Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study
Abstract
1. Introduction
2. Results
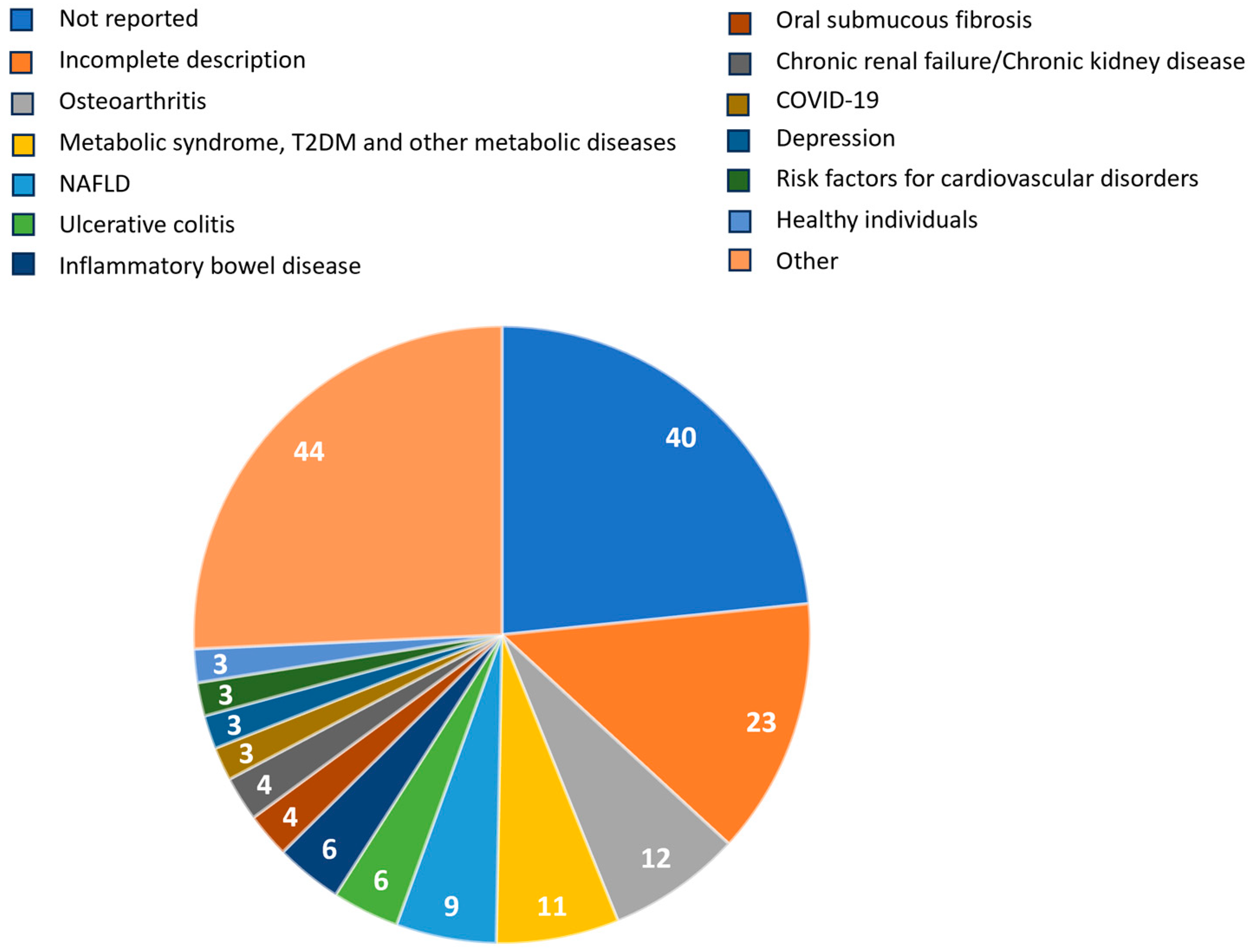
2.1. Characteristics of Included Systematic Reviews
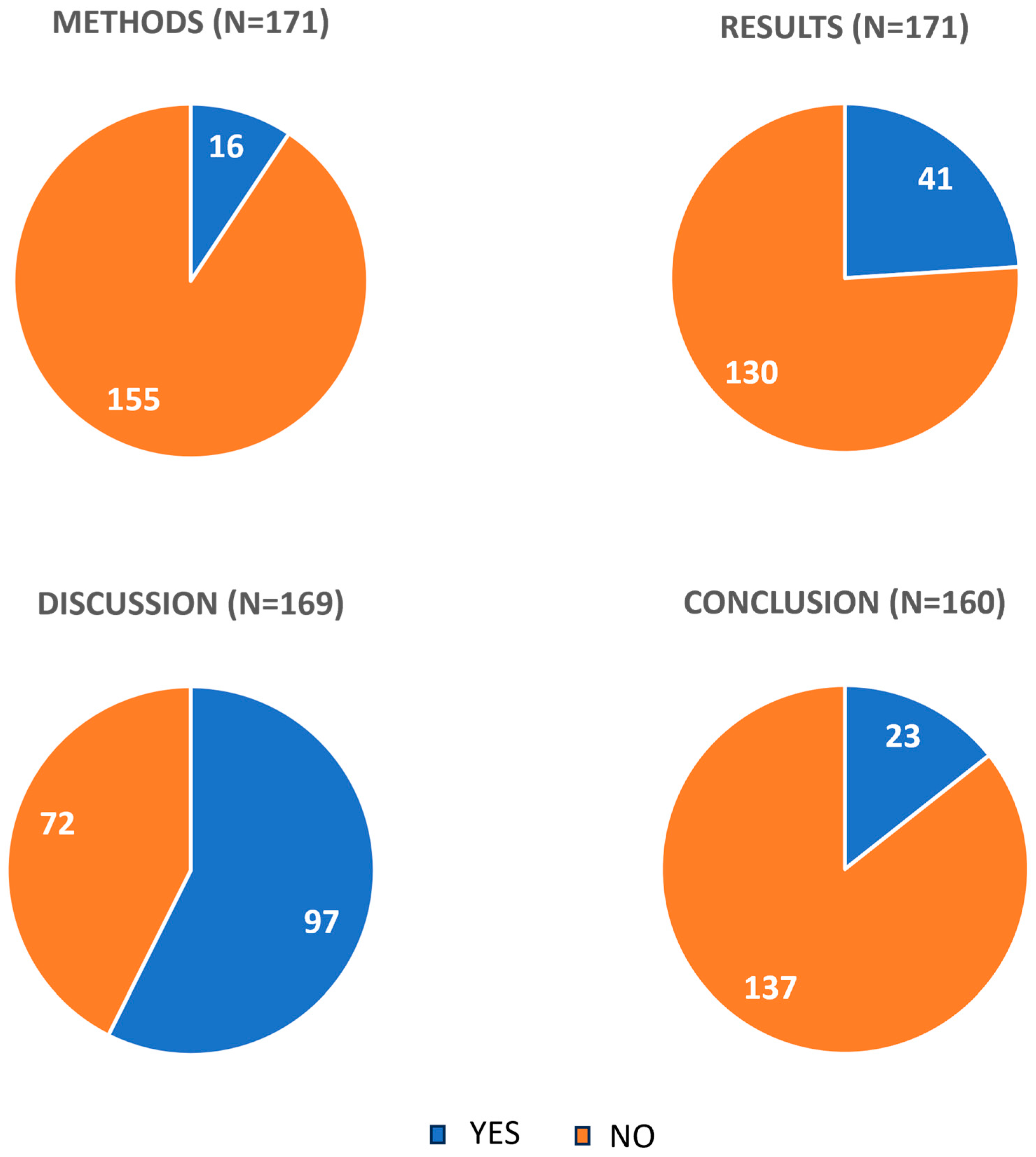
2.2. Mentions of Bioavailability
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Protocol Registration
4.3. Inclusion Criteria
4.4. Exclusion Criteria
4.5. Changes between the Protocol and the Review
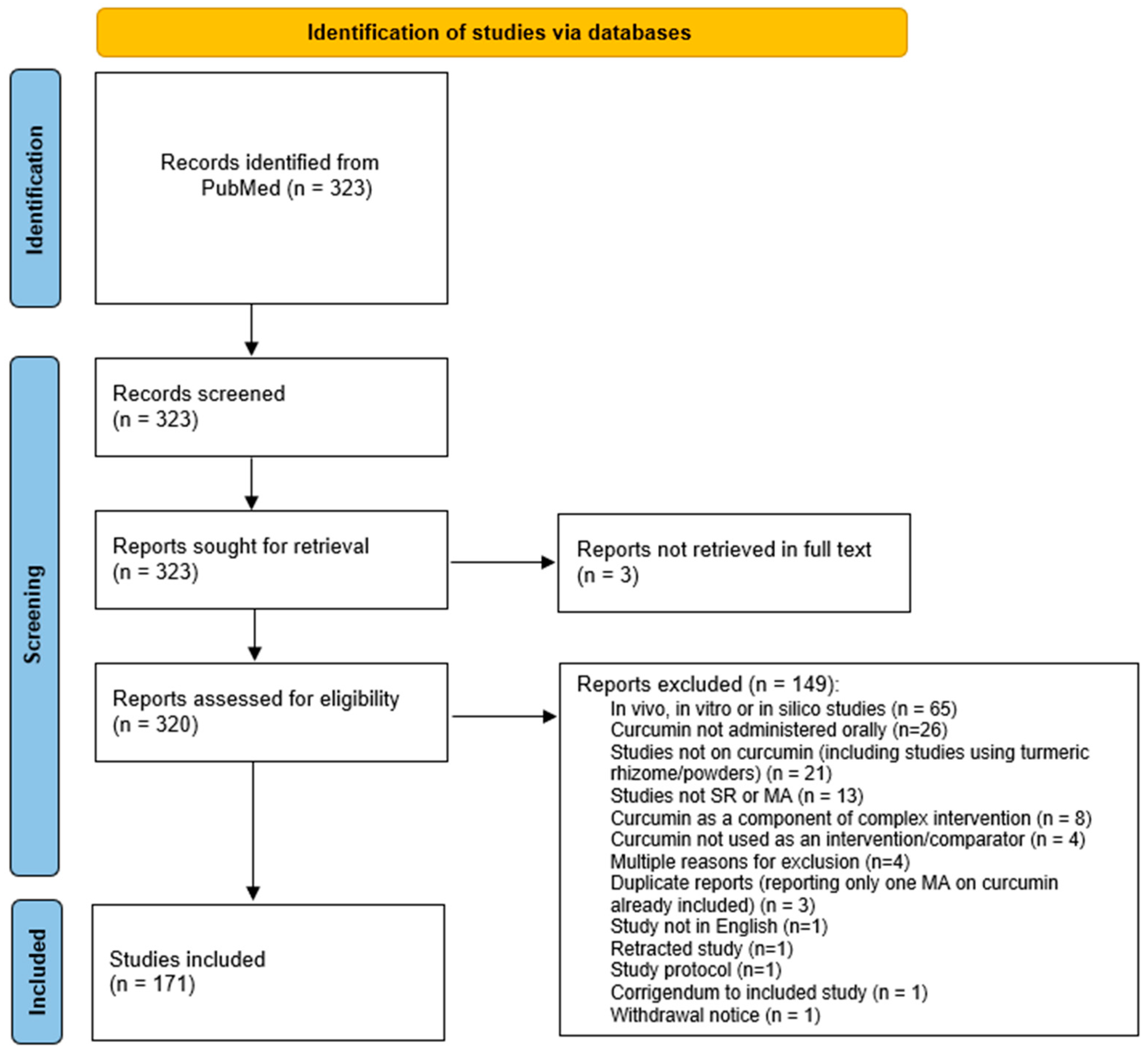
4.6. Search
4.7. Screening
4.8. Data Extraction
4.9. Statistics
4.10. Raw Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef] [PubMed]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Racz, L.Z.; Racz, C.P.; Pop, L.-C.; Tomoaia, G.; Mocanu, A.; Barbu, I.; Sárközi, M.; Roman, I.; Avram, A.; Tomoaia-Cotisel, M.; et al. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules 2022, 27, 6854. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Hao, M.; Chu, Y.; Lei, J.; Yao, Z.; Wang, P.; Chen, Z.; Wang, K.; Sang, X.; Han, X.; Wang, L.; et al. Pharmacological Mechanisms and Clinical Applications of Curcumin: Update. Aging Dis. 2023, 14, 716. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Olotu, F.; Agoni, C.; Soremekun, O.; Soliman, M.E.S. An Update on the Pharmacological Usage of Curcumin: Has It Failed in the Drug Discovery Pipeline? Cell Biochem. Biophys. 2020, 78, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Mimica, B.; Bučević Popović, V.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Methods Used for Enhancing the Bioavailability of Oral Curcumin in Randomized Controlled Trials: A Meta-Research Study. Pharmaceuticals 2022, 15, 939. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Curcumin for Neuropsychiatric Disorders: A Review of in Vitro, Animal and Human Studies. J. Psychopharmacol. 2017, 31, 287–302. [Google Scholar] [CrossRef]
- Briskey, D.; Sax, A.; Mallard, A.R.; Rao, A. Increased Bioavailability of Curcumin Using a Novel Dispersion Technology System (LipiSperse®). Eur. J. Nutr. 2019, 58, 2087–2097. [Google Scholar] [CrossRef]
- Liao, S.-C.; Hsu, W.-H.; Huang, Z.-Y.; Chuang, K.-L.; Lin, K.-T.; Tseng, C.-L.; Tsai, T.-H.; Dao, A.-H.; Su, C.-L.; Huang, C.-Y.F. Bioactivity Evaluation of a Novel Formulated Curcumin. Nutrients 2019, 11, 2982. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical Strategies of Improving Oral Systemic Bioavailability of Curcumin for Clinical Application. J. Control Release 2019, 316, 359–380. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef]
- De Oliveira, T.V.; Stein, R.; De Andrade, D.F.; Beck, R.C.R. Preclinical Studies of the Antitumor Effect of Curcumin-loaded Polymeric Nanocapsules: A Systematic Review and Meta-analysis. Phytother. Res. 2022, 36, 3202–3214. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Beltzig, L.; Frumkina, A.; Schwarzenbach, C.; Kaina, B. Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin. Nutrients 2021, 13, 2385. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Pan-On, S.; Dilokthornsakul, P.; Tiyaboonchai, W. Trends in Advanced Oral Drug Delivery System for Curcumin: A Systematic Review. J. Control Release 2022, 348, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Górnicka, J.; Mika, M.; Wróblewska, O.; Siudem, P.; Paradowska, K. Methods to Improve the Solubility of Curcumin from Turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Penedones, A.; Alves, C.; Batel-Marques, F. Recommendations to Conduct and Report Systematic Reviews in Medical Literature: A Scoping Review. BMC Med. Res. Methodol. 2019, 19, 234. [Google Scholar] [CrossRef]
- Krnic Martinic, M.; Pieper, D.; Glatt, A.; Puljak, L. Definition of a Systematic Review Used in Overviews of Systematic Reviews, Meta-Epidemiological Studies and Textbooks. BMC Med. Res. Methodol. 2019, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Al-Karawi, D.; Al Mamoori, D.A.; Tayyar, Y. The Role of Curcumin Administration in Patients with Major Depressive Disorder: Mini Meta-Analysis of Clinical Trials: Clinical Effect of Curcumin on Depression. Phytother. Res. 2016, 30, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.J.A.; Abbott, K.A.; Garg, M.L. Anti-Inflammatory Effects of Oral Supplementation with Curcumin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2021, 79, 1043–1066. [Google Scholar] [CrossRef]
- Sadeghian, M.; Rahmani, S.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. The Effect of Oral Curcumin Supplementation on Health-Related Quality of Life: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Affect. Disord. 2021, 278, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Singh, A.; Jones, G.; Winzenberg, T.; Ding, C.; Chopra, A.; Das, S.; Danda, D.; Laslett, L.; Antony, B. Efficacy and Safety of Turmeric Extracts for the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Curr. Rheumatol. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Al-Eid, F.; Wang, C. Efficacy of Curcumin and Boswellia for Knee Osteoarthritis: Systematic Review and Meta-Analysis. Semin. Arthritis Rheum. 2018, 48, 416–429. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Simental-Mendía, L.E.; Bo, S.; Sahebkar, A. Effect of Curcumin on Circulating Interleukin-6 Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2016, 111, 394–404. [Google Scholar] [CrossRef]
- Sahebkar, A. Are Curcuminoids Effective C-Reactive Protein-Lowering Agents in Clinical Practice? Evidence from a Meta-Analysis: CURCUMINOIDS AND CRP. Phytother. Res. 2014, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Cicero, A.F.G.; Simental-Mendía, L.E.; Aggarwal, B.B.; Gupta, S.C. Curcumin Downregulates Human Tumor Necrosis Factor-α Levels: A Systematic Review and Meta-Analysis Ofrandomized Controlled Trials. Pharmacol. Res. 2016, 107, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Henrotin, Y. Analgesic Efficacy and Safety of Curcuminoids in Clinical Practice: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Med. 2016, 17, 1192–1202. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, X.; Bai, J.; Li, W.; Yang, J.; Deng, Z.; Wu, M.; Ying, T.; He, G. The Effects of Curcumin on Anthropometric and Cardiometabolic Parameters of Patients with Metabolic Related Diseases: A Systematic Review and Dose-Effect Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 9282–9298. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Abbasi, F.; Ebrahimzadeh, A.; Jibril, A.T.; Milajerdi, A. Effects of Curcumin Supplementation on Inflammatory Biomarkers in Patients with Rheumatoid Arthritis and Ulcerative Colitis: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2021, 61, 102773. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.D.A.; Araújo, A.C.; Bechara, M.D. Curcumin, Autoimmune and Inflammatory Diseases: Going beyond Conventional Therapy—A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Mashhadi, N.; Bagherniya, M. Askari, G.; Sathyapalan, T.; Sahebkar, A. A Systematic Review of the Clinical Use of Curcumin for the Treatment of Osteoarthritis. In Studies on Biomarkers and New Targets in Aging Research in Iran; Guest, P.C., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1291, pp. 265–282. ISBN 978-3-030-56152-9. [Google Scholar]
- Hsiao, A.-F.; Lien, Y.-C.; Tzeng, I.-S.; Liu, C.-T.; Chou, S.-H.; Horng, Y.-S. The Efficacy of High- and Low-Dose Curcumin in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2021, 63, 102775. [Google Scholar] [CrossRef]
- Gusenbauer, M.; Haddaway, N.R. Which Academic Search Systems Are Suitable for Systematic Reviews or Meta-analyses? Evaluating Retrieval Qualities of Google Scholar, PubMed, and 26 Other Resources. Res. Syn. Meth. 2020, 11, 181–217. [Google Scholar] [CrossRef]
| Variable | Result | |
|---|---|---|
| Type of included studies, N (%) * | RCTs | 144 (84) |
| Both RCTs and NRSs | 27 (16) | |
| Number of studies included, median (range) | 6 (1 to 29) | |
| Number of participants included, median (range) | 380 (8 to 13,594) | |
| Type of curcumin product specified in methods, N (%) | Curcumin | 56 (33) |
| Not reported | 36 (21) | |
| Curcumin/turmeric | 17 (9.9) | |
| Turmeric | 3 (1.8) | |
| Other | 59 (35) | |
| Statement about review funding reported? N (%) | Yes | 119 (70) |
| No | 52 (30) | |
| Category of funding, N (%) | Commercial | 1 (0.6) |
| Non-commercial | 57 (33) | |
| Mixed | 3 (1.8) | |
| No funding | 58 (34) | |
| Not applicable | 52 (30) | |
| A “conflict of interest” statement reported? N (%) | Yes | 147 (86) |
| No | 24 (14) | |
| If “conflict of interest” reported, was at least one author funded/supported in any way by a sponsor of the investigated product? N (%) | Yes | 7 (4.1) |
| No | 140 (82) |
| Study Section Mentioning Bioavailability | Categorization | N (%) |
|---|---|---|
| Methods * | Inclusion criteria | 6 (3.5) |
| Statistics regarding bioavailability | 6 (3.5) | |
| Heterogeneity | 2 (1.2) | |
| Categorization of interventions | 1 (1.2) | |
| Data on bioavailability in included studies not available | 1 (0.6) | |
| Assessing strength of study methodology | 1 (0.6) | |
| Results ** | Intervention description | 19 (11) |
| Results of subgroup analysis for bioavailability | 10 (5.8) | |
| Results for studies using bioavailability enhancement | 7 (4.1) | |
| Reporting plasma levels of curcumin | 2 (1.2) | |
| Results of a sensitivity analysis for bioavailability | 1 (0.6) | |
| Methodological assessment | 1 (0.6) | |
| Lack of studies that have evaluated bioavailability | 1 (0.6) | |
| Stratification of results | 1 (0.6) | |
| Explaining low oral availability of curcumin | 1 (0.6) | |
| Discussion *** | General comments about curcumin bioavailability | 68 (40) |
| Description of included interventions | 22 (13) | |
| Results of included studies | 19 (11) | |
| Recommendations for future trials | 15 (8.8) | |
| Heterogeneity of studies in terms of bioavailability | 10 (5.8) | |
| Limitations due to using low bioavailability product | 4 (2.3) | |
| Lack of studies analyzing bioavailability/bioavailability-enhanced formulations | 2 (1.7) | |
| Lack of bioavailability evaluation in included studies | 2 (1.7) | |
| Conclusions **** | Summary of results regarding bioavailability | 8 (4.7) |
| More studies are needed with products with high bioavailability | 7 (4.1) | |
| More bioavailable product forms are needed | 5 (2.9) | |
| Future research needs to compare various formulations regarding bioavailability | 4 (2.3) | |
| Low bioavailability hinders effectiveness of the studied intervention | 1 (0.6) | |
| Future studies need to explore the effect of availability | 1 (0.6) | |
| Curcumin in more bioavailable form can produce effect | 1 (0.6) | |
| Poor availability of curcumin is an issue | 1 (0.6) |
| Study | Outcomes Measured | Subgroup Analysis | Result | |||
|---|---|---|---|---|---|---|
| Subgroup 1 | Subgroup 2 | |||||
| Derosa, 2016 [30] | Interleukin-6 concentration | Bioavailability-enhanced curcumin plus piperine (6), Meriva (1) | Unformulated curcumin unformulated curcumin (3) | Formulation does not significantly affect the IL-6-lowering effect of curcuminoids | ||
| Ferguson, 2021 [26] | Proinflammatory markers | Bioavailability-enhanced CRP: curcumin plus piperine (5), Longvida (3), Acumin (2), nanomicelle curcumin (2), nanocurcumin (1), BCM-95 curcumin (1), curcumin micelle (1), Meriva (1) IL-6: curcumin plus piperine (1), nanocurcumin (1), nanomicelle curcumin (1), curcumin micelles (1), Longvida (1) TNF-α: curcumin plus piperine (4), nanomicelle curcumin (2), nanocurcumin (1), Longvida (1) IL-8: 3 curcumin plus piperine (1) | Nonbioavailability-enhanced CRP: unformulated curcumin (4), turmeric (2) IL-6: unformulated curcumin (2), turmeric (2) TNF-α: unformulated curcumin (2), turmeric (1) IL-8: curcumin (1), turmeric (1) | Bioavailability-enhanced formulations result in larger extent of reduction in CRP and comparable reductions in IL-6 and TNF-α; significant reduction in IL-8 was observed only for nonbioavailability enhanced interventions | ||
| Sahebkar, 2014b [31] | CRP concentration | Bioavailability-improved formulations curcumin plus piperine (2), Longvida (1), Meriva (1) | Non-improved formulations unformulated curcumin (2) | Significant CRP lowering effects observed only for bioavailability improved formulations | ||
| Sahebkar, 2016a [32] | TNF-α concentration | Bioavailability-enhanced forumulations cucumin plus piperine (5) | Unformulated curcumin unformulated curcumin (3), turmeric (1) | Larger TNF-α-lowering effect size for bioavailability-improved formulations, but the difference is not of statistical significance | ||
| Sahebkar, 2016 [33] | Analgesic effect | Bioavailability-improved formulations curcumin plus piperine (2), Meriva (1) | All products unformulated curcumin (5), curcumin plus piperine (2), Meriva (1) | Larger effect size for pain reduction for bioavailability improved formulations | ||
| Sadeghian, 2021 [27] | HRQOL scores * | High bioavailability curcumin plus piperine (3), SinaCurmin (1), BCM-95 (2), CurQfen (2) | Low bioavailability unformulated curcumin (4) | Curcumin increased HRQOL for high-bioavailability formulations | ||
| Subgroup 1 | Subgroup 2 | Subgroup 3 | ||||
| Sun, 2022 [34] | Anthropometric and cardiometabolic parameters ** | Low-bioavailability unformulated curcumin (11), turmeric powder (2) | High-bioavailability phospholipid curcumin/Meriva (5), curcumin plus piperine (4), curcumin micelles (1), Theracurmin (1), BCM-95 (1), curcumin β-cyclodextrin complex (1) | Nanocurcumin nanocurcumin (5) | Improvements in BW, BMI, and the levels of FPG, HbA1c, INS, HOMA-IR, HDL-C and Hs-CRP; greater reductions for nanocurcumin group were observed in FPG, INS, TC and LDL-C *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bučević Popović, V.; Karahmet Farhat, E.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals 2024, 17, 164. https://doi.org/10.3390/ph17020164
Bučević Popović V, Karahmet Farhat E, Banjari I, Jeličić Kadić A, Puljak L. Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals. 2024; 17(2):164. https://doi.org/10.3390/ph17020164
Chicago/Turabian StyleBučević Popović, Viljemka, Esma Karahmet Farhat, Ines Banjari, Antonia Jeličić Kadić, and Livia Puljak. 2024. "Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study" Pharmaceuticals 17, no. 2: 164. https://doi.org/10.3390/ph17020164
APA StyleBučević Popović, V., Karahmet Farhat, E., Banjari, I., Jeličić Kadić, A., & Puljak, L. (2024). Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals, 17(2), 164. https://doi.org/10.3390/ph17020164







