Evaluation of Hippocampal Microanatomy and Neuro-Biomarkers Following Administration of Silver Nanoparticles Conjugated with Tenofovir Disoproxil Fumarate in Experimental Diabetic Rats
Abstract
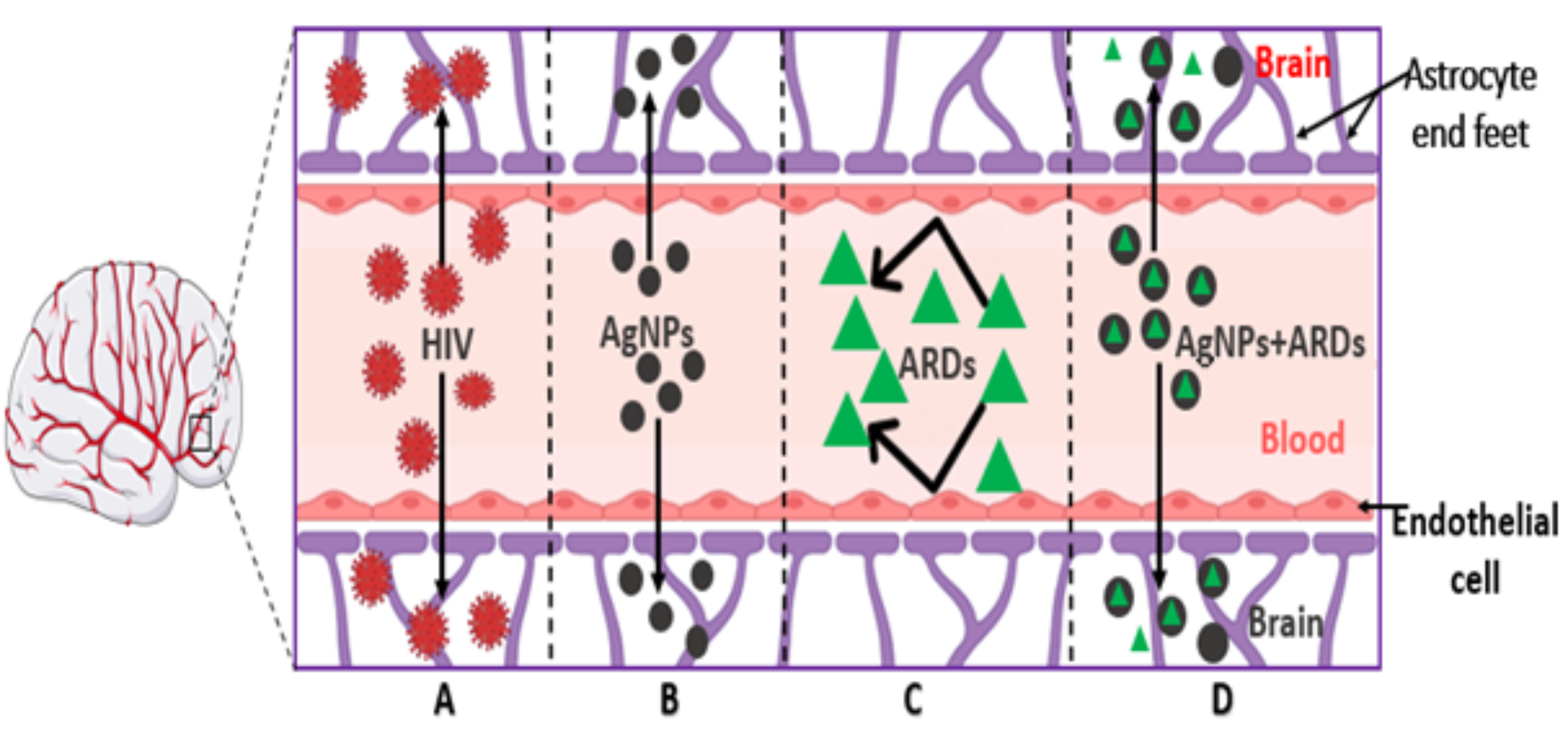
1. Introduction
2. Results
2.1. The Results of AgNPs-TDF Characterisation
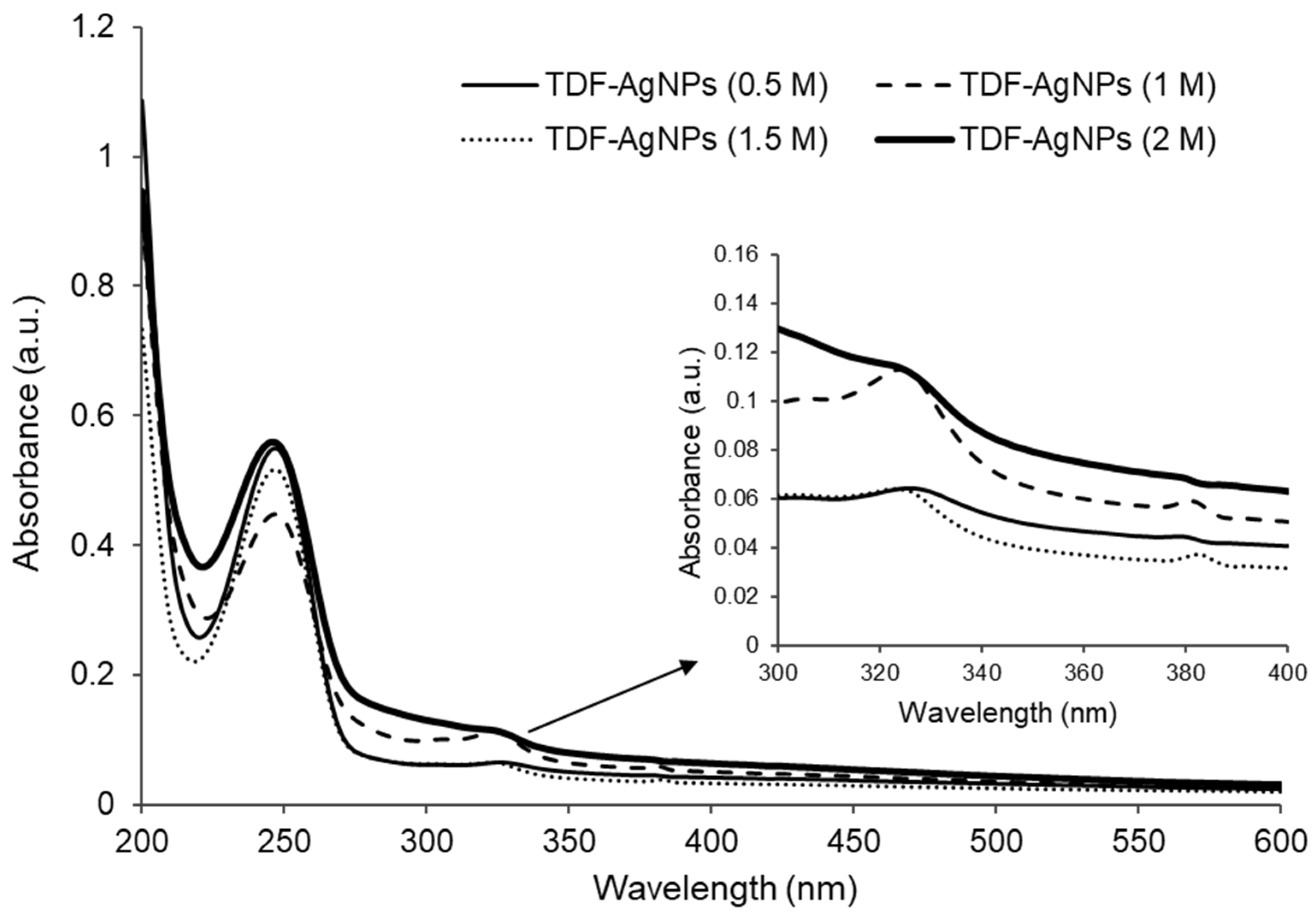
2.2. Ultraviolet–Visible Spectroscopy Analysis
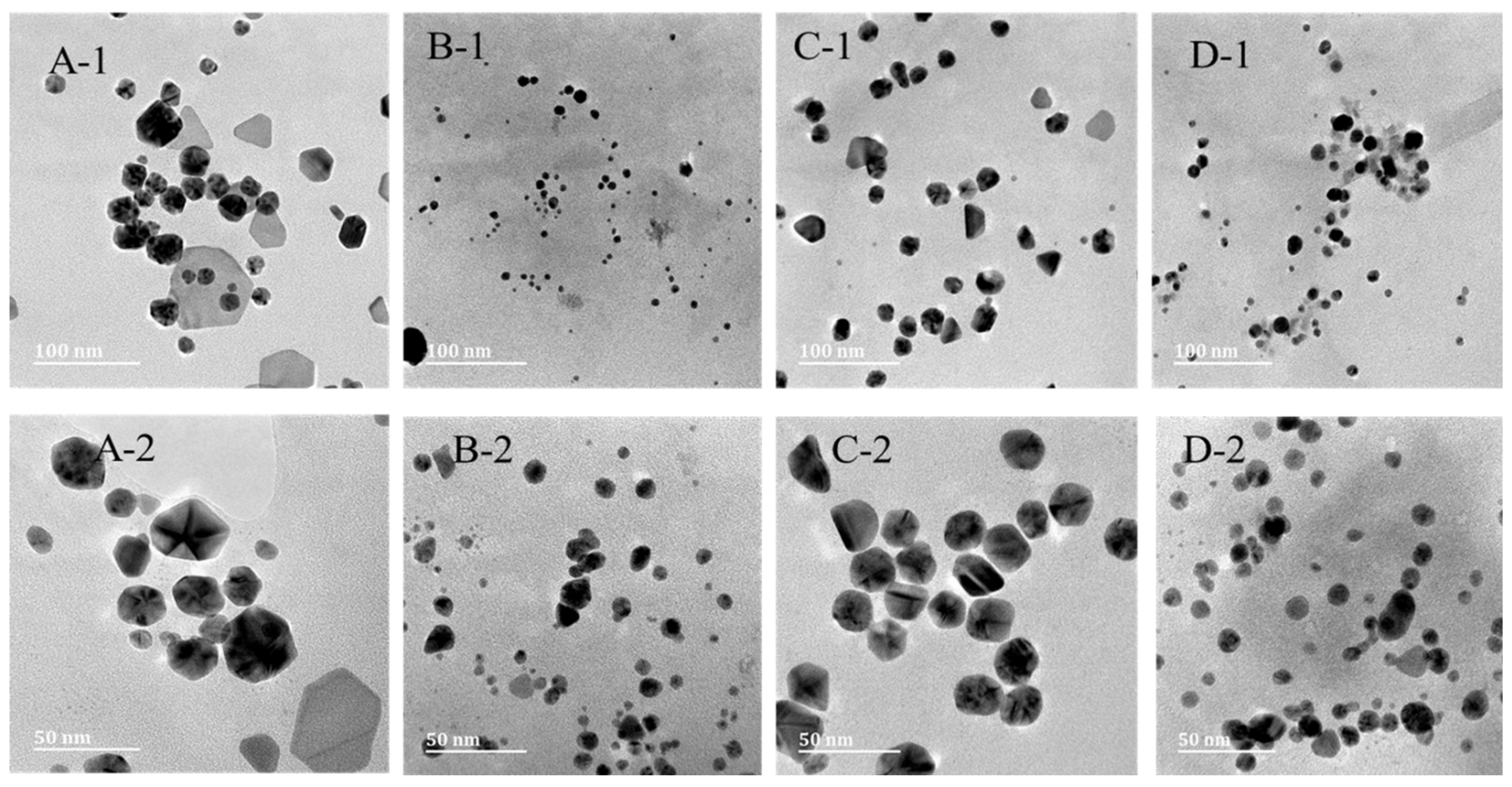
2.3. Transmission Electron Microscopy (TEM) Analysis
2.4. Energy Dispersive X-Ray (EDX) Analysis
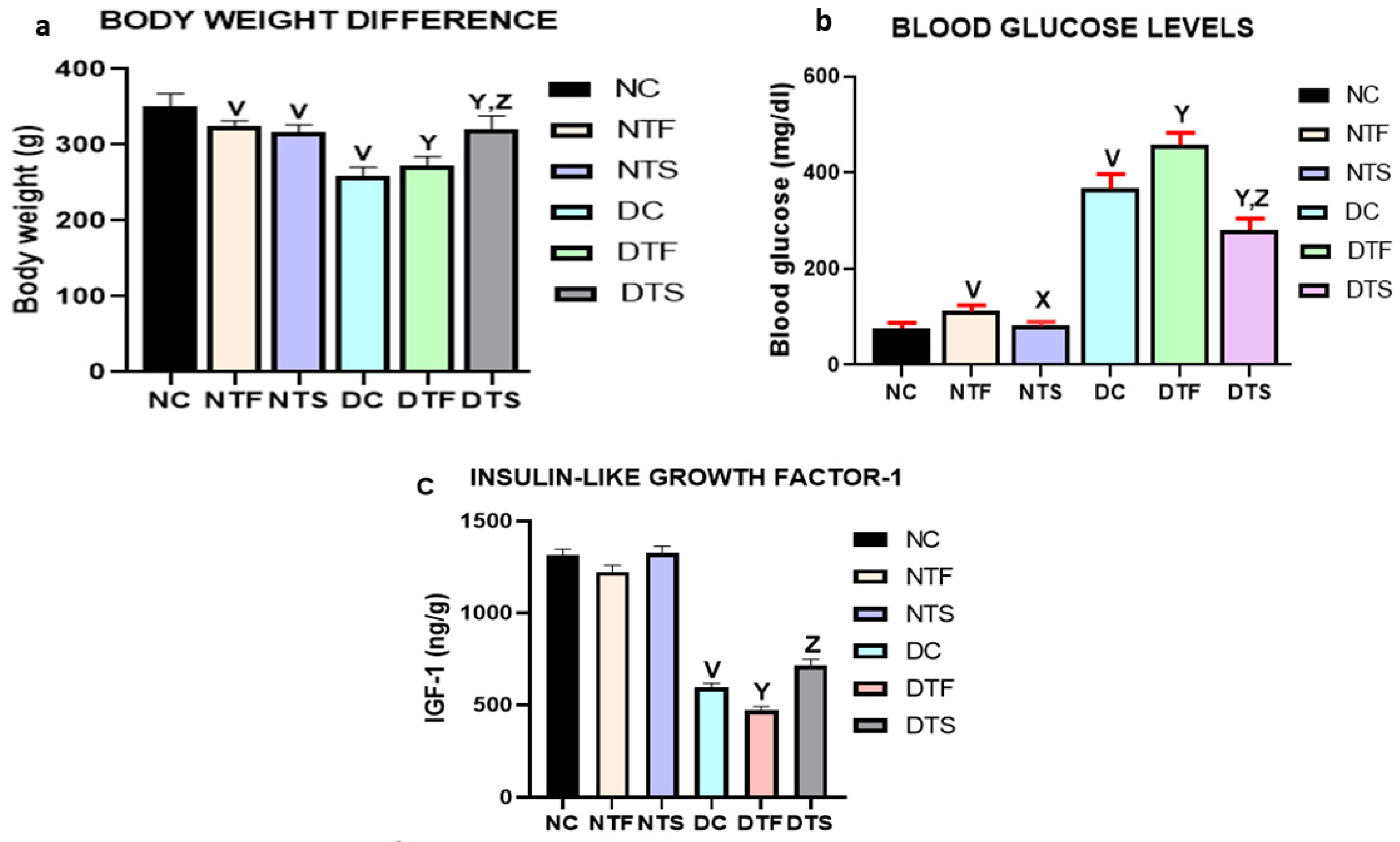
2.5. Effects of AgNPs-TDF on Bodyweight Difference and Blood Glucose Levels
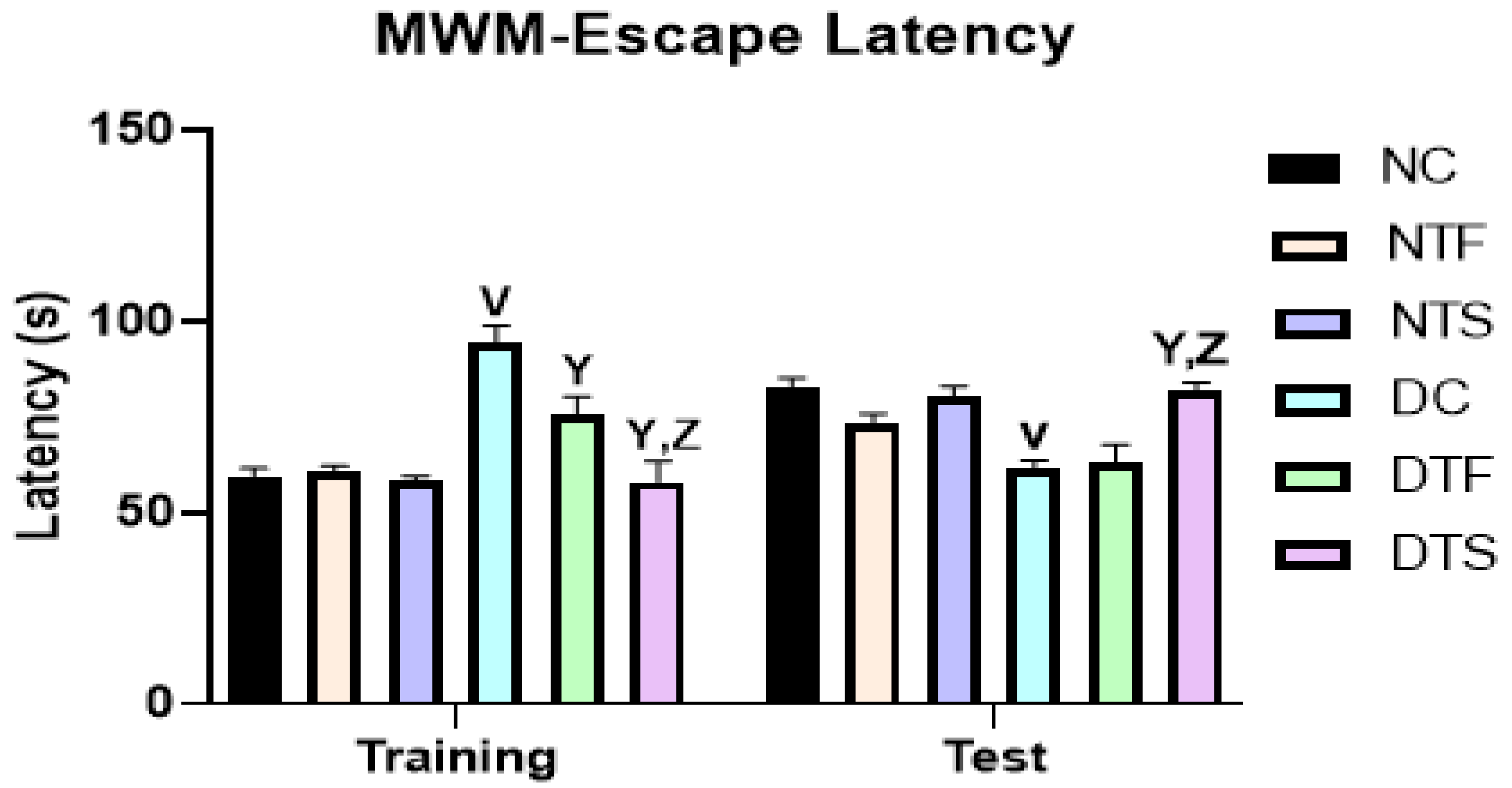
2.6. Effects of AgNPs-TDF on Spatial Working Memory
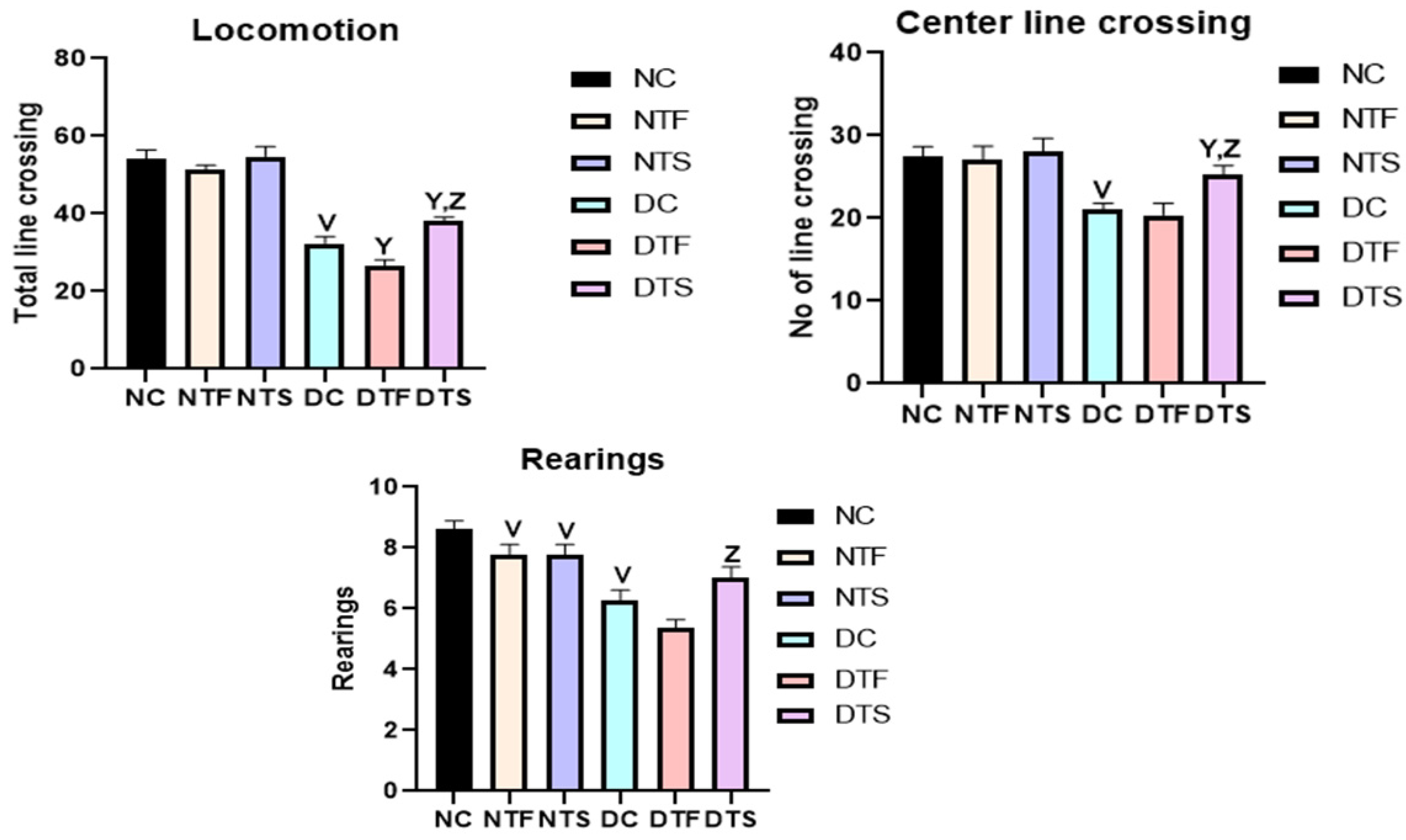
2.7. Effects of AgNPs-TDF on Locomotion and Explorative Behavior
2.8. Effects of AgNPs-TDF on Hippocampal Oxidative Stress Markers
2.9. Effects of AgNPs-TDF on Hippocampal Inflammatory Markers
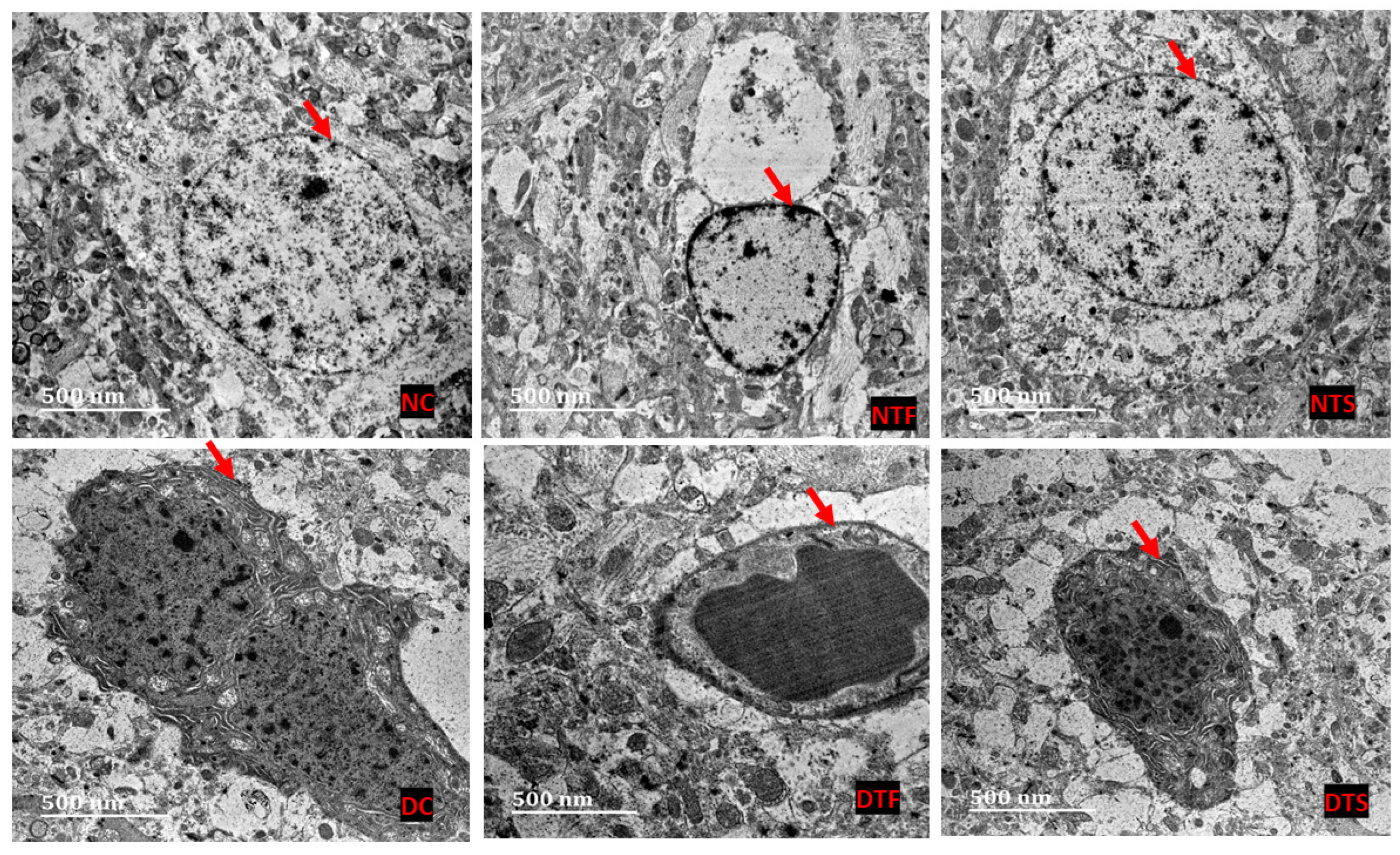
2.10. Effects of AgNPs-TDF on Hippocampal Ultrastructure—Neuronal Nuclear Membrane
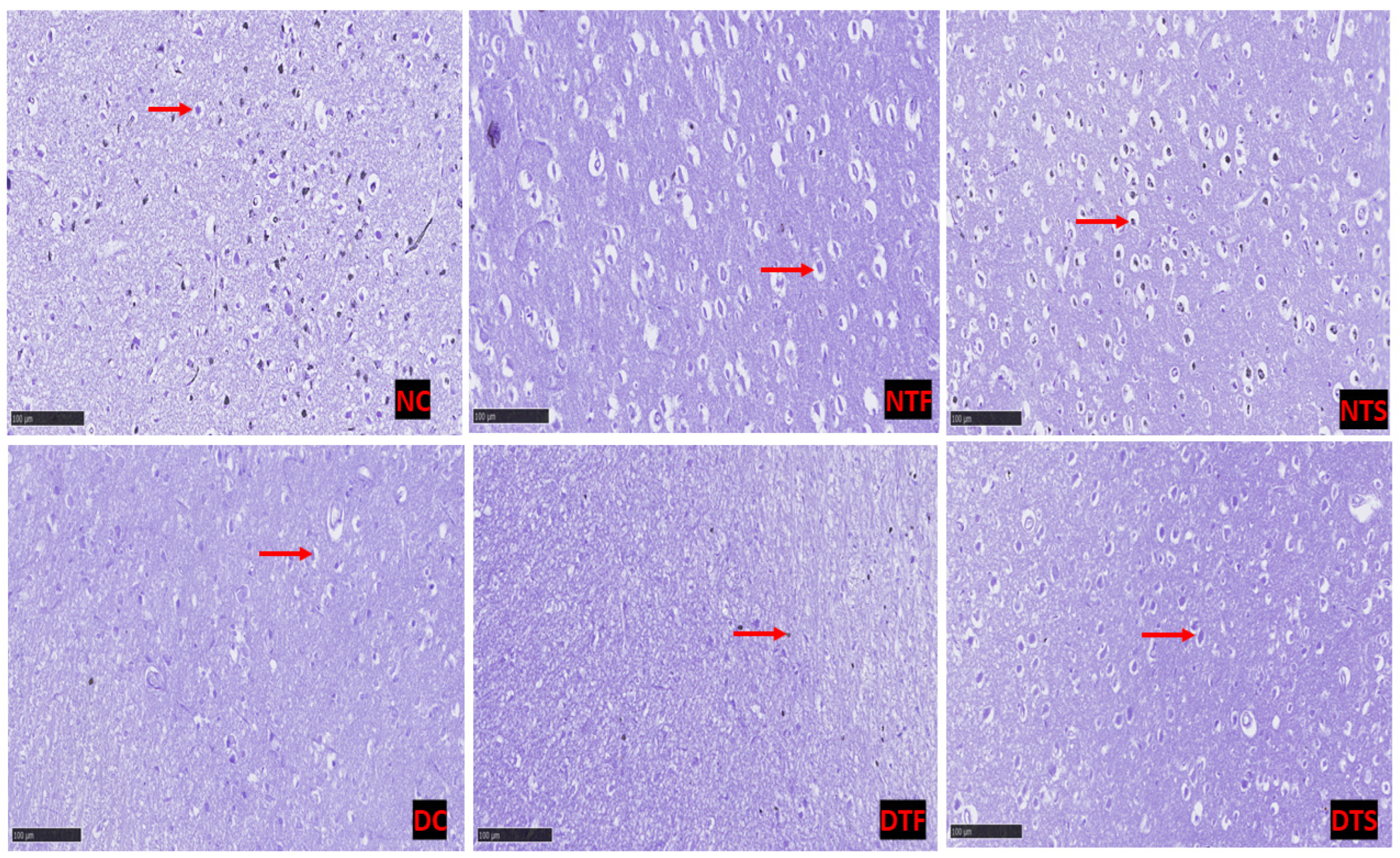
2.11. Effects of AgNPs-TDF on Hippocampal Neuronal Nissl Bodies
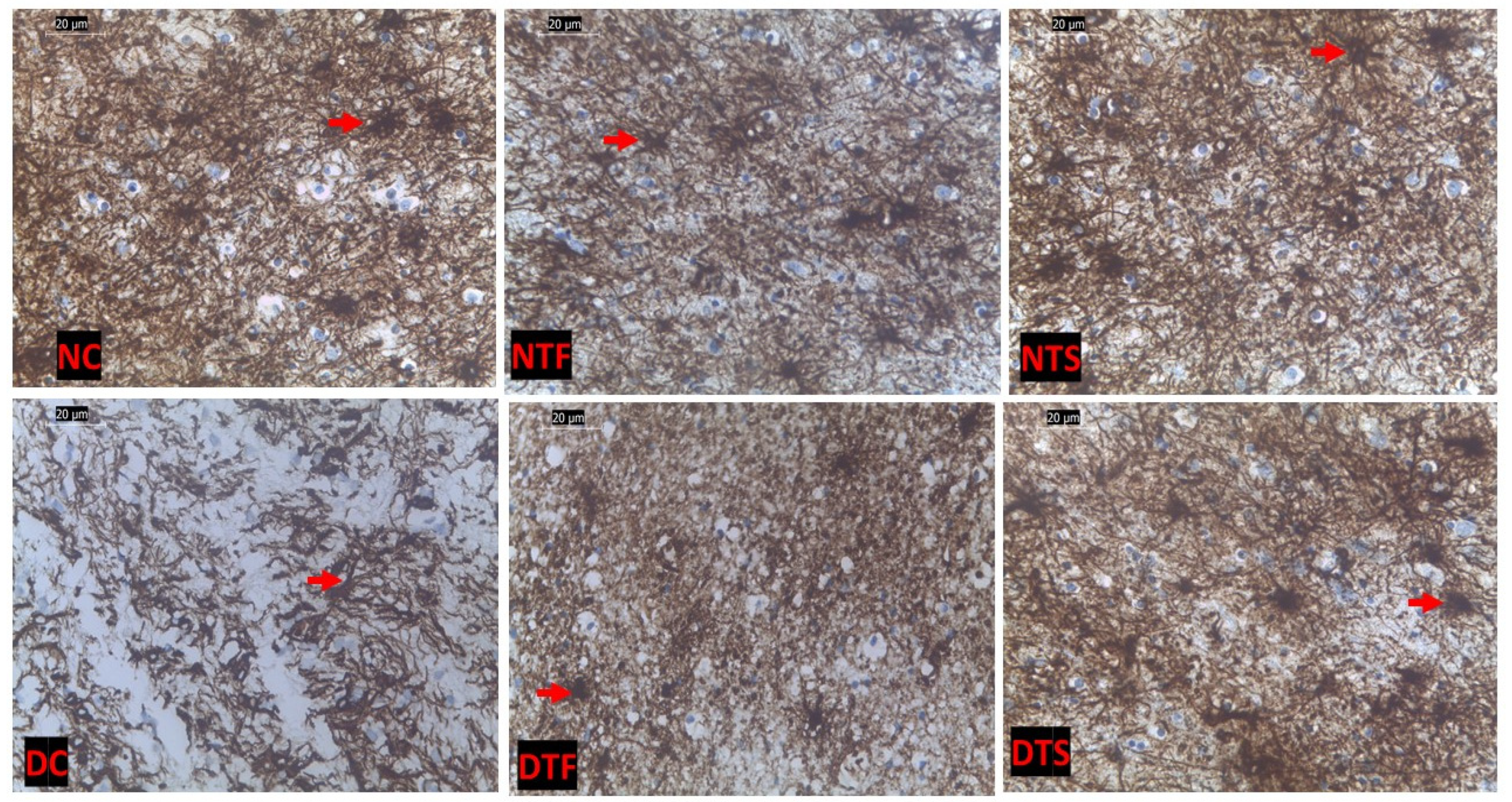
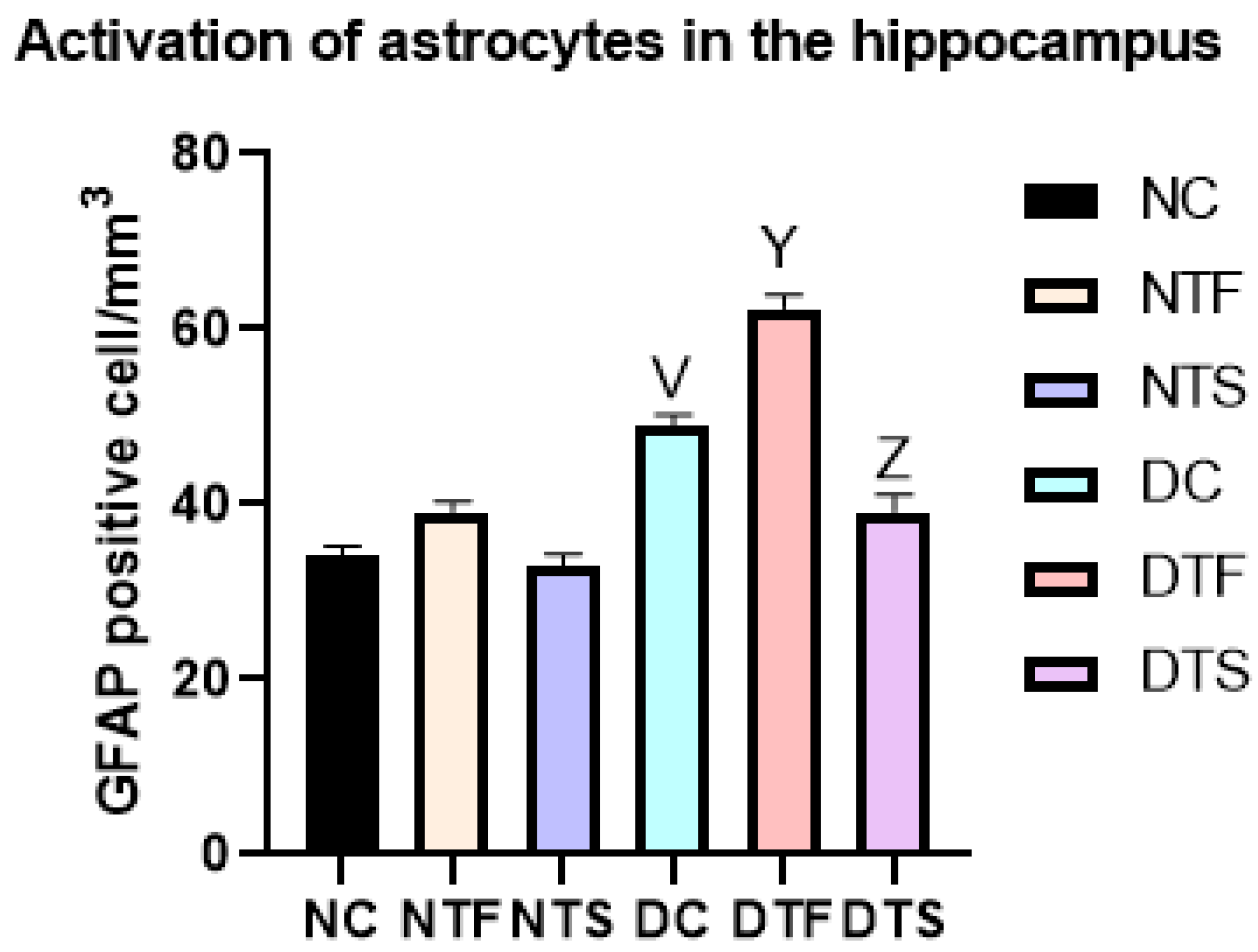
2.12. Effects of AgNPs-TDF on Hippocampal GFAP-Astrocytic Cells
2.13. Effects of AgNPs-TDF on Hippocampal Astrogliosis
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Animal
4.3. Ethical Approval
4.4. Experimental Design
4.5. Induction of Type II Diabetes Mellitus in Experimental Rats
4.5.1. Synthesis of AgNPs and Formulations of AgNPs-TDF
4.5.2. Characterization of AgNPs and AgNPs-TDF
4.6. Blood Glucose and Body Weight Difference Measurement
4.7. Behavioral Studies
4.7.1. Morris Water Maze for Spatial Working Memory
4.7.2. Open Field Test for Locomotion and Explorative Behavior
4.8. Euthanasia Procedure and Tissue Harvesting
4.9. Determination of Antioxidant Levels and Other Biochemical Analyses
4.10. Determination of Inflammatory Biomarkers
4.11. Histopathological and Immunohistological Studies
4.12. Transmission Electron Microscopic Analysis of Hippocampal Tissue
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, P.; Yoon, B.; Colby, D.; Kroon, E.; Sacdalan, C.; Sriplienchan, S.; Pinyakorn, S.; Ananworanich, J.; Valcour, V.; Vasan, S.; et al. Immunological, Cognitive, and Psychiatric Outcomes After Initiating Efavirenz- and Dolutegravir-based Antiretroviral Therapy During Acute Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2023, 76, e718–e726. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Eaton, J.W.; Mahy, M.; Sabin, K.; Autenrieth, C.S.; Wanyeki, I.; Daher, J.; Ghys, P.D. Global, regional and country-level 90–90–90 estimates for 2018: Assessing progress towards the 2020 target. Aids 2019, 33, S213–S226. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.A.; Swinton, M.K.; Carson, A.; Soontornniyomkij, B.; Lindsay, C.; Han, M.M.; Frizzi, K.; Sambhwani, S.; Murphy, A.; Achim, C.L.; et al. Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice. Sci. Rep. 2019, 9, 17158. [Google Scholar] [CrossRef]
- Petruccelli, K.C.S.; Baía-Da-Silva, D.C.; Val, F.; Valões, M.S.; Cubas-Vega, N.; Silva-Neto, A.V.; Sampaio, V.; Alencar, A.; Pecoits-Filho, R.; Moreira, R.C.; et al. Kidney function and daily emtricitabine/tenofovir disoproxil fumarate pre-exposure prophylaxis against HIV: Results from the real-life multicentric demonstrative project PrEP Brazil. AIDS Res. Ther. 2022, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Lanman, T.; Letendre, S.; Ma, Q.; Bang, A.; Ellis, R. CNS Neurotoxicity of Antiretrovirals. J. Neuroimmune Pharmacol. 2021, 16, 130–143. [Google Scholar] [CrossRef]
- Turner, D.; Drak, D.; O’connor, C.C.; Templeton, D.J.; Gracey, D.M. Renal function change after switching tenofovir disoproxil fumarate for tenofovir alafenamide in the HIV-positive patients of a metropolitan sexual health service. AIDS Res. Ther. 2019, 16, 40. [Google Scholar] [CrossRef]
- Patel, R.R.; Presti, R.; Harrison, L.C.; Powderly, W.G.; Chan, P.A. Tenofovir disoproxil fumarate as pre-exposure prophylaxis for HIV prevention in women with osteoporosis: A case report and review of the literature. Antivir. Ther. 2018, 23, 379–382. [Google Scholar] [CrossRef]
- Abdool Karim, S.S.; Baxter, C.; Abdool Karim, Q. Advancing HIV prevention using tenofovir-based pre-exposure prophylaxis. Antivir. Ther. 2022, 27, 13596535211067589. [Google Scholar] [CrossRef]
- Saberi, P.; Scott, H.M. On-Demand Oral Pre-exposure Prophylaxis with Tenofovir/Emtricitabine: What Every Clinician Needs to Know. J. Gen. Intern. Med. 2020, 35, 1285–1288. [Google Scholar] [CrossRef]
- Glidden, D.V.; Mulligan, K.; McMahan, V.; Anderson, P.L.; Guanira, J.; Chariyalertsak, S.; Buchbinder, S.P.; Bekker, L.-G.; Schechter, M.; Grinsztejn, B.; et al. Metabolic Effects of Preexposure Prophylaxis with Coformulated Tenofovir Disoproxil Fumarate and Emtricitabine. Clin. Infect. Dis. 2018, 67, 411–419. [Google Scholar] [CrossRef]
- Ozturk, N.B.; Akyuz, M.; Gulluoglu, M.; Akyuz, F. S3176° Tenofovir Disoproxil Fumarate-Related Metabolic Syndrome and Steatohepatitis in a Case of Chronic Hepatitis B. Am. J. Gastroenterol. 2022, 117, e2036. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, K.; Lan, Z.; Song, W.; Cheng, L.; Chi, W.; Chen, J.; Huo, Y.; Xu, L.; Liu, X.; et al. Tenofovir and adefovir down-regulate mitochondrial chaperone TRAP1 and succinate dehydrogenase subunit B to metabolically reprogram glucose metabolism and induce nephrotoxicity. Sci Rep 2017, 7, 46344. [Google Scholar] [CrossRef] [PubMed]
- Lawal, S.; Olojede, S.O.; Sulaiman, S.O.; Aladeyelu, O.S.; Moodley, R.; Naidu, E.C.S.; Rennie, C.O.; Azu, O.O. Tenofovir-silver nanoparticles conjugate ameliorates neurocognitive disorders and protects ultrastructural and cytoarchitectonic properties of the prefrontal cortex in diabetic rats. Bosn. J. Basic Med. Sci. 2022, 22, 569. [Google Scholar] [CrossRef] [PubMed]
- Best, B.M.; Letendre, S.L.; Koopmans, P.; Rossi, S.S.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Marra, C.M.; McArthur, J.C.; McCutchan, J.A.; et al. Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 59, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ntshangase, S.; Mdanda, S.; Naicker, T.; Kruger, H.G.; Baijnath, S.; Govender, T. Spatial distribution of elvitegravir and tenofovir in rat brain tissue: Application of matrix-assisted laser desorption/ionization mass spectrometry imaging and liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 33, 1643–1651. [Google Scholar] [CrossRef]
- Lawal, S.K.; Lawal, S.K.; Olojede, S.O.; Faborode, O.S.; Aladeyelu, O.S.; Matshipi, M.N.; Sulaiman, S.O.; Naidu, E.C.S.; Rennie, C.O.; Azu, O.O. Nanodelivery of antiretroviral drugs to nervous tissues. Front. Pharmacol. 2022, 3, 1025160. [Google Scholar] [CrossRef]
- Bowen, A.; Sweeney, E.E.; Fernandes, R. Nanoparticle-Based Immunoengineered Approaches for Combating HIV. Front. Immunol. 2020, 11, 789. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Alkaladi, A.; Abdelazim, A.M.; Afifi, M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef]
- Bedlovicova, Z.; Strapac, I.; Balaz, M.; Salayova, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Khashan, A.A.; Dawood, Y.; Khalaf, Y.H. Green chemistry and anti-inflammatory activity of silver nanoparticles using an aqueous curcumin extract. Results Chem. 2023, 5, 100913. [Google Scholar] [CrossRef]
- Olojede, S.O.; Lawal, S.K.; Mahlangeni, N.; Shelembe, B.; Matshipi, M.N.; Moodley, R.; Rennie, C.O.; Naidu, E.C.; Azu, O.O. Synthesis and characterization of a conjugate of silver nanoparticles loaded with tenofovir disoproxil fumarate. Next Nanotechnol. 2024, 5, 100058. [Google Scholar] [CrossRef]
- Menon, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour.-Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Kosuda, K.; Bingham, J.; Wustholz, K.; Van Duyne, R. Nanostructures and surfaceenhanced Raman spectroscopy. Handb. Nanoscale Opt. Electron. 2010, 309, 117–152. [Google Scholar]
- Uddin, A.R.; Siddique, M.A.; Rahman, F.; Ullah, A.A.; Khan, R. Cocos nucifera Leaf Extract Mediated Green Synthesis of Silver Nanoparticles for Enhanced Antibacterial Activity. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3305–3316. [Google Scholar] [CrossRef]
- Mondal, N.; Singh, Y. Development and validation of different spectrophotometric methods for estimation of tenofovir disoproxil fumarate from bulk drug and tablets. Int. J. Pharm. Sci. Res. 2014, 5, 623. [Google Scholar]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef]
- Yang, Z.; Huck, W.T.S.; Clarke, S.M.; Tajbakhsh, A.R.; Terentjev, E.M. Shape-memory nanoparticles from inherently non-spherical polymer colloids. Nat. Mater. 2005. 4, 486–490. [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. 2019, 4, 20170082. [Google Scholar] [CrossRef]
- Kanters, S.; Renaud, F.; Rangaraj, A.; Zhang, K.; Limbrick-Oldfield, E.; Hughes, M.; Ford, N.; Vitoria, M. Evidence synthesis evaluating body weight gain among people treating HIV with antiretroviral therapy—A systematic literature review and network meta-analysis. EClinicalMedicine 2022, 48, 101412. [Google Scholar] [CrossRef] [PubMed]
- Hocqueloux, L.; Menard, A.; Arvieux, C.; Joly, V.; Becker, A.; Chéret, A.; Duvivier, C.; Cabié, A.; Delpierre, C.; Allavena, C.; et al. Weight gain following the single substitution of tenofovir disoproxil fumarate by tenofovir alafenamide in HIV-infected people from the French Dat’AIDS cohort: A propensity score-matched analysis. HIV Med. 2023, 24, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.; Akpomiemie, G.; Chandiwana, N.; Sokhela, S.; Hill, A.; McCann, K.; Qavi, A.; Mirchandani, M.; Venter, W.D. Weight and Metabolic Changes After Switching from Tenofovir Alafenamide/Emtricitabine (FTC) + Dolutegravir (DTG), Tenofovir Disoproxil Fumarate (TDF)/FTC + DTG, and TDF/FTC/Efavirenz to TDF/Lamivudine/DTG. Clin. Infect. Dis. 2023, 76, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.; Bhatti, A.; John, P. Evaluation of Antidiabetic Activity of Biogenic Silver Nanoparticles Using Thymus serpyllum on Streptozotocin-Induced Diabetic BALB/c Mice. Polymers 2022, 14, 3138. [Google Scholar] [CrossRef] [PubMed]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Gluckman, P.D.; Feldman, E.L.; Werther, G.A. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr. Rev. 2005, 26, 916–943. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Biadgo, B.; Tamir, W.; Ambachew, S. Insulin-like Growth Factor and its Therapeutic Potential for Diabetes Complications—Mechanisms and Metabolic Links: A Review. Rev. Diabet. Stud. 2020, 16, 24–34. [Google Scholar] [CrossRef]
- Todorova, M.; Milusheva, M.; Kaynarova, L.; Georgieva, D.; Delchev, V.; Simeonova, S.; Pilicheva, B.; Nikolova, S. Drug-Loaded Silver Nanoparticles-A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment. Biomedicines 2023, 11, 1593. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.A.; Leo, B.F.; Ruenraroengsak, P.; Chen, S.; Goode, A.E.; Theodorou, I.G.; Chung, K.F.; Carzaniga, R.; Shaffer, M.S.P.; Dexter, D.T.; et al. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci. Rep. 2017, 7, 42871. [Google Scholar] [CrossRef]
- Olojede, S.O.; Lawal, S.K.; Dare, A.; Naidu, E.C.; Rennie, C.O.; Azu, O.O. Evaluation of tenofovir disoproxil fumarate loaded silver nanoparticle on testicular morphology in experimental type-2 diabetic rats. Artif. Cells Nanomed. Biotechnol. 2022, 50, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zulu, S.S.; Simola, N.; Mabandla, M.V.; Daniels, W.M. Effect of long-term administration of antiretroviral drugs (Tenofovir and Nevirapine) on neuroinflammation and neuroplasticity in mouse hippocampi. J. Chem. Neuroanat. 2018, 94, 86–92. [Google Scholar] [CrossRef]
- Khawaja, A.A.; Taylor, K.A.; Lovell, A.O.; Nelson, M.; Gazzard, B.; Boffito, M.; Emerson, M. HIV Antivirals Affect Endothelial Activation and Endothelial-Platelet Crosstalk. Circ. Res. 2020, 127, 1365–1380. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.-K.; Rai, V.R.; Ramachandra, Y. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Bold, B.E.; Urnukhsaikhan, E.; Mishig-Ochir, T. Biosynthesis of silver nanoparticles with antibacterial, antioxidant, anti-inflammatory properties and their burn wound healing efficacy. Front. Chem. 2022, 10, 972534. [Google Scholar] [CrossRef] [PubMed]
- Fehaid, A.; Fujii, R.; Sato, T.; Taniguchi, A. Silver Nanoparticles Affect the Inflammatory Response in a Lung Epithelial Cell Line. Open Biotechnol. J. 2020, 14, 113–123. [Google Scholar] [CrossRef]
- Foghi, K.; Ahmadpour, S. Diabetes mellitus type1 and neuronal degeneration in ventral and dorsal hippocampus. Iran. J. Pathol. 2014, 9, 33–37. [Google Scholar]
- Piotrowski, P.; Wierzbicka, K.; Smialek, M. Neuronal death in the rat hippocampus in experimental diabetes and cerebral ischaemia treated with antioxidants. Folia Neuropathol. 2001, 39, 147–154. [Google Scholar]
- Nagayach, A.; Patro, N.; Patro, I. Astrocytic and microglial response in experimentally induced diabetic rat brain. Metab. Brain Dis. 2014, 29, 747–761. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010, 41, 242–247. [Google Scholar] [CrossRef]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical modifications in the hippocampus, cortex and hypothalamus of mice exposed to long-term high-fat diet. Front. Neurosci. 2019, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, Y.; Qin, Z.; Qiu, L.; Zhang, M.; Huang, Y.; Zheng, J.C. Combined medication of antiretroviral drugs tenofovir disoproxil fumarate, emtricitabine, and raltegravir reduces neural progenitor cell proliferation in vivo and in vitro. J. Neuroimmune Pharmacol. 2017, 12, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Akay, C.; Cooper, M.; Odeleye, A.; Jensen, B.K.; White, M.G.; Vassoler, F.; Gannon, P.J.; Mankowski, J.; Dorsey, J.L.; Buch, A.M.; et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J. Neurovirology 2014, 20, 39–53. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Lawal, S.K.; Olojede, S.O.; Dare, A.; Faborode, O.S.; Naidu, E.C.S.; Rennie, C.O.; Azu, O.O. Silver nanoparticles conjugate attenuates highly active antiretroviral therapy-induced hippocampal nissl substance and cognitive deficits in diabetic rats. J. Diabetes Res. 2021, 2021, 2118538. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Leung, L.Y.; Hardy, W.; Mason, M.; Yang, K.H.; King, A.I. Up-regulation of reactivity and survival genes in astrocytes after exposure to short duration overpressure. Neurosci. Lett. 2008, 434, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Sajja, V.S.; Hlavac, N.; VandeVord, P.J. Role of glia in memory deficits following traumatic brain injury: Biomarkers of glia dysfunction. Front. Integr. Neurosci. 2016, 10, 7. [Google Scholar] [CrossRef]
- Powers, C.M. Developmental Neurotoxicity of Silver and Silver Nanoparticles Modeled In Vitro and In Vivo. Ph.D. Thesis, Duke University, Durham, NC, USA, 2010. [Google Scholar]
- Yin, N.; Yao, X.; Zhou, Q.; Faiola, F.; Jiang, G. Vitamin E attenuates silver nanoparticle-induced effects on body weight and neurotoxicity in rats. Biochem. Biophys. Res. Commun. 2015, 458, 405–410. [Google Scholar] [CrossRef][Green Version]
- Skalska, J.; Strużyńska, L. Toxic effects of silver nanoparticles in mammals–does a risk of neurotoxicity exist? Folia Neuropathol. 2015, 53, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Strużyńska, L.; Skalska, J. Mechanisms underlying neurotoxicity of silver nanoparticles. Cell. Mol. Toxicol. Nanoparticles 2018, 1048, 227–250. [Google Scholar]
- Suthar, J.K.; Vaidya, A.; Ravindran, S. Toxic implications of silver nanoparticles on the central nervous system: A systematic literature review. J. Appl. Toxicol. 2023, 43, 4–21. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Ren. Fail. 2014, 36, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Alqahtani, A.A.; Alotaibi, A.F.; Abdulla, A.M.; Bukelly, A.T.; Alsobyani, F.M.; Alharbi, G.H.; Alkiyumi, I.S.; Aldawish, M.M.; Alshahrani, T.F.; et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice. Int. J. Environ. Res. Public Health 2019, 16, 148. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Eilam, D. Open-field behavior withstands drastic changes in arena size. Behav. Brain Res. 2003, 142, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bădescu, S.; Tătaru, C.; Kobylinska, L.; Georgescu, E.; Zahiu, D.; Zăgrean, A.; Zăgrean, L. Effects of caffeine on locomotor activity in streptozotocin-induced diabetic rats. J. Med. Life 2016, 9, 275. [Google Scholar]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behaviour recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Biancotti, J.C.; Kumar, S.; De Vellis, J.; Drapeau, V.; Després, J.P.; Bouchard, C.; Allard, L.; Fournier, G.; Leblanc, C.; Bancroft, J.D.; et al. Theory and Practice of Histological Techniques, Edinburgh: Churchill Livingstone. Cogn. Impair. Sleep-Deprived Ovariectomized (OVX) Female Rats Volunt. Exerc. 2020, 11, 2615–2628. [Google Scholar]
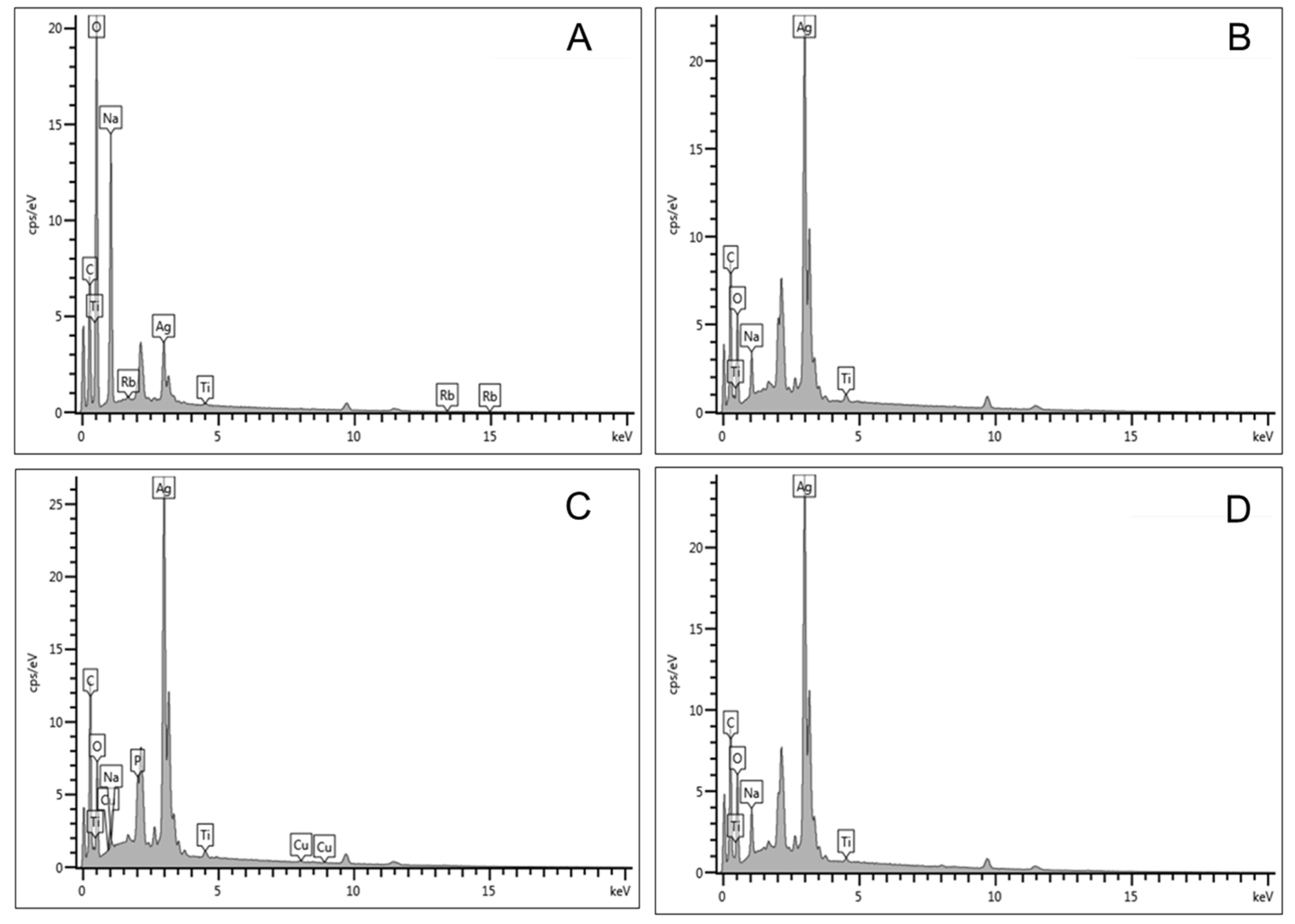
| Elements | Sample 0.5 M (Wt%) | Sample 1.0 M (Wt%) | Sample 1.5 M (Wt%) | Sample 2.0 M (Wt%) |
|---|---|---|---|---|
| C | 30.64 | 25.32 | 30.76 | 25.51 |
| O | 49.76 | 27.63 | 27.51 | 27.76 |
| Na | 13.95 | 3.40 | 0.47 | 3.69 |
| Ti | 0.09 | 0.62 | 0.52 | 0.34 |
| Ag | 5.45 | 43.03 | 38.59 | 42.70 |
| Rb | 0.11 | 00.00 | 00.00 | 0.00 |
| Cu | 00.00 | 00.00 | 0.30 | 0.00 |
| P | 00.00 | 00.00 | 1.85 | 0.00 |
| Total | 100 | 100 | 100 | 100 |
| Hippocampal Oxidative Markers | Animal Grouping | |||||
|---|---|---|---|---|---|---|
| NC | NTF | NTS | DC | DTF | DTS | |
| MDA (nmol/mg) | 30 ± 1.34 | 41.42 ± 1.57 V | 30.46 ± 1.71 X | 65.73 ± 1.79 V | 72.64 ± 1.47 Y | 45.30 ± 0.97 Y,Z |
| SOD (u/mg) | 10.06 ± 0.23 | 9.26 ± 0.22 | 9.19 ± 0.39 | 4.87 ± 0.17 V | 3.54 ± 0.32 Y | 6.29 ± 0.18 Y,Z |
| CAT (nmol/mg) | 16.50 ± 0.70 | 12.83 ± 0.78 | 14.12 ± 0.56 X | 7.51 ± 0.36 V | 7.70 ± 0.59 | 9.93 ± 0.42 Y |
| GSH (nmol/mg) | 91.26 ± 3.12 | 76.38 ± 3.92 | 86.23 ± 3.30 V | 49.02 ± 1.77 V | 37.34 ± 1.03 Y | 62.85 ± 2.16 Y,Z |
| Groups/Parameters | TNF-α (pg/mL) | IL-1β (pg/mL) (n = 5) |
|---|---|---|
| NC | 185.4 ± 4.71 | 35.19 ± 1.00 |
| NTF | 234.50 ± 6.29 V | 43.48 ± 2.83 |
| NTS | 204.60 ± 6.09 | 40.55 ± 2.52 |
| DC | 339.90 ± 13.85 V | 72.84 ± 1.98 V |
| DTF | 412.30 ± 14.14 Y | 88.96 ± 1.76 Y |
| DTS | 309.50 ± 4.908 Y,Z | 55.13 ± 1.53 Y,Z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawal, S.K.; Olojede, S.O.; Alabi, B.A.; Dithole, K.S.; Matula, S.T.; Naidu, E.C.; Rennie, C.O.; Azu, O.O. Evaluation of Hippocampal Microanatomy and Neuro-Biomarkers Following Administration of Silver Nanoparticles Conjugated with Tenofovir Disoproxil Fumarate in Experimental Diabetic Rats. Pharmaceuticals 2024, 17, 1635. https://doi.org/10.3390/ph17121635
Lawal SK, Olojede SO, Alabi BA, Dithole KS, Matula ST, Naidu EC, Rennie CO, Azu OO. Evaluation of Hippocampal Microanatomy and Neuro-Biomarkers Following Administration of Silver Nanoparticles Conjugated with Tenofovir Disoproxil Fumarate in Experimental Diabetic Rats. Pharmaceuticals. 2024; 17(12):1635. https://doi.org/10.3390/ph17121635
Chicago/Turabian StyleLawal, Sodiq Kolawole, Samuel Oluwaseun Olojede, Babatunde Adebola Alabi, Kafalotse Sylvia Dithole, Samuel Thopho Matula, Edwin Coleridge Naidu, Carmen Olivia Rennie, and Onyemaechi Okpara Azu. 2024. "Evaluation of Hippocampal Microanatomy and Neuro-Biomarkers Following Administration of Silver Nanoparticles Conjugated with Tenofovir Disoproxil Fumarate in Experimental Diabetic Rats" Pharmaceuticals 17, no. 12: 1635. https://doi.org/10.3390/ph17121635
APA StyleLawal, S. K., Olojede, S. O., Alabi, B. A., Dithole, K. S., Matula, S. T., Naidu, E. C., Rennie, C. O., & Azu, O. O. (2024). Evaluation of Hippocampal Microanatomy and Neuro-Biomarkers Following Administration of Silver Nanoparticles Conjugated with Tenofovir Disoproxil Fumarate in Experimental Diabetic Rats. Pharmaceuticals, 17(12), 1635. https://doi.org/10.3390/ph17121635







