Application of LC-MS/MS for the Identification of Drugs of Abuse in Driver’s License Regranting Procedures
Abstract
1. Introduction
2. Results
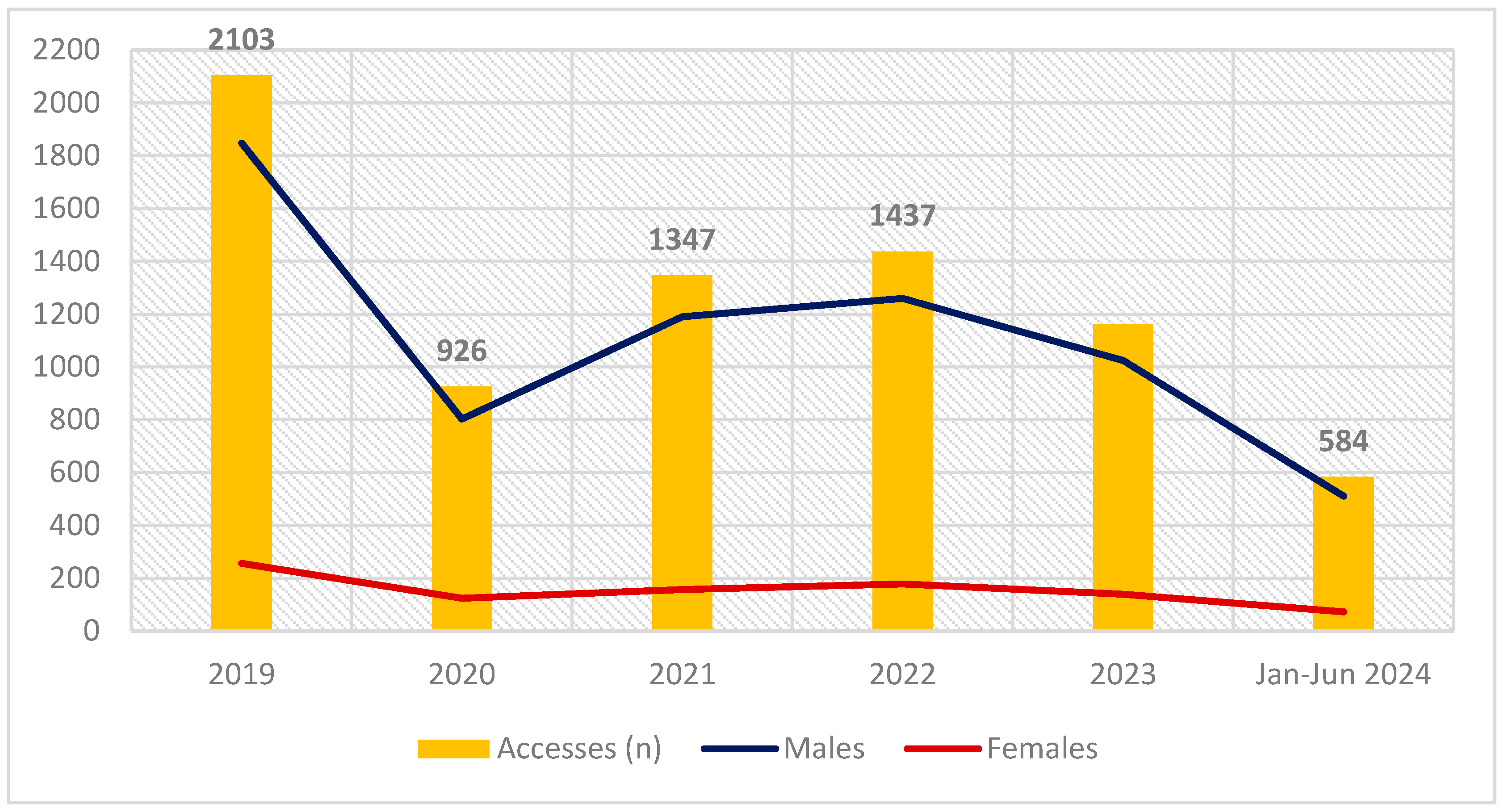
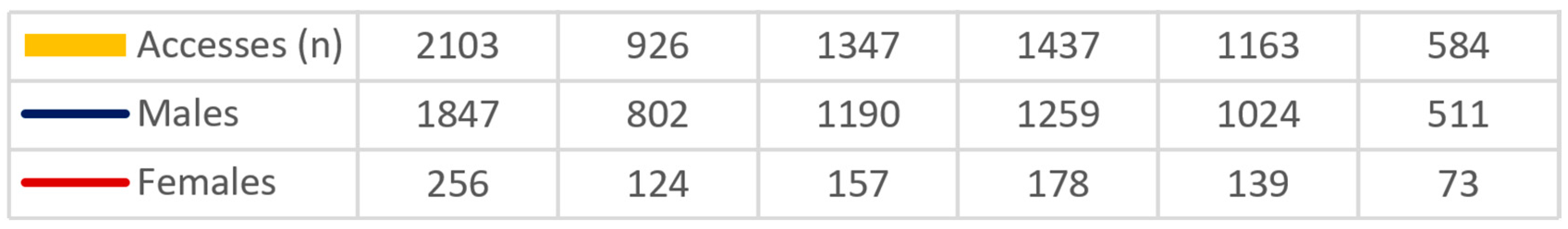
2.1. Epidemiological Data
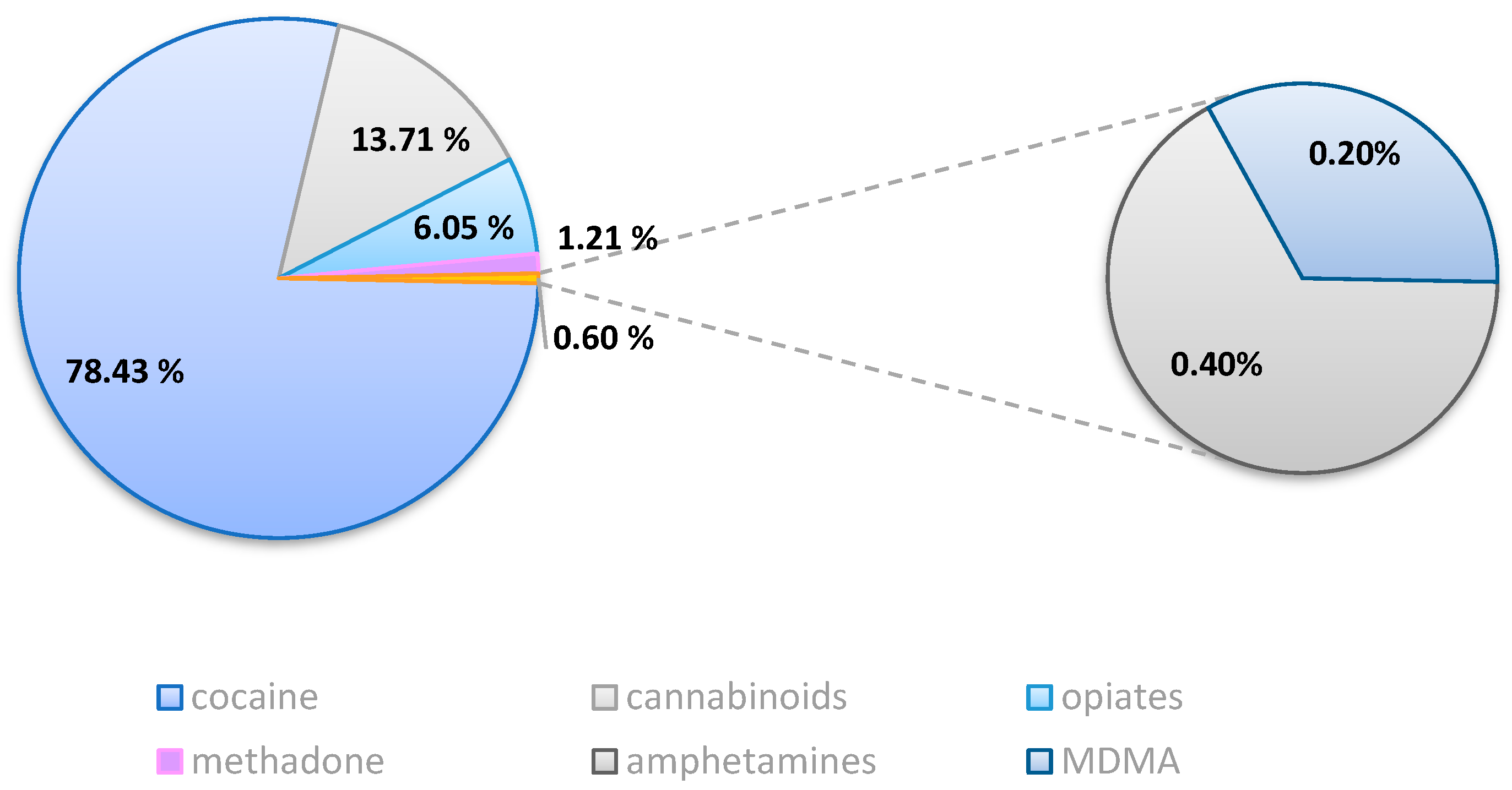
2.2. Toxicological Results
2.2.1. Group A
2.2.2. Group B
2.3. Hair Color Characteristics
3. Discussion
4. Materials and Methods
4.1. Hair Samples Collection
Hair Sample Treatment
4.2. UPLC-MS/MS Analysis
4.2.1. Calibrators and Quality Control Solutions
4.2.2. Instrumentation
4.2.3. Method Validation
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Global Status Report on Road Safety 2023. 2024. Available online: https://www.who.int/teams/social-determinants-of-health/safety-and-mobility/global-status-report-on-road-safety-2023 (accessed on 22 October 2024).
- World Health Organization (WHO). Road Traffic Injuries. Available online: https://www.who.int/news-room/fact-sheets/detail/road-traffic-injuries (accessed on 21 June 2021).
- United Nations on Drugs and Crime (UNODC). Current NPS Threats. October 2022. Available online: https://www.unodc.org/documents/scientific/Current_NPS_Threats_V.pdf (accessed on 22 October 2024).
- Italian National Statistics Institute (ISTAT). Road Accidents—Year 2022. 2023. Available online: https://www.istat.it/wp-content/uploads/2023/07/REPORT_INCIDENTI_STRADALI_2022_EN.pdf (accessed on 23 October 2024).
- D.P.R. 495/92, Regolamento di Esecuzione e di Attuazione del nuovo Codice della Strada. (GU n.303 del 28-12-1992—Suppl. Or-dinario n. 134). Available online: https://www.gazzettaufficiale.it/atto/vediMenuHTML?atto.dataPubblicazioneGazzetta=1992-12-28&atto.codiceRedazionale=092G0531&tipoSerie=serie_generale&tipoVigenza=originario (accessed on 22 October 2024).
- Tassoni, G.; Cippitelli, M.; Mietti, G.; Cerioni, A.; Buratti, E.; Bury, E.; Cingolani, M. Hair Analysis to Evaluate Polydrug Use. Healthcare 2021, 9, 972. [Google Scholar] [CrossRef]
- Pragst, F.; Balikova, M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 2006, 370, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Pascali, J.P.; Vaiano, F.; Palumbo, D.; Umani Ronchi, F.; Mari, F.; Bertol, E. Psychotropic substance abuse and fitness to hold a driving license in Italy. Traffic Inj. Prev. 2019, 20, 244–248. [Google Scholar] [CrossRef]
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Naseer, A.; Baig, Y.; Jamshaid, T.; Shahwar, M.; Khurshuid, S. Forensic toxicological analysis of hair: A review. Egypt. J. Forensic Sci. 2019, 9, 17. [Google Scholar] [CrossRef]
- Kintz, P.; Villain, M.; Cirimele, V. Hair Analysis for Drug Detection. Ther. Drug Monit. 2006, 28, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Favretto, D.; Cooper, G.; Andraus, M.; Sporkert, F.; Agius, R.; Appenzeller, B.; Baumgartner, M.; Binz, T.; Cirimele, V.; Kronstrand, R.; et al. The Society of Hair Testing consensus on general recommendations for hair testing and drugs of abuse testing in hair. Drug Test. Anal. 2023, 15, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, A.; Álvarez-Freire, I.; Bermejo-Barrera, A.; Cabarcos-Fernández, P.; Tabernero-Duque, M. Disappearance of codeine, morphine and 6-MAM in hair after cessation of abuse. Forensic Sci. Int. 2023, 352, 111855. [Google Scholar] [CrossRef] [PubMed]
- Mantinieks, D.; Gerostamoulos, D.; Wright, P.; Drummer, O. The effectiveness of decontamination procedures used in forensic hair analysis. Forensic Sci. Med. Pathol. 2018, 14, 349–357. [Google Scholar] [CrossRef]
- de Campos, E.G.; da Costa, B.R.B.; dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Santos Junior, W.J.; De Martinis, B.S. Alternative matrices in forensic toxicology: A critical review. Forensic Toxicol. 2021, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cone, E.J. Mechanisms of Drug Incorporation into Hair. Ther. Drug Monit. 1996, 18, 438–443. [Google Scholar] [CrossRef]
- Ferreira, C.; Paulino, C.; Quintas, A. Extraction Procedures for Hair Forensic Toxicological Analysis: A Mini-Review. Chem. Res. Toxicol. 2019, 32, 2367–2381. [Google Scholar] [CrossRef]
- Xiang, P.; Shen, M.; Drummer, O.H. Review: Drug concentrations in hair and their relevance in drug facilitated crimes. J. Forensic Leg. Med. 2015, 36, 126–135. [Google Scholar] [CrossRef]
- Rollins, D.E.; Wilkins, D.G.; Krueger, G.G.; Augsburger, M.P.; Mizuno, A.; O’Neal, C.; Borges, C.R.; Slawson, M.H. The Effect of Hair Color on the Incorporation of Codeine into Human Hair. J. Anal. Toxicol. 2003, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, T.; Kruger, M. Interpreting the color effect of melanin on cocaine and benzoylecgonine assays for hair analysis: Brown and black samples compared. J. Forensic Leg. Med. 2007, 14, 7–15. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of Mixed Melanogenesis—Pivotal Roles of Dopaquinone. Photochem. Photobiol. 2007, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.A.; Kronstrand, R.; Kintz, P. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci. Int. 2011, 218, 20–24. [Google Scholar] [CrossRef]
- Kuwayama, K.; Miyaguchi, H.; Kanamori, T.; Tsujikawa, K.; Yamamuro, T.; Segawa, H.; Okada, Y.; Iwata, Y.T. Micro-segmental hair analysis: Detailed procedures and applications in forensic toxicology. Forensic Toxicol. 2022, 40, 215–233. [Google Scholar] [CrossRef]
- European Union Drugs Agency (EUDA). European Drug Report 2024: Trends and Developments. 2024. Available online: https://www.euda.europa.eu/publications/european-drug-report/2024_en (accessed on 23 October 2024).
- United Nations Office on Drugs and Crime (UNODC). UNODC Research—Data Portal—Drug Use & Treatment. 2022. Available online: https://dataunodc.un.org/dp-drug-use-prevalence (accessed on 23 October 2024).
- Gómez-Talegón, T.; Fierro, I.; González-Luque, J.C.; Colás, M.; López-Rivadulla, M.; Álvarez, F.J. Prevalence of psychoactive substances, alcohol, illicit drugs, and medicines, in Spanish drivers: A roadside study. Forensic Sci. Int. 2012, 223, 106–113. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime (UNODC). Global Report on Cocaine 2023—Local Dynamics, Global Challenges. 2023. Available online: https://www.unodc.org/documents/data-and-analysis/cocaine/Global_cocaine_report_2023.pdf (accessed on 23 October 2024).
- Lukas, S.E.; Sholar, M.; Kouri, E.; Fukuzako, H.; Mendelson, J.H. Marihuana smoking increases plasma cocaine levels and subjective reports of euphoria in male volunteers. Pharmacol. Biochem. Behav. 1994, 48, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Strano Rossi, S.; Frison, G.; Chericoni, S.; Bertol, E.; Favretto, D.; Pichini, S.; Salomone, A.; Tagliaro, F.; Vignali, C. Linee guida per la determinazione di sostanze stupefacenti e psicotrope su campioni biologici con finalità tossicologico-forensi e medico-legali. Riv. Ital. Med. Lab. 2023, 19, 192–205. [Google Scholar] [CrossRef]
- Perry, F. Recommendations for Collecting Hair Samples for Toxicology. Available online: https://fflm.ac.uk/wp-content/uploads/2020/12/ARCHIVED-Recommendations-for-collecting-hair-samples-for-toxicology-Dr-F-Perry-June-2017.pdf (accessed on 22 October 2024).
- Peters, F.T.; Wissenbach, D.K.; Busardo, F.P.; Marchei, E.; Pichini, S. Method Development in Forensic Toxicology. Curr. Pharm. Des. 2018, 23, 5455–5467. [Google Scholar] [CrossRef]
- Wille, S.M.; Coucke, W.; De Baere, T.; Peters, F.T. Update of Standard Practices for New Method Validation in Forensic Toxicology. Curr. Pharm. Des. 2018, 23, 5442–5454. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; van den Wildenberg, W.P.M.; Hommel, B. Reduced Attentional Scope in Cocaine Polydrug Users. PLoS ONE 2009, 4, e6043. [Google Scholar] [CrossRef]

| Group | Target Analyte | Cut-Off (pg/mg) | Comments |
|---|---|---|---|
| Cannabinoids | Δ9-THC | 500 | Detection of THC-COOH strongly supports THC use/intake |
| CBD | |||
| Cocaine Group | Cocaine | 500 | The presence of BZE, NC, CE, hydroxyl-cocaine, or hydroxy-benzoylecgonine must be considered to confirm use. For crack cocaine use, anhydroecgonine methyl ester must be considered |
| Opiates | Morphine | 200 | Heroin consumption must be differentiated from codeine or morphine use by the presence of 6-acetylmorphine and/or heroin |
| Codeine | |||
| 6-MAM | |||
| Methadone | Methadone | 200 | Confirmation of EDDP definitively proves the use of methadone |
| Amphetamine Group | Amphetamine | 200 | |
| Methamphetamine | |||
| MDA | |||
| MDMA | |||
| MDEA |
| Substance | Frequency (n) |
|---|---|
| Cocaine+Δ9-THC | 24 |
| Cocaine + Codeine | 2 |
| Cocaine + MDMA | 1 |
| Δ9-THC + MDMA | 1 |
| MDMA + Cocaine + Δ9-THC | 1 |
| Hair Color | |||
|---|---|---|---|
| Substances | Dark | Gray | Light |
| Cocaine | 230 | 59 | 18 |
| Δ9-THC | 56 | 4 | 7 |
| Opiates | 17 | 7 | 2 |
| Amphetamine | 2 | ||
| Methadone | 1 | 2 | |
| MDMA | 2 | ||
| Analyte | Retention Time (min) | CV (V) | Quantifier MRM Transitions (m/z) | CE (eV) | Qualifier MRM Transition (m/z) | CE (eV) |
|---|---|---|---|---|---|---|
| Ecgonine Methyl Ester-d3 | 0.57 | 33.00 | 203.2 > 185.1 | 17.00 | – | – |
| Ecgonine Methyl Ester | 0.57 | 33.00 | 200.2 > 82.1 | 23.00 | 200.2 > 182.1 | 17.00 |
| Morphine-d3 | 0.60 | 35.00 | 289.2 > 61 | 28.00 | 289.2 > 201 | 40.00 |
| Morphine | 0.60 | 35.00 | 286 > 165.1 | 40.00 | 286 > 153 | 40.00 |
| Codeine-d3 | 0.88 | 30.00 | 303 > 215.1 | 25.00 | 303 > 61.1 | 27.00 |
| Codeine | 0.88 | 30.00 | 300.1 > 215.1 | 25.00 | 300.1 > 199.2 | 27.00 |
| Amphetamine-d6 | 1.14 | 20.00 | 150.1 > 91.1 | 12.00 | – | – |
| Amphetamine | 1.14 | 20.00 | 136.1 > 119.1 | 8.00 | 136.1 > 91.1 | 15.00 |
| Methamphetamine-d5 | 1.20 | 20.00 | 154.8 > 91.8 | 12.00 | 154.8 > 121.1 | 10.00 |
| Methamphetamine | 1.20 | 20.00 | 150.1 > 91.1 | 12.00 | 150.1 > 119.1 | 10.00 |
| MDA-d5 | 1.23 | 20.00 | 185.1 > 110 | 26.00 | 185.1 > 163.1 | 20.00 |
| MDA | 1.23 | 20.00 | 180.1 > 133.1 | 18.00 | 180.1 > 163.1 | 10.00 |
| 6-MAM-d3 | 1.25 | 30.00 | 331 > 61.1 | 30.00 | 331 > 195.1 | 36.00 |
| 6-MAM | 1.25 | 30.00 | 328.1 > 165.1 | 40.00 | 328.1 > 181.2 | 40.00 |
| MDMA-d5 | 1.28 | 20.00 | 199.1 > 165.1 | 12.00 | 199.1 > 135.2 | 20.00 |
| MDMA | 1.28 | 20.00 | 194.1 > 163 | 12.00 | 199.1 > 135.2 | 20.00 |
| MDEA-d5 | 1.51 | 20.00 | 213.1 > 163.1 | 14.00 | 213.1 > 105.1 | 26.00 |
| MDEA | 1.51 | 20.00 | 208.1 > 163.2 | 14.00 | 208.1 > 135.1 | 14.00 |
| Cocaine-d3 | 2.06 | 30.00 | 307 > 184.7 | 20.00 | 307 > 84.8 | 30.00 |
| Cocaine | 2.06 | 30.00 | 304.2 > 182.2 | 20.00 | 304.2 > 82.3 | 28.00 |
| Norcocaine-d3 | 2.36 | 35.00 | 304.2 > 182.2 | 20.00 | 304.2 > 82.3 | 28.00 |
| Norcocaine | 2.36 | 30.00 | 290.2 > 136.1 | 20.00 | 290.2 > 168.2 | 14.00 |
| Benzoylecgonine-d3 | 3.10 | 30.00 | 293.1 > 171.1 | 20.00 | 293.1 > 105.1 | 32.00 |
| Benzoylecgonine | 3.11 | 30.00 | 290.1 > 168.1 | 20.00 | 290.1 > 105.1 | 33.00 |
| EDDP-d3 | 3.31 | 30.00 | 281.3 > 235.1 | 30.00 | 281.3 > 250.2 | 22.00 |
| EDDP | 3.31 | 45.00 | 278.2 > 234.2 | 26.00 | 278.2 > 186.2 | 35.00 |
| Methadone-d3 | 4.11 | 30.00 | 313.3 > 268.2 | 14.00 | – | – |
| Methadone | 4.11 | 30.00 | 310.3 > 265.2 | 14.00 | 310.3 > 105.1 | 32.00 |
| CBD-d3 | 6.61 | 40.00 | 318.3 > 123.2 | 32.00 | 318.3 > 196.2 | 22.00 |
| CBD | 6.62 | 35.00 | 315.15 > 123.1 | 32.00 | 315.1 > 193.1 | 20.00 |
| CBN-d3 | 6.81 | 40.00 | 314.3 > 241.2 | 16.00 | 314.3 > 223.2 | 18.00 |
| CBN | 7.13 | 30.00 | 311.2 > 241 | 20.00 | 311.2 > 223 | 20.00 |
| Delta-9-THC-d3 | 7.35 | 35.00 | 318.2 > 123 | 34.00 | 318.2 > 196.1 | 22.00 |
| Delta-9-THC | 7.35 | 35.00 | 315.21 > 123 | 34.00 | 315.21 > 193.1 | 22.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tittarelli, R.; Stefani, L.; Romani, L.; Mineo, F.; Vernich, F.; Mannocchi, G.; Pellecchia, M.R.; Russo, C.; Marsella, L.T. Application of LC-MS/MS for the Identification of Drugs of Abuse in Driver’s License Regranting Procedures. Pharmaceuticals 2024, 17, 1728. https://doi.org/10.3390/ph17121728
Tittarelli R, Stefani L, Romani L, Mineo F, Vernich F, Mannocchi G, Pellecchia MR, Russo C, Marsella LT. Application of LC-MS/MS for the Identification of Drugs of Abuse in Driver’s License Regranting Procedures. Pharmaceuticals. 2024; 17(12):1728. https://doi.org/10.3390/ph17121728
Chicago/Turabian StyleTittarelli, Roberta, Lucrezia Stefani, Leonardo Romani, Federico Mineo, Francesca Vernich, Giulio Mannocchi, Maria Rosaria Pellecchia, Carmelo Russo, and Luigi Tonino Marsella. 2024. "Application of LC-MS/MS for the Identification of Drugs of Abuse in Driver’s License Regranting Procedures" Pharmaceuticals 17, no. 12: 1728. https://doi.org/10.3390/ph17121728
APA StyleTittarelli, R., Stefani, L., Romani, L., Mineo, F., Vernich, F., Mannocchi, G., Pellecchia, M. R., Russo, C., & Marsella, L. T. (2024). Application of LC-MS/MS for the Identification of Drugs of Abuse in Driver’s License Regranting Procedures. Pharmaceuticals, 17(12), 1728. https://doi.org/10.3390/ph17121728








