposure of CYP2D6 substrates, e.g., propafenone and flecainide, tricyclic antidepressive and typical psychotropic drugs, metoprolol, nebivolol; QTc prolongation may result in additive effects and an increased risk of ventricular arrhythmias, including torsade de pointes and sudden death with QTc prolongating drugs, e.g., voriconazole | not in severe hepatic dysfunction; not with MAOIs (and relapse phase of 14 days required); linezolid; pimozide; serotonergic potentiation with St. John’s wort, tramadol, sumatriptan, fenfluramine; unstable epilepsy; not with grapefruit juice; not with strong CYP3A4 inhibitors; alcohol | caution in patients with renal and hepatic impairment; 98% bound to plasma proteins; risk of serotonin syndrome with CsA |
| Simvastatin [100,101,102] | myopathy manifested as muscle pain, tenderness, or weakness with increased CK; rhabdomyolysis; in pre-disposing factors for rhabdomyolysis, CK level measurement before starting a treatment in elderly patient >70 years, renal impairment, uncontrolled hypothyroidism, personal or familial history of hereditary muscular disorders, previous history of muscular toxicity with a statin or fibrate, alcohol abuse; myasthenia gravis; myopathy sometimes takes the form of rhabdomyolysis, with or without acute renal failure, secondary to myoglobinuria; diabetes mellitus; interstitial lung disease | risk of rhabdomyolysis is increased with CsA, TAC, SIR, EVR, erythromycin, clarithromycin, fluconazole, itraconazole, ketoconazole, posaconazole, voriconazole, nefazodone, niacin, gemfibrozil, other fibric acid derivates or HIV-protease inhibitors, grapefruit juice, or amiodarone; oral contraceptives increase in plasma concentrations of norethindrone and ethinyl estradiol; with daptomycin risk of myopathy and/or rhabdomyolysis; daily ticagrelor doses greater than 40 mg simvastatin are not recommended; other fibrates (except fenofibrate) do not exceed 10 mg simvastatin daily nicotinic acid ≥ 1 g/day for Asian patients, not recommended with simvastatin; with amiodarone, amlodipine, verapamil, diltiazem, elbasvir, grazoprevir, do not exceed 20 mg simvastatin daily; rifampicin is a potent CYP3A4 inducer with a loss of efficacy of simvastatin | not in active liver disease or unexplained persistent elevations of serum transaminases; potent CYP3A4 inhibitors, that increase the AUC by at least approx. fivefold, e.g., itraconazole, ketoconazole, posaconazole, voriconazole, HIV protease inhibitors, ritonavir, erythromycin, clarithromycin, telithromycin, CsA, gemfibrozil, danazol, cobicistat, and nefazodone; fusidic acid; grapefruit juice; with ticagrelor or lomitapide max. 40 mg/d | all patients starting therapy with simvastatin, or whose dose of simvastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness, or weakness; simvastatin should be temporarily stopped a few days prior to elective major surgery, and when any major medical or surgical condition, supervene; in patients with severe renal impairment (creatinine clearance < 30 mL/min), dosages above 10 mg/day should be carefully considered; pitavastatin and pravastatin may be safer alternatives, since they are not metabolized by CYP3A4 or fluvastatin (only partially via CYP3A) |
| Sirolimus (SIR) [3,4,5,6,7] | impaired or delayed wound healing in patients receiving SIR, including lymphocele in renal transplant patients and wound dehiscence, patients with a BMI greater than 30 kg/m2 may be at increased risk of abnormal wound healing; fluid accumulation, includeing peripheral oedema, lymphoedema, pleural effusion, and pericardial effusions; hepatotoxicity increasing SIR exposure; proteinuria; arthralgia; microbial infections; thrombocytopenia; anemia; leucopenia; hypokalemia; hypophosphatemia; hyperlipidemia with increased serum cholesterol and triglycerides that may require treatment, risk/benefit to be considered in patients with established hyperlipidemia, and to be re-evaluated in patients with severe refractory hyperlipidemia.; hyperglycemia; diabetes mellitus; ovarian cysts; thrombosis and pulmonary embolism; skin cancer; lymphoma; monitor for elevated lipids; increased susceptibility to infection, and the possible development of lymphoma and other malignancies, particularly of the skin; increases susceptibility to infection, including opportunistic infections (bacterial, fungal, viral, and protozoal), fatal infections, and sepsis, in renal transplant patients BK virus-associated nephropathy and JC virus-associated progressive multifocal leukoencephalopathy (PML) to be considered in differential diagnosis in immunosuppressed patients with deteriorating renal function or neurological symptoms; antimicrobial prophylaxis for Pneumocystis carinii pneumonia should be administered for the first 12 months following transplantation, CMV prophylaxis is recommended for 3 months after renal transplantation, particularly for patients at increased risk of CMV disease | extensively metabolized by the CYP3A4 isozyme in the intestinal wall and liver, also a substrate for the multidrug efflux pump P-gp located in the small intestine; coadministration with voriconazole increases SIR 7-11-fold, with ketoconazole not recommended; avoid grapefruit juice; rifampicin decreases SIR (5,5-fold increased clearance of SIR) and is not recommended; renal function to be monitored with CsA; the concomitant use of SIR with a CNI may increase the risk of CNI-induced hemolytic uremic syndrome/thrombotic thrombocytopenic purpura/thrombotic microangiopathy (HUS/TTP/TMA); monitor for rhabdomyolysis with statins; with (ACE) inhibitors angioneurotic oedema-type reactions, elevated SIR levels, for example, due to interaction with strong CYP3A4 inhibitors, may also potentiate angioedema; increased rates of biopsy-confirmed acute rejection (BCAR) in renal transplant patients have been observed with concomitant use of SIR with ACE inhibitors | continued coadministration of CsA and SIR as maintenance therapy cannot be recommended considering increased nephrotoxicity and malignancies; SIR, MMF, and corticosteroids, in combination with IL-2 receptor antibody (IL2R Ab) induction, is not recommended in the de novo renal transplant setting; not with strong inhibitors of CYP3A4 (such as ketoconazole, voriconazole, itraconazole, telithromycin, or clarithromycin) which severely increase SIR exposure; not with inducers of CYP3A4 (such as rifampin and rifabutin), rifampicin increased the clearance of SIR by approximately 5.5-fold, coadministration of SIR, and rifampicin is not recommended; vaccination may be less effective, the use of live vaccines should be avoided during SIR; safety and efficacy of SIR as immunosuppressive therapy have not been established in liver or lung transplant patients, and therefore such use is not recommended | dose reduction in hepatic impairment; periodic quantitative monitoring of proteinuria; conversion from the TAC to SIR in maintenance renal transplant patients was associated with an unfavorable safety profile without efficacy benefits, and can therefore not be recommended; in hepatically impaired patients, SIR whole blood trough levels to be closely monitored, in patients with severe hepatic impairment, reduction in maintenance dose by one-half is recommended with close monitoring after a loading dose or a change of dose for a prolonged period of time, until stable concentrations are reached; in de novo liver transplant patients, the use of SIR plus CsA or TAC was associated with an increase in hepatic artery thrombosis, mostly leading to graft loss or death; a clinical study in liver transplant patients randomized to conversion from a CNI-based regimen to a SIR-based regimen versus continuation of a CNI-based regimen 6-144 months post-liver transplantation failed to demonstrate superiority in baseline-adjusted GFR at 12 months; cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when SIR has been used as part of an immunosuppressive regimen |
| Sitagliptin [103] | acute pancreatitis; hypoglycemia when used in combination with other anti-hyperglycemic drug (e.g., sulphonylurea, insulin); headache | CYP3A4 metabolism may play a role in the elimination of sitagliptin in the setting of severe renal impairment or end-stage renal disease (ESRD); for this reason, potent CYP3A4 inhibitors (i.e., ketoconazole, itraconazole, ritonavir, clarithromycin) might increase exposure | should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis | dose adjustment based upon renal function, the assessment of renal function is recommended prior to initiation and periodically thereafter |
| Sulfamethoxazole/Trimethoprim [104] | hyperkalemia and hyponatremia; metabolic acidosis; headache; nausea, diarrhea; caution in patients with severe atopy or bronchial asthma; very rare, due to severe Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, other blood dyscrasias, and hypersensitivity of the respiratory tract; very rarely hemophagocytic lymphohistiocytosis (HLH); very rarely respiratory toxicity; very rarely sulphonamide crystals are noted in cooled urine, in patients suffering from malnutrition, the risk may be increased; asymptomatic changes in hematological laboratory indices due to the lack of available folate, supplementation with folinic acid may be considered, but this should be initiated with caution due to possible interferences with antimicrobial efficacy; overgrowth fungal; jaundice, cholestatic, and hepatic necrosis; cough, dyspnea and lung infiltration; rhabdomyolysis reported in HIV patients with prophylaxis or the treatment of PJP | deterioration in renal function with CsA; zidovudine may increase the risk of hematological adverse reactions; possibility of competitive inhibition and increased exposure with drugs that form cations at physiological pH, and are also partly excreted by active renal secretion (e.g., procainamide, amantadine), there is the possibility of competitive inhibition; elderly patients concurrently receiving diuretics, mainly thiazides, appear to be at an increased risk of thrombocytopenia; megaloblastic anemia with pyrimethamine; potentiates the anticoagulant activity of warfarin; increased exposure of phenytoin; increased digoxin exposure; may increase the free plasma levels of methotrexate; with methotrexate, a folate supplement should be considered; increased lamivudine exposure; likely potentiation with sulphonylurea hypoglycemic agents; risk of hyperkalemia with ACE inhibitors, angiotensin receptor blockers, and potassium-sparing diuretics, such as spironolactone; increased exposure of repaglinide (hypoglycemia); folinic acid supplementation interferes with the antimicrobial efficacy; with azathioprine risk of serious hematological abnormalities; oral contraceptive failures have been reported with antibiotics | not in severe hepatic impairment; not in severe renal insufficiency; not with a history of drug-induced immune thrombocytopenia with the use of trimethoprim and/or sulphonamides; except under careful supervision, it should not be given to patients with serious hematological disorders | care is always advisable in elderly patients due to higher levels of susceptibility to ADRs, e.g., with impaired kidney and/or liver function and/or concomitant use of other drugs; effects associated with high dose pneumocystis jirovecii pneumonitis (PJP) management are severe hypersensitivity reactions, rash, pyrexia, neutropenia, thrombocytopenia, hepatic enzyme increased, hyperkalemia, hyponatremia, rhabdomyolysis |
| Tacrolimus (TAC) [2] | acute renal impairment without active intervention may progress to chronic renal impairment, patients with impaired renal function should be monitored closely as the dosage of TAC may need to be reduced, the risk for nephrotoxicity may increase when TAC is administered with drugs associated with nephrotoxicity; renal failure, renal failure acute, oliguria, renal tubular necrosis, nephropathy toxic, urinary abnormalities, bladder, and urethral symptoms; hypertension, ischemic coronary artery disorders, tachycardia; hemorrhage, thromboembolic, and ischemic events, peripheral vascular disorders, vascular hypotensive disorders; increased risk for infections (viral, bacterial, fungal, protozoal), the course of preexisting infections may be aggravated, both generalized and localized infections can occur, CMV infection, BK virus-associated nephropathy, JC virus-associated progressive multifocal leukoencephalopathy (PML); gastrointestinal disorders incl. diarrhea, nausea, gastrointestinal inflammatory conditions, gastrointestinal ulceration and perforation, gastrointestinal hemorrhages, stomatitis and ulceration, ascites, vomiting, gastrointestinal and abdominal pains, dyspeptic signs and symptoms, constipation, flatulence, bloating and distension, and loose stools; TAC blood levels may significantly decrease during diarrhea episodes; anemia, leukopenia, thrombocytopenia, leukocytosis, red blood cell analyses may be abnormal; dyspnea; benign as well as malignant neoplasms including EBV-associated lymphoproliferative disorders, skin malignancies; parenchymal lung disorders, pleural effusion, pharyngitis, cough, nasal congestion, and inflammations; pain in lower extremities reported as calcineurin-inhibitor induced pain syndrome (CIPS); primary graft dysfunction with medication errors; abnormal liver function tests, cholestasis and jaundice, hepatocellular damage and hepatitis, cholangitis; asthenic conditions, febrile disorders, oedema, pain and discomfort, body temperature perception disturbed; arthralgia, muscle spasms, pain in extremity, back pain; pruritus, rash, alopecia, acne, sweating increased; tinnitus; vision blurred, photophobia, eye disorders; tremor, headache, seizures, disturbances in consciousness, paraesthesia and dysesthesia, peripheral neuropathies, dizziness, writing impaired, nervous system disorders; insomnia, anxiety symptoms, confusion and disorientation, depression, depressed mood, mood disorders and disturbances, nightmares, hallucinations, mental disorders; hyperglycemic conditions, diabetes mellitus, hyperkalemia, hypomagnesemia, hypophosphatemia, hypokalemia, hypocalcemia, hyponatremia, fluid overload, yperuricemia, decreased appetite, metabolic acidosis, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, other electrolyte abnormalities | CYP3A4 inhibitors increase TAC blood levels, which could lead to serious adverse reactions, including nephrotoxicity, neurotoxicity, and QTc prolongation; increase in TAC blood levels when coadministered with inhibitors of CYP3A4 is mainly a result of an increase in the oral bioavailability of TAC, owing to the inhibition of gastrointestinal metabolism, the effect on hepatic clearance is less pronounced; P-glycoprotein inhibitors increase TAC blood levels; CYP3A4 inducers reduce TAC exposure; high-potassium intake or potassium-sparing diuretics should be avoided; TAC with drugs known to have neurotoxic effects may increase the risk of these effects; the risk for nephrotoxicity may increase when TAC is administered with drugs associated with nephrotoxicity, combination should be avoided; with MMF, caution when switching combination therapy from CsA, which interferes with enterohepatic recirculation reducing MPA, to TAC, which is devoid of this effect, resulting in elevated mycophenolic acid exposure; as TAC may be associated with hyperkalemia, or may increase pre-existing hyperkalemia, high-potassium intake, or potassium-sparing diuretics (e.g., amiloride, triamterene, or spironolactone) should be avoided, care when TAC is coadministered with other agents that increase serum potassium, such as trimethoprim and cotrimoxazole (trimethoprim/sulfamethoxazole), as trimethoprim acts as a potassium-sparing diuretic like amiloride, the close monitoring of serum potassium is recommended; concomitant administration TAC with SIR or EVR may increase the risk of thrombotic microangiopathy (incl. hemolytic uremic syndrome and thrombotic thrombocytopenic purpura); enhanced nephrotoxic or neurotoxic effect with aminoglycosides, gyrase inhibitors, vancomycin, sulfamethoxazole and trimethoprim, NSAIDs, ganciclovir, acyclovir, cidofovir, foscarnet, amphotericin B; avoid grapefruit or grapefruit juice associated with TAC increase; moderate or weak CYP3A4 inhibitors: antifungal agents (e.g., fluconazole, isavuconazole, clotrimazole, miconazole), the macrolide antibiotics (e.g., azithromycin), calcium channel blockers (e.g., nifedipine, nicardipine, diltiazem, verapamil), amiodarone, danazol, ethinylestradiol, lansoprazole, omeprazole, the HCV antivirals elbasvir/grazoprevir and glecaprevir/pibrentasvir, the CMV antiviral letermovir, and the tyrosine kinase inhibitors nilotinib, crizotinib, imatinib, and (Chinese) herbal remedies containing extracts of Schisandra sphenanthera may increase TAC whole blood trough concentrations and increase the risk of serious ADRs (e.g., neurotoxicity, QTc prolongation); in vitro, the following substances have been shown to be potential inhibitors of TAC metabolism: bromocriptine, cortisone (although in vivo, we also found a decrease in TAC and CsA with cortisone), dapsone, ergotamine, gestodene, lidocaine, mephenytoin, midazolam, nilvadipine, norethisterone, quinidine, tamoxifen; these may increase TAC whole blood trough concentrations and increase the risk of serious adverse reactions (e.g., neurotoxicity, QTc prolongation); graft function closely; moderate CYP3A4 inducers: metamizole, phenobarbital, isoniazid, rifabutin, efavirenz, etravirine, nevirapin, and weak CYP3A4 inducers: flucloxacillin, decrease TAC whole blood trough concentrations and increase the risk of rejection, it is strongly recommended to closely monitor TAC trough levels during treatment and up to 2 weeks after discontinuation; cannabinol increases TAC exposure; DDI with drugs with high affinity for plasma proteins, e.g.,: NSAIDs, oral anticoagulants, oral antidiabetics since TAC is extensively bound to plasma proteins; prokinetic agents: metoclopramide, cimetidine, and magnesium-aluminum-hydroxide may increase TAC whole blood trough concentrations; maintenance doses of corticosteroids decrease TAC whole blood trough concentrations and increase the risk of rejection; direct-acting antiviral (DAA) therapy may have an impact on the pharmacokinetics of TAC by impacting liver function during DAA therapy, related to the clearance of hepatitis virus, a decrease in TAC blood levels may occur; however, the CYP3A4-inhibiting potential of some DAAs may counteract that effect or lead to increased TAC blood levels; TAC is a CYP3A4 inhibitor, thus, the concomitant use of TAC with drugs known to be metabolized by CYP3A4 may affect the metabolism of such drugs, e.g., an increase in the exposure of CsA, phenytoin, steroid-based contraceptives, leading to increased hormone exposure; particular care should be exercised when deciding upon contraceptive measures; limited knowledge of interactions between TAC and statins is available (see Section 3.3.8 for more information); pentobarbital and phenazone half-life is increased | the half-life of CsA is prolonged when TAC is given concomitantly, in addition, synergistic/additive nephrotoxic effects, coadministration of CsA and TAC is not recommended; medication errors, including inadvertent, unintentional, or unsupervised substitution of the immediate- or prolonged-release TAC formulations, have been observed with serious adverse events, including graft rejection, or other side effects, which could be a consequence of either under- or overexposure to TAC, patients should be maintained on a single formulation of TAC with the corresponding daily dosing regimen, alterations in formulation or regimen should only take place under the close supervision of a transplant specialist; during the initial posttransplant period, monitor blood pressure, ECG, neurological and visual status, fasting blood glucose levels, electrolytes (particularly potassium), liver and renal function tests, hematology parameters, coagulation values, and plasma protein determinations for dose adjustments; strong CYP3A4 inhibitors: antifungal agents (e.g., ketoconazole, itraconazole, posaconazole, voriconazole), the macrolide antibiotics (e.g., telithromycin, troleandomycin, clarithromycin, josamycin, erythromycin), HIV protease inhibitors (e.g., ritonavir, nelfinavir, saquinavir), HCV protease inhibitors (e.g., telaprevir, boceprevir, and the combination of ombitasvir and paritaprevir with ritonavir, when used with and without dasabuvir), nefazodone, the pharmacokinetic enhancer cobicistat, and the kinase inhibitors idelalisib and ceritinib should be avoided, if unavoidable, monitor TAC blood levels, renal function, blood pressure, ECG, QTC intervals, and the clinical condition of the patient and adjust immediately, overall TAC exposure may increase >5-fold, with ritonavir >50-fold; the effect on TAC blood concentrations may remain for several days after coadministration is completed; similarly, the discontinuation of CYP3A4 inhibitors may lead to subtherapeutic blood levels of TAC, and therefore requires the close monitoring and supervision of a transplant specialist; CYP3A4 inducers decrease TAC blood levels, potentially increasing the risk of transplant rejection, the recommended that the concomitant use of strong CYP3A4 inducers (such as rifampicin, phenytoin, carbamazepine apalutamide, enzalutamide, mitotane, or St. John’s wort) with TAC should be avoided; if unavoidable, TAC blood levels and graft function should be monitored closely, maximal effect on TAC blood concentrations may be achieved after 1–2 weeks (we find about 5 days) after coadministration, and may remain for 1–2 weeks (we find about 5 days) after the completion of the treatment; similarly, the discontinuation of CYP3A4 inducers may lead to supratherapeutic blood levels TAC, and therefore requires the close monitoring and supervision of a transplant specialist; St. John’s wort or other herbal preparations should be avoided, due to decreases in TAC blood concentrations and reduced clinical effect, or an increase in TAC blood concentrations and TAC toxicity; not with CsA | 27% bioavailability decrease with food consumption, administer at least 1 h before or 2 h after meals; grapefruit juice increases exposure, St. John’s wort decreases exposure, TAC target whole blood trough concentration recommendations; dosing should primarily be based on clinical assessments of rejection and tolerability in each individual patient; to optimize dosing, several immunoassays are available for determining TAC concentrations in the whole blood, comparisons of concentrations from the published literature to individual values in clinical practice should be assessed with the care and knowledge of the assay methods employed; when dosed orally, blood trough levels should be drawn approximately 12 h post-dosing, just prior to the next dose; the frequency of blood level monitoring should be based on clinical needs, as TAC is with low clearance; adjustments to the dosage regimen may take several days before changes in blood levels are apparent; vaccination during treatment with TAC may be less effective, the use of live attenuated vaccines should be avoided |
| Tamsulosin [105] | hypotension, rarely syncope can occur, at the first signs of orthostatic hypotension (dizziness, weakness), the patient should sit or lie down until the symptoms have disappeared; dizziness, headache; palpitations; ejaculation disorder; angioedema; ‘intraoperative floppy iris syndrome’ (IFIS, a variant of small pupil syndrome), benefits of treatment discontinuation have not yet been established | strong inhibitors of CYP3A4 may lead to the increased exposure of tamsulosin hydrochloride, e.g., with ketoconazole 2.8-fold AUC, caution in combination with strong and moderate inhibitors of CYP3A4; diclofenac and warfarin may increase the elimination rate; concurrent other α1-adrenoceptor antagonists could lead to hypotensive effects | not with a history of orthostatic hypotension, not in severe hepatic insufficiency, micturition syncope history; not to be given in combination with strong inhibitors of CYP3A4 in patients with poor metabolizer CYP2D6 phenotype | caution with severe renal impairment (creatinine clearance of <10 mL/min), not yet studied |
| Ticagrelor [106] | blood disorder bleedings; hyperuricemia, gout/gouty arthritis; dyspnea (with asthma/chronic obstructive pulmonary disease (COPD) increased absolute risk of experiencing dyspnea), respiratory system bleedings; dizziness, syncope, headache; vertigo; hypotension; gastrointestinal hemorrhage; diarrhea, nausea, dyspepsia, constipation; urinary tract bleeding; blood creatinine increased (attention to patients ≥ 75 years, patients with moderate/severe renal impairment, and those receiving concomitant treatment with an angiotensin receptor blocker); post-procedural hemorrhage, traumatic bleedings; bradyarrhythmia events and AV block (primarily in patients with ACS, where cardiac ischemia and concomitant drugs reducing the heart rate or affecting cardiac conduction are potential confounders), ventricular pauses with ticagrelor were higher in patients with chronic heart failure (CHF); central sleep apnea including Cheyne–Stokes respiration; thrombotic thrombocytopenic purpura (TTP) | caution with the concomitant administration of medicinal products that may increase the risk of bleeding (e.g NSAIDs, oral anticoagulants and/or fibrinolytics); antifibrinolytic therapy (aminocaproic acid or tranexamic acid) and/or recombinant factor VIIa therapy may increase haemostasias; caution with bradycardia-inducing drugs; moderate CYP3A4 inhibitors (e.g., amprenavir, aprepitant, diltiazem, erythromycin, and fluconazole) only slightly change AUC; twofold-increase with grapefruit juice; decreased exposure with CYP3A inducers (e.g., rifampicin, phenytoin, carbamazepine, and phenobarbital), do not combine; caution increased exposure with potent P-gp inhibitors and moderate CYP3A4 inhibitors (e.g., CsA, verapamil, quinidine); reduced ticagrelor efficacy in patients who have been coadministered ticagrelor and morphine (not with parenteral); haemostasias altering drugs with caution in combination (with heparin, enoxaparin, and ASA or desmopressin possible); not with doses of simvastatin or lovastatin > 40 mg; not with CYP3A4 substrates with narrow therapeutic indices (i.e., cisapride or ergot alkaloids); monitoring in narrow therapeutic index P-gp-dependent drugs like digoxin; might reduce the renal excretion of rosuvastatin; cutaneous bleeding abnormalities with SSRIs (e.g., paroxetine, sertraline, and citalopram) | not with active pathological bleeding; history of intracranial hemorrhage; not with severe hepatic impairment; substantial increase in exposure with strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin, nefazodone, ritonavir, and atazanavir) | discontinue 5 days prior to surgery; platelet transfusion did not reverse the antiplatelet effect, unlikely to be of clinical benefit in patients with bleeding; desmopressin unlikely to be effective in managing clinical bleeding events; caution in moderate hepatic impairment, in sick sinus syndrome, 2nd or 3rd degree AV block, or bradycardic-related syncope; false negative results in a platelet function test (to include, but may not be limited to the HIPA test) for HIT have been reported in patients administered ticagrelor |
| Tigecycline [107] | prolonged both prothrombin time (PT) and activated partial thromboplastin time (aPTT), hypofibrinogenemia; nausea, vomiting, diarrhea; impaired healing; in complicated skin and soft tissue infections (cSSTI), complicated intra-abdominal infections (cIAI), diabetic foot infections, nosocomial pneumonia, and studies in resistant pathogens, a numerically higher mortality rate among tigecycline-treated patients has been observed as compared to the comparator treatment; liver injury with a predominantly cholestatic pattern up to hepatic failure; photosensitivity, pseudotumor cerebri, pancreatitis, and anti-anabolic action with increased BUN, azotemia, acidosis, and hyperphosphatemia; acute pancreatitis; AST and ALT abnormalities, hyperbilirubinemia | tigecycline is a P-gp substrate—the coadministration of P-gp inhibitors (e.g., ketoconazole or CSA) or P-gp inducers (e.g., rifampicin) could affect the pharmacokinetics of tigecycline; increase in trough concentrations of CsA and TAC; as it may prolong both the prothrombin time (PT) and activated partial thromboplastin time (aPTT), the relevant coagulation tests should be closely monitored when tigecycline is coadministered with anticoagulants | not in children < 8 years because of teeth discoloration | only where alternative antibiotics are not suitable; caution and monitor response with severe hepatic impairment (Child Pugh C), the dose of tigecycline should be reduced by 50%; the impaired healing of the surgical wound has been associated with superinfection, e.g., nosocomial pneumonia associated with poorer outcomes; there were a limited number of patients with severe underlying disease, such as immune-compromi-sed patients, patients with APACHE II scores > 15 (3.3%), or with surgically apparent multiple intra-abdominal abscesses (11.4%); limited experience is also available in treating patients with concurrent bacteremia (5.6%), therefore, caution is advised when treating such patients; consideration should be given to the use of combination antibacterial therapy whenever tigecycline is to be administered to severely ill patients with cIAI secondary to clinically apparent intestinal perforation or patients with incipient sepsis or septic shock; biliary excretion accounts for approximately 50% of the total tigecycline excretion, monitor in cholestasis |
| Valganciclovir (see Ganciclovir) [62,108] | to ensure maximal oral absorption, administer with or immediately after a meal | |||
| Vancomycin i.v. [109,110] | nephrotoxicity, ototoxicity from high serum concentrations, transitory or permanent, elderly more susceptible; monitor auditory function esp. in the elderly; rapid bolus administration may be associated with exaggerated hypotension, including shock and, rarely, cardiac arrest, histamine-like responses and maculopapular or erythematous rash (“red man’s syndrome”); thrombocytopenia, neutropenia, agranulocytosis, eosinophilia; phlebitis, redness of the upper body and the face, pain and spasms in the chest and back muscles | with an aminoglycoside (e.g., gentamycin), patients should be monitored carefully for signs of neurotoxicity and ototoxicity; dosage to be adjusted when renal disturbance occurs; the concurrent, sequential systemic, or topical use of other potentially neurotoxic or nephrotoxic drugs, such as gentamycin, amphotericin B, streptomycin, neomycin, kanamycin, amikacin, tobramycin, bacitracin, polymyxin B, colistin, viomycin, or cisplatin, may potentiate the nephrotoxicity and/or ototoxicity of vancomycin, and consequently requires careful monitoring; increased potential of neuromuscular blockade with concomitant neuromuscular blocking agents | should be avoided in patients with previous hearing loss; avoid the concomitant administration of vancomycin and anesthetic agents with risk of histamine-like flushing and anaphylactoid reactions | infusions should be given over at least 60 min; TDM—the measurement of levels to aid dose adjustments; dose adjustment and regular monitoring of renal function, neutropenia, agranulocytosis, eosinophilia, thrombocytopenia, pancytopenia |
| Voriconazol [111,112] | most common symptoms are as follows: visual impairment, pyrexia, rash, vomiting, nausea, diarrhea, headache, peripheral oedema; cheilitis, dyspepsia, constipation, gingivitis; liver function test abnormalities, respiratory distress, and abdominal pain; sinusitis; agranulocytosis, pancytopenia, thrombocytopenia, leukopenia, anemia; oedema peripheral; hypoglycemia, hypokalemia, hyponatremia; depression, hallucination, anxiety, insomnia, agitation, confusional state; convulsion, syncope, tremor, hypertonia, paresthesia, somnolence, dizziness; hypotension, phlebitis; arrhythmia supraventricular, tachycardia, bradycardia; QTc prolongation, risk of torsades de pointes increased with a history of cardiotoxic chemotherapy, cardiomyopathy, hypokalemia, hypomagnesemia, hypocalcemia, and concomitant QTc-prolonging drugs; caution with potentially proarrhythmic conditions, such as congenital or acquired QTc prolongation, cardiomyopathy, particularly when heart failure is present, sinus bradycardia, existing symptomatic arrhythmias; acute respiratory distress syndrome, pulmonary oedema; jaundice, jaundice cholestatic, hepatitis, hepatic toxicity, primarily in patients with serious underlying medical conditions (predominantly hematological malignancy); transient hepatic reactions, including hepatitis and jaundice, among patients with no other identifiable risk factors, must be carefully monitored for hepatic toxicity; phototoxicity, avoid exposure to direct sunlight and use protection; squamous cell carcinoma of the skin (including cutaneous SCC in situ, or Bowen’s disease); adrenal insufficiency (the direct inhibition of steroidogenesis); non-infectious periostitis (skeletal pain) with elevated fluoride and alkaline phosphatase levels has been reported in transplant patients; blurred vision, optic neuritis, and papilledema, retinal hemorrhage; pyrexia; chest pain, face oedema, asthenia, chills; acute renal failure, more likely with nephrotoxic drugs and concurrent conditions that may result in decreased renal function, monitor renal function, hematuria; with risk factors for acute pancreatitis (e.g., recent chemotherapy, hematopoietic stem cell transplantation [HSCT], serum amylase, or lipase should be monitored closely; hepatic function should be monitored | increased exposure of CsA and TAC with risk of renal impairment, dose be halved in CsA and reduced to one third in TAC, closely monitor levels, with the discontinuation of voriconazole, increase the CsA and TAC doses as necessary, according to TDM; increased exposure of NSAIDs (up to 100% in ibuprofen AUC) requires dose reduction, avoid NSAIDs generally because of additive nephrotoxicity; adrenal insufficiency in patients receiving azoles with corticosteroids (corticosteroid excess and adrenal suppression), Cushing’s syndrome with and without subsequent adrenal insufficiency has been reported with corticosteroids; on long-term treatment with corticosteroids (including inhaled corticosteroids, e.g., budesonide and intranasal corticosteroids) carefully monitor for adrenal cortex dysfunction, both during treatment and when voriconazole is discontinued; monitoring of phenytoin levels; increased glasdegib plasma concentrations with risks of QTc; increased tyrosine kinase inhibitor plasma concentrations and the risk of ADRs; monitoring of full blood counts ADRs to rifabutin (e.g., uveitis), combination should be avoided; low-dose ritonavir (100 mg twice daily) should be avoided; with methadone QTc prolongation, a dose reduction of methadone is recommended; reduction in the dose of alfentanil, fentanyl, and other short-acting opiates similar in structure to alfentanil and metabolized by CYP3A4 (e.g., sufentanil) should be considered; the half-life of alfentanil is prolonged fourfold; frequent monitoring for opiate-associated adverse reactions (including a longer respiratory monitoring period); where there are reductions in the dose of oxycodone and other long-acting opiates metabolized by CYP3A4 (e.g., hydrocodone), monitoring for opiate-associated adverse reactions may be necessary; oral fluconazole resulted in a significant increase in voriconazole exposure, monitoring for voriconazole-associated ADRs if voriconazole is used sequentially after fluconazole; decreased exposure (about 50%) with Letermovir, monitor for loss of voriconazole effectiveness; with flucloxacillin, decrease exposure; tyrosine kinase inhibitors (e.g., axitinib, bosutinib, cabozantinib, ceritinib, cobimetinib, dabrafenib, dasatinib, nilotinib, sunitinib, ibrutinib, ribociclib) require dose reduction because of increased exposure with voriconazole; warfarin and other oral coumarins monitor for increased prothrombin time to adjust; increased exposure of ivacaftor recommends dose reduction; increased exposure of midazolam and other benzodiazepines with a prolonged sedative effect require dose reduction; with increased PPI exposure, doses are probably to be halved; increased oral contraceptives exposure besides voriconazole exposure, monitor for ADRs; CYP3A4 metabolized statins (simvastatin, lovastatin) require dose reduction because of the risk of rhabdomyolysis; increased sulfonylureas (e.g., tolbutamide, glipizide, glyburide), dose reduction due to the risk of hypoglycemia; increased exposure of vinca alkaloids (e.g., vincristine and vinblastine) requires dose reduction, due to the risk of neurotoxicity; this may inhibit the metabolism of HIV protease inhibitors and the metabolism of voriconazole may also be inhibited by HIV protease inhibitors, dose adjustment will be required; with other non-nucleoside reverse transcriptase inhibitors (NNRTIs) (e.g., delavirdine, nevirapine) not studied, monitor for toxicity and inefficacy, as well to adjust; increase in tretinoin concentrations risk of pseudotumor cerebri, hypercalcemia; the effect on either erythromycin or azithromycin is unknown | not with CYP3A4 substrates, terfenadine, astemizole, cisapride, pimozide, or quinidine, due to QTc prolongation and the risk of torsades de pointes; not with rifampicin, carbamazepine, phenobarbital, and St. John’s Wort because of the decrease in plasma voriconazole exposure; not with efavirenz because of the decreased plasma voriconazole exposure and increased efavirenz; not with high-doses of ritonavir (400 mg and above twice daily) because of the decreased voriconazole exposure; not with ergot alkaloids (ergotamine, dihydroergotamine), which are CYP3A4 substrates, as this can lead to ergotism; not with SIR or EVR because of significantly elevated SIR or EVR exposure; not with naloxegol, because the increased naloxegol exposure precipitates opioid withdrawal symptoms; not with tolvaptan, lurasidone, because of the increased exposure; not with apixan, edoxaban, and rivaroxaban; caution with dabigatran due to the increased exposure and bleeding risk | is metabolized by, and inhibits the activity of, CYP2C19, CYP2C9, and, strongly, CYP3A4; must be carefully monitored for hepatic toxicity including laboratory evaluation of hepatic function (specifically AST and ALT) at the initiation of treatment and at least weekly for the first month of treatment; treatment duration should be as short as possible; long-term exposure (treatment or prophylaxis) greater than 6 months requires careful assessment of the benefit–risk balance, e.g., squamous cell carcinoma of the skin (SCC) (including cutaneous SCC in situ, or Bowen’s disease) has been reported in relation to long-term treatment; monitor renal function; to ensure maximal oral absorption, voriconazole tablets and oral suspension should be taken at least one hour before or after a meal |
| Zolpidem [113] | myorelaxant effect with a risk of falls and consequent injuries, particularly for elderly patients when they are active at night; anxiety or agitation have been described as signs of decompensated respiratory insufficiency; tolerance with some loss of efficacy to the hypnotic effects of short-acting benzodiazepines and benzodiazepine-like agents may develop after repeated use for a few weeks; abuse and physical and psychological dependence; withdrawal symptoms; rebound insomnia; decrease the dose gradually; somnambulism—sleep walking and other associated behaviors; next-day psychomotor impairment further increased with other CNS depressants or with other drugs that increase the blood levels of zolpidem, or with alcohol or illicit drugs; anterograde amnesia—ensure that they will be able to have an uninterrupted sleep of 8 h; psychiatric and “paradoxical” reactions more likely in the elderly; drowsiness and a decreased level of consciousness, which may lead to falls and consequently to severe injuries; consider congenital long QTc syndrome; may precipitate encephalopathy; increased incidence of suicidal ideation, suicide attempts, and suicide in patients with or without depression; pre-existing depression may be disclosed during any use of zolpidem, since insomnia may be a symptom of depression; the patient should be revaluated if insomnia persists; respiratory tract infection; hallucinations, agitation, nightmares, depression; somnolence, drowsiness during the following day, numbed emotions, reduced alertness, headache, dizziness, ataxia, exacerbated insomnia, cognitive disorder, amnesia; vertigo | concomitant opioids may result in sedation, respiratory depression, coma, and death; the enhancement of the central depressive effect may occur in cases of concomitant use with antipsychotics (neuroleptics), hypnotics, anxiolytics/sedatives, muscle relaxants, antidepressant agents, narcotic analgesics, antiepileptic drugs, anesthetics, and sedative antihistamines may increase drowsiness and next-day psychomotor impairment; visual hallucinations in patients taking zolpidem with antidepressants including bupropion, desipramine, fluoxetine, sertraline, and venlafaxine; in the case of narcotic analgesics, the enhancement of euphoria may also occur, leading to an increase in psychological dependence; rifampicin, carbamazepine, phenytoin, and St. John’s wort decrease blood levels of zolpidem; CYP3A4 inhibitors (ketoconazole) increase plasma concentrations and require dose reduction | contraindicated in patients with severe hepatic impairment; 5 mg in mild-to-moderate hepatic impairment; not with sleep apnea syndrome, previously known complex sleep behaviors after taking zolpidem, myasthenia gravis, acute or severe respiratory insufficiency; not with alcohol; not with fluvoxamine; not with ciprofloxacin due to increased blood levels | only indicated when insomnia is severe, disabling, or subjecting the individual to extreme distress; the duration of treatment varies from a few days to two weeks with a maximum, including the tapering-off process of four weeks; the tapering off process should be tailored to the individual; a single intake, should not be re-administered during the same night, lowest effective daily dose of zolpidem should be used and must not exceed 10 mg; in elderly or debilitated patients who may be especially sensitive to the effects of zolpidem, a dose of 5 mg is recommended; psychotic illness benzodiazepines and benzodiazepine-like agents not recommended for the primary treatment; extreme caution in patients with a history of alcohol or drug abuse; in elderly patients, the bioavailability of zolpidem is increased with reduced clearance, the maximal plasma concentration is increased by approximately 80% in a patient group aged 81–95 years; in hepatic impairment, the bioavailability of zolpidem is increased by 80%, and the half-life is increased from 2.4 h in healthy individuals to 9.9 h; in patients with liver cirrhosis, a fivefold increase in AUC and a threefold increase was observed in the half-life |
| Others | Interactions | CI | Other Aspects | |
| Red blood cell concentrate [114] | increased blood levels of CsA, TAC, SIR, EVR | |||
| Grapefruit juice | increased exposure of CsA, TAC, SIR, EVR, simvastatin, atorvastatin, lovastatin | |||
| St. John’s wort | decreased exposure of CsA, TAC, SIR, EVR, simvastatin, atorvastatin, lovastatin | |||
| Tyramine-rich foods [115] | red wine, many beers, aged cheeses, processed meats, and smoked fish; chocolate containing an amino acid derivative called tyramine—not with monoamine oxidase inhibitors, such as tranylcypromine, selegiline and phenelzine, or linezolid, due to the risk of hypertensive crisis | |||
| Vaccination [116] | never apply live vaccines with immunosuppressants; live vaccines include the nasal flu vaccine, measles, mumps, rubella vaccines, small pox vaccine, and the chicken pox vaccine (but not the Shingrix/zoster vaccine); the oral polio vaccine is contraindicated in patients and household members; vaccinia is contraindicated in patients, household members, and healthcare workers in transplant centers | |||
| Ginkgo biloba [117] | may influence the effect of anticoagulant drugs, such as warfarin and aspirin NSAIDs, and may potentiate the risk of bleeding; a lack of information on DDIs with DOACs, but since they may modulate P-gp activity, their ability to enhance the anticoagulant potential of DOACs needs to be analyzed; may reduce the effectiveness of antiepileptic drugs, SSRIs, and SNRIs | Ginkgolides, the major chemical components of Ginkgo biloba, have anti-inflammatory and anti-platelet properties | ||
| Cannabis [118] | CYP3A4 inhibitor ketoconazole nearly doubled the THC and cannabidiol concentrations; similar interactions could occur with other CYP3A4 inhibitors, including macrolides and verapamil, thus augmenting the psychoactive effects of THC and the dose-related adverse effects of cannabidiol (e.g., somnolence, transaminase elevation), CYP2C9 inhibitors, such as cotrimoxazole, fluoxetine, and amiodarone, would also be expected to increase the THC exposure and psychoactive effects; cannabidiol inhibits CYP2C19, increasing levels of the active metabolite of clobazam 3-fold; threefold increase in TAC with cannabidiol shows that CYP3A4/5 inhibition can also occur. Cave: this would identically affect CsA, SIR, EVR and numerous other drugs, e.g., most statins and calcium channel blockers. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, U. A Drug Safety Briefing (II) in Transplantation from Real-World Individual Pharmacotherapy Management to Prevent Patient and Graft from Polypharmacy Risks at the Very Earliest Stage. Pharmaceuticals 2024, 17, 294. https://doi.org/10.3390/ph17030294
Wolf U. A Drug Safety Briefing (II) in Transplantation from Real-World Individual Pharmacotherapy Management to Prevent Patient and Graft from Polypharmacy Risks at the Very Earliest Stage. Pharmaceuticals. 2024; 17(3):294. https://doi.org/10.3390/ph17030294
Chicago/Turabian StyleWolf, Ursula. 2024. "A Drug Safety Briefing (II) in Transplantation from Real-World Individual Pharmacotherapy Management to Prevent Patient and Graft from Polypharmacy Risks at the Very Earliest Stage" Pharmaceuticals 17, no. 3: 294. https://doi.org/10.3390/ph17030294
APA StyleWolf, U. (2024). A Drug Safety Briefing (II) in Transplantation from Real-World Individual Pharmacotherapy Management to Prevent Patient and Graft from Polypharmacy Risks at the Very Earliest Stage. Pharmaceuticals, 17(3), 294. https://doi.org/10.3390/ph17030294






