Mouse Type-I Interferon-Mannosylated Albumin Fusion Protein for the Treatment of Chronic Hepatitis
Abstract
1. Introduction
2. Results
2.1. Production and Biological Activity of Man-MSA-mIFNα2
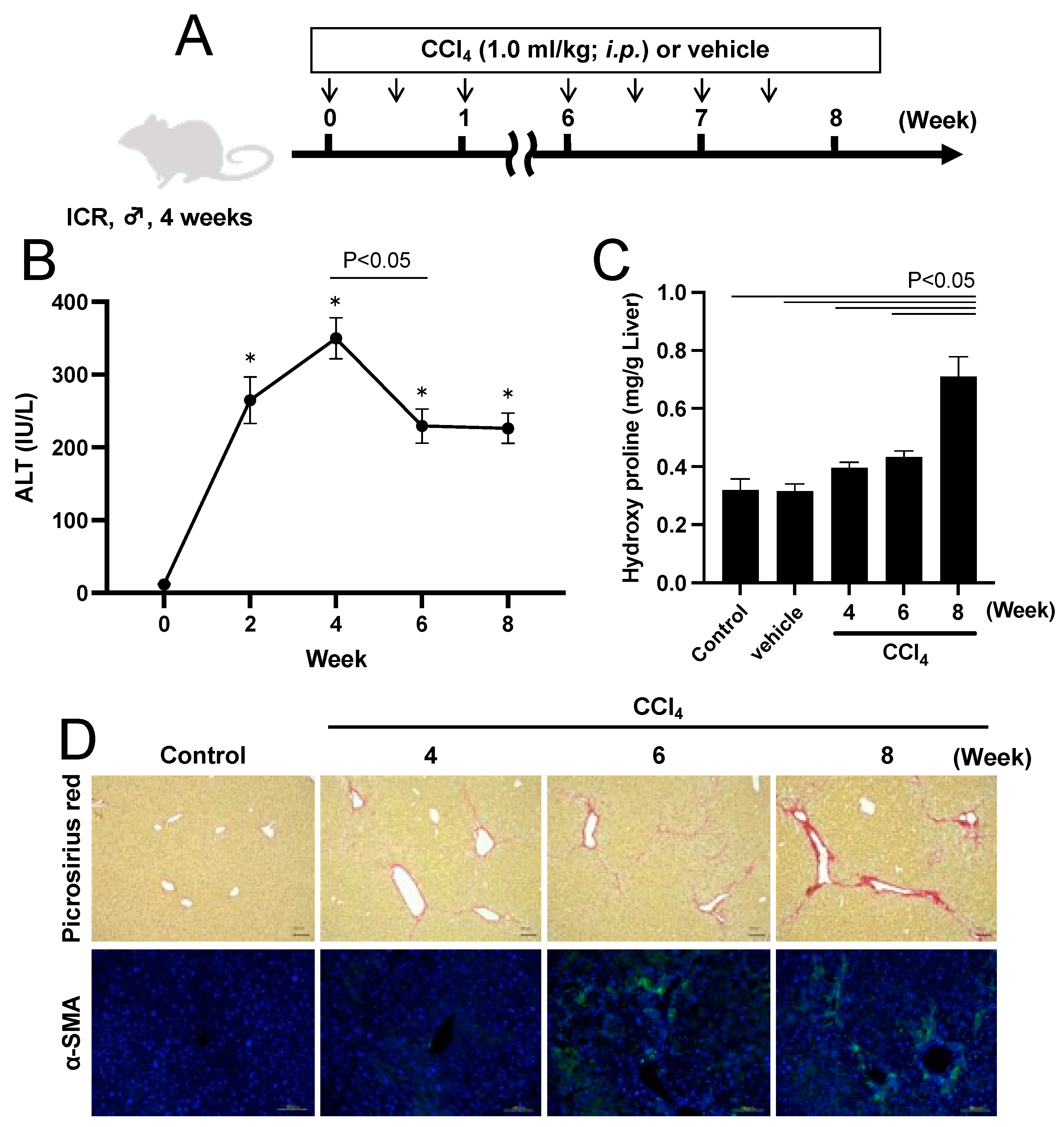
2.2. CCl4-Induced Chronic Hepatitis Model Mouse
2.3. Effect of Man-MSA-mIFNα2 on Hepatocellular Damage in CCl4-Induced Chronic Hepatitis Mice
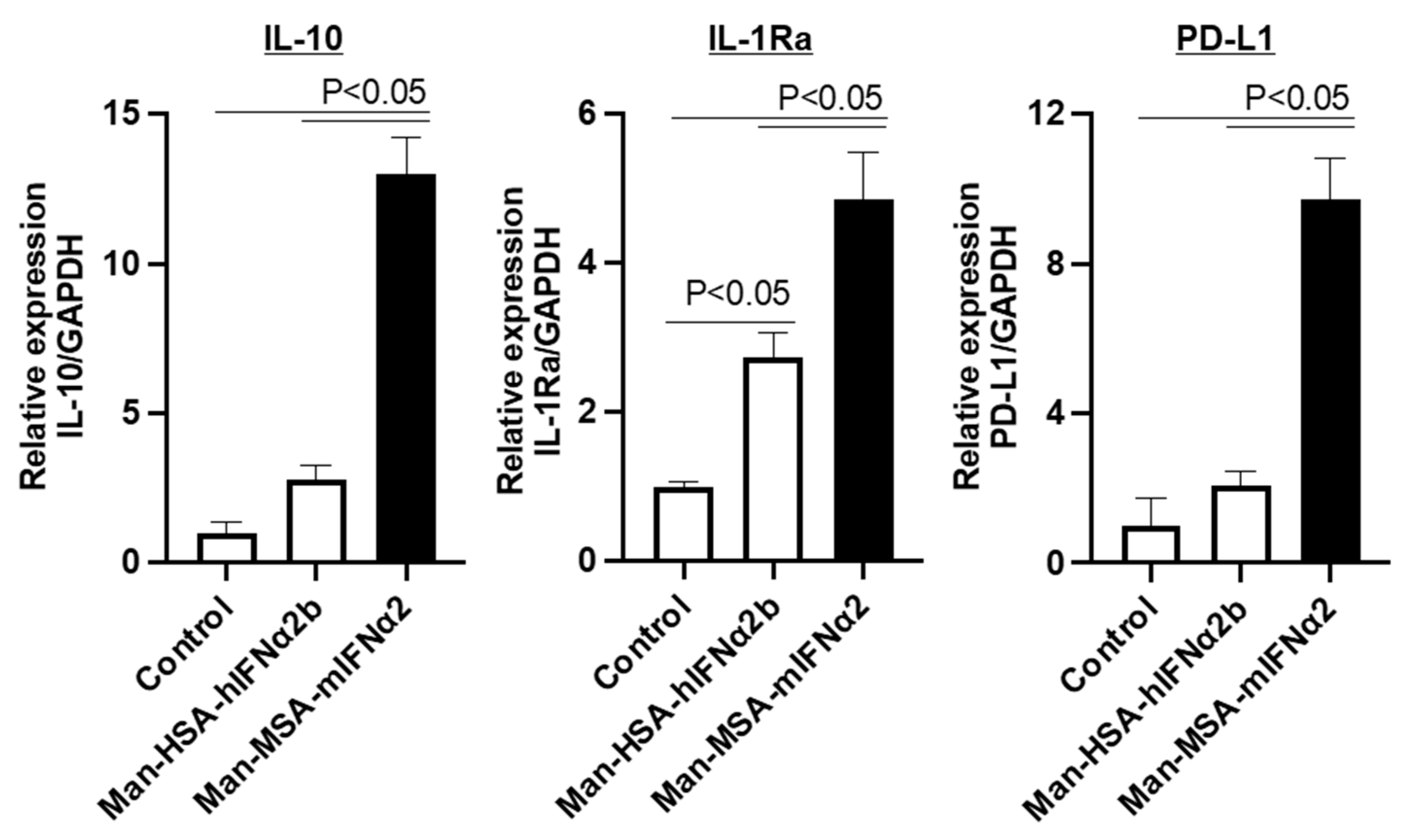
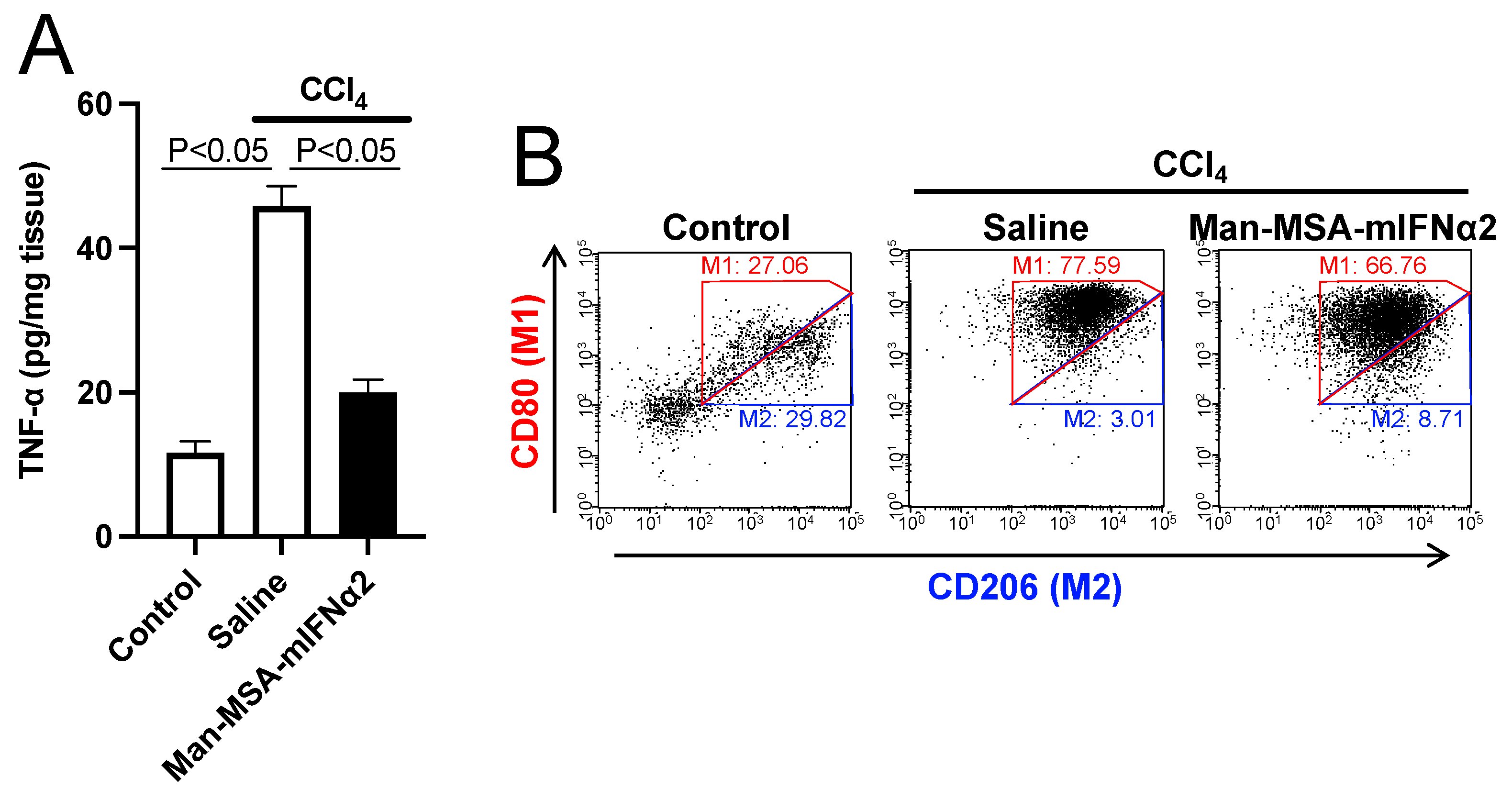
2.4. Hepatoprotective Mechanism of Man-MSA-mIFNα2 against CCl4-Induced Chronic Hepatitis Mice
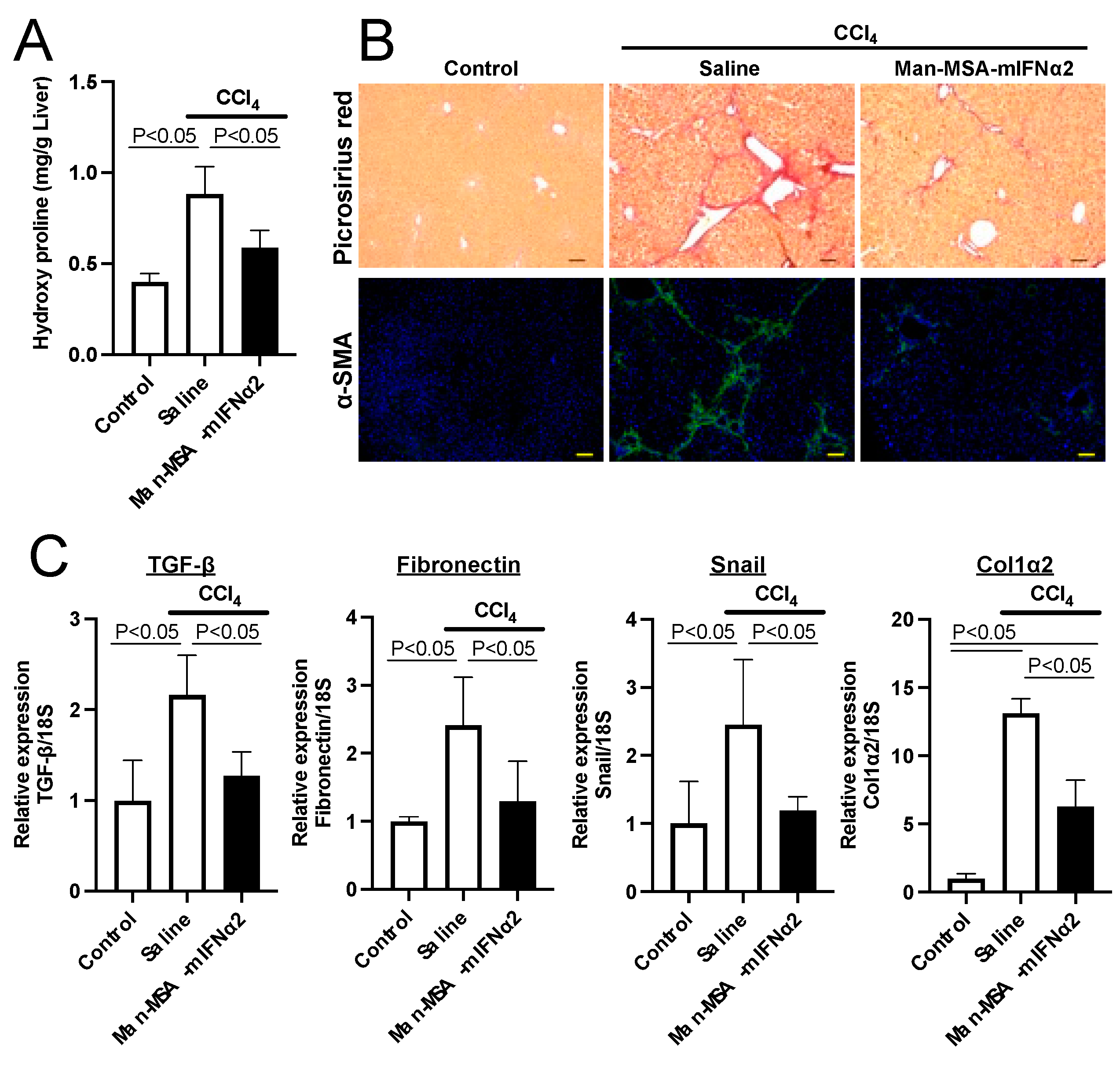
2.5. Effect of Man-MSA-mIFNα2 on Liver Fibrosis in CCl4-Induced Chronic Hepatitis Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Cell Culture
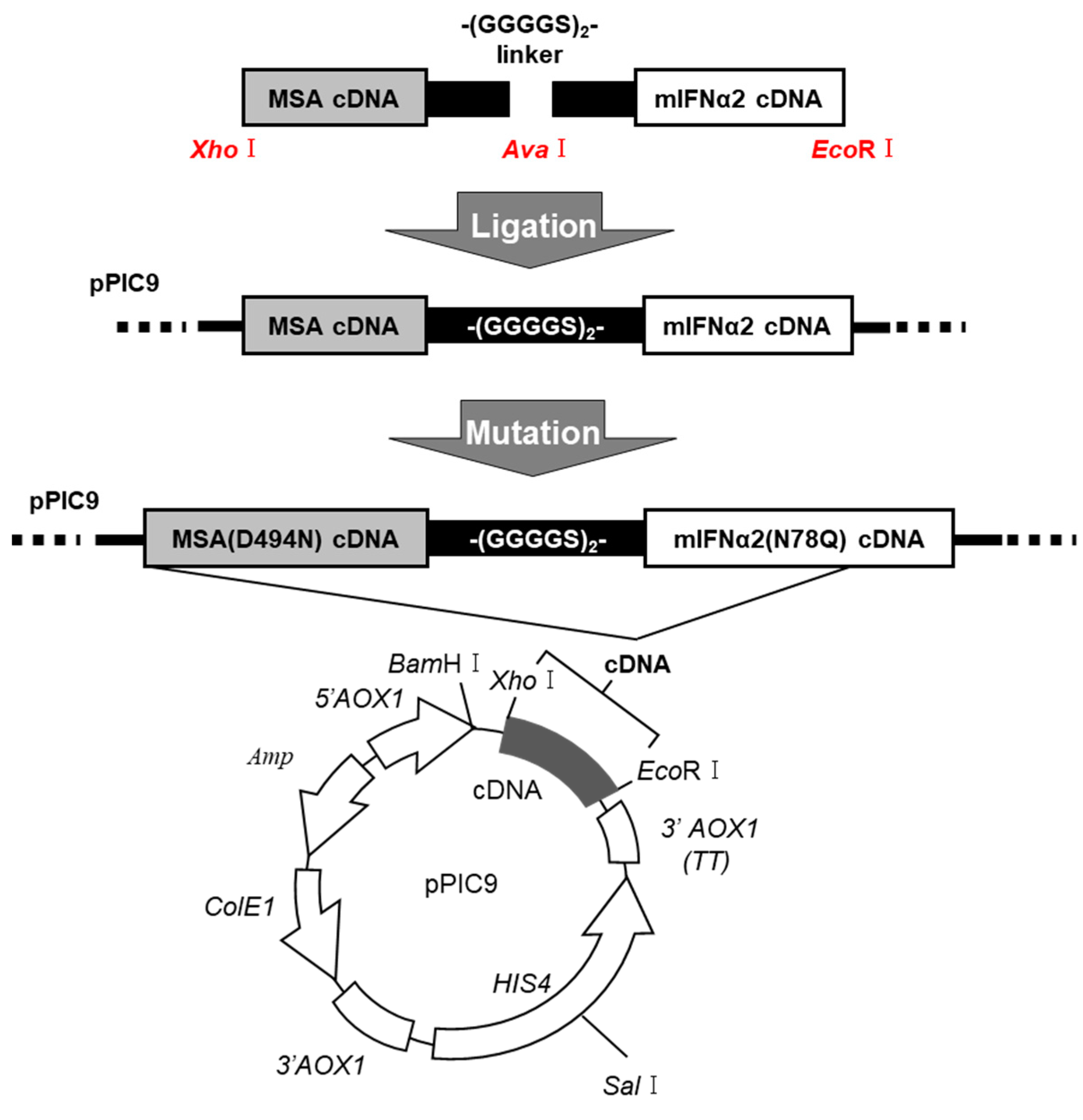
4.4. DNA Recombination of MSA(D494N)-mIFNα2(N78Q) Fusion Protein
4.5. Expression and Purification of the Fusion Protein
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Measurement of Alanine Aminotransferase (ALT)
4.8. Measurement of Hepatic Hydroxyproline Contents
4.9. Histological Analyses of Liver Tissue
4.10. Immunofluorescence Staining of Liver Tissue
4.11. Quantification of Hepatic TNF-α Levels
4.12. Analysis of M1/M2 Macrophage Polarization
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 11033–11053. [Google Scholar] [CrossRef]
- Sun, F.; Zhuang, Z.; Zhang, D.; Chen, Y.; Liu, S.; Gao, N.; Shi, J.; Wang, B. Chronic moderate alcohol consumption relieves high-fat high-cholesterol diet-induced liver fibrosis in a rat model. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1046–1055. [Google Scholar] [CrossRef]
- Schuppan, D.; Surabattula, R.; Wang, X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018, 68, 238–250. [Google Scholar] [CrossRef]
- Krawitt, E.L. Autoimmune hepatitis. N. Engl. J. Med. 2006, 354, 54–66. [Google Scholar] [CrossRef]
- Pinzani, M.; Rombouts, K.; Colagrande, S. Fibrosis in chronic liver diseases: Diagnosis and management. J. Hepatol. 2005, 42, S22–S36. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Tacke, F.; Zimmermann, H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.a. Science in medicine Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Kojima, Y. Immunizing property of vaccinia virus inactivated by ultraviolets rays. Comptes Rendus Seances Soc. Biol. Ses Fil. 1954, 148, 1700–1702. [Google Scholar]
- Holicek, P.; Guilbaud, E.; Klapp, V.; Truxova, I.; Spisek, R.; Galluzzi, L.; Fucikova, J. Type I interferon and cancer. Immunol. Rev. 2023, 321, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.L.; Grace, M.J.; Bordens, R.W. Interferon-alpha as an immunotherapeutic protein. J. Leukoc. Biol. 2002, 71, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Syedbasha, M.; Bonfiglio, F.; Linnik, J.; Stuehler, C.; Wüthrich, D.; Egli, A. Interferon-λ Enhances the Differentiation of Naive B Cells into Plasmablasts via the mTORC1 Pathway. Cell Rep. 2020, 33, 108211. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Dolganiuc, A.; Csak, T.; Nath, B.; Hritz, I.; Kodys, K.; Catalano, D.; Kurt-Jones, E.; Mandrekar, P.; Szabo, G. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 2011, 53, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Park, S.; Kim, J.W.; Lim, C.W.; Seki, E.; Kim, B. Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis. Hepatology 2014, 60, 237–249. [Google Scholar] [CrossRef]
- Xiao, W.; Klement, J.D.; Lu, C.; Ibrahim, M.L.; Liu, K. IFNAR1 Controls Autocrine Type I IFN Regulation of PD-L1 Expression in Myeloid-Derived Suppressor Cells. J. Immunol. 2018, 201, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Bazhin, A.V.; von Ahn, K.; Fritz, J.; Werner, J.; Karakhanova, S. Interferon-α Up-Regulates the Expression of PD-L1 Molecules on Immune Cells Through STAT3 and p38 Signaling. Front. Immunol. 2018, 9, 2129. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Teixeira, L.; Mayer-Barber, K.; Sher, A.; O’Garra, A. Type I interferons in tuberculosis: Foe and occasionally friend. J. Exp. Med. 2018, 215, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Type I interferon: Friend or foe? J. Exp. Med. 2010, 207, 2053–2063. [Google Scholar] [CrossRef]
- Yasui, S.; Fujiwara, K.; Yokosuka, O. Autoimmune fulminant hepatic failure in chronic hepatitis C during Peg-interferon-alpha 2b plus ribavirin treatment showing histological heterogeneity. Dig. Liver Dis. 2011, 43, 666–667. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Chuang, V.T.; Kragh-Hansen, U.; Otagiri, M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm. Res. 2002, 19, 569–577. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert. Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef]
- Kragh-Hansen, U. Human serum albumin: A multifunctional protein. In Albumin Medicine: Pathological and Clinical Applications; Springer: Singapore, 2016; pp. 1–24. [Google Scholar]
- Loureiro, A.; Azoia, N.G.; Gomes, A.C.; Cavaco-Paulo, A. Albumin-Based Nanodevices as Drug Carriers. Curr. Pharm. Des. 2016, 22, 1371–1390. [Google Scholar] [CrossRef]
- Kratz, F.; Elsadek, B. Clinical impact of serum proteins on drug delivery. J. Control. Release 2012, 161, 429–445. [Google Scholar] [CrossRef]
- Hirata, K.; Maruyama, T.; Watanabe, H.; Maeda, H.; Nakajou, K.; Iwao, Y.; Ishima, Y.; Katsumi, H.; Hashida, M.; Otagiri, M. Genetically engineered mannosylated-human serum albumin as a versatile carrier for liver-selective therapeutics. J. Control. Release 2010, 145, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hirata, K.; Watanabe, H.; Ishima, Y.; Chuang, V.T.; Taguchi, K.; Inatsu, A.; Kinoshita, M.; Tanaka, M.; Sasaki, Y.; et al. Polythiol-containing, recombinant mannosylated-albumin is a superior CD68+/CD206+ Kupffer cell-targeted nanoantioxidant for treatment of two acute hepatitis models. J. Pharmacol. Exp. Ther. 2015, 352, 244–257. [Google Scholar] [CrossRef]
- Maeda, H.; Minayoshi, Y.; Ichimizu, S.; Mizuta, Y.; Nagasaki, T.; Matsusaka, K.; Oshiro, S.; Oniki, K.; Saruwatari, J.; Ishima, Y.; et al. Repeated Administration of Kupffer Cells-Targeting Nanoantioxidant Ameliorates Liver Fibrosis in an Experimental Mouse Model. Biol. Pharm. Bull. 2020, 43, 93–101. [Google Scholar] [CrossRef]
- Maeda, H.; Ishima, Y.; Saruwatari, J.; Mizuta, Y.; Minayoshi, Y.; Ichimizu, S.; Yanagisawa, H.; Nagasaki, T.; Yasuda, K.; Oshiro, S.; et al. Nitric oxide facilitates the targeting Kupffer cells of a nano-antioxidant for the treatment of NASH. J. Control. Release 2022, 341, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Mesev, E.V.; LeDesma, R.A.; Ploss, A. Decoding type I and III interferon signalling during viral infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Minayoshi, Y.; Maeda, H.; Yanagisawa, H.; Hamasaki, K.; Mizuta, Y.; Nishida, K.; Kinoshita, R.; Enoki, Y.; Imafuku, T.; Chuang, V.T.G.; et al. Development of Kupffer cell targeting type-I interferon for the treatment of hepatitis via inducing anti-inflammatory and immunomodulatory actions. Drug Deliv. 2018, 25, 1067–1077. [Google Scholar] [CrossRef]
- Scholten, D.; Trebicka, J.; Liedtke, C.; Weiskirchen, R. The carbon tetrachloride model in mice. Lab. Anim. 2015, 49, 4–11. [Google Scholar] [CrossRef]
- Takano, M.; Watanabe, H.; Toda, S.; Nishida, K.; Imafuku, T.; Minayoshi, Y.; Nakano, T.; Maeda, H.; Maruyama, T. Therapeutic Effects of Albumin-Fused BMP7 on 2 Experimental Models of Liver Fibrosis. Biol. Pharm. Bull. 2023, 46, 1421–1426. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A.; Britton, R.S. Free radical damage and lipid peroxidation. In Hepatotoxicology; CRC Press: Boca Raton, FL, USA, 2020; pp. 401–436. [Google Scholar]
- Simeonova, P.P.; Gallucci, R.M.; Hulderman, T.; Wilson, R.; Kommineni, C.; Rao, M.; Luster, M.I. The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol. Appl. Pharmacol. 2001, 177, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Lai, C.Y.; Yeh, D.W.; Liu, Y.L.; Su, Y.W.; Hsu, L.C.; Chang, C.H.; Catherine Jin, S.L.; Chuang, T.H. Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation. Mediat. Inflamm. 2018, 2018, 3523642. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef]
- Weiskirchen, R. Hepatoprotective and Anti-fibrotic Agents: It’s Time to Take the Next Step. Front. Pharmacol. 2015, 6, 303. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, V.; Russo, M.L.; Carbone, M.; Bruno, S. Treatment discontinuation with peg-interferon: What to consider. Expert Rev. Clin. Pharmacol. 2015, 8, 761–768. [Google Scholar] [CrossRef]
- Huang, Y.W.; Hsu, C.W.; Lu, S.N.; Yu, M.L.; Su, C.W.; Su, W.W.; Chien, R.N.; Hsu, C.S.; Hsu, S.J.; Lai, H.C.; et al. Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for patients with chronic hepatitis B. Hepatol. Int. 2020, 14, 997–1008. [Google Scholar] [CrossRef]
- Qin, A.; Wu, C.R.; Ho, M.C.; Tsai, C.Y.; Chen, P.J. Sequential Therapy with Ropeginterferon Alfa-2b and Anti-Programmed Cell Death 1 Antibody for Inhibiting the Recurrence of Hepatitis B-Related Hepatocellular Carcinoma: From Animal Modeling to Phase I Clinical Results. Int. J. Mol. Sci. 2023, 25, 433. [Google Scholar] [CrossRef]
- Miwa, S.; Kadono, Y.; Sugata, T.; Mizokami, A.; Namiki, M. Successful treatment for metastases from renal cell carcinoma with alternation of interferon-alpha subtypes. Int. J. Clin. Oncol. 2010, 15, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Rudick, R.A.; Goelz, S.E. Beta-interferon for multiple sclerosis. Exp. Cell Res. 2011, 317, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Kotredes, K.P.; Thomas, B.; Gamero, A.M. The Protective Role of Type I Interferons in the Gastrointestinal Tract. Front. Immunol. 2017, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.M.; Chang, Y.; Jia, A. Effects of interferon-alpha on expression of hepatic stellate cell and transforming growth factor-beta1 and alpha-smooth muscle actin in rats with hepatic fibrosis. World J. Gastroenterol. 2005, 11, 2634–2636. [Google Scholar] [CrossRef] [PubMed]
- Fort, J.; Pilette, C.; Veal, N.; Oberti, F.; Gallois, Y.; Douay, O.; Rosenbaum, J.; Calès, P. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J. Hepatol. 1998, 29, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Nemoto, T.; Kushida, M.; Sheng, Y.; Higashi, K.; Ikeda, K.; Kawada, N.; Shirasaki, F.; Takehara, K.; Sugiyama, K.; et al. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology 2003, 38, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Mallat, A.; Preaux, A.M.; Blazejewski, S.; Rosenbaum, J.; Dhumeaux, D.; Mavier, P. Interferon alfa and gamma inhibit proliferation and collagen synthesis of human Ito cells in culture. Hepatology 1995, 21, 1003–1010. [Google Scholar] [CrossRef]
- Ogawa, T.; Kawada, N.; Ikeda, K. Effect of natural interferon α on proliferation and apoptosis of hepatic stellate cells. Hepatol. Int. 2009, 3, 497–503. [Google Scholar] [CrossRef][Green Version]
- Shen, H.; Zhang, M.; Minuk, G.Y.; Gong, Y. Different effects of rat interferon alpha, beta and gamma on rat hepatic stellate cell proliferation and activation. BMC Cell Biol. 2002, 3, 9. [Google Scholar] [CrossRef]
- Tanabe, J.; Izawa, A.; Takemi, N.; Miyauchi, Y.; Torii, Y.; Tsuchiyama, H.; Suzuki, T.; Sone, S.; Ando, K. Interferon-beta reduces the mouse liver fibrosis induced by repeated administration of concanavalin A via the direct and indirect effects. Immunology 2007, 122, 562–570. [Google Scholar] [CrossRef]
- Carlson, J.; Sakamoto, Y.; Laurell, C.B.; Madison, J.; Watkins, S.; Putnam, F.W. Alloalbuminemia in Sweden: Structural study and phenotypic distribution of nine albumin variants. Proc. Natl. Acad. Sci. USA 1992, 89, 8225–8229. [Google Scholar] [CrossRef]
- Brennan, S.O.; Rollo, C.; Potter, H.C. Novel silent albumin variant (191Ala ⟶ Thr) detected by TOF MS of whole plasma. Clin. Chim. Acta 2015, 440, 40–43. [Google Scholar] [CrossRef]
- Peach, R.J.; Brennan, S.O. Structural characterization of a glycoprotein variant of human serum albumin: Albumin Casebrook (494 Asp ⟶ Asn). Biochim. Biophys. Acta 1991, 1097, 49–54. [Google Scholar] [CrossRef]
- Brennan, S.O.; Myles, T.; Peach, R.J.; Donaldson, D.; George, P.M. Albumin Redhill (-1 Arg, 320 Ala ⟶ Thr): A glycoprotein variant of human serum albumin whose precursor has an aberrant signal peptidase cleavage site. Proc. Natl. Acad. Sci. USA 1990, 87, 26–30. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yonezawa, M.; Okamoto, T.; Fujiwara, Y.; Suzuki, A.; Suzuki, Y.; Endo, K.; Kakisaka, K.; Oikawa, T.; Kuroda, H.; et al. Occurrence of hepatocellular carcinoma 24 years after successful interferon therapy in a patient with chronic hepatitis C: A case report. Clin. J. Gastroenterol. 2019, 12, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Eguchi, A.; McGeough, M.D.; Pena, C.A.; Johnson, C.D.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014, 59, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Joosten, L.A.B.; Koenen, T.; van Tits, B.; van Diepen, J.A.; van den Berg, S.A.A.; Rensen, P.C.N.; Voshol, P.J.; Fantuzzi, G.; Hijmans, A.; et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ismael, S.; Nasoohi, S.; Sakata, K.; Liao, F.F.; McDonald, M.P.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) Associated NLRP3 Inflammasome Activation in Human Alzheimer’s Disease Brain. J. Alzheimer’s Dis. 2019, 68, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Roos, P.M.; Larsson, S.C. Interleukin-1 receptor antagonist, interleukin-2 receptor alpha subunit and amyotrophic lateral sclerosis. Eur. J. Neurol. 2020, 27, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M. [IL-1Ra: Its role in rheumatoid arthritis]. Reumatismo 2004, 56, 41–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vastert, S.J.; de Jager, W.; Noordman, B.J.; Holzinger, D.; Kuis, W.; Prakken, B.J.; Wulffraat, N.M. Effectiveness of first-line treatment with recombinant interleukin-1 receptor antagonist in steroid-naive patients with new-onset systemic juvenile idiopathic arthritis: Results of a prospective cohort study. Arthritis Rheumatol. 2014, 66, 1034–1043. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, Y.; Zhao, N.; Zhou, S.; Wang, X.; Han, W.; Yu, Y.; Zhao, X.; Cui, Y. Pharmacokinetics and Safety of Recombinant Human Interleukin-1 Receptor Antagonist GR007 in Healthy Chinese Subjects. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 353–360. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Liu, L.; Ruan, Q.; Zhang, X.; Hong, W.; Wu, S.; Jin, G.; Bai, Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 2018, 154, 203–212. [Google Scholar] [CrossRef]
- Zhang, M.; Nakamura, K.; Kageyama, S.; Lawal, A.O.; Gong, K.W.; Bhetraratana, M.; Fujii, T.; Sulaiman, D.; Hirao, H.; Bolisetty, S.; et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kanda, T.; Nakamura, M.; Yasui, S.; Arai, M.; Kato, N. Acutely exacerbated chronic hepatitis C after administration of nivolumab: A case report. Kanzo 2019, 60, 459–465. [Google Scholar] [CrossRef][Green Version]
- de Andrade, K.Q.; Moura, F.A.; dos Santos, J.M.; de Araújo, O.R.; de Farias Santos, J.C.; Goulart, M.O. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef] [PubMed]
- Center, S.A. Metabolic, antioxidant, nutraceutical, probiotic, and herbal therapies relating to the management of hepatobiliary disorders. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 67–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.C.; Zhang, Q.B.; Qiao, L. Pathogenesis of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7312–7324. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, N.; Kanai, T. Role of toll-like receptors in immune activation and tolerance in the liver. Front. Immunol. 2014, 5, 221. [Google Scholar] [CrossRef]
- Moore, S.M.; Kremer, M.; Sanderlin, E.J.; Wheeler, M.D.; Hines, I.N. Emerging roles for lipids in the hepatic innate immune response. Hum. Nutr. Food. Sci. 2013, 1, 1–9. [Google Scholar]
- Ziegler-Heitbrock, L.; Lötzerich, M.; Schaefer, A.; Werner, T.; Frankenberger, M.; Benkhart, E. IFN-alpha induces the human IL-10 gene by recruiting both IFN regulatory factor 1 and Stat3. J. Immunol. 2003, 171, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Förster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P.; et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef] [PubMed]

| Group | M1 (%) | M2 (%) | M1/M2 |
|---|---|---|---|
| Control | 27.06 | 29.82 | 0.90 |
| Saline | 77.59 | 3.01 | 25.78 |
| Man-MSA-mIFNα2 | 66.76 | 8.71 | 7.66 |
| Mutation | Forward | Reverse |
|---|---|---|
| MSA(D494N) | ACATATGTTTCATTAACTGTCAGAG | CTCTGACAGTTAATGAAACATATGT |
| mIFNα2(N78Q) | AGGAGGGTTGCCTGCCAAGCAGCAG | CTGCTGCTTGGCAGGCAACCCTCCT |
| Target Gene | Forward | Reverse |
|---|---|---|
| IL-10 | GGACAACATACTGCTAACCGACTC | AAAATCACTCTTCACCTGCTCCAC |
| IL-1Ra | TCAGATCTGCACTCAATGCC | CTGGTGTTTGACCTGGGAGT |
| PD-L1 | TCAGCTACGGTGGTGCGGACT | AGCTTCTGGATAACCCTCGGCCT |
| GAPDH | AACTTTGGCATTGTGGAAGG | ACACATTGGGGGTAGGAACA |
| TGF-β | GGATACCAACTATTGCTTCAGCTCC | AGGCTCCAAATATAGGGGCAGGGTC |
| Fibronectin | GGCCACACCTACAACCAGTA | TCGTCTCTGTCAGCTTGCAC |
| Snail | CAACTATAGCGAGCTGCAGGA | ACTTGGGGTACCAGGAGAGAGT |
| Col1α2 | CACCCCAGCGAAGAACTCATA | GCCACCATTGATAGTCTCTCCTAAC |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minayoshi, Y.; Maeda, H.; Hamasaki, K.; Nagasaki, T.; Takano, M.; Fukuda, R.; Mizuta, Y.; Tanaka, M.; Sasaki, Y.; Otagiri, M.; et al. Mouse Type-I Interferon-Mannosylated Albumin Fusion Protein for the Treatment of Chronic Hepatitis. Pharmaceuticals 2024, 17, 260. https://doi.org/10.3390/ph17020260
Minayoshi Y, Maeda H, Hamasaki K, Nagasaki T, Takano M, Fukuda R, Mizuta Y, Tanaka M, Sasaki Y, Otagiri M, et al. Mouse Type-I Interferon-Mannosylated Albumin Fusion Protein for the Treatment of Chronic Hepatitis. Pharmaceuticals. 2024; 17(2):260. https://doi.org/10.3390/ph17020260
Chicago/Turabian StyleMinayoshi, Yuki, Hitoshi Maeda, Keisuke Hamasaki, Taisei Nagasaki, Mei Takano, Ryo Fukuda, Yuki Mizuta, Motohiko Tanaka, Yutaka Sasaki, Masaki Otagiri, and et al. 2024. "Mouse Type-I Interferon-Mannosylated Albumin Fusion Protein for the Treatment of Chronic Hepatitis" Pharmaceuticals 17, no. 2: 260. https://doi.org/10.3390/ph17020260
APA StyleMinayoshi, Y., Maeda, H., Hamasaki, K., Nagasaki, T., Takano, M., Fukuda, R., Mizuta, Y., Tanaka, M., Sasaki, Y., Otagiri, M., Watanabe, H., & Maruyama, T. (2024). Mouse Type-I Interferon-Mannosylated Albumin Fusion Protein for the Treatment of Chronic Hepatitis. Pharmaceuticals, 17(2), 260. https://doi.org/10.3390/ph17020260








