Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain
Abstract
1. Introduction
- Analyze the safety of treatments in the clinical trials studied.
- Elucidate the mechanism by which CBD interacts to produce therapeutic effects through preclinical trials.
- Determine possible disease treatments based on their analgesic properties.
2. Results
3. Discussion
4. Materials and Methods
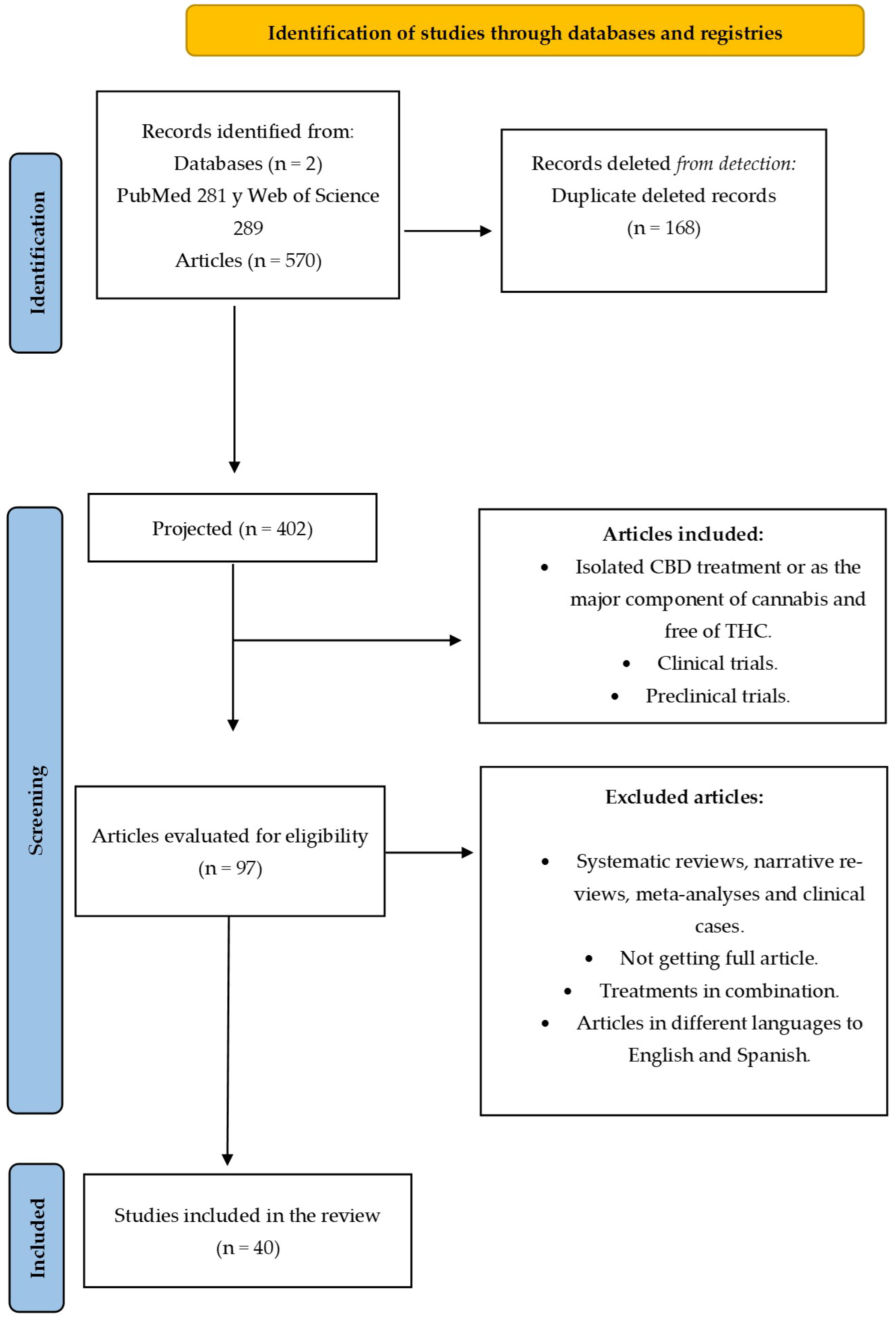
4.1. Search Strategy and Data Resources
4.2. Eligibility Criteria
4.3. Selection Procedure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Earleywine, M. Understanding Marijuana: A New Look at the Scientific Evidence; Oxford University Press: Oxford, UK, 2002; p. 326. [Google Scholar]
- Crocq, M.A. History of Cannabis and the Endocannabinoid System. Dialogues Clin. Neurosci. 2020, 22, 223. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.; Kayser, O. The Cannabis Plant: Botanical Aspects. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–12. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef] [PubMed]
- Olafuyi, O.; Kapusta, K.; Reed, A.; Kolodziejczyk, W.; Saloni, J.; Hill, G.A. Investigation of Cannabidiol’s Potential Targets in Limbic Seizures. In-Silico Approach. J. Biomol. Struct. Dyn. 2023, 41, 7744–7756. [Google Scholar] [CrossRef] [PubMed]
- Etemad, L.; Karimi, G.; Alavi, M.S.; Roohbakhsh, A. Pharmacological Effects of Cannabidiol by Transient Receptor Potential Channels. Life Sci. 2022, 300, 120582. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis Sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol Goes Nuclear: The Role of PPARγ. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic. Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Mlost, J.; Kędziora, M.; Starowicz, K. Computational Approach Reveals Pronociceptive Potential of Cannabidiol in Osteoarthritis: Role of Transient Receptor Potential Channels. Pharmaceuticals 2021, 14, 964. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled Clinical Trial of Cannabidiol in Huntington’s Disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of Cannabinoids in the Treatment of Epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Newmeyer, M.N.; Swortwood, M.J.; Barnes, A.J.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Free and Glucuronide Whole Blood Cannabinoids’ Pharmacokinetics after Controlled Smoked, Vaporized, and Oral Cannabis Administration in Frequent and Occasional Cannabis Users: Identification of Recent Cannabis Intake. Clin. Chem. 2016, 62, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, K.A.; Szeto, I.; Setnik, B.; Sellers, E.M.; Levy-Cooperman, N.; Mills, C.; Etges, T.; Sommerville, K. Abuse Potential Assessment of Cannabidiol (CBD) in Recreational Polydrug Users: A Randomized, Double-Blind, Controlled Trial. Epilepsy Behav. 2018, 88, 162–171. [Google Scholar] [CrossRef]
- Haney, M.; Malcolm, R.J.; Babalonis, S.; Nuzzo, P.A.; Cooper, Z.D.; Bedi, G.; Gray, K.M.; McRae-Clark, A.; Lofwall, M.R.; Sparenborg, S.; et al. Oral Cannabidiol Does Not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology 2015, 41, 1974–1982. [Google Scholar] [CrossRef]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug–Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Clauw, D.J.; Häuser, W. Cautious Hope for Cannabidiol (CBD) in Rheumatology Care. Arthritis Care Res. 2023, 75, 1371–1375. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Cuñetti, L.; Manzo, L.; Peyraube, R.; Arnaiz, J.; Curi, L.; Orihuela, S. Chronic Pain Treatment with Cannabidiol in Kidney Transplant Patients in Uruguay. Transplant. Proc. 2018, 50, 461–464. [Google Scholar] [CrossRef]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef] [PubMed]
- Capano, A.; Weaver, R.; Burkman, E. Evaluation of the Effects of CBD Hemp Extract on Opioid Use and Quality of Life Indicators in Chronic Pain Patients: A Prospective Cohort Study. Postgrad. Med. 2020, 132, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Bebee, B.; Taylor, D.M.; Bourke, E.; Pollack, K.; Foster, L.; Ching, M.; Wong, A. The CANBACK Trial: A Randomised, Controlled Clinical Trial of Oral Cannabidiol for People Presenting to the Emergency Department with Acute Low Back Pain. Med. J. Aust. 2021, 214, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Cochrane-Snyman, K.C.; Cruz, C.; Morales, J.; Coles, M. The Effects of Cannabidiol Oil on Noninvasive Measures of Muscle Damage in Men. Med. Sci. Sports Exerc. 2021, 53, 1460–1472. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A Randomized, Double-Blind, Placebo-Controlled Study of Daily Cannabidiol for the Treatment of Canine Osteoarthritis Pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Xu, D.H.; Cullen, B.D.; Tang, M.; Fang, Y. The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Curr. Pharm. Biotechnol. 2019, 21, 390–402. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Tan, Y.; Liu, W.; Ouaddi, S.; McCoy, J.; Kovacevic, M.; Situm, M.; Stanimirovic, A.; Li, M.; et al. Novel Cannabidiol Aspartame Combination Treatment (JW-100) Significantly Reduces ISGA Score in Atopic Dermatitis: Results from a Randomized Double-Blinded Placebo-Controlled Interventional Study. J. Cosmet. Dermatol. 2022, 21, 1647–1650. [Google Scholar] [CrossRef]
- Heineman, J.T.; Forster, G.L.; Stephens, K.L.; Cottler, P.S.; Timko, M.P.; DeGeorge, B.R. A Randomized Controlled Trial of Topical Cannabidiol for the Treatment of Thumb Basal Joint Arthritis. J. Hand Surg. Am. 2022, 47, 611–620. [Google Scholar] [CrossRef]
- Van Orten-Luiten, A.-C.B.; De Roos, N.M.; Majait, S.; Witteman, B.J.M.; Witkamp, R.F. Effects of Cannabidiol Chewing Gum on Perceived Pain and Well-Being of Irritable Bowel Syndrome Patients: A Placebo-Controlled Crossover Exploratory Intervention Study with Symptom-Driven Dosing. Cannabis Cannabinoid Res. 2022, 7, 436–444. [Google Scholar] [CrossRef]
- Bawa, Z.; Lewis, D.; Gavin, P.D.; Libinaki, R.; Joubran, L.; El-Tamimy, M.; Taylor, G.; Meltzer, R.; Bedoya-Pérez, M.; Kevin, R.C.; et al. An Open-Label Feasibility Trial of Transdermal Cannabidiol for Hand Osteoarthritis. Sci. Rep. 2024, 14, 11792. [Google Scholar] [CrossRef]
- Alaia, M.J.; Li, Z.I.; Chalem, I.; Hurley, E.T.; Vasavada, K.; Gonzalez-Lomas, G.; Rokito, A.S.; Jazrawi, L.M.; Kaplan, K. Cannabidiol for Postoperative Pain Control After Arthroscopic Rotator Cuff Repair Demonstrates No Deficits in Patient-Reported Outcomes Versus Placebo: 1-Year Follow-up of a Randomized Controlled Trial. Orthop. J. Sports Med. 2024, 12, 23259671231222265. [Google Scholar] [CrossRef] [PubMed]
- Walczyńska-Dragon, K.; Kurek-Górecka, A.; Niemczyk, W.; Nowak, Z.; Baron, S.; Olczyk, P.; Nitecka-Buchta, A.; Kempa, W.M. Cannabidiol Intervention for Muscular Tension, Pain, and Sleep Bruxism Intensity—A Randomized, Double-Blind Clinical Trial. J. Clin. Med. 2024, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The Non-Psychoactive Cannabis Constituent Cannabidiol Is an Orally Effective Therapeutic Agent in Rat Chronic Inflammatory and Neuropathic Pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.J.; David Ramirez, M.; Neelakantan, H.; Walker, E.A. Cannabidiol Prevents the Development of Cold and Mechanical Allodynia in Paclitaxel-Treated Female C57Bl6 Mice. Anesth. Analg. 2011, 113, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal Cannabidiol Reduces Inflammation and Pain-Related Behaviours in a Rat Model of Arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. A New Formulation of Cannabidiol in Cream Shows Therapeutic Effects in a Mouse Model of Experimental Autoimmune Encephalomyelitis. DARU J. Pharm. Sci. 2015, 23, 48. [Google Scholar] [CrossRef]
- Lehmann, C.; Fisher, N.B.; Tugwell, B.; Szczesniak, A.; Kelly, M.; Zhou, J. Experimental Cannabidiol Treatment Reduces Early Pancreatic Inflammation in Type 1 Diabetes. Clin. Hemorheol. Microcirc. 2016, 64, 655–662. [Google Scholar] [CrossRef]
- Sajjadian, M.; Kashani, I.R.; Pasbakhsh, P.; Hassani, M.; Omidi, A.; Takzare, N.; Clarner, T.; Beyer, C.; Zendedel, A. Protective Effects of Cannabidiol on Cuprizone-Induced Demyelination in C57BL/6 Mice. J. Contemp. Med. Sci. 2017, 3, 278–283. [Google Scholar]
- Genaro, K.; Fabris, D.; Arantes, A.L.F.; Zuardi, A.W.; Crippa, J.A.S.; Prado, W.A. Cannabidiol Is a Potential Therapeutic for the Affective-Motivational Dimension of Incision Pain in Rats. Front. Pharmacol. 2017, 8, 391. [Google Scholar] [CrossRef]
- Philpott, H.T.; O’Brien, M.; McDougall, J.J. Attenuation of Early Phase Inflammation by Cannabidiol Prevents Pain and Nerve Damage in Rat Osteoarthritis. Pain 2017, 158, 2442–2451. [Google Scholar] [CrossRef]
- Li, H.; Kong, W.; Chambers, C.R.; Yu, D.; Ganea, D.; Tuma, R.F.; Ward, S.J. The Non-Psychoactive Phytocannabinoid Cannabidiol (CBD) Attenuates pro-Inflammatory Mediators, T Cell Infiltration, and Thermal Sensitivity Following Spinal Cord Injury in Mice. Cell. Immunol. 2018, 329, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol Modulates Serotonergic Transmission and Reverses Both Allodynia and Anxiety-like Behavior in a Model of Neuropathic Pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.H.A.; Redivo, D.D.B.; Gasparin, A.T.; Sotomaior, B.B.; de Carvalho, M.C.; Genaro, K.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Zanoveli, J.M.; et al. Cannabidiol Attenuates Mechanical Allodynia in Streptozotocin-Induced Diabetic Rats via Serotonergic System Activation through 5-HT1A Receptors. Brain Res. 2019, 1715, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Belardo, C.; Iannotta, M.; Boccella, S.; Rubino, R.C.; Ricciardi, F.; Infantino, R.; Pieretti, G.; Stella, L.; Paino, S.; Marabese, I.; et al. Oral Cannabidiol Prevents Allodynia and Neurological Dysfunctions in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2019, 10, 444120. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, G.C.; Ferrari, D.P.; Guimaraes, F.S.; Del Bel, E.A.; Bortolanza, M.; Ferreira-Junior, N.C. Cannabidiol Increases the Nociceptive Threshold in a Preclinical Model of Parkinson’s Disease. Neuropharmacology 2020, 163, 107808. [Google Scholar] [CrossRef]
- Silva-Cardoso, G.K.; Lazarini-Lopes, W.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Garcia-Cairasco, N.; Leite-Panissi, C.R.A. Cannabidiol Effectively Reverses Mechanical and Thermal Allodynia, Hyperalgesia, and Anxious Behaviors in a Neuropathic Pain Model: Possible Role of CB1 and TRPV1 Receptors. Neuropharmacology 2021, 197, 108712. [Google Scholar] [CrossRef]
- Malvestio, R.B.; Medeiros, P.; Negrini-Ferrari, S.E.; Oliveira-Silva, M.; Medeiros, A.C.; Padovan, C.M.; Luongo, L.; Maione, S.; Coimbra, N.C.; de Freitas, R.L. Cannabidiol in the Prelimbic Cortex Modulates the Comorbid Condition between the Chronic Neuropathic Pain and Depression-like Behaviour in Rats: The Role of Medial Prefrontal Cortex 5-HT1A and CB1 Receptors. Brain Res. Bull. 2021, 174, 323–338. [Google Scholar] [CrossRef]
- Vivanco-Estela, A.N.; dos-Santos-Pereira, M.; Guimaraes, F.S.; Del-Bel, E.; Nascimento, G.C. do Cannabidiol Has Therapeutic Potential for Myofascial Pain in Female and Male Parkinsonian Rats. Neuropharmacology 2021, 196, 108700. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Wang, J.; Kumar, V.; Ciaccio, J.; Dakhel, S.; Tan, C.; Kim, J.; Lee, S.; Katz-Lichtenstein, H.; Gironda, Z.; et al. Evidence For Cannabidiol Modulation of Serotonergic Transmission in a Model of Osteoarthritis via In Vivo PET Imaging and Behavioral Assessment. Int. J. Innov. Res. Med. Sci. 2022, 7, 254–271. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Morris, D.P.; Raup-Konsavage, W.M.; Sun, D.; Vrana, K.E.; Graziane, N.M. Evaluating the Antinociceptive Efficacy of Cannabidiol Alone or in Combination with Morphine Using the Formalin Test in Male and Female Mice. Cannabis Cannabinoid Res. 2022, 7, 648–657. [Google Scholar] [CrossRef]
- Li, H.Y.; Ward, S.J.; Basavarajappa, S.; Laks, E.Y.; Li, H.; Ward, S.J. Non-Psychoactive Cannabinoid Modulation of Nociception and Inflammation Associated with a Rat Model of Pulpitis. Biomolecules 2023, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.J.; Kim, S.; Shin, D.; Lee, H.J.; Jeon, K.-H.; Tian, W.J.; Hur, K.J.; Kang, J.S.; Park, H.-J.; Cha, J.Y.; et al. Cannabidiol Alleviates Chronic Prostatitis and Chronic Pelvic Pain Syndrome via CB2 Receptor Activation and TRPV1 Desensitization. World J. Men’s Health 2024, 42. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.V.L.; Braga, A.V.; Silva, I.R.; de Souza, A.R.B.; Kohlhoff, M.; César, I.C.; Machado, R.R.; Oliveira, R.B. Synthesis and Antiallodynic Activity of Cannabidiol Analogue on Peripheral Neuropathy in Mice. Chem. Biodivers. 2024, 21, e202301935. [Google Scholar] [CrossRef] [PubMed]
- Arantes, A.L.F.; Carvalho, M.C.; Brandão, M.L.; Prado, W.A.; Crippa, J.A.; de Souza Crippa, J.A.; Lovick, T.A.; Genaro, K. Antinociceptive Action of Cannabidiol on Thermal Sensitivity and Post-Operative Pain in Male and Female Rats. Behav. Brain Res. 2024, 459, 114793. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Tavares, L.R.; Petrilli, L.A.; Baptista-De-Souza, D.; Canto-De-Souza, L.; da Silva Planeta, C.; Guimarães, F.S.; Nunes-De-Souza, R.L.; Canto-De-Souza, A. Cannabidiol Treatment Shows Therapeutic Efficacy in a Rodent Model of Social Transfer of Pain in Pair-Housed Male Mice. Cannabis Cannabinoid Res. 2024, 9, 699–713. [Google Scholar] [CrossRef]
- Jelínek, P.; Roušarová, J.; Ryšánek, P.; Ježková, M.; Havlůjová, T.; Pozniak, J.; Kozlík, P.; Křížek, T.; Kučera, T.; Šíma, M.; et al. Application of Oil-in-Water Cannabidiol Emulsion for the Treatment of Rheumatoid Arthritis. Cannabis Cannabinoid Res. 2024, 9, 147–159. [Google Scholar] [CrossRef]
- Escobar-Espinal, D.M.; Vivanco-Estela, A.N.; Barros, N.; dos Santos Pereira, M.; Guimaraes, F.S.; Del Bel, E.; Nascimento, G.C. Cannabidiol and It Fluorinate Analog PECS-101 Reduces Hyperalgesia and Allodynia in Trigeminal Neuralgia via TRPV1 Receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 132, 110996. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, S.; Qin, X.; Bai, W.; Hao, J.; Xu, X.; Guo, H.; Bai, H.; Yang, Z.; Wang, S.; et al. Exploring the Therapeutic Potential of Cannabidiol for Sleep Deprivation-Induced Hyperalgesia. Neuropharmacology 2024, 249, 109893. [Google Scholar] [CrossRef]
- Samara, E.; Bialer, M.; Mechoulam, R. Pharmacokinetics of Cannabidiol in Dogs. Drug Metab. Dispos. 1988, 16, 469–472. [Google Scholar]
- Coelho, M.P.R.C.; Leme, F.d.O.P.; Moreira, F.A.; Branco, S.E.M.T.; Melo, M.M.; de Melo, E.G. Current Review of Hemp-Based Medicines in Dogs. J. Vet. Pharmacol. Ther. 2021, 44, 870–882. [Google Scholar] [CrossRef]
- Di Salvo, A.; Conti, M.B.; della Rocca, G. Pharmacokinetics, Efficacy, and Safety of Cannabidiol in Dogs: An Update of Current Knowledge. Front. Vet. Sci. 2023, 10, 1204526. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lindgren, J.-E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-Dose Kinetics of Deuterium-Labelled Cannabidiol in Man after Smoking and Intravenous Administration. Biomed. Environ. Mass. Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human Skin Permeation of Delta8-Tetrahydrocannabinol, Cannabidiol and Cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and Therapeutic Targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef]
- Greco, R.; Francavilla, M.; Demartini, C.; Zanaboni, A.M.; Sodergren, M.H.; Facchetti, S.; Pacchetti, B.; Palmisan, M.; Franco, V.; Tassorelli, C. Characterization of the Biochemical and Behavioral Effects of Cannabidiol: Implications for Migraine. J. Headache Pain 2023, 24, 48. [Google Scholar] [CrossRef]
- Macêdo-Souza, C.; Maisonnette, S.S.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Landeira-Fernandez, J.; Leite-Panissi, C.R.A. Systemic Chronic Treatment with Cannabidiol in Carioca High- and Low-Conditioned Freezing Rats in the Neuropathic Pain Model: Evaluation of Pain Sensitivity. Pharmaceuticals 2023, 16, 1003. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
| Authors | Treatment | Number of Patients | Randomization | Masking | Jadad | Result |
|---|---|---|---|---|---|---|
| Cuñetti 2018 [22] | CBD 50–150 mg/2 day for 3 weeks. | 7 patients with transplanted kidney. | No. | No. | 1 | Well-tolerated treatment; 6/7 patients had improvement in chronic pain; longer follow-up would be necessary. |
| Capano 2020 [24] | Oil rich in CBD, 60 mg daily. | 131 patients with chronic pain. | Yes. | Double blind. | 5 | CBD is an effective analgesic that decreases opioid use in patients with chronic pain. |
| Xu 2019 [28] | Topical oil containing 250 mg of CBD. | 29 patients with peripheral neuropathy sympathy. | Yes | Double blind | 4 | Topical application of CBD manages to significantly improve the pain and symptoms of peripheral neuropathy |
| Bebee 2021 [25] | 400 mg CBD oral. | 143 lower back pain patients. | Yes. | Double blind. | 5 | CBD was not superior to placebo as an adjunct medication to treat lower back pain. |
| Cochrane-Snyman 2021 [26] | 150 mg CBD oil 24 and 48 h. | 30 people who do not practice exercise. | No. | Double blind. | 3 | The results indicate that this dose and treatment time is not beneficial for muscle pain caused by exercise. |
| Gao 2022 [29] | JW-100 (pure CBD + aspartame) topical formulation for 14 days/twice a day. | 18 patients with atopic dermatitis (AD). | Yes. | Double blind. | 5 | JW-100 showed statistically significant enhancements in AD symptoms after 14 days of topical application applied twice daily |
| Heineman 2022 [30] | 1 mL of topical CBD (6.2 mg/mL) with shea butter. | 18 participants with thumb basal joint arthritis. | Yes. | Double blind. | 5 | Topical treatment with CBD showed notable enhancements in pain and disability associated with thumb basal joint arthritis. |
| van Orten-Luiten 2022 [31] | 50 mg CBD chewing gum. | 32 females with irritable bowel syndrome (IBS). | Yes | Double blind. | 5 | Results show no significant differences in pain scores suggesting that perceived benefits did not outweigh practical obstacles such as prolonged chewing |
| Walczyńska-Dragon 2024 [34] | 5 and 10% CBD formulations. | 60 patients with temporo-mandibular disorders (TMD). | Yes. | Double blind. | 5 | Intraoral administration of CBD formulations demonstrated effectiveness in alleviating pain, reducing muscle tension, and decreasing bruxism activity in individuals with sleep bruxism and muscle-related TMDs. |
| Alaia 2024 [33] | 25 mg and 50 mg of CBD 3 times/day for 14 days | 83 patients with arthroscopic rotator cuff repair (ARCR). | Yes. | Double blind. | 5 | Perioperative CBD use for pain control in ARCR patients did not show significant deficits in pain, satisfaction, or patient-reported outcomes at one-year post-surgery compared to a placebo group. |
| Bawa 2024 [32] | Transdermal CBD gel (4% w/w) 3 times/day for four weeks. | 15 patients with hand osteoarthritis. | No. | No. | 1 | Transdermal CBD gel potentially ameliorates pain and grip strength in individuals with symptomatic hand osteoarthritis (OA). |
| Authors | Treatment | Number of Patients | Randomization | Masking | Jadad | Result |
|---|---|---|---|---|---|---|
| Gamble 2018 [23] | CBD oil 2 mg/kg. | 14 companion dogs. | Yes. | Double blind. | 4 | Pharmacokinetic and clinical studies indicate that 2 mg/kg of CBD oil twice daily increases the comfort and activity of dogs with osteoarthritis. |
| Verrico 2020 [27] | Encapsulated CBD (20 mg day) and unencapsulated (50 mg day). | 20 companion dogs with osteoarthritis. | Yes. | Double blind. | 5 | The results show that CBD has a high bioavailability and that it exerts anti-inflammatory properties in a solid and quantifiable way. |
| Authors | Model | Treatment | Main Result |
|---|---|---|---|
| Costa 2007 [35] | Male Wistar rats subjected to constriction of the sciatic nerve in right hind leg. | Oral CBD (20 mg/kg) | CBD reduced inflammatory mediators such as PGE2, iNOs, and lipid peroxide in several tissues. |
| Ward 2011 [36] | C57Bl/6 mice. | CBD (5.0 or 10.0 mg/kg IP) | CBD ameliorates allodynia and hyperalgesia in paclitaxel-induced neuropathic pain mice. |
| Hammel 2015 [37] | Male Sprague Dawley rats as a model of monoarthritic knee joint induced by Freund’s adjuvant. | Gel CBD (0.6-3.1-6.2-62.3 mg/day) | These data indicate that topical application of CBD has therapeutic potential for the relief of arthritis pain-related behaviors and inflammation without obvious side effects. |
| Giacoppo 2015 [38] | C57BL/6 mice experimental model of autoimmune encephalomyelitis (EAE). | 1% of CBD-cream | Daily application of topical 1% CBD cream has the potential to provide neuroprotective benefits against the series of processes (inflammation, oxidative damage, and neuronal cell death) linked to the development of EAE. |
| Lehmann 2016 [39] | Non-obese diabetic female mice. | 5 mg/kg CBD daily and five times weekly for ten weeks | CBD-treated mice with T1D exhibited delayed onset of the condition and demonstrated notably decreased inflammatory markers along with heightened functional capillary density (FCD) in the microcirculation of the pancreas. |
| Sajjadian 2017 [40] | Cuprizone-induced demyelination model in C57/ BL6 mice. | CBD injection 5 mg/kg | The results obtained that CBD attenuates the destructive effects of cuprizone on CC by decreasing oxidative stress and microglia. Microglia suppression will potentially reduce inflammatory lesions and limit demyelination within the CNS. |
| Genaro 2017 [41] | Wistar rats with an incision (pain model). | CBD intraperitoneal 3–10 mg/kg | There is evidence that CBD modulates the sensory and affective dimension of pain in a differential way. |
| Philpott 2017 [42] | Male Wistar rats with osteoarthritis (150–175 g) sodium monoiodoacetate model of osteoarthritis. | Local administration CBD 100–300 μg. Topical treatment with CBD | Local administration of CBD inhibits pain and peripheral sensitization in osteoarthritis. Topical CBD can be a safe treatment to treat pain in OA, as well as block acute inflammatory flare-ups. |
| Li 2018 [43] | Sham or contusion injury model in C57/BL6 mice. | Intraperitoneal CBD (1.5 mg/kg) | CBD significantly reduces
|
| De Gregorio 2019 [44] | Male Wistar rats (250–260 g) spared nerve injury model. | Oral CBD (10–40 mg/kg) | Low-dose CBD produces analgesia through the activation of TRPV1 receptors and reduces neuropathic pain through 5-HT. |
| Jesus 2019 [45] | Model of neuropathic pain induced by chronic constriction nerve injury in male Wistar rats. | Intraperitoneal CBD (0.1–0.3 mg/kg) | CBD can be a treatment for neuropathic pain in diabetics, acting through the activation of 5-HT1A. |
| Belardo 2019 [46] | Mild traumatic brain injury (TBI) induction in C57/BL6 mice. | 10% CBD oil (30 μL) | Daily treatment with CBD significantly reduced pain, disappearing within 30 days. |
| Crivelaro do Nascimento 2020 [47] | Parkinson model by 6-OHDA in C57/BL6 mice. | Oral CBD (10, 20 and 100 mg) | CBD may be a useful drug to prevent parkinsonism-induced nociceptive pain by lowering its threshold. They also suggest that CB1 and TRPV1 receptors are important for CBD-induced analgesia. CBD could produce these analgesic effects by increasing endogenous anandamide levels. |
| Silva-cardoso 2021 [48] | Male Wistar rats (250 g) exposed to chronic constriction injury of the sciatic nerve. | CBD intraperitoneal 0.3, 3, 10, 30 mg/kg | The results may be clinically relevant for using CBD in the treatment of chronic pain and associated comorbidities due to interaction with CB1 and TRPV1 receptors. |
| Malvestio 2021 [49] | Albino Wistar rat model of neuropathic pain. | CBD injection (15, 30 and 60 nmol) | CBD could be a potential medication for pain and depression in patients with neuropathic pain. Due to its interaction with CB1 and 5-HT1A receptors. |
| Vivanco-Estela 2021 [50] | Parkinsonism-induced orofacial allodynia in Wistar rats. | CBD injection (10, 50 and 100 μg) | Local CBD treatment reduces the increase in allodynia and orofacial hyperalgesia in both sexes, although it responds differently to the same doses. |
| MLOST 2021 [10] | OA-induced male Wistar rats. | CBD injection (50 mg/kg) | The beneficial effect of CBD for osteoarthritis is mediated by the PPARy receptor, and the activation of the TRP channel by CBD is necessary to produce the analgesic effects. |
| Ding 2022 [51] | OA-induced adult mice (C57BL/6J). | CBD (1.0 mg/kg, i.v.) | The engagement of CBD with the serotonin 5-HT1A receptor plays a role in its pain-relieving and anxiety-reducing effects in the MIA-induced OA animal model. |
| Sepulveda 2022 [52] | C57BL/6 wild-type mice. | CBD (10 mg/kg, i.p.) | Treatment with CBD could potentially offer beneficial pain-relieving effects during the initial stage of chronic pain in formalin assay. |
| Li 2023 [53] | Sprague Dawley rats, model of pulpitis. | CBD (5 mg/kg i.p.) | The group treated with CBD did not show significantly higher sensitivity compared to the sham controls. Furthermore, CBD only attenuated a marker of macrophage activation (AIF-1). |
| Piao 2024 [54] | Sprague Dawley male rats with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). | Oral CBD (50, 100 and 150 mg/kg) | CBD exhibits a notable ability to attenuate the pain associated with prostatitis, with a more pronounced enhancement observed at higher CBD dosage levels. |
| Marques 2024 [55] | Male Swiss mice. | CBD (20 mg/kg, i.p.) | Cannabidiol significantly reduced mechanical allodynia induced by paclitaxel. |
| Arantes 2024 [56] | Male and female Wistar rats. | Intraperitoneal CBD (0.3 and 3 mg/kg) | CBD demonstrates the capacity to mitigate enduring pain in individuals of both genders. In females, the responsiveness to CBD exhibits notable variations throughout the estrous cycle. |
| Rodrigues-Tavares 2024 [57] | Male Swiss mice. | Intraperitoneal CBD (0.3, 1, 10, or 30 mg/kg) | CBD administration attenuated pain hypersensitivity. |
| Jelínek 2024 [58] | Rheumatoid Arthritis rat model. | CBD-containing emulsion | Beneficial Impact of Cannabidiol (CBD) in a Rat Model of Rheumatoid Arthritis (RA). |
| Escobar-Espinar 2024 [59] | Male Wistar rats. | CBD (30 mg/kg) | CBD may provide therapeutic benefits for trigeminal neuralgia without inducing motor coordination deficits. |
| Zhu 2024 [60] | Male C57BL/6N mice. | CBD (30 mg/kg i.p.) | CBD exhibits antinociceptive effects by activating dopamine receptors and promoting wakefulness under sleep deprivation conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cásedas, G.; Yarza-Sancho, M.d.; López, V. Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain. Pharmaceuticals 2024, 17, 1438. https://doi.org/10.3390/ph17111438
Cásedas G, Yarza-Sancho Md, López V. Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain. Pharmaceuticals. 2024; 17(11):1438. https://doi.org/10.3390/ph17111438
Chicago/Turabian StyleCásedas, Guillermo, Martín de Yarza-Sancho, and Víctor López. 2024. "Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain" Pharmaceuticals 17, no. 11: 1438. https://doi.org/10.3390/ph17111438
APA StyleCásedas, G., Yarza-Sancho, M. d., & López, V. (2024). Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain. Pharmaceuticals, 17(11), 1438. https://doi.org/10.3390/ph17111438







