[18F]Fluspidine—A PET Tracer for Imaging of σ1 Receptors in the Central Nervous System
Abstract
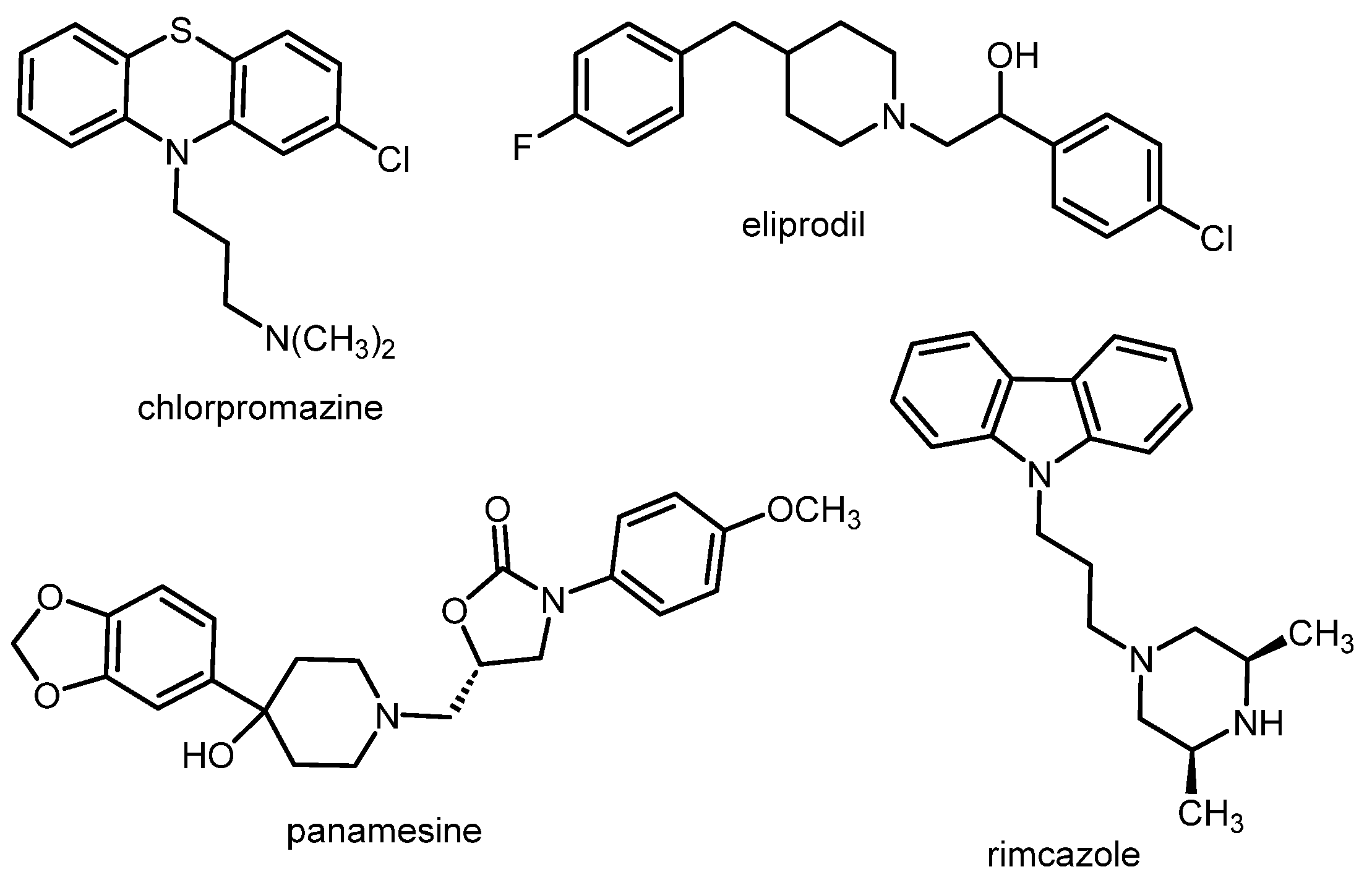
1. Introduction: The Role of σ1 Receptors in Some Brain Diseases
1.1. Pain
1.2. Psychosis
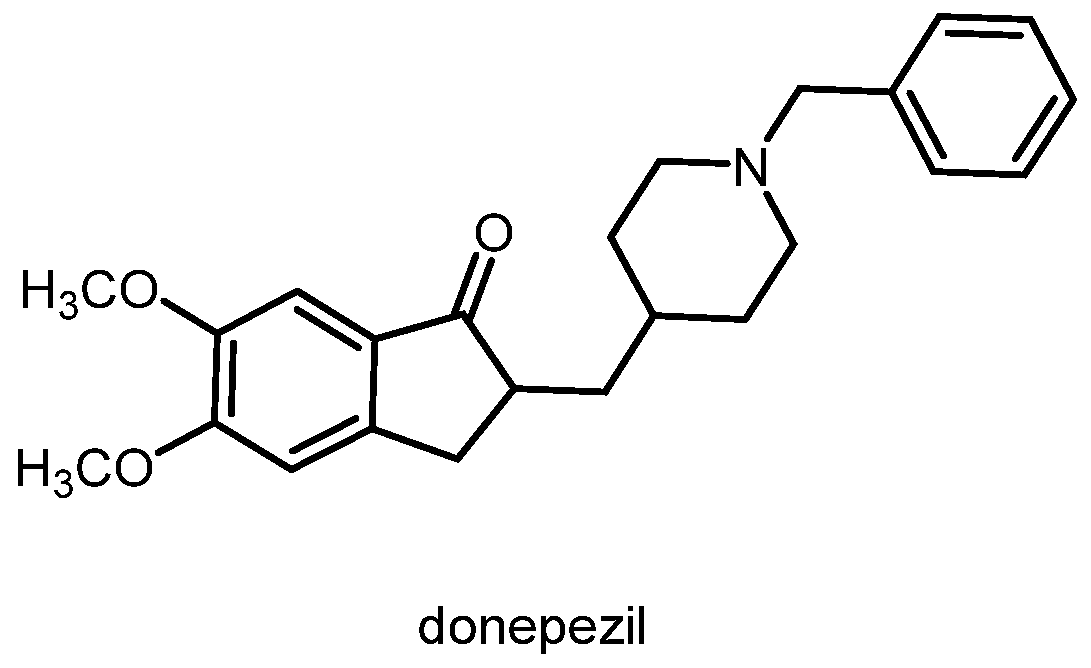
1.3. Alzheimer’s Disease
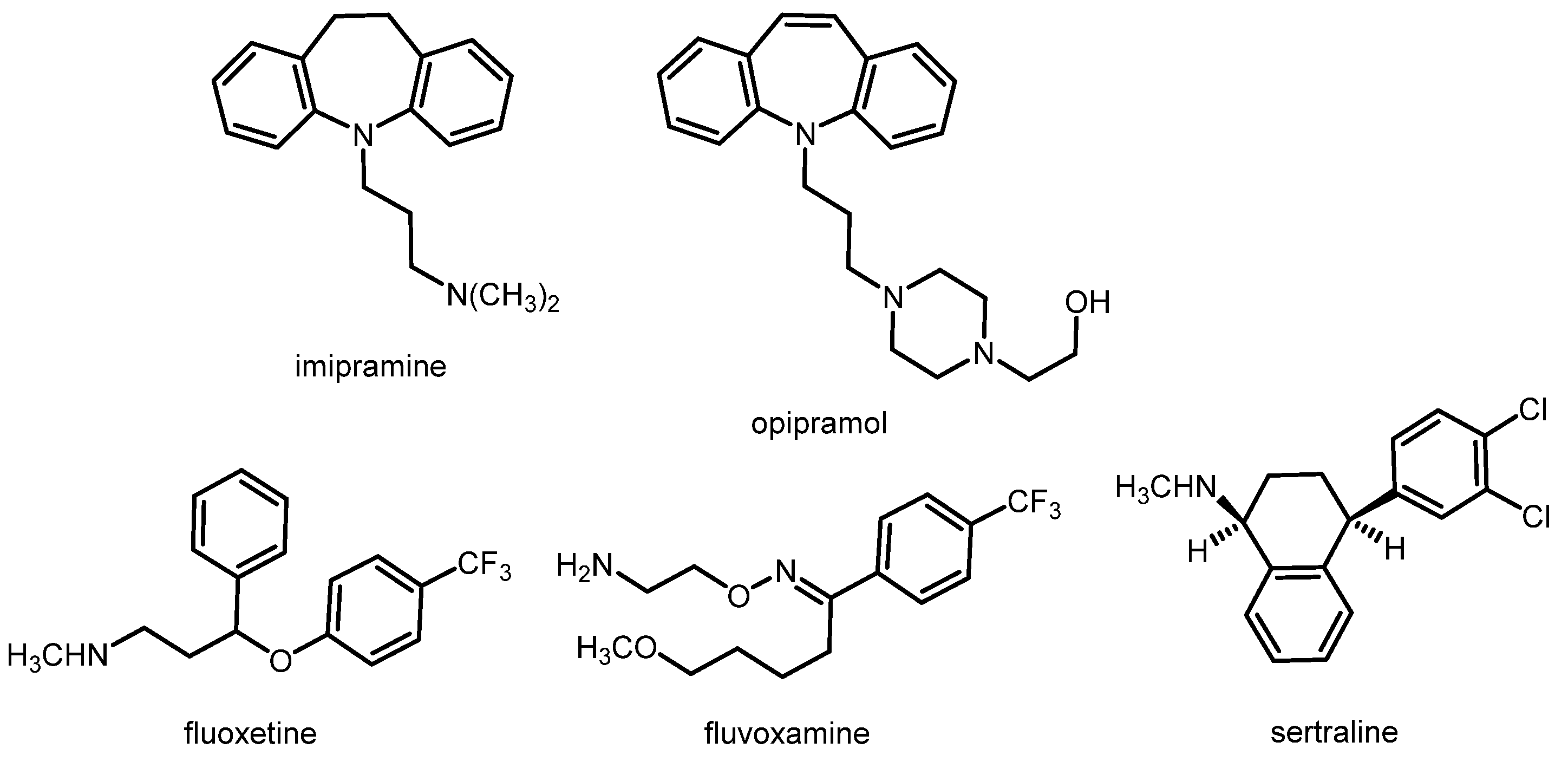
1.4. Depression
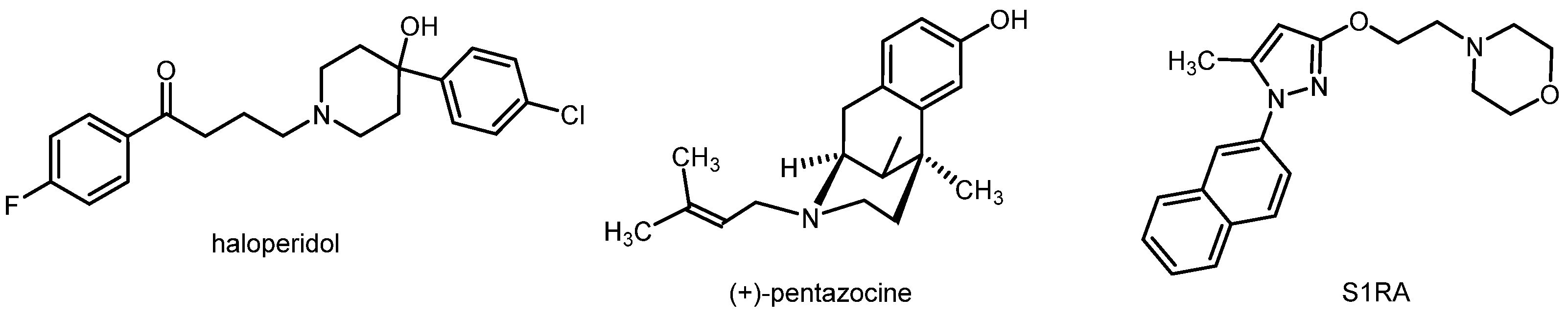
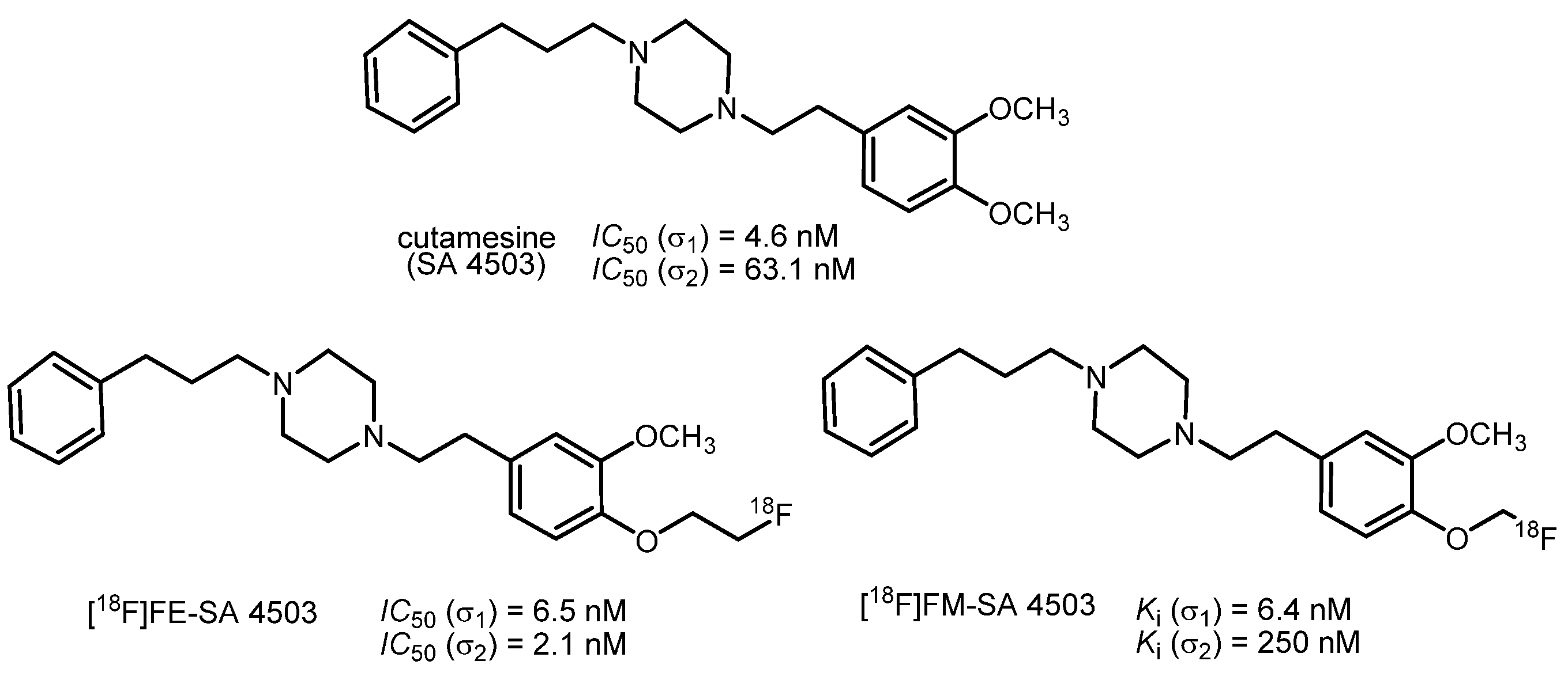
2. Fluorinated PET Tracers Derived from the Promising σ1 Ligand Cutamesine (SA 4503)
3. Spirocyclic σ1 Receptor Ligands Designed for PET Studies: Structure Affinity Relationships
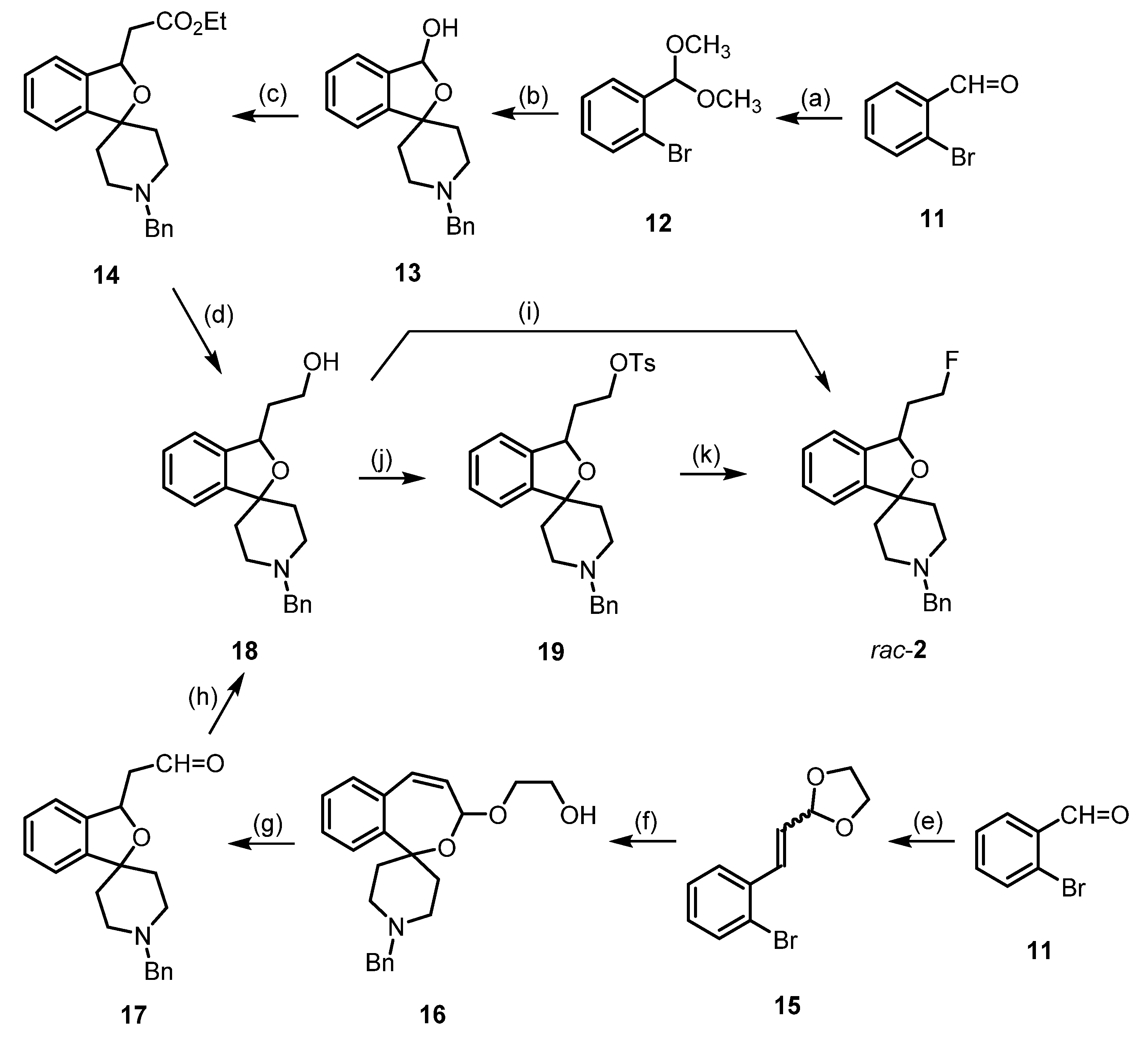
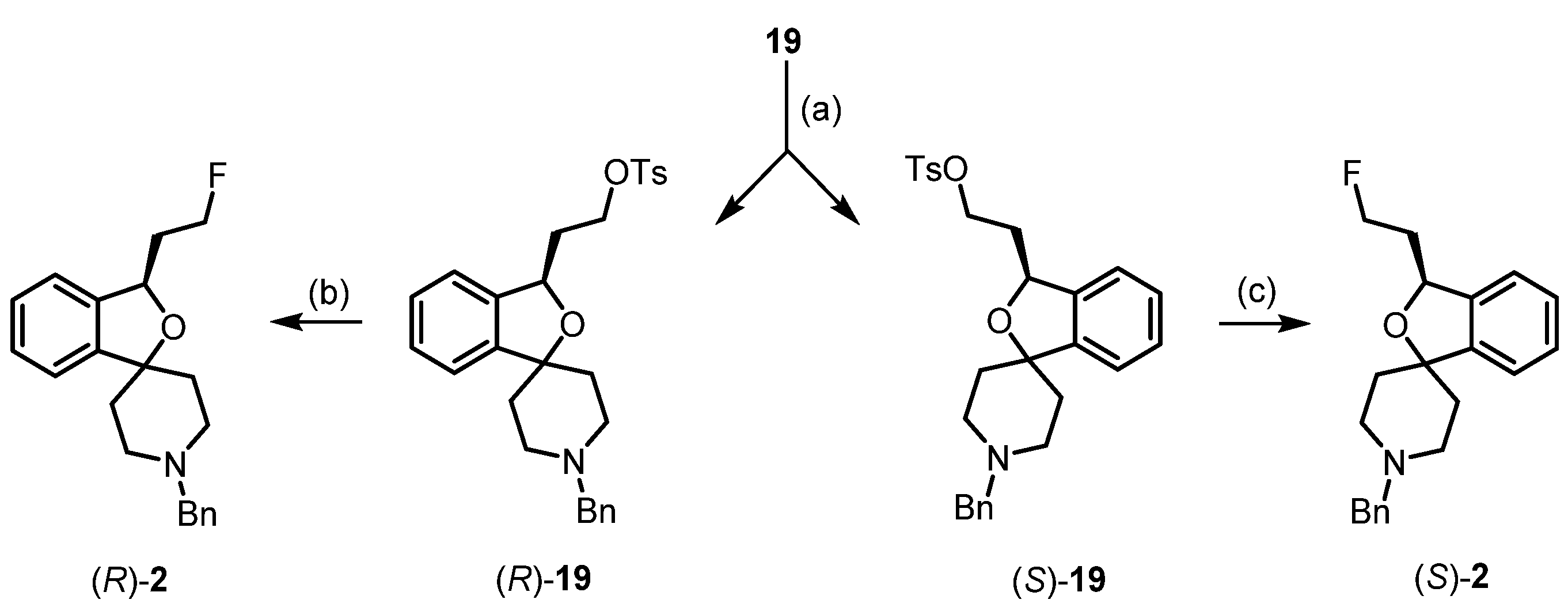
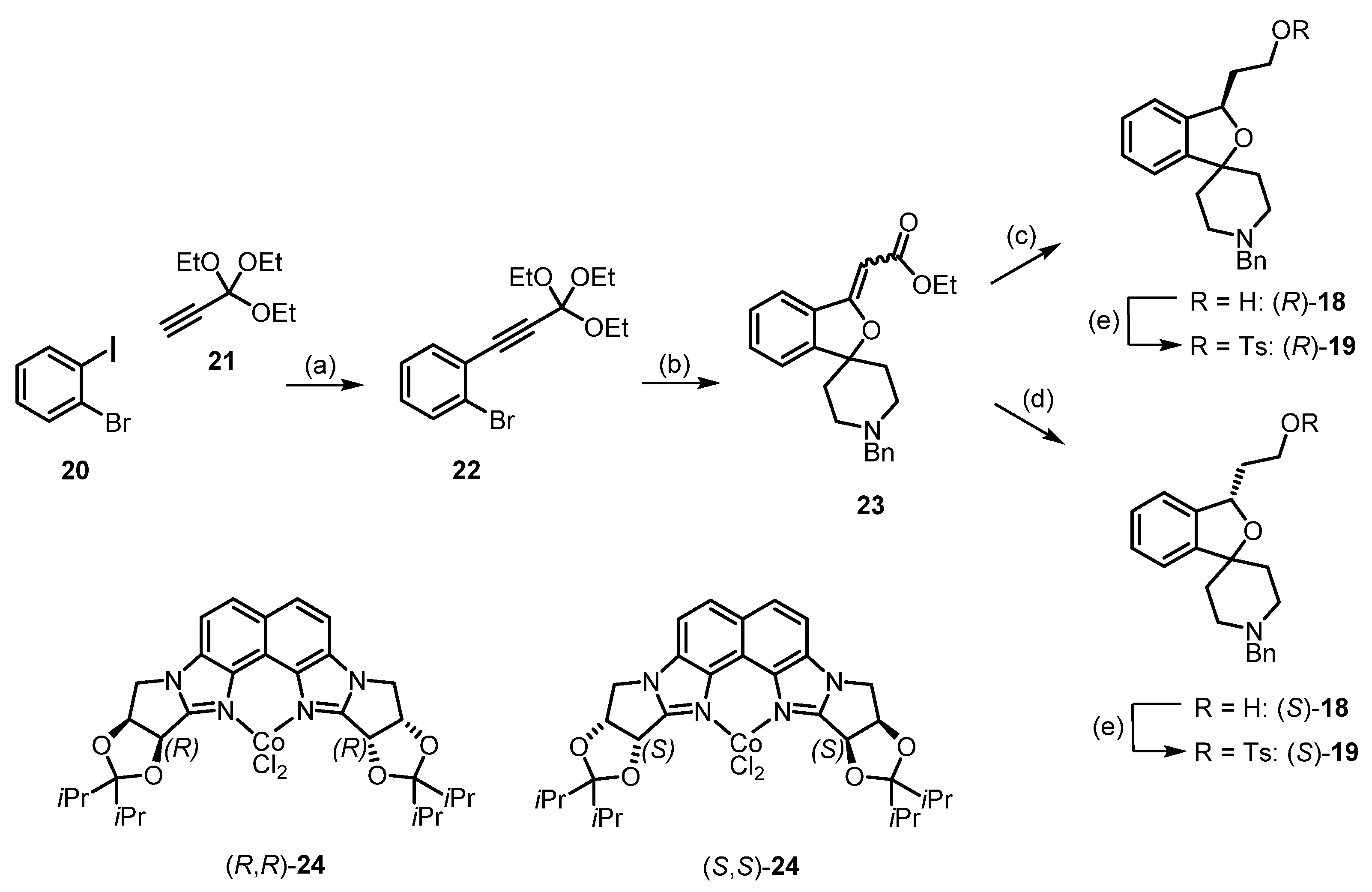
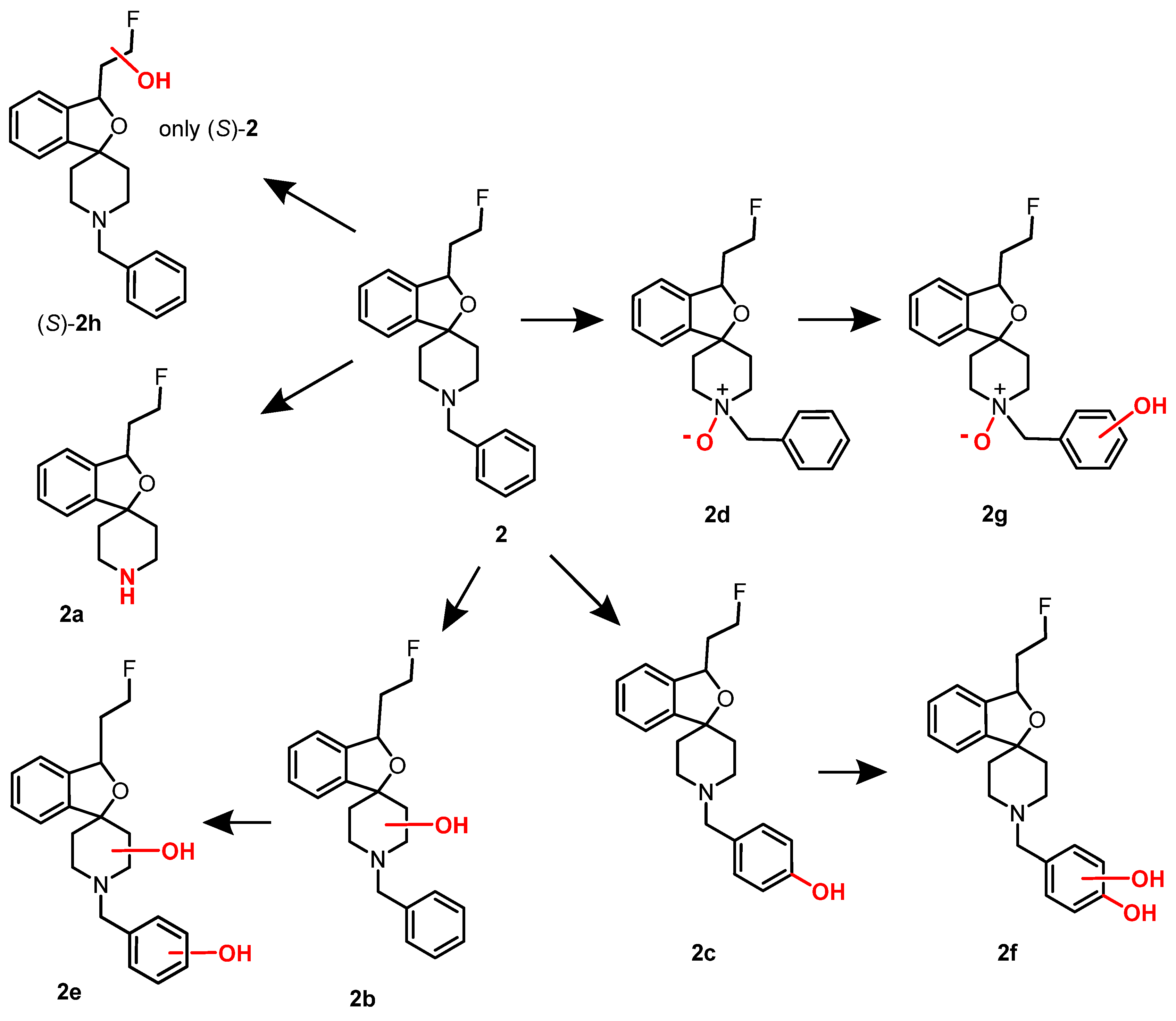
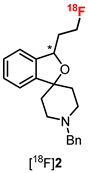
4. Synthesis of Racemic and Enantiomerically Pure Fluspidine (2, (S)-2 and (R)-2)
5. In Vitro Characterization of Fluspidine and Its Enantiomers
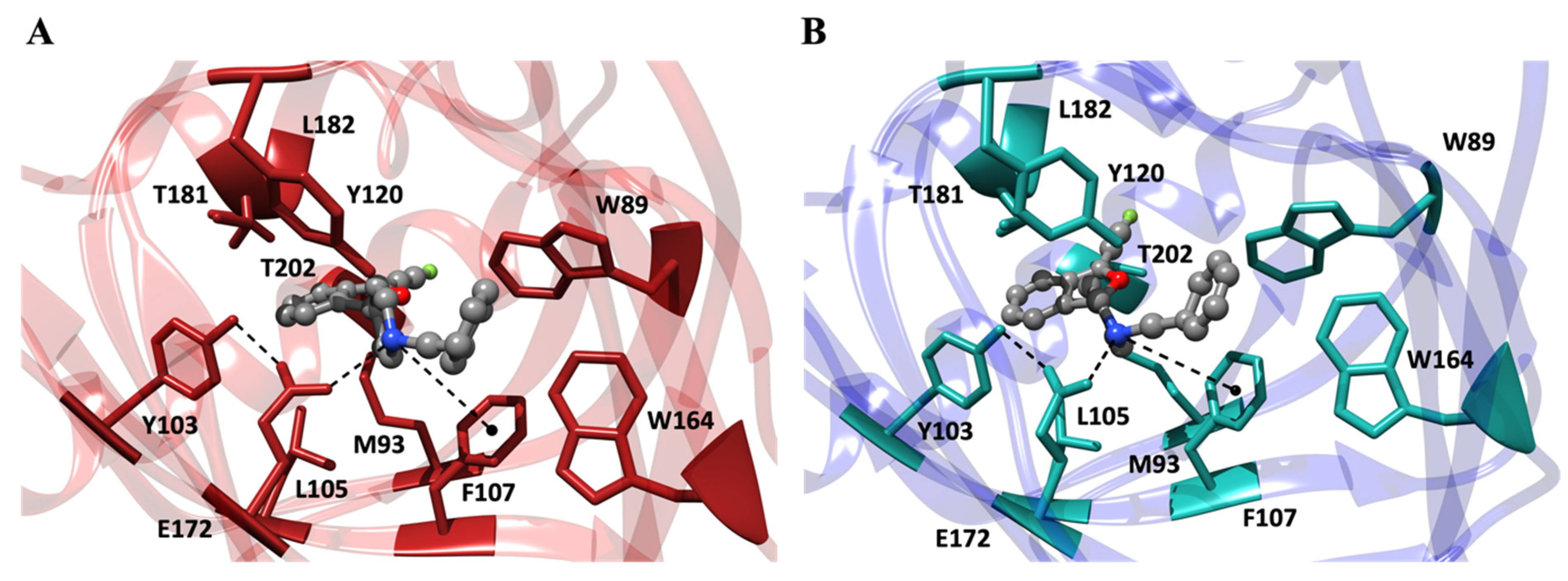
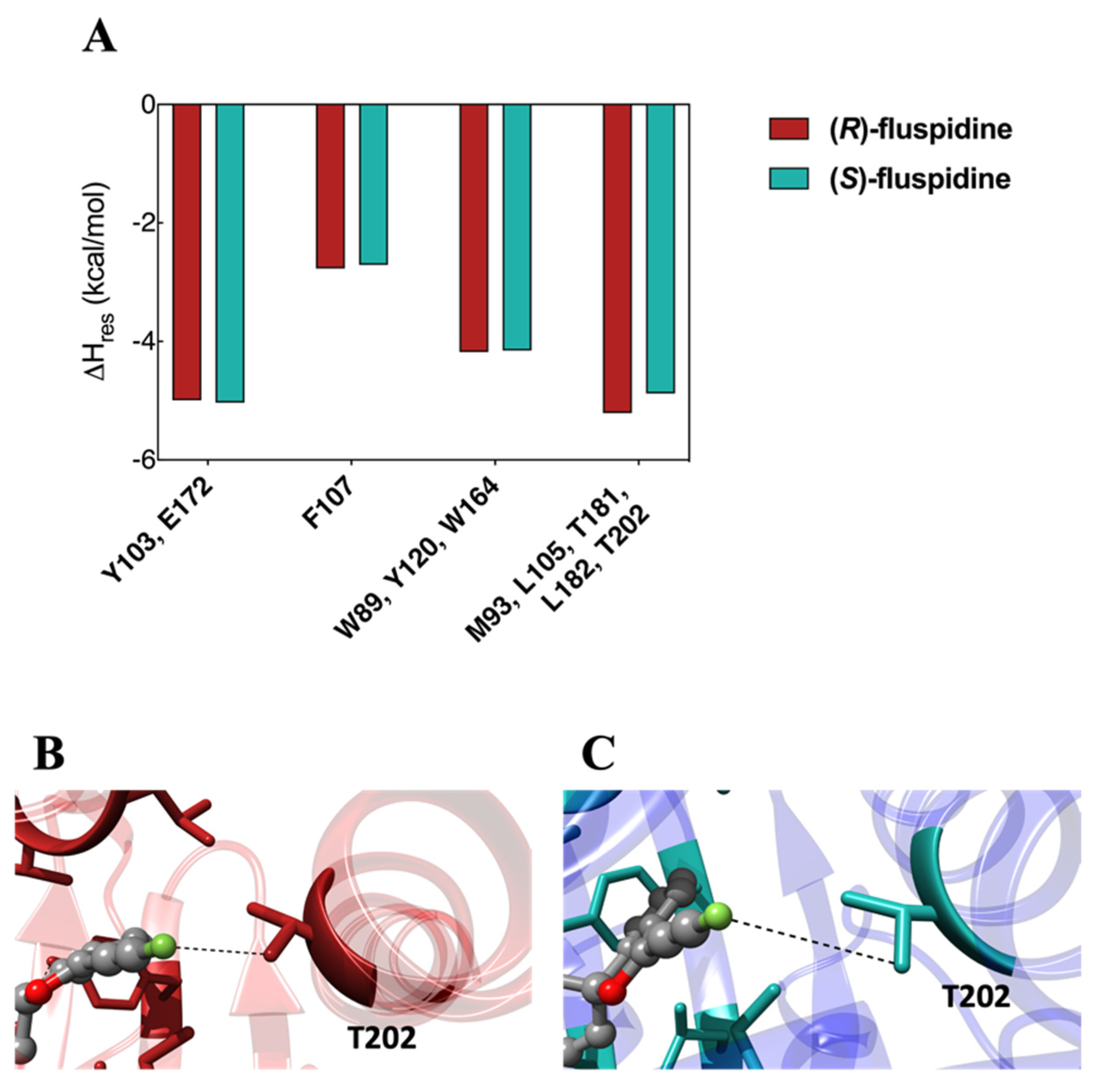
6. Molecular Interactions of Fluspidine Enantiomers with the σ1 Receptor
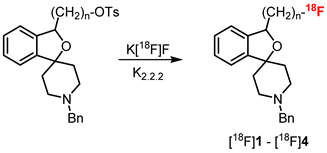
7. Radiosynthesis
8. Preclinical In Vivo Studies of Racemic and Enantiomerically Pure [18F]Fluspidine [18F]2, (R)-[18F]2 and (S)-[18F]2
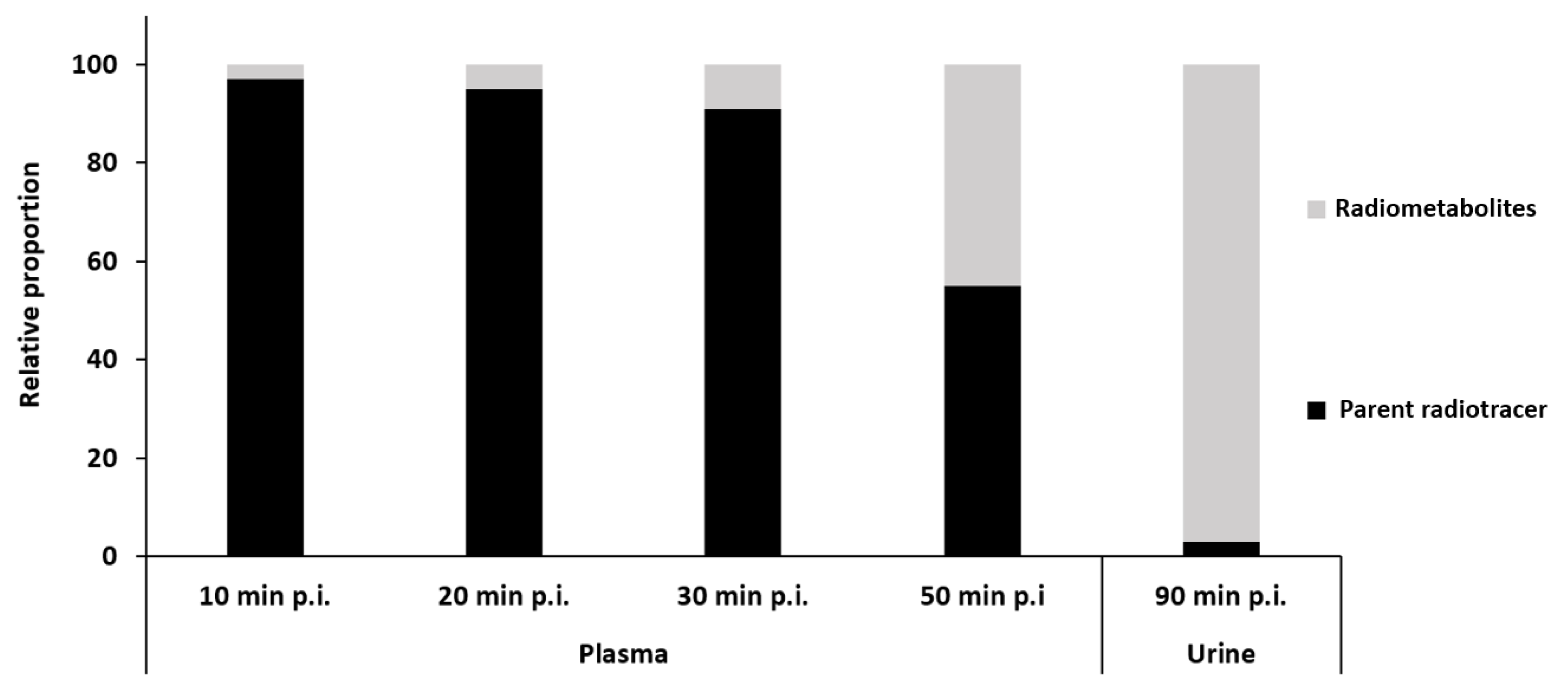
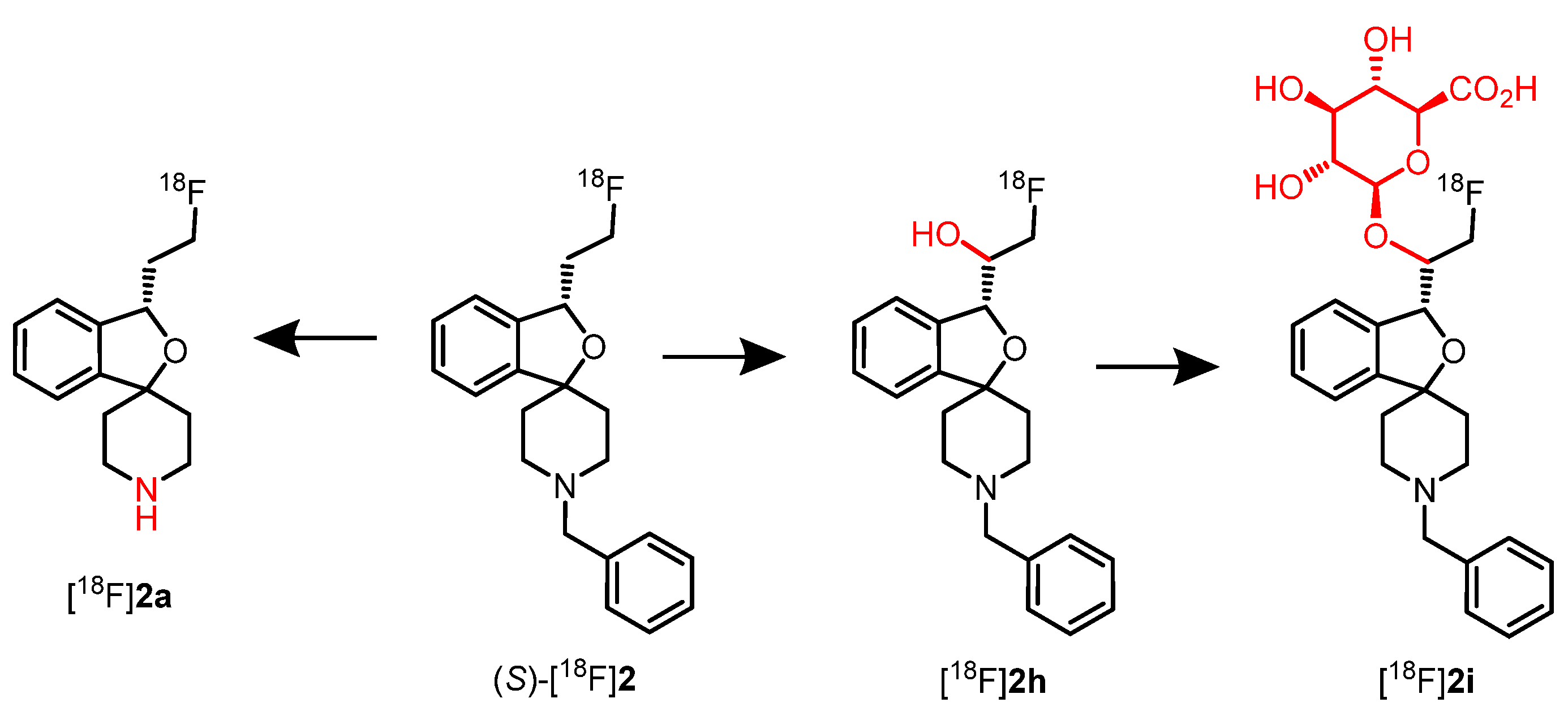
8.1. Radiometabolites of rac-[18F]2, (R)-[18F]2 and (S)-[18F]2 In Vivo
8.2. Organ Distribution in Mice, Piglets and Non-Human Primates
9. Human Studies with (S)-[18F]Fluspidine ((S)-[18F]2)
Imaging of σ1 Receptors in a Clinical Study (Major Depressive Disorder Patients)
10. Computational Details
11. Determination of In Vitro Pharmacokinetic Parameters
Author Contributions
Funding
Conflicts of Interest
References
- Cobos, E.J.; Entrena, J.M.; Nieto, F.R.; Cendan, C.M.; Del Pozo, E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr. Neuropharmacol. 2008, 6, 344–366. [Google Scholar] [CrossRef]
- Maurice, T.; Su, T.-P. The Pharmacology of Sigma-1 Receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef]
- Smith, S. Sigma Receptors: Their Role in Disease and as Therapeutic Targets; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2016. [Google Scholar]
- Chien, C.C.; Pasternak, G.W. Selective antagonism of opioid analgesia by a sigma system. J. Pharmacol. Exp. Ther. 1994, 271, 1583–1590. [Google Scholar]
- Entrena, J.M.; Cobos, E.J.; Nieto, F.R.; Cendan, C.M.; Gris, G.; Del Pozo, E.; Zamanillo, D.; Baeyens, J.M. σ1 receptors are essential for capsaicin-induced mechanical hypersensitivity: Studies with selective σ1 ligands and σ1 knockout mice. Pain 2009, 143, 252–261. [Google Scholar] [CrossRef]
- Diaz, J.L.; Cuberes, R.; Berrocal, J.; Contijoch, M.; Christmann, U.; Fernandez, A.; Port, A.; Holenz, J.; Buschmann, H.; Laggner, C.; et al. Synthesis and biological evaluation of the 1-arylpyrazole class of σ1 receptor antagonists: Identification of 4-{2-[5-methyl-1-(naphthalen-2-yl)-1H-pyrazol-3-yloxy]ethyl}morpholine (S1RA, E-52862). J. Med. Chem. 2012, 55, 8211–8224. [Google Scholar] [CrossRef]
- Wünsch, B. The σ1 receptor antagonist S1RA is a promising candidate for the treatment of neurogenic pain. J. Med. Chem. 2012, 55, 8209–8210. [Google Scholar] [CrossRef]
- Richelson, E.; Souder, T. Binding of antipsychotic drugs to human brain receptors Focus on newer generation compounds. Life Sci. 2000, 68, 29–39. [Google Scholar] [CrossRef]
- Blicker, L.; Gonzáles-Cano, R.; Laurini, E.; Nieto, F.R.; Schmidt, J.; Schepmann, D.; Pricl, S.; Wünsch, B. Conformationally restricted σ1 receptor antagonists from (−)-isopulegol. J. Med. Chem. 2023, 66, 4999–5020. [Google Scholar] [CrossRef]
- Milenina, L.S.; Krutetskaya, Z.I.K.; Antonov, V.G.; Krutetskaya, N.I. Sigma-1 receptor ligands chlorpromazine and trifluoperazine attenuate Ca2+ responses in rat peritoneal macrophage. Cell Tissue Biol. 2022, 16, 233–244. [Google Scholar] [CrossRef]
- Ohi, K.; Hashimoto, R.; Yasuda, Y.; Fukumoto, M.; Yamamori, H.; Umeda-Yano, S.; Kamino, K.; Ikezawa, K.; Azechi, M.; Iwase, M.; et al. The SIGMAR1 gene is associated with a risk of schizophrenia and activation of the prefrontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psych. 2011, 35, 1309–1315. [Google Scholar] [CrossRef]
- Hayashi, T.; Tsai, S.Y.; Mori, T.; Fujimoto, M.; Su, T.P. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin. Ther. Targets 2011, 15, 557–577. [Google Scholar] [CrossRef]
- Urani, A.; Romieu, P.; Roman, F.J.; Maurice, T. Enhanced antidepressant effect of sigma1 (σ1) receptor agonists in σ25–35-amyloid peptide-treated mice. Behav. Brain Res. 2002, 134, 239–247. [Google Scholar] [CrossRef]
- Urani, A.; Romieu, P.; Roman, F.J.; Yamada, K.; Noda, Y.; Kamei, H.; Manh Tran, H.; Nagai, T.; Nabeshima, T.; Maurice, T. Enhanced antidepressant efficacy of σ1 receptor agonists in rats after chronic intracerebroventricular infusion of σ-amyloid-(1–40) protein. Eur. J. Pharmacol. 2004, 486, 151–161. [Google Scholar] [CrossRef]
- Meunier, J.; Ieni, J.; Maurice, T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25–35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br. J. Pharmacol. 2006, 149, 998–1012. [Google Scholar] [CrossRef]
- Wang, T.; Jia, H. The sigma receptors in Alzheimer’s Disease: New potential targets for diagnosis and therapy. Int. J. Mol. Sci. 2023, 24, 12025. [Google Scholar] [CrossRef]
- Sabino, V.; Cottone, P.; Parylak, S.L.; Steardo, L.; Zorrilla, E.P. σ1 receptor knockout mice display a depressive-like phenotype. Behav. Brain Res. 2009, 198, 472–476. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. An update on the development of drugs for neuropsychiatric disorders: Focusing on the σ1 receptor ligand. Expert. Opin. Ther. Targets 2008, 12, 45–58. [Google Scholar] [CrossRef]
- Lucas, G.; Rymar, V.V.; Sadikot, A.F.; Debonnel, G. Further evidence for an antidepressant potential of the selective σ1 agonist SA 4503: Electrophysiological, morphological and behavioural studies. Int. J. Neuropsychopharmacol. 2008, 11, 485–495. [Google Scholar] [CrossRef]
- Shirayama, Y.; Nishikawa, T.; Umino, A.; Takahashi, K. p-chlorophenylalanine-reversible reduction of σ binding sites by chronic imipramine treatment in rat brain. Eur. J. Pharmacol. 1993, 237, 117–126. [Google Scholar] [CrossRef]
- Shirayama, Y.; Nishikawa, T.; Takahashi, K. Differential effects of repeated dl-pentazocine treatment on σ binding sites in discrete brain areas of the rat. Neurosci. Lett. 1994, 165, 219–222. [Google Scholar] [CrossRef]
- Brust, P.; Deuther-Conrad, W.; Lehmkuhl, K.; Jia, H.; Wünsch, B. Molecular Imaging of σ1 Receptors In Vivo: Current Status and Perspectives. Curr. Med. Chem. 2014, 21, 35–69. [Google Scholar] [CrossRef]
- Weber, F.; Brust, P.; Laurini, E.; Pricl, S.; Wünsch, B. Fluorinated PET tracers for molecular imaging of σ1 receptors in the central nervous system. In Sigma Receptors: Their Role in Disease and as Therapeutic Targets; Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 964, pp. 31–48. [Google Scholar]
- Xu, R.; Lord, S.A.; Peterson, R.M.; Fergason-Cantrell, E.A.; Lever, J.R.; Lever, S.Z. Ether modifications to 1-[2-(3,4-dimethoxyphenyl)ethyl]-4-(3-phenylpropyl)piperazine (SA4503): Effects on binding affinity and selectivity for sigma receptors and monoamine transporters. Bioorg. Med. Chem. 2015, 23, 222–230. [Google Scholar] [CrossRef]
- Matsuno, K.; Senda, T.; Kobayashi, T.; Okamoto, K.; Nakata, K.; Mita, S. SA4503, a novel cognitive enhancer, with sigma 1 receptor agonistic properties. Behav. Brain Res. 1997, 83, 221–224. [Google Scholar] [CrossRef]
- Ramakrishnan, N.K.; Schepers, M.; Luurtsema, G.; Nyakas, C.J.; Elsinga, P.H.; Ishiwata, K.; Dierckx, R.A.J.O.; van Waarde, A. Cutamesine Overcomes REM Sleep Deprivation-Induced Memory Loss: Relationship to Sigma-1 Receptor Occupancy. Mol. Imaging Biol. 2015, 17, 364–372. [Google Scholar] [CrossRef]
- Skuza, G. Potential antidepressant activity of sigma ligands. Pol. J. Pharmacol. 2003, 55, 923–934. [Google Scholar]
- Elsinga, P.H.; Kawamura, K.; Kobayashi, T.; Tsukada, H.; Senda, M.; Vaalburg, W.; Ishiwata, K. Synthesis and evaluation of [18F]fluoroethyl SA4503 as a PET ligand for the σ receptor. Synapse 2002, 43, 259–267. [Google Scholar] [CrossRef]
- Kawamura, K.; Tsukada, H.; Shiba, K.; Tsuji, C.; Harada, N.; Kimura, Y.; Ishiwata, K. Synthesis and evaluation of fluorine-18-labeled SA4503 as a selective σ1 receptor ligand for positron emission tomography. Nucl. Med. Biol. 2007, 34, 571–577. [Google Scholar] [CrossRef]
- Maisonal, A.; Maestrup, E.G.; Wiese, C.; Hiller, A.; Schepmann, D.; Fischer, S.; Deuther-Conrad, W.; Steinbach, J.; Brust, P.; Wünsch, B. Synthesis, radiofluorination and pharmacological evaluation of a fluoromethyl spirocyclic PET tracer for central σ1 receptors and comparison with fluoroalkyl homologs. Bioorg. Med. Chem. 2012, 20, 257–269. [Google Scholar] [CrossRef]
- Maestrup, E.G.; Wiese, C.; Schepmann, D.; Brust, P.; Wünsch, B. Synthesis, pharmacological activity and structure affinity relationships of spirocyclic σ1 receptor ligands with a (2-fluoroethyl) residue in 3-position. Bioorg. Med. Chem. 2011, 19, 393–405. [Google Scholar] [CrossRef]
- Fischer, S.; Wiese, C.; Maestrup, E.G.; Hiller, A.; Deuther-Conrad, W.; Scheunemann, W.; Schepmann, D.; Steinbach, J.; Wünsch, B.; Brust, P. Molecular imaging of σ receptors: Synthesis and evaluation of the potent σ1 selective radioligand [18F]fluspidine. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 540–551. [Google Scholar] [CrossRef]
- Große Maestrup, E.; Fischer, S.; Wiese, C.; Schepmann, D.; Hiller, A.; Deuther-Conrad, W.; Steinbach, J.; Wünsch, B.; Brust, P. Evaluation of spirocyclic 3-(3-fluoropropyl)-2-benzofurans as σ1 receptor ligands for neuroimaging with positron emission tomography. J. Med. Chem. 2009, 52, 6062–6072. [Google Scholar] [CrossRef]
- Maisonial, A.; Maestrup, E.G.; Fischer, S.; Hiller, A.; Scheunemann, M.; Wiese, C.; Schepmann, D.; Steinbach, J.; Deuther-Conrad, W.; Wünsch, B.; et al. A 18F-labeled fluorobutyl-substituted spirocyclic piperidine derivative as a selective radioligand for PET imaging of sigma1 receptors. ChemMedChem 2011, 6, 1401–1410. [Google Scholar] [CrossRef]
- Maier, C.A.; Wünsch, B. Novel σ receptor ligands. part 2. SAR of spiro[[2]benzopyran-1,4′-piperidines] and spiro[[2]benzofuran-1,4′-piperidines] with carbon substituents in position 3. J. Med. Chem. 2002, 45, 4923–4930. [Google Scholar] [CrossRef]
- Holl, K.; Falck, E.; Köhler, J.; Schepmann, D.; Humpf, H.-U.; Brust, P.; Wünsch, B. Synthesis, characterization, and metabolism studies of fluspidine enantiomers. Chem. Med. Chem. 2013, 12, 2047–2056. [Google Scholar] [CrossRef]
- Bunse, P.; Würthwein, E.-U.; Wünsch, B. Synthesis of substituted 1-alkylidenephthalanes via lithium-promoted 5-exo-dig cyclization. Eur. J. Org. Chem. 2018, 1806–1812. [Google Scholar] [CrossRef]
- Shuto, Y.; Yamamura, T.; Tanaka, S.; Yoshimura, M.; Kitamura, M. Asymmetric NaBH41,4-Reduction of C3-Disubstituted 2-Propenoates Catalyzed by a Diamidine Cobalt Complex. ChemCatChem 2015, 7, 1547–1550. [Google Scholar] [CrossRef]
- Bunse, P.; Schlepphorst, C.; Glorius, F.; Kitamura, M.; Wünsch, B. Short and atom-economic enantioselective synthesis of the σ1 receptor ligands (S)- and (R)-fluspidine—Important tools for positron emission tomography studies. J. Org. Chem. 2019, 84, 13744–13754. [Google Scholar] [CrossRef]
- Nakane, S.; Yoshinaka, S.; Iwase, S.; Shuto, Y.; Bunse, P.; Wünsch, B.; Tanaka, S.; Kitamura, M. Synthesis of fluspidine via asymmetric NaBH4 reduction of silicon enolates of β-keto esters. Tetrahedron 2018, 74, 5069–5084. [Google Scholar] [CrossRef]
- Galla, F.; Bourgeois, C.; Lehmkuhl, K.; Schepmann, D.; Soeberdt, M.; Lotts, T.; Abels, C.; Ständer, S.; Wünsch, B. Effects of polar κ receptor agonists designed for the periphery on ATP-induced Ca2+ release from keratinocytes. Med. Chem. Commun. 2016, 7, 317–326. [Google Scholar] [CrossRef]
- Börgel, F.; Galla, F.; Lehmkuhl, K.; Schepmann, D.; Ametamey, S.M.; Wünsch, B. Pharmacokinetic properties of enantiomerically pure GluN2B selective NMDA receptor antagonists with 3-benzazepine scaffold. J. Pharm. Biomed. Anal. 2019, 172, 214–222. [Google Scholar] [CrossRef]
- Falck, E.; Begrow, F.; Verspohl, E.; Wünsch, B. In vitro and in vivo biotransformation of WMS-1410, a potent GluN2B selective NMDA receptor antagonist. J. Pharm. Biomed. Anal. 2014, 94, 36–44. [Google Scholar] [CrossRef]
- Falck, E.; Begrow, F.; Verspohl, E.; Wünsch, B. Metabolism studies of ifenprodil, a potent GluN2B receptor antagonist. J. Pharm. Biomed. Anal. 2014, 88, 96–105. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Zheng, S.D.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human σ1 receptor. Nature 2016, 532, 527. [Google Scholar] [CrossRef]
- Massova, I.; Kollman, P.A. Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to predict ligand binding. Perspect. Drug Discov. 2000, 18, 113–135. [Google Scholar] [CrossRef]
- Kopp, N.; Holtschulte, C.; Börgel, F.; Lehmkuhl, K.; Friedland, K.; Civenni, G.; Laurini, E.; Catapano, C.V.; Pricl, S.; Humpf, H.-U.; et al. Novel σ1 antagonists designed for tumor therapy: Structure—Activity relationships of aminoethyl substituted cyclohexanes. Eur. J. Med. Chem. 2021, 210, 112950. [Google Scholar] [CrossRef]
- Holtschulte, C.; Börgel, F.; Westphälinger, S.; Schepmann, D.; Civenni, G.; Laurini, E.; Marson, D.; Catapano, C.V.; Pricl, S.; Wünsch, B. Synthesis of Aminoethyl-Substituted Piperidine Derivatives as σ1 Receptor Ligands with Antiproliferative Properties. ChemMedChem 2022, 17, e202100735. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.; Shi, H.; Cheng, D. Fluorine-18: Radiochemistry and Target-Specific PET Molecular Probes Design. Front. Chem. 2022, 10, 884517. [Google Scholar] [CrossRef]
- Rong, J.; Haider, A.; Jeppesen, T.E.; Josephson, L.; Liang, S.H. Radiochemistry for positron emission tomography. Nat. Commun. 2023, 14, 3257. [Google Scholar] [CrossRef]
- Maisonial-Besset, A.; Funke, U.; Wenzel, B.; Fischer, S.; Holl, K.; Wünsch, B.; Steinbach, J.; Brust, P. Automation of the radiosynthesis and purification procedures for [18F]Fluspidine preparation, a new radiotracer for clinical investigations in PET imaging of σ1 receptors in brain. Appl. Radiat. Isot. 2014, 84, 1–7. [Google Scholar] [CrossRef]
- Brust, P.; Deuther-Conrad, W.; Becker, G.; Patt, M.; Donat, C.K.; Stittsworth, S.; Fischer, S.; Hiller, A.; Wenzel, B.; Dukic-Stefanovic, S.; et al. Distinctive in vivo kinetics of the new σ1 receptor ligands (R)-(+)- and (S)-(−)-18F-fluspidine in porcine brain. J. Nucl. Med. 2014, 55, 1730–1736. [Google Scholar] [CrossRef]
- Wiese, C.; Maestrup, E.G.; Galla, F.; Schepmann, D.; Hiller, A.; Fischer, S.; Ludwig, F.-A.; Deuther-Conrad, W.; Donat, C.K.; Brust, P.; et al. Comparison of in silico, electrochemical, in vitro and in vivo metabolism of a homologous series of (radio)fluorinated σ1 receptor ligands designed for positron emission tomography. ChemMedChem 2016, 11, 2445–2458. [Google Scholar] [CrossRef]
- Baum, E.; Cai, Z.; Bois, F.; Holden, D.; Lin, S.-F.; Lara-Jaime, T.; Kapinos, M.; Chen, Y.; Deuther-Conrad, W.; Fischer, S.; et al. PET imaging evaluation of four σ1 radiotracers in nonhuman primates. J. Nucl. Med. 2017, 58, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.L.; Urani, A.; Sandillon, F.; Privat, A.; Maurice, T. Preserved sigma1 (sigma1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol. Aging. 2003, 24, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.; Fowler, J.S.; Volkow, N.D.; Wolf, A.P.; Dewey, S.L.; Schlyer, D.J.; MacGregor, R.R.; Hitzemann, R.; Bendriem, B.; Gatley, S.J.; et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990, 10, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Kranz, M.; Sattler, B.; Deuther-Conrad, W.; Fischer, S.; Hiller, A.; Donat, C.; Wünsch, B.; Steinbach, J.; Sabri, O.; Brust, P. Preclinical dose assessment of (S)-(−)-[18F]fluspidine and (R)-(+)-[18F]fluspidine, new PET tracers for imaging of σ1 receptors. J. Nucl. Med. 2013, 54 (Suppl. 2), 1028. [Google Scholar]
- Kranz, M.; Sattler, B.; Wüst, N.; Deuther-Conrad, W.; Patt, M.; Meyer, P.M.; Fischer, S.; Donat, C.K.; Wünsch, B.; Hesse, S.; et al. Evaluation of the Enantiomer Specific Biokinetics and Radiation Doses of [18F]Fluspidine-A New Tracer in Clinical Translation for Imaging of σ1 Receptors. Molecules 2016, 21, 1164. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, M.; Deuther-Conrad, W.; Kranz, M.; Fischer, S.; Ludwig, F.-A.; Juratli, T.A.; Patt, M.; Wünsch, B.; Schackert, G.; Sabri, O.; et al. Sigma-1 receptor Positron Emission Tomography: A new molecular imaging approach using (S)-(−)-[18F]fluspidine in glioblastoma. Molecules 2020, 25, 2170. [Google Scholar] [CrossRef] [PubMed]
- Sattler, B.; Kranz, M.; Wuest, N.; Patt, M.; Meyer, P.; Deuther-Conrad, W.; Fischer, S.; Wuensch, B.; Brust, P.; Sabri, O. First-in-man incorporation dosimetry of (S)-(−)-[18F]fluspidine. J. Nucl. Med. 2016, 57 (Suppl. 2), 1022. [Google Scholar]
- Wang, Y.M.; Xia, C.Y.; Jia, H.M.; He, J.; Lian, W.W.; Yan, Y.; Wang, W.P.; Zhang, W.K.; Xu, J.K. Sigma-1 receptor: A potential target for the development of antidepressants. Neurochem Int. 2022, 159, 105390. [Google Scholar] [CrossRef]
- Meyer, P.; Strauss, M.; Becker, G.; Hesse, S.; Bednasch, K.; Ettrich, B.; Zientek, F.; Rullmann, M.; Wilke, S.; Luthardt, J.; et al. Increased sigma-1 receptor (Sig-1R) binding in the brain of unmedicated patients with acute major depressive disorder (MDD) using the novel Sig-1R-specific radioligand (−)-[18F]Fluspidine and PET. J. Nucl. Med. 2018, 59 (Suppl. 1), 551. [Google Scholar]
- Guo, Y.; Zhang, C.; Chen, X.; Liu, X.; Ye, T.; Fo, Y.; Shi, S.; Qu, C.; Liang, J.; Shen, B.; et al. Sigma-1 receptor ligands improves ventricular repolarization-related ion remodeling in rats with major depression disorder. Psychopharmacology 2021, 238, 487–499. [Google Scholar] [CrossRef]
- Ludwig, F.-A.; Fischer, S.; Houska, R.; Hoepping, A.; Deuther-Conrad, W.; Schepmann, D.; Patt, M.; Meyer, P.M.; Hesse, S.; Becker, G.-A.; et al. In vitro and in vivo Human Metabolism of (S)-[18F]Fluspidine—A Radioligand for Imaging σ1 Receptors With Positron Emission Tomography (PET). Front. Pharmacol. 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.L. Experimental hypoglutamatergia model for cognitive deficits of schizophrenia: Effects of classical neuroleptics, 5-HT 2Areceptor blocking antipsychotics, the ampakine CX516 and the dopaminergic stabiliser ACR16. Eur. Neuropsychopharmacol. 2002, 12, 167–168. [Google Scholar] [CrossRef]
- A. Carlsson Research Ab. New Modulators of Dopamine Neurotransmission. WO/2001/046145; PCT/SE2000/002674, 4 October 2001. [Google Scholar]
- Carlsson, M.L.; Carlsson, A.; Nilsson, M. Schizophrenia: From dopamine to glutamate and back. Curr. Med. Chem. 2004, 11, 267–277. [Google Scholar] [CrossRef] [PubMed]
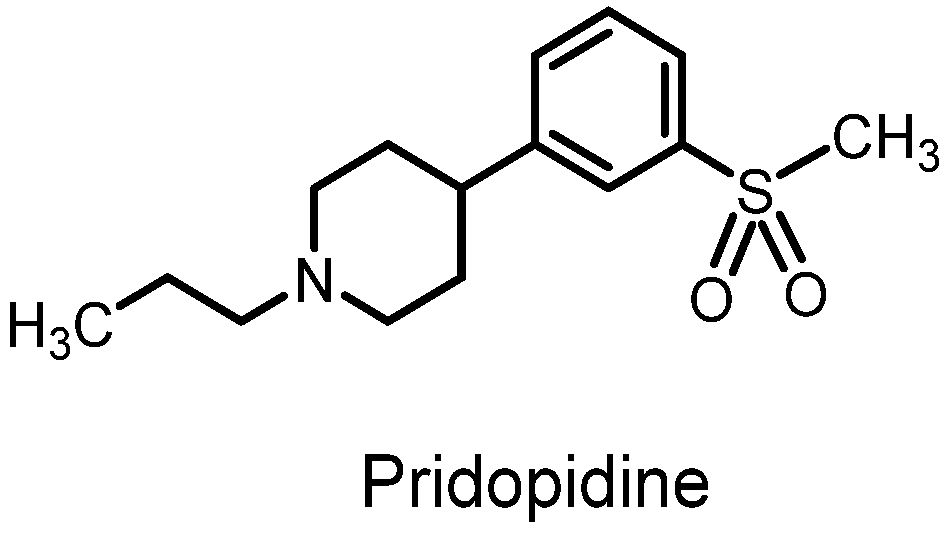
- Squitieri, F.; Di Pardo, A.; Favellato, M.; Amico, E.; Maglione, V.; Frati, L. Pridopidine, a dopamine stabilizer, improves motor performance and shows neuroprotective effects in Huntington disease R6/2 mouse model. J. Cell. Mol. Med. 2015, 19, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Sahlholm, K.; Sijbesma, J.W.; Maas, B.; Kwizera, C.; Marcellino, D.; Ramakrishnan, N.K.; Dierckx, R.A.; Elsinga, P.H.; van Waarde, A. Pridopidine selectively occupies sigma-1 rather than dopamine D2 receptors at behaviorally active doses. Psychopharmacology 2015, 232, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Dreymann, J.; Geva, M.; Ross, J.; Cha, Y.; Kusko, R.; Escalante-Chong, R.; Zeskind, B.; Laifenfeld, B.; Grossman, I.; Hayden, M. Loss of the Sigma-1 receptor disrupts pridopidine-induced gene expression. Neurology 2018, 90, P4.048. [Google Scholar] [CrossRef]
- Grachev, I.D.; Meyer, P.M.; Becker, G.A.; Bronzel, M.; Marsteller, D.; Pastino, G.; Voges, O.; Rabinovich, L.; Knebel, H.; Zientek, F.; et al. Sigma-1 and dopamine D2/D3 receptor occupancy of pridopidine in healthy volunteers and patients with Huntington disease: A [18F] fluspidine and [18F] fallypride PET study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Onufriev, A.; Bashford, D.; Case, D.A. Modification of the generalized born model suitable for macromolecules. J. Phys. Chem. 2000, 104, 3712–3720. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cisneros, G.A.; Cruzeiro, V.W.D.; Darden, T.A.; et al. Amber 2021; University of California: San Francisco, CA, USA, 2021. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]


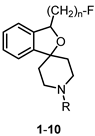 | |||||
|---|---|---|---|---|---|
| Compd. | n | R | Ki(σ1) (nM) | Ki(σ2) (nM) | σ1/σ2 Selectivity |
| 1 | 1 | -CH2C6H5 | 0.74 | 550 | 743 |
| 2 | 2 | -CH2C6H5 | 0.59 | 785 | 1331 |
| 3 | 3 | -CH2C6H5 | 1.4 | 837 | 598 |
| 4 | 4 | -CH2C6H5 | 1.2 | 489 | 408 |
| 5 | 2 | -CH2C6H4-p-F | 0.57 | 481 | 844 |
| 6 | 2 | -CH2C6H11 | 0.71 | 57 | 80 |
| 7 | 2 | -CH(CH3)C6H5 | 1.0 | >1 µM | >1000 |
| 8 | 2 | n-butyl | 3.9 | 878 | 225 |
| 9 | 2 | n-octyl | 15 | 118 | 7.9 |
| 10 | 2 | -CH2CH=C(CH3)2 | 1.5 | >1 µM | 667 |
 | ||||||
|---|---|---|---|---|---|---|
| Compd. | n | Ki(σ1) (nM) | logD7.4 (a) Micro Shake Flask | logD7.2 (b) PET Tracer | Plasma Protein Binding (%) | In Vitro Metabolism—Intact Compd. after 90 min (%) |
| 1 | 1 | 0.74 | 2.80 ± 0.04 | 2.39 ± 0.04 | 84 ± 0.5 | 53 ± 3.9 |
| 2 | 2 | 0.59 | 3.33 ± 0.07 | 2.57 ± 0.32 | 88 ± 0.4 | 34 ± 8.8 |
| 3 | 3 | 1.4 | 3.60 ± 0.12 | 2.78 ± 0.06 | 91 ± 0.4 | 33 ± 7.9 |
| 4 | 4 | 1.2 | 3.71 ± 0.14 | 3.11 ± 0.14 | 95 ± 0.3 | 28 ± 4.5 |
 | ||||||
|---|---|---|---|---|---|---|
| PET Tracer | Reaction Conditions (a) | RCYni (b) (%) | RCYfi (c) (%) | RCP (d) (%) | Am (e) (GBq/µmol) | Time (f) (min) |
| [18F]1 | DMSO, 150 °C, | 67–86 | 38–50 | >99.1 | 173–412 | 80–100 |
| (n = 1) | 10–15 min | (N = 4) | (N = 3) | |||
| [18F]2 | CH3CN, 85 °C, | 60–70 | 35–45 | >99.6 | 150–350 | 90–120 |
| (n = 2) | 25 min | (N = 10) | (N = 6) | |||
| [18F]3 | CH3CN, 85 °C, | 60–70 | 35–48 | >99.5 | 150–238 | 90–120 |
| (n = 3) | 30 min | (N = 11) | (N = 7) | |||
| [18F]4 | CH3CN, 83 °C, | 60–88 | 45–51 | >98.6 | 201–528 | 90–120 |
| (n = 4) | 20 min | (N = 8) | (N = 7) | |||
 | |||||
|---|---|---|---|---|---|
| PET Tracer | Reaction Conditions (a) | RCYfi (b) | RCP (c) | Am (d) (GBq/µmol) | Time (e) |
| (%) | (%) | (min) | |||
| [18F]2 | CH3CN, 85 °C, | 37 ± 8 | 99.4 ± 0.5 | 177 ± 52 | 59 ± 4 |
| 25 min | (N = 9) | (N = 11) | (N = 5) | (N = 8) | |
| (R)-[18F]2 | CH3CN, 85 °C, | 35–45 | >99 | 650–870 | 70 |
| 15 min | |||||
| (S)-[18F]2 | CH3CN, 85 °C, | 35–45 | >99 | 650–870 | 70 |
| 15 min | |||||
| Species | Parameter (Experimental Details) | Radiotracer | Brain Region (Values) |
|---|---|---|---|
| Mouse [32] | Ratio of activity concentration (target region vs. olfactory bulb at 45 min p.i.; N = 1) | rac-[18F]2 | Facial nucleus (4.69), cerebellum (1.75), superficial grey layer of superior colliculus (1.57), cortex (1.45), thalamus (1.24), hippocampus (1.21), striatum (1.11) |
| Piglet [52] | VT in mL/g (Logan plot; baseline conditions; N = 3) | (S)-[18F]2 | Midbrain (15.4), colliculi (15.2), cerebellum (14.8), thalamus (14.1), striatum (13.5), hippocampus (13.5), cortex (9.8–13.1) |
| (R)-[18F]2 | Cerebellum (140), midbrain (132), colliculi (123), thalamus (106), striatum (100), hippocampus (95.2), cortex (67.8–98.4) | ||
| Rhesus monkey [54] | VT in mL/g (1-Tissue compartment model; baseline conditions; N = 1) | (S)-[18F]2 | Cortex (14.6–19.6), hippocampus (16.4), putamen (14.9), amygdala (14.6), caudate (13.5), cerebellum (13.6), caudate (13.5), thalamus (12.2) |
| (R)-[18F]2 | Cortex (174–291), putamen (199), hippocampus (193), caudate (181), cerebellum (175), amygdala (153), thalamus (128) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludwig, F.-A.; Laurini, E.; Schmidt, J.; Pricl, S.; Deuther-Conrad, W.; Wünsch, B. [18F]Fluspidine—A PET Tracer for Imaging of σ1 Receptors in the Central Nervous System. Pharmaceuticals 2024, 17, 166. https://doi.org/10.3390/ph17020166
Ludwig F-A, Laurini E, Schmidt J, Pricl S, Deuther-Conrad W, Wünsch B. [18F]Fluspidine—A PET Tracer for Imaging of σ1 Receptors in the Central Nervous System. Pharmaceuticals. 2024; 17(2):166. https://doi.org/10.3390/ph17020166
Chicago/Turabian StyleLudwig, Friedrich-Alexander, Erik Laurini, Judith Schmidt, Sabrina Pricl, Winnie Deuther-Conrad, and Bernhard Wünsch. 2024. "[18F]Fluspidine—A PET Tracer for Imaging of σ1 Receptors in the Central Nervous System" Pharmaceuticals 17, no. 2: 166. https://doi.org/10.3390/ph17020166
APA StyleLudwig, F.-A., Laurini, E., Schmidt, J., Pricl, S., Deuther-Conrad, W., & Wünsch, B. (2024). [18F]Fluspidine—A PET Tracer for Imaging of σ1 Receptors in the Central Nervous System. Pharmaceuticals, 17(2), 166. https://doi.org/10.3390/ph17020166






