Effect of Hydration Forms and Polymer Grades on Theophylline Controlled-Release Tablet: An Assessment and Evaluation
Abstract
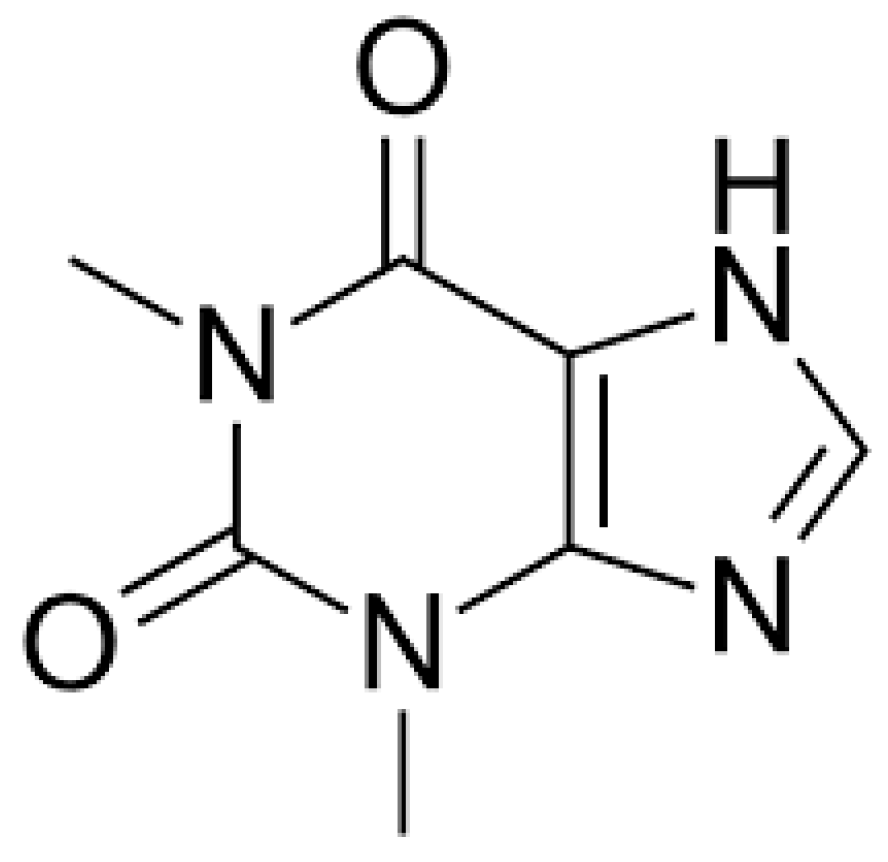
1. Introduction
2. Results
2.1. Quality Control Assessment
2.2. Calibration Curve
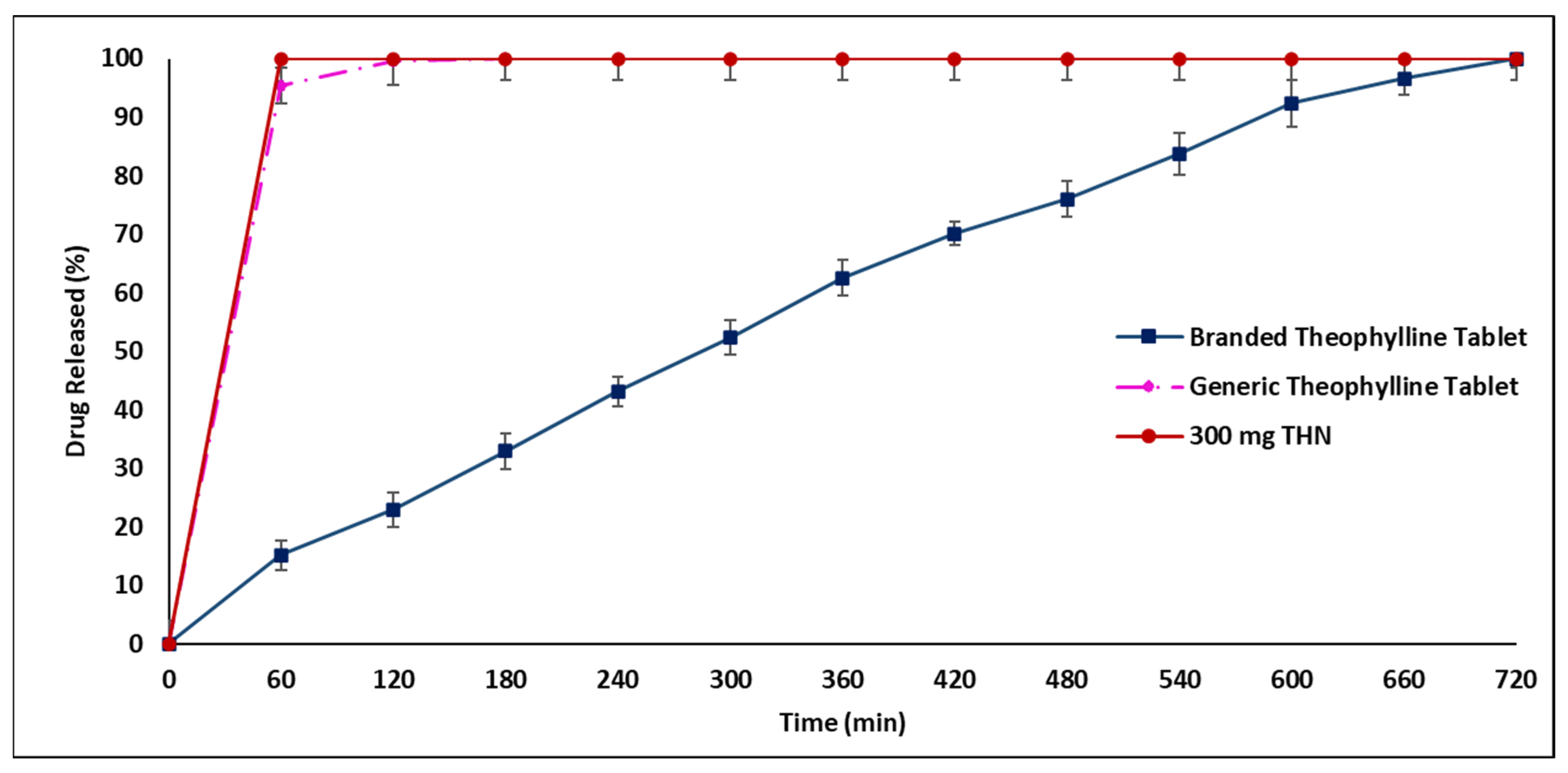
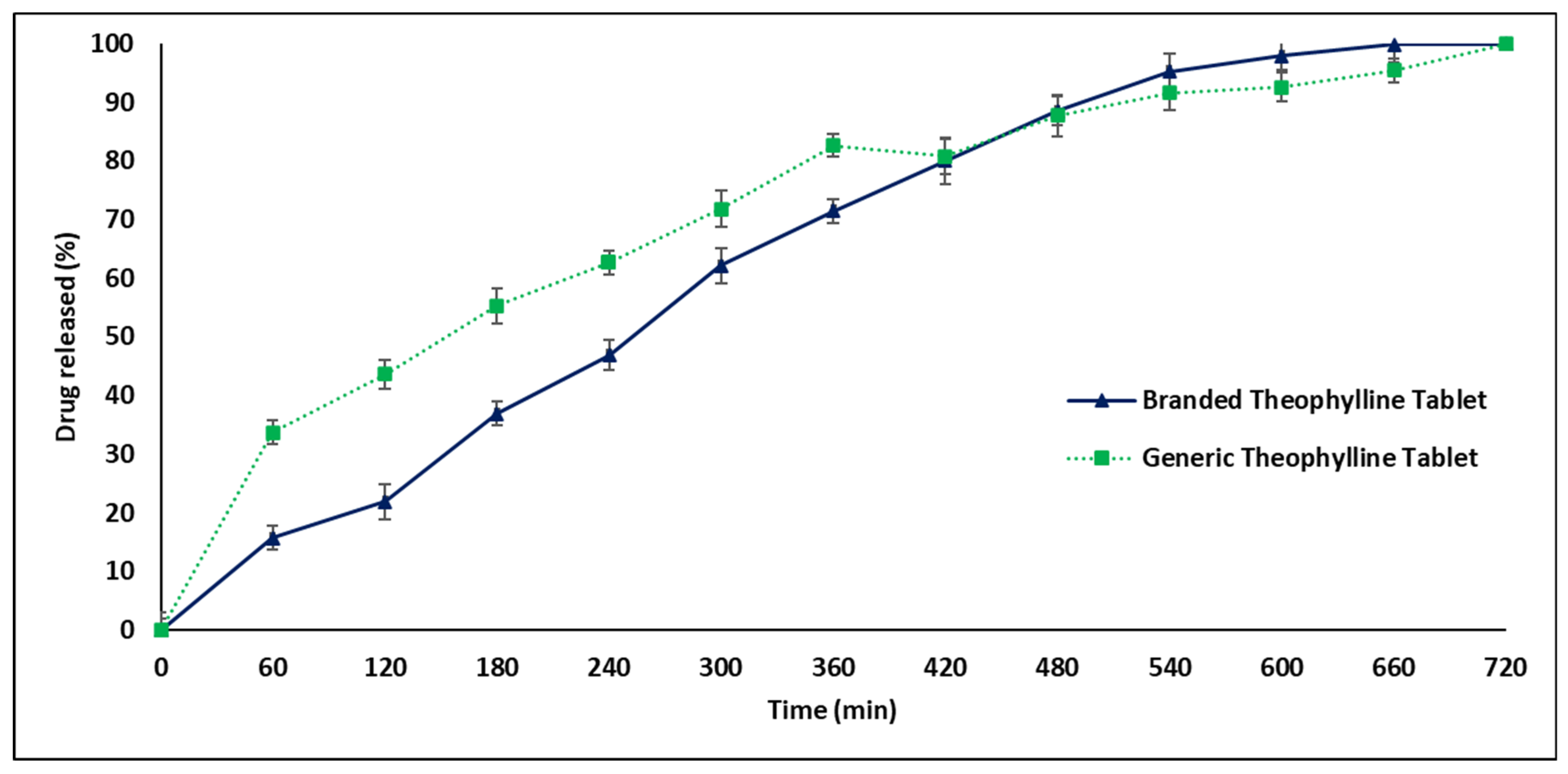
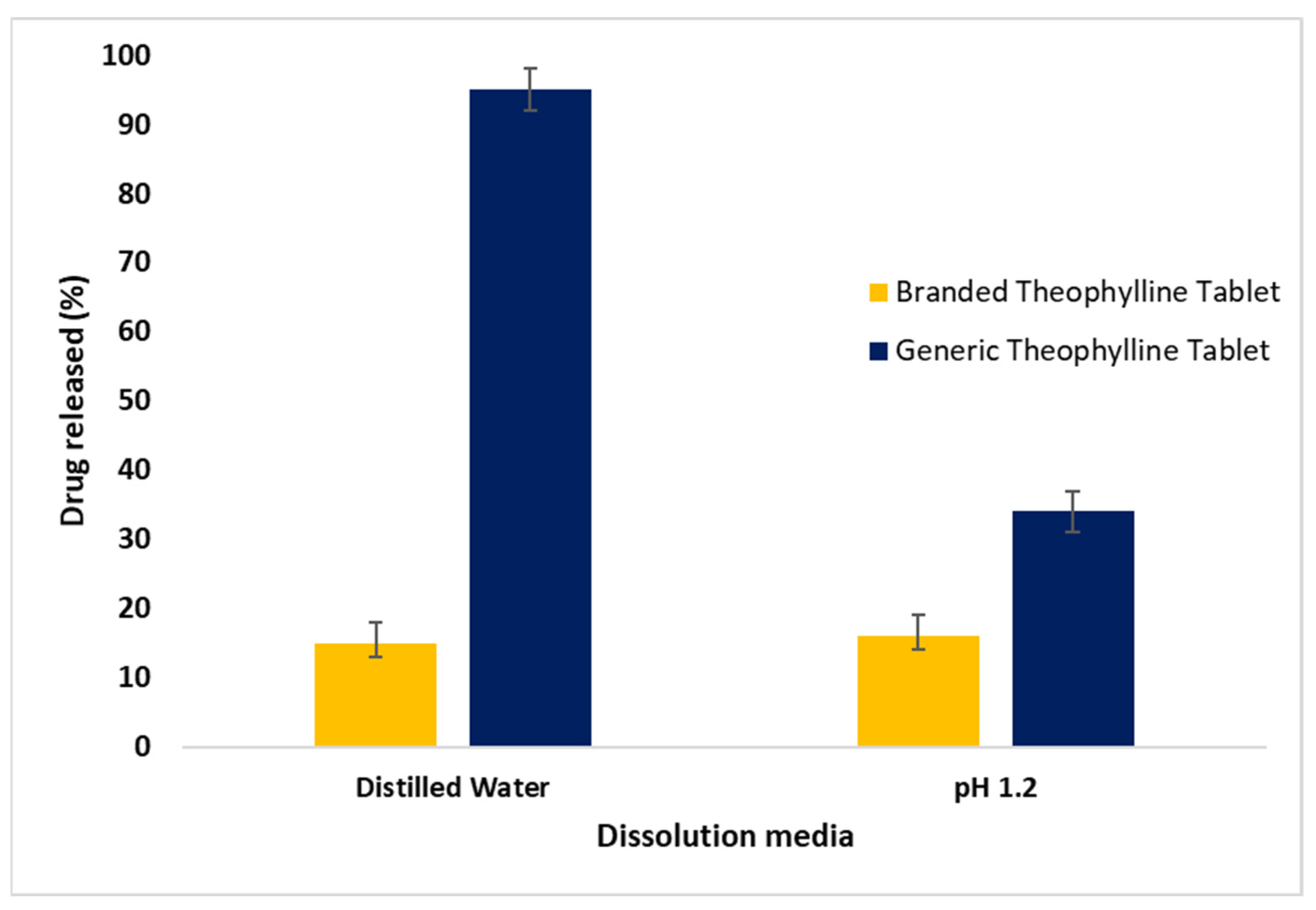
2.3. In Vitro Drug Release
2.4. Characterization Using Thermal and Analytical Techniques
2.4.1. X-ray Diffraction
2.4.2. Scanning Electron Microscopy
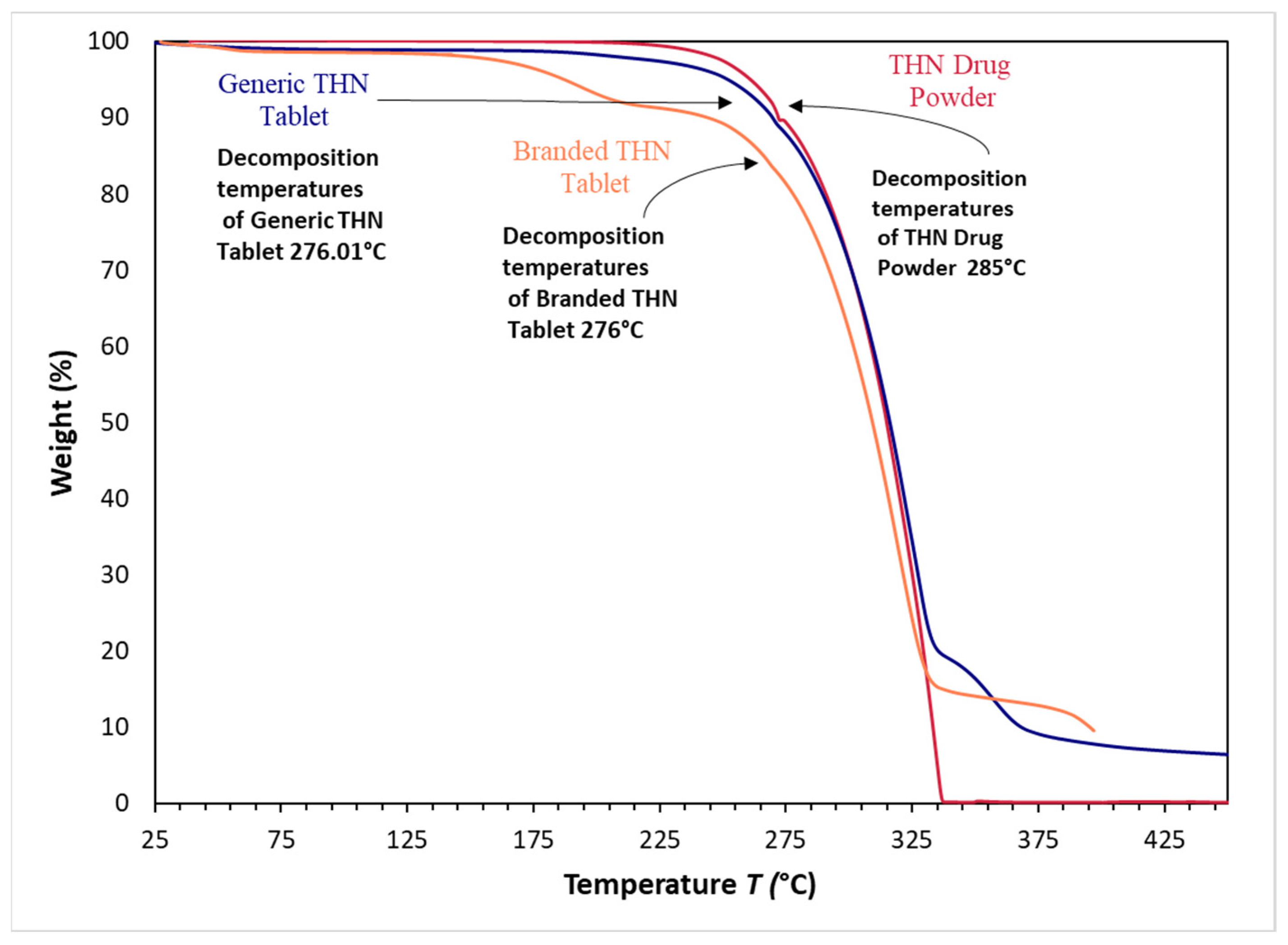
2.4.3. Thermal Analysis
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Quality Control Assessments
4.2.1. Weight Variation Analysis of Samples
4.2.2. Tablet Friability Testing
4.2.3. Assessment of Tablet Crushing Strength
4.3. Calibration Curve
4.4. In Vitro Dissolution Test
4.5. Characterization Using Thermal and Analytical Techniques
4.5.1. X-ray Diffraction
4.5.2. Scanning Electron Microscopy
4.5.3. Differential Scanning Calorimeter
4.5.4. Thermogravimetric Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, C.; Wu, M.; Xu, S.; Wang, X.; Shi, W.; Dong, Y.; Yang, L.; He, W.; Han, X.; Yin, L. Design and Optimization of Gastro-Floating Sustained-Release Tablet of Pregabalin: In vitro and in vivo Evaluation. Int. J. Pharm. 2018, 545, 37–44. [Google Scholar] [CrossRef]
- Mosab Arafat View of Approaches to Achieve an Oral Controlled Release Drug Delivery System Using Polymers: A Recent Review|International Journal of Pharmacy and Pharmaceutical Sciences. Available online: https://journals.innovareacademics.in/index.php/ijpps/article/view/4568/7060 (accessed on 1 December 2023).
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Ojoe, E.; Miyauchi, E.M.; Viviani, T.C.; Consiglieri, V.O. Formulation and in vitro Evaluation of Theophylline-Eudragit® Sustained-Release Tablets. Rev. Bras. Ciências Farm. 2005, 41, 377–384. [Google Scholar] [CrossRef][Green Version]
- THOMAS, J. Approach to the Management of Cancer Pain. In Essentials of Pain Medicine and Regional Anesthesia; Elsevier: Amsterdam, The Netherlands, 2005; pp. 525–534. [Google Scholar]
- Jin, G.; Ngo, H.V.; Wang, J.; Cui, J.-H.; Cao, Q.-R.; Park, C.; Jung, M.; Lee, B.-J. Design and Evaluation of in vivo Bioavailability in Beagle Dogs of Bilayer Tablet Consisting of Immediate Release Nanosuspension and Sustained Release Layers of Rebamipide. Int. J. Pharm. 2022, 619, 121718. [Google Scholar] [CrossRef]
- Montaño, L.; Sommer, B.; Gomez-Verjan, J.; Morales-Paoli, G.; Ramírez-Salinas, G.; Solís-Chagoyán, H.; Sanchez-Florentino, Z.; Calixto, E.; Pérez-Figueroa, G.; Carter, R.; et al. Theophylline: Old Drug in a New Light, Application in COVID-19 through Computational Studies. Int. J. Mol. Sci. 2022, 23, 4167. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Magistri, C.; Mellini, C.; Sarli, G.; Baldessarini, R.J. Comparison of Immediate and Sustained Release Formulations of Lithium Salts. Int. Rev. Psychiatry 2022, 34, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Heng, P.W.S. Controlled Release Drug Delivery Systems. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.I.; Limone, B.; Sobieraj, D.M.; Lee, S.; Roberts, M.S.; Kaur, R.; Alam, T. Dosing Frequency and Medication Adherence in Chronic Disease. J. Manag. Care Pharm. 2012, 18, 527–539. [Google Scholar] [CrossRef]
- Bouriche, S.; Alonso-García, A.; Cárceles-Rodríguez, C.M.; Rezgui, F.; Fernández-Varón, E. An in vivo Pharmacokinetic Study of Metformin Microparticles as an Oral Sustained Release Formulation in Rabbits. BMC Vet. Res. 2021, 17, 315. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Fahelelbom, K.; Sarfraz, M.; Bostanudin, M.; Sharif, Q.-A.; Esmaeil, A.; AL Hanbali, O.; Aburuz, S. Comparison between Branded and Generic Furosemide 40 Mg Tablets Using Thermal Gravimetric Analysis and Fourier Transform Infrared Spectroscopy. J. Pharm. Bioallied Sci. 2020, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Wichitnithad, W.; Nantaphol, S.; Noppakhunsomboon, K.; Thitikornpong, W.; Rojsitthisak, P. Current Status and Prospects of Development of Analytical Methods for Determining Nitrosamine and N-Nitroso Impurities in Pharmaceuticals. Talanta 2023, 254, 124102. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, M.R.; Zhang, F.; Ross-Degnan, D.; Wharam, J.F. Rates of Opioid Dispensing and Overdose After Introduction of Abuse-Deterrent Extended-Release Oxycodone and Withdrawal of Propoxyphene. JAMA Intern. Med. 2015, 175, 978. [Google Scholar] [CrossRef]
- Foglia, R.; Cooperman, N.; Mattern, D.; Borys, S.; Kline, A. Predictors of Intentional Fentanyl Use: Market Availability vs Consumer Demand. Int. J. Drug Policy 2021, 95, 103403. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Setnik, B.; Lee, K.; Cleveland, J.M.; Roland, C.L.; Wase, L.; Webster, L. Effectiveness and Safety of Morphine Sulfate Extended-Release Capsules in Patients with Chronic, Moderate-to-Severe Pain in a Primary Care Setting. J. Pain Res. 2011, 4, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Wolny, D.; Gruchlik, A.; Chodurek, E.W.A.; Szara, M. Comparison of Dissolution Profiles of Theophylline Extended-Release Dosage Forms. Acta Pol. Pharm. 2012, 69, 1384–1386. [Google Scholar]
- Karamchand, S.; Williams, M.; Naidoo, P.; Decloedt, E.; Allwood, B. Post-Tuberculous Lung Disease: Should We Be Using Theophylline? J. Thorac. Dis. 2021, 13, 1230–1238. [Google Scholar] [CrossRef]
- Viskin, S.; Havakuk, O.; Antzelevitch, C.; Ph, D.; Rosso, R.; Aviv, T. Theophylline: The Forgotten Antiarrhythmic Drug …. Now for Malignant Early Repolarization. Pacing Clin. Electrophysiol. PACE 2017, 41, 441–443. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline: New perspectives on an old drug. Am. J. Respir. Crit. Care Med. 2003, 167, 813–818. [Google Scholar] [CrossRef]
- Arafat, M.; Sarfraz, M.; AbuRuz, S. Development and In vitro Evaluation of Controlled Release Viagra® Containing Poloxamer-188 Using Gastroplus™ PBPK Modeling Software for In vivo Predictions and Pharmacokinetic Assessments. Pharmaceuticals 2021, 14, 479. [Google Scholar] [CrossRef]
- Bucklin, M.H.; Groth, C.M. Theophylline. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 530–532. [Google Scholar]
- Bostanudin, M.F.; Salam, A.; Mahmood, A.; Arafat, M.; Kaharudin, A.N.; Sahudin, S.; Lazim, A.M.; Azfaralariff, A. Formulation and In-Vitro Characterisation of Cross-Linked Amphiphilic Guar Gum Nanocarriers for Percutaneous Delivery of Arbutin. J. Pharm. Sci. 2021, 12, 3918. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hardy, R.J.; Gu, X. Effect of Drug Solubility on Polymer Hydration and Drug Dissolution from Polyethylene Oxide (PEO) Matrix Tablets. AAPS PharmSciTech 2008, 9, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Sakkal, M.; Bostanudin, M.F.; Alhanbali, O.A.; Yuvaraju, P.; Beiram, R.; Sadek, B.; Akour, A.; AbuRuz, S. Enteric-Coating Film Effect on the Delayed Drug Release of Pantoprazole Gastro-Resistant Generic Tablets. F1000Research 2023, 12, 1325. [Google Scholar] [CrossRef]
- Al-Hanbali, O.A.; Hamed, R.; Arafat, M.; Bakkour, Y.; Al-Matubsi, H.; Mansour, R.; Al-Bataineh, Y.; Aldhoun, M.; Sarfraz, M.; Dardas, A.K.Y. Formulation and Evaluation of Diclofenac Controlled Release Matrix Tablets Made of HPMC and Poloxamer 188 Polymer: An Assessment on Mechanism of Drug Release. Pak. J. Pharm. Sci. 2018, 31, 345–351. [Google Scholar] [PubMed]
- Hartzke, D.; Pössl, A.; Schlupp, P.; Runkel, F.E. Evaluation of Hydroxyethyl Cellulose Grades as the Main Matrix Former to Produce 3D-Printed Controlled-Release Dosage Forms. Pharmaceutics 2022, 14, 2103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, X.; Jasti, B.R. Role of Physicochemical Properties of Some Grades of Hydroxypropyl Methylcellulose on in vitro Mucoadhesion. Int. J. Pharm. 2021, 609, 121218. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Kamal, T.; Sarfraz, M.; Arafat, M.; Mikov, M.; Rahman, N. Cross-Linked Guar Gum and Sodium Borate Based Microspheres as Colon-Targeted Anticancer Drug Delivery Systems for 5-Fluorouracil. Pak. J. Pharm. Sci. 2017, 30, 2329–2336. [Google Scholar]
- Jannin, V.; Rosiaux, Y.; Doucet, J. Exploring the Possible Relationship between the Drug Release of Compritol®-Containing Tablets and Its Polymorph Forms Using Micro X-ray Diffraction. J. Control. Release 2015, 197, 158–164. [Google Scholar] [CrossRef]
- Mishra, D.; Nagpal, K.; Singh, S.; Mathur, V. Comparative Release Profile of Sustained Release Matrix Tablets of Verapamil HCl. Int. J. Pharm. Investig. 2013, 3, 60. [Google Scholar] [CrossRef]
- Roy, H.; Brahma, C.; Nandi, S.; Parida, K. Formulation and Design of Sustained Release Matrix Tablets of Metformin Hydrochloride: Influence of Hypromellose and Polyacrylate Polymers. Int. J. Appl. Basic Med. Res. 2013, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Mofizur Rahman, M.; Kumar Jha, M. Evaluation of Various Grades of Hydroxypropylmethylcellulose Matrix Systems as Oral Sustained Release Drug Delivery Systems Hindbrain Estrogenreceptor View Project Effect of Careya Arborea on Obesity Induced Diabetes in Wistar Albino Rats View Project. Artic. J. Pharm. Sci. Res. 2011, 03, 930–938. [Google Scholar]
- Ritschel, W.A.; Koch, H.P.; Alcorn, G.J. In Vivo-in vitro Correlations with Sustained-Release Theophylline Preparations. Methods Find. Exp. Clin. Pharmacol. 1984, 6, 609–618. [Google Scholar] [PubMed]
- Wilson, B.; Babubhai, P.P.; Sajeev, M.S.; Jenita, J.L.; Priyadarshini, S.R.B. Sustained Release Enteric Coated Tablets of Pantoprazole: Formulation, in vitro and in Vivo Evaluation. Acta Pharm. 2013, 63, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.-X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms; Elsevier: Amsterdam, The Netherlands, 2017; pp. 181–223. [Google Scholar]
- Asrade, B.; Tessema, E.; Tarekegn, A. In vitro comparative quality evaluation of different brands of carbamazepine tablets commercially available in Dessie town, Northeast Ethiopia. BMC Pharmacol. Toxicol. 2023, 24, 35. [Google Scholar] [CrossRef]
- Usuda, S.; Masukawa, N.; Leong, K.H.; Okada, K.; Onuki, Y. Effects of Manufacturing Process Variables on the Tablet Weight Variation of Mini-Tablets Clarified by a Definitive Screening Design. Chem. Pharm. Bull. 2021, 69, c21-00427. [Google Scholar] [CrossRef]
- Njega, E.K.; Maru, S.M.; Tirop, L.J. The Binder Effect of Povidone on the Mechanical Properties of Paracetamol Containing Tablets. East Cent. Afr. J. Pharm. Sci. 2018, 21, 3–9. [Google Scholar]
- Bobrovs, R.; Seton, L.; Dempster, N. The Reluctant Polymorph: Investigation into the Effect of Self-Association on the Solvent Mediated Phase Transformation and Nucleation of Theophylline. CrystEngComm 2015, 17, 5237–5251. [Google Scholar] [CrossRef]
- Tian, F.; Qu, H.; Zimmermann, A.; Munk, T.; Jørgensen, A.C.; Rantanen, J. Factors Affecting Crystallization of Hydrates. J. Pharm. Pharmacol. 2010, 62, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, E.; Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M. Pharmaceutical Hydrates Analysis—Overview of Methods and Recent Advances. Pharmaceutics 2020, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Zaworotko, M.J. Polymorphic Crystal Forms and Cocrystals in Drug Delivery (Crystal Engineering). In Burger’s Medicinal Chemistry and Drug Discovery; Wiley: Hoboken, NJ, USA, 2010; pp. 187–218. [Google Scholar]
- Adeli, E. Preparation and Evaluation of Azithromycin Binary Solid Dispersions Using Various Polyethylene Glycols for the Improvement of the Drug Solubility and Dissolution Rate. Braz. J. Pharm. Sci. 2016, 52, 1–13. [Google Scholar] [CrossRef]
- Cantin, O.; Siepmann, F.; Danede, F.; Willart, J.F.; Karrout, Y.; Siepmann, J. PEO Hot Melt Extrudates for Controlled Drug Delivery: Importance of the Molecular Weight. J. Drug Deliv. Sci. Technol. 2016, 36, 130–140. [Google Scholar] [CrossRef]
- Murugesan, S.; Gowramma, B.; Lakshmanan, K.; Reddy Karri, V.V.S.; Radhakrishnan, A. Oral Modified Drug Release Solid Dosage Form with Special Reference to Design; An Overview. Curr. Drug Res. Rev. 2020, 12, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Samie, M.; Bashir, S.; Abbas, J.; Khan, S.; Aman, N.; Jan, H.; Muhammad, N. Design, Formulation and In-Vitro Evaluation of Sustained Release Tablet Formulations of Levosulpiride. Turk. J. Pharm. Sci. 2018, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Baishya, H. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Gorajana, A.; Garg, S.; Koh, P.; Chuah, J.; Talekar, M. Formulation Development and Dissolution Rate Enhancement of Efavirenz by Solid Dispersion Systems. Indian J. Pharm. Sci. 2013, 75, 291. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Itkor, P.; Sirieawphikul, C.; Lee, Y.S. Characterization of Natural Anthocyanin Indicator Based on Cellulose Bio-Composite Film for Monitoring the Freshness of Chicken Tenderloin. Molecules 2022, 27, 2752. [Google Scholar] [CrossRef]
- Nunes, C.; Mahendrasingam, A.; Suryanarayanan, R. Investigation of the Multi-Step Dehydration Reaction of Theophylline Monohydrate Using 2-Dimensional Powder X-ray Diffractometry. Pharm. Res. 2006, 23, 2393–2404. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, A.; Ullah, R.; Ullah, M.; Alotaibi, A.; Ullah, R.; Haider, A. Preparation and Characterization of Hydrophilic Polymer Based Sustained-Release Matrix Tablets of a High Dose Hydrophobic Drug. Polymers 2022, 14, 1985. [Google Scholar] [CrossRef]
- Arafat, M.; Sakkal, M.; Yuvaraju, P.; Esmaeil, A.; Poulose, V.; Aburuz, S. Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets. Pharmaceuticals 2023, 16, 539. [Google Scholar] [CrossRef] [PubMed]




| Quality Assessment Parameter | The Generic Product of THN | The Branded Product of THN |
|---|---|---|
| Average weight (mg) | 394.43 ± 5.5 * | 414.2 ± 4.76 |
| Weight variation range (%) | 0.95 ± 0.93 | 0.76 ± 0.80 |
| Tablet friability (%) | 0.17 ± 0.02 | 0.16 ± 0.001 |
| Mean resistance force (N) | 120.83 ± 7.78 * | 492 ± 2.83 |
| Mean tablet diameter (mm) | 12.03 ± 0.01 * | 6.02 ± 0.01 |
| Sample | Melting Temperature (°C) | Enthalpy of Melting (J/g) | Onset Temperature (°C) | Maximum Decomposition Temperature (°C) |
|---|---|---|---|---|
| THN Drug Powder | 272.39 | 153.43 | 269.89 | 285.00 * |
| Generic THN Tablet | 272.04 | 178.50 | 268.26 | 276.01 |
| Branded THN Tablet | 269.69 | 86.036 * | 265.59 | 276.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakkal, M.; Arafat, M.; Yuvaraju, P.; Beiram, R.; Ali, L.; Altarawneh, M.; Hajamohideen, A.R.; AbuRuz, S. Effect of Hydration Forms and Polymer Grades on Theophylline Controlled-Release Tablet: An Assessment and Evaluation. Pharmaceuticals 2024, 17, 271. https://doi.org/10.3390/ph17030271
Sakkal M, Arafat M, Yuvaraju P, Beiram R, Ali L, Altarawneh M, Hajamohideen AR, AbuRuz S. Effect of Hydration Forms and Polymer Grades on Theophylline Controlled-Release Tablet: An Assessment and Evaluation. Pharmaceuticals. 2024; 17(3):271. https://doi.org/10.3390/ph17030271
Chicago/Turabian StyleSakkal, Molham, Mosab Arafat, Priya Yuvaraju, Rami Beiram, Labeeb Ali, Mohammednoor Altarawneh, Abdul Razack Hajamohideen, and Salahdein AbuRuz. 2024. "Effect of Hydration Forms and Polymer Grades on Theophylline Controlled-Release Tablet: An Assessment and Evaluation" Pharmaceuticals 17, no. 3: 271. https://doi.org/10.3390/ph17030271
APA StyleSakkal, M., Arafat, M., Yuvaraju, P., Beiram, R., Ali, L., Altarawneh, M., Hajamohideen, A. R., & AbuRuz, S. (2024). Effect of Hydration Forms and Polymer Grades on Theophylline Controlled-Release Tablet: An Assessment and Evaluation. Pharmaceuticals, 17(3), 271. https://doi.org/10.3390/ph17030271








