Efficacy of Ceragenins in Controlling the Growth of Oral Microorganisms: Implications for Oral Hygiene Management
Abstract
1. Introduction
2. Results
2.1. Potent Antimicrobial Activity of Ceragenins against All Tested Fungal and Bacterial Microorganisms Commonly Found in the Oral Cavity
2.2. Inhibition of the Formation of Mono- and Dual-Species Fungal and Bacterial Biofilms by Ceragenins
2.3. Low Toxicity of Ceragenins against Host Cells
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Fungal and Bacterial Strains
4.3. Antimicrobial Testing
4.4. Biofilm Viability Measurements
4.5. Biocompatibility Assessment
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sjögren, P.; Wårdh, I.; Zimmerman, M.; Almståhl, A.; Wikström, M. Oral Care and Mortality in Older Adults with Pneumonia in Hospitals or Nursing Homes: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2016, 64, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Koulenti, D.; Blot, S.; Sierra, R.; Diaz, E.; De Waele, J.J.; Macor, A.; Agbaht, K.; Rodriguez, A. Oral care practices in intensive care units: A survey of 59 European ICUs. Intensive Care Med. 2007, 33, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Salamone, K.; Yacoub, E.; Mahoney, A.M.; Edward, K.L. Oral care of hospitalised older patients in the acute medical setting. Nurs. Res. Pract. 2013, 2013, 827670. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.; Ruppé, E.; Harbarth, S.; Asehnoune, K.; Poulakou, G.; Luyt, C.E.; Rello, J.; Klompas, M.; Depuydt, P.; Eckmann, C.; et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit. Care Nurs. 2022, 70, 103227. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral. Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, F.; Dubois, D.; Pronnier, P.; Herbecq, P.; Leroy, O.; Desmettre, T.; Pottier-Cau, E.; Boutigny, H.; Di Pompéo, C.; Durocher, A.; et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: A double-blind placebo-controlled multicenter study. Crit. Care Med. 2005, 33, 1728–1735. [Google Scholar] [CrossRef]
- Hellyer, T.P.; Ewan, V.; Wilson, P.; Simpson, A.J. The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J. Intensive Care Soc. 2016, 17, 238–243. [Google Scholar] [CrossRef]
- Maryani, N.; Octavia, A.; Budiyantoro, C.; Ulfa, M. Prevention of Pneumonia due to Ventilator in Critical Patients with U Shape Oral Hygiene Model: A Systematic Review. Rom. J. Anaesth. Intensive Care 2023, 30, 1–9. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Bélanger, M.; Rodrigues, P.H.; Simpson-Haidaris, P.J.; Akin, D.; Dunn, W.A.; Progulske-Fox, A.; Burne, R.A. Invasion of human coronary artery endothelial cells by Streptococcus mutans OMZ175. Oral. Microbiol. Immunol. 2009, 24, 141–145. [Google Scholar] [CrossRef]
- Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms 2021, 9, 1218. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Thornhill, M.; Michalowicz, B.S.; Noll, J.; Bahrani-Mougeot, F.K.; Sasser, H.C. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Dent. Assoc. 2009, 140, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriotis, G.; Marangos, M.; Lagadinou, M.; Bhagani, S.; Assimakopoulos, S.F. Oral Antibiotics for Bacteremia and Infective Endocarditis: Current Evidence and Future Perspectives. Microorganisms 2023, 11, 3004. [Google Scholar] [CrossRef] [PubMed]
- Linden, G.J.; Lyons, A.; Scannapieco, F.A. Periodontal systemic associations: Review of the evidence. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.F.; de Paula, R.M.; de Castro Piau, C.G.; Costa, P.P.; Bezerra, A.C. Oral care practices for patients in Intensive Care Units: A pilot survey. Indian. J. Crit. Care Med. 2016, 20, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef]
- Dias, C.; Rauter, A.P. Membrane-targeting antibiotics: Recent developments outside the peptide space. Future Med. Chem. 2019, 11, 211–228. [Google Scholar] [CrossRef]
- Neves, A.R.; Freitas-Silva, J.; Durães, F.; Silva, E.R.; Rodrigues, I.C.; Mergulhão, F.; Gomes, M.; Teixeira-Santos, R.; Bernardes André, M.; Silva, R.; et al. Insights into the antimicrobial properties of a cationic steroid and antibiofilm performance in PDMS-based coatings to potentially treat urinary infections. J. Mater. Chem. B 2023, 11, 8697–8716. [Google Scholar] [CrossRef]
- Wang, J.; Ghali, S.; Xu, C.; Mussatto, C.C.; Ortiz, C.; Lee, E.C.; Tran, D.H.; Jacobs, J.P.; Lagishetty, V.; Faull, K.F.; et al. Ceragenin CSA13 Reduces Clostridium difficile Infection in Mice by Modulating the Intestinal Microbiome and Metabolites. Gastroenterology 2018, 154, 1737–1750. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Pham, T.X.; Farnsworth, R.W.; Williams, D.L.; Loc-Carrillo, C.; Horne, L.A.; Ingebretsen, S.H.; Bloebaum, R.D. Development of a broad spectrum polymer-released antimicrobial coating for the prevention of resistant strain bacterial infections. J. Biomed. Mater. Res. A 2012, 100, 2732–2738. [Google Scholar] [CrossRef] [PubMed]
- Isogai, E.; Isogai, H.; Takahashi, K.; Okumura, K.; Savage, P.B. Ceragenin CSA-13 exhibits antimicrobial activity against cariogenic and periodontopathic bacteria. Oral. Microbiol. Immunol. 2009, 24, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Olekson, M.A.; You, T.; Savage, P.B.; Leung, K.P. Antimicrobial ceragenins inhibit biofilms and affect mammalian cell viability and migration. FEBS Open Bio 2017, 7, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, K.P.; Bourgault, A.M.; Sole, M.L. Oral Microbes in Hospital-Acquired Pneumonia: Practice and Research Implications. Crit. Care Nurse 2022, 42, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.; Hogendoorn, G.K.; Rijneveld, R.; Luijten, S.; van Alewijk, D.C.J.G.; van den Munckhof, E.H.A.; de Kam, M.L.; Feiss, G.L.; Prens, E.P.; et al. Pharmacodynamic Effects of Topical Omiganan in Patients With Mild to Moderate Atopic Dermatitis in a Randomized, Placebo-Controlled, Phase II Trial. Clin. Transl. Sci. 2020, 13, 994–1003. [Google Scholar] [CrossRef]
- Delisle, M.S.; Williamson, D.R.; Albert, M.; Perreault, M.M.; Jiang, X.; Day, A.G.; Heyland, D.K. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can. Respir. J. 2011, 18, 131–136. [Google Scholar] [CrossRef]
- Sands, K.M.; Twigg, J.A.; Lewis, M.A.O.; Wise, M.P.; Marchesi, J.R.; Smith, A.; Wilson, M.J.; Williams, D.W. Microbial profiling of dental plaque from mechanically ventilated patients. J. Med. Microbiol. 2016, 65, 147–159. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(E); Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 22 September 2023).
- Dagnew, Z.A.; Abraham, I.A.; Beraki, G.G.; Tesfamariam, E.H.; Mittler, S.; Tesfamichael, Y.Z. Nurses’ attitude towards oral care and their practicing level for hospitalized patients in Orotta National Referral Hospital, Asmara-Eritrea: A cross-sectional study. BMC Nurs. 2020, 19, 63. [Google Scholar] [CrossRef]
- Janto, M.; Iurcov, R.; Daina, C.M.; Neculoiu, D.C.; Venter, A.C.; Badau, D.; Cotovanu, A.; Negrau, M.; Suteu, C.L.; Sabau, M.; et al. Oral Health among Elderly, Impact on Life Quality, Access of Elderly Patients to Oral Health Services and Methods to Improve Oral Health: A Narrative Review. J. Pers. Med. 2022, 12, 372. [Google Scholar] [CrossRef]
- Kelly, N.; Blackwood, B.; Credland, N.; Stayt, L.; Causey, C.; Winning, L.; McAuley, D.F.; Lundy, F.T.; El Karim, I. Oral health care in adult intensive care units: A national point prevalence study. Nurs Crit Care. 2023, 28, 773–780. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, X.; Zhang, Q.; Li, C.; Worthington, H.V.; Hua, F. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2020, 12, CD008367. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A. Supragingival Biofilm: Toothpaste and Toothbrushes. Monogr. Oral. Sci. 2021, 29, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Burgos-Morón, E.; López-Lázaro, M. A 30-s exposure to ethanol 20% is cytotoxic to human keratinocytes: Possible mechanistic link between alcohol-containing mouthwashes and oral cancer. Clin. Oral. Investig. 2018, 22, 2943–2946. [Google Scholar] [CrossRef] [PubMed]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, K.; Namiot, D.; Byfield, F.J.; Cruz, K.; Zendzian-Piotrowska, M.; Fein, D.E.; Savage, P.B.; Diamond, S.; McCulloch, C.A.; Janmey, P.A.; et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J. Antimicrob. Chemother. 2013, 68, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Rapala-Kozik, M.; Bochenska, O.; Zawrotniak, M.; Wolak, N.; Trebacz, G.; Gogol, M.; Ostrowska, D.; Aoki, W.; Ueda, M.; Kozik, A. Inactivation of the antifungal and immunomodulatory properties of human cathelicidin LL-37 by aspartic proteases produced by the pathogenic yeast Candida albicans. Infect. Immun. 2015, 83, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.A.; Macfarlane, G.T. Studies on the proteolytic activity of Bacteroides fragilis. J. Gen. Microbiol. 1988, 134, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, Y.C.; Jeng, S.L.; Chang, J.J.; Wang, J.Y.; Lin, C.H.; Tsai, P.F.; Ko, N.Y.; Ko, W.C.; Wang, J.L. Short-term effects of Chlorhexidine mouthwash and Listerine on oral microbiome in hospitalized patients. Front. Cell Infect. Microbiol. 2023, 13, 1056534. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Devine, D.; Marsh, P.D. Oral biofilms: Molecular analysis, challenges, and future prospects in dental diagnostics. Clin. Cosmet. Investig. Dent. 2013, 5, 11–19. [Google Scholar] [CrossRef]
- Bucki, R.; Namiot, D.B.; Namiot, Z.; Savage, P.B.; Janmey, P.A. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J. Antimicrob. Chemother. 2008, 62, 329–335. [Google Scholar] [CrossRef]
- Abouassi, T.; Hannig, C.; Mahncke, K.; Karygianni, L.; Wolkewitz, M.; Hellwig, E.; Al-Ahmad, A. Does human saliva decrease the antimicrobial activity of chlorhexidine against oral bacteria? BMC Res. Notes 2014, 7, 711. [Google Scholar] [CrossRef]
- Spijkervet, F.K.; van Saene, J.J.; van Saene, H.K.; Panders, A.K.; Vermey, A.; Fidler, V. Chlorhexidine inactivation by saliva. Oral. Surg. Oral. Med. Oral. Pathol. 1990, 69, 444–449. [Google Scholar] [CrossRef]
- Ustrell-Borràs, M.; Traboulsi-Garet, B.; Gay-Escoda, C. Alcohol-based mouthwash as a risk factor of oral cancer: A systematic review. Med. Oral. Patol. Oral. Cir. Bucal 2020, 25, e1–e12. [Google Scholar] [CrossRef]
- Tokajuk, J.; Deptuła, P.; Chmielewska, S.J.; Skłodowski, K.; Mierzejewska, Ż.; Grądzka-Dahlke, M.; Tołstoj, A.; Daniluk, T.; Paprocka, P.; Savage, P.B.; et al. Ceragenin CSA-44 as a Means to Control the Formation of the Biofilm on the Surface of Tooth and Composite Fillings. Pathogens 2022, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Piktel, E.; Michalak, G.; Gu, X.; Kułakowska, A.; Savage, P.B.; Bucki, R. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci. Rep. 2017, 7, 4610. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.E.; Walczak, M.A.; Hameed, R.; Coonan, P. Chlorhexidine resistance in antibiotic-resistant bacteria isolated from the surfaces of dispensers of soap containing chlorhexidine. Infect. Control Hosp. Epidemiol. 2002, 23, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Ziegler, E.; Palmer, K.L. Chlorhexidine Induces VanA-Type Vancomycin Resistance Genes in Enterococci. Antimicrob. Agents Chemother. 2016, 60, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Holden, B.S.; Coburn, J.; Taylor, M.F.; Weber, S.; Hilton, B.; Zaugg, A.L.; McEwan, C.; Carson, R.; Andersen, J.L.; et al. Proteomic Analysis of Resistance of Gram-Negative Bacteria to Chlorhexidine and Impacts on Susceptibility to Colistin, Antimicrobial Peptides, and Ceragenins. Front. Microbiol. 2019, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Noto, M.J.; Domenico, H.J.; Byrne, D.W.; Talbot, T.; Rice, T.W.; Bernard, G.R.; Wheeler, A.P. Chlorhexidine bathing and health care-associated infections: A randomized clinical trial. JAMA 2015, 313, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Spałek, J.; Daniluk, T.; Godlewski, A.; Deptuła, P.; Wnorowska, U.; Ziembicka, D.; Cieśluk, M.; Fiedoruk, K.; Ciborowski, M.; Krętowski, A.; et al. Assessment of Ceragenins in Prevention of Damage to Voice Prostheses Caused by. Pathogens 2021, 10, 1371. [Google Scholar] [CrossRef]
- Pollard, J.E.; Snarr, J.; Chaudhary, V.; Jennings, J.D.; Shaw, H.; Christiansen, B.; Wright, J.; Jia, W.; Bishop, R.E.; Savage, P.B. In vitro evaluation of the potential for resistance development to ceragenin CSA-13. J. Antimicrob. Chemother. 2012, 67, 2665–2672. [Google Scholar] [CrossRef]
- McCullough, M.J.; Farah, C.S. The role of alcohol in oral carcinogenesis with particular reference to alcohol-containing mouthwashes. Aust. Dent. J. 2008, 53, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.E.; Kristen, U. Toxicity assessment of mouthwashes in the pollen tube growth test. Anticancer. Res. 2003, 23, 941–947. [Google Scholar] [PubMed]
- Hostiuc, S.; Ionescu, I.V.; Drima, E. Mouthwash Use and the Risk of Oral, Pharyngeal, and Laryngeal Cancer. A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 8215. [Google Scholar] [CrossRef] [PubMed]
- Lanzetti, J.; Finotti, F.; Savarino, M.; Gassino, G.; Dell’Acqua, A.; Erovigni, F.M. Management of Oral Hygiene in Head-Neck Cancer Patients Undergoing Oncological Surgery and Radiotherapy: A Systematic Review. Dent. J. 2023, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.P.; Li, M.; Budhathoki, C.; Yeh, C.H.; Ruble, K. Anti-inflammatory mouthwashes for the prevention of oral mucositis in cancer therapy: An integrative review and meta-analysis. Support. Care Cancer 2022, 30, 7205–7218. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Boschetti, C.E.; Vitagliano, R.; Colella, C.; Jiao, L.; King-Smith, N.; Li, C.; Nuoh Lau, Y.; Lai, Z.; Mohammed, A.I.; et al. Interventions for the Prevention of Oral Mucositis in Patients Receiving Cancer Treatment: Evidence from Randomised Controlled Trials. Curr. Oncol. 2023, 30, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal activity of ceragenin CSA-13 in cell culture and an animal model of peritoneal infection. Antimicrob. Agents Chemother. 2015, 59, 6274–6282. [Google Scholar] [CrossRef]
- Wnorowska, U.; Piktel, E.; Deptuła, P.; Wollny, T.; Król, G.; Głuszek, K.; Durnaś, B.; Pogoda, K.; Savage, P.B.; Bucki, R. Ceragenin CSA-13 displays high antibacterial efficiency in a mouse model of urinary tract infection. Sci. Rep. 2022, 12, 19164. [Google Scholar] [CrossRef]
- Yousefimanesh, H.; Amin, M.; Robati, M.; Goodarzi, H.; Otoufi, M. Comparison of the Antibacterial Properties of Three Mouthwashes Containing Chlorhexidine Against Oral Microbial Plaques: An in vitro Study. Jundishapur J. Microbiol. 2015, 8, e17341. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.M.; Gharaibeh, S.F.; Alzoubi, K.H.; Al-Azzam, S.I.; Obeidat, W.M. Antimicrobial activity of common mouthwash solutions on multidrug-resistance bacterial biofilms. J. Clin. Med. Res. 2013, 5, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics 2022, 11, 727. [Google Scholar] [CrossRef]
- Zaugg, A.; Sherren, E.; Yi, R.; Larsen, T.; Dyck, B.; Stump, S.; Pauga, F.; Linder, A.; Takara, M.; Gardner, E.; et al. Incorporating Ceragenins into Coatings Protects Peripherally Inserted Central Catheter Lines against Pathogen Colonization for Multiple Weeks. Int. J. Mol. Sci. 2023, 24, 14923. [Google Scholar] [CrossRef]
- Yazicioglu, O.; Ucuncu, M.K.; Guven, K. Ingredients in Commercially Available Mouthwashes: A Review. Int. Dent. J. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Guan, Q.; Walsh, J.P.; Boswell, J.S.; Winter, T.W.; Winter, E.S.; Boyd, S.S.; Li, C.; Savage, P.B. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 2002, 45, 663–669. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Sheehan, D.J.; Rex, J.H. Determination of fungicidal activities against yeasts and molds: Lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 2004, 17, 268–280. [Google Scholar] [CrossRef]
- Sandberg, M.E.; Schellmann, D.; Brunhofer, G.; Erker, T.; Busygin, I.; Leino, R.; Vuorela, P.M.; Fallarero, A. Pros and cons of using resazurin staining for quantification of viable Staphylococcus aureus biofilms in a screening assay. J. Microbiol. Methods 2009, 78, 104–106. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Targeting Biofilm Associated Staphylococcus aureus Using Resazurin Based Drug-susceptibility Assay. J. Vis. Exp. 2016, 111, e53925. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Vishnepolsky, B.; Zaalishvili, G.; Karapetian, M.; Nasrashvili, T.; Kuljanishvili, N.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M.; Grigolava, M.; et al. De Novo Design and In Vitro Testing of Antimicrobial Peptides against Gram-Negative Bacteria. Pharmaceuticals 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Verma, U.P.; Gupta, A.; Yadav, R.K.; Tiwari, R.; Sharma, R.; Balapure, A.K. Cytotoxicity of chlorhexidine and neem extract on cultured human gingival fibroblasts through fluorescence-activated cell sorting analysis: An. Eur. J. Dent. 2018, 12, 344–349. [Google Scholar] [CrossRef] [PubMed]
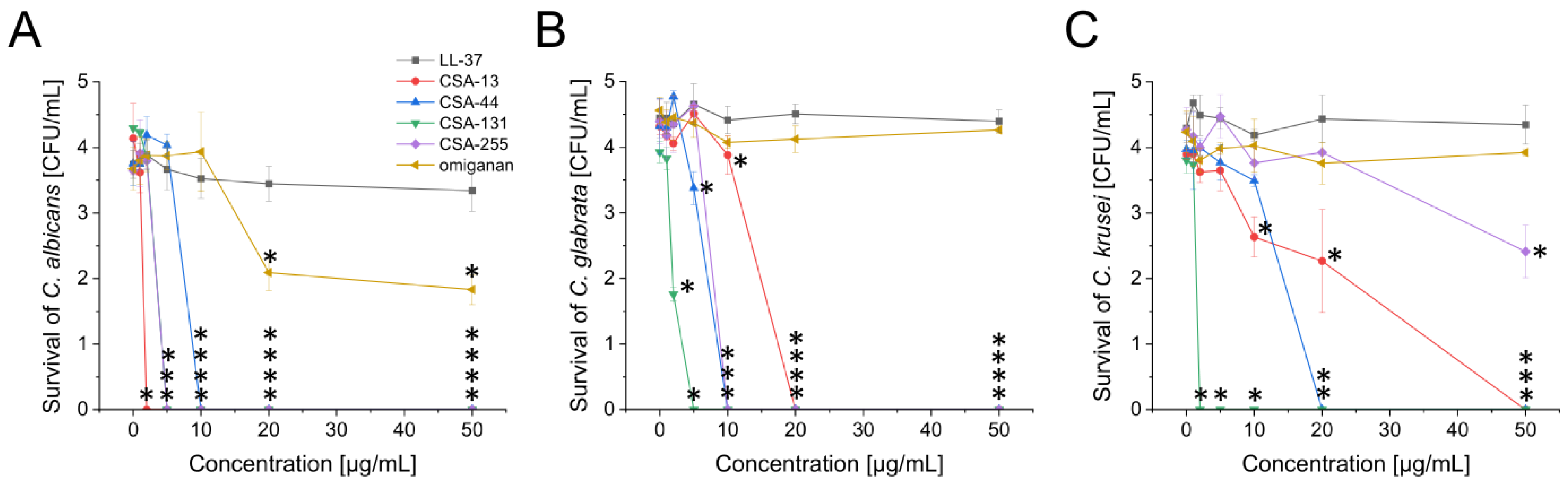
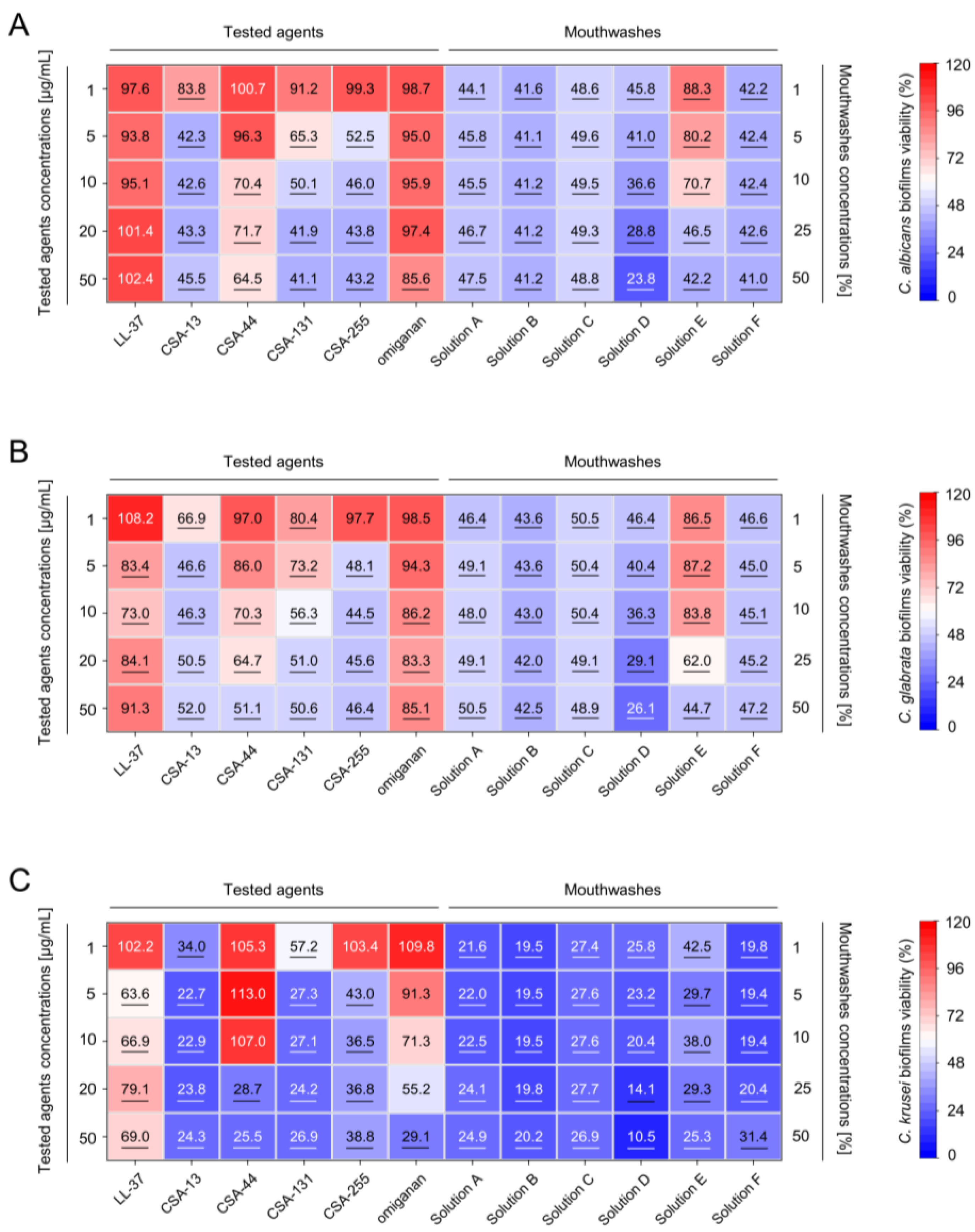
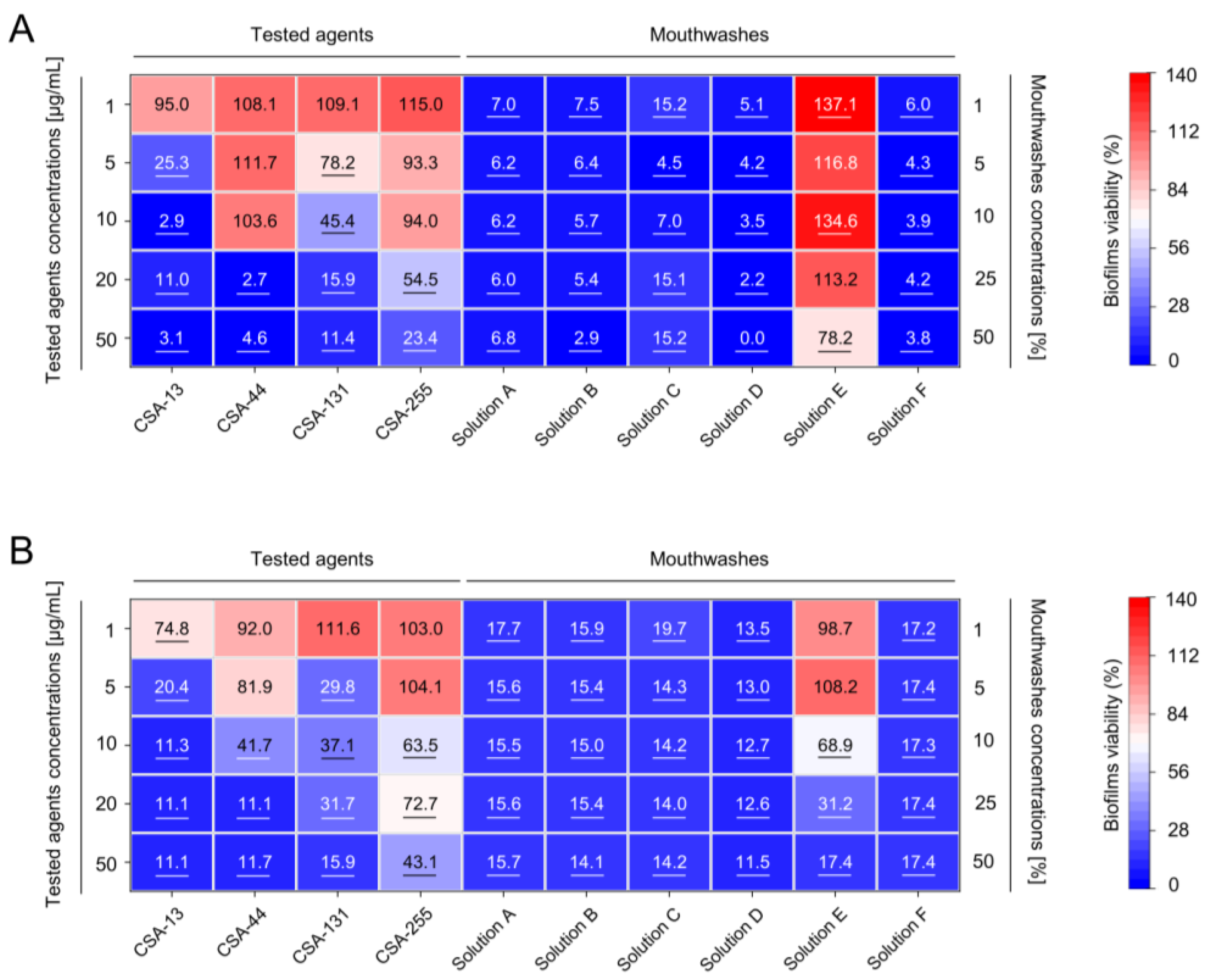
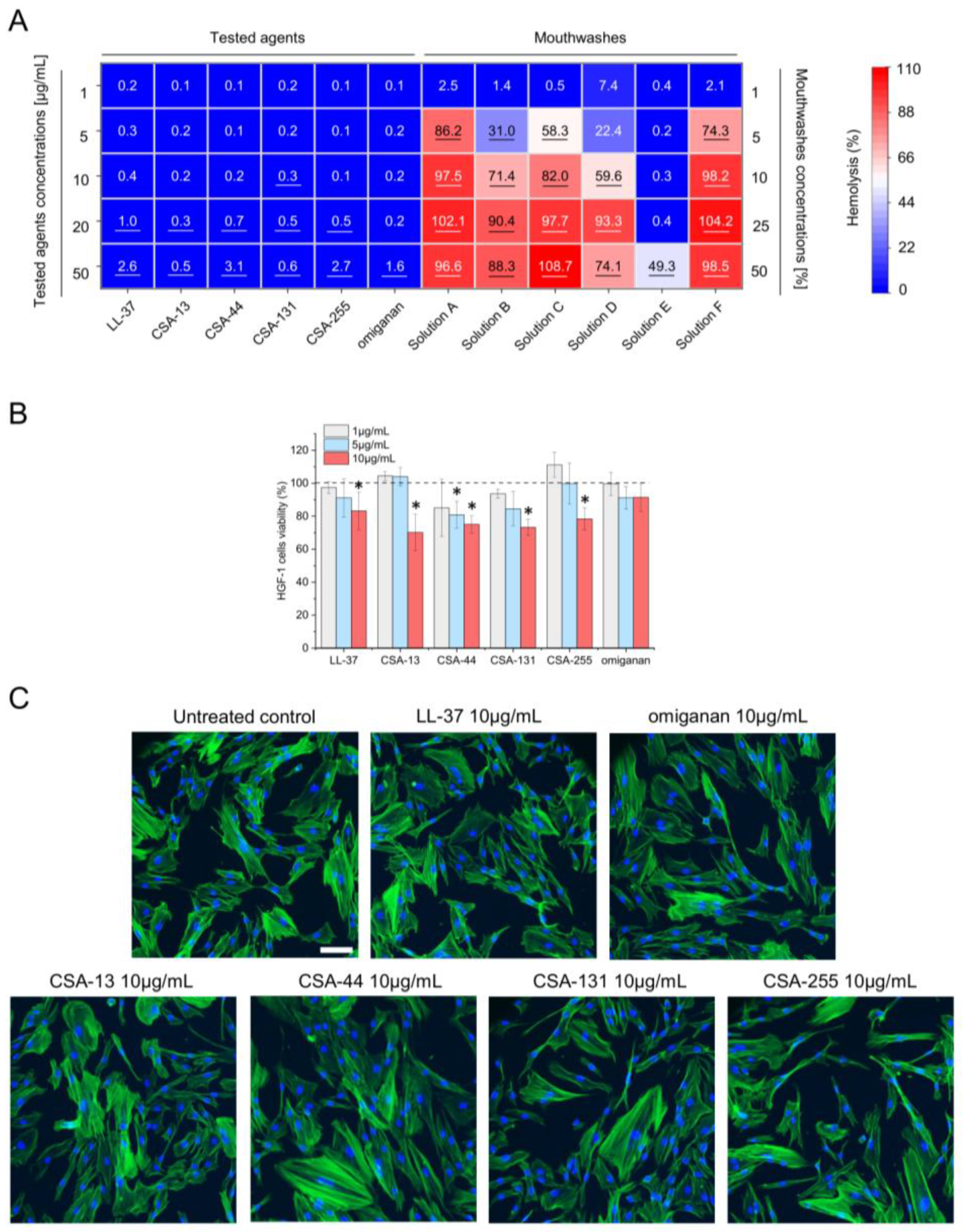
| LL-37 | CSA-13 | CSA-44 | CSA-131 | CSA-255 | Omiganan | |
|---|---|---|---|---|---|---|
| C. albicans 1408 | >128/>128 | 4/4 | 64/64 | 16/16 | 8/8 | >128/>128 |
| C. albicans * | >128/>128 | 4/4 | 32/32 | 16/64 | 16/16 | 128/128 |
| C. albicans * | >128/>128 | 2/2 | 16/16 | 4/4 | 8/8 | 128/128 |
| C. albicans * | >128/>128 | 2/2 | 64/64 | 4/4 | 8/8 | 128/128 |
| C. albicans * | >128/>128 | 2/2 | 16/32 | 4/4 | 8/8 | 128/128 |
| C. albicans * | >128/>128 | 2/2 | 16/32 | 2/2 | 8/8 | 128/128 |
| C. albicans * | >128/>128 | 2/2 | 16/64 | 4/4 | 8/8 | 128/128 |
| C. albicans * | >128/>128 | 2/16 | 16/32 | 4/4 | 8/8 | 128/128 |
| C. albicans * | >128/>128 | 4/4 | 32/128 | 32/32 | 32/32 | 128/128 |
| C. albicans * | >128/>128 | 2/2 | 64/64 | 8/32 | 4/32 | 32/64 |
| C. albicans * | >128/>128 | 2/16 | 16/>128 | 4/4 | 8/8 | 128/128 |
| C. albicans * | >128/>128 | 4/4 | 32/32 | 8/8 | 8/8 | 128/128 |
| C. glabrata * | >128/>128 | 4/4 | 128/128 | 4/4 | 8/8 | >128/>128 |
| C. glabrata * | >128/>128 | 2/2 | 128/128 | 2/32 | 8/32 | 128/128 |
| C. glabrata * | >128/>128 | 1/8 | 8/8 | 4/16 | 4/4 | 128/128 |
| C. glabrata * | >128/>128 | 1/4 | 16/32 | 2/2 | 8/8 | 128/>128 |
| C. glabrata * | >128/>128 | 1/1 | 8/8 | 2/2 | 8/8 | >128/>128 |
| C. glabrata * | >128/>128 | 1/1 | 16/16 | 128/128 | 8/8 | >128/>128 |
| C. glabrata * | >128/>128 | 1/8 | 8/64 | 2/2 | 8/64 | >128/>128 |
| C. krusei * | >128/>128 | 1/1 | 8/8 | 2/2 | 4/8 | 64/64 |
| C. krusei * | >128/>128 | 1/1 | 8/8 | 2/4 | 4/4 | 64/64 |
| C. krusei * | >128/>128 | 1/4 | 8/8 | 2/2 | 4/4 | 32/32 |
| LL-37 | CSA-13 | CSA-44 | CSA-131 | CSA-255 | |
|---|---|---|---|---|---|
| E. faecalis ATCC 45477 | >128/>128 | 2/4 | 64/64 | 8/8 | >128/>128 |
| E. faecalis ATCC 51575 | >128/>128 | 2/16 | 64/64 | 8/8 | 64/128 |
| E. faecalis ATCC 29212 | >128/>128 | 0.5/>4 | 64/128 | 4/8 | 64/64 |
| E. faecalis * | >128/>128 | 2/16 | 64/64 | 4/4 | 128/128 |
| E. faecalis * | >128/>128 | 0.25/2 | 64/64 | 4/8 | 32/128 |
| S. mutans ATCC 35668 | >128/>128 | 1/>8 | 64/64 | 16/16 | 64/64 |
| S. mutans * | >128/>128 | 1/8 | 128/128 | 128/128 | 128/128 |
| E. hirae ATCC 10541 | >128/>128 | 0.5/>4 | 64/64 | 4/4 | 64/64 |
| B. fragilis * | >128/>128 | 0.5/4 | 64/>128 | 8/64 | 32/>128 |
| B. fragilis * | 8/>64 | 1/1 | 64/64 | 4/8 | 8/16 |
| B. fragilis * | 4/8 | 1/1 | 64/64 | 16/32 | 16/32 |
| Capnocytophaga * | 8/>64 | 0.5/1 | 32/128 | 4/8 | 8/64 |
| Prevotella * | 8/>64 | 1/2 | 32/128 | 4/8 | 16/16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnowski, M.; Słowińska, M.; Sawieljew, M.; Wnorowska, U.; Daniluk, T.; Król, G.; Karasiński, M.; Okła, S.; Savage, P.B.; Piktel, E.; et al. Efficacy of Ceragenins in Controlling the Growth of Oral Microorganisms: Implications for Oral Hygiene Management. Pharmaceuticals 2024, 17, 204. https://doi.org/10.3390/ph17020204
Czarnowski M, Słowińska M, Sawieljew M, Wnorowska U, Daniluk T, Król G, Karasiński M, Okła S, Savage PB, Piktel E, et al. Efficacy of Ceragenins in Controlling the Growth of Oral Microorganisms: Implications for Oral Hygiene Management. Pharmaceuticals. 2024; 17(2):204. https://doi.org/10.3390/ph17020204
Chicago/Turabian StyleCzarnowski, Michał, Monika Słowińska, Mariusz Sawieljew, Urszula Wnorowska, Tamara Daniluk, Grzegorz Król, Maciej Karasiński, Sławomir Okła, Paul B. Savage, Ewelina Piktel, and et al. 2024. "Efficacy of Ceragenins in Controlling the Growth of Oral Microorganisms: Implications for Oral Hygiene Management" Pharmaceuticals 17, no. 2: 204. https://doi.org/10.3390/ph17020204
APA StyleCzarnowski, M., Słowińska, M., Sawieljew, M., Wnorowska, U., Daniluk, T., Król, G., Karasiński, M., Okła, S., Savage, P. B., Piktel, E., & Bucki, R. (2024). Efficacy of Ceragenins in Controlling the Growth of Oral Microorganisms: Implications for Oral Hygiene Management. Pharmaceuticals, 17(2), 204. https://doi.org/10.3390/ph17020204







