Exploring Benzo[h]chromene Derivatives as Agents against Protozoal and Mycobacterial Infections
Abstract
1. Introduction
2. Results and Discussion
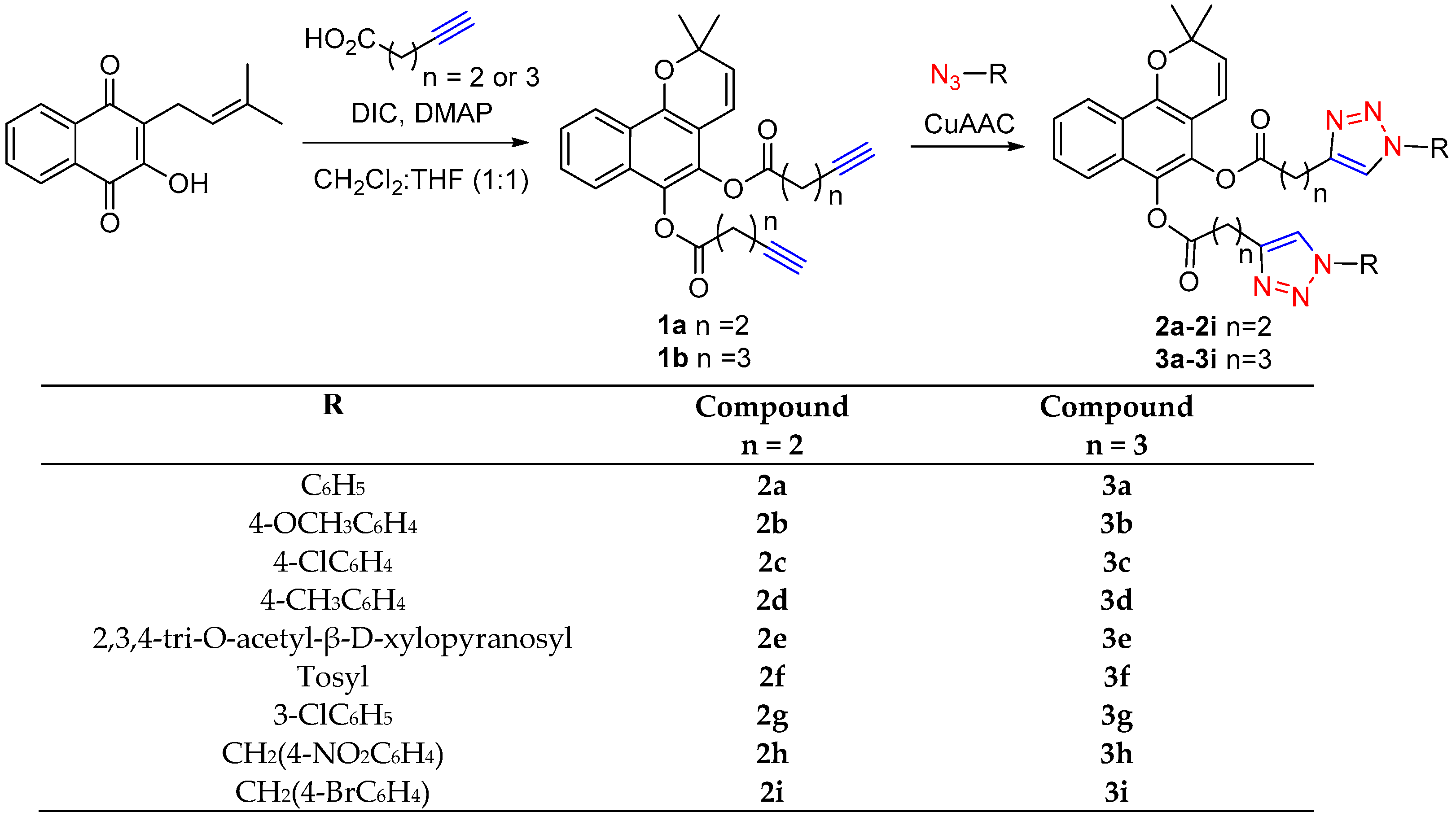
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antiprotozoal Activity
2.2.2. Antibacterial Activity
2.3. ADME In Silico Approach
| Physicochemical Properties | Lipophilicity | Water Solubility | Pharmacokinetics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MW 1 | RB 2 | HBA 3 | HBD 4 | TPSA 5 | Log Po/w 6 | (mol/L) | GI abs 7 | BBB 8 | log Kp 9 | |
| 1a | 402.44 | 8 | 5 | 0 | 61.83 | 4.73 | 9.14 × 10−4 | High | Yes | −5.47 |
| 1b | 430.49 | 10 | 5 | 0 | 61.83 | 5.23 | 1.79 × 10−4 | High | No | −5.14 |
| 2a | 640.69 | 12 | 9 | 0 | 123.25 | 5.39 | 1.35 × 10−6 | Low | No | −5.74 |
| 3a | 668.74 | 14 | 9 | 0 | 123.25 | 6.16 | 2.52 × 10−7 | Low | No | −5.40 |
| 2b | 700.74 | 14 | 11 | 0 | 141.71 | 5.51 | 6.97 × 10−7 | Low | No | −6.14 |
| 3b | 728.79 | 16 | 11 | 0 | 141.71 | 5.87 | 1.30 × 10−7 | Low | No | −5.80 |
| 2c | 709.58 | 12 | 9 | 0 | 123.25 | 6.48 | 7.35 × 10−8 | Low | No | −5.26 |
| 3c | 737.63 | 14 | 9 | 0 | 123.25 | 7.10 | 1.40 × 10−8 | Low | No | −4.93 |
| 2d | 668.74 | 12 | 9 | 0 | 123.28 | 5.97 | 2.49 × 10−7 | Low | No | −5.39 |
| 3d | 696.79 | 14 | 9 | 0 | 123.25 | 6.64 | 4.70 × 10−8 | Low | No | −5.06 |
| 2e | 1004.94 | 24 | 23 | 0 | 299.51 | 2.92 | 9.60 × 10−6 | Low | No | −10.94 |
| 3e | 1033.00 | 26 | 23 | 0 | 299.51 | 3.18 | 1.77 × 10−6 | Low | No | −10.60 |
| 2f | 796.87 | 14 | 13 | 0 | 208.29 | 5.66 | 3.75 × 10−8 | Low | No | −6.87 |
| 3f | 840.96 | 16 | 13 | 0 | 208.29 | 5.50 | 1.53 × 10−9 | Low | No | −6.08 |
| 2g | 709.58 | 12 | 9 | 0 | 123.25 | 6.60 | 7.35 × 10−8 | Low | No | −5.26 |
| 3g | 737.63 | 14 | 9 | 0 | 123.25 | 7.19 | 1.40 × 10−8 | Low | No | −4.93 |
| 2h | 758.74 | 16 | 13 | 0 | 214.89 | 4.49 | 5.84 × 10−8 | Low | No | −6.79 |
| 3h | 786.79 | 18 | 13 | 0 | 214.89 | 5.03 | 1.08 × 10−8 | Low | No | −6.45 |
| 2i | 826.53 | 14 | 9 | 0 | 123.25 | 6.67 | 8.57 × 10−8 | Low | No | −5.97 |
| 3i | 854.59 | 16 | 9 | 0 | 123.25 | 7.47 | 1.62 ×10−8 | Low | No | −5.64 |
3. Materials and Methods
3.1. Chemistry
3.1.1. General Overview
3.1.2. General Procedure Followed for Esterification
3.2. Biology
3.2.1. Antiprotozoal Assays
3.2.2. Cytotoxicity
3.2.3. Bacterial Strains and Culture Conditions
3.2.4. Determination of the Minimum Inhibitory Concentration (MIC)
3.3. ADME In Silico Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conteh, L.; Engels, T.; Molyneux, D.H. Socioeconomic aspects of neglected tropical diseases. Lancet 2010, 375, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Health in the Americas 2007. In Regional, Scientific and Technical Publication 622; Pan American Health Organization: Washington, DC, USA, 2007. [Google Scholar]
- World Health Organization. First WHO Report on Neglected Tropical Diseases. In Working to Overcome the Global Impact Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-1564090. [Google Scholar]
- Yamagata, Y.; Nakagawa, J. Control of Chagas disease. Adv. Parasitol. 2006, 61, 129–165. [Google Scholar]
- World Health Organization. Global Health Estimates 2014 Summary Tables: DALY by Cause, Age and Sex, by WHO Region, 2000–2012; World Health Organization: Geneva, Switzerland, 2014; Available online: http://www.who.int/news-room/facts-in-pictures/detail/chagas-disease (accessed on 10 October 2024).
- Meymandi, S.K.; Forsyth, C.J.; Soverow, J.; Hernandez, S.; Sanchez, D.; Montgomery, S.P.; Traina, M. Prevalence of Chagas disease in the Latin American-born population of Los Angeles. Clin. Infect. Dis. 2017, 64, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; O’Connor, P.D.; Yardley, V.; Maes, L.; Launay, D.; Braillard, S.; Chatelain, E.; Wan, B.; Franzblau, S.G.; Ma, Z.; et al. Novel linker variants of Antileishmanial/Antitubercular 7-substituted 2-nitroimidazooxazines offer enhanced solubility. ACS Med. Chem. Lett. 2021, 12, 275–281. [Google Scholar] [CrossRef]
- Roldos, V.; Nakayama, H.; Rolo, M.; Trucco, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G.; Go, A.; et al. Activity of a hydroxybibenzyl bryophyte constituent against Leishmania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef]
- Vega, C.; Rolón, M.; Martínez-Fernández, A.R.; Escario, J.A.; Gómez-Barrio, A. A new pharmacological screening assay with Trypanosoma cruzi epimastigotes expressing beta-galactosidase. Parasitol. Res. 2005, 95, 296–298. [Google Scholar] [CrossRef]
- Santos, S.S.; de Araújo, R.V.; Giarolla, J.; Seoud, O.E.; Ferreira, E.I. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: A review. Int. J. Antimicrob. Agents 2020, 55, 105906. [Google Scholar] [CrossRef]
- Lepesheva, G.I. Design or screening of drugs for the treatment of Chagas disease: What shows the most promise? Expert Opin. Drug Discov. 2013, 8, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Scarim, C.B.; Jornada, D.H.; Chelucci, R.C.; de Almeida, L.; Dos Santos, J.L.; Chung, M.C. Current advances in drug discovery for Chagas disease. Eur. J. Med. Chem. 2018, 155, 824–838. [Google Scholar] [CrossRef]
- Lamim, M.; Domeneghini, L.; Rosaria, L.; Dias, S.; Kramer, L.; Regina, T.; Mascarello, A.; Steindel, M.; Augusto, R.; Carla, H.; et al. Trimethoxy-chalcone derivatives inhibit growth of Leishmania braziliensis: Synthesis, biological evaluation, molecular modeling and structure-activity relationship (SAR). Bioorg. Med. Chem. 2011, 19, 5046–5052. [Google Scholar]
- WHO. Research and Development to Meet Health Needs in Developing Countries; Report of the Consultative Expert Working Group on Research and Development: Strengthening Global Financing and Coordination; Financing and Coordination; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Ortiz-Brizuela, E.; Menzies, D.; Behr, M.A. Testing and Treating Mycobacterium tuberculosis Infection. Med. Clin. N. Am. 2022, 106, 929–947. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Murugaiyan, J.; Hammoudi Halat, D.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic discovery and resistance: The chase and the race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (accessed on 10 October 2024).
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Hooker, S.C. The constitution of lapachol and its derivatives. Part V. The structure of paternd’s “Isolapachone”. J. Am. Chem. Soc. 1936, 58, 1190–1197. [Google Scholar] [CrossRef]
- de la Torre, A.F.; Ali, A.; Westermann, B.; Schmeda-Hirschmann, G.; Pertino, M.W. An efficient cyclization of lapachol to new benzo[h]chromene hybrid compounds: A stepwise vs. one-pot esterification-click (CuAAC) study. New J. Chem. 2018, 42, 19591–19599. [Google Scholar] [CrossRef]
- Da Silva, E.N.; Menna-Barreto, R.F.S.; Pinto, M.d.C.F.R.; Silva, R.S.F.; Teixeira, D.V.; de Souza, M.C.B.V.; de Simone, C.A.; de Castro, S.L.; Ferreira, V.F.; Pinto, A.V. Naphthoquinoidal [1,2,3]-triazole, a new structural moiety active against Trypanosoma cruzi. Eur. J. Med. Chem. 2008, 43, 1774–1780. [Google Scholar] [CrossRef]
- Da Silva, E.N., Jr.; de Melo, I.M.M.; Diogo, E.B.T.; Costa, V.A.; Filho, J.D.S.; Valença, W.O.; Camara, C.A.; de Oliveira, R.N.; de Araujo, A.S.; Emery, F.S.; et al. On the search for potential anti-Trypanosoma cruzi drugs: Synthesis and biological evaluation of 2-hydroxy-3-methylamino and 1,2,3-triazolic naphthoquinoidal compounds obtained by click chemistry reactions. Eur. J. Med. Chem. 2012, 52, 304–312. [Google Scholar] [CrossRef]
- Da Silva, E.N., Jr.; Guimarães, T.T.; Menna-Barreto, R.F.S.; Pinto, M.C.F.R.; de Simone, C.A.; Pessoa, C.; Cavalcanti, B.C.; Sabino, J.R.; Andrade, C.K.Z.; Goulart, M.O.F.; et al. The evaluation of quinonoid compounds against Trypanosoma cruzi: Synthesis of imidazolic anthraquinones, nor-β-lapachone derivatives and β-lapachone-based 1,2,3-triazoles. Bioorg. Med. Chem. 2010, 18, 3224–3230. [Google Scholar] [CrossRef]
- Diogo, E.B.T.; Dias, G.G.; Rodrigues, B.L.; Guimarães, T.T.; Valença, W.O.; Camara, C.A.; de Oliveira, R.N.; da Silva, M.G.; Ferreira, V.F.; Paiva, Y.G.; et al. Synthesis and anti-Trypanosoma cruzi activity of naphthoquinone-containing triazoles: Electrochemical studies on the effects of the quinoidal moiety. Bioorg. Med. Chem. 2013, 21, 6337–6348. [Google Scholar] [CrossRef]
- Guimarães, T.T.; do Carmo, M.; Pinto, F.R.; Lanza, J.S.; Melo, M.N.; Monte-Neto, R.L.; de Melo, I.M.M.; Diogo, E.B.T.; Ferreira, V.F.; Camara, C.A.; et al. Potent naphthoquinones against antimony-sensitive and -resistant Leishmania parasites: Synthesis of novel α- and nor-α-lapachone based 1,2,3-triazoles by copper-catalyzed azide-alkyne cycloaddition. Eur. J. Med. Chem. 2013, 63, 523–530. [Google Scholar] [CrossRef]
- Pertino, M.W.; de la Torre, A.F.; Schmeda-Hirschmann, G.; Vega, C.; Rolon, M.; Coronel, C.; Rojas de Arias, A.; Leal Lopez, K.; Carranza-Rosales, P.; Viveros Valdez, E. Synthesis, trypanocidal and anti-leishmania activity of new triazole-lapachol and nor-lapachol hybrids. Bioorg. Chem. 2020, 103, 104122. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, L.; Sádlová, J.; Suau, H.A.; Hendrickx, S.; Meneses, C.; Kamhawi, S.; Volf, P.; Maes, L.; Caljon, G. Impaired development of a miltefosine-resistant Leishmania infantum strain in the sand fly vectors Phlebotomus perniciosus and Lutzomyia longipalpis. IJP Drugs Drug Resist. 2019, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing failure of miltefosine in the treatment of Kalaazar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Dhameliya, T.M.; Vekariya, D.D.; Patel, H.Y.; Patel, J.T. Comprehensive coverage on anti-mycobacterial endeavour reported during 2022. Eur. J. Med. Chem. 2023, 5, 255. [Google Scholar] [CrossRef]
- Copp, B.R. Antimycobacterial natural products. Nat. Prod. Rep. 2003, 20, 535–557. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Azevedo-Ximenes, E.; Luzzati, R.; Garcia, R.C. The hydroxy-naphthoquinone lapachol arrests mycobacterial growth and immunomodulates host macrophages. Int. Immunopharmacol. 2010, 10, 1463–1473. [Google Scholar] [CrossRef]
- Ieque, A.L.; Carvalho, H.C.; Baldin, V.P.; Santos, N.C.S.; Costacurta, G.F.; Sampiron, E.G.; Fernandez de Andrade, C.M.M.; Siqueira, V.L.D.; Ferracioli, K.R.C.; Cardoso, R.F.; et al. Antituberculosis activities of lapachol and β-lapachone in combination with other drugs in acidic pH. Microb. Drug Resist. 2021, 27, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.M.; Johnson, R.J. Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance. Drug Dev. Res. 2019, 80, 33–47. [Google Scholar] [CrossRef]
- Coll, P. Fármacos con actividad frente a Mycobacterium tuberculosis [Active drugs against Mycobacterium tuberculosis]. Enferm. Infecc. Microbiol. Clin. 2009, 27, 474–480. [Google Scholar] [CrossRef]
- Wu, M.L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef]
- Akram, S.M.; Attia, F.N. Mycobacterium avium Complex. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431110/# (accessed on 10 October 2024).
- Lin, S.; Hua, W.; Wang, S.; Zhang, Y.; Chen, X.; Liu, H.; Shao, L.; Chen, J.; Zhang, W. In vitro assessment of 17 antimicrobial agents against clinical Mycobacterium avium complex isolates. BMC Microbiol. 2022, 22, 175. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Dortet, L.; Caseris, M.; Raymond, J.; Lorrot, M.; Toubiana, J. Treatment of Resistant Gram-negative bacilli in children. Infect. Dis. Now 2023, 53, 104794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, J.; Kaushik, C.P.; Kumar, L.; Sindhu, J.; Chahal, M. Design, synthesis and antimicrobial activities of 1,2,3-triazole hybrids with amine-ester functionality. Med. Chem. Res. 2024, 33, 77–88. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Iombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef]
- Meanwell, N.A. Improving drug candidates by design: A focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Rolón, M.; Seco, E.M.; Vega, C.; Nogal, J.J.; Escario, A.; Gómez-Barrio, A.; Malpartida, F. Selective activity of polyene macrolides produced by genetically modified Streptomyces on Trypanosoma cruzi. Int. J. Antimicrob. Agents 2006, 28, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Telenti, A.; Marchesi, F.; Balz, M.; Bally, F.; Böttger, E.C.; Bodmer, T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 1993, 31, 175–178. [Google Scholar] [CrossRef] [PubMed]
| Compound | Leishmanicidal Activity (µM) | Trypanocidal Activity (µM) | Cytotoxicity (µM) | Selectivity Index * | |||
|---|---|---|---|---|---|---|---|
| IC50—L. b | IC50—L. i | IC50 T. cruzi | CC50—L929 | L929/ L. b | L929/ L. i | L929/ T. cruzi | |
| 1a | 10.7 | 24.9 | 19.2 | 106.4 | 10 | 4 | 6 |
| 1b | 6.7 | 30.5 | 37.3 | 128 | 19 | 4 | 3 |
| 2a | >256 | >256 | 68.7 | >256 | NE | NE | >4 |
| 3a | >256 | >256 | >256 | >256 | NE | NE | NE |
| 2b | >256 | >256 | 204.3 | >256 | NE | NE | >1 |
| 3b | >256 | >256 | >256 | >256 | NE | NE | NE |
| 2c | >256 | >256 | >256 | >256 | NE | NE | NE |
| 3c | >256 | >256 | >256 | >256 | NE | NE | NE |
| 2d | >256 | >256 | >256 | >256 | NE | NE | NE |
| 3d | >256 | >256 | >256 | >256 | NE | NE | NE |
| 2e | >256 | >256 | >256 | >256 | NE | NE | NE |
| 3e | >256 | >256 | >256 | >256 | NE | NE | NE |
| 2f | 14.5 | 38.2 | 21.1 | 31.8 | 2 | 1 | 2 |
| 3f | 17.6 | 46.6 | 24.7 | 312.2 | 18 | 7 | 13 |
| 2g | >256 | >256 | >256 | >384 | >2 | >2 | >2 |
| 3g | >256 | >256 | 146.5 | >384 | >2 | >2 | >3 |
| 2h | >256 | >256 | >256 | >256 | NE | NE | NE |
| 3h | >256 | >256 | >256 | >256 | NE | NE | NE |
| 2i | >256 | >256 | >256 | >256 | NE | NE | NE |
| 3i | >256 | >256 | >256 | >256 | NE | NE | NE |
| Miltefosine | 64.0 | 25.1 | NA | 2754 | 43.0 | 109.7 | NA |
| Benznidazole | NA | NA | 54.7 | 769 | NA | NA | 14.0 |
| Strain | 1a | 3e | 2f | 3f | 3h | * Rifampicin | * Linezolid | * Imipenem |
|---|---|---|---|---|---|---|---|---|
| M. tuberculosis CIPTIR -D152 | >200 | >200 | >200 | >200 | >200 | 32 | NA | NA |
| M. tuberculosis CIPTIR-F296 | >200 | >200 | >200 | >200 | >200 | 1 | NA | NA |
| M. tuberculosis ATCC H37Rv | >200 | >200 | >200 | >200 | >200 | 2 | NA | NA |
| M. abscessus LIID-01 | >200 | >200 | >200 | 100 | 100 | NA | 1 | 32 |
| M. abscessus LIID-02 | >200 | >200 | >200 | 100 | 100 | NA | 1 | 32 |
| M. abscessus LIID-03 | >200 | >200 | >200 | 100 | 100 | NA | 1 | 8 |
| M. fortuitum LIID-01 | >200 | >200 | >200 | 100 | >200 | NA | 8 | 4 |
| M. intracellulare LIID-01 | 50 | 50 | 50 | 100 | >200 | NA | 2 | 1 |
| M. intracellulare LIID-02 | >200 | >200 | >200 | >200 | >200 | NA | 8 | >64 |
| Strain | 1a | 3e | 2f | 3f | 3h | * Gentamicin |
|---|---|---|---|---|---|---|
| E. coli (G−) | >200 | >200 | >200 | 100 | >200 | 0.2 |
| P. aeruginosa (G−) | >200 | >200 | >200 | 100 | >200 | 0.78 |
| S. typhi (G−) | >200 | 100 | >200 | >200 | 100 | 0.1 |
| S. aureus (G+) | 100 | >200 | >200 | 100 | 100 | 0.1 |
| L. innocua (G+) | >200 | >200 | >200 | 100 | >200 | 1.56 |
| L. monocytogenes (G+) | >200 | >200 | >200 | 100 | >200 | 0.2 |
| E. faecalis (G+) | >200 | >200 | >200 | >200 | >200 | 0.78 |
| B. cereus (G+) | >200 | >200 | >200 | 100 | >200 | 0.78 |
| M. luteus (G+) | >200 | >200 | >200 | 100 | >200 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertino, M.W.; F. de la Torre, A.; Schmeda-Hirschmann, G.; Vega Gómez, C.; Rolón, M.; Coronel, C.; Rojas de Arias, A.; Molina-Torres, C.A.; Vera-Cabrera, L.; Viveros-Valdez, E. Exploring Benzo[h]chromene Derivatives as Agents against Protozoal and Mycobacterial Infections. Pharmaceuticals 2024, 17, 1375. https://doi.org/10.3390/ph17101375
Pertino MW, F. de la Torre A, Schmeda-Hirschmann G, Vega Gómez C, Rolón M, Coronel C, Rojas de Arias A, Molina-Torres CA, Vera-Cabrera L, Viveros-Valdez E. Exploring Benzo[h]chromene Derivatives as Agents against Protozoal and Mycobacterial Infections. Pharmaceuticals. 2024; 17(10):1375. https://doi.org/10.3390/ph17101375
Chicago/Turabian StylePertino, Mariano Walter, Alexander F. de la Torre, Guillermo Schmeda-Hirschmann, Celeste Vega Gómez, Miriam Rolón, Cathia Coronel, Antonieta Rojas de Arias, Carmen A. Molina-Torres, Lucio Vera-Cabrera, and Ezequiel Viveros-Valdez. 2024. "Exploring Benzo[h]chromene Derivatives as Agents against Protozoal and Mycobacterial Infections" Pharmaceuticals 17, no. 10: 1375. https://doi.org/10.3390/ph17101375
APA StylePertino, M. W., F. de la Torre, A., Schmeda-Hirschmann, G., Vega Gómez, C., Rolón, M., Coronel, C., Rojas de Arias, A., Molina-Torres, C. A., Vera-Cabrera, L., & Viveros-Valdez, E. (2024). Exploring Benzo[h]chromene Derivatives as Agents against Protozoal and Mycobacterial Infections. Pharmaceuticals, 17(10), 1375. https://doi.org/10.3390/ph17101375








