A Multi-Residue Analytical Method for Assessing the Effects of Stacking Treatment on Antimicrobial and Coccidiostat Degradation in Broiler Litter
Abstract
1. Introduction
2. Results
2.1. Method Validation
2.1.1. Suitability (Repeatability)
2.1.2. Inter-Day Recovery (Reproducibility)
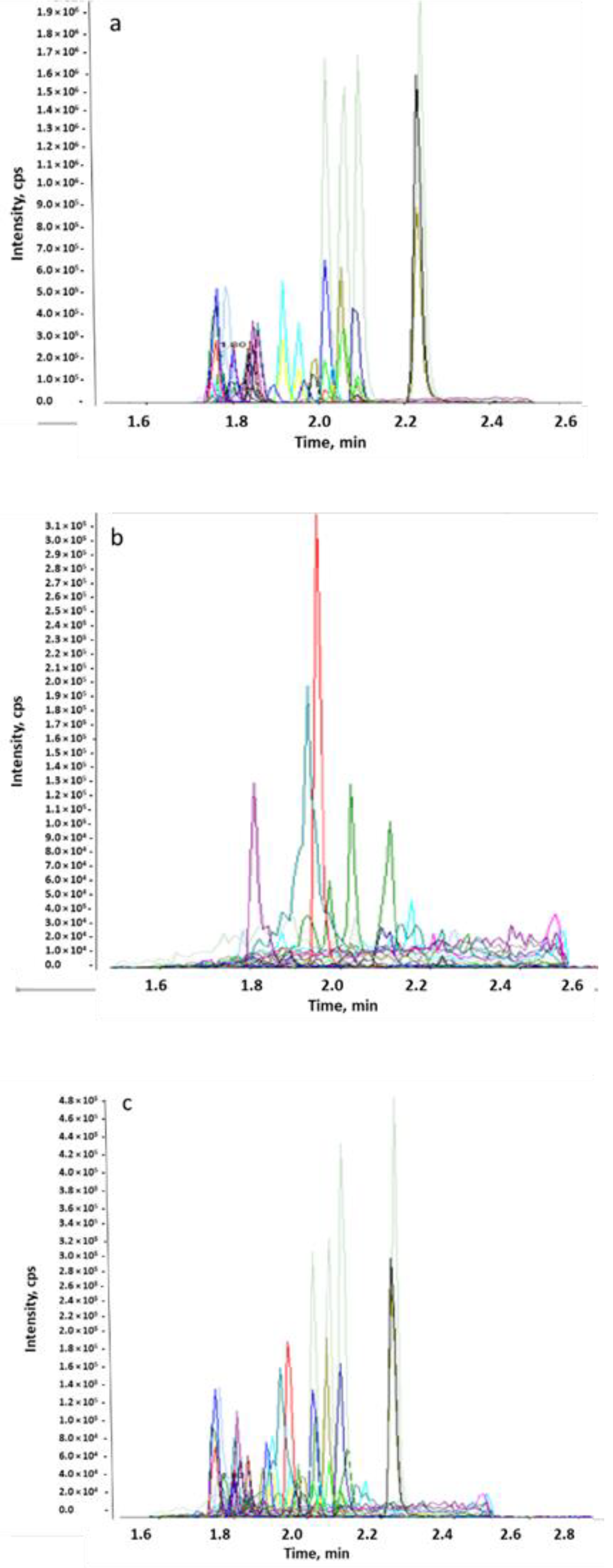
2.1.3. Specificity and Selectivity
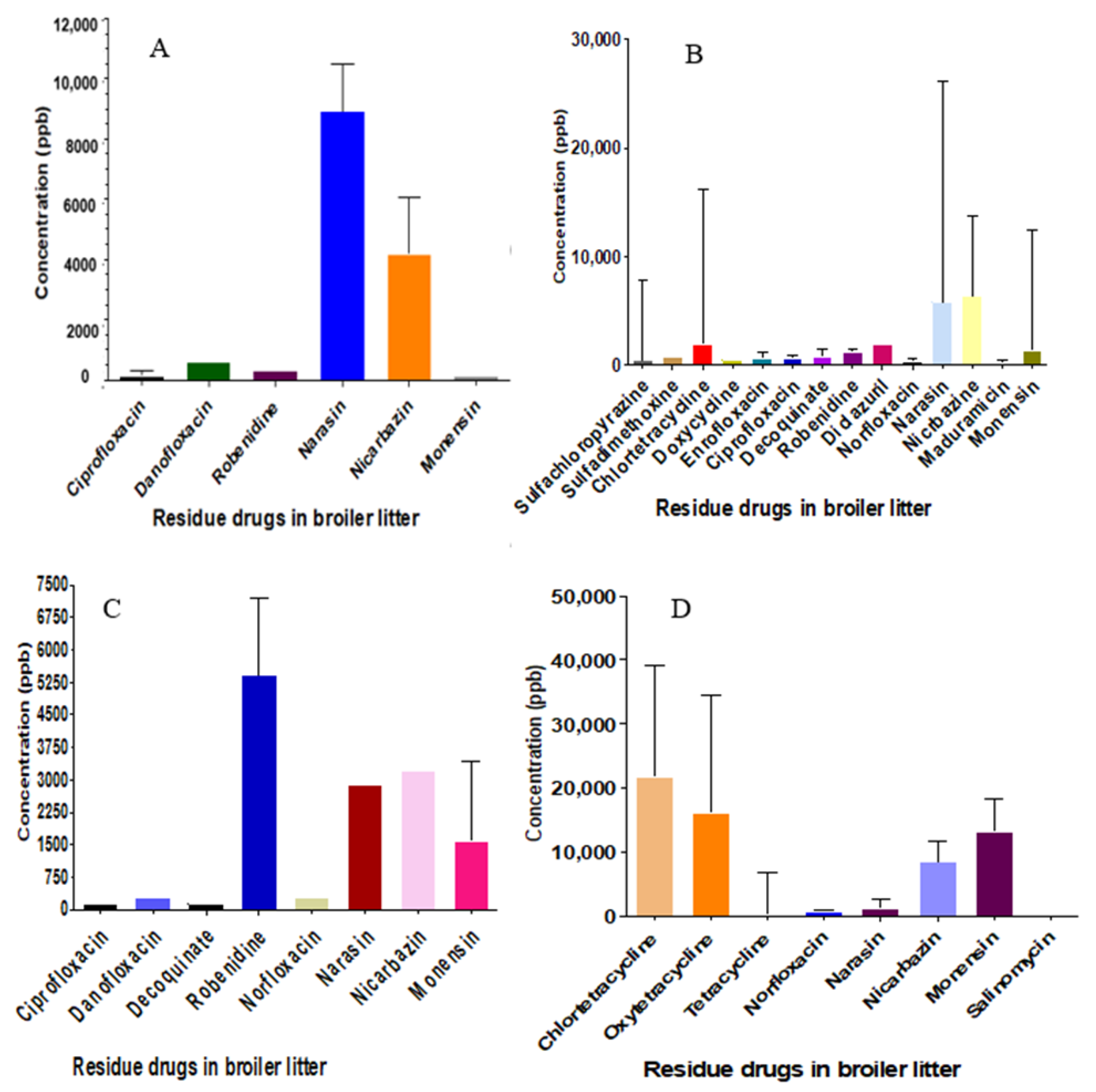
2.2. Identification and Quantification of BL Samples
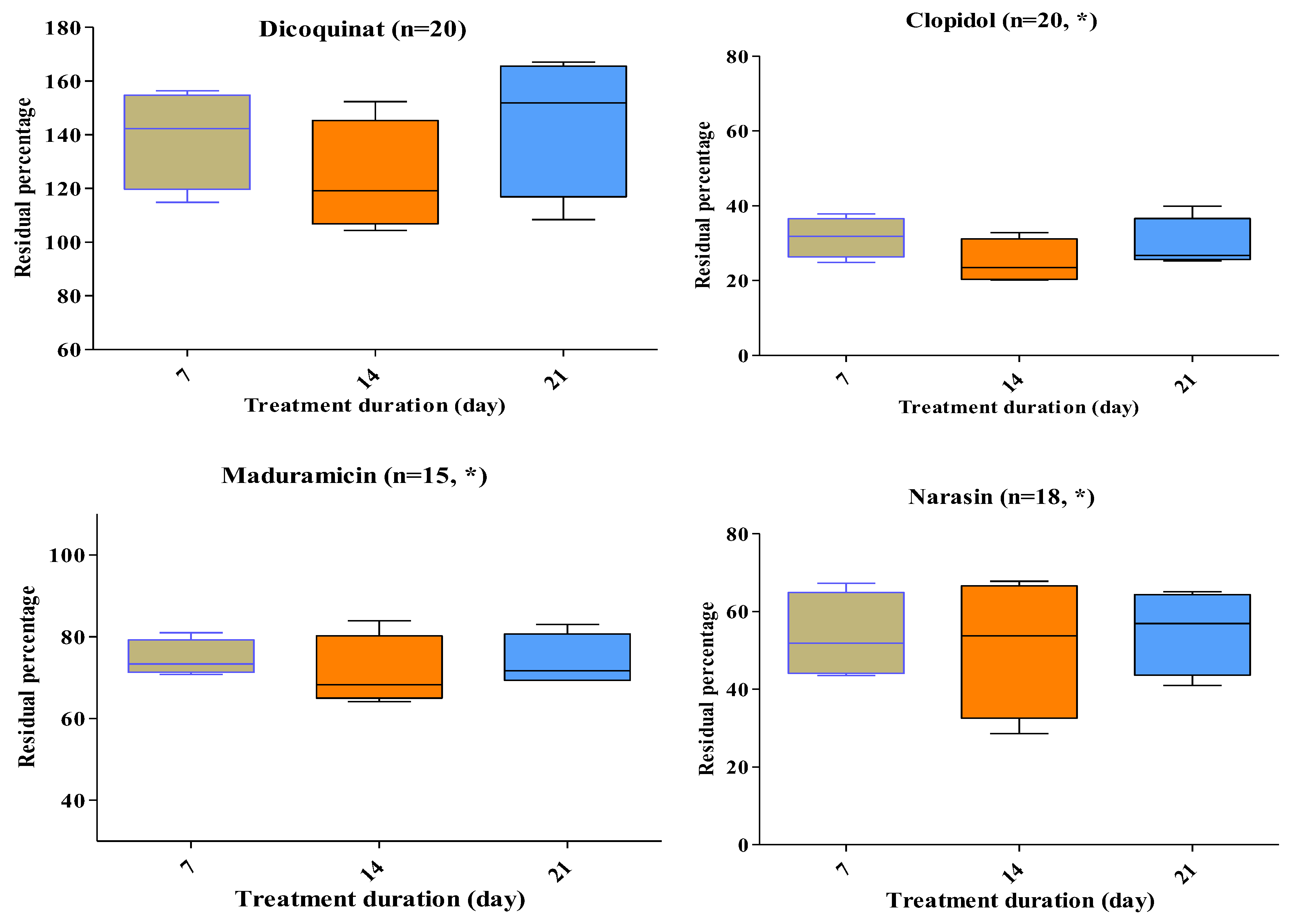
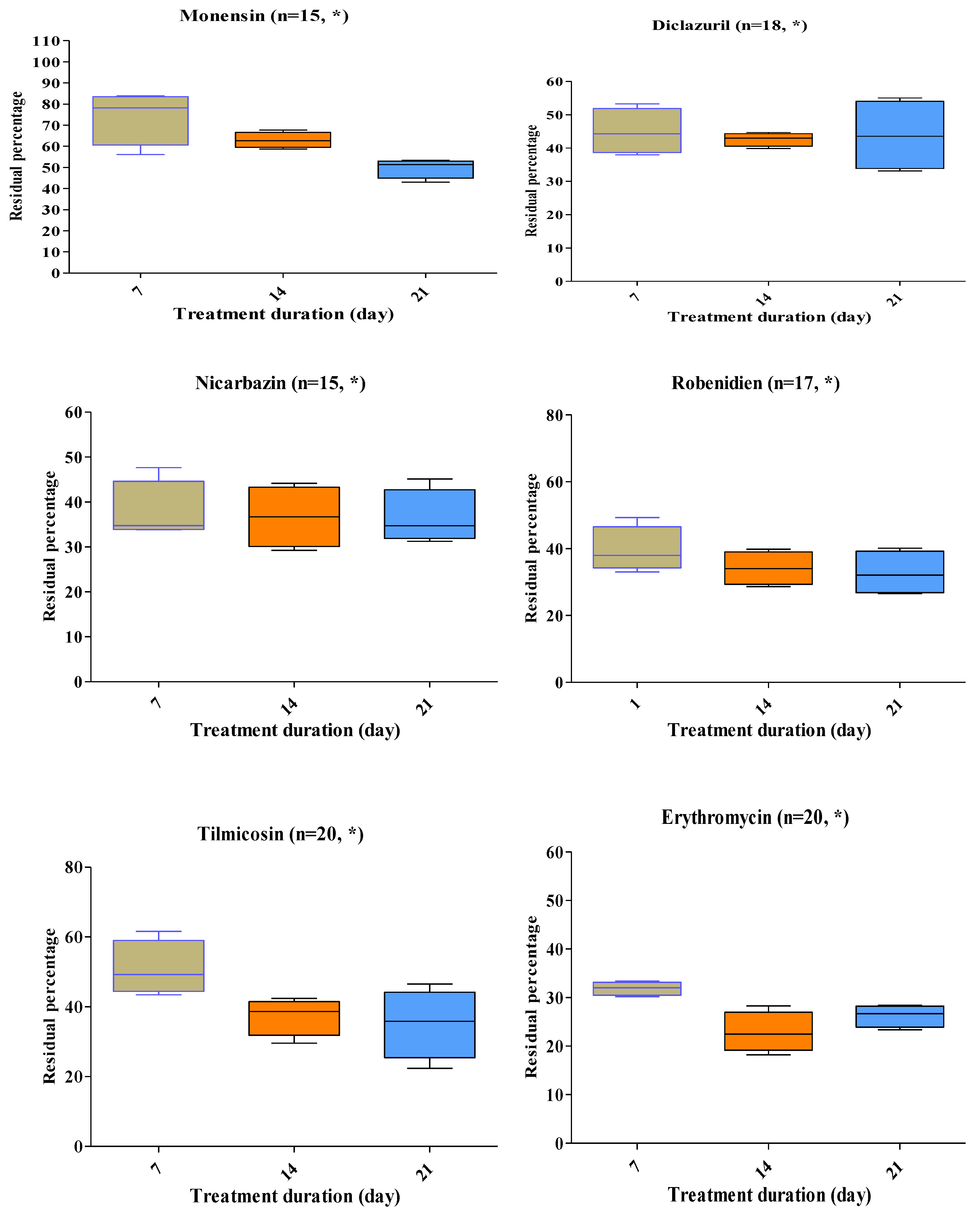
2.3. Antimicrobial and Coccidiostat Residue Degradation upon Stacking Treatment
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Collection
4.3. Pre-Treatment of Raw BL
4.4. Sample Extraction
4.5. LC/MS/MS Analysis
4.6. Method Validation
4.7. Calibration
4.8. Recovery
4.9. Suitability (Repeatability) and Inter-Day Recovery (Reproducibility)
4.10. Specificity and Selectivity
4.11. Matrix Effect
4.12. Designing a Method for Stacking BL Treatment Processes in the Laboratory
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Bishop, E.; Wilke, P.; Nash, W.; Nell, J.; Macdonald, D.; Compaan, J.; Grobler, J.; Kingman, E. Poultry manure as a livestock feed. Farming S. Afr. 1971, 46, 34. [Google Scholar]
- Lyle, A.; Muller, J.; Dreyer, B. Over-Wintering of Oxen on Rested Veld Using Four Different Licks: Winter 1975; Report; Kokstad Research Station: Kokstad, South Africa, 1975.
- Rocha, A.G.; Dilkin, P.; Neto, R.M.; Schaefer, C.; Mallmann, C.A. Growth performance of broiler chickens fed on feeds with varying mixing homogeneity. Vet. Anim. Sci. 2022, 17, 100263. [Google Scholar] [CrossRef]
- Hansen, M.; Björklund, E.; Krogh, K.A.; Halling-Sørensen, B. Analytical strategies for assessing ionophores in the environment. TrAC Trends Anal. Chem. 2009, 28, 521–533. [Google Scholar] [CrossRef]
- Sun, P.; Cabrera, M.L.; Huang, C.-H.; Pavlostathis, S.G. Biodegradation of veterinary ionophore antibiotics in broiler litter and soil microcosms. Environ. Sci. Technol. 2014, 48, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; Department of Health and Human Services: Washington, DC, USA, 2019.
- Furtula, V.; Farrell, E.; Diarrassouba, F.; Rempel, H.; Pritchard, J.; Diarra, M. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult. Sci. 2010, 89, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hribar, C. Understanding Concentrated Animal Feeding Operations and Their Impact on Communities; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Oehme, F.; Pickrell, J. An analysis of the chronic oral toxicity of polyether ionophore antibiotics in animals. Vet. Hum. Toxicol. 1999, 41, 251–257. [Google Scholar] [PubMed]
- Bohn, P.; Bak, S.A.; Björklund, E.; Krogh, K.A.; Hansen, M. Abiotic degradation of antibiotic ionophores. Environ. Pollut. 2013, 182, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, B.; Reyns, T.; Devreese, M.; De Backer, P.; Van Loco, J.; Croubels, S. Determination of selected veterinary antimicrobials in poultry excreta by UHPLC-MS/MS, for application in Salmonella control programs. Anal. Bioanal. Chem. 2015, 407, 4447–4457. [Google Scholar] [CrossRef]
- Karcı, A.; Balcıoğlu, I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2007, 41, 50–57. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Dolliver, H.; Gupta, S.; Noll, S. Antibiotic degradation during manure composting. J. Environ. Qual. 2008, 37, 1245–1253. [Google Scholar] [CrossRef]
- Capleton, A.C.; Courage, C.; Rumsby, P.; Holmes, P.; Stutt, E.; Boxall, A.B.; Levy, L.S. Prioritising veterinary medicines according to their potential indirect human exposure and toxicity profile. Toxicol. Lett. 2006, 163, 213–223. [Google Scholar] [CrossRef]
- Hao, C.; Lissemore, L.; Nguyen, B.; Kleywegt, S.; Yang, P.; Solomon, K. Determination of pharmaceuticals in environmental waters by liquid chromatography/electrospray ionization/tandem mass spectrometry. Anal. Bioanal. Chem. 2006, 384, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Harter, T.H.; Bergamaschi, B.A. Environmental occurrence and shallow ground water detection of the antibiotic monensin from dairy farms. J. Environ. Qual. 2008, 37, S-78–S-85. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Mazhar, S.H.; Zeng, Q.; Kiki, C.; Yu, C.-P.; Sun, Q. Simultaneous analysis of multiclass antibiotic residues in complex environmental matrices by liquid chromatography with tandem quadrupole mass spectrometry. J. Chromatogr. B 2020, 1145, 122103. [Google Scholar] [CrossRef] [PubMed]
- Xian-Gang, H.; Yi, L.; Qi-Xing, Z.; Lin, X. Determination of thirteen antibiotics residues in manure by solid phase extraction and high performance liquid chromatography. Chin. J. Anal. Chem. 2008, 36, 1162–1166. [Google Scholar]
- Filigenzi, M.S.; Ehrke, N.; Aston, L.S.; Poppenga, R.H. Evaluation of a rapid screening method for chemical contaminants of concern in four food-related matrices using QuEChERS extraction, UHPLC and high resolution mass spectrometry. Food Addit. Contam. Part A 2011, 28, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Poudel, U.; Dahal, U.; Dhakal, S. Review of poultry production and poultry vaccine manufacture in Nepal. Glob. J. Agric. Allied Sci. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Svahn, O.; Björklund, E. Thermal stability assessment of antibiotics in moderate temperature and subcriticalwater using a pressurized dynamic flow-through system. Int. J. Innov. Appl. Stud. 2015, 11, 872–880. [Google Scholar]
- Usui, K.; Hayashizaki, Y.; Minagawa, T.; Hashiyada, M.; Nakano, A.; Funayama, M. Rapid determination of disulfoton and its oxidative metabolites in human whole blood and urine using QuEChERS extraction and liquid chromatography–tandem mass spectrometry. Leg. Med. 2012, 14, 309–316. [Google Scholar] [CrossRef]
- Guo, C.; Wang, M.; Xiao, H.; Huai, B.; Wang, F.; Pan, G.; Liao, X.; Liu, Y. Development of a modified QuEChERS method for the determination of veterinary antibiotics in swine manure by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2016, 1027, 110–118. [Google Scholar] [CrossRef]
- Arikan, O.A.; Mulbry, W.; Rice, C. The effect of composting on the persistence of four ionophores in dairy manure and poultry litter. Waste Manag. 2016, 54, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.J.; Wallace, J.S.; Aga, D.S. Method development for the analysis of ionophore antimicrobials in dairy manure to assess removal within a membrane-based treatment system. Chemosphere 2018, 197, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Theivaraj, S.D.; Boovaragamoorthy, G.M.; Veeramani, V.; Brindhadevi, K.; Al-Dhabi, N.A.; Arasu, M.V.; Kaliannan, T. Impact on degradation of antibiotics from poultry litter using Autothermal Thermophilic Aerobic Digestion (ATAD). Saudi J. Biol. Sci. 2021, 28, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Yévenes, K.; Pokrant, E.; Trincado, L.; Lapierre, L.; Galarce, N.; Martín, B.S.; Maddaleno, A.; Hidalgo, H.; Cornejo, J. Detection of antimicrobial residues in poultry litter: Monitoring a risk through a selective and sensitive HPLC–MS/MS method. Animals 2021, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Van Alstine, W.; Ficken, M.; Miskimins, D.; Carson, T.; Osweiler, G. Acute monensin toxicosis in sheep: Light and electron microscopic changes. Am. J. Vet. Res. 1984, 45, 1142–1147. [Google Scholar] [PubMed]
- Sun, P.; Liu, B.; Ahmed, I.; Yang, J.; Zhang, B. Composting effect and antibiotic removal under a new temperature control strategy. J. Waste Manag. 2022, 153, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Majors, E. Sample Preparation Fundamentals for Chromatography; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2013; Volume 13. [Google Scholar]
- Wohde, M.; Berkner, S.; Junker, T.; Sabine Konradi, S.; Schwarz, L.; Düring, A. Occurrence and transformation of veterinary pharmaceuticals and biocides in manure: A literature review. Environ. Sci. Eur. 2016, 28, 23. [Google Scholar] [CrossRef]
- Dias, B.O.; Silva, C.A.; Higashikawa, F.S.; Roig, A.; Sánchez-Monedero, M.A. Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour. Technol. 2010, 101, 1239–1246. [Google Scholar] [CrossRef]
- Kiss, N.É.; Tamás, J.; Szőllősi, N.; Gorliczay, E.; Nagy, A. Assessment of composted pelletized poultry litter as an alternative to chemical fertilizers based on the environmental impact of their production. Agriculture 2021, 11, 1130. [Google Scholar] [CrossRef]
- Leal, R.M.P.; Figueira, R.F.; Tornisielo, V.L.; Regitano, J.B. Occurrence and sorption of fluoroquinolones in poultry litters and soils from São Paulo State, Brazil. Sci. Total Environ. 2012, 432, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Van Epps, A.; Blaney, L. Antibiotic residues in animal waste: Occurrence and degradation in conventional agricultural waste management practices. Curr. Pollut. Rep. 2016, 2, 135–155. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef]
- Bastianello, S.S.; Fourie, N.; Nel, P.; Kellerman, T.S.; Prozesky, L. Cardiomyopathy of ruminants induced by the litter of poultry fed on rations containing the ionophore antibiotic, maduramicin. II. Macropathology and histopathology. Onderstepoort. J. Vet. Res. 1995, 62, 5–18. [Google Scholar] [PubMed]
- Confer, A.; Reavis, D.; Panciera, R. Light and electron microscopic changes in cardiac and skeletal muscle of sheep with experimental monensin toxicosis. Vet. Pathol. 1983, 20, 590–602. [Google Scholar] [CrossRef]
- Gonzalez, M.; Barkema, H.W.; Keefe, G.P. Monensin toxicosis in a dairy herd. Can. Vet. J. 2005, 46, 910. [Google Scholar]
- Huyben, M.; Sol, J.; Counotte, G.; Roumen, M.; Borst, G.H.A. Salinomycin poisoning in veal calves. Vet. Rec. 2001, 149, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Esperón, F.; Albero, B.; Ugarte-Ruíz, M.; Domínguez, L.; Carballo, M.; Tadeo, J.L.; del Mar Delgado, M.; Moreno, M.Á.; de la Torre, A. Assessing the benefits of composting poultry manure in reducing antimicrobial residues, pathogenic bacteria, and antimicrobial resistance genes: A field-scale study. Environ. Sci. Pollut. Res. 2020, 27, 27738–27749. [Google Scholar] [CrossRef]
- Subirats, J.; Murray, R.; Scott, A.; Lau, C.H.-F.; Topp, E. Composting of chicken litter from commercial broiler farms reduces the abundance of viable enteric bacteria, Firmicutes, and selected antibiotic resistance genes. Sci. Total Environ. 2020, 746, 141113. [Google Scholar] [CrossRef]
- Tappe, W.; Herbst, M.; Hofmann, D.; Koeppchen, S.; Kummer, S.; Thiele, B.; Groeneweg, J. Degradation of sulfadiazine by Microbacterium lacus strain SDZm4, isolated from lysimeters previously manured with slurry from sulfadiazine-medicated pigs. Appl. Environ. Microbiol. 2013, 79, 2572–2577. [Google Scholar] [CrossRef]
- Šabić, M.; Čižmek, L.; Vuković Domanovac, M.; Meštrović, E. Biodegradation of erythromycin with environmental microorganism Pseudomonas aeruginosa 3011. Chem. Biochem. Eng. Q. 2015, 29, 367–373. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour. Technol. 2013, 131, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, J.; Prasher, S.O.; Patel, R.M.; Hussain, S.A.; Barrington, S.F. The effect of composting on the degradation of a veterinary pharmaceutical. Bioresour. Technol. 2010, 101, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Munaretto, J.S.; Yonkos, L.; Aga, D.S. Transformation of ionophore antimicrobials in poultry litter during pilot-scale composting. Environ. Pollut. 2016, 212, 392–400. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives, Products or Substances used in Animal Feed (EFSA FEEDAP Panel); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S. Safety and efficacy of Deccox® (decoquinate) for chickens for fattening. EFSA J. 2019, 17, e05541. [Google Scholar]

| Group | Analyte | 0.1 mg kg−1 | RSD % | 1.5 mg kg−1 | RSD % |
|---|---|---|---|---|---|
| Coccidiostats | Narasin | 76 | 10 | 92 | 20 |
| Diclazuril | 95 | 4 | 96 | 5 | |
| Clopidol | 101 | 9 | 97 | 7 | |
| Nicarbazine | 80 | 20 | 95 | 4 | |
| Maduramycin | 88 | 6 | 84 | 11 | |
| Monensin | 87 | 11 | 96 | 4 | |
| Robenidine | 116 | 10 | 105 | 2 | |
| Salinomycin | 70 | 12 | 95 | 4 | |
| Semduramycin | 112 | 11 | 101 | 9 | |
| Lasalocid | 113 | 6 | 101 | 0 | |
| Decoquinate | 105 | 9 | 105 | 2 | |
| Sulfonamides | Sulfachloropyrazine | 101 | 20 | 102 | 5 |
| Sulfachloropyridazine | 110 | 8 | 101 | 2 | |
| Sulfadiazine | 112 | 17 | 109 | 2 | |
| Sulfadimidine | 130 | 15 | 99 | 12 | |
| Sulfaquinoxaline | 87 | 4 | 98 | 5 | |
| Sulfadoxine | 124 | 7 | 107 | 2 | |
| Macrolides | Tilmicosin | 103 | 12 | 104 | 2 |
| Tylosin | 84 | 12 | 94 | 1 | |
| Erythromycin | 116 | 19 | 104 | 8 | |
| Fluoroquinolones | Danofloxacin | 124 | 15 | 103 | 2 |
| Ciprofloxacin | 128 | 17 | 105 | 0 | |
| Norfloxacin | 85 | 19 | 101 | 4 | |
| Enrofloxacin | 114 | 17 | 103 | 2 | |
| Tetracycline | Doxycycline Hyclate | 100 | 14 | 111 | 7 |
| Oxytetracycline | 102 | 5 | 97 | 24 | |
| Chlortetracycline | 116 | 19 | 104 | 8 | |
| Tetracycline | 84 | 5 | 102 | 4 | |
| b-Lactams | Amoxicillin trihydrate | 102 | 14 | 112 | 18 |
| Ampicillin | 96 | 10 | 102 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efriem, S.; Britzi, M.; Soback, S.; Sabastian, C.; Mabjeesh, S.J. A Multi-Residue Analytical Method for Assessing the Effects of Stacking Treatment on Antimicrobial and Coccidiostat Degradation in Broiler Litter. Pharmaceuticals 2024, 17, 203. https://doi.org/10.3390/ph17020203
Efriem S, Britzi M, Soback S, Sabastian C, Mabjeesh SJ. A Multi-Residue Analytical Method for Assessing the Effects of Stacking Treatment on Antimicrobial and Coccidiostat Degradation in Broiler Litter. Pharmaceuticals. 2024; 17(2):203. https://doi.org/10.3390/ph17020203
Chicago/Turabian StyleEfriem, Solomon, Malka Britzi, Stefan Soback, Chris Sabastian, and Sameer J. Mabjeesh. 2024. "A Multi-Residue Analytical Method for Assessing the Effects of Stacking Treatment on Antimicrobial and Coccidiostat Degradation in Broiler Litter" Pharmaceuticals 17, no. 2: 203. https://doi.org/10.3390/ph17020203
APA StyleEfriem, S., Britzi, M., Soback, S., Sabastian, C., & Mabjeesh, S. J. (2024). A Multi-Residue Analytical Method for Assessing the Effects of Stacking Treatment on Antimicrobial and Coccidiostat Degradation in Broiler Litter. Pharmaceuticals, 17(2), 203. https://doi.org/10.3390/ph17020203






