Advancing Pain Understanding and Drug Discovery: Insights from Preclinical Models and Recent Research Findings
Abstract
1. Introduction
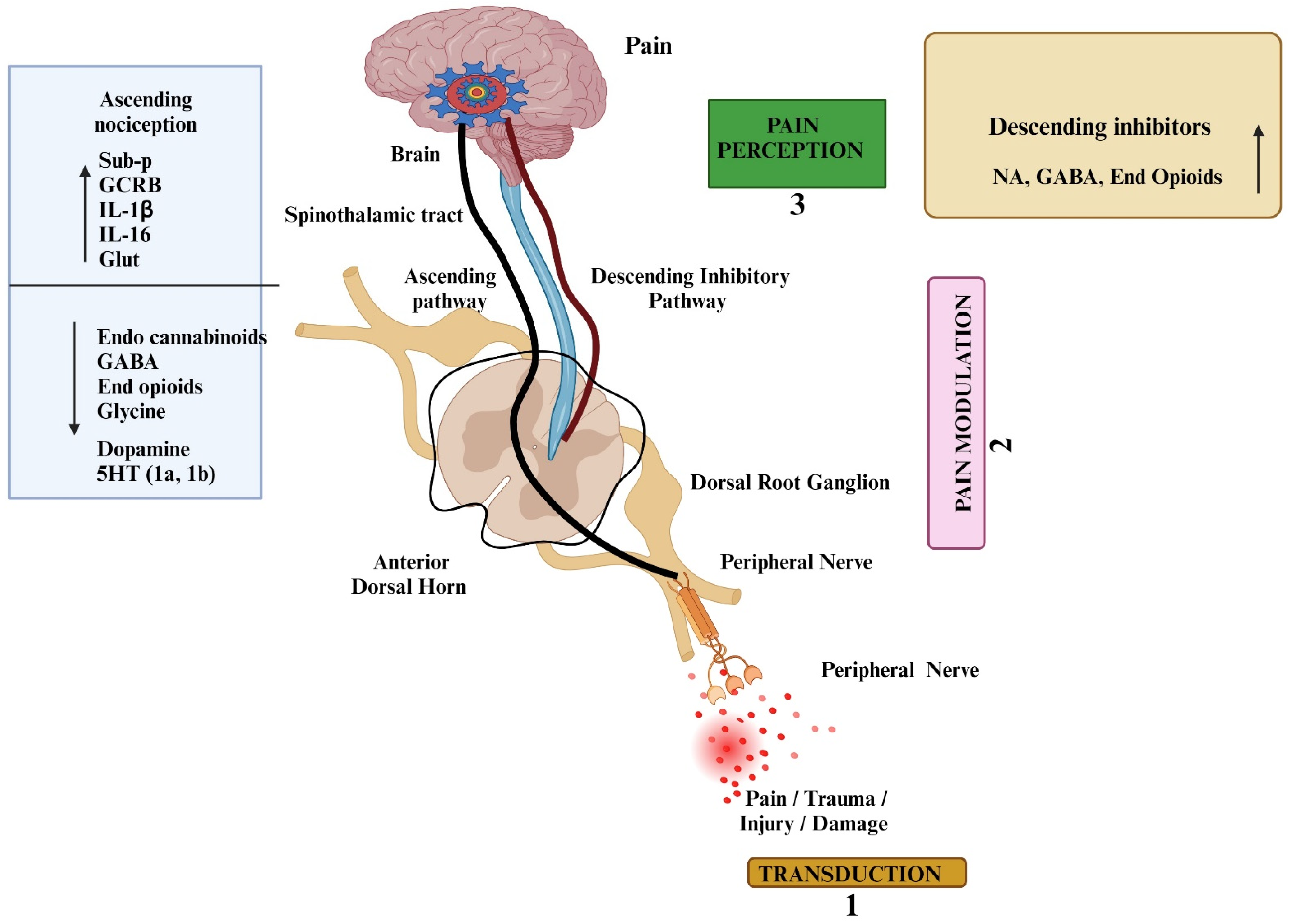
2. Signaling Mechanism of Pain
3. Epigenetic Pathway of Pain
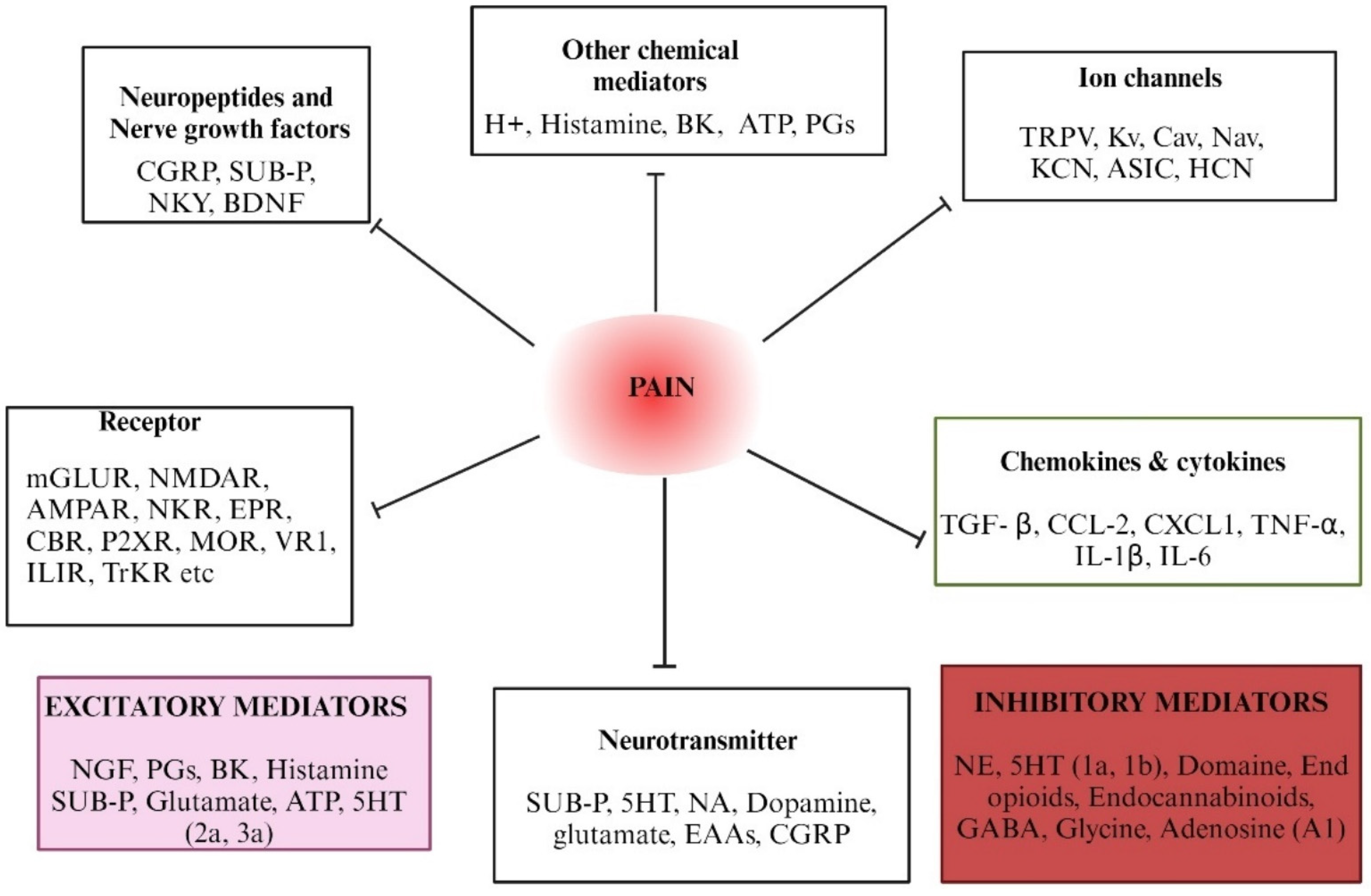
4. Mediators and Molecular Targets of Pain—An Overview
5. Classical Pain Models—An Overview
5.1. Inflammatory Pain Models
5.1.1. Complete Freund’s Adjuvant (CFA) Induced Inflammatory Pain Model
5.1.2. Formalin-Induced Nociceptive Pain Model
5.2. Visceral Pain Mechanisms Through Animal Models
The Colorectal Distension (CRD) Model
5.3. Mechanisms and Models of Neuropathic Pain
5.3.1. The Chronic Constriction Injury (CCI)
5.3.2. Spinal Nerve Ligation Model
5.3.3. Chronic Constriction of the Infraorbital Nerve Model
5.3.4. The Chronic Compression of Dorsal Root Ganglion (CCD) Model
5.3.5. STZ-Induced Diabetic Neuropathic Pain Model
5.3.6. Burn Injury-Induced Pain
5.4. Cancer Pain Mechanisms Through Animal Models
5.4.1. Syngeneic Tumor Implantation Model of Pain
5.4.2. Bone Metastasis Model of Pain
5.4.3. Development of Colorectal Carcinoma Metastasis Model of Pain
5.5. Genetically Modified Pain Models
5.5.1. TRPV1 Knockout Mice
5.5.2. OPRM1 Knockout Mice
5.5.3. TRPV1 Overexpression Mice
5.5.4. Nav1.7 Knock-In Mice
5.5.5. P2X3 Knockout Mice
5.5.6. Application of CRISPR in Pain Research: A Promising Frontier
5.5.7. CRISPR on Identification of Novel Pain Targets and Elucidating Pain Pathways
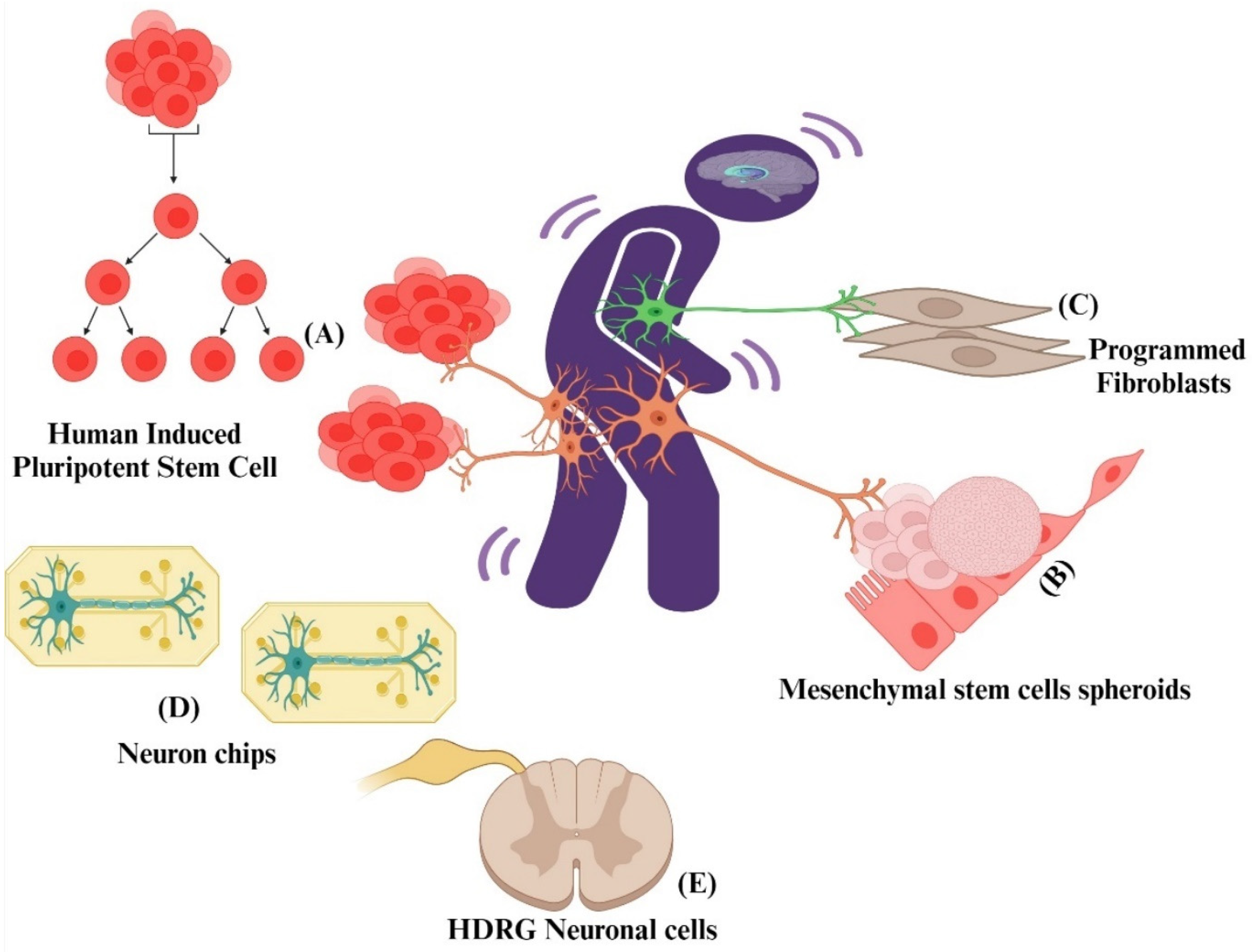
5.6. Cellular Models of Pain
5.6.1. Reprogrammed Nociceptor Neurons from Fibroblasts
5.6.2. Human-Induced Pluripotent Stem Cells (HiPSCs)
5.6.3. Human and Rat hDRG Neuronal Cultures for Pain Drug Discovery
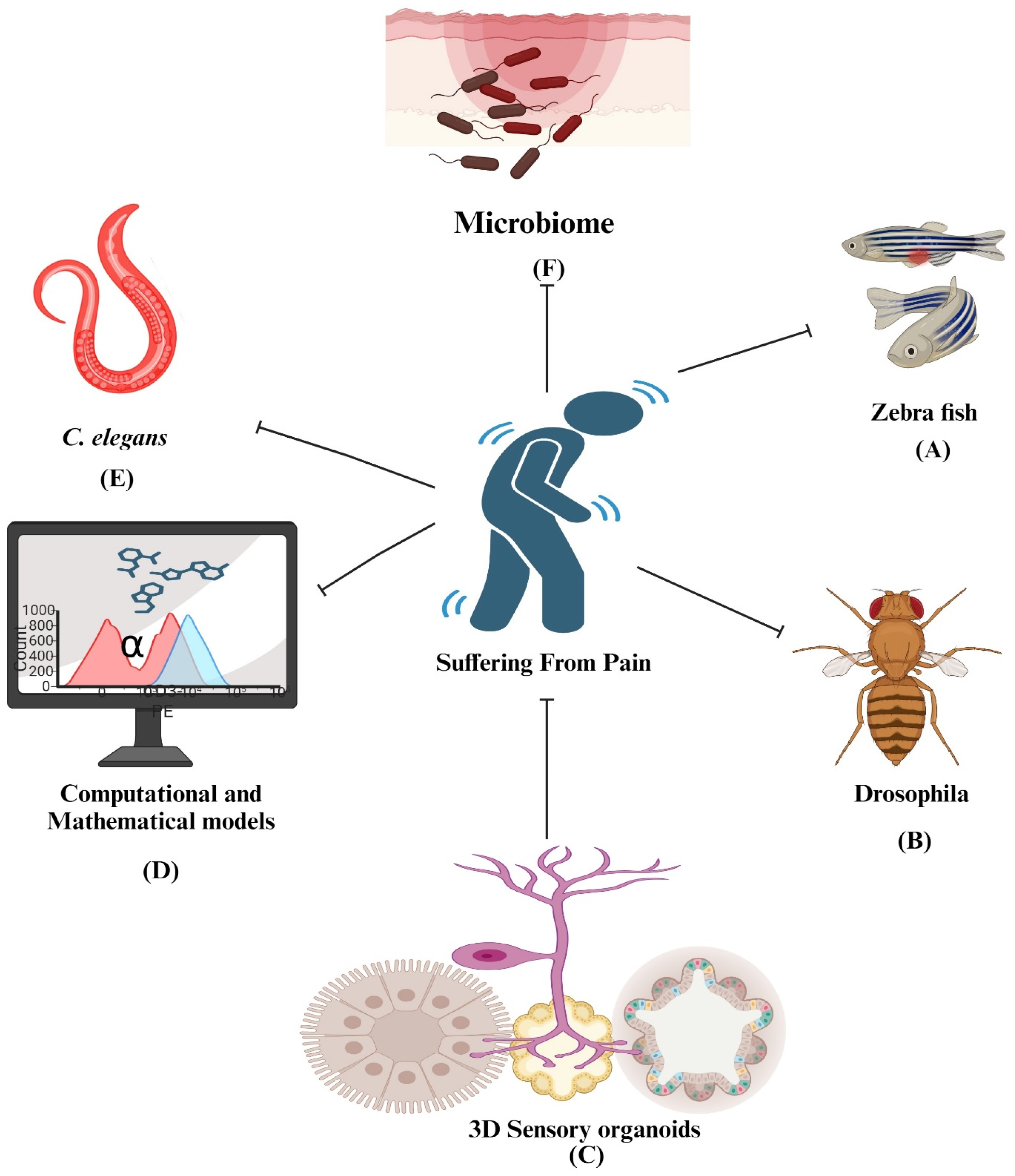
5.7. Alternative to Animal Models of Pain
5.7.1. Zebrafish (Danio rerio)—A Fish Model
5.7.2. Drosophila melanogaster: A Fruit Fly Model
5.7.3. Caenorhabditis elegans: A Nematode Model
5.8. Human Experimental Models of Pain
5.8.1. The Heat/Capsaicin Sensitization Model
5.8.2. Intradermal Capsaicin Model
5.8.3. The Cold Pressor Model
5.8.4. The Ultraviolet Light UV-B Pain Model
5.9. Role Human Volunteers in Advancing Pain Research and Ethical Considerations
5.10. Neuroimaging Techniques in Pain Research
5.10.1. Functional Magnetic Resonance Imaging (fMRI)
5.10.2. Positron Emission Tomography (PET)
5.10.3. Neuroimaging on Brain Circuitry, Plasticity and Pain Modulation
5.11. Microbiome in Pain Research
5.12. Computational and Mathematical Models in Pain Research
6. Animal Models of Pain: A Critical Evaluation of Validity
7. Progress and Challenges in Translational Pain Research
8. Pain Models and Analgesic Drug Discovery
Novel Pain Drug Development Using Animal Models
9. Review Summary
- Key areas of focus include:
- Molecular pain research: This includes investigating pain at the molecular level using techniques like genomics and proteomics, including employing gene editing, omics, and imaging to enhance pain research. It also includes utilizing high-resolution imaging to study pain pathways in real-time and at a cellular resolution, as well as studying the role of ion channel modulation in pain neurons for potential therapeutic targets.
- Genetically Modified Models: Advances in genetic engineering have led to the development of transgenic and knockout mice, allowing researchers to study the role of specific genes in pain pathways.
- In vitro models: These involve utilizing human sensory neurons (such as HiPSCs) and stem cell-derived nerves for preclinical research.
- Behavioral Assessments: These allow for improved methods for assessing pain-related behaviours in animals, including non-reflexive and voluntary behaviors, to better capture the complexity of pain.
- Bridging the gap: This entails addressing the disparity between animal and human pain through refined methods and measures, including developing models that reflect the genetic and phenotypic diversity of human populations, to better understand individual differences in pain perception and treatment responses
- Multimodal Approaches: These entail combining different types of pain models (e.g., inflammatory, neuropathic) to study the interplay between various pain mechanisms, as well as combining animal models with genomics, proteomics, and metabolomics to identify novel pain biomarkers and therapeutic targets.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stucky, C.L.; Gold, M.S.; Zhang, X. Mechanisms of Pain. Proc. Natl. Acad. Sci. USA 2001, 98, 11845–11846. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Watson, C.P.N. The Pharmacotherapy of Chronic Pain: A Review. Pain. Res. Manag. 2006, 11, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, P.; Stillman, A.M. Pathogenesis of Pain. Semin. Pediatr. Neurol. 2016, 23, 201–208. [Google Scholar] [CrossRef]
- Khalid, S.; Tubbs, R.S. Neuroanatomy and Neuropsychology of Pain. Cureus 2017, 9, e1754. [Google Scholar] [CrossRef]
- Borsook, D.; Hargreaves, R.; Bountra, C.; Porreca, F. Lost but Making Progress—Where Will New Analgesic Drugs Come from? Sci. Transl. Med. 2014, 6, 249sr3. [Google Scholar] [CrossRef]
- Woolf, C.J. Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Caudle, R.M.; Smith, M.T.; Romero-Sandoval, E.A. Editorial: Verification of Animal Pain Models by Reverse Translation. Front. Pharmacol. 2021, 12, 778880. [Google Scholar] [CrossRef]
- Herzberg, D.E.; Bustamante, H.A.; Herzberg, D.E.; Bustamante, H.A. Animal Models of Chronic Pain. Are Naturally Occurring Diseases a Potential Model for Translational Research? Austral J. Vet. Sci. 2021, 53, 47–54. [Google Scholar] [CrossRef]
- Clark, J.D. Preclinical Pain Research: Can We Do Better? Anesthesiology 2016, 125, 846–849. [Google Scholar] [CrossRef]
- Moran, C.J.; Ramesh, A.; Brama, P.A.J.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The Benefits and Limitations of Animal Models for Translational Research in Cartilage Repair. J. Exp. Orthop. 2016, 3, 1. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; Negus, S.S.; Martin, T.J. Editorial: Preclinical Animal Models and Measures of Pain: Improving Predictive Validity for Analgesic Drug Development. Front. Pain Res. 2022, 3, 867786. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, G.; Villano, I.; Ilardi, C.R.; Messina, A.; Monda, V.; Iodice, A.C.; Porro, C.; Panaro, M.A.; Chieffi, S.; Messina, G.; et al. Mechanisms of Transmission and Processing of Pain: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3064. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Morales-Lázaro, S.L.; Islas, L.D. TRP Channels: A Journey towards a Molecular Understanding of Pain. Nat. Rev. Neurosci. 2022, 23, 596–610. [Google Scholar] [CrossRef]
- Sneddon, L.U. Comparative Physiology of Nociception and Pain. Physiology 2018, 33, 63–73. [Google Scholar] [CrossRef]
- Tracey, W.D. Nociception. Curr. Biol. 2017, 27, R129–R133. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef]
- Jardín, I.; López, J.J.; Diez, R.; Sánchez-Collado, J.; Cantonero, C.; Albarrán, L.; Woodard, G.E.; Redondo, P.C.; Salido, G.M.; Smani, T.; et al. TRPs in Pain Sensation. Front. Physiol. 2017, 8, 392. [Google Scholar] [CrossRef]
- Woller, S.A.; Eddinger, K.A.; Corr, M.; Yaksh, T.L. An Overview of Pathways Encoding Nociception. Clin. Exp. Rheumatol. 2017, 35, 40–46. [Google Scholar]
- Luo, D.; Li, X.; Tang, S.; Song, F.; Li, W.; Xie, G.; Liang, J.; Zhou, J. Epigenetic Modifications in Neuropathic Pain. Mol. Pain 2021, 17, 17448069211056767. [Google Scholar] [CrossRef]
- Dhar, G.A.; Saha, S.; Mitra, P.; Nag Chaudhuri, R. DNA Methylation and Regulation of Gene Expression: Guardian of Our Health. Nucleus 2021, 64, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Villicaña, S.; Bell, J.T. Genetic Impacts on DNA Methylation: Research Findings and Future Perspectives. Genome Biol. 2021, 22, 127. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yao, Y.; Tao, Y.-X. Chapter 5—Role of DNA Methylation in Chronic Pain. In Epigenetics of Chronic Pain; Bai, G., Ren, K., Eds.; Translational Epigenetics; Academic Press: Cambridge, MA, USA, 2019; Volume 7, pp. 99–110. [Google Scholar]
- Møller Johansen, L.; Gerra, M.C.; Arendt-Nielsen, L. Time Course of DNA Methylation in Pain Conditions: From Experimental Models to Humans. Eur. J. Pain 2021, 25, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Costello, J. DNA Methylation: An Epigenetic Mark of Cellular Memory. Exp. Mol. Med. 2017, 49, e322. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Xiong, H.-Y.; Hendrix, J.; Schabrun, S.; Wyns, A.; Campenhout, J.V.; Nijs, J.; Polli, A. The Role of the Brain-Derived Neurotrophic Factor in Chronic Pain: Links to Central Sensitization and Neuroinflammation. Biomolecules 2024, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Shen, L.; Hou, Y. Epigenetic Modification of BDNF Mediates Neuropathic Pain via miR-30a-3p/EP300 Axis in CCI Rats. Biosci. Rep. 2020, 40, BSR20194442. [Google Scholar] [CrossRef]
- Crow, M.; Denk, F.; McMahon, S.B. Genes and Epigenetic Processes as Prospective Pain Targets. Genome Med. 2013, 5, 12. [Google Scholar] [CrossRef]
- Dourson, A.J.; Willits, A.; Raut, N.G.R.; Kader, L.; Young, E.; Jankowski, M.P.; Chidambaran, V. Genetic and Epigenetic Mechanisms Influencing Acute to Chronic Postsurgical Pain Transitions in Pediatrics: Preclinical to Clinical Evidence. Can. J. Pain 2022, 6, 85–107. [Google Scholar] [CrossRef]
- Jin, M.L.; Jeong, K.W. Histone Modifications in Drug-Resistant Cancers: From a Cancer Stem Cell and Immune Evasion Perspective. Exp. Mol. Med. 2023, 55, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, L.-X.; Tan, X.-Y.; Yu, P.; Dong, M. Inflammation and Histone Modification in Chronic Pain. Front. Immunol. 2023, 13, 1087648. [Google Scholar] [CrossRef]
- Diamantopoulos, M.A.; Tsiakanikas, P.; Scorilas, A. Non-Coding RNAs: The Riddle of the Transcriptome and Their Perspectives in Cancer. Ann. Transl. Med. 2018, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhong, W.; Gong, C.; Chen, B.; Guo, J. Global Research Trends on Epigenetics and Neuropathic Pain: A Bibliometric Analysis. Front. Mol. Neurosci. 2023, 16, 1145393. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Obeng, S.; Hiranita, T.; León, F.; McMahon, L.R.; McCurdy, C.R. Novel Approaches, Drug Candidates, and Targets in Pain Drug Discovery. J. Med. Chem. 2021, 64, 6523–6548. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thyagarajan, B. Pain Pathways and Potential New Targets for Pain Relief. Biotechnol. Appl. Biochem. 2022, 69, 110–123. [Google Scholar] [CrossRef]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of Inflammatory Mediators with Pain Perception. Biomed. Pharmacother. 2017, 96, 1445–1452. [Google Scholar] [CrossRef]
- Ji, J.; Huh, Y.; Ji, R.-R. Inflammatory Mediators, Nociceptors, and Their Interactions in Pain. In Neuroimmune Interactions in Pain: Mechanisms and Therapeutics; Ji, R.-R., Cheng, J., Ji, J., Eds.; Springer International Publishing: Cham, Switerland, 2023; pp. 87–119. ISBN 978-3-031-29231-6. [Google Scholar]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP1 and IP Reveals Peripheral Nociceptive Mechanism of Prostaglandins. Mol. Pain 2005, 1, 3. [Google Scholar] [CrossRef]
- Molyva, D. Neuropeptides and Pain. Ann. Gen. Psychiatry 2010, 9, S3. [Google Scholar] [CrossRef]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Li Puma, S.; Landini, L.; Portelli, F.; Innocenti, A.; de Araujo, D.S.M.; Janal, M.N.; Patacchini, R.; Bunnett, N.W.; Geppetti, P.; et al. Schwann Cells Expressing Nociceptive Channel TRPA1 Orchestrate Ethanol-Evoked Neuropathic Pain in Mice. J. Clin. Investig. 2019, 129, 5424–5441. [Google Scholar] [CrossRef] [PubMed]
- Ulugöl, A. The Endocannabinoid System as a Potential Therapeutic Target for Pain Modulation. Balkan Med. J. 2014, 31, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Abboud, C.; Duveau, A.; Bouali-Benazzouz, R.; Massé, K.; Mattar, J.; Brochoire, L.; Fossat, P.; Boué-Grabot, E.; Hleihel, W.; Landry, M. Animal Models of Pain: Diversity and Benefits. J. Neurosci. Methods 2021, 348, 108997. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.E.; Mogil, J.S.; Stucky, C.L. Innovations and Advances in Modelling and Measuring Pain in Animals. Nat. Rev. Neurosci. 2022, 23, 70–85. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999, 40, 111–118. [Google Scholar] [CrossRef]
- White, M. Mediators of Inflammation and the Inflammatory Process. J. Allergy Clin. Immunol. 1999, 103, S378–S381. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiiflammatory Drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Moncada, S.; Vane, J.R. Indomethacin and Aspirin Abolish Prostaglandin Release from the Spleen. Nat. New Biol. 1971, 231, 237–239. [Google Scholar] [CrossRef]
- Maleškić Kapo, S.; Rakanović-Todić, M.; Burnazović-Ristić, L.; Kusturica, J.; Kulo Ćesić, A.; Ademović, E.; Loga-Zec, S.; Sarač-Hadžihalilović, A.; Aganović-Mušinović, I. Analgesic and Anti-Inflammatory Effects of Diclofenac and Ketoprofen Patches in Two Different Rat Models of Acute Inflammation. J. King Saud Univ.-Sci. 2023, 35, 102394. [Google Scholar] [CrossRef]
- Gelderman, K.A.; Hultqvist, M.; Pizzolla, A.; Zhao, M.; Nandakumar, K.S.; Mattsson, R.; Holmdahl, R. Macrophages Suppress T Cell Responses and Arthritis Development in Mice by Producing Reactive Oxygen Species. J. Clin. Investig. 2007, 117, 3020–3028. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Wen, C.; Yan, Z.; Olatunji, O.J.; Yin, Z. Dehydrozingerone Alleviates Hyperalgesia, Oxidative Stress and Inflammatory Factors in Complete Freund’s Adjuvant-Induced Arthritic Rats. DDDT 2022, 16, 3015–3022. [Google Scholar] [CrossRef]
- Peng, S.; Hu, C.; Liu, X.; Lei, L.; He, G.; Xiong, C.; Wu, W. Rhoifolin Regulates Oxidative Stress and Proinflammatory Cytokine Levels in Freund’s Adjuvant-Induced Rheumatoid Arthritis via Inhibition of NF-κB. Braz. J. Med. Biol. Res. 2020, 53, e9489. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 Mediates Formalin-Induced Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y.; Fukuoka, T.; Yamanaka, H.; Kobayashi, K.; Obata, K.; Cui, X.; Tominaga, M.; Noguchi, K. Phospholipase C and Protein Kinase A Mediate Bradykinin Sensitization of TRPA1: A Molecular Mechanism of Inflammatory Pain. Brain 2008, 131, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rojas, V.A.; García, G.; Noriega-Navarro, R.; Guzmán-Priego, C.G.; Torres-López, J.E.; Granados-Soto, V.; Murbartián, J. Peripheral and Spinal TRPA1 Channels Contribute to Formalin-Induced Long-Lasting Mechanical Hypersensitivity. J. Pain Res. 2017, 11, 51–60. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The Formalin Test: A Quantitative Study of the Analgesic Effects of Morphine, Meperidine, and Brain Stem Stimulation in Rats and Cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Abbott, F.V.; Franklin, K.B.J.; Westbrook, F.R. The Formalin Test: Scoring Properties of the First and Second Phases of the Pain Response in Rats. Pain 1995, 60, 91–102. [Google Scholar] [CrossRef]
- Hoffmann, T.; Klemm, F.; I Kichko, T.; Sauer, S.K.; Kistner, K.; Riedl, B.; Raboisson, P.; Luo, L.; Babes, A.; Kocher, L.; et al. The Formalin Test Does Not Probe Inflammatory Pain but Excitotoxicity in Rodent Skin. Physiol. Rep. 2022, 10, e15194. [Google Scholar] [CrossRef]
- López-Cano, M.; Fernández-Dueñas, V.; Llebaria, A.; Ciruela, F. Formalin Murine Model of Pain. Bio-Protocol 2017, 7, e2628. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.M.; Cater, H.L.; Thakur, M.; Wells, S.; McMahon, S.B. A Refinement to the Formalin Test in Mice. F1000Res 2019, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Tramullas, M.; Fitzgerald, P.; Cryan, J.F. Rodent Models of Colorectal Distension. Curr. Protoc. Neurosci. 2012, 61, 9–40. [Google Scholar] [CrossRef] [PubMed]
- Moshiree, B.; Zhou, Q.; Price, D.D.; Verne, G.N. Central Sensitisation in Visceral Pain Disorders. Gut 2006, 55, 905–908. [Google Scholar] [CrossRef]
- Takezawa, K.; Kondo, M.; Nonomura, N.; Shimada, S. Urothelial ATP Signaling: What Is Its Role in Bladder Sensation? Neurourol. Urodyn. 2017, 36, 966–972. [Google Scholar] [CrossRef]
- Chen, X.; Gebhart, G.F. Differential Purinergic Signaling in Bladder Sensory Neurons of Naïve and Bladder-Inflamed Mice. Pain 2010, 148, 462–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birder, L.; Andersson, K.-E. Urothelial Signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, L.; Chen, H.; Hill, W.G.; Robson, S.C.; Zeidel, M.L.; Yu, W. Targetable Purinergic Receptors P2Y12 and A2b Antagonistically Regulate Bladder Function. JCI Insight 2019, 4, e122112. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The Neuropathic Pain: An Overview of the Current Treatment and Future Therapeutic Approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Hameed, S. Nav1.7 and Nav1.8: Role in the Pathophysiology of Pain. Mol. Pain 2019, 15, 1744806919858801. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.-D.; Zubieta, J.-K. Human Brain Mechanisms of Pain Perception and Regulation in Health and Disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Marziniak, M.; Schäfers, M.; Toyka, K.V.; Sommer, C. Thalidomide Treatment in Chronic Constrictive Neuropathy Decreases Endoneurial Tumor Necrosis Factor-Alpha, Increases Interleukin-10 and Has Long-Term Effects on Spinal Cord Dorsal Horn Met-Enkephalin. Pain 2000, 88, 267–275. [Google Scholar] [CrossRef]
- Da Silva, L.F.; Desantana, J.M.; Sluka, K.A. Activation of NMDA Receptors in the Brainstem, Rostral Ventromedial Medulla, and Nucleus Reticularis Gigantocellularis Mediates Mechanical Hyperalgesia Produced by Repeated Intramuscular Injections of Acidic Saline in Rats. J. Pain 2010, 11, 378–387. [Google Scholar] [CrossRef]
- Li, Y.; Dorsi, M.J.; Meyer, R.A.; Belzberg, A.J. Mechanical Hyperalgesia after an L5 Spinal Nerve Lesion in the Rat Is Not Dependent on Input from Injured Nerve Fibers. Pain 2000, 85, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Kim, H.K.; Chung, K. Segmental Spinal Nerve Ligation Model of Neuropathic Pain. Methods Mol. Med. 2004, 99, 35–45. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Ji, R.-R. Chemokines, Neuronal-Glial Interactions, and Central Processing of Neuropathic Pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial Activation: A Driving Force for Pathological Pain. Trends Neurosci. 2001, 24, 450–455. [Google Scholar] [CrossRef]
- LaBuda, C.J.; Little, P.J. Pharmacological Evaluation of the Selective Spinal Nerve Ligation Model of Neuropathic Pain in the Rat. J. Neurosci. Methods 2005, 144, 175–181. [Google Scholar] [CrossRef]
- Deseure, K.; Hans, G.H. Chronic Constriction Injury of the Rat’s Infraorbital Nerve (IoN-CCI) to Study Trigeminal Neuropathic Pain. J. Vis. Exp. 2015, 103, e53167. [Google Scholar] [CrossRef]
- Luo, Y.; Suttle, A.; Zhang, Q.; Wang, P.; Chen, Y. Transient Receptor Potential (TRP) Ion Channels in Orofacial Pain. Mol. Neurobiol. 2021, 58, 2836–2850. [Google Scholar] [CrossRef] [PubMed]
- Staikopoulos, V.; Sessle, B.J.; Furness, J.B.; Jennings, E.A. Localization of P2X2 and P2X3 Receptors in Rat Trigeminal Ganglion Neurons. Neuroscience 2007, 144, 208–216. [Google Scholar] [CrossRef]
- Jara-Oseguera, A.; Simon, S.A.; Rosenbaum, T. TRPV1: On the Road to Pain Relief. Curr. Mol. Pharmacol. 2008, 1, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Kernisant, M.; Gear, R.W.; Jasmin, L.; Vit, J.-P.; Ohara, P.T. Chronic Constriction Injury of the Infraorbital Nerve in the Rat Using Modified Syringe Needle. J. Neurosci. Methods 2008, 172, 43–47. [Google Scholar] [CrossRef]
- Fan, N.; Donnelly, D.F.; LaMotte, R.H. Chronic Compression of Mouse Dorsal Root Ganglion Alters Voltage-Gated Sodium and Potassium Currents in Medium-Sized Dorsal Root Ganglion Neurons. J. Neurophysiol. 2011, 106, 3067–3072. [Google Scholar] [CrossRef][Green Version]
- Wu, G.; Ringkamp, M.; Hartke, T.V.; Murinson, B.B.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Early Onset of Spontaneous Activity in Uninjured C-Fiber Nociceptors after Injury to Neighboring Nerve Fibers. J. Neurosci. 2001, 21, RC140. [Google Scholar] [CrossRef] [PubMed]
- Devor, M.; Govrin-Lippmann, R.; Rappaport, Z.H. Mechanism of Trigeminal Neuralgia: An Ultrastructural Analysis of Trigeminal Root Specimens Obtained during Microvascular Decompression Surgery. J. Neurosurg. 2002, 96, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Sewell, R.D.E. Neuropathic Pain Models and Outcome Measures: A Dual Translational Challenge. Ann. Transl. Med. 2018, 6, S42. [Google Scholar] [CrossRef]
- Velázquez-Flores, M.Á.; Sánchez-Chávez, G.; Morales-Lázaro, S.L.; Ruiz Esparza-Garrido, R.; Canizales-Ontiveros, A.; Salceda, R. Streptozotocin-Induced Diabetic Rats Showed a Differential Glycine Receptor Expression in the Spinal Cord: A GlyR Role in Diabetic Neuropathy. Neurochem. Res. 2024, 49, 684–691. [Google Scholar] [CrossRef]
- Pham, V.M.; Matsumura, S.; Katano, T.; Funatsu, N.; Ito, S. Diabetic Neuropathy Research: From Mouse Models to Targets for Treatment. Neural Regen. Res. 2019, 14, 1870–1879. [Google Scholar] [CrossRef]
- Chen, S.-R.; Zhang, J.; Chen, H.; Pan, H.-L. Streptozotocin-Induced Diabetic Neuropathic Pain Is Associated with Potentiated Calcium-Permeable AMPA Receptor Activity in the Spinal Cord. J. Pharmacol. Exp. Ther. 2019, 371, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zheng, Z.M. Animal Models of Diabetic Neuropathic Pain. Exp. Clin. Endocrinol. Diabetes 2014, 122, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Subhan, F.; Abbas, M.; Zeb, J.; Shahid, M.; Sewell, R.D.E. A Streptozotocin-Induced Diabetic Neuropathic Pain Model for Static or Dynamic Mechanical Allodynia and Vulvodynia: Validation Using Topical and Systemic Gabapentin. Naunyn-Schmiedebergs Arch. Pharmacol. 2015, 388, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Hidmark, A.S.; Sturm, V.; Fischer, M.; Milford, D.; Hausser, I.; Sahm, F.; Breckwoldt, M.O.; Agarwal, N.; Kuner, R.; et al. Characterization of Experimental Diabetic Neuropathy Using Multicontrast Magnetic Resonance Neurography at Ultra High Field Strength. Sci. Rep. 2020, 10, 7593. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kang, D.-W.; Kim, J.; Lee, M.; Choi, S.-R.; Park, J.B.; Kim, H.-W. Burn Injury-Induced Pain and Depression-Like Behavior in Mice. J. Vis. Exp. 2021, 175, e62817. [Google Scholar] [CrossRef]
- Hao, D.; Nourbakhsh, M. Recent Advances in Experimental Burn Models. Biology 2021, 10, 526. [Google Scholar] [CrossRef]
- Mészár, Z.; Erdei, V.; Szücs, P.; Varga, A. Epigenetic Regulation and Molecular Mechanisms of Burn Injury-Induced Nociception in the Spinal Cord of Mice. Int. J. Mol. Sci. 2024, 25, 8510. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Shields, S.D.; Cheng, X.; Uçeyler, N.; Sommer, C.; Dib-Hajj, S.D.; Waxman, S.G. Sodium Channel Na(v)1.7 Is Essential for Lowering Heat Pain Threshold after Burn Injury. J. Neurosci. 2012, 32, 10819–10832. [Google Scholar] [CrossRef]
- Summer, G.J.; Puntillo, K.A.; Miaskowski, C.; Dina, O.A.; Green, P.G.; Levine, J.D. TrkA and PKC-Epsilon in Thermal Burn-Induced Mechanical Hyperalgesia in the Rat. J. Pain 2006, 7, 884–891. [Google Scholar] [CrossRef]
- Roy, T.K.; Uniyal, A.; Akhilesh; Tiwari, V. Multifactorial Pathways in Burn Injury-Induced Chronic Pain: Novel Targets and Their Pharmacological Modulation. Mol. Biol. Rep. 2022, 49, 12121–12132. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.L.; Sena, E.S.; Fallon, M.T.; Macleod, M.R.; Colvin, L.A. Using Animal Models to Understand Cancer Pain in Humans. Curr. Pain Headache Rep. 2014, 18, 423. [Google Scholar] [CrossRef] [PubMed]
- Pacharinsak, C.; Beitz, A. Animal Models of Cancer Pain. Comp. Med. 2008, 58, 220–233. [Google Scholar]
- Schmidt, B.L.; Hamamoto, D.T.; Simone, D.A.; Wilcox, G.L. Mechanism of Cancer Pain. Mol. Interv. 2010, 10, 164–178. [Google Scholar] [CrossRef]
- Heussner, M.J.; Folger, J.K.; Dias, C.; Massri, N.; Dahdah, A.; Vermeer, P.D.; Laumet, G. A Novel Syngeneic Immunocompetent Mouse Model of Head and Neck Cancer Pain Independent of Interleukin-1 Signaling. Anesth. Analg. 2021, 132, 1156–1163. [Google Scholar] [CrossRef]
- Horan, N.L.; McIlvried, L.A.; Atherton, M.A.; Yuan, M.M.; Dolan, J.C.; Scheff, N.N. The Impact of Tumor Immunogenicity on Cancer Pain Phenotype Using Syngeneic Oral Cancer Mouse Models. Front. Pain Res. 2022, 3, 991725. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Farias, J.B.; Saloman, J.L.; Scheff, N.N. Animal Models of Cancer-Related Pain: Current Perspectives in Translation. Front. Pharmacol. 2020, 11, 610894. [Google Scholar] [CrossRef]
- Slosky, L.M.; Largent-Milnes, T.M.; Vanderah, T.W. Use of Animal Models in Understanding Cancer-Induced Bone Pain. Cancer Growth Metastasis 2015, 8, 47–62. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wordliczek, J. Bone Pain in Cancer Patients: Mechanisms and Current Treatment. Int. J. Mol. Sci. 2019, 20, 6047. [Google Scholar] [CrossRef]
- Mantyh, P.W. Bone Cancer Pain: From Mechanism to Therapy. Curr. Opin. Support. Palliat. Care 2014, 8, 83–90. [Google Scholar] [CrossRef]
- Liu, Z.; Murphy, S.F.; Huang, J.; Zhao, L.; Hall, C.C.; Schaeffer, A.J.; Schaeffer, E.M.; Thumbikat, P. A Novel Immunocompetent Model of Metastatic Prostate Cancer-Induced Bone Pain. Prostate 2020, 80, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Jin, X.; Zhang, Q.; Zhou, Y.; Zhu, C.; Yang, Y.; Tang, Z.; Yu, G.; Wang, C. A Mouse Model of Cancer Induced Bone Pain: From Pain to Movement. Front. Behav. Neurosci. 2022, 16, 873750. [Google Scholar] [CrossRef] [PubMed]
- DE ROSA, M.; PACE, U.; REGA, D.; COSTABILE, V.; DURATURO, F.; IZZO, P.; DELRIO, P. Genetics, Diagnosis and Management of Colorectal Cancer (Review). Oncol. Rep. 2015, 34, 1087–1096. [Google Scholar] [CrossRef]
- Terracina, K.P.; Aoyagi, T.; Huang, W.-C.; Nagahashi, M.; Yamada, A.; Aoki, K.; Takabe, K. Development of a Metastatic Murine Colon Cancer Model. J. Surg. Res. 2015, 199, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Strelez, C.; Chilakala, S.; Ghaffarian, K.; Lau, R.; Spiller, E.; Ung, N.; Hixon, D.; Yoon, A.Y.; Sun, R.X.; Lenz, H.-J.; et al. Human Colorectal Cancer-on-Chip Model to Study the Microenvironmental Influence on Early Metastatic Spread. iScience 2021, 24, 102509. [Google Scholar] [CrossRef]
- Antoniazzi, C.T.d.D.; Ruviaro, N.A.; Peres, D.S.; Rodrigues, P.; Viero, F.T.; Trevisan, G. Targeting TRPV4 Channels for Cancer Pain Relief. Cancers 2024, 16, 1703. [Google Scholar] [CrossRef]
- de Almeida, A.S.; Bernardes, L.d.B.; Trevisan, G. TRP Channels in Cancer Pain. Eur. J. Pharmacol. 2021, 904, 174185. [Google Scholar] [CrossRef]
- Scuteri, D.; Hamamura, K.; Watanabe, C.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Transgenic Mice for the Translational Study of Neuropathic Pain and Dystonia. Int. J. Mol. Sci. 2022, 23, 8580. [Google Scholar] [CrossRef]
- Wotton, J.M.; Peterson, E.; Flenniken, A.M.; Bains, R.S.; Veeraragavan, S.; Bower, L.R.; Bubier, J.A.; Parisien, M.; Bezginov, A.; Haselimashhadi, H.; et al. Identifying Genetic Determinants of Inflammatory Pain in Mice Using a Large-Scale Gene-Targeted Screen. Pain 2022, 163, 1139–1157. [Google Scholar] [CrossRef]
- Christoph, T.; Bahrenberg, G.; De Vry, J.; Englberger, W.; Erdmann, V.A.; Frech, M.; Kögel, B.; Röhl, T.; Schiene, K.; Schröder, W.; et al. Investigation of TRPV1 Loss-of-Function Phenotypes in Transgenic shRNA Expressing and Knockout Mice. Mol. Cell. Neurosci. 2008, 37, 579–589. [Google Scholar] [CrossRef]
- Bölcskei, K.; Helyes, Z.; Szabó, Á.; Sándor, K.; Elekes, K.; Németh, J.; Almási, R.; Pintér, E.; Pethő, G.; Szolcsányi, J. Investigation of the Role of TRPV1 Receptors in Acute and Chronic Nociceptive Processes Using Gene-Deficient Mice. Pain 2005, 117, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.R.; Beyer, C.E.; Stahl, S.M. TRPV1 Antagonists and Chronic Pain: Beyond Thermal Perception. Pharmaceuticals 2012, 5, 114–132. [Google Scholar] [CrossRef]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2021, 81, 7–27. [Google Scholar] [CrossRef]
- Maldonado, R.; Baños, J.E.; Cabañero, D. Usefulness of Knockout Mice to Clarify the Role of the Opioid System in Chronic Pain. Br. J. Pharmacol. 2018, 175, 2791–2808. [Google Scholar] [CrossRef]
- Erbs, E.; Faget, L.; Scherrer, G.; Matifas, A.; Filliol, D.; Vonesch, J.-L.; Koch, M.; Kessler, P.; Hentsch, D.; Birling, M.-C.; et al. A Mu-Delta Opioid Receptor Brain Atlas Reveals Neuronal Co-Occurrence in Subcortical Networks. Brain Struct. Funct. 2015, 220, 677–702. [Google Scholar] [CrossRef]
- Zhang, Y.; Picetti, R.; Butelman, E.R.; Ho, A.; Blendy, J.A.; Kreek, M.J. Mouse Model of the OPRM1 (A118G) Polymorphism: Differential Heroin Self-Administration Behavior Compared with Wild-Type Mice. Neuropsychopharmacology 2015, 40, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Degrandmaison, J.; Rochon-Haché, S.; Parent, J.-L.; Gendron, L. Knock-In Mouse Models to Investigate the Functions of Opioid Receptors in Vivo. Front. Cell. Neurosci. 2022, 16, 807549. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kim, M.; Ali, Z.; Ong, K.; Pae, E.-K.; Chung, M.-K. TRPV1 and TRPV1-Expressing Nociceptors Mediate Orofacial Pain Behaviors in a Mouse Model of Orthodontic Tooth Movement. Front. Physiol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Duo, L.; Hu, L.; Tian, N.; Cheng, G.; Wang, H.; Lin, Z.; Wang, Y.; Yang, Y. TRPV1 Gain-of-Function Mutation Impairs Pain and Itch Sensations in Mice. Mol. Pain 2018, 14, 1744806918762031. [Google Scholar] [CrossRef]
- Treat, A.; Henri, V.; Liu, J.; Shen, J.; Gil-Silva, M.; Morales, A.; Rade, A.; Tidgewell, K.J.; Kolber, B.; Shen, Y. Novel TRPV1 Modulators with Reduced Pungency Induce Analgesic Effects in Mice. ACS Omega 2022, 7, 2929–2946. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Waxman, S.G. Sodium Channels in Human Pain Disorders: Genetics and Pharmacogenomics. Annu. Rev. Neurosci. 2019, 42, 87–106. [Google Scholar] [CrossRef]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-Specific Gene Deletion Reveals a Major Role for Nav1.7 (PN1) in Acute and Inflammatory Pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar] [CrossRef] [PubMed]
- Gingras, J.; Smith, S.; Matson, D.J.; Johnson, D.; Nye, K.; Couture, L.; Feric, E.; Yin, R.; Moyer, B.D.; Peterson, M.L.; et al. Global Nav1.7 Knockout Mice Recapitulate the Phenotype of Human Congenital Indifference to Pain. PLoS ONE 2014, 9, e105895. [Google Scholar] [CrossRef]
- Minett, M.S.; Nassar, M.A.; Clark, A.K.; Passmore, G.; Dickenson, A.H.; Wang, F.; Malcangio, M.; Wood, J.N. Distinct Nav1.7-Dependent Pain Sensations Require Different Sets of Sensory and Sympathetic Neurons. Nat. Commun. 2012, 3, 791. [Google Scholar] [CrossRef]
- Cockayne, D.A.; Hamilton, S.G.; Zhu, Q.M.; Dunn, P.M.; Zhong, Y.; Novakovic, S.; Malmberg, A.B.; Cain, G.; Berson, A.; Kassotakis, L.; et al. Urinary Bladder Hyporeflexia and Reduced Pain-Related Behaviour in P2X3-Deficient Mice. Nature 2000, 407, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.-H.; Shyu, B.-C. Nociceptive Transmission and Modulation via P2X Receptors in Central Pain Syndrome. Mol. Brain 2016, 9, 58. [Google Scholar] [CrossRef]
- Vlaskovska, M.; Kasakov, L.; Rong, W.; Bodin, P.; Bardini, M.; Cockayne, D.A.; Ford, A.P.; Burnstock, G. P2X3 Knock-out Mice Reveal a Major Sensory Role for Urothelially Released ATP. J. Neurosci. 2001, 21, 5670–5677. [Google Scholar] [CrossRef]
- Jarvis, M.F.; Burgard, E.C.; McGaraughty, S.; Honore, P.; Lynch, K.; Brennan, T.J.; Subieta, A.; Van Biesen, T.; Cartmell, J.; Bianchi, B.; et al. A-317491, a Novel Potent and Selective Non-Nucleotide Antagonist of P2X3 and P2X2/3 Receptors, Reduces Chronic Inflammatory and Neuropathic Pain in the Rat. Proc. Natl. Acad. Sci. USA 2002, 99, 17179–17184. [Google Scholar] [CrossRef]
- Sun, L.; Lutz, B.M.; Tao, Y.-X. The CRISPR/Cas9 System for Gene Editing and Its Potential Application in Pain Research. Transl. Perioper. Pain Med. 2016, 1, 22–33. [Google Scholar]
- Remmel, A. CRISPR-Based Gene Therapy Dampens Pain in Mice. Nature 2021, 591, 359. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Cambria, E.; Besse, L.; Besse, A.; Bowles, R.; Wuertz-Kozak, K. The Potential of CRISPR/Cas9 Genome Editing for the Study and Treatment of Intervertebral Disc Pathologies. JOR Spine 2018, 1, e1003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, J.; Fan, Y.; Li, J.; You, T.; He, S.; Xiao, G.; Chen, D. Exploration of CRISPR/Cas9-Based Gene Editing as Therapy for Osteoarthritis. Ann. Rheum. Dis. 2019, 78, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, L.; Lai, Y.; Lu, K.; Huang, J. CRISPR-Cas9-Mediated Loss of Function of β-Catenin Attenuates Intervertebral Disc Degeneration. Mol. Ther. Nucleic Acids 2022, 28, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tang, M.; Xu, Z.; Wang, Y.; Li, M.; Zheng, S.; Zhu, J.; Lin, Z.; Zhang, M. CRISPR/Cas9 System and Its Applications in Nervous System Diseases. Genes Dis. 2024, 11, 675–686. [Google Scholar] [CrossRef]
- Koga, K.; Kobayashi, K.; Tsuda, M.; Kubota, K.; Kitano, Y.; Furue, H. Voltage-Gated Calcium Channel Subunit A2δ-1 in Spinal Dorsal Horn Neurons Contributes to Aberrant Excitatory Synaptic Transmission and Mechanical Hypersensitivity after Peripheral Nerve Injury. Front. Mol. Neurosci. 2023, 16, 1099925. [Google Scholar] [CrossRef]
- Larson, M.H.; Gilbert, L.A.; Wang, X.; Lim, W.A.; Weissman, J.S.; Qi, L.S. CRISPR Interference (CRISPRi) for Sequence-Specific Control of Gene Expression. Nat. Protoc. 2013, 8, 2180–2196. [Google Scholar] [CrossRef]
- Farhang, N.; Brunger, J.M.; Stover, J.D.; Thakore, P.I.; Lawrence, B.; Guilak, F.; Gersbach, C.A.; Setton, L.A.; Bowles, R.D. CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments. Tissue Eng. Part A 2017, 23, 738–749. [Google Scholar] [CrossRef]
- Renthal, W.; Chamessian, A.; Curatolo, M.; Davidson, S.; Burton, M.; Dib-Hajj, S.; Dougherty, P.M.; Ebert, A.D.; Gereau, R.W.; Ghetti, A.; et al. Human Cells and Networks of Pain: Transforming Pain Target Identification and Therapeutic Development. Neuron 2021, 109, 1426–1429. [Google Scholar] [CrossRef]
- Tsuda, M.; Koga, K.; Chen, T.; Zhuo, M. Neuronal and Microglial Mechanisms for Neuropathic Pain in the Spinal Dorsal Horn and Anterior Cingulate Cortex. J. Neurochem. 2017, 141, 486–498. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, P.; Wen, Q. Optimization Strategies for Mesenchymal Stem Cell-Based Analgesia Therapy: A Promising Therapy for Pain Management. Stem Cell Res. Ther. 2024, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Negro, S.; Pirazzini, M.; Rigoni, M. Models and Methods to Study Schwann Cells. J. Anat. 2022, 241, 1235–1258. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell. Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Furusho, M.; Dupree, J.L.; Bryant, M.; Bansal, R. Disruption of Fibroblast Growth Factor Receptor Signaling in Nonmyelinating Schwann Cells Causes Sensory Axonal Neuropathy and Impairment of Thermal Pain Sensitivity. J. Neurosci. 2009, 29, 1608–1614. [Google Scholar] [CrossRef]
- Hartlehnert, M.; Derksen, A.; Hagenacker, T.; Kindermann, D.; Schäfers, M.; Pawlak, M.; Kieseier, B.C.; Meyer Zu Horste, G. Schwann Cells Promote Post-Traumatic Nerve Inflammation and Neuropathic Pain through MHC Class II. Sci. Rep. 2017, 7, 12518. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.-Y.; Ling, Z.-M.; Chen, G.; Wei, Z.-Y. Inhibition of Schwann Cell Pannexin 1 Attenuates Neuropathic Pain through the Suppression of Inflammatory Responses. J. Neuroinflamm. 2022, 19, 244. [Google Scholar] [CrossRef]
- Abdo, H.; Calvo-Enrique, L.; Lopez, J.M.; Song, J.; Zhang, M.-D.; Usoskin, D.; El Manira, A.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized Cutaneous Schwann Cells Initiate Pain Sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef]
- Bremer, M.; Fröb, F.; Kichko, T.; Reeh, P.; Tamm, E.R.; Suter, U.; Wegner, M. Sox10 Is Required for Schwann-Cell Homeostasis and Myelin Maintenance in the Adult Peripheral Nerve. Glia 2011, 59, 1022–1032. [Google Scholar] [CrossRef]
- Ojeda-Alonso, J.; Calvo-Enrique, L.; Paricio-Montesinos, R.; Kumar, R.; Zhang, M.-D.; Poulet, J.F.A.; Ernfors, P.; Lewin, G.R. Sensory Schwann Cells Set Perceptual Thresholds for Touch and Selectively Regulate Mechanical Nociception. Nat. Commun. 2024, 15, 898. [Google Scholar] [CrossRef]
- Hu, X.; Agarwal, N.; Zhang, M.-D.; Ernfors, P.; Kuner, R.; Nyengaard, J.R.; Karlsson, P. Identification and Quantification of Nociceptive Schwann Cells in Mice with and without Streptozotocin-Induced Diabetes. J. Chem. Neuroanat. 2022, 123, 102118. [Google Scholar] [CrossRef]
- Xu, Z.; Su, S.; Zhou, S.; Yang, W.; Deng, X.; Sun, Y.; Li, L.; Li, Y. How to Reprogram Human Fibroblasts to Neurons. Cell Biosci. 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.W.; Eade, K.T.; Szűcs, A.; Lo Sardo, V.; Tsunemoto, R.K.; Williams, D.; Sanna, P.P.; Baldwin, K.K. Selective Conversion of Fibroblasts into Peripheral Sensory Neurons. Nat. Neurosci. 2015, 18, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wainger, B.J.; Buttermore, E.D.; Oliveira, J.T.; Mellin, C.; Lee, S.; Saber, W.A.; Wang, A.J.; Ichida, J.K.; Chiu, I.M.; Barrett, L.; et al. Modeling Pain in Vitro Using Nociceptor Neurons Reprogrammed from Fibroblasts. Nat. Neurosci. 2015, 18, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.G.; Daley, G.Q. Induced Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, S.; Guo, X.; Colón, A.; Jackson, M.; Akanda, N.; Patel, A.; Grillo, M.; Hickman, J.J. Development of a Functional Human Induced Pluripotent Stem Cell-Derived Nociceptor MEA System as a Pain Model for Analgesic Drug Testing. Front. Cell. Dev. Biol. 2023, 11, 1011145. [Google Scholar] [CrossRef] [PubMed]
- Fakoya, A.O.J.; Omole, A.E.; Satyadev, N.; Haider, K.H. Induced Pluripotent Stem Cells. In Handbook of Stem Cell Therapy; Haider, K.H., Ed.; Springer Nature: Singapore, 2022; pp. 895–919. ISBN 978-981-19265-5-6. [Google Scholar]
- Alsaloum, M.; Waxman, S.G. iPSCs and DRGs: Stepping Stones to New Pain Therapies. Trends Mol. Med. 2022, 28, 110–122. [Google Scholar] [CrossRef]
- Labau, J.I.R.; Andelic, M.; Faber, C.G.; Waxman, S.G.; Lauria, G.; Dib-Hajj, S.D. Recent Advances for Using Human Induced-Pluripotent Stem Cells as Pain-in-a-Dish Models of Neuropathic Pain. Exp. Neurol. 2022, 358, 114223. [Google Scholar] [CrossRef]
- Chrysostomidou, L.; Cooper, A.H.; Weir, G.A. Cellular Models of Pain: New Technologies and Their Potential to Progress Preclinical Research. Neurobiol. Pain 2021, 10, 100063. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Chen, J.-C. Dorsal Root Ganglia Isolation and Primary Culture to Study Neurotransmitter Release. J. Vis. Exp. 2018, 140, 57569. [Google Scholar] [CrossRef]
- Liu, P.; Chen, T.; Tan, F.; Tian, J.; Zheng, L.; Deng, Y.; Chen, J.; Chi, X. Dexmedetomidine Alleviated Neuropathic Pain in Dorsal Root Ganglion Neurons by Inhibition of Anaerobic Glycolysis Activity and Enhancement of ROS Tolerance. Biosci. Rep. 2020, 40, BSR20191994. [Google Scholar] [CrossRef]
- Wangzhou, A.; McIlvried, L.A.; Paige, C.; Barragan-Iglesias, P.; Shiers, S.; Ahmad, A.; Guzman, C.A.; Dussor, G.; Ray, P.R.; Gereau, R.W.; et al. Pharmacological Target-Focused Transcriptomic Analysis of Native vs Cultured Human and Mouse Dorsal Root Ganglia. Pain 2020, 161, 1497–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Priest, B.T.; Belfer, I.; Gold, M.S. Voltage-Gated Na+ Currents in Human Dorsal Root Ganglion Neurons. Elife 2017, 6, e23235. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The Current Status and Biomedical Applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development. Int. J. Mol. Sci. 2023, 24, 3113. [Google Scholar] [CrossRef]
- Pereira, J.D.; DuBreuil, D.M.; Devlin, A.-C.; Held, A.; Sapir, Y.; Berezovski, E.; Hawrot, J.; Dorfman, K.; Chander, V.; Wainger, B.J. Human Sensorimotor Organoids Derived from Healthy and Amyotrophic Lateral Sclerosis Stem Cells Form Neuromuscular Junctions. Nat. Commun. 2021, 12, 4744. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Malafoglia, V.; Colasanti, M.; Raffaeli, W.; Balciunas, D.; Giordano, A.; Bellipanni, G. Extreme Thermal Noxious Stimuli Induce Pain Responses in Zebrafish Larvae. J. Cell. Physiol. 2014, 229, 300–308. [Google Scholar] [CrossRef]
- Costa, F.V.; Rosa, L.V.; Quadros, V.A.; de Abreu, M.S.; Santos, A.R.S.; Sneddon, L.U.; Kalueff, A.V.; Rosemberg, D.B. The Use of Zebrafish as a Non-Traditional Model Organism in Translational Pain Research: The Knowns and the Unknowns. Curr. Neuropharmacol. 2022, 20, 476–493. [Google Scholar] [CrossRef]
- Campos-Sánchez, J.C.; Esteban, M.Á. Review of Inflammation in Fish and Value of the Zebrafish Model. J. Fish Dis. 2021, 44, 123–139. [Google Scholar] [CrossRef]
- Bier, E. Drosophila, the Golden Bug, Emerges as a Tool for Human Genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef]
- Boonen, B.; Startek, J.B.; Milici, A.; López-Requena, A.; Beelen, M.; Callaerts, P.; Talavera, K. Activation of Drosophila Melanogaster TRPA1 Isoforms by Citronellal and Menthol. Int. J. Mol. Sci. 2021, 22, 10997. [Google Scholar] [CrossRef] [PubMed]
- Tiffin, H. Do Insects Feel Pain? Anim. Stud. J. 2016, 5, 80–96. [Google Scholar]
- Carney, G.E.; Bender, M. The Drosophila Ecdysone Receptor (EcR) Gene Is Required Maternally for Normal Oogenesis. Genetics 2000, 154, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Lim, J.Y.; Kang, S.; Kim, M.; Hwang, S.W.; Kim, C. Drosophila Ppk19 Encodes a Proton-Gated and Mechanosensitive Ion Channel. Sci. Rep. 2022, 12, 18346. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Morikawa, R.K.; Hasegawa, E.; Emoto, K. Neural Circuitry That Evokes Escape Behavior upon Activation of Nociceptive Sensory Neurons in Drosophila Larvae. Curr. Biol. 2017, 27, 2499–2504.e3. [Google Scholar] [CrossRef]
- He, J.; Li, B.; Han, S.; Zhang, Y.; Liu, K.; Yi, S.; Liu, Y.; Xiu, M. Drosophila as a Model to Study the Mechanism of Nociception. Front. Physiol. 2022, 13, 854124. [Google Scholar] [CrossRef]
- Manev, H.; Dimitrijevic, N. Fruit Flies for Anti-Pain Drug Discovery. Life Sci. 2005, 76, 2403–2407. [Google Scholar] [CrossRef]
- Lopez-Bellido, R.; Puig, S.; Huang, P.J.; Tsai, C.-R.; Turner, H.N.; Galko, M.J.; Gutstein, H.B. Growth Factor Signaling Regulates Mechanical Nociception in Flies and Vertebrates. J. Neurosci. 2019, 39, 6012–6030. [Google Scholar] [CrossRef]
- Santos-Silva, T.; Lopes, C.F.B.; Gumarães, J.D.S.; Valer, F.B.; Kuhn, G.C.S.; Romero, T.R.L.; Naves, L.A.; Duarte, I.D.G. Classical Analgesic Drugs Modulate Nociceptive-like Escape Behavior in Drosophila Melanogaster Larvae. Res. Results Pharmacol. 2022, 8, 185–196. [Google Scholar] [CrossRef]
- Tracey, W.D.; Wilson, R.I.; Laurent, G.; Benzer, S. Painless, a Drosophila Gene Essential for Nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef]
- Glauser, D.A.; Chen, W.C.; Agin, R.; Macinnis, B.L.; Hellman, A.B.; Garrity, P.A.; Tan, M.-W.; Goodman, M.B. Heat Avoidance Is Regulated by Transient Receptor Potential (TRP) Channels and a Neuropeptide Signaling Pathway in Caenorhabditis Elegans. Genetics 2011, 188, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Wittenburg, N.; Baumeister, R. Thermal Avoidance in Caenorhabditis Elegans: An Approach to the Study of Nociception. Proc. Natl. Acad. Sci. USA 1999, 96, 10477–10482. [Google Scholar] [CrossRef] [PubMed]
- Carr, F.B.; Zachariou, V. Nociception and Pain: Lessons from Optogenetics. Front. Behav. Neurosci. 2014, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Husson, S.J.; Costa, W.S.; Wabnig, S.; Stirman, J.N.; Watson, J.D.; Spencer, W.C.; Akerboom, J.; Looger, L.L.; Treinin, M.; Miller, D.M.; et al. Optogenetic Analysis of a Nociceptor Neuron and Network Reveals Ion Channels Acting Downstream of Primary Sensors. Curr. Biol. 2012, 22, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.M.; Bargmann, C.I. Invertebrate Nociception: Behaviors, Neurons and Molecules. J. Neurobiol. 2004, 61, 161–174. [Google Scholar] [CrossRef]
- Bray, N. A Long Pain-Free Life. Nat. Rev. Drug Discov. 2014, 13, 495. [Google Scholar] [CrossRef]
- Leung, K.; Mohammadi, A.; Ryu, W.S.; Nemenman, I. Stereotypical Escape Behavior in Caenorhabditis Elegans Allows Quantification of Effective Heat Stimulus Level. PLoS Comput. Biol. 2016, 12, e1005262. [Google Scholar] [CrossRef]
- Jordan, A.; Glauser, D.A. Distinct Clusters of Human Pain Gene Orthologs in Caenorhabditis Elegans Regulate Thermo-Nociceptive Sensitivity and Plasticity. Genetics 2023, 224, iyad047. [Google Scholar] [CrossRef]
- Nkambeu, B.; Salem, J.B.; Beaudry, F. Capsaicin and Its Analogues Impede Nocifensive Response of Caenorhabditis Elegans to Noxious Heat. Neurochem. Res. 2020, 45, 1851–1859. [Google Scholar] [CrossRef]
- Cho, C.; Deol, H.K.; Martin, L.J. Bridging the Translational Divide in Pain Research: Biological, Psychological and Social Considerations. Front. Pharmacol. 2021, 12, 603186. [Google Scholar] [CrossRef]
- kumar Reddy, K.S.; Naidu, M.U.R.; Rani, P.U.; Rao, T.R.K. Human Experimental Pain Models: A Review of Standardized Methods in Drug Development. J. Res. Med. Sci. 2012, 17, 587–595. [Google Scholar]
- Langley, C.K.; Aziz, Q.; Bountra, C.; Gordon, N.; Hawkins, P.; Jones, A.; Langley, G.; Nurmikko, T.; Tracey, I. Volunteer Studies in Pain Research--Opportunities and Challenges to Replace Animal Experiments: The Report and Recommendations of a Focus on Alternatives Workshop. Neuroimage 2008, 42, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. From Genes to Pain: Nav1.7 and Human Pain Disorders. Trends Neurosci. 2007, 30, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand Spiciness: Mechanism of TRPV1 Channel Activation by Capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Hugosdottir, R.; Kasting, M.; Mørch, C.D.; Andersen, O.K.; Arendt-Nielsen, L. Priming of Central and Peripheral Mechanisms with Heat and Cutaneous Capsaicin Facilitates Secondary Hyperalgesia to High-Frequency Electrical Stimulation. J. Neurophysiol. 2022, 127, 651–659. [Google Scholar] [CrossRef]
- Petersen, K.L.; Rowbotham, M.C. A New Human Experimental Pain Model: The Heat/Capsaicin Sensitization Model. Neuroreport 1999, 10, 1511–1516. [Google Scholar] [CrossRef]
- Price, R.C.; Gandhi, W.; Nadeau, C.; Tarnavskiy, R.; Qu, A.; Fahey, E.; Stone, L.; Schweinhardt, P. Characterization of a Novel Capsaicin/Heat Ongoing Pain Model. Eur. J. Pain 2018, 22, 370–384. [Google Scholar] [CrossRef]
- Dirks, J.; Petersen, K.L.; Dahl, J.B. The Heat/Capsaicin Sensitization Model: A Methodologic Study. J. Pain 2003, 4, 122–128. [Google Scholar] [CrossRef]
- Petersen, K.L.; Jones, B.; Segredo, V.; Dahl, J.B.; Rowbotham, M.C. Effect of Remifentanil on Pain and Secondary Hyperalgesia Associated with the Heat--Capsaicin Sensitization Model in Healthy Volunteers. Anesthesiology 2001, 94, 15–20. [Google Scholar] [CrossRef]
- Cavallone, L.F.; Frey, K.; Montana, M.C.; Joyal, J.; Regina, K.J.; Petersen, K.L.; Gereau, R.W. Reproducibility of the Heat/Capsaicin Skin Sensitization Model in Healthy Volunteers. J. Pain Res. 2013, 6, 771–784. [Google Scholar] [CrossRef]
- Jensen, M.T.; Petersen, K.L. Gender Differences in Pain and Secondary Hyperalgesia after Heat/Capsaicin Sensitization in Healthy Volunteers. J. Pain 2006, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.-K.; Campbell, J.N. Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals 2016, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Max, M.B.; Robinovitz, E.; Gracely, R.H.; Bennett, G.J. The Human Capsaicin Model of Allodynia and Hyperalgesia: Sources of Variability and Methods for Reduction. J. Pain Symptom Manag. 1998, 16, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Staahl, C.; Olesen, A.E.; Andresen, T.; Arendt-Nielsen, L.; Drewes, A.M. Assessing Analgesic Actions of Opioids by Experimental Pain Models in Healthy Volunteers—An Updated Review. Br. J. Clin. Pharmacol. 2009, 68, 149–168. [Google Scholar] [CrossRef]
- Wallace, M.S.; Barger, D.; Schulteis, G. The Effect of Chronic Oral Desipramine on Capsaicin-Induced Allodynia and Hyperalgesia: A Double-Blinded, Placebo-Controlled, Crossover Study. Anesth. Analg. 2002, 95, 973–978. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Andersen, O.K. Capsaicin in Human Experimental Pain Models of Skin, Muscle and Visceral Sensitization. In Turning Up the Heat on Pain: TRPV1 Receptors in Pain and Inflammation; Malmberg, A.B., Bley, K.R., Eds.; Birkhäuser: Basel, Switerland, 2005; pp. 117–144. ISBN 978-3-7643-7379-5. [Google Scholar]
- Balabathula, P.; Bhattacharjee, H.; Thoma, L.A.; Nolly, R.J.; Horton, F.P.; Stornes, G.D.; Wan, J.Y.; Brooks, I.M.; Bachmann, G.A.; Foster, D.C.; et al. Potency and Stability of Intradermal Capsaicin: Implications for Use as a Human Model of Pain in Multicenter Clinical Trials. Clin. Exp. Pharmacol. 2014, 4, 142. [Google Scholar] [CrossRef]
- Lötsch, J.; Walter, C.; Zunftmeister, M.; Zinn, S.; Wolters, M.; Ferreiros, N.; Rossmanith, T.; Oertel, B.G.; Geisslinger, G. A Data Science Approach to the Selection of Most Informative Readouts of the Human Intradermal Capsaicin Pain Model to Assess Pregabalin Effects. Basic Clin. Pharmacol. Toxicol. 2020, 126, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Aykanat, V.; Gentgall, M.; Briggs, N.; Williams, D.; Yap, S.; Rolan, P. Intradermal Capsaicin as a Neuropathic Pain Model in Patients with Unilateral Sciatica. Br. J. Clin. Pharmacol. 2012, 73, 37–45. [Google Scholar] [CrossRef]
- Scanlon, G.C.; Wallace, M.S.; Ispirescu, J.S.; Schulteis, G. Intradermal Capsaicin Causes Dose-Dependent Pain, Allodynia, and Hyperalgesia in Humans. J. Investig. Med. 2006, 54, 238–244. [Google Scholar] [CrossRef]
- von Baeyer, C.L.; Piira, T.; Chambers, C.T.; Trapanotto, M.; Zeltzer, L.K. Guidelines for the Cold Pressor Task as an Experimental Pain Stimulus for Use with Children. J. Pain 2005, 6, 218–227. [Google Scholar] [CrossRef]
- Babes, A. Ion Channels Involved in Cold Detection in Mammals: TRP and Non-TRP Mechanisms. Biophys. Rev. 2009, 1, 193–200. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D. How Cold Is It? TRPM8 and TRPA1 in the Molecular Logic of Cold Sensation. Mol. Pain 2005, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Wasner, G.; Schattschneider, J.; Binder, A.; Baron, R. Topical Menthol--a Human Model for Cold Pain by Activation and Sensitization of C Nociceptors. Brain 2004, 127, 1159–1171. [Google Scholar] [CrossRef]
- Birnie, K.A.; Caes, L.; Wilson, A.C.; Williams, S.E.; Chambers, C.T. A Practical Guide and Perspectives on the Use of Experimental Pain Modalities with Children and Adolescents. Pain Manag. 2014, 4, 97–111. [Google Scholar] [CrossRef]
- McIntyre, M.H.; Kless, A.; Hein, P.; Field, M.; Tung, J.Y. Validity of the Cold Pressor Test and Pain Sensitivity Questionnaire via Online Self-Administration. PLoS ONE 2020, 15, e0231697. [Google Scholar] [CrossRef]
- Modir, J.G.; Wallace, M.S. Human Experimental Pain Models 2: The Cold Pressor Model. Methods Mol. Biol. 2010, 617, 165–168. [Google Scholar] [CrossRef]
- Vidal Rodriguez, S.; Castillo Aguilar, I.; Cuesta Villa, L.; Serrano Saenz de Tejada, F. TRPA1 Polymorphisms in Chronic and Complete Spinal Cord Injury Patients with Neuropathic Pain: A Pilot Study. Spinal Cord. Ser. Cases 2017, 3, 17089. [Google Scholar] [CrossRef] [PubMed]
- Fontanillas, P.; Kless, A.; Bothmer, J.; Tung, J.Y.; 23andMe Research Team. Genome-Wide Association Study of Pain Sensitivity Assessed by Questionnaire and the Cold Pressor Test. Pain 2022, 163, 1763–1776. [Google Scholar] [CrossRef]
- Nathan, P.W.; Rudge, P. Testing the Gate-Control Theory of Pain in Man. J. Neurol. Neurosurg. Psychiatry 1974, 37, 1366–1372. [Google Scholar] [CrossRef]
- Weinkauf, B.; Main, M.; Schmelz, M.; Rukwied, R. Modality-Specific Nociceptor Sensitization Following UV-B Irradiation of Human Skin. J. Pain 2013, 14, 739–746. [Google Scholar] [CrossRef]
- Moore, C.; Cevikbas, F.; Pasolli, H.A.; Chen, Y.; Kong, W.; Kempkes, C.; Parekh, P.; Lee, S.H.; Kontchou, N.-A.; Yeh, I.; et al. UVB Radiation Generates Sunburn Pain and Affects Skin by Activating Epidermal TRPV4 Ion Channels and Triggering Endothelin-1 Signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E3225–E3234. [Google Scholar] [CrossRef] [PubMed]
- Rössler, B.; Paul, A.; Schuch, M.; Schulz, M.; Sycha, T.; Gustorff, B. Central Origin of Pinprick Hyperalgesia Adjacent to an UV-B Induced Inflammatory Skin Pain Model in Healthy Volunteers. Scand. J. Pain 2013, 4, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Siebenga, P.S.; van Amerongen, G.; Klaassen, E.S.; de Kam, M.L.; Rissmann, R.; Groeneveld, G.J. The Ultraviolet B Inflammation Model: Postinflammatory Hyperpigmentation and Validation of a Reduced UVB Exposure Paradigm for Inducing Hyperalgesia in Healthy Subjects. Eur. J. Pain 2019, 23, 874–883. [Google Scholar] [CrossRef]
- Taylor, S.L.; Kaur, M.; LoSicco, K.; Willard, J.; Camacho, F.; O’Rourke, K.S.; Feldman, S.R. Pilot Study of the Effect of Ultraviolet Light on Pain and Mood in Fibromyalgia Syndrome. J. Altern. Complement. Med. 2009, 15, 15–23. [Google Scholar] [CrossRef]
- Bishop, T.; Marchand, F.; Young, A.R.; Lewin, G.R.; McMahon, S.B. Ultraviolet-B-Induced Mechanical Hyperalgesia: A Role for Peripheral Sensitisation. Pain 2010, 150, 141–152. [Google Scholar] [CrossRef]
- Mørch, C.D.; Gazerani, P.; Nielsen, T.A.; Arendt-Nielsen, L. The UVB Cutaneous Inflammatory Pain Model: A Reproducibility Study in Healthy Volunteers. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 203–215. [Google Scholar] [PubMed]
- Ao, Z.; Cai, H.; Wu, Z.; Krzesniak, J.; Tian, C.; Lai, Y.Y.; Mackie, K.; Guo, F. Human Spinal Organoid-on-a-Chip to Model Nociceptive Circuitry for Pain Therapeutics Discovery. Anal. Chem. 2022, 94, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Martucci, K.T.; Mackey, S.C. Neuroimaging of Pain: Human Evidence and Clinical Relevance of Central Nervous System Processes and Modulation. Anesthesiology 2018, 128, 1241–1254. [Google Scholar] [CrossRef]
- Smith, S.B.; Maixner, D.W.; Fillingim, R.B.; Slade, G.; Gracely, R.H.; Ambrose, K.; Zaykin, D.V.; Hyde, C.; John, S.; Tan, K.; et al. Large Candidate Gene Association Study Reveals Genetic Risk Factors and Therapeutic Targets for Fibromyalgia. Arthritis Rheum. 2012, 64, 584–593. [Google Scholar] [CrossRef]
- Steele, V.R. Transcranial Magnetic Stimulation and Addiction: Toward Uncovering Known Unknowns. EBioMedicine 2020, 57, 102839. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The Biopsychosocial Approach to Chronic Pain: Scientific Advances and Future Directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef] [PubMed]
- Casarett, D.J.; Karlawish, J. Beyond Informed Consent: The Ethical Design of Pain Research. Pain Med. 2001, 2, 138–146. [Google Scholar] [CrossRef]
- Borsook, D.; Becerra, L.; Hargreaves, R. A Role for fMRI in Optimizing CNS Drug Development. Nat. Rev. Drug Discov. 2006, 5, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.T.; Seminowicz, D.A. Neuroimaging of Pain in Animal Models: A Review of Recent Literature. Pain Rep. 2019, 4, e732. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.D.; Flor, H.; Greely, H.T.; Iannetti, G.D.; Mackey, S.; Ploner, M.; Pustilnik, A.; Tracey, I.; Treede, R.-D.; Wager, T.D. Brain Imaging Tests for Chronic Pain: Medical, Legal and Ethical Issues and Recommendations. Nat. Rev. Neurol. 2017, 13, 624–638. [Google Scholar] [CrossRef]
- Morton, D.L.; Sandhu, J.S.; Jones, A.K. Brain Imaging of Pain: State of the Art. J. Pain Res. 2016, 9, 613–624. [Google Scholar] [CrossRef]
- Carmichael, O.; Schwarz, A.J.; Chatham, C.H.; Scott, D.; Turner, J.A.; Upadhyay, J.; Coimbra, A.; Goodman, J.A.; Baumgartner, R.; English, B.A.; et al. The Role of fMRI in Drug Development. Drug Discov. Today 2018, 23, 333–348. [Google Scholar] [CrossRef]
- Schweinhardt, P.; Bountra, C.; Tracey, I. Pharmacological FMRI in the Development of New Analgesic Compounds. NMR Biomed. 2006, 19, 702–711. [Google Scholar] [CrossRef]
- Wager, T.D.; Woo, C.-W. fMRI in Analgesic Drug Discovery. Sci. Transl. Med. 2015, 7, 274fs6. [Google Scholar] [CrossRef]
- Ledermann, K.; Jenewein, J.; Sprott, H.; Hasler, G.; Schnyder, U.; Warnock, G.; Johayem, A.; Kollias, S.; Buck, A.; Martin-Soelch, C. Relation of Dopamine Receptor 2 Binding to Pain Perception in Female Fibromyalgia Patients with and without Depression—A [11C] Raclopride PET-Study. Eur. Neuropsychopharmacol. 2016, 26, 320–330. [Google Scholar] [CrossRef]
- Kang, D.H.; Son, J.H.; Kim, Y.C. Neuroimaging Studies of Chronic Pain. Korean J. Pain 2010, 23, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Juárez, J.V.; Chiquete, E.; Estañol, B.; Aceves, J. de J. Optogenetic and Chemogenic Control of Pain Signaling: Molecular Markers. Int. J. Mol. Sci. 2023, 24, 10220. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, C.; Wu, Z.; Wang, Z.; Peng, Y.; Li, Z.; Zhang, Z.; Lin, H.; Chen, Z. Fecal Microbiota Transplantation Revealed a Pain-Related Gut Microbiota Community in Ovariectomized Mice. J. Pain 2023, 24, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Morreale, C.; Bresesti, I.; Bosi, A.; Baj, A.; Giaroni, C.; Agosti, M.; Salvatore, S. Microbiota and Pain: Save Your Gut Feeling. Cells 2022, 11, 971. [Google Scholar] [CrossRef]
- Pane, K.; Boccella, S.; Guida, F.; Franzese, M.; Maione, S.; Salvatore, M. Role of Gut Microbiota in Neuropathy and Neuropathic Pain States: A Systematic Preclinical Review. Neurobiol. Dis. 2022, 170, 105773. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Johnson, A.C. Stress-Induced Chronic Visceral Pain of Gastrointestinal Origin. Front. Syst. Neurosci. 2017, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Stress-Induced Visceral Pain: Toward Animal Models of Irritable-Bowel Syndrome and Associated Comorbidities. Front. Psychiatry 2015, 6, 15. [Google Scholar] [CrossRef]
- Chichlowski, M.; Rudolph, C. Visceral Pain and Gastrointestinal Microbiome. J. Neurogastroenterol. Motil. 2015, 21, 172–181. [Google Scholar] [CrossRef]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.R.; Cook, T.M.; Gavini, C.K.; White, C.R.; Jones, J.R.; Bovo, E.; Zima, A.V.; Brown, I.A.; Dugas, L.R.; Zakharian, E.; et al. Fecal Transplantation and Butyrate Improve Neuropathic Pain, Modify Immune Cell Profile, and Gene Expression in the PNS of Obese Mice. Proc. Natl. Acad. Sci. USA 2020, 117, 26482–26493. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Choi, S.-W. Dietary Modulation of Gut Microbiota for the Relief of Irritable Bowel Syndrome. Nutr. Res. Pract. 2021, 15, 411–430. [Google Scholar] [CrossRef]
- Chen, Z.S.; Wang, J. Pain, from Perception to Action: A Computational Perspective. iScience 2023, 26, 105707. [Google Scholar] [CrossRef]
- Pellicer-Valero, O.J.; Martín-Guerrero, J.D.; Cigarán-Méndez, M.I.; Écija-Gallardo, C.; Fernández-de-las-Peñas, C.; Navarro-Pardo, E. Mathematical Modeling for Neuropathic Pain: Bayesian Linear Regression and Self-Organizing Maps Applied to Carpal Tunnel Syndrome. Symmetry 2020, 12, 1581. [Google Scholar] [CrossRef]
- Mancini, F.; Zhang, S.; Seymour, B. Computational and Neural Mechanisms of Statistical Pain Learning. Nat. Commun. 2022, 13, 6613. [Google Scholar] [CrossRef]
- Su, J.; Du, Y.; Bevers, K.; Xiao, P.; Licciardone, J.; Brotto, M.; Gatchel, R.J. Transitioning from Acute to Chronic Pain: A Simulation Study of Trajectories of Low Back Pain. J. Transl. Med. 2019, 17, 306. [Google Scholar] [CrossRef]
- Argüello, E.J.; Silva, R.J.; Huerta, M.K.; Avila, R.S. Computational Modeling of Peripheral Pain: A Commentary. Biomed. Eng. Online 2015, 14, 56. [Google Scholar] [CrossRef]
- Ishikawa, R.; Izawa, J. The Computational Neuroanatomy of Predictive Dynamics of Pain Perception. bioRxiv 2022, 2022.04.13.488260. [Google Scholar]
- Drusko, A.; Baumeister, D.; McPhee Christensen, M.; Kold, S.; Fisher, V.L.; Treede, R.-D.; Powers, A.; Graven-Nielsen, T.; Tesarz, J. A Novel Computational Approach to Pain Perception Modelling within a Bayesian Framework Using Quantitative Sensory Testing. Sci. Rep. 2023, 13, 3196. [Google Scholar] [CrossRef]
- Lang, V.A.; Lundh, T.; Ortiz-Catalan, M. Mathematical and Computational Models for Pain: A Systematic Review. Pain Med. 2021, 22, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, G.T.; Adedoyin, A.; Leventhal, L. Predictive Validity of Animal Pain Models? A Comparison of the Pharmacokinetic-Pharmacodynamic Relationship for Pain Drugs in Rats and Humans. Neuropharmacology 2008, 54, 767–775. [Google Scholar] [CrossRef] [PubMed]
- van der Staay, F.J.; Arndt, S.S.; Nordquist, R.E. Evaluation of Animal Models of Neurobehavioral Disorders. Behav. Brain Funct. 2009, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Berge, O.-G. Predictive Validity of Behavioural Animal Models for Chronic Pain. Br. J. Pharmacol. 2011, 164, 1195–1206. [Google Scholar] [CrossRef]
- Burek, D.J.; Massaly, N.; Yoon, H.J.; Doering, M.; Morón, J.A. Behavioral Outcomes of Complete Freund Adjuvant-Induced Inflammatory Pain in the Rodent Hind Paw: A Systematic Review and Meta-Analysis. Pain 2022, 163, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Oliva, A.; Mota-Rojas, D.; Hernández-Avalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Casas-Alvarado, A.; Whittaker, A.L. The Neurobiology of Pain and Facial Movements in Rodents: Clinical Applications and Current Research. Front. Vet. Sci. 2022, 9, 1016720. [Google Scholar] [CrossRef]
- van der Wal, S.; Cornelissen, L.; Behet, M.; Vaneker, M.; Steegers, M.; Vissers, K. Behavior of Neuropathic Pain in Mice Following Chronic Constriction Injury Comparing Silk and Catgut Ligatures. Springerplus 2015, 4, 225. [Google Scholar] [CrossRef]
- Cheng, Z.; Feng, S.; Yang, L.; Huang, J.; Chen, X.; Guo, Y.; Xiang, Y.; Peng, B. Rat Model of Neuropathic Pain Induced by Spinal Nerve Ligation: A New Approach via an Oblique Lateral Incision. J. Pain Res. 2024, 17, 2443–2454. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Naseem, N.; Rahman, I.; Imam, N.; Younus, H.; Pandey, S.K.; Siddiqui, W.A. Naringin Attenuates the Diabetic Neuropathy in STZ-Induced Type 2 Diabetic Wistar Rats. Life 2022, 12, 2111. [Google Scholar] [CrossRef]
- Medhurst, S.J.; Walker, K.; Bowes, M.; Kidd, B.L.; Glatt, M.; Muller, M.; Hattenberger, M.; Vaxelaire, J.; O’Reilly, T.; Wotherspoon, G.; et al. A Rat Model of Bone Cancer Pain. Pain 2002, 96, 129–140. [Google Scholar] [CrossRef]
- Gui, Q.; Xu, C.; Zhuang, L.; Xia, S.; Chen, Y.; Peng, P.; Yu, S. A New Rat Model of Bone Cancer Pain Produced by Rat Breast Cancer Cells Implantation of the Shaft of Femur at the Third Trochanter Level. Cancer Biol. Ther. 2013, 14, 193–199. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zambelli, V.O.; Sinharoy, P.; Brabenec, L.; Bian, Y.; Rwere, F.; Hell, R.C.; Stein Neto, B.; Hung, B.; Yu, X.; et al. A Human TRPV1 Genetic Variant within the Channel Gating Domain Regulates Pain Sensitivity in Rodents. J. Clin. Investig. 2023, 133, e163735. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Evans, S.R.; Mbowe, O.; McDermott, M.P. Essential Statistical Principles of Clinical Trials of Pain Treatments. Pain Rep. 2020, 6, e863. [Google Scholar] [CrossRef]
- Mogil, J.S. Animal Models of Pain: Progress and Challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef]
- Schott, G.D. Pain Past, Present and Future: The Unhappy Paradox of Scientific Advances and Therapeutic Standstill. Brain 2007, 130, 1704–1708. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Pereira, E.W.M.; Campos, A.R.; Quintans-Júnior, L.J.; Quintans, J.S.S. Chronic Orofacial Pain Animal Models—Progress and Challenges. Expert Opin. Drug Discov. 2018, 13, 949–964. [Google Scholar] [CrossRef]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An Overview of Animal Models of Pain: Disease Models and Outcome Measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef]
- Taylor, N.E.; Ferrari, L. Discovering Chronic Pain Treatments: Better Animal Models Might Help Us Get There. J. Clin. Investig. 2023, 133, e167814. [Google Scholar] [CrossRef]
- Kaliyaperumal, S.; Wilson, K.; Aeffner, F.; Dean, C. Animal Models of Peripheral Pain: Biology Review and Application for Drug Discovery. Toxicol. Pathol. 2020, 48, 202–219. [Google Scholar] [CrossRef]
- Gewandter, J.S.; Dworkin, R.H.; Turk, D.C.; Devine, E.G.; Hewitt, D.; Jensen, M.P.; Katz, N.P.; Kirkwood, A.A.; Malamut, R.; Markman, J.D.; et al. Improving Study Conduct and Data Quality in Clinical Trials of Chronic Pain Treatments: IMMPACT Recommendations. J. Pain 2020, 21, 931–942. [Google Scholar] [CrossRef]
- Hafko, R.; Villapol, S.; Nostramo, R.; Symes, A.; Sabban, E.L.; Inagami, T.; Saavedra, J.M. Commercially Available Angiotensin II At2 Receptor Antibodies Are Nonspecific. PLoS ONE 2013, 8, e69234. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T. Nonopioid Analgesics Discovery and the Valley of Death: EMA401 from Concept to Clinical Trial. Pain 2022, 163, S15–S28. [Google Scholar] [CrossRef] [PubMed]
- Zanata, G.C.; Pinto, L.G.; da Silva, N.R.; Lopes, A.H.P.; de Oliveira, F.F.B.; Schivo, I.R.S.; Cunha, F.Q.; McNaughton, P.; Cunha, T.M.; Silva, R.L. Blockade of Bradykinin Receptors or Angiotensin II Type 2 Receptor Prevents Paclitaxel-Associated Acute Pain Syndrome in Mice. Eur. J. Pain 2021, 25, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S.C.; Dworkin, R.H.; Finnerup, N.B.; Attal, N.; Anand, P.; Freeman, R.; Piaia, A.; Callegari, F.; Doerr, C.; Mondal, S.; et al. Efficacy and Safety of EMA401 in Peripheral Neuropathic Pain: Results of 2 Randomised, Double-Blind, Phase 2 Studies in Patients with Postherpetic Neuralgia and Painful Diabetic Neuropathy. Pain 2021, 162, 2578–2589. [Google Scholar] [CrossRef] [PubMed]
- Eagles, D.A.; Chow, C.Y.; King, G.F. Fifteen Years of NaV 1.7 Channels as an Analgesic Target: Why Has Excellent in Vitro Pharmacology Not Translated into in Vivo Analgesic Efficacy? Br. J. Pharmacol. 2022, 179, 3592–3611. [Google Scholar] [CrossRef]
- Mesch, S.; Walter, D.; Laux-Biehlmann, A.; Basting, D.; Flanagan, S.; Miyatake Ondozabal, H.; Bäurle, S.; Pearson, C.; Jenkins, J.; Elves, P.; et al. Discovery of BAY-390, a Selective CNS Penetrant Chemical Probe as Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonist. J. Med. Chem. 2023, 66, 1583–1600. [Google Scholar] [CrossRef]
- Pergolizzi, J.; Varrassi, G. The Emerging Role of Sigma Receptors in Pain Medicine. Cureus 2023, 15, e42626. [Google Scholar] [CrossRef]
- Raffa, R.B.; Pergolizzi, J.V. Recent Developments in Sigma-2 Receptor Compounds for Pain. Cureus 2024, 16, e59882. [Google Scholar] [CrossRef]
- Zhuang, T.; Xiong, J.; Hao, S.; Du, W.; Liu, Z.; Liu, B.; Zhang, G.; Chen, Y. Bifunctional μ Opioid and Σ1 Receptor Ligands as Novel Analgesics with Reduced Side Effects. Eur. J. Med. Chem. 2021, 223, 113658. [Google Scholar] [CrossRef]
- García, M.; Virgili, M.; Alonso, M.; Alegret, C.; Farran, J.; Fernández, B.; Bordas, M.; Pascual, R.; Burgueño, J.; Vidal-Torres, A.; et al. Discovery of EST73502, a Dual μ-Opioid Receptor Agonist and Σ1 Receptor Antagonist Clinical Candidate for the Treatment of Pain. J. Med. Chem. 2020, 63, 15508–15526. [Google Scholar] [CrossRef]
- Rossino, G.; Marra, A.; Listro, R.; Peviani, M.; Poggio, E.; Curti, D.; Pellavio, G.; Laforenza, U.; Dondio, G.; Schepmann, D.; et al. Discovery of RC-752, a Novel Sigma-1 Receptor Antagonist with Antinociceptive Activity: A Promising Tool for Fighting Neuropathic Pain. Pharmaceuticals 2023, 16, 962. [Google Scholar] [CrossRef] [PubMed]
- Djouhri, L. PG110, A Humanized Anti-NGF Antibody, Reverses Established Pain Hypersensitivity in Persistent Inflammatory Pain, but Not Peripheral Neuropathic Pain, Rat Models. Pain Med. 2016, 17, 2082–2094. [Google Scholar] [CrossRef]
- Luan, Y.; Luo, Y.; Deng, M. New Advances in Nrf2-Mediated Analgesic Drugs. Phytomedicine 2023, 110, 154598. [Google Scholar] [CrossRef]
- Lane, N.E.; Schnitzer, T.J.; Birbara, C.A.; Mokhtarani, M.; Shelton, D.L.; Smith, M.D.; Brown, M.T. Tanezumab for the Treatment of Pain from Osteoarthritis of the Knee. N. Engl. J. Med. 2010, 363, 1521–1531. [Google Scholar] [CrossRef]
- McDonnell, C.; Leánez, S.; Pol, O. The Induction of the Transcription Factor Nrf2 Enhances the Antinociceptive Effects of Delta-Opioid Receptors in Diabetic Mice. PLoS ONE 2017, 12, e0180998. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C. Anti-Nociceptive and Anti-Inflammatory Actions of Sulforaphane in Chronic Constriction Injury-Induced Neuropathic Pain Mice. Inflammopharmacology 2017, 25, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Giffin, N.J.; Kowacs, F.; Libri, V.; Williams, P.; Goadsby, P.J.; Kaube, H. Effect of the Adenosine A1 Receptor Agonist GR79236 on Trigeminal Nociception with Blink Reflex Recordings in Healthy Human Subjects. Cephalalgia 2003, 23, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Choong, K.-C.; Dima, S.; Henze, D.A.; Kane, S.A.; Urban, M.O. HC-030031, a TRPA1 Selective Antagonist, Attenuates Inflammatory- and Neuropathy-Induced Mechanical Hypersensitivity. Mol. Pain 2008, 4, 48. [Google Scholar] [CrossRef]
- de David Antoniazzi, C.T.; De Prá, S.D.-T.; Ferro, P.R.; Silva, M.A.; Adamante, G.; de Almeida, A.S.; Camponogara, C.; da Silva, C.R.; de Bem Silveira, G.; Silveira, P.C.L.; et al. Topical Treatment with a Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonist Reduced Nociception and Inflammation in a Thermal Lesion Model in Rats. Eur. J. Pharm. Sci. 2018, 125, 28–38. [Google Scholar] [CrossRef]
- Anand, S.; Rajagopal, S. A Comprehensive Review on the Regulatory Action of TRP Channels: A Potential Therapeutic Target for Nociceptive Pain. Neurosci. Insights 2023, 18, 26331055231220340. [Google Scholar] [CrossRef]
- Javed, H.; Johnson, A.M.; Challagandla, A.K.; Emerald, B.S.; Shehab, S. Cutaneous Injection of Resiniferatoxin Completely Alleviates and Prevents Nerve-Injury-Induced Neuropathic Pain. Cells 2022, 11, 4049. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, Y.; Kawamata, T.; Yamamoto, J.; Furuse, S.; Namiki, A. SB366791, a TRPV1 Antagonist, Potentiates Analgesic Effects of Systemic Morphine in a Murine Model of Bone Cancer Pain. Br. J. Anaesth. 2009, 102, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ossola, C.A.; Balcarcel, N.B.; Astrauskas, J.I.; Bozzini, C.; Elverdin, J.C.; Fernández-Solari, J. A New Target to Ameliorate the Damage of Periodontal Disease: The Role of Transient Receptor Potential Vanilloid Type-1 in Contrast to That of Specific Cannabinoid Receptors in Rats. J. Periodontol. 2019, 90, 1325–1335. [Google Scholar] [CrossRef]
- Gomez, K.; Santiago, U.; Nelson, T.S.; Allen, H.N.; Calderon-Rivera, A.; Hestehave, S.; Rodríguez Palma, E.J.; Zhou, Y.; Duran, P.; Loya-Lopez, S.; et al. A Peptidomimetic Modulator of the CaV2.2 N-Type Calcium Channel for Chronic Pain. Proc. Natl. Acad. Sci. USA 2023, 120, e2305215120. [Google Scholar] [CrossRef]
- Lagard, C.; Chevillard, L.; Guillemyn, K.; Risède, P.; Laplanche, J.-L.; Spetea, M.; Ballet, S.; Mégarbane, B. Bifunctional Peptide-Based Opioid Agonist/Nociceptin Antagonist Ligand for Dual Treatment of Nociceptive and Neuropathic Pain. Pain 2017, 158, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Conibear, A.E.; Asghar, J.; Hill, R.; Henderson, G.; Borbely, E.; Tekus, V.; Helyes, Z.; Palandri, J.; Bailey, C.; Starke, I.; et al. A Novel G Protein-Biased Agonist at the δ Opioid Receptor with Analgesic Efficacy in Models of Chronic Pain. J. Pharmacol. Exp. Ther. 2020, 372, 224–236. [Google Scholar] [CrossRef]
- Ullrich, A.; Schneider, J.; Braz, J.M.; Neu, E.; Staffen, N.; Stanek, M.; Bláhová, J.; Hove, T.; Albert, T.; Allikalt, A.; et al. Discovery of a Functionally Selective Serotonin 5-HT1A Receptor Agonist for the Treatment of Pain. bioRxiv 2023, 2023.09.11.557127. [Google Scholar]
- Kim, M.S.; Kim, B.Y.; Saghetlians, A.; Zhang, X.; Okida, T.; Kim, S.Y. Anti-Nociceptive Effects of Dual Neuropeptide Antagonist Therapy in Mouse Model of Neuropathic and Inflammatory Pain. Korean J. Pain 2022, 35, 173. [Google Scholar] [CrossRef]

| Pain Models | Applications | Limitations | Possible Underlying Mechanisms and Pathways | |
|---|---|---|---|---|
| Inflammatory Pain | Complete Freund’s Adjuvant (CFA) |
| Variability in response based on species difference |
|
| Formalin tests |
| It may not fully replicate all types of clinical pain conditions. |
| |
| Visceral Pain | Colorectal Distension (CRD) |
| Invasiveness |
|
| Neuropathic Pain | The Chronic Constriction Injury (CCI) |
| Technically challenge, may not fully replicate all aspects of human neuropathic pain. Variability in response Invasiveness |
|
| Spinal Nerve Ligation Model | Neuropathic pain research and Drug screening | Model complexity Species differences |
| |
| Chronic Constriction of the Infraorbital Nerve Model |
| Variability in response | STAT3 pathways in astrocytes via IL6 TRPV channels | |
| The Chronic Compression of Dorsal Root Ganglion (CCD) | Drug Development and Neuropathic Pain Research | Variability in response | CXCL12/CXCR4 Signaling | |
| STZ-induced DNP model | To study mechanisms of painful diabetic neuropathy and to evaluate potential therapies | Various differences in how the model works and how real diabetes happens compared to human diabetes | Increased expression of Nav1.3, Nav1.6, and Nav1.9 channels in DRG neurons | |
| Cancer Pain |
|
|
|
|
| Genetically Modified |
|
|
|
|
| Cellular Models |
|
| Cell viability (The cells may not fully replicate the in vivo environment. | Voltage-Gated Sodium Channels (Nav) |
| Compound Name | Target/Class of Drug | The Type of Pain Studied | The Type of Animal Model Used |
|---|---|---|---|
| EMA401 (Olodanrigan) | Selective AT2R receptor antagonist | Neuropathic Pain | Chronic constriction injury |
| GR79236 IB-MECA (Piclidenoson) | A1R agonist A3R agonist | Neuropathic Pain | Neuropathic pain models |
| Pn3a | Nav1.7 antagonist | Nociception Neuropathic Pain Inflammatory pain | Hotplate, formalin, carrageenan, CFA |
| BAY-390 | TRPA1 Agonist | Neuropathic Pain | Cinnamaldehyde |
| HC-030031 | TRPA1 Agonist | Neuropathic pain Inflammatory pain | UV-light B-induced burn injury model (Sun-burn thermal injury) |
| GRCI7536 | TRPA1 Agonist | Diabetic neuropathic pain | Neuropathic pain model |
| Resiniferatoxin (RTX) | TRPV1 Agonist | Neuropathic pain | L5 Nerve Injury model |
| CBD3063 | Cav2.2 antagonist | Neuopathic pan Inflammatory pain | The spinal nerve injury model, Hot plate, cold plate tests, Chronic constriction injury |
| EST73502 (Bifunctional ligand) | MOR (µ) agonist Sigma (σ) Receptor antagonist | Neuropathic Pain Cancer Pain Arthritic pain | Partial sciatic nerve ligation Paw Pressure test |
| Sulforaphane | MOR agonist | Neuropathic Pain | Chronic constriction injury Chronic constriction injury |
| SB366791 PN6047 | VR1/TRPV1 antagonist MOR agonist | Neuropathic Pain Cancer Pain Inflammatory pain Acute dental Pain | The spinal nerve ligation; L5 Nerve injury model Rat model of Periodontal, mono-iodoacetate–-induced osteoarthritic pain model and carrageenan-induced acute inflammatory model |
| ST171 | 5HT1AR agonist | Neuropathic pain (Acute and Chronic Pain) | Hot plate, Tail flick, CFA, Spinal Nerve injury models |
| PG-110 (Antibody) | Anti-NGF | Inflammatory pain | CFA, L5 spinal nerve axotomy (SNA) model |
| Tanezumab (Antibody) | Anti-Nrf2 | Neuropathic pain | Diabetic neuropathic models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asiri, Y.I.; Moni, S.S.; Ramar, M.; Chidambaram, K. Advancing Pain Understanding and Drug Discovery: Insights from Preclinical Models and Recent Research Findings. Pharmaceuticals 2024, 17, 1439. https://doi.org/10.3390/ph17111439
Asiri YI, Moni SS, Ramar M, Chidambaram K. Advancing Pain Understanding and Drug Discovery: Insights from Preclinical Models and Recent Research Findings. Pharmaceuticals. 2024; 17(11):1439. https://doi.org/10.3390/ph17111439
Chicago/Turabian StyleAsiri, Yahya I., Sivakumar S. Moni, Mohankumar Ramar, and Kumarappan Chidambaram. 2024. "Advancing Pain Understanding and Drug Discovery: Insights from Preclinical Models and Recent Research Findings" Pharmaceuticals 17, no. 11: 1439. https://doi.org/10.3390/ph17111439
APA StyleAsiri, Y. I., Moni, S. S., Ramar, M., & Chidambaram, K. (2024). Advancing Pain Understanding and Drug Discovery: Insights from Preclinical Models and Recent Research Findings. Pharmaceuticals, 17(11), 1439. https://doi.org/10.3390/ph17111439







