Abstract
Owing to the spread of resistance between pathogenic bacteria, searching for novel compounds with antibacterial activity is essential. Here, we investigated the potential antibacterial activity of Greek clover or Trigonella foenum-graecum herb extract on Salmonella typhimurium clinical isolates. The chemical profile of the herb was initially determined using LC-ESI-MS/MS, which explored 36 different compounds. Interestingly, the fenugreek extract possessed antibacterial action in vitro with minimum inhibitory concentrations of 64 to 512 µg/mL. The potential mechanism of action was studied by elucidating the effect of the fenugreek extract on the membrane properties of S. typhimurium bacteria, including the inner and outer membrane permeability and membrane integrity. Remarkably, the fenugreek extract had detrimental effects on the membrane properties in 40–60% of the isolates. Moreover, the in vivo antibacterial action was studied using a gastrointestinal infection model with S. typhimurium bacteria. Interestingly, the fenugreek extract (200 mg/kg) improved the infection outcomes in the tested mice. This was represented by the noteworthy decrease (p < 0.05) in the bacterial count in the small intestine and caecum tissues. The survival rate of the fenugreek-extract-treated mice significantly increased compared to the S. typhimurium-infected group. Additionally, there was an improvement in the histological and immunohistochemical features of tumor necrosis factor-alpha. In addition, using an ELISA and qRT-PCR, there was an improvement in the proinflammatory and oxidative stress markers in the fenugreek-extract-treated group. Consequently, fenugreek extract should be investigated further on other food pathogens.
1. Introduction
Many foodborne infections are triggered by ingesting food and beverages contaminated with pathogenic microorganisms like bacteria, fungi, and parasites [1]. Such foodborne diseases are considered a significant problem to public health worldwide. Salmonellosis infection is regarded as one of the central sources of foodborne diseases, and this infection is brought on by Salmonella species [2].
Salmonella enterica serovar Typhimurium belongs to the Enterobacteriaceae family. Such Gram-negative, facultative anaerobic flagellated rods cannot produce spores [3]. The most common clinical symptom of salmonellosis in animals and humans is gastrointestinal (GIT) disease. However, it may progress to several other disorders like acute septicemia [4].
Antibiotics are frequently used in food animal production in developing countries to promote the well-being and growth of animals [5]. Unfortunately, such countries have major concerns regarding the escalation of antibiotic resistance. Antibiotic resistance can be defined as a failure of antibiotics to affect bacterial progression [6]. Such resistance becomes more complicated if the bacteria are multidrug resistance (MDR). The speedy development of MDR amongst bacteria is triggered by the continued selective pressure and progression of novel survival mechanisms in bacteria [7]. Consequently, there are unlimited struggles to tackle such antibacterial resistance and generate efficient, safe, and eco-friendly antibacterial therapeutic methods [8].
Natural sources, like plants, are considered a treasure as they contain various phytochemical compounds with various potent pharmacological actions [9]. Such plants could be used as therapeutic alternatives to combat infections caused by MDR bacterial pathogens [10]. The phytochemicals of plants could exert their action against bacteria by inhibiting efflux pumps, affecting membrane properties, impairing bacterial virulence, and influencing antibiotic-degrading enzymes [11].
Trigonella foenum-graecum, family Fabaceae [12], was traditionally used in ancient times [13]. The seeds, leaves, and sprouts were used as nutraceuticals and in medicinal applications. They are crucial in preventing gastrointestinal disorders and managing diabetes, microbial infections, hypercholesterolemia, and other ailments. Fenugreek has anticancer [14], antioxidant, anti-inflammatory, antimicrobial [15], antilipidemic, demulcent, wound healing [16] and immunological activities [17]. It is a valuable nutraceutical and medicinal plant in various food products [18]. The leaves contain graecunins, which are saponins (glycosides of diosgenin) [19], phenolic acids and flavonoids [20]. Fenugreek seeds are a good source of galactomannan (complex heteropolysaccharides) and saponins such as diosgenin, yamogenin, tigogenin, neotigogens, and gitogenin [19]. The seeds also contain alkaloids (choline and trigonilline), mucilage, flavonoids, phenolic acid derivatives [21], steroids, amino acids, and volatile oils [17]. The fenugreek seed extract possesses antimicrobial and anti-inflammatory activities, as reported previously [22].
This investigation aims to reveal the chemical profile of Trigonella foenum-graecum herb extract and discover the potential antibacterial action of fenugreek extract on S. typhimurium bacteria in vitro and in vivo.
2. Results
2.1. Phytochemical Analysis of Trigonella foenum-graecum Herb Using LC-ESI-MS/MS
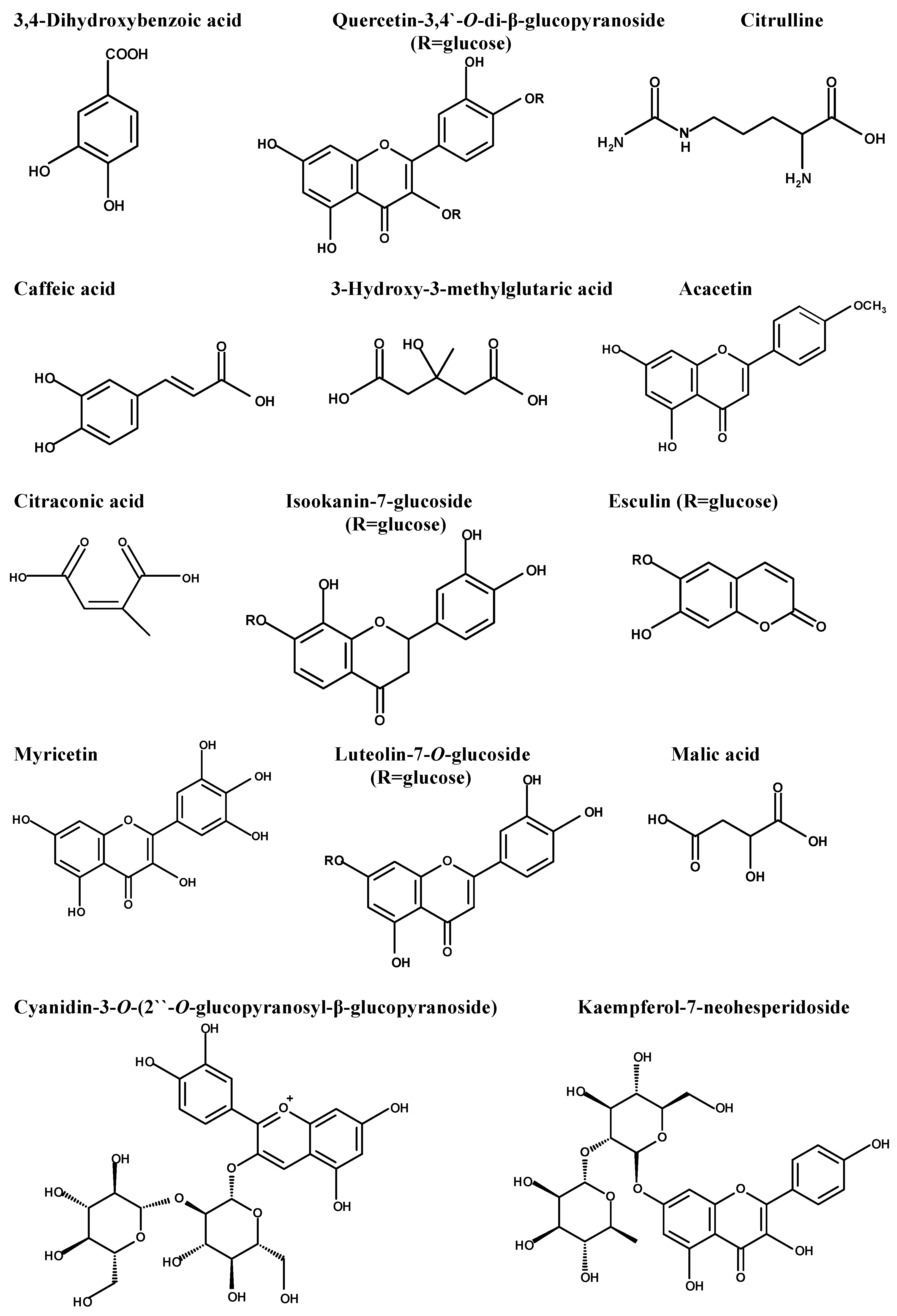
The diversity of the phytochemical content of fenugreek herb was initially determined using LC-ESI-MS/MS, which recorded the presence of 36 compounds. The major compounds, which were displayed using the negative mode ESI, were 3,4-dihydroxy benzoic acid, quercetin-3,4’-O-di-β-glucopyranoside, caffeic acid, citrulline, 3-hydroxy-3-methyl glutaric acid, acacetin, citraconic acid, isookanin-7-glucoside, esculin, myricetin, luteolin-7-O-glucoside, and malic acid. Table 1 represents the initial compounds detected using the in-house database and supported by the reported data. Figure 1 represents the major compounds identified in the herb extract using LC-ESI-MS/MS. Figure S1 illustrates the total ion chromatogram in the Supplementary Materials.

Table 1.
Phytochemical analysis of Trigonella foenum-graecum using LC-ESI-MS/MS (negative mode).
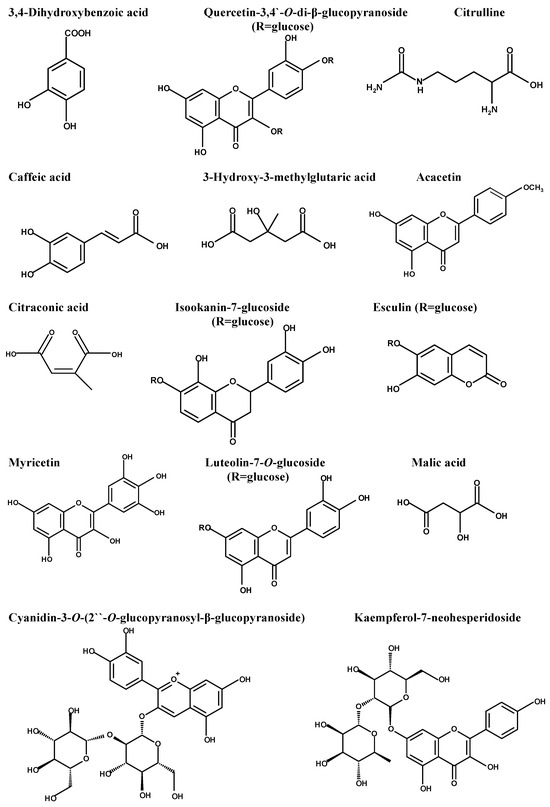
Figure 1.
Major compounds detected in Trigonella foenum-graecum using LC-ESI-MS/MS.
2.2. Antibacterial Action (In Vitro)
The fenugreek extract showed antibacterial action towards S. typhimurium by revealing inhibition zones around the wells in the Muller–Hinton agar (MHA) plates. The broth microdilution technique recorded the minimum inhibitory concentrations (MICs). They ranged from 64 to 512 µg/mL (Table 2).

Table 2.
MICs of the fenugreek extract on the S. typhimurium isolates.
Membrane Properties
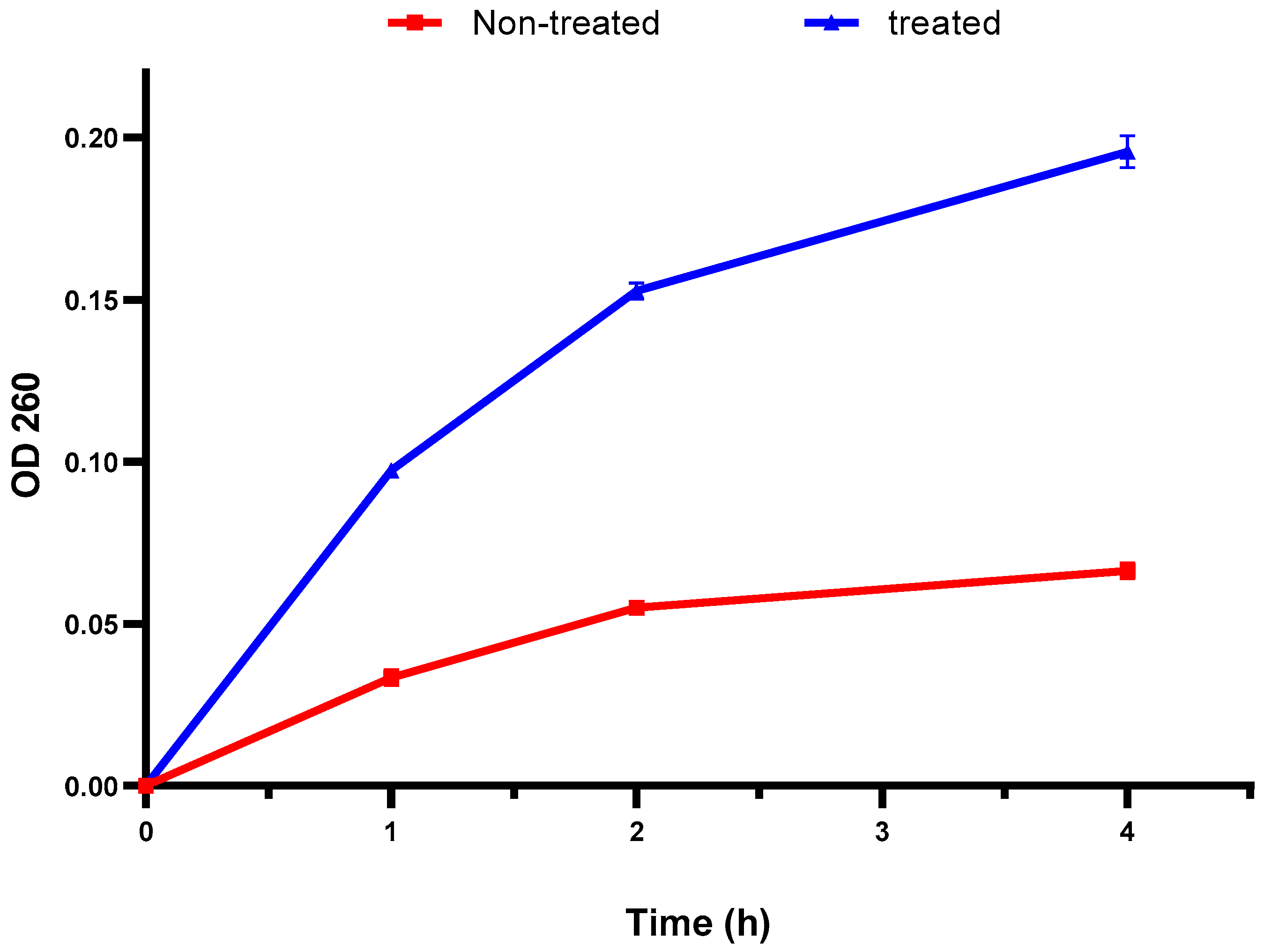
The bacterial membrane integrity was explored by elucidating the DNA and RNA discharge from the cellular interior. There was a remarkable increase in the absorbance at 260 nm after treatment with the fenugreek extract in 60% of the isolates (Figure 2). This finding designates the detrimental impact of the fenugreek extract on the bacterial membrane.
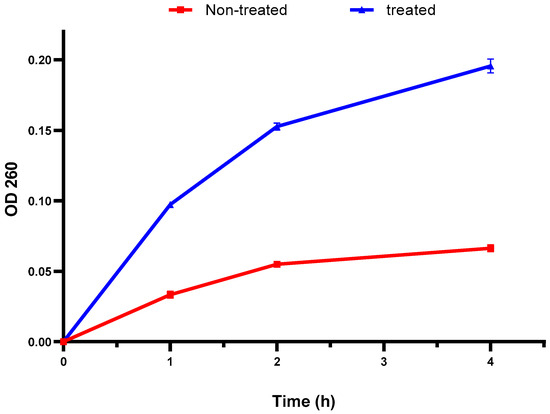
Figure 2.
An illustration of the increased discharge of DNA and RNA (with absorbance at 260 nm) after treatment with the fenugreek extract. The red line represents the absorbance before treatment, and the blue line represents the absorbance after treatment with the fenugreek extract. The measured optical density (OD) 260 reveals the release of DNA and RNA from the cellular interior.
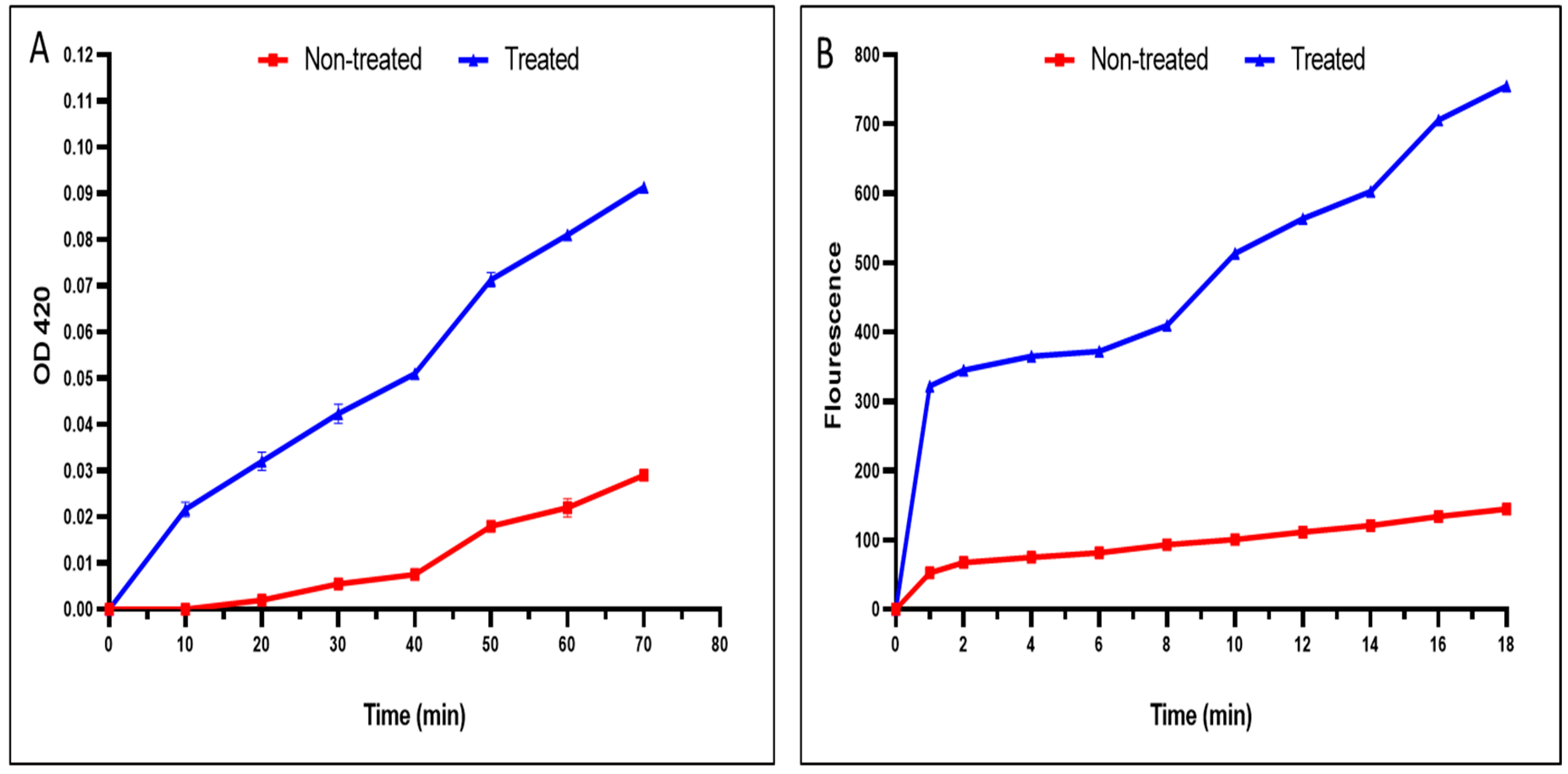
A noticeable rise in the inner and outer membrane permeability of the tested S. typhimurium isolates treated the fenugreek extract was recorded in 40% and 50% of the isolates, respectively (Figure 3).
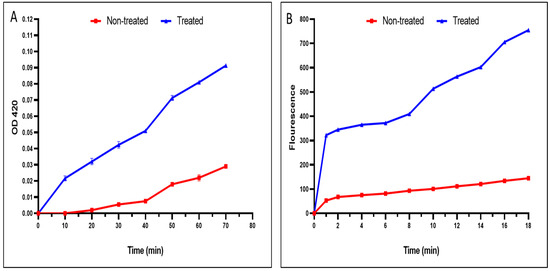
Figure 3.
An illustration of the damage caused by the fenugreek extract on the (A) inner and (B) outer membrane permeability. The red line represents the absorbance before treatment, and the blue line represents the absorbance after treatment with the fenugreek extract.
2.3. Antibacterial Action (In Vivo)
The antibacterial impact of the fenugreek extract on the infection caused by S. typhimurium was studied in vivo to reveal its clinical significance.
2.3.1. Bacterial Burden and Survival Curve
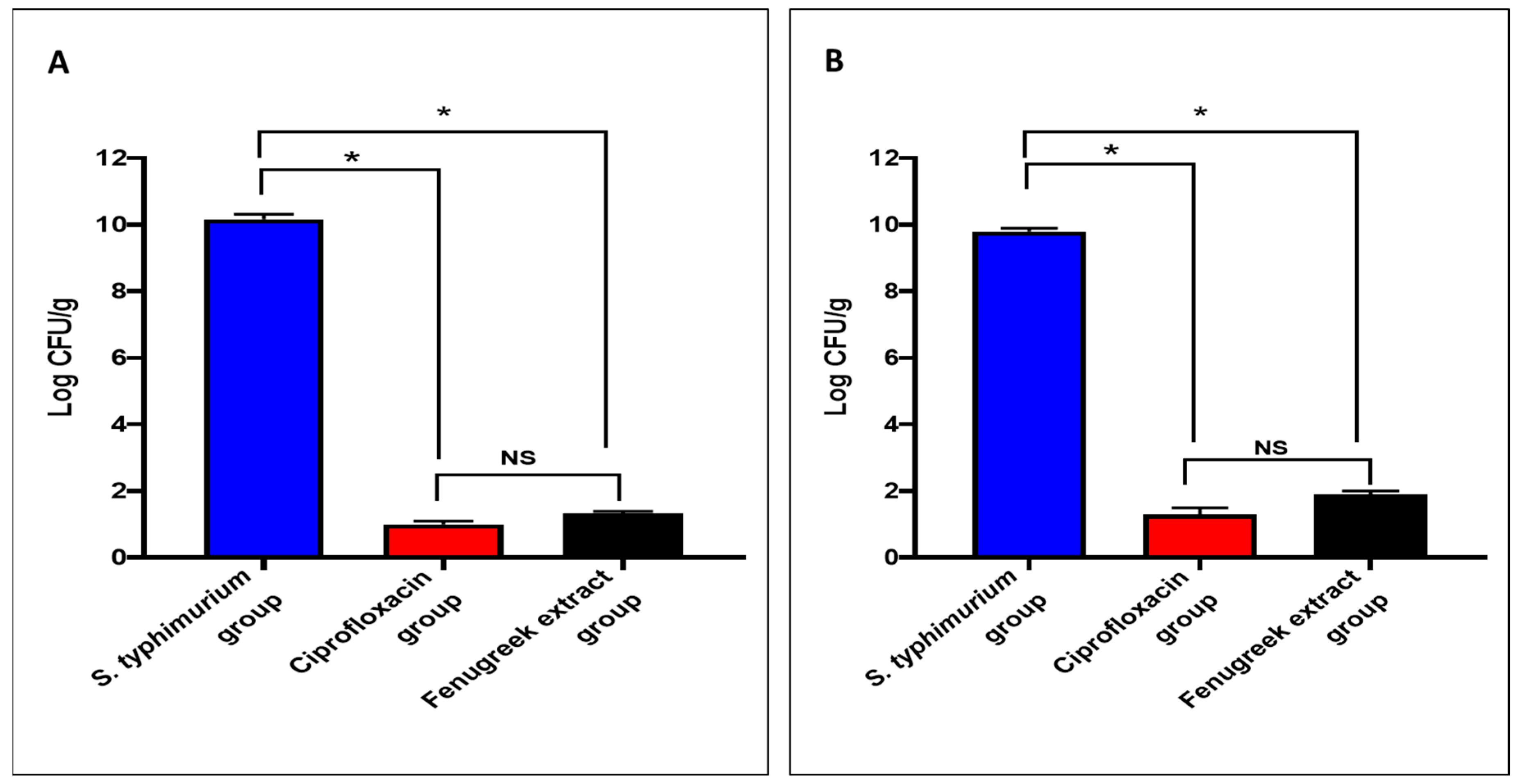
The fenugreek extract produced a noteworthy decrease (p < 0.05) in the number of colony-forming units per gram (CFU/g) in the small intestine and caecum tissues (Figure 4).
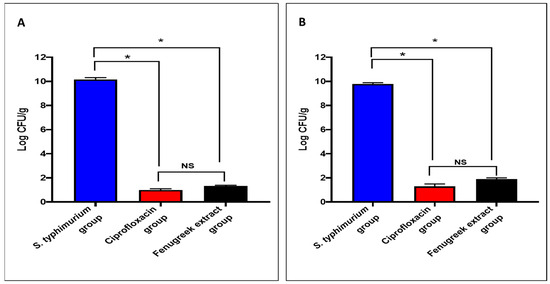
Figure 4.
The CFU/mL number in (A) the small intestine and (B) the caecum. (*) symbolizes a significant difference (p < 0.05), and NS denotes a non-significant (p > 0.05) difference.
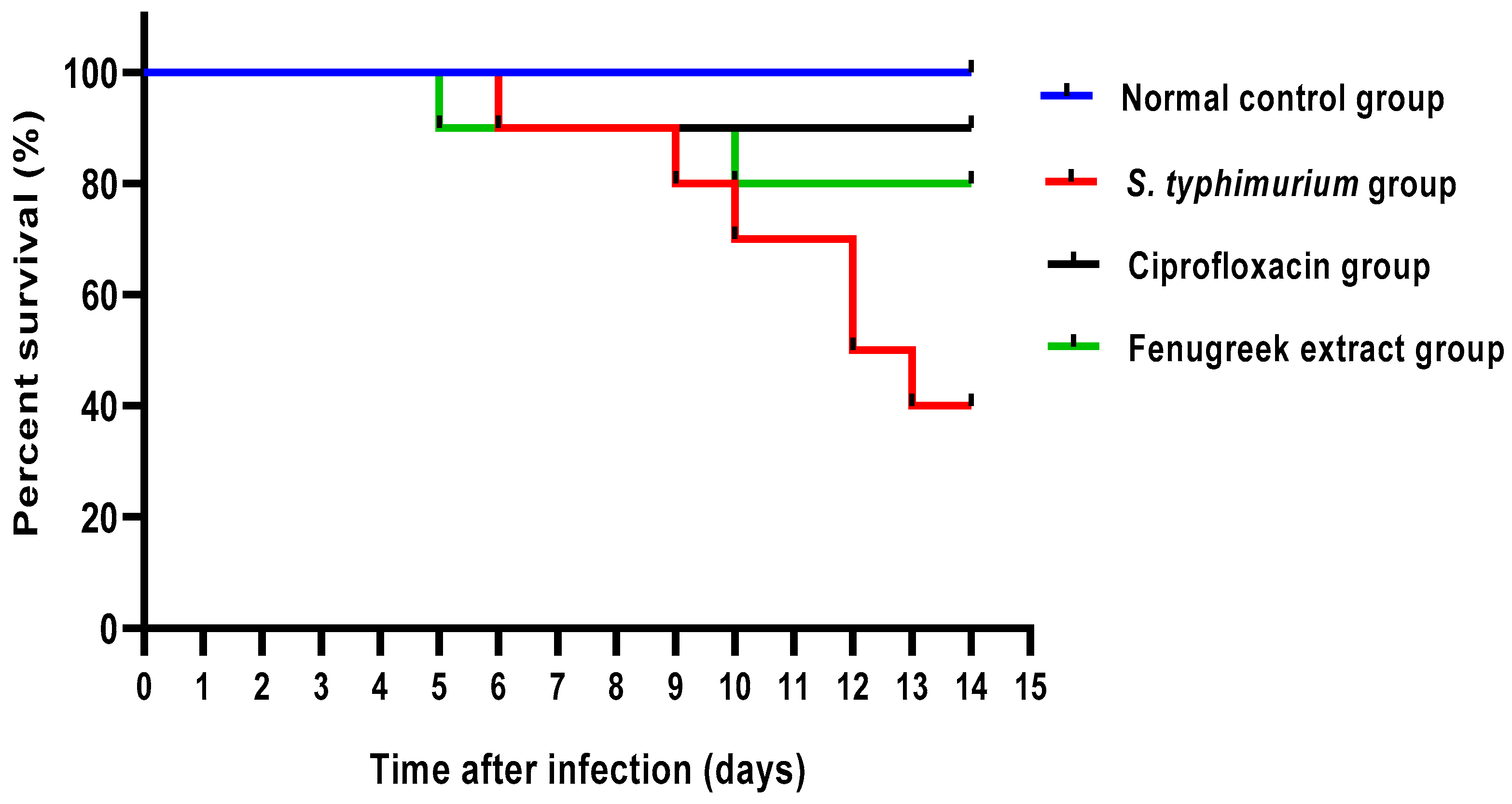
As revealed in Figure 5, the fenugreek extract improved the survival rate of the infected mice. All mice in the normal control group were alive till the end of the experiment. In the S. typhimurium-infected group, one mouse died on the sixth, ninth, tenth, and thirteenth days, and two died on the twelfth day. Regarding the ciprofloxacin-treated group, one mouse died on the sixth day. In the fenugreek-extract-treated group, only one mouse died on the fifth and tenth days.
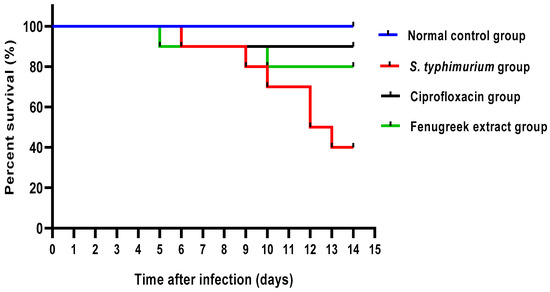
Figure 5.
Mice survival curve. All mice were alive till the end of the experiment in the control group. In the S. typhimurium-infected group, one mouse died in the sixth, ninth, tenth, and thirteenth days, and two mice died in the twelfth day. In the ciprofloxacin-treated group, only one mouse died on the sixth day. In the fenugreek-extract-treated group, one mouse died on the fifth and tenth days.
2.3.2. Histological Features
The effect of the treatment with the fenugreek extract on the histological features was investigated after staining the tissues with hematoxylin and eosin (H&E) stain (Figure 6 and Figure 7).
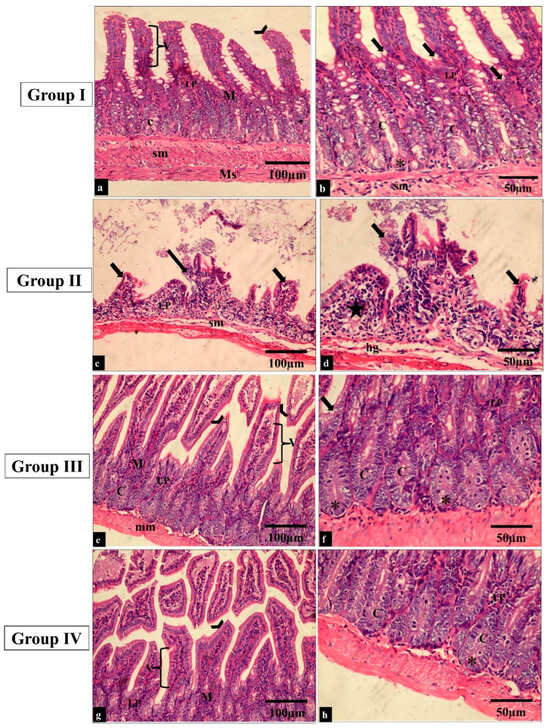
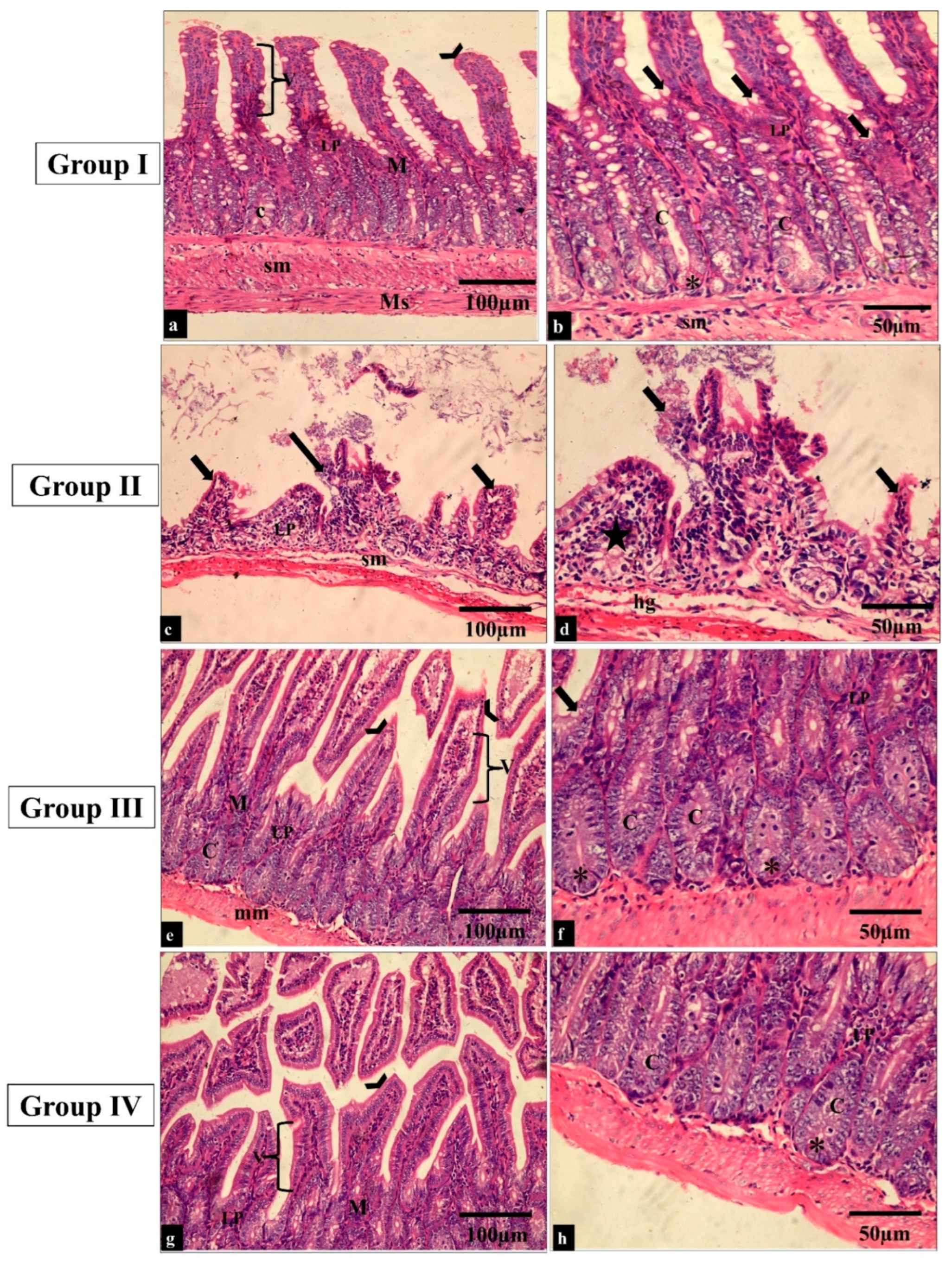
Figure 6.
Light microscopic images of H&E-stained sections of the small intestine sections in all the studied groups. (a–f) Groups I and III show normal histological structure of the small intestine. The small intestine is formed of the mucosa (M), submucosa (sm) and muscularis propria (Ms). The surface epithelial cells are arranged in villi (V) lined by absorptive and goblet cells (thick arrows). The lamina propria (LP) underlies the epithelium; just beneath this is a thin muscularis mucosae of smooth muscle (mm). The intestinal crypts (C) with paneth cells (*) can be seen. (c,b) Group II shows a shortening of the villi and dense infiltration of the lamina propria by mononuclear cells (star). (g,h) Group IV shows restoration to the normal histological appearance of the small intestine (H&E × 200, scale bar = 100 μm; H&E × 400, scale bar = 50 μm).
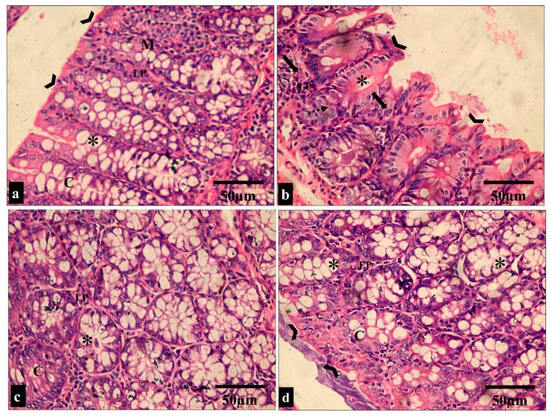
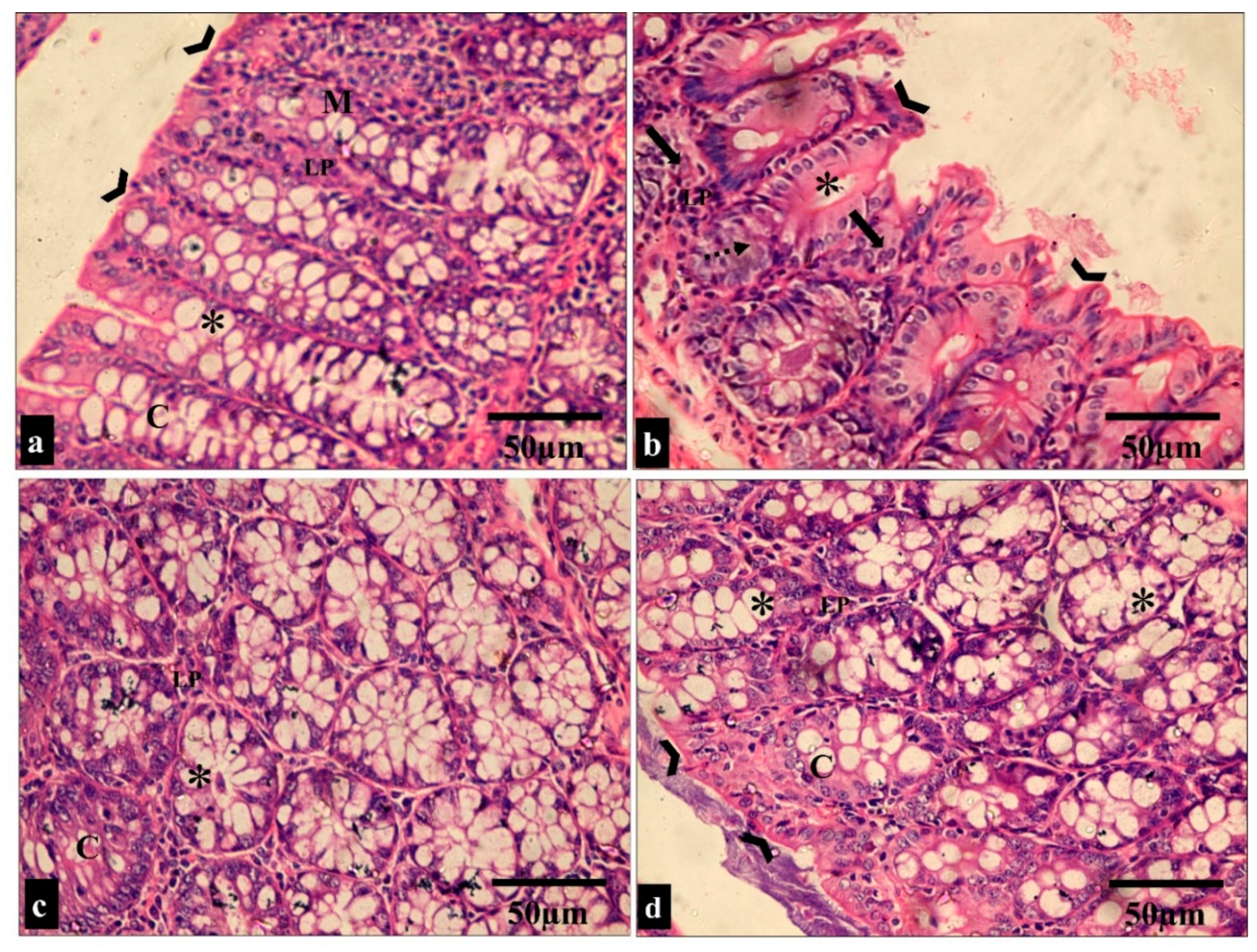
Figure 7.
Light microscopic images of the H&E-stained large intestine sections in all the studied groups. (a,c) Groups I and III show normal colonic mucosa, with the surface of the mucosa covered with a striated brush border (arrowheads). The lamina propria (LP) of the mucosa contains simple tubular intestinal glands (crypts of Lieberkühn) (C). The crypts are lined by numerous goblet cells (*). (b) Group II shows loss of surface epithelium (arrowheads) with mononuclear cellular infiltration in the lamina propria of the glandular tissue (arrows). Additionally, there is an apparent reduction in the number of goblet cells (*) and crypt abscess formation (dashed arrow). (d) Group IV shows restoration to the normal histological appearance of the colonic mucosa, with straight intact brush borders (arrowheads) and numerous goblet cells in the glandular tissue (*). (H&E × 400, scale bar = 50 μm).
2.3.3. Immunohistochemical Features
The influence of the fenugreek extract on the level of the inflammatory marker tumor necrosis factor-alpha (TNF-α) in the studied tissues was investigated (Figure 8 and Figure 9).
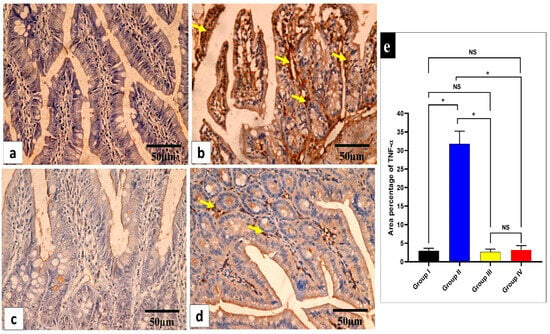
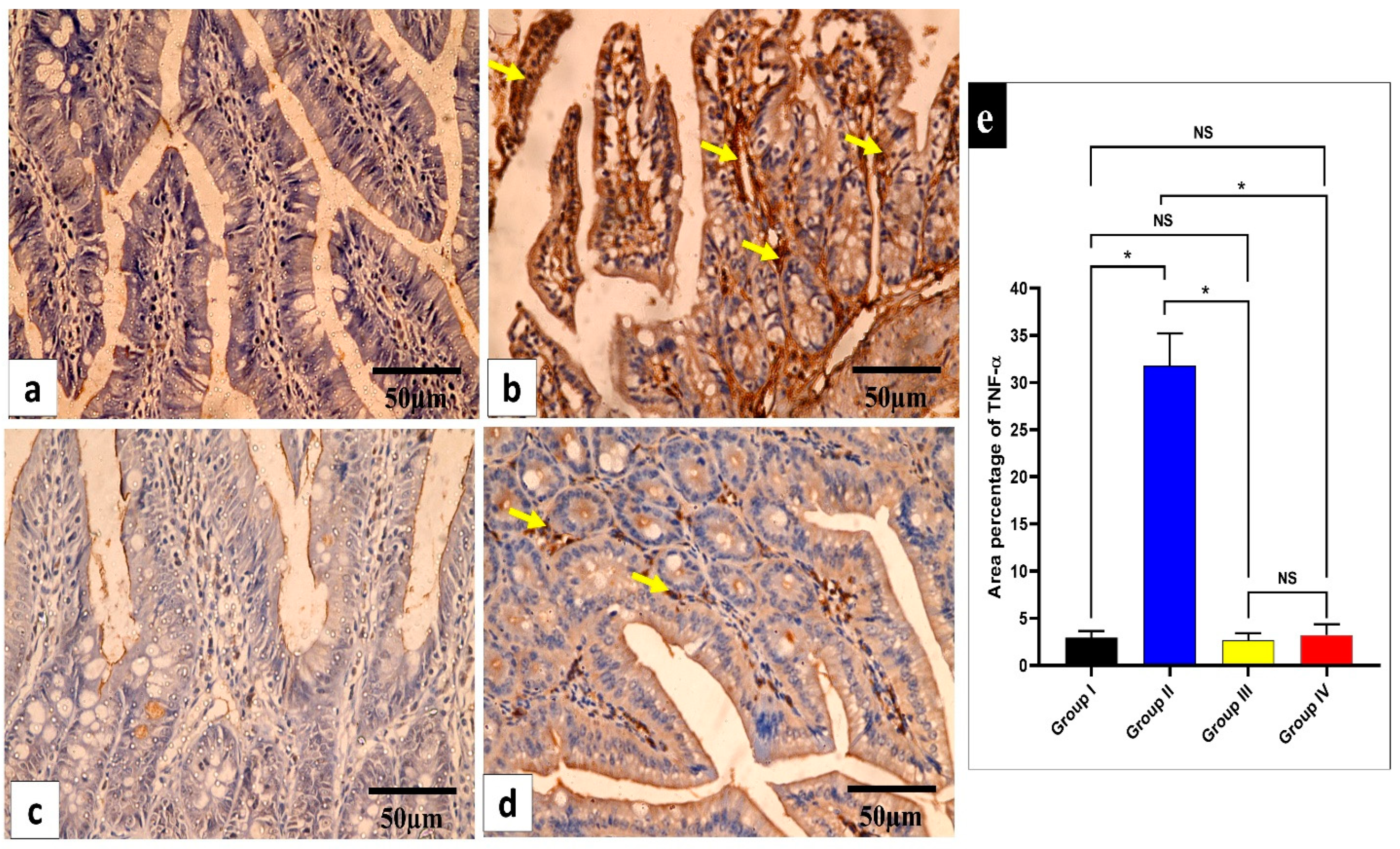
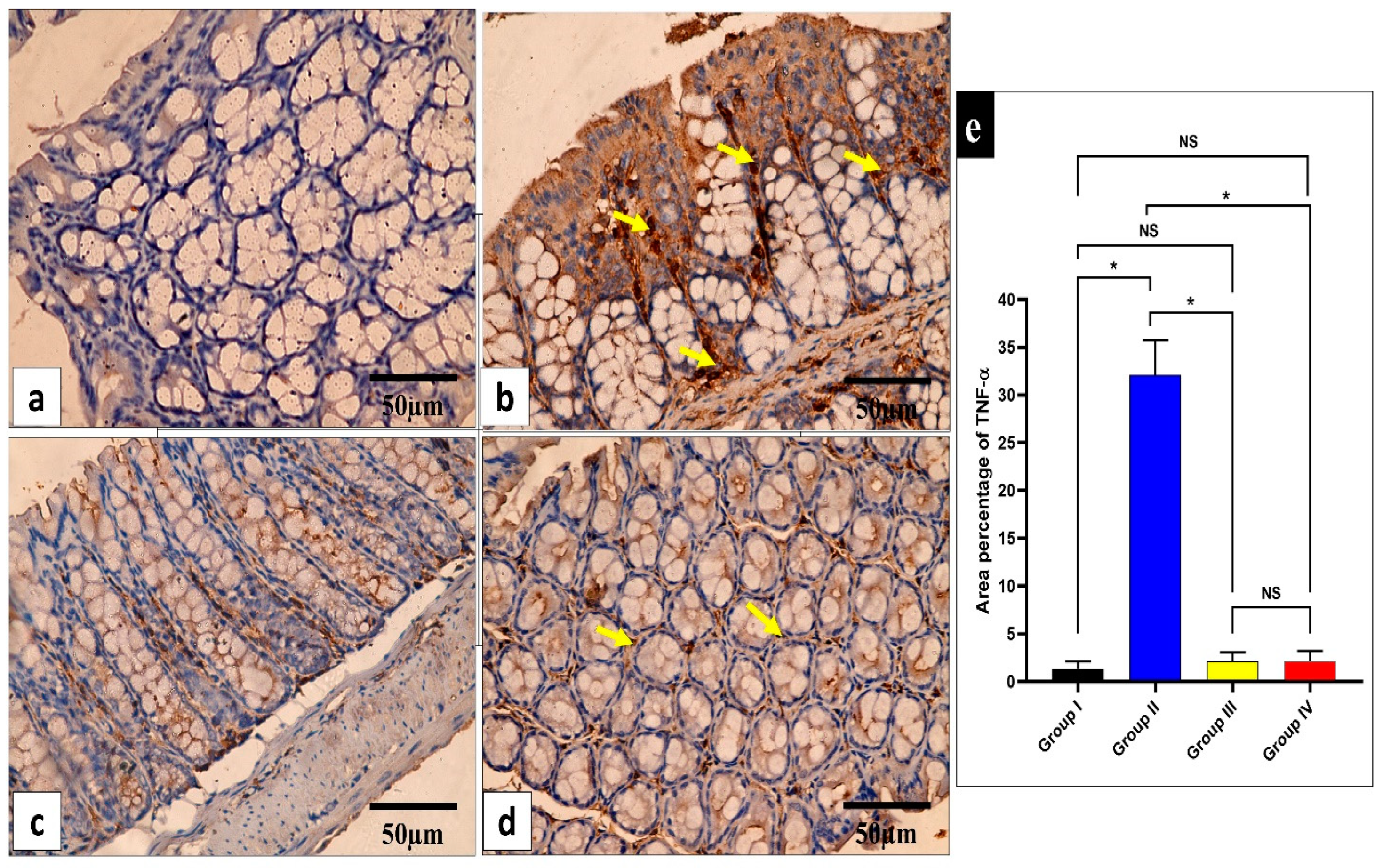
Figure 8.
Light microscopic images of TNF-α-stained sections of the small intestine in the different studied groups. (a) Group I shows a negative TNF-α immune reaction in the intestinal mucosa cells. (b) Group II shows a strong positive TNF-α immune reaction in the intestinal mucosa cells (yellow arrows). (c) Group III shows a negative TNF-α immune reaction expression in all intestinal mucosa cells. (d) Group IV shows no TNF-α immune reaction in most cells and a weak immune reaction in a few cells (arrows). (e) The rea percentage of TNF-α in all the groups. Mean ± standard deviation (SD) is used to express the data. Statistical comparison was performed using a one-way ANOVA and Tukey’s post hoc test for multiple comparisons. The single asterisk represents a significant change, and the abbreviation NS represents a non-significant change (p < 0.05). (TNF-α immune reaction in cell immunostaining × 400, scale bar = 50 μm).
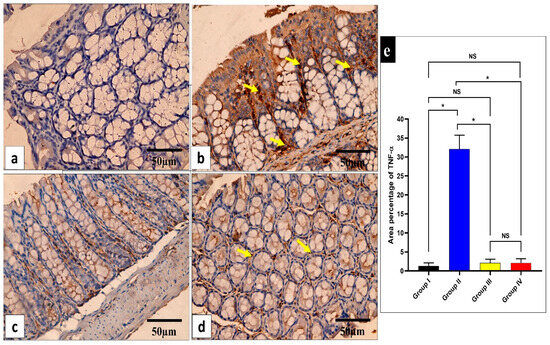
Figure 9.
Light microscopic images of TNF-α-stained large intestine sections in the different studied groups. (a) Group I shows a negative TNF-α immune reaction in the surface epithelium and all colonic glandular cells. (b) Group II shows a strong positive TNF-α immune reaction in the form of a brown cytoplasm of the surface epithelial and glandular cells (yellow arrows). (c) Group III exhibits a negative TNF-α immune reaction expression in all mucosal cells. (d) Group IV shows no TNF-α immune reaction in the majority of cells and a weak immune reaction in a few glandular cells (arrows). (e) The area percentage of TNF-α in all groups. Mean ± SD is used to express the data. Statistical comparison was performed using a one-way ANOVA and Tukey’s post hoc test for multiple comparisons. The single asterisk represents a significant change, and the abbreviation NS represents a non-significant change (p < 0.05). (TNF-α immune reaction in cell immunostaining × 400, scale bar = 50 μm).
2.3.4. ELISA and qRT-PCR
The fenugreek extract was found to significantly (p < 0.05) decrease interleukin 6 and 1β (IL-6 and IL-1β), which are proinflammatory interleukins (Table 3).

Table 3.
Levels of IL-6 and IL-1β in the studied tissues.
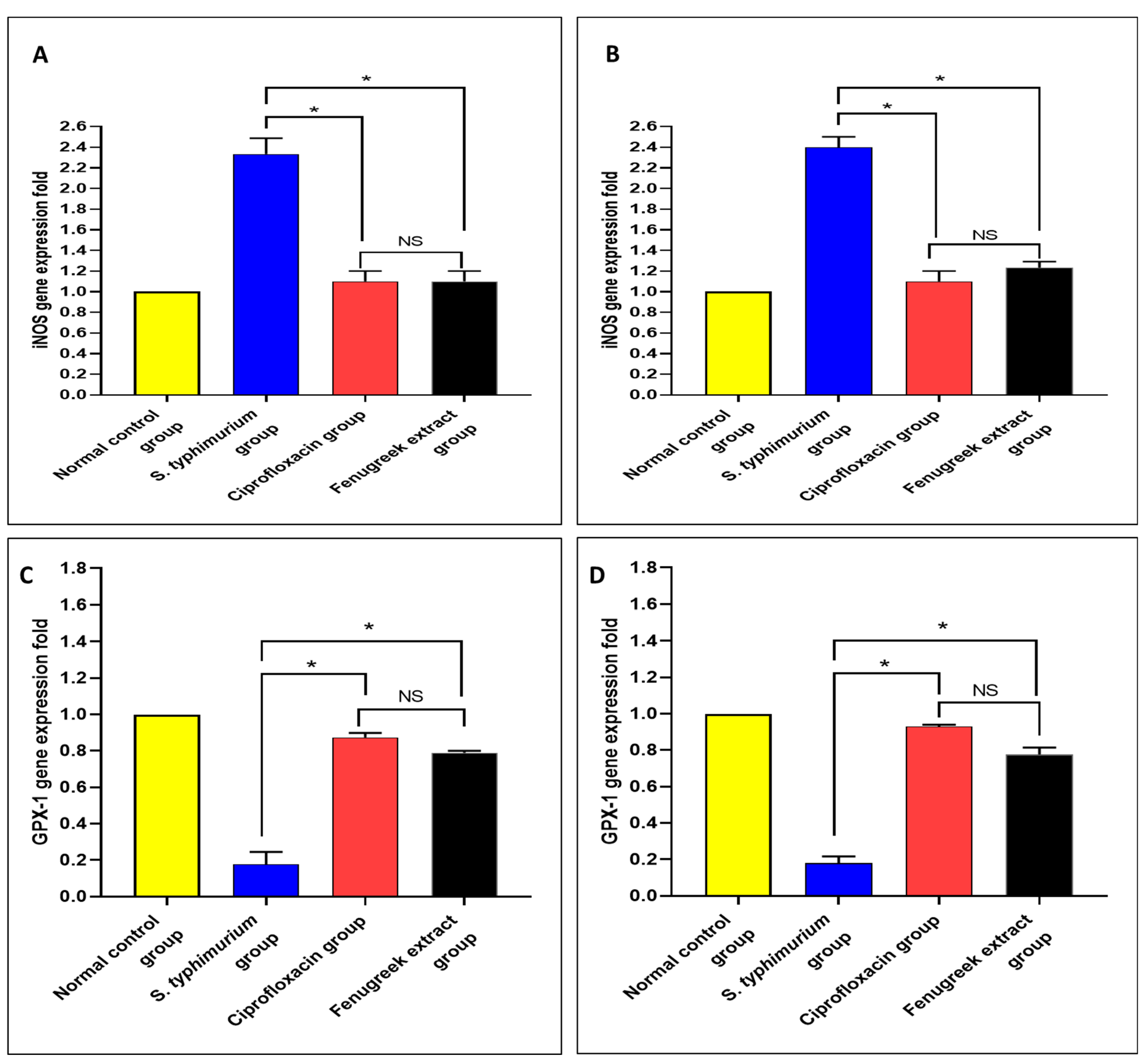
Using qRT-PCR, the gene expressions of nitric oxide synthase (iNOS) and glutathione peroxidase 1 (GPX-1) were determined (Figure 10). There was a significant reduction (p < 0.05) in iNOS gene expression in the fenugreek-treated group. Moreover, there was a significant increase (p < 0.05) in the gene expression of GPX-1 in the fenugreek-treated group.
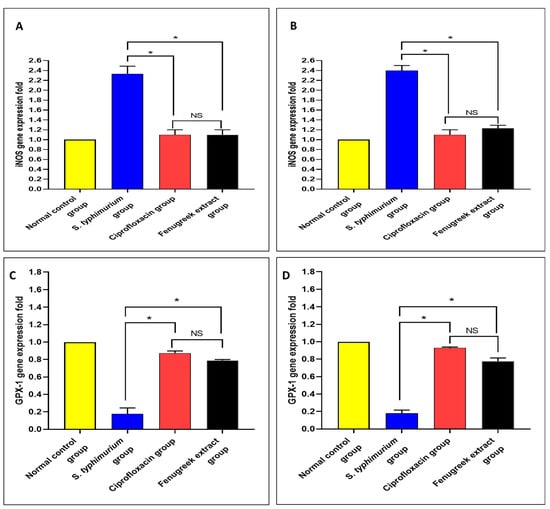
Figure 10.
The effect of fenugreek extract on the expression levels of (A) iNOS in the small intestine, (B) iNOS in the caecum, (C) GPX-1 in the small intestine, and (D) GPX-1 in the caecum. (*) represents a significant difference (p < 0.05), and NS denotes a non-significant (p > 0.05) difference.
3. Discussion
The identification and characterization of the chemical content of Trigonella foenum-graecum herb extract was initially performed using LC-ESI-MS/MS, which recorded the existence of 36 compounds of different chemical groups. The initially identified compounds belonged to hydroxy fatty acids, flavonoid glycosides, phenyl propanoic acids, amino acids, purine nucleosides, coumarin, anthocyanidin glycosides, aurone glycoside, and isoflavonoid glycosides. The major compounds were determined as 3,4-dihydroxy benzoic acid, quercetin-3,4’-O-di-β-glucopyranoside, caffeic acid, citrulline, 3-hydroxy-3-methyl glutaric acid, acacetin, citraconic acid, isookanin-7-glucoside, esculin, myricetin, luteolin-7-O-glucoside, malic acid, and kaempferol-7-neohesperidoside. Several studies have documented the potential antibacterial activity of these bioactive compounds in fenugreek extract [54,55,56,57,58].
Various antibacterials work by disturbing the bacterial membranes to exert their action. The membranes of bacteria are an important barrier that protect them from harmful agents [59,60]. Here, membrane integrity was inspected before and after treatment with the fenugreek extract. A noticeable reduction (p < 0.05) in membrane integrity was noticed in 60% of the isolates. The integrity of the bacterial membrane is an indication of its quality. If the membrane is negatively affected, its integrity decreases, and the discharge of DNA and RNA to the bacterial exterior increases [59].
Additionally, membrane permeability is an essential character of the bacterial membrane. Gram-negative bacteria such as S. typhimurium have inner and outer membranes [61,62]. We elucidated the impact of fenugreek extract on membrane permeability. Remarkably, the fenugreek extract (p < 0.05) significantly augmented inner and outer membrane permeability in 40% and 50% of the S. typhimurium bacteria, respectively. These findings suggest that the probable mechanism of action of fenugreek extract on the tested bacteria is related to its detrimental effects on the bacterial membrane.
As previously mentioned, S. typhimurium is a common pathogen that can trigger gastroenteritis; therefore, we used a GIT infection model in vivo to illuminate the antibacterial potential of fenugreek extract. Remarkably, there was an improvement in the histological features of the small intestine and caecum after staining with H&E.
The inflammatory mediators in the small intestine and caecum were studied in this study. The immunohistochemical experiments revealed a significant decline (p < 0.05) in TNF-α in the fenugreek-treated group. Additionally, using an ELISA, IL-6 and IL-1β were found to considerably decline (p < 0.05) in the fenugreek-extract-treated group. TNF-α, IL-6, and IL-1β usually rise in the infected tissues to start the inflammatory process [63,64], which was inhibited by the treatment with fenugreek extract. We utilized qRT-PCR to illuminate the gene expression of iNOS and GPX-1. The fenugreek extract caused a noteworthy reduction in the gene expression of iNOS, which increases the release of reactive oxygen species that trigger cytotoxicity [65]. On the other hand, GPX-1 is an antioxidant enzyme [66], and its gene expression was found to increase significantly in the fenugreek-treated group.
The significant actions of fenugreek may explain its antibacterial effects against S. typhimurium. It was found that caffeic acid (a derivative of p-hydroxybenzoic) exerted an antimutagenic effect against Salmonella typhimurium [67]. Another study showed that caffeic acid, myricetin, and kaempferol have antioxidant, anti-inflammatory, and antiseptic effects [68]. Additionally, p-hydroxy benzoic acid and its derivatives negatively affect Gram-positive and Gram-negative bacteria [69]. In addition, p-hydroxybenzoic acid showed an anti-inflammatory effect [70,71]. Quercetin, luteolin, and kaempferol glycosides exerted antibacterial activity, as reported by Akroum et al. [72]. Antibacterial effects have also been revealed for citrulline [73], acacetin [74], esculin [75], and anthocyanin as cyanidin glycosides [76].
4. Materials and Methods
4.1. Collection of Plant Materials and the Formation of the Crude Extract
The herbs (leaves and flowers) of Trigonella foenum-graecum were collected from agricultural fields in November 2022 in Tanta, El Gharbia Governorate, Egypt. The collected herbs were desiccated. The dried plants were powdered (2 kg) and macerated in 95% methanol (5 L × 4 times). The combined methanol extract was evaporated using a rotary evaporator under vacuum at 40 °C to give 118.6 gm of the crude residue. Professor El-Sayed Hamid El-Seidy, Faculty of Agriculture, Agronomy Department, Tanta University, authenticated the herb extracts. The Herbarium of the Pharmacognosy Department, Faculty of Pharmacy, Tanta University, own the herb representative sample (PG-A-00123).
4.2. LC/ESI-MS/MS of the Fenugreek Herb
4.2.1. Sample Preparation and Injection
The herbs (leaves and flowers) of T. foenum-graecum were exposed to LC-ESI-MS/MS analysis, as described by Tsugawa et al. [77]. The sample residue (50 mg) was solubilized in one milliliter of water/methanol/acetonitrile (50:25:25, v/v%) via vortexing and ultrasonication for two minutes and ten minutes, respectively. Then, 10 µL of a 2.5 µg/mL solution was injected for comparison against a blank sample (the solvent).
4.2.2. Acquisition Method and Analytical Parameters
In-line filter disks (0.5 µm × 3.0 mm, Phenomenex®, Torrance, CA, USA) and X select HSS T3 (2.5 µm, 2.1 × 150 mm, Waters®, Milford, MA, USA, 40 °C) were employed as a pre-column and analytical column, respectively. The mobile phases were composed of buffer A (5 mM ammonium formate buffer pH 3 with 1% methanol), buffer B (5 mM ammonium formate buffer pH 8 with 1% methanol), and buffer C (100% acetonitrile). The flow rate was set at 0.3 mL/min. The composition of the mobile phase began with 90 (A or B) to 10 (C) for the first 20 min, then inversed from minute 21 to 25, and lastly returned back for the last three minutes until the end at 28 min. Furthermore, the instrument was coupled with the Triple TOF 5600+ (Sciex, Framingham, MA, USA) for IDA acquisition and Analyst TF 1.7.1 (Sciex®) for LC-Triple TOF control.
4.2.3. Data Processing
MasterView was utilized for feature (peaks) extraction from the total ion chromatogram (TIC) based on a signal-to-noise ratio of more than five (non-targeted analysis) and sample-to-blank intensities of greater than three. Moreover, a Reifycs Abf (Analysis Base File) Converter (Reifycs®, Tokyo, Japan) was employed for Wiff file conversion and MS-DIAL 4.6 (RIKEN® Tokyo, Japan) for data analysis. Metabolite annotation was carried out using the ReSpect Database and fragmentation patterns and retention times mentioned in the previous reports for metabolites isolated from the investigated plant or others.
4.3. Bacteria, Chemicals, and Media
Ten S. typhimurium bacteria (S1–S10) were included in the current study from the Department of Microbiology and Immunology, Tanta University. The chemicals dimethyl sulfoxide (DMSO), O-nitrophenyl-β-galactopyranoside (ONPG), 1-N-phenylnapthylamine (NPN), and methanol were from Merck, Philadelphia, PA, USA, and the media MHA, Muller–Hinton broth (MHB), and MacConkey agar were from Oxoid, Hampshire, UK.
4.3.1. Antibacterial Potential
Using the agar well diffusion method, the antibacterial potential of the fenugreek extract was investigated, as previously reported [77]. Ciprofloxacin was employed as a positive control, and DMSO was a negative control. Fenugreek extract was added to the wells of the Petri dish plates containing MHA, with the tested bacteria spread on their surfaces at a concentration of 2000 µg/mL. The plates were incubated at 37 °C for 24 h and inspected for the appearance of inhibition zones to indicate antibacterial action. Then, the MICs were recorded using the broth microdilution method, as previously explained [78]. In each microtitration plate, the fenugreek extract was serially diluted in MHB from 2048 µg/mL to 0.5 µg/mL, using ciprofloxacin as a positive control and DMSO as a negative control. Bacterial suspensions were added to each well, and the plates were incubated for 18 h at 37 °C. The lowest concentration that hindered bacterial growth was documented as the MIC value.
4.3.2. Impact on the Bacterial Membranes
The impact of fenugreek extract on the membrane integrity of bacteria by measuring the discharge of DNA and RNA, with absorbances at 260 nm, was recorded using a UV spectrophotometer (SHIMADZU, Kyoto, Japan). Additionally, its impact on inner and outer membrane permeability was inspected.
Inner membrane permeability was investigated by tracking the discharge of the β-galactosidase enzyme from the cell by adding ONPG, which is converted by the enzyme to o-nitrophenol (ONP). Then, the absorbance of the formed ONP was measured at 420 nm using an ELISA reader (Sunrise, VA, USA) [79]. The β-galactosidase enzyme under normal conditions is present in the cell interior. When inner membrane integrity is impaired, the enzyme is released from the cell interior to the outside. Upon adding its substrate, ONPG is converted to ONP, which has an absorption at 420 nm.
The outer membrane permeability was investigated using NPN, a fluorescent compound, using a fluorescence spectrophotometer (SHIMADZU, Kyoto, Japan). The fluorescence was detected at 340 and 420 nm [79].
4.3.3. In Vivo Antibacterial Action
The potential antibacterial action of fenugreek extract was revealed in vivo using a GIT infection model using forty male albino mice (22–26 gm). They were provided with filtered water and a standard pellet diet. The experimental protocol was approved by the Research Ethics Committee of the Faculty of Pharmacy, Tanta University, Egypt (number of TP/RE/12/23 p-059).
4.3.4. Experiment Protocol
The animals were divided into four groups (each with ten mice) as follows [80]:
- Normal control (I): not infected.
- S. typhimurium-infected group (II): infected with S. typhimurium (1 × 106 CFU/mL) via the oral route.
- Ciprofloxacin-treated group (III): infected with S. typhimurium and treated with ciprofloxacin (20 mg/kg), taken orally for three constitutive days.
- Fenugreek-extract-treated group (IV): infected with S. typhimurium and treated with fenugreek extract (200 mg/kg), taken orally for three constitutive days [81].
The first doses of ciprofloxacin and fenugreek extract were administered after infection within 12 h. Then, mice were monitored for two weeks to calculate their survival rate. After two weeks, the animals were euthanized, and the small intestine and the caecum were withdrawn for histopathological, immunohistochemical, and biochemical studies. Furthermore, the bacterial burden was recorded in the studied tissues by calculating the number of colony-forming units (CFU/g) [62].
4.3.5. Histopathological and Immunohistochemical Investigations
For histological evaluation, the small intestine and caecum of the experimental groups were preserved in 10% formalin saline and prepared for paraffin blocks. Serial sections were obtained on a rotary microtome at 5–6 μm in thickness. Sections were placed in hematoxylin for one minute, washed in water for one minute, and then differentiated in 1% hydrochloric acid ethanol for 10–30 s. Later, they were soaked in water for 20 min, placed in eosin for 5–10 min at room temperature, and then dehydrated using an ethanol gradient. Slides were visualized under a light microscope [82].
Immunohistochemical staining was performed on six-micrometer tissue sections. The sections were dewaxed, rehydrated using a decreasing alcohol series, and then treated with 10% hydrogen peroxide in methanol for 10 min. The sections were then microwaved for 10 min in 0.01 M sodium citrate buffer (pH 6.0), allowed to cool at room temperature, and then given three PBS washes for five minutes. Antigens were recovered by autoclaving in citrate buffer for 11 min following washing. Following this, sections were incubated overnight at 4 °C with primary antibodies. The tissues were then treated for 30 min at room temperature with the rabbit polyclonal TNF-α antibody and 3, 3-diaminobenzidine (Invitrogen, Waltham, MA, USA). Finally, the tissue sections were cleaned in xylene (Sigma, Washington, WA, USA), mounted for visibility, and faintly counterstained with hematoxylin (Sigma, Washington, USA). Slides were observed at ×400 magnification using a light microscope. Morphometric analysis was performed using image analysis tools (Image J, 1.46a, NIH, Bethesda, MD, USA). The mean area percentage of TNF-α protein expression of the different experimental groups was assessed in ten non-overlapping fields of each section at ×400 magnification [83].
4.3.6. Inflammatory and Oxidative Stress Markers Using ELISA and qRT-PCR
IL-6 and IL-1β were quantified in the small intestine and caecum using ELISA kits (Abcam, Cambridge, United Kingdom). The gene expression of iNOS and GPX-1 was determined after extraction of the total RNA using an RNA extraction kit (Invitrogen, Waltham, MA, USA). The cDNA was synthesized, and qRT-PCR was ran using a SYBR green master mix (Qiagen, Hilden, Germany). The employed primers are listed in Table S1.
5. Statistical Analysis
Data are shown as mean ± SD after performing the experiments three times. An ANOVA was utilized to evaluate the differences between the groups. If p < 0.05, the results were judged to be significant. Kaplan–Meier survival curves were assembled to assess mice survival using the Prism software (GraphPad, La Jolla, CA, USA).
6. Conclusions
Trigonella foenum-graecum herb extract contains 36 different compounds. The herb exerts antibacterial action on S. typhimurium isolates both in vitro and in vivo. This conclusion was attained using various molecular techniques, including ELISA, qRT-PCR, and histopathological and immunohistochemical data. The probable mechanism of action of fenugreek herb extract on the tested bacteria is its harmful impact on the membrane properties of the bacteria. The chemical content of fenugreek herb could explain its antibacterial effect. However, further investigations are crucial to understand the antibacterial mode of action and disclosing its likely influence on other food pathogens, as well as to discovering the underlying mechanism of action.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17020259/s1, Figure S1: Negative mode total ion chromatogram of LC-ESI-MS/MS of Trigonella foenum-graecum herb extract; Table S1: Sequences of the primers.
Author Contributions
Conceptualization, W.A.N., E.E. and S.A.E.-S.; formal analysis, J.A., E.E. and M.J.A.; investigation, J.A., E.M., W.A.N., E.E. and S.A.E.-S.; methodology, W.A.N., E.E. and S.A.E.-S.; project administration, J.A.; software, W.A.N., E.M. and S.A.E.-S.; validation, J.A., M.J.A. and S.I.; writing—original draft, W.A.N., E.E., E.M., S.I. and S.A.E.-S.; writing—review and editing, W.A.N., E.E. and S.A.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Institutional Review Board Statement
The experimental protocol was approved by the Research Ethics Committee of the Faculty of Pharmacy, Tanta university, Egypt (number of TP/RE/12/23 p-059).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request.
Acknowledgments
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Popa, G.L.; Papa, M.I. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 11, 88. [Google Scholar] [CrossRef]
- Aljahdali, N.H.; Sanad, Y.M.; Han, J.; Foley, S.L. Current knowledge and perspectives of potential impacts of Salmonella enterica on the profile of the gut microbiota. BMC Microbiol. 2020, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Moreno Switt, A.I.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 34. [Google Scholar] [CrossRef]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic stewardship in food-producing animals: Challenges, progress, and opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Jadimurthy, R.; Jagadish, S.; Nayak, S.C.; Kumar, S.; Mohan, C.D.; Rangappa, K.S. Phytochemicals as invaluable sources of potent antimicrobial agents to combat antibiotic resistance. Life 2023, 13, 948. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Ashraf, M.V.; Pant, S.; Khan, M.H.; Shah, A.A.; Siddiqui, S.; Jeridi, M.; Alhamdi, H.W.S.; Ahmad, S. Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 881. [Google Scholar] [CrossRef]
- Basu, S.K.; Zandi, P.; Cetzal-Ix, W. Chapter 28—Fenugreek (Trigonella foenum-graecum L.): Distribution, Genetic Diversity, and Potential to Serve as an Industrial Crop for the Global Pharmaceutical, Nutraceutical, and Functional Food Industries. In The Role of Functional Food Security in Global Health; Singh, R.B., Watson, R.R., Takahashi, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 471–497. [Google Scholar]
- Snehlata, H.S.; Payal, D.R. Fenugreek (Trigonella foenum-graecum L.): An overview. Int. J. Curr. Pharm. Rev. Res. 2012, 2, 169–187. [Google Scholar]
- Almalki, D.A.; Naguib, D.M. Anticancer activity of aqueous fenugreek seed extract against pancreatic cancer, histological evidence. J. Gastrointest. Cancer 2022, 53, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, P.; Rani, A. Antimicrobial activity of Trigonella foenum-graecum L. (Fenugreek). Eur. Exp. Biol. 2016, 7, 4. [Google Scholar]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Mariod, A.A.; Saeed Mirghani, M.E.; Hussein, I. Chapter 22—Trigonella foenum-graecum Fenugreek, Bird’s Foot, Greek Hayseed. In Unconventional Oilseeds and Oil Sources; Mariod, A.A., Saeed Mirghani, M.E., Hussein, I., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 125–130. [Google Scholar]
- Akhlaghi, N.; Najafpour-Darzi, G. Phytochemical analysis, antioxidant activity, and pancreatic lipase inhibitory effect of ethanolic extract of Trigonella foenumgraceum L. leaves. Biocatal. Agric. Biotechnol. 2021, 32, 101961. [Google Scholar] [CrossRef]
- Saxena, S.; Karwa, S.; Saxena, R.; Sharma, T.; Sharma, Y.; Kakani, R.; Anwer, M. Analysis of antioxidant activity, phenolic and flavanoids content of fenugreek (Trigonellafoenum-graecum L.) seed extracts. Int. J. Seed Spices 2011, 1, 38–43. [Google Scholar]
- Sindhusha, V.B.; Rajasekar, A. Preparation and Evaluation of Antimicrobial Property and Anti-inflammatory Activity of Fenugreek Gel Against Oral Microbes: An Invitro Study. Cureus 2023, 15, e47659. [Google Scholar] [CrossRef]
- Václavík, J.; Coene, K.L.; Vrobel, I.; Najdekr, L.; Friedecký, D.; Karlíková, R.; Mádrová, L.; Petsalo, A.; Engelke, U.F.; van Wegberg, A. Structural elucidation of novel biomarkers of known metabolic disorders based on multistage fragmentation mass spectra. J. Inherit. Metab. Dis. 2018, 41, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.A.; van den Bos, A.A.; Kite, G.C.; Veitch, N.C.; Simmonds, M.S. Flavonol glycosides acylated with 3-hydroxy-3-methylglutaric acid as systematic characters in Rosa. Phytochemistry 2012, 81, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.T.; Ramesh, P.R.; Mahesh, K.; Madhu, K.M.; Anandan, E.M.; Praveen, M.; Balachandran, I. Chemical profiling of a polyherbal formulation by tandem mass spectroscopic analysis with multiple ionization techniques. Future J. Pharm. Sci. 2020, 6, 40. [Google Scholar]
- Shahzad, M.N.; Ahmad, S.; Tousif, M.I.; Ahmad, I.; Rao, H.; Ahmad, B.; Basit, A. Profiling of phytochemicals from aerial parts of Terminalia neotaliala using LC-ESI-MS2 and determination of antioxidant and enzyme inhibition activities. PLoS ONE 2022, 17, e0266094. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, B.; Li, Z.; Hong, T.; Chen, M.; Tan, Y.; Jiang, J.; Huang, C. Metabolite identification of myricetin in rats using HPLC coupled with ESI-MS. Chromatographia 2012, 75, 655–660. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Liang, W.; Fitzloff, J.F.; van Breemen, R.B. Identification of caffeic acid derivatives in Actea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 978–982. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Obama, T.; Itabe, H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 8312. [Google Scholar] [CrossRef]
- Asen, S.; Plimmer, J.R. 4,6,4′-Trihydroxyaurone and other flavonoids from Limonium. Phytochemistry 1972, 11, 2601–2603. [Google Scholar] [CrossRef]
- Kumar, K.K.; Goodwin, C.R.; Uhouse, M.A.; Bornhorst, J.; Schwerdtle, T.; Aschner, M.; McLean, J.A.; Bowman, A.B. Untargeted metabolic profiling identifies interactions between Huntington’s disease and neuronal manganese status. Metallomics 2015, 7, 363–370. [Google Scholar] [CrossRef]
- Benoit, F.; Holmes, J.; Isaacs, N. The mass spectra of carboxylic acids—I: Fragmentation mechanisms in maleic and fumaric acids and related compounds. Org. Mass Spectrom. 1969, 2, 591–601. [Google Scholar] [CrossRef]
- Grossert, J.S.; Fancy, P.D.; White, R.L. Fragmentation pathways of negative ions produced by electrospray ionization of acyclic dicarboxylic acids and derivatives. Can. J. Chem. 2005, 83, 1878–1890. [Google Scholar] [CrossRef]
- Hengel, S.M.; Goodlett, D.R. A review of tandem mass spectrometry characterization of adenosine diphosphate-ribosylated peptides. Int. J. Mass Spectrom. 2012, 312, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Buzgaia, N.; Lee, S.Y.; Rukayadi, Y.; Abas, F.; Shaari, K. Antioxidant activity, α-glucosidase inhibition and UHPLC–ESI–MS/MS profile of shmar (Arbutus pavarii Pamp). Plants 2021, 10, 1659. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.J.; Elekhnawy, E.; Negm, W.A.; Mahgoub, S.; Hussein, I.A. Encephalartos villosus Lem. Displays a strong in vivo and in vitro antifungal potential against Candida glabrata clinical isolates. J. Fungi 2022, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Vagula, J.M.; Sinosaki, N.M.; Ribeiro, M.A.; Magon, T.; Bertozzi, J.; Meurer, E.C.; Santos Junior, O.O.; Visentainer, J.V. Simple and fast method for identification and quantification of anthocyanidins in berries by ultra performance liquid chromatography-mass spectrometry. J. Braz. Chem. Soc. 2018, 29, 38–44. [Google Scholar] [CrossRef]
- El-Aal, A.; Mohammed, H.; Ibrahim, M.; Ismail, L. Chemical profiling of polyphenols in Thunbergia alata and in silico virtual screening of their antiviral activities against COVID-19. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 94–100. [Google Scholar]
- Mekky, R.H.; del Mar Contreras, M.; El-Gindi, M.R.; Abdel-Monem, A.R.; Abdel-Sattar, E.; Segura-Carretero, A. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Negm, W.A.; Elekhnawy, E.; Elmongy, E.I.; Altwaijry, N.; El-Haroun, H.; El-Masry, T.A.; El-Sherbeni, S.A. Elucidation of Phytochemical Content of Cupressus macrocarpa Leaves: In Vitro and In Vivo Antibacterial Effect against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Antibiotics 2021, 10, 890. [Google Scholar] [CrossRef]
- Ablajan, K.; Tuoheti, A. Fragmentation characteristics and isomeric differentiation of flavonol O-rhamnosides using negative ion electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 451–460. [Google Scholar] [CrossRef]
- Prasain, J.K.; Jones, K.; Kirk, M.; Wilson, L.; Smith-Johnson, M.; Weaver, C.; Barnes, S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2003, 51, 4213–4218. [Google Scholar] [CrossRef]
- Mekky, R.H.; Abdel-Sattar, E.; Segura-Carretero, A.; del Mar Contreras, M. Metabolic profiling of the oil of Sesame of the Egyptian cultivar ‘Giza 32’ employing LC-MS and tandem ms-based untargeted method. Foods 2021, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Amal, D.; Kawashty, S.; Shamso, E.; Hosni, H.; Hussein, S. Chemical profiling of Oxalis species growing wild in Egypt using HRLC/MS Spectrometry. Int. J. Second. Metab. 2022, 9, 426–439. [Google Scholar]
- March, R.E.; Miao, X.S.; Metcalfe, C.D. A fragmentation study of a flavone triglycoside, kaempferol-3-O-robinoside-7-O-rhamnoside. Rapid Commun. Mass Spectrom. 2004, 18, 931–934. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Verardo, V.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Exploratory characterization of phenolic compounds with demonstrated anti-diabetic activity in guava leaves at different Oxidation States. Int. J. Mol. Sci. 2016, 17, 699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shang, Z.; Li, Q.; Huang, M.; He, W.; Wang, Z.; Zhang, J. Rapid screening and identification of daidzein metabolites in rats based on UHPLC-LTQ-orbitrap mass spectrometry coupled with data-mining technologies. Molecules 2018, 23, 151. [Google Scholar] [CrossRef]
- Sayed, D.; Afifi, A.; Temraz, A.; Ahmed, A. Metabolic Profiling of Mimusops elengi Linn. Leaves extract and in silico anti-inflammatory assessment targeting NLRP3 inflammasome. Arab. J. Chem. 2023, 16, 104753. [Google Scholar] [CrossRef]
- Mele, A.; Panzeri, W.; Selva, A. Fast-atom bombardment mass spectrometric and tandem mass spectrometric study of (−)-menthol-β-(d)-glucopyranoside, neohesperidin dihydrochalcone and their non-covalent association with β-cyclodextrin. Two examples of interaction of a carbohydrate host with glycoconjugate guests. Eur. Mass Spectrom. 1997, 3, 347–354. [Google Scholar]
- Wang, Y.; Zhao, M.; Ou, Y.; Zeng, B.; Lou, X.; Wang, M.; Zhao, C. Metabolic profile of esculin in rats by ultra high performance liquid chromatography combined with Fourier transform ion cyclotron resonance mass spectrometry. J. Chromatogr. B 2016, 1020, 120–128. [Google Scholar] [CrossRef]
- Elmongy, E.I.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Attallah, N.G.; Altwaijry, N.; Batiha, G.E.-S.; El-Sherbeni, S.A. Antidiarrheal and antibacterial activities of monterey cypress phytochemicals: In vivo and in vitro approach. Molecules 2022, 27, 346. [Google Scholar] [CrossRef]
- Tiepo, A.N.; Coutinho, I.D.; Machado, G.O.; Oliveira, H.C.; Pimenta, J.A.; Henning, L.M.M.; Colnago, L.A.; Stolf-Moreira, R. Phenolic Compounds from Leaves of Cariniana estrellensis (Raddi) Kuntze (Lecythidaceae): A Brazilian Atlantic Forest Tree. J. Braz. Chem. Soc. 2023, 34, 146–152. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G.; Henriques, M. Evaluation of antibacterial activity of caffeic acid encapsulated by β-cyclodextrins. J. Microencapsul. 2015, 32, 804–810. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Cusumano, Z.T.; Caparon, M.G. Citrulline protects Streptococcus pyogenes from acid stress using the arginine deiminase pathway and the F1Fo-ATPase. J. Bacteriol. 2015, 197, 1288–1296. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella enteritidis and Escherichia coli O157: H7 in apple, pear and melon juices. Food Control 2009, 20, 105–112. [Google Scholar] [CrossRef]
- Pinto, H.B.; Brust, F.R.; Macedo, A.J.; Trentin, D.S. The antivirulence compound myricetin possesses remarkable synergistic effect with antibacterials upon multidrug resistant Staphylococcus aureus. Microb. Pathog. 2020, 149, 104571. [Google Scholar] [CrossRef] [PubMed]
- Chitemerere, T.A.; Mukanganyama, S. Evaluation of cell membrane integrity as a potential antimicrobial target for plant products. BMC Complement. Altern. Med. 2014, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Almukainzi, M.; El-Masry, T.A.; Negm, W.A.; Elekhnawy, E.; Saleh, A.; Sayed, A.E.; Ahmed, H.M.; Abdelkader, D.H. Co-delivery of gentiopicroside and thymoquinone using electrospun m-PEG/PVP nanofibers: In-vitro and In vivo studies for antibacterial wound dressing in diabetic rats. Int. J. Pharm. 2022, 625, 122106. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability barriers of Gram-negative pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.; Al-Fakhrany, O.M.; Elekhnawy, E.; Hussein, I.A.; Shaldam, M.A.; Altwaijry, N.; Alqahtani, M.J.; Negm, W.A. Anti-biofilm and antibacterial activities of Cycas media R. Br secondary metabolites: In silico, in vitro, and in vivo approaches. Antibiotics 2022, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Alotaibi, B.; El-Masry, T.A.; Elekhnawy, E.; El-Kadem, A.H.; Saleh, A.; Negm, W.A.; Abdelkader, D.H. Aqueous core epigallocatechin gallate PLGA nanocapsules: Characterization, antibacterial activity against uropathogens, and in vivo reno-protective effect in cisplatin induced nephrotoxicity. Drug Deliv. 2022, 29, 1848–1862. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Zhang, G.; Chen, F.; Cao, Y.; Kalyanaraman, B.; See, W.A. iNOS expression and NO production contribute to the direct effects of BCG on urothelial carcinoma cell biology. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2014; pp. 45.e1–45.e9. [Google Scholar]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Tomita, Y. Antimutagenic activity of caffeic acid and related compounds. Biosci. Biotechnol. Biochem. 1996, 60, 328–329. [Google Scholar] [CrossRef]
- Choi, K.-C.; Son, Y.-O.; Hwang, J.-M.; Kim, B.-T.; Chae, M.; Lee, J.-C. Antioxidant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated from Lolium multiflorum. Pharm. Biol. 2017, 55, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Luecha, P.; Umehara, K.; Miyase, T.; Noguchi, H. Antiestrogenic constituents of the Thai medicinal plants Capparis flavicans and Vitex glabrata. J. Nat. Prod. 2009, 72, 1954–1959. [Google Scholar] [CrossRef]
- Akroum, S.; Bendjeddou, D.; Satta, D.; Lalaoui, K. Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia. Am.–Eurasian J. Sci. Res. 2009, 4, 93–96. [Google Scholar]
- Ho, S.; Shah, N. The antibacterial effect of addition of citrulline in fermented milk against foodborne pathogens. In Proceedings of the ADSA Annual Meeting, Pittsburgh, PA, USA, 25–28 June 2017; American Dairy Science Association: Champaign, IL, USA, 2017. [Google Scholar]
- Li, S.; Lv, Q.; Sun, X.; Tang, T.; Deng, X.; Yin, Y.; Li, L. Acacetin inhibits Streptococcus pneumoniae virulence by targeting pneumolysin. J. Pharm. Pharmacol. 2020, 72, 1092–1100. [Google Scholar] [CrossRef]
- Duncan, S.H.; Leitch, E.C.M.; Stanley, K.N.; Richardson, A.J.; Laven, R.A.; Flint, H.J.; Stewart, C.S. Effects of esculin and esculetin on the survival of Escherichia coli O157 in human faecal slurries, continuous-flow simulations of the rumen and colon and in calves. Br. J. Nutr. 2004, 91, 749–755. [Google Scholar] [CrossRef]
- Coelho, M.; Silva, S.; Costa, E.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M. Anthocyanin recovery from grape by-products by combining ohmic heating with food-grade solvents: Phenolic composition, antioxidant, and antimicrobial properties. Molecules 2021, 26, 3838. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Sonbol, F.; Elbanna, T.; El-Ekhnawy, E. Exposure to sublethal concentrations of benzalkonium chloride induces antimicrobial resistance and cellular changes in Klebsiellae pneumoniae clinical isolates. Microb. Drug Resist. 2019, 25, 631–638. [Google Scholar] [CrossRef]
- Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Elekhnawy, E. Investigation of the Antibacterial Activity and Efflux Pump Inhibitory Effect of Cycas thouarsii R. Br. Extract against Klebsiella pneumoniae Clinical Isolates. Pharmaceuticals 2021, 14, 756. [Google Scholar] [CrossRef]
- Elekhnawy, E.A.; Sonbol, F.I.; Elbanna, T.E.; Abdelaziz, A.A. Evaluation of the impact of adaptation of Klebsiella pneumoniae clinical isolates to benzalkonium chloride on biofilm formation. Egypt. J. Med. Hum. Genet. 2021, 22, 51. [Google Scholar] [CrossRef]
- Elekhnawy, E.; Negm, W.A. The potential application of probiotics for the prevention and treatment of COVID-19. Egypt. J. Med. Hum. Genet. 2022, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Abd El-Rahman, H.S.M. Anti-shigellosis activity of the aqueous extract of garlic, clove and fenugreek. J. Food Saf. 2022, 42, e12978. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Miotto, A.; Lins, T.A.; Montero, E.; Oshima, C.; Alonso, L.G.; Perfeito, J. Immunohistochemical analysis of the COX-2 marker in acute pulmonary injury in rats. Ital. J. Anat. Embryol. Arch. Ital. Anat. Embriol. 2009, 114, 193–199. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).