Multicenter Experience with Good Manufacturing Practice Production of [11C]PiB for Amyloid Positron Emission Tomography Imaging
Abstract
1. Introduction
2. Results
3. Discussion
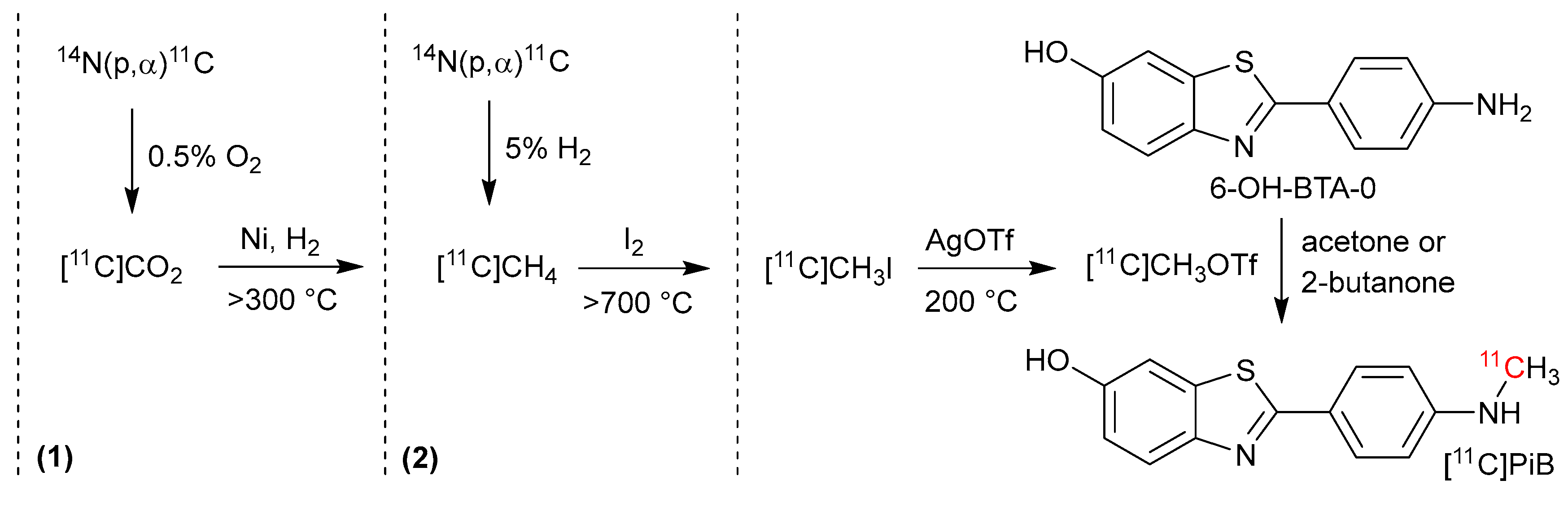
3.1. Production and Separation of [11C]PiB
3.1.1. [11C]CO2 vs. [11C]CH4 Methods
3.1.2. Radiolysis Caused by High Am
3.1.3. Radiochemical and Chemical Impurities
3.1.4. Semi-Preparative HPLC Purification
3.1.5. AgOTf Column and Sterile Filtration
3.1.6. Summary of [11C]PiB Production
3.2. Clinical Use of [11C]PiB
4. Materials and Methods
4.1. Radiosynthesis of [11C]PiB
4.1.1. AUH
4.1.2. HUH
4.1.3. OUH
4.1.4. RH
4.2. Product Quality Control and Analyses
4.3. Statistical Methods
4.4. Ethical Treatment of Humans and Animals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.-F.; Estrada, S.; et al. Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- van Oostveen, W.M.; de Lange, E.C.M. Imaging Techniques in Alzheimer’s Disease: A Review of Applications in Early Diagnosis and Longitudinal Monitoring. Int. J. Mol. Sci. 2021, 22, 2110. [Google Scholar] [CrossRef]
- Jack, C.R.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association; Thies, W.; Bleiler, L. 2013 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2013, 9, 208–245. [Google Scholar] [CrossRef] [PubMed]
- Ruangma, A.; Panpitpat, S.; Saonam, T.; Kijprayoon, S.; Ngokpol, S.; Tanasirimanon, M.; Khajitkhajonwong, S.; Tuamputsa, S.; Rachadara, S.; Chinvarun, Y. Challenges in Production of Alzheimer’s Tracer C-11 PiB. Bangk. Med. J. 2015, 9, 70. [Google Scholar] [CrossRef]
- Pemberton, H.G.; Collij, L.E.; Heeman, F.; Bollack, A.; Shekari, M.; Salvadó, G.; Alves, I.L.; Garcia, D.V.; Battle, M.; Buckley, C.; et al. Quantification of Amyloid PET for Future Clinical Use: A State-of-the-Art Review. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3508–3528. [Google Scholar] [CrossRef] [PubMed]
- Mathis, C.A.; Bacskai, B.J.; Kajdasz, S.T.; McLellan, M.E.; Frosch, M.P.; Hyman, B.T.; Holt, D.P.; Wang, Y.; Huang, G.-F.; Debnath, M.L.; et al. A Lipophilic Thioflavin-T Derivative for Positron Emission Tomography (PET) Imaging of Amyloid in Brain. Bioorganic Med. Chem. Lett. 2002, 12, 295–298. [Google Scholar] [CrossRef]
- Mathis, C.A.; Wang, Y.; Holt, D.P.; Huang, G.-F.; Debnath, M.L.; Klunk, W.E. Synthesis and Evaluation of 11C-Labeled 6-Substituted 2-Arylbenzothiazoles as Amyloid Imaging Agents. J. Med. Chem. 2003, 46, 2740–2754. [Google Scholar] [CrossRef]
- Gómez-Vallejo, V.; Gaja, V.; Koziorowski, J.; Llop, J. Specific Activity of 11C-Labelled Radiotracers: A Big Challenge for PET Chemists. Positron Emiss. Tomogr.—Curr. Clin. Res. Asp. 2012, 2, 183–210. [Google Scholar]
- Taddei, C.; Gee, A.D. Recent Progress in [11C] Carbon Dioxide ([11C]CO2) and [11C] Carbon Monoxide ([11C]CO) Chemistry. J. Label. Compd. Radiopharm. 2018, 61, 237–251. [Google Scholar] [CrossRef]
- Fukumura, T.; Nakao, R.; Yamaguchi, M.; Suzuki, K. Stability of 11C-Labeled PET Radiopharmaceuticals. Appl. Radiat. Isot. 2004, 61, 1279–1287. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Coliva, A.; Monterisi, C.; Apollaro, A.; Gatti, D.; Penso, M.; Gianolli, L.; Perani, D.; Gilardi, M.C.; Carpinelli, A. Synthesis Optimization of 2-(4-N-[11C]Methylaminophenyl)-6-Hydroxybenzothiazole ([11C]PIB), β-Amyloid PET Imaging Tracer for Alzheimer’s Disease Diagnosis. Appl. Radiat. Isot. 2015, 105, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Solbach, C.; Uebele, M.; Reischl, G.; Machulla, H.J. Efficient Radiosynthesis of Carbon-11 Labelled Uncharged Thioflavin T Derivatives using [11C]Methyl Triflate for β-Amyloid Imaging in Alzheimer’s Disease with PET. Appl. Radiat. Isot. 2005, 62, 591–595. [Google Scholar] [CrossRef]
- Wilson, A.A.; Garcia, A.; Chestakova, A.; Kung, H.; Houle, S. A Rapid One-Step Radiosynthesis of the β-Amyloid Imaging Radiotracer N-Methyl-[11C]2-(4′-Methylaminophenyl)-6-Hydroxybenzothiazole ([11C]-6-OH-BTA-1). J. Label. Compd. Radiopharm. 2004, 47, 679–682. [Google Scholar] [CrossRef]
- Långström, B.; Antoni, G.; Gullberg, P.; Halldin, C.; Malmborg, P.; Någren, K.; Rimland, A.; Svärd, H. Synthesis of L- and D-[Methyl-11C]Methionine. J. Nucl. Med. 1987, 28, 1037–1040. [Google Scholar] [PubMed]
- Larsen, P.; Ulin, J.; Dahlstrøm, K.; Jensen, M. Synthesis of [11C]Iodomethane by Iodination of [11C]Methane. Appl. Radiat. Isot. 1997, 48, 153–157. [Google Scholar] [CrossRef]
- Jewett, D.M. A Simple Synthesis of [11C]Methyl Triflate. Int. J. Radiat. Appl. Instrum. Part A Appl. Radiat. Isot. 1992, 43, 1383–1385. [Google Scholar] [CrossRef]
- Liger, F.; Eijsbouts, T.; Cadarossanesaib, F.; Tourvieille, C.; Le Bars, D.; Billard, T. Direct [11C]Methylation of Amines from [11C]CO2 for the Synthesis of PET Radiotracers. Eur. J. Org. Chem. 2015, 2015, 6434–6438. [Google Scholar] [CrossRef]
- Buccino, P.; Savio, E.; Porcal, W. Fully-Automated Radiosynthesis of the Amyloid Tracer [11C]PiB via Direct [11C]CO2 Fixation-Reduction. EJNMMI Radiopharm. Chem. 2019, 4, 14. [Google Scholar] [CrossRef]
- Gómez-Vallejo, V.; Llop, J. Fully Automated and Reproducible Radiosynthesis of High Apecific Activity [11C] Raclopride and [11C] Pittsburgh Compound-B using the Combination of two Commercial Synthesizers. Nucl. Med. Commun. 2011, 32, 1011–1017. [Google Scholar] [CrossRef]
- Boudjemeline, M.; Hopewell, R.; Rochon, P.-L.; Jolly, D.; Hammami, I.; Villeneuve, S.; Kostikov, A. Highly Efficient Solid Phase Supported Radiosynthesis of [11C]PiB using tC18 Cartridge as a “3-in-1” Production Entity. J. Label. Compd. Radiopharm. 2017, 60, 632–638. [Google Scholar] [CrossRef]
- Zhang, W.; Oya, S.; Kung, M.-P.; Hou, C.; Maier, D.L.; Kung, H.F. F-18 Polyethyleneglycol Stilbenes as PET Imaging Agents Targeting Aβ Aggregates in the Brain. Nucl. Med. Biol. 2005, 32, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Dean, F.W.; Paul, B.R.; Yun, Z.; Anil, K.; Vanessa, R.; Hayden, T.R.; Robert, F.D.; Ayon, N.; James, R.B.; Weiguo, Y.; et al. In Vivo Imaging of Amyloid Deposition in Alzheimer Disease using the Radioligand 18F-AV-45 (Florbetapir F 18). J. Nucl. Med. 2010, 51, 913. [Google Scholar] [CrossRef]
- Vandenberghe, R.; Van Laere, K.; Ivanoiu, A.; Salmon, E.; Bastin, C.; Triau, E.; Hasselbalch, S.; Law, I.; Andersen, A.; Korner, A.; et al. 18F-Flutemetamol Amyloid Imaging in Alzheimer Disease and Mild Cognitive Impairment: A Phase 2 Trial. Ann. Neurol. 2010, 68, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Cselényi, Z.; Jönhagen, M.E.; Forsberg, A.; Halldin, C.; Julin, P.; Schou, M.; Johnström, P.; Varnäs, K.; Svensson, S.; Farde, L. Clinical Validation of 18F-AZD4694, an Amyloid-β–Specific PET Radioligand. J. Nucl. Med. 2012, 53, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.; Chalkidou, A.; Hammers, A.; Peacock, J.; Summers, J.; Keevil, S. Diagnostic Accuracy of 18F Amyloid PET Tracers for the Diagnosis of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Buckley, K.R.; Huser, J.M.; Jivan, S.; Chun, K.S.; Ruth, T.J. 11C-Methane Production in Small Volume, High Pressure Gas Targets. Radiochim. Acta 2000, 88, 201–206. [Google Scholar] [CrossRef]
- Patt, M.; Solbach, C.; Habermann, B.; Schildan, A.; Baur, B.; Sabri, O. Influence of Additives to the Formulation of n.c.a. [11C]PiB on Sterile Filter Performance. Appl. Radiat. Isot. 2013, 82, 289–292. [Google Scholar] [CrossRef]
- An, H.H.; Moon, B.S.; Park, H.S.; Lee, H.J.; Lee, S.J.; Oh, S.J.; Kim, B.S.; Lee, B.C.; Lee, W.W.; Kim, S.E. Comparative Study in Different Filters for Efficient Sterile Filtration. Bull. Korean Chem. Soc. 2020, 41, 824–828. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Boccardi, M.; Barkhof, F.; Blennow, K.; Cappa, S.; Chiotis, K.; Démonet, J.-F.; Garibotto, V.; Giannakopoulos, P.; Gietl, A.; et al. Strategic Roadmap for an Early Diagnosis of Alzheimer’s Disease Based on Biomarkers. Lancet Neurol. 2017, 16, 661–676. [Google Scholar] [CrossRef]
- Madsen, L.S.; Parbo, P.; Ismail, R.; Gottrup, H.; Østergaard, L.; Brooks, D.J.; Eskildsen, S.F. Capillary Dysfunction Correlates with Cortical Amyloid Load in Early Alzheimer’s Disease. Neurobiol. Aging 2023, 123, 1–9. [Google Scholar] [CrossRef]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The Relationships between Neuroinflammation, Beta-Amyloid and Tau Deposition in Alzheimer’s Disease: A Longitudinal PET Study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef]
- Rosengren, S.; Skibsted Clemmensen, T.; Tolbod, L.; Granstam, S.-O.; Eiskjær, H.; Wikström, G.; Vedin, O.; Kero, T.; Lubberink, M.; Harms Hendrik, J.; et al. Diagnostic Accuracy of [11C]PIB Positron Emission Tomography for Detection of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 1337–1347. [Google Scholar] [CrossRef]



| AUH | HUH | OUH | RH | |
|---|---|---|---|---|
| n | 74 | 105 | 84 | 252 |
| 11C-precursor | [11C]CO2 a | [11C]CH4 | [11C]CO2 | [11C]CO2 a |
| TNi [°C] | 375 | N/A | 350 | 350 |
| TIodine [°C] | 100 | 60 | 100 | 20–25 |
| TQuartz [°C] | 740 | 720 | 700 | 760 |
| m [mg] | 2.0 | 1.0 | 1.0 | 1.0 |
| Solvent | anhydrous acetone | dried 2-butanone | acetone | 2-butanone |
| VSol [μL] | 300 | 150 | 300 | 300 |
| Ttrap [°C] | −20 | −10 | 5 | −10 |
| TR [°C] | 50 | 85 | 60 | 80 |
| tR [min] | 2.5 | 2.5 | 2.0 | 3.0 |
| Yield EOS [GBq] | 1.98 ± 1.00 | 0.83 ± 0.29 | 3.17 ± 1.20 | 1.46 ± 0.64 |
| Am [GBq/μmol] | 21.2 ± 16.8 | 98.0 ± 61.4 | 95.6 ± 44.2 b | 55.0 ± 50.4 |
| AUH | HUH | OUH | RH | |
|---|---|---|---|---|
| Pump(s) | Knauer P 4.1S | 2 × Knauer 100 on valve dock | SYKAM S1021 | Knauer 100 Knauer P.4.1S |
| Column | Phenomonex Luna C18, 250 × 10 mm a | Phenomonex Kinetex 2.6 μm C18, 100 Å, 50 × 4.6 mm | Merck Chromolith Performance RP-18e, 100 × 10 mm | Phenomenex Onyx Monolithic C18, 100 × 10 mm |
| UV detector | Knauer K-2501 | Knauer Smartline UV 200 | Knauer K-2001 | Knauer UV 120 Knauer Azura UVD 215 |
| Gamma detector | TRACERlab FX c built-in | TracerMaker built-in (GM tube) | TRACERlab FX c built-in | Gilson 401 dilutor |
| Method | Isocratic | Isocratic | Isocratic | Isocratic |
| Eluent ratio (A:B) | 45:55 b | 30:70 | 25:75 b | 30:70 b |
| Eluent A | Ethanol | Ethanol | Ethanol | Ethanol |
| Eluent B | 70 mM NaH2PO4 | 0.1% H3PO4/25 mM ascorbic acid/1% 2-butanone | 15 mM H3PO4 | 0.1% H3PO4/25 mM ascorbic acid |
| Rate (mL/min) | ≈6 | 8 | 6.5 | 6 |
| RT (min) | 7–8 | 3–4 | ≈8 | 5–6 |
| Vfrac [mL] | ≈6 | 2.67 | 6.5 | 6 |
| AUH | HUH | OUH | RH | |
|---|---|---|---|---|
| Cyclotron | GE PETtrace 600 a GE PETtrace 800 a IBA Cyclone 18/18 | IBA Cyclone 18/18 | GE PETtrace | CTI XP b Scanditronix c |
| Target gas | 99.5% N2 + 0.5% O2 95% N2 + 5% H2 | 95% N2 + 5% H2 | 99.5% N2 + 0.5% O2 | 99.5% N2 + 0.5% O2 95% N2 + 5% H2 |
| Irradiation time [min] | 44.5 | 25–30 | 30–60 | 44.5 |
| Beam current [μA] | 60.0 a, 20.0 | 27.0 | 60 | 52.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, A.B.A.; Lehel, S.; Grove, E.K.; Langkjaer, N.; Fuglø, D.; Huynh, T.H.V. Multicenter Experience with Good Manufacturing Practice Production of [11C]PiB for Amyloid Positron Emission Tomography Imaging. Pharmaceuticals 2024, 17, 217. https://doi.org/10.3390/ph17020217
Andersen ABA, Lehel S, Grove EK, Langkjaer N, Fuglø D, Huynh THV. Multicenter Experience with Good Manufacturing Practice Production of [11C]PiB for Amyloid Positron Emission Tomography Imaging. Pharmaceuticals. 2024; 17(2):217. https://doi.org/10.3390/ph17020217
Chicago/Turabian StyleAndersen, Anders Bruhn Arndal, Szabolcs Lehel, Ebbe Klit Grove, Niels Langkjaer, Dan Fuglø, and Tri Hien Viet Huynh. 2024. "Multicenter Experience with Good Manufacturing Practice Production of [11C]PiB for Amyloid Positron Emission Tomography Imaging" Pharmaceuticals 17, no. 2: 217. https://doi.org/10.3390/ph17020217
APA StyleAndersen, A. B. A., Lehel, S., Grove, E. K., Langkjaer, N., Fuglø, D., & Huynh, T. H. V. (2024). Multicenter Experience with Good Manufacturing Practice Production of [11C]PiB for Amyloid Positron Emission Tomography Imaging. Pharmaceuticals, 17(2), 217. https://doi.org/10.3390/ph17020217






