Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Oils’ Chemical Composition
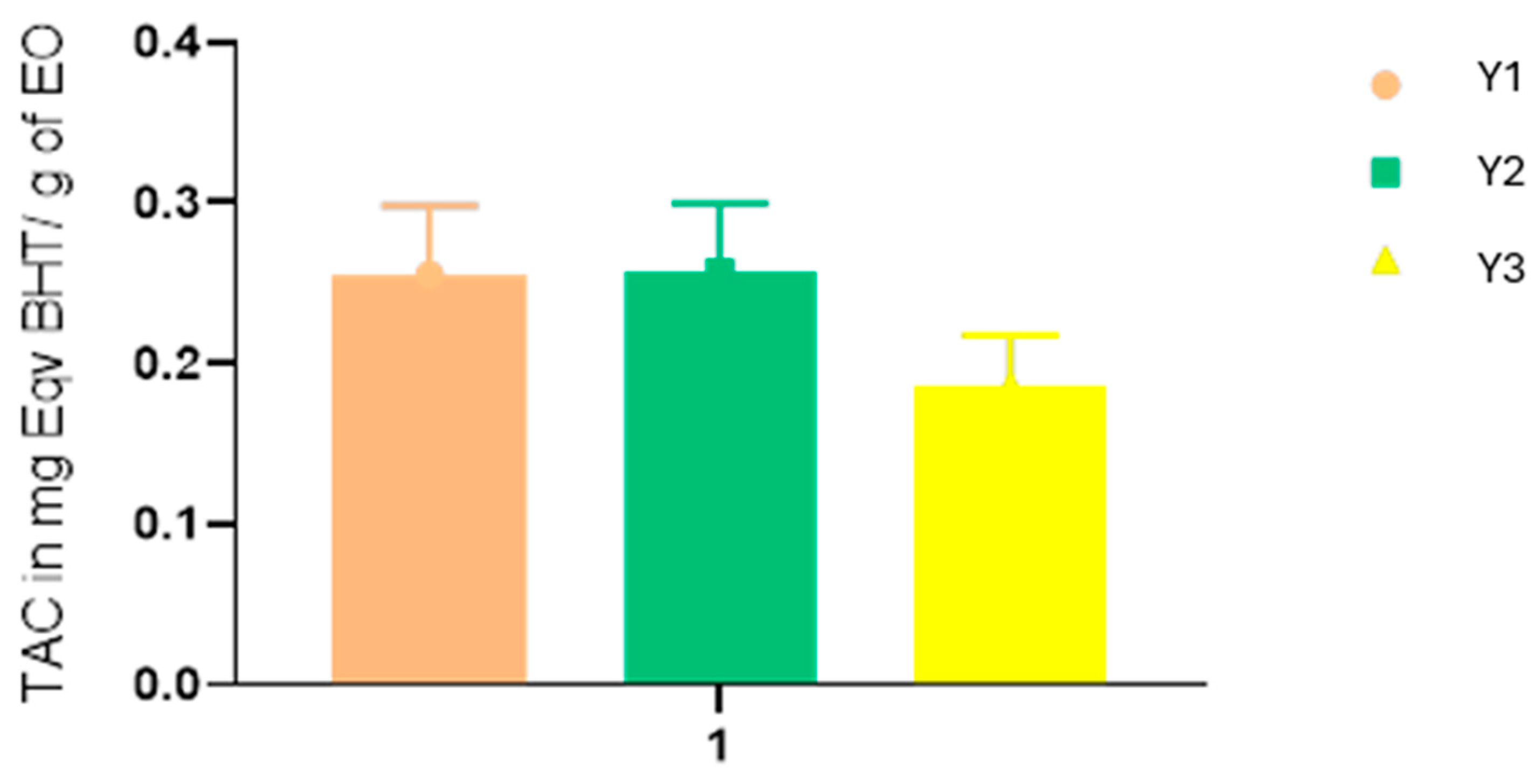
2.2. Antioxidant Activities
2.3. Antibacterial Activity
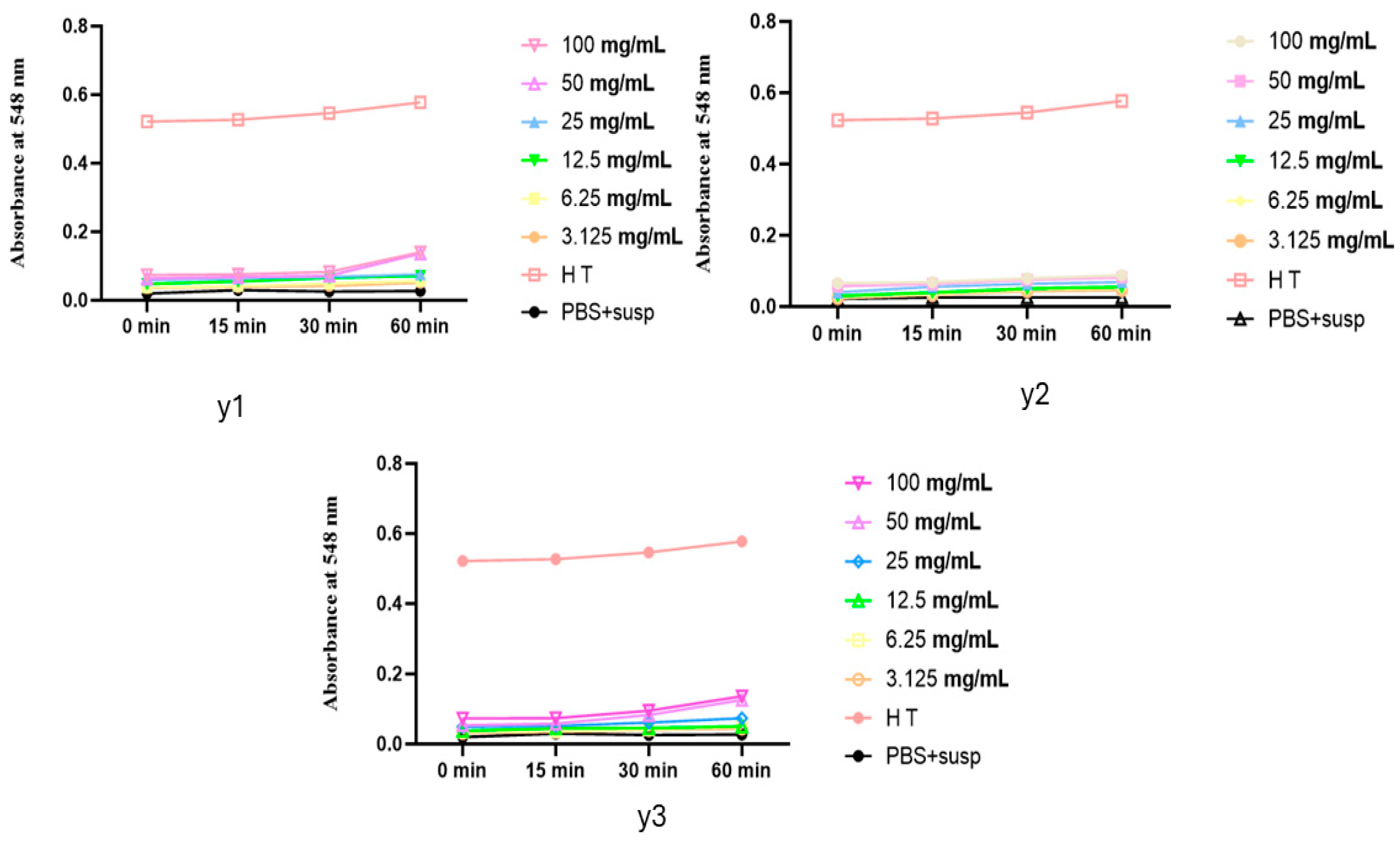
2.4. Hemolytic Test
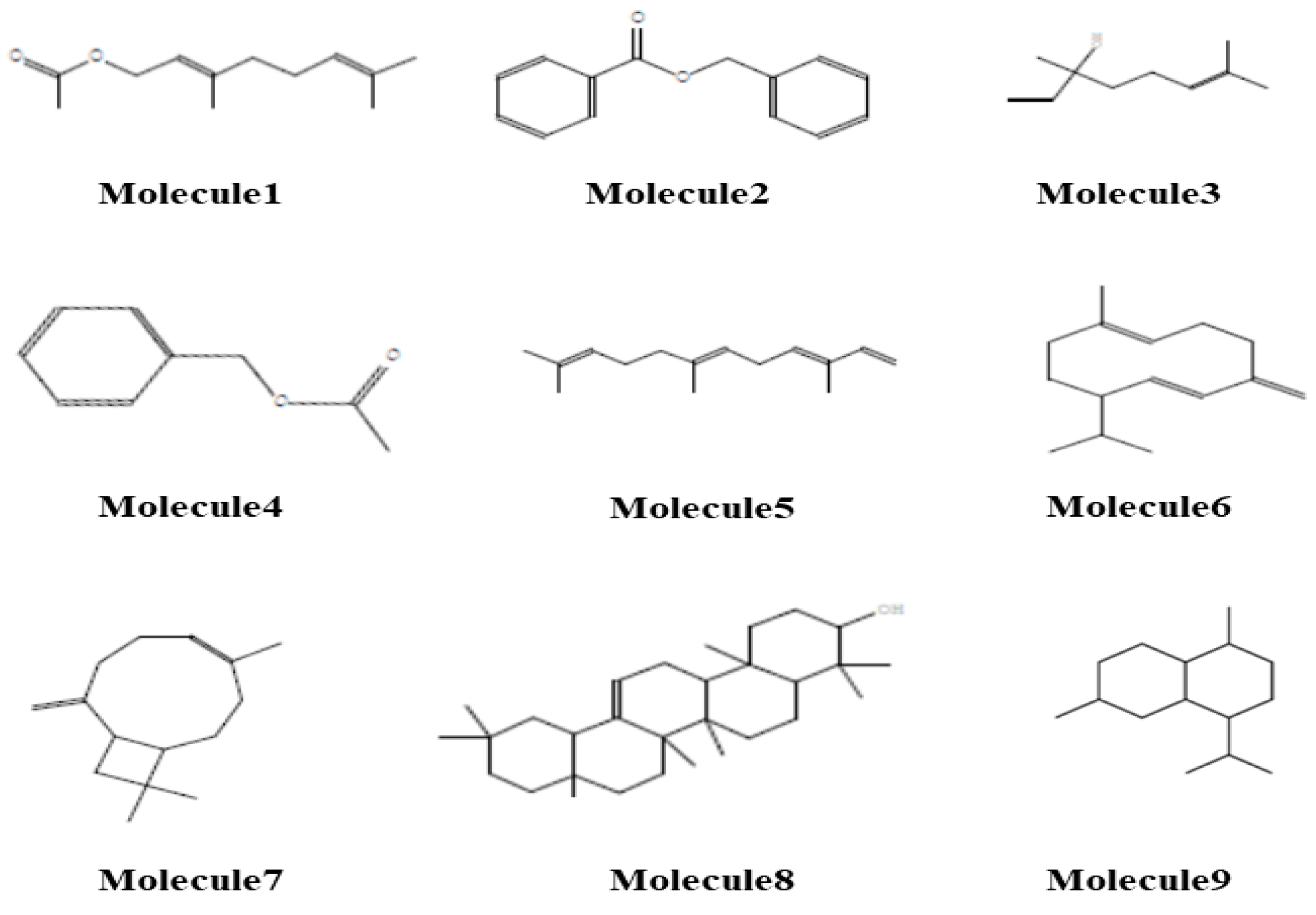
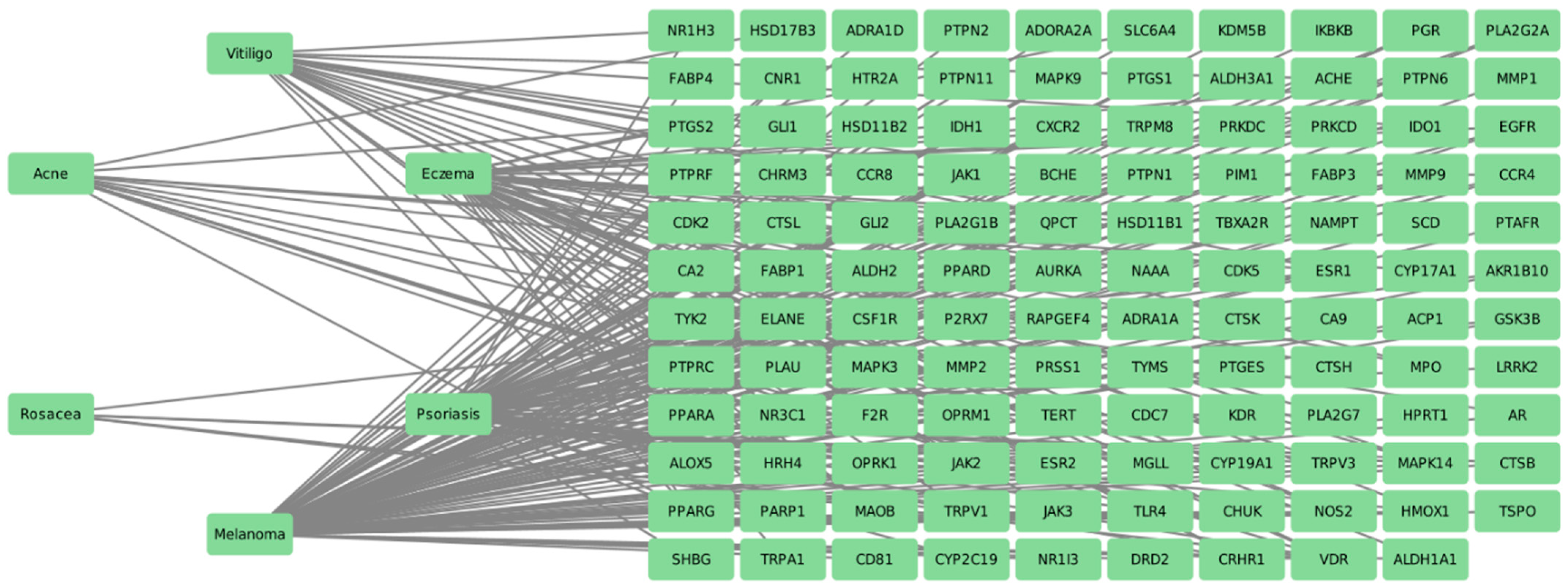
2.5. Identification of Therapeutic Targets for Dermatological Diseases via Molecular Docking
3. Material and Methods
3.1. Plant Material
3.2. Phytochemical Analysis
3.3. Antioxidant Activity
- a.
- 2,2-diphenylpicrylhydrazyl (DPPH) Method
- b.
- Ferric Reducing Antioxidant Power (FRAP) Test
- c.
- Total Antioxidant Capacity (TAC) Test
- d.
- Beta-Carotene Bleaching Inhibition Assay
3.4. Antibacterial Activity
3.5. Hemolytic Test
3.6. Molecular Docking
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef] [PubMed]
- EL Moussaoui, A.; Zouirech, O.; Zahra Jawhari, F.; Bari, A. Propagation method and germination condition of an arid and semi-arid species: Withania frutescens (L.). J. Biol. Biomed. Res. 2024, 1, 62–69. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Mssillou, I.; Agour, A.; Lefrioui, Y.; Chebaibi, M. LC-TOFMS analysis, in vitro and in silico antioxidant activity on NADPH oxidase, and toxicity assessment of an extract mixture based on Marrubium vulgare L. and Dittrichia viscosa L. J. Biol. Biomed. Res. 2024, 1, 31–45. [Google Scholar] [CrossRef]
- El Abdali, Y.; Meryem, M.J.; Agour, A.; Allali, A.; Chebaibi, M.; Bouia, A. Chemical composition, free radicals, pathogenic microbes, α-amylase and α-glucosidase suppressant proprieties of essential oil derived from Moroccan Mentha pulegium: In silico and in vitro approaches. J. Biol. Biomed. Res. 2024, 1, 46–61. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef]
- Agour, A.; Mssillou, I.; El Abdali, Y.; Bari, A.; Lyoussi, B.; Derwich, E. Phytochemical characterization, acute toxicity, and hemolytic activity of Cotula cinerea (Del.) aqueous and ethanolic extracts. J. Biol. Biomed. Res. 2024, 1, 70–78. [Google Scholar] [CrossRef]
- Irshad, M.; Subhani, M.A.; Ali, S.; Hussain, A. Biological Importance of Essential Oils; BoD–Books on Demand: Norderstedt, The Netherlands, 2020. [Google Scholar]
- El Barnossi, A.; Moubchir, T.; Beniaich, G.; Saghrouchni, H.; Allali, A.; Housseini, A.I. Isolation, conventional and molecular identification of Fusarium proliferatum responsible to bulb rot of garlic and potential biological control by new bacterial strains. J. Biol. Biomed. Res. 2024, 1, 1–9. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Giordani, C.; Cappellacci, L.; Petrelli, R.; Canale, A. Insecticidal activity of two essential oils used in perfumery (ylang ylang and frankincense). Nat. Prod. Res. 2021, 35, 4746–4752. [Google Scholar] [CrossRef]
- Chebaibi, M.; Mssillou, I.; Allali, A.; Bourhia, M.; Bousta, D.; Gonçalves, R.F.B.; Hoummani, H.; Aboul-Soud, M.A.M.; Augustyniak, M.; Giesy, J.P.; et al. Antiviral Activities of Compounds Derived from Medicinal Plants against SARS-CoV-2 Based on Molecular Docking of Proteases. J. Biol. Biomed. Res. 2024, 1, 10–30, ISSN 3009-5522. [Google Scholar] [CrossRef]
- Azzouni, D.; Mrani, S.A.; Bertani, R.; Alanazi, M.M.; En-Nabety, G.; Taleb, M. Experimental and Theoretical Investigation of the Inhibitor Efficiency of Eucalyptus globulus Leaf Essential Oil (EuEO) on Mild Steel Corrosion in a Molar Hydrochloric Acid Medium. Molecules 2024, 29, 3323. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A. Essential Oil as Antimicrobial Agents: Efficacy, Stability, and Safety Issues for Food Application; BoD–Books on Demand: Norderstedt, The Netherlands, 2020. [Google Scholar]
- Butnariu, M. Plants as Source of Essential Oils and Perfumery Applications; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar]
- Ng, F.; Basri, N.; Wu, W.; Thong, A.; Thong, G.; Chew, W.; Dharmawan, J. Characterization of volatile compounds in Ylang-Ylang essential oils from Comoros and Madagascar by gas chromatography and principal component analysis. Flavour. Fragr. J. 2021, 36, 159–166. [Google Scholar] [CrossRef]
- Tubachi, S.S.; Rasal, V.P.; Ugare, S.R.; Khatib, N.A.; Ojha, P.S.; Patil, V.S. Evaluation of Ylang Ylang essential oil on alcohol induced hepatotoxicity in rats. Adv. Tradit. Med. 2023, 23, 575–588. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional uses, phytochemistry, and bioactivities of Cananga odorata (ylang-ylang). Evid.-Based Complement. Altern. Med. 2015, 2015, 896314. [Google Scholar] [CrossRef]
- Denkova, Z.; Goranov, B.; Blazheva, D.; Tomova, T.; Teneva, D.; Denkova-Kostova, R.; Slavchev, A.; Pagán, R.; Degraeve, P.; Kostov, G. Chemical Composition and Antimicrobial Activity of Lavender (Lavandula angustifolia Mill.), Peppermint (Mentha piperita L.), Raspberry Seed (Rubus idaeus L.), and Ylang-Ylang (Cananga odorata (Lam.) Essential Oils—Towards Hurdle Technologies in the Production of Chocolate Mousse. Appl. Sci. 2023, 13, 11281. [Google Scholar] [CrossRef]
- Elkenawy, N.M.; Soliman, M.A.W.; El-behery, R.R. In-vitro Antimicrobial Study of Non/irradiated Ylang-ylang Essential Oil Against Multi Drug Resistant Pathogens with Reference to Microscopic Morphological Alterations. Indian. J. Microbiol. 2023, 63, 621–631. [Google Scholar] [CrossRef]
- Rizal Eh Suk, V.; Khalid, K.; Misran, M.; Mai Sci, C.J. Preparation and Characterization of Ylang-Ylang (Cananga odorata) Essential Oil and Ascorbic Acid Loaded Olive Oil-in-Water Emulsion. Chiang Mai J. Sci. 2019, 46, 353–360. [Google Scholar]
- Maddheshiya, S.; Ahmad, A.; Ahmad, W.; Zakir, F.; Aggarwal, G. Essential oils for the treatment of skin anomalies: Scope and potential. S. Afr. J. Bot. 2022, 151, 187–197. [Google Scholar] [CrossRef]
- Higaki, S. Lipase inhibitors for the treatment of acne. J. Mol. Catal. B Enzym. 2003, 22, 377–384. [Google Scholar] [CrossRef]
- Abozeid, D.; Fawzy, G.; Issa, M.; Abdeltawab, N.; Soliman, F. Medicinal Plants and their Constituents in the Treatment of Acne vulgaris. Biointerface Res. Appl. Chem. 2023, 13, 189. [Google Scholar] [CrossRef]
- Takahashi, K.; Miyake, K.; Ito, J.; Shimamura, H.; Suenaga, T.; Karasuyama, H.; Ohashi, K. Topical Application of a PDE4 Inhibitor Ameliorates Atopic Dermatitis through Inhibition of Basophil IL-4 Production. J. Investig. Dermatol. 2024, 144, 1048–1057.e8. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.E.; Chan, V.P.; Leung, A.K. Experimental Drugs with the Potential to Treat Atopic Eczema. J. Exp. Pharmacol. 2021, 13, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ehst, B.; Wang, Z.; Leitenberger, J.; McClanahan, D.; De La Torre, R.; Sawka, E.; Ortega-Loayza, A.G.; Strunck, J.; Greiling, T.; Simpson, E.; et al. Synergistic induction of IL-23 by TNFα, IL-17A, and EGF in keratinocytes. Cytokine 2021, 138, 155357. [Google Scholar] [CrossRef]
- Bhoi, A.K.; Grover, C.; Singal, A.; Kashyap, B.; Dibyashree, D. Serum levels of tumour necrosis factor (TNF-α) and interleukin-17 (IL-17) in patients with nail psoriasis: A cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2024, 90, 453–457. [Google Scholar] [CrossRef]
- Fisher, G.W.; Travers, J.B.; Rohan, C.A. Rosacea pathogenesis and therapeutics: Current treatments and a look at future targets. Front. Med. 2023, 10, 1292722. [Google Scholar] [CrossRef]
- Qi, F.; Liu, F.; Gao, L. Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front. Immunol. 2021, 12, 790125. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dummer, R.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Contribution of MEK Inhibition to BRAF/MEK Inhibitor Combination Treatment of BRAF-Mutant Melanoma: Part 2 of the Randomized, Open-Label, Phase III COLUMBUS Trial. J. Clin. Oncol. 2023, 41, 4621–4631. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2019, 48, D845–D855. [Google Scholar] [CrossRef]
- Loucif, K.; Benabdallah, H.; Benchikh, F.; Mehlous, S.; Souici, C.B.; Amira, S. Total Phenolic Contents, DPPH Radical Scavenging and β-Carotene Bleaching Activities of Aqueous Extract from Ammoides atlantica. J. Drug Deliv. Ther. 2020, 10, 196–198. [Google Scholar] [CrossRef]
- Zejli, H.; El Amrani, B.; Zafra Bousseraf, F.; Fitat, A.; Taleb, M.; Abdellaoui, A. Comparative assessment of total phenolics content and in vitro antioxidant capacity variations of leaf extracts of Origanum grossii and Thymus pallidus. Mor. J. Chem. 2024, 12, 1–448. [Google Scholar] [CrossRef]
- Maškovič, P.Z.; Manojlovič, N.T.; Mandič, A.I.; Mišan, A.Ç.; Milovanovic, I.L.; Radojkovič, M.M.; Cvijovič, M.S.; Solujič, S.R. Fitohemija i biološka aktivnost ekstrakata biljne vrste Halacsya sendtneri (Boiss.) Dörfl. Hem. Ind. 2012, 66, 43–51. [Google Scholar] [CrossRef]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Furtado, G.L.; Medeiros, A.A. Single-Disk Diffusion Testing (Kirby-Bauer) of Susceptibility of Proteus mirabilis to Chloramphenicol: Significance of the Intermediate Category. J. Clin. Microbiol. 1980, 12, 550–553. [Google Scholar] [CrossRef]
- Zejli, H.; Fitat, A.; Lefrioui, Y.; Siddique, F.; Bourhia, M.; Bousseraf, F.Z.; Salamatullah, A.M.; Nafidi, H.-A.; Mekonnen, A.B.; Gourch, A.; et al. Phytochemical analysis and biological activities of essential oils extracted from Origanum grossii and Thymus pallidus: In vitro and in silico analysis. Sci. Rep. 2023, 13, 20021. [Google Scholar] [CrossRef]
- Almeida, T.S.; Arantes, M.R.; Lopes Neto, J.J.; Souza, T.M.; Pessoa, I.P.; Medeiros, J.L.; Tabosa, P.M.S.; Moreira, T.B.; Farias, D.F.; Carvalho, A.F.U. Evaluation of seeds ethanolic extracts of Triplaris gardneriana Wedd. using in vitro and in vivo toxicological methods. J. Toxicol. Environ. Health A 2020, 83, 135–152. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
| Name | Chemical Formula | Retention Index | Area % | ||
|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | |||
| Hexanal | C6H12O | 800 | - | 1.36 | - |
| 2,4-Dimethylheptane | C9H20 | 1022 | - | 0.91 | - |
| 3-Methoxy-toluene | C8H10O | 1031 | 1.73 | 4.78 | - |
| Benzoic acid, methyl ester | C8H8O2 | 1079 | 1.46 | 3.76 | - |
| Linalool | C10H18O | 1095 | 4.19 | 10.52 | - |
| Benzyl acetate | C9H10O2 | 1160 | - | - | 0.44 |
| Benzyl acetate | C9H10O2 | 1165 | 2.82 | 5.02 | 0.55 |
| Dodecane, 2,6,11-trimethyl | C15H32 | 1200 | 0.97 | 5.41 | 1.47 |
| Nerol | C10H18O | 1228 | 0.29 | - | - |
| Cinnamyl alcohol | C9H10O | 1259 | 1.96 | - | - |
| Elemene | C15H24 | 1340 | 0.47 | - | - |
| α-Copaene | C15H24 | 1377 | 1.06 | - | 1.90 |
| Farnesan | C15H32 | 1381 | - | 2.19 | 1.15 |
| Geranyl acetate | C12H20O2 | 1384 | 5.24 | 4.18 | 1.40 |
| β-Funebrene | C15H24 | 1416 | - | - | 0.86 |
| β-Caryophyllene | C15H24 | 1418 | 5.27 | 1.17 | 8.83 |
| α-Guaiene | C15H24 | 1440 | - | - | 0.73 |
| Phenol acetate | C10H12O4 | 1445 | - | 2.93 | 0.82 |
| β-Farnesene | C15H24 | 1448 | 0.78 | - | - |
| Muurola-4(14),5-diene | C15H24 | 1470 | 0.36 | - | 0.63 |
| α-Caryophyllene | C15H24 | 1478 | 1.74 | - | - |
| Germacrene D | C15H24 | 1490 | 7.26 | 2.93 | 15.30 |
| α-Muurolene | C15H24 | 1503 | 0.25 | - | 0.43 |
| γ-Muurolene | C15H24 | 1504 | 0.85 | - | - |
| α-Muurolene | C15H24 | 1507 | 1.11 | - | 2.09 |
| α-Farnesene | C15H24 | 1510 | 13.65 | 3.03 | 24.80 |
| α-Amorphene | C15H24 | 1513 | 1.13 | - | 0.47 |
| γ-Cadinene | C15H24 | 1517 | 1.66 | - | 6.34 |
| δ-Cadinene | C15H24 | 1525 | 3.72 | - | 6.00 |
| Zonarene | C14H22 | 1528 | 0.25 | - | - |
| Copaen-11-ol | C15H24O | 1544 | 0.62 | - | 1.17 |
| Junenol | C15H26O | 1618 | 0.48 | - | 0.47 |
| α-Muurolol | C15H26O | 1648 | 7.73 | - | 5.45 |
| Farnesol | C15H26O | 1686 | 0.65 | - | - |
| Benzyl Benzoate | C14H12O2 | 1766 | 10.52 | 3.16 | 4.54 |
| Octadecane | C18H38 | 1800 | 0.95 | - | - |
| Farnesyl acetate (2Z,6E) | C17H28O2 | 1823 | 2.90 | - | 3.15 |
| Benzyl salicylate | C14H12O3 | 1857 | 4.70 | - | 1.18 |
| β-Amyrin | C30H50O | 1980 | - | 25.84 | - |
| Eicosane | C20H42 | 2000 | 0.80 | 5.97 | 2.57 |
| Dotriacontane | C32H66 | 3204 | 2.08 | 15.72 | 3.06 |
| Betunal | C30H48O3 | 3628 | 4.58 | - | - |
| TOTAL | 94.23 | 98.88 | 98.39 | ||
| Y1 | Y2 | Y3 | BHT | Quercetin | |
|---|---|---|---|---|---|
| DPPH (IC50 mg/mL) | 3.5 ± 0.03 | 1.57 ± 0.08 | 1.91 ± 0.04 | 0.11 ± 0.001 | - |
| FRAP (EC50 mg/mL) | 0.21 ± 0.01 | 0.17 ± 0.04 | 0.19 ± 0.01 | - | 0.03 ± 0.004 |
| Relative antioxidant activity in % | 56.67% | 58.67% | 57.32% | 100% | - |
| E. coli | S. aureus | B. subtilis | P. aeruginosa | |||||
|---|---|---|---|---|---|---|---|---|
| ID (mm) | MIC mg/mL | ID (mm) | MIC mg/mL | ID (mm) | MIC mg/mL | ID (mm) | MIC mg/mL | |
| Y1 | NF | NF | 14.5±0.45 | 0.04 | 11.00±1.00 | 0.04 | NF | NF |
| Y2 | 17.11±0.00 | 0.02 | 12.5±1.11 | 0.01 | 18.05±1.25 | 0.02 | NF | NF |
| Y3 | NF | NF | 14.5±1.00 | 0.04 | 14.00±0.5 | 0.04 | NF | NF |
| Kanamycin | 19.3±1.56 | 0.002 | 21.4±1.2 | 0.016 | 19.3±1.5 | 0.004 | 17.00±0.00 | 0.004 |
| Molecule | Formula | MW | RT | HA | HD | MR | TPSA | MlogP | Lipinski | Ghose | Veber |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Violations | |||||||||||
| Molecule 1 (Geranyl acetate) | C12H20O2 | 196.29 | 6 | 2 | 0 | 60.13 | 26.3 | 2.95 | 0 | 0 | 0 |
| Molecule 2 (benzyl benzoate) | C14H12O2 | 212.24 | 4 | 2 | 0 | 62.21 | 26.3 | 3.41 | 0 | 0 | 0 |
| Molecule 3 (Linalool) | C10H18O | 154.25 | 4 | 1 | 1 | 50.44 | 20.23 | 2.59 | 0 | 1 | 0 |
| Molecule 4 (Benzyl acetate) | C9H10O2 | 150.17 | 3 | 2 | 0 | 42.31 | 26.3 | 1.98 | 0 | 1 | 0 |
| Molecule 5 (α-Farnesene) | C15H24 | 204.35 | 6 | 0 | 0 | 72.32 | 0 | 4.84 | 1 | 0 | 0 |
| Molecule 6 (Germacrene-D) | C15H24 | 204.35 | 1 | 0 | 0 | 70.68 | 0 | 4.53 | 1 | 0 | 0 |
| Molecule 7 (Caryophylene) | C15H24 | 204.35 | 0 | 0 | 0 | 68.78 | 0 | 4.63 | 1 | 0 | 0 |
| Molecule 8 (β-Amyrin) | C30H50O | 426.72 | 0 | 1 | 1 | 134.88 | 20.23 | 6.92 | 1 | 3 | 0 |
| Molecule 9 (δ-Cadinene) | C15H24 | 204.35 | 1 | 0 | 0 | 69.04 | 0 | 4.63 | 1 | 0 | 0 |
| Disease | Gene | Molecule | PDB ID |
|---|---|---|---|
| Acne | AR | Molecule 3, Molecule 6, Molecule 8 | 1E3G |
| CYP17A1 | Molecule 1, Molecule 2, Molecule 8 | 1E6A | |
| CYP19A1 | Molecule 4, Molecule 6, Molecule 8 | 3S7R | |
| Eczema | CA2 | Molecule 1, Molecule 2, Molecule 3, Molecule 4 | 1CA2 |
| JAK2 | Molecule 1, Molecule 3, Molecule 4 | 3KCK | |
| JAK3 | Molecule 1, Molecule 3, Molecule 4 | 4Z16 | |
| PPARA | Molecule 6, Molecule 8, Molecule 9 | 1K7L | |
| Psoriasis | CA2 | Molecule 1, Molecule 2, Molecule 3, Molecule 4 | 1CA2 |
| ESR2 | Molecule 2, Molecule 6, Molecule 8 | 3OLS | |
| JAK2 | Molecule 1, Molecule 3, Molecule 4 | 3KCK | |
| PPARA | Molecule 6, Molecule 8, Molecule 9 | 1K7L | |
| Rosacea | CYP19A1 | Molecule 4, Molecule 6, Molecule 8 | 3S7R |
| Vitiligo | PRSS1 | Molecule 1, Molecule 2, Molecule 4 | 3R43 |
| Melanoma | AR | Molecule 3, Molecule 6, Molecule 8 | 1E3G |
| CA2 | Molecule 1, Molecule 2, Molecule 3, Molecule 4 | 1CA2 | |
| CYP19A1 | Molecule 4, Molecule 6, Molecule 8 | 3S7R | |
| ESR2 | Molecule 2, Molecule 6, Molecule 8 | 3OLS | |
| JAK2 | Molecule 1, Molecule 3, Molecule 4 | 3KCK | |
| JAK3 | Molecule 1, Molecule 3, Molecule 4 | 4Z16 | |
| PPARA | Molecule 6, Molecule 8, Molecule 9 | 1K7L | |
| PRSS1 | Molecule 1, Molecule 2, Molecule 4 | 3R43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrani, S.A.; Zejli, H.; Azzouni, D.; Fadili, D.; Alanazi, M.M.; Hassane, S.O.S.; Sabbahi, R.; Kabra, A.; Moussaoui, A.E.; Hammouti, B.; et al. Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology. Pharmaceuticals 2024, 17, 1376. https://doi.org/10.3390/ph17101376
Mrani SA, Zejli H, Azzouni D, Fadili D, Alanazi MM, Hassane SOS, Sabbahi R, Kabra A, Moussaoui AE, Hammouti B, et al. Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology. Pharmaceuticals. 2024; 17(10):1376. https://doi.org/10.3390/ph17101376
Chicago/Turabian StyleMrani, Soukaina Alaoui, Hind Zejli, Dounia Azzouni, Driss Fadili, Mohammed M. Alanazi, Said Omar Said Hassane, Rachid Sabbahi, Atul Kabra, Abdelfattah El Moussaoui, Belkheir Hammouti, and et al. 2024. "Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology" Pharmaceuticals 17, no. 10: 1376. https://doi.org/10.3390/ph17101376
APA StyleMrani, S. A., Zejli, H., Azzouni, D., Fadili, D., Alanazi, M. M., Hassane, S. O. S., Sabbahi, R., Kabra, A., Moussaoui, A. E., Hammouti, B., & Taleb, M. (2024). Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology. Pharmaceuticals, 17(10), 1376. https://doi.org/10.3390/ph17101376








