Natural Deep Eutectic Solvents Combined with Supercritical Carbon Dioxide for the Extraction of Curcuminoids from Turmeric
Abstract
1. Introduction
2. Results
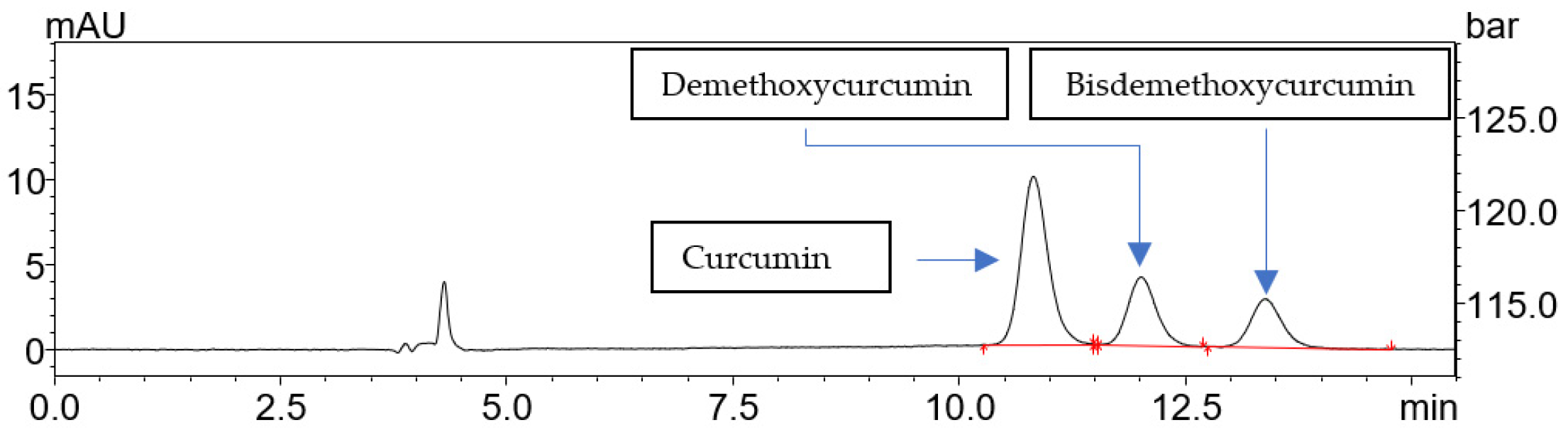
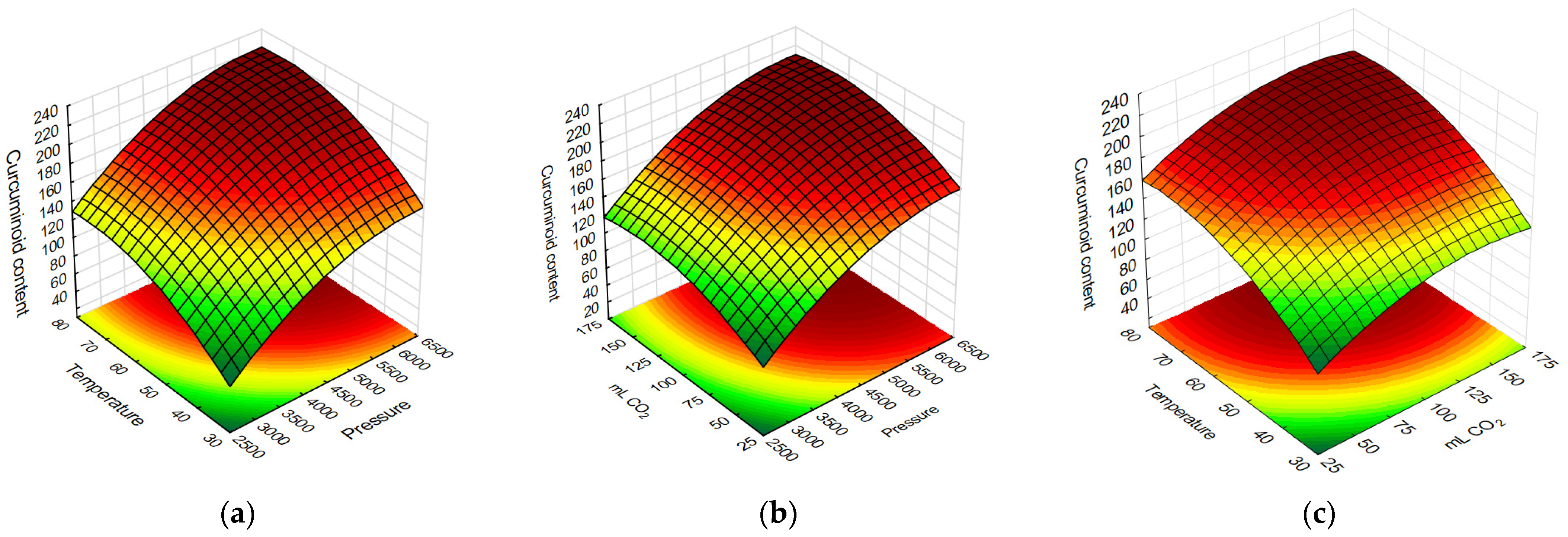
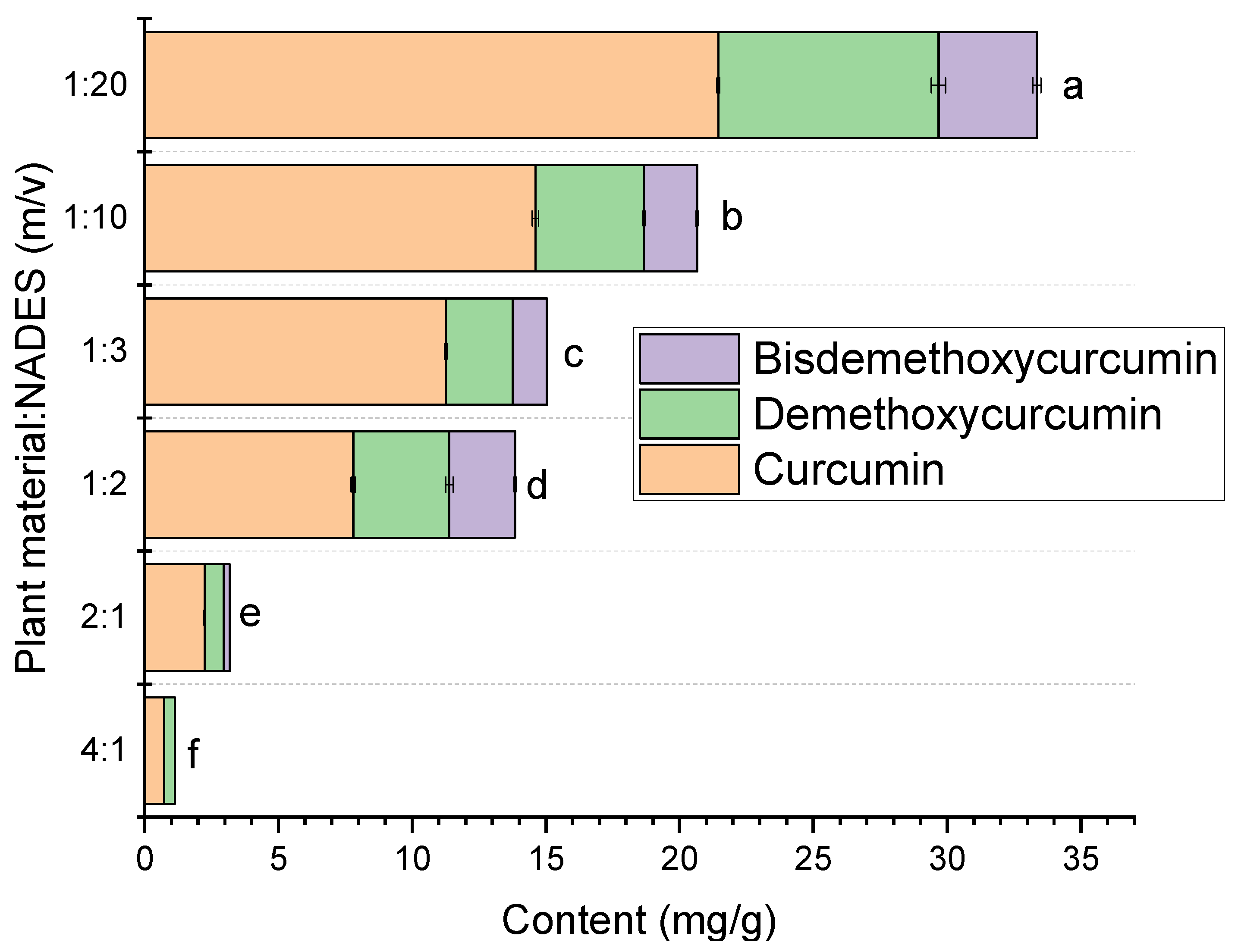
2.1. Extraction of Curcuminoids
2.2. Biological Activity Studies
2.3. Biotoxicity Studies
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemicals
4.2. High-Performance Liquid Chromatography
4.3. Extraction of Curcuminoids
4.4. Biological Activity Studies
4.4.1. Antioxidant Activity Studies
4.4.2. Anticholinergic Activity Studies
4.4.3. Tyrosinase Inhibitory Activity Studies
4.5. Biotoxicity Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuandani; Jantan, I.; Rohani, A.S.; Sumantri, I.B. Immunomodulatory Effects and Mechanisms of Curcuma Species and Their Bioactive Compounds: A Review. Front. Pharmacol. 2021, 12, 643119. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Omosa, L.K.; Midiwo, J.O.; Kuete, V. Curcuma Longa. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 425–435. ISBN 978-0-12-809286-6. [Google Scholar]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef]
- Febriza, A.; Zahrah, A.A.; Andini, N.S.; Usman, F.; Idrus, H.H. Potential Effect of Curcumin in Lowering Blood Glucose Level in Streptozotocin-Induced Diabetic Rats. Diabetes Metab. Syndr. Obes. 2024, 17, 3305. [Google Scholar] [CrossRef]
- Moon, D.-O. Curcumin in Cancer and Inflammation: An In-Depth Exploration of Molecular Interactions, Therapeutic Potentials, and the Role in Disease Management. Int. J. Mol. Sci. 2024, 25, 2911. [Google Scholar] [CrossRef]
- Jafarzadeh, E.; Shoeibi, S.; Bahramvand, Y.; Nasrollahi, E.; Maghsoudi, A.S.; Yazdi, F.; KarkonShayan, S.; Hassani, S. Turmeric for Treatment of Irritable Bowel Syndrome: A Systematic Review of Population-Based Evidence. Iran. J. Public Health 2022, 51, 1223. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, Purification and Applications of Curcumin from Plant Materials—A Comprehensive Review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.; Ocampo, M.; Jiménez-Aparicio, A. Microwave-Assisted Extraction of Functional Compounds from Plants: A Review. BioResources 2023, 18, 6614. [Google Scholar] [CrossRef]
- Nanda, B.; Sailaja, M.; Mohapatra, P.; Pradhan, R.K.; Nanda, B.B. Green Solvents: A Suitable Alternative for Sustainable Chemistry. Mater. Today Proc. 2021, 47, 1234–1240. [Google Scholar] [CrossRef]
- Puzeryte, V.; Martusevice, P.; Sousa, S.; Balciunaitiene, A.; Viskelis, J.; Gomes, A.M.; Viskelis, P.; Cesoniene, L.; Urbonaviciene, D. Optimization of Enzyme-Assisted Extraction of Bioactive Compounds from Sea Buckthorn (Hippophae rhamnoides L.) Leaves: Evaluation of Mixed-Culture Fermentation. Microorganisms 2023, 11, 2180. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Mantiniotou, M.; Kalompatsios, D.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Optimization of Pressurized Liquid Extraction (PLE) Parameters for Extraction of Bioactive Compounds from Moringa Oleifera Leaves and Bioactivity Assessment. Int. J. Mol. Sci. 2024, 25, 4628. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical Carbon Dioxide Extraction of Plant Phytochemicals for Biological and Environmental Applications—A Review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Widmann, A.-K.; Wahl, M.A.; Kammerer, D.R.; Daniels, R. Supercritical Fluid Extraction with CO2 of Curcuma longa L. in Comparison to Conventional Solvent Extraction. Pharmaceutics 2022, 14, 1943. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Paula, J.T.; Kayano, A.C.A.V.; Queiroga, C.L.; Magalhães, P.M.; Costa, F.T.M.; Cabral, F.A. Composition and Antimalarial Activity of Extracts of Curcuma longa L. Obtained by a Combination of Extraction Processes Using Supercritical CO2, Ethanol and Water as Solvents. J. Supercrit. Fluids 2017, 119, 122–129. [Google Scholar] [CrossRef]
- Wakte, P.S.; Sachin, B.S.; Patil, A.A.; Mohato, D.M.; Band, T.H.; Shinde, D.B. Optimization of Microwave, Ultra-Sonic and Supercritical Carbon Dioxide Assisted Extraction Techniques for Curcumin from Curcuma Longa. Sep. Purif. Technol. 2011, 79, 50–55. [Google Scholar] [CrossRef]
- Sutarsi; Jati, P.T.; Wiradiestia, D.; Altway, A.; Winardi, S.; Wahyudiono; Machmudah, S. Extraction Process Optimization of Curcumin from Curcuma xanthorrhiza Roxb. with Supercritical Carbon Dioxide Using Ethanol as a Cosolvent. ACS Omega 2024, 9, 1251–1264. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.P.; Phan, T.H.; Nguyen, T.H.P.; Nguyen, V.K.; Dang, T.C.T.; Nguyen, L.G.K.; Chung, T.Q.; Nguyen, H.Q.; Chau, P.T.T.; Thinh, L.D.A.; et al. Green Extraction of Phenolics and Terpenoids from Passion Fruit Peels Using Natural Deep Eutectic Solvents. J. Food Process Eng. 2024, 47, e14503. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Kljakić, A.C.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Duarte, A.R.C.; Mišan, A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef]
- Doldolova, K.; Bener, M.; Lalikoğlu, M.; Aşçı, Y.S.; Arat, R.; Apak, R. Optimization and Modeling of Microwave-Assisted Extraction of Curcumin and Antioxidant Compounds from Turmeric by Using Natural Deep Eutectic Solvents. Food Chem. 2021, 353, 129337. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced Extraction of Natural Pigments from Curcuma longa L. Using Natural Deep Eutectic Solvents. Ind. Crops Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Kongpol, K.; Sermkaew, N.; Makkliang, F.; Khongphan, S.; Chuaboon, L.; Sakdamas, A.; Sakamoto, S.; Putalun, W.; Yusakul, G. Extraction of Curcuminoids and Ar-Turmerone from Turmeric (Curcuma longa L.) Using Hydrophobic Deep Eutectic Solvents (HDESs) and Application as HDES-Based Microemulsions. Food Chem. 2022, 396, 133728. [Google Scholar] [CrossRef]
- Abouheif, S.; Sallam, S.; El Sohafy, S.; Kassem, F.; Shawky, E. A Green Extraction Approach Using Natural Deep Eutectic Solvents Enhances the In-Vivo Bioavailability of Curcuminoids from Turmeric Extracts. Ind. Crops Prod. 2023, 189, 115790. [Google Scholar] [CrossRef]
- Patil, S.; Rathod, V. Extraction and Purification of Curcuminoids from Curcuma Longa Using Microwave Assisted Deep Eutectic Solvent Based System and Its Cost Estimation. Process Biochem. 2022, 126, 61–71. [Google Scholar] [CrossRef]
- Nagavekar, N.; Singhal, R.S. Supercritical Fluid Extraction of Curcuma longa and Curcuma amada Oleoresin: Optimization of Extraction Conditions, Extract Profiling, and Comparison of Bioactivities. Ind. Crops Prod. 2019, 134, 134–145. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Lukk, T.; Karpichev, Y.; Gathergood, N.; Singh, B.N.; Thakur, V.K.; Tabatabaei, M.; Gupta, V.K. Biobased Natural Deep Eutectic System as Versatile Solvents: Structure, Interaction and Advanced Applications. Sci. Total Environ. 2023, 881, 163002. [Google Scholar] [CrossRef] [PubMed]
- Shekaari, H.; Mokhtarpour, M.; Faraji, S.; Zafarani-Moattar, M.T. Enhancement of Curcumin Solubility by Some Choline Chloride-Based Deep Eutectic Solvents at Different Temperatures. Fluid Phase Equilibria 2021, 532, 112917. [Google Scholar] [CrossRef]
- Patil, S.S.; Pathak, A.; Rathod, V.K. Optimization and Kinetic Study of Ultrasound Assisted Deep Eutectic Solvent Based Extraction: A Greener Route for Extraction of Curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021, 70, 105267. [Google Scholar] [CrossRef]
- Le, N.T.; Hoang, N.T.; Van, V.T.T.; Nguyen, T.P.D.; Chau, N.H.T.; Le, N.T.N.; Le, H.B.T.; Phung, H.T.; Nguyen, H.T.; Nguyen, H.M. Extraction of Curcumin from Turmeric Residue (Curcuma longa L.) Using Deep Eutectic Solvents and Surfactant Solvents. Anal. Methods 2022, 14, 850–858. [Google Scholar] [CrossRef]
- Oliveira, G.; Marques, C.; de Oliveira, A.; de Almeida dos Santos, A.; do Amaral, W.; Ineu, R.P.; Leimann, F.V.; Peron, A.P.; Igarashi-Mafra, L.; Mafra, M.R. Extraction of Bioactive Compounds from Curcuma longa L. Using Deep Eutectic Solvents: In Vitro and in Vivo Biological Activities. Innov. Food Sci. Emerg. Technol. 2021, 70, 102697. [Google Scholar] [CrossRef]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Kongpol, K.; Chaihao, P.; Shuapan, P.; Kongduk, P.; Chunglok, W.; Yusakul, G. Therapeutic Hydrophobic Deep Eutectic Solvents of Menthol and Fatty Acid for Enhancing Anti-Inflammation Effects of Curcuminoids and Curcumin on RAW264.7 Murine Macrophage Cells. RSC Adv. 2022, 12, 17443–17453. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, S.H.; Monhemi, H.; Hojjatipour, M.; Hojjatipour, M.; Eftekhari, M.; Vafaeei, M. Supercritical CO2/Deep Eutectic Solvent Biphasic System as a New Green and Sustainable Solvent System for Different Applications: Insights from Molecular Dynamics Simulations. J. Phys. Chem. B 2023, 127, 8057–8065. [Google Scholar] [CrossRef]
- Roy, D.; Miller, L. Exploring the Utility of Natural Deep Eutectic Solvents as Additives in Super/Subcritical Fluid Chromatography- Insights into Chiral Recognition Mechanism. Anal. Chim. Acta 2022, 1200, 339584. [Google Scholar] [CrossRef]
- Vladić, J.; Kovačević, S.; Aladić, K.; Jokić, S.; Radman, S.; Podunavac-Kuzmanović, S.; Duarte, A.R.C.; Jerković, I. Innovative Strategy for Aroma Stabilization Using Green Solvents: Supercritical CO2 Extracts of Satureja Montana Dispersed in Deep Eutectic Solvents. Biomolecules 2023, 13, 1126. [Google Scholar] [CrossRef] [PubMed]
- Vladić, J.; Jakovljević Kovač, M.; Pavić, V.; Jokić, S.; Simić, S.; Paiva, A.; Jerković, I.; Duarte, A.R. Towards a Greener Approach for Biomass Valorization: Integration of Supercritical Fluid and Deep Eutectic Solvents. Antibiotics 2023, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Grisales-Mejía, J.F.; Cedeño-Fierro, V.; Ortega, J.P.; Torres-Castañeda, H.G.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A.; Álvarez-Rivera, G.; Mendiola, J.A.; Cifuentes, A.; Ibañez, E. Advanced NADES-Based Extraction Processes for the Recovery of Phenolic Compounds from Hass Avocado Residues: A Sustainable Valorization Strategy. Sep. Purif. Technol. 2024, 351, 128104. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Méndez-Albiñana, P.; Mendiola, J.A.; Villamiel, M.; Cifuentes, A.; Martínez, J.; Ibáñez, E. An Eco-Friendly Extraction Method to Obtain Pectin from Passion Fruit Rinds (Passiflora edulis sp.) Using Subcritical Water and Pressurized Natural Deep Eutectic Solvents. Carbohydr. Polym. 2024, 326, 121578. [Google Scholar] [CrossRef]
- Plaza, A.; Tapia, X.; Yañez, C.; Vilches, F.; Candia, O.; Cabezas, R.; Romero, J. Obtaining Hydroxytyrosol from Olive Mill Waste Using Deep Eutectic Solvents and Then Supercritical CO2. Waste Biomass Valor 2020, 11, 6273–6284. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, M.I.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef]
- Rachkeeree, A.; Kantadoung, K.; Puangpradab, R.; Suksathan, R. Phytochemicals, Antioxidants and Anti-Tyrosinase Analyses of Selected Ginger Plants. Pharmacogn. J. 2020, 12, 872–883. [Google Scholar] [CrossRef]
- Gawron-Gzella, A. Aktywność antyoksydacyjna popularnych przypraw. Post Fitoter 2021, 22, 179–188. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.-H. Inhibitory Effect of Curcuminoids on Acetylcholinesterase Activity and Attenuation of Scopolamine-Induced Amnesia May Explain Medicinal Use of Turmeric in Alzheimer’s Disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef]
- HV, S.; Raj, A.; K, G.; S, C.; K, S. Kinetics and Computational Analysis of Cholinesterase Inhibition by REVERC3, a Bisdemethoxycurcumin-Rich Curcuma longa Extract: Relevance to the Treatment of Alzheimer’s Disease. SAGE Open Med. 2020, 8, 2050312120973499. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Gazioğlu, I.; Erim, F.B. Comparison of Antioxidant, Anticholinesterase, and Antidiabetic Activities of Three Curcuminoids Isolated from Curcuma longa L. Nat. Prod. Res. 2017, 31, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, D.; Sumiwi, S.A.; Saptarini, N.M.; Levita, J. Curcuma Longa Extract Inhibits the Activity of Mushroom Tyrosinase and the Growth of Murine Skin Cancer B16F10 Cells. J. Herbmed. Pharmacol. 2022, 12, 153–158. [Google Scholar] [CrossRef]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cai, Y.; Ma, Q.; Zhao, Z.; Yang, D.; Xu, X. Optimization of Extraction of Bioactive Compounds from Baphicacanthus Cusia Leaves by Hydrophobic Deep Eutectic Solvents. Molecules 2021, 26, 1729. [Google Scholar] [CrossRef] [PubMed]
- Jayarani, R.; Chatterjee, N.S.; Lekshmi, R.G.K.; Dara, P.K.; Anandan, R. Fucoxanthin Content and Antioxidant Activity in Supercritical CO2, Enzymatic and Natural Hydrophobic Deep Eutectic Solvent Extracts of Sargassum Wightii Seaweed. Fish. Technol. 2021, 58, 155–159. [Google Scholar]
- Vieira, C.; Rebocho, S.; Craveiro, R.; Paiva, A.; Duarte, A.R.C. Selective Extraction and Stabilization of Bioactive Compounds from Rosemary Leaves Using a Biphasic NADES. Front. Chem. 2022, 10, 954835. [Google Scholar] [CrossRef]
- Macchioni, V.; Carbone, K.; Cataldo, A.; Fraschini, R.; Bellucci, S. Lactic Acid-Based Deep Natural Eutectic Solvents for the Extraction of Bioactive Metabolites of Humulus lupulus L.: Supramolecular Organization, Phytochemical Profiling and Biological Activity. Sep. Purif. Technol. 2021, 264, 118039. [Google Scholar] [CrossRef]
- Kisanthia, R.; Hunt, A.J.; Sherwood, J.; Somsakeesit, L.; Phaosiri, C. Impact of Conventional and Sustainable Solvents on the Yield, Selectivity, and Recovery of Curcuminoids from Turmeric. ACS Sustain. Chem. Eng. 2022, 10, 104–114. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2022, 12, 56. [Google Scholar] [CrossRef]
- Jauregi, P.; Esnal-Yeregi, L.; Labidi, J. Natural Deep Eutectic Solvents (NADES) for the Extraction of Bioactives: Emerging Opportunities in Biorefinery Applications. PeerJ Anal. Chem. 2024, 6, e32. [Google Scholar] [CrossRef]
- Isci, A.; Martin, K. Recovery and Recycling of Deep Eutectic Solvents in Biomass Conversions: A Review. Biomass Convers. Biorefin. 2022, 12, 197–226. [Google Scholar] [CrossRef]
- Green, C.E.; Mitchell, S.A. The Effects of Blanching, Harvest Time and Location (with a Minor Look at Postharvest Blighting) on Oleoresin Yields, Percent Curcuminoids and Levels of Antioxidant Activity of Turmeric (Curcuma longa) Rhizomes Grown in Jamaica. Mod. Chem. Appl. 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Pan, Y.; Ju, R.; Cao, X.; Pei, H.; Zheng, T.; Wang, W. Optimization Extraction and Purification of Biological Activity Curcumin from Curcuma longa L by High-performance Counter-current Chromatography. J. Sep. Sci. 2020, 43, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Manap, M.Y.A.; Khatib, A.; Razis, A.F.A. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-Cyclodextrin as an Effective Carrier of Curcumin–Piperine Nutraceutical System with Improved Enzyme Inhibition Properties. J. Enzym. Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Rosiak, N.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Tykarska, E.; Cielecka-Piontek, J. Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics 2022, 14, 2098. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]






| Extract | Curcuminoid Content (μg/g) |
|---|---|
| Extract 1 | 7.5 |
| Extract 2 | 216.6 |
| Extract 3 | 91.9 |
| Extract 4 | 152.4 |
| Extract 5 | 183.5 |
| Extract 6 | 236.9 |
| Extract 7 | 163.1 |
| Extract 8 | 99.6 |
| Extract 9 | 107.4 |
| Extract 10 | 130.3 |
| Extract 11 | 164.0 |
| Extract 12 | 175.8 |
| Extract 13 | 199.3 |
| Extract 14 | 179.2 |
| Extract 15 | 181.7 |
| Solvent | Hydrogen Bond Acceptor (HBA) | Hydrogen Bond Donor (HBD) | Molar Ratio (HBA:HBD) | Curcuminoid Content in the Extract (mg/g) |
|---|---|---|---|---|
| NADES_1 | choline chloride | lactic acid | 1:1 | 13.77 ± 0.17 g |
| NADES_2 | choline chloride | citric acid | 1:1 | 8.22 ± 0.03 i |
| NADES_3 | choline chloride | urea | 1:2 | 12.46 ± 0.13 h |
| NADES_4 | choline chloride | propylene glycol | 1:2 | 23.12 ± 0.18 d |
| NADES_5 | menthol | lactic acid | 1:2 | 30.50 ± 0.39 a |
| NADES_6 | menthol | lauric acid | 2:1 | 17.89 ± 0.20 f |
| NADES_7 | menthol | stearic acid | 8:1 | 19.31 ± 0.23 e |
| NADES_8 | menthol | myristic acid | 8:1 | 25.94 ± 0.12 c |
| 80% ethanol | - | - | - | 26.42 ± 0.08 b |
| 80% methanol | - | - | - | 22.95 ± 0.02 d |
| Invertebrate | Concentration (µL/mL) | CUR–scCO2–NADES_1:20 | ||
|---|---|---|---|---|
| y = a ln(x) + b | R2 | IT50 (s) | ||
| Daphnia pulex | 0.04 | y = 2.3491ln(x) − 11.017 | 0.9205 | 914 |
| 0.10 | y = 3.6549ln(x) − 18.838 | 0.9529 | 680 | |
| 0.20 | y = 3.7233ln(x) − 18.203 | 0.9148 | 509 | |
| 1.00 | y = 4.1438ln(x) − 21.891 | 0.901 | 658 | |
| 4.00 | y = 5.6058ln(x) − 30.771 | 0.9113 | 591 | |
| 10.00 | y = 5.3741ln(x) − 27.693 | 0.9355 | 439 | |
| Chironomus aprilinus | 0.04 | y = 45.974ln(x) − 518.66 | 0.8911 | 88,464 * |
| 0.10 | y = 19.781ln(x) − 220.27 | 0.7598 | 88,274 * | |
| 0.20 | y = 14.221ln(x) − 158.17 | 0.6445 | 96,170 * | |
| 1.00 | y = 1.8697ln(x) − 16.515 | 0.7253 | 99,428 * | |
| 4.00 | y = 2.0262ln(x) − 13.344 | 0.609 | 8548 | |
| 10.00 | y = 1.7811ln(x) − 7.1898 | 0.9066 | 938 | |
| CUR–scCO2–NADES_1:20 | |||
|---|---|---|---|
| y = a ln(x) + b | R2 | EC50 (µL/mL) | |
| Daphnia pulex | - | - | h.t. |
| Chironomus aprilinus | y = 1.1462ln(x) + 7.6575 | 0.9649 | 0.098 |
| Extract | Pressure (PSI) | CO2 Volume (mL) | Temperature (°C) |
|---|---|---|---|
| Extract 1 | 2500 | 100 | 30 |
| Extract 2 | 6500 | 175 | 55 |
| Extract 3 | 2500 | 25 | 55 |
| Extract 4 | 6500 | 100 | 30 |
| Extract 5 | 2500 | 100 | 80 |
| Extract 6 | 6500 | 100 | 80 |
| Extract 7 | 6500 | 25 | 55 |
| Extract 8 | 2500 | 175 | 55 |
| Extract 9 | 4500 | 25 | 30 |
| Extract 10 | 4500 | 25 | 80 |
| Extract 11 | 4500 | 175 | 30 |
| Extract 12 | 4500 | 175 | 80 |
| Extract 13 | 4500 | 100 | 55 |
| Extract 14 | 4500 | 100 | 55 |
| Extract 15 | 4500 | 100 | 55 |
| Hydrogen Bond Acceptor | Hydrogen Bond Donor | Molar Ratio | |
|---|---|---|---|
| NADES_1 | choline chloride | lactic acid | 1:1 |
| NADES_2 | choline chloride | citric acid | 1:1 |
| NADES_3 | choline chloride | urea | 1:2 |
| NADES_4 | choline chloride | propylene glycol | 1:2 |
| NADES_5 | menthol | lactic acid | 1:2 |
| NADES_6 | menthol | lauric acid | 2:1 |
| NADES_7 | menthol | stearic acid | 8:1 |
| NADES_8 | menthol | myristic acid | 8:1 |
| Extract | Plant Material to NADES_5 Ratio (m/v) |
|---|---|
| CUR–scCO2–NADES_4:1 | 4:01 |
| CUR–scCO2–NADES_2:1 | 2:01 |
| CUR–scCO2–NADES_2:1 | 2:01 |
| CUR–scCO2–NADES_1:2 | 1:02 |
| CUR–scCO2–NADES_1:3 | 1:03 |
| CUR–scCO2–NADES_1:10 | 1:10 |
| CUR–scCO2–NADES_1:20 | 1:20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiłowicz-Krzemień, A.; Wójcik, J.; Gościniak, A.; Szymański, M.; Szulc, P.; Górecki, K.; Cielecka-Piontek, J. Natural Deep Eutectic Solvents Combined with Supercritical Carbon Dioxide for the Extraction of Curcuminoids from Turmeric. Pharmaceuticals 2024, 17, 1596. https://doi.org/10.3390/ph17121596
Stasiłowicz-Krzemień A, Wójcik J, Gościniak A, Szymański M, Szulc P, Górecki K, Cielecka-Piontek J. Natural Deep Eutectic Solvents Combined with Supercritical Carbon Dioxide for the Extraction of Curcuminoids from Turmeric. Pharmaceuticals. 2024; 17(12):1596. https://doi.org/10.3390/ph17121596
Chicago/Turabian StyleStasiłowicz-Krzemień, Anna, Julia Wójcik, Anna Gościniak, Marcin Szymański, Piotr Szulc, Krzysztof Górecki, and Judyta Cielecka-Piontek. 2024. "Natural Deep Eutectic Solvents Combined with Supercritical Carbon Dioxide for the Extraction of Curcuminoids from Turmeric" Pharmaceuticals 17, no. 12: 1596. https://doi.org/10.3390/ph17121596
APA StyleStasiłowicz-Krzemień, A., Wójcik, J., Gościniak, A., Szymański, M., Szulc, P., Górecki, K., & Cielecka-Piontek, J. (2024). Natural Deep Eutectic Solvents Combined with Supercritical Carbon Dioxide for the Extraction of Curcuminoids from Turmeric. Pharmaceuticals, 17(12), 1596. https://doi.org/10.3390/ph17121596













