Fast Clinical Response of Bimekizumab in Nail Psoriasis: A Retrospective Multicenter 36-Week Real-Life Study
Abstract
1. Introduction
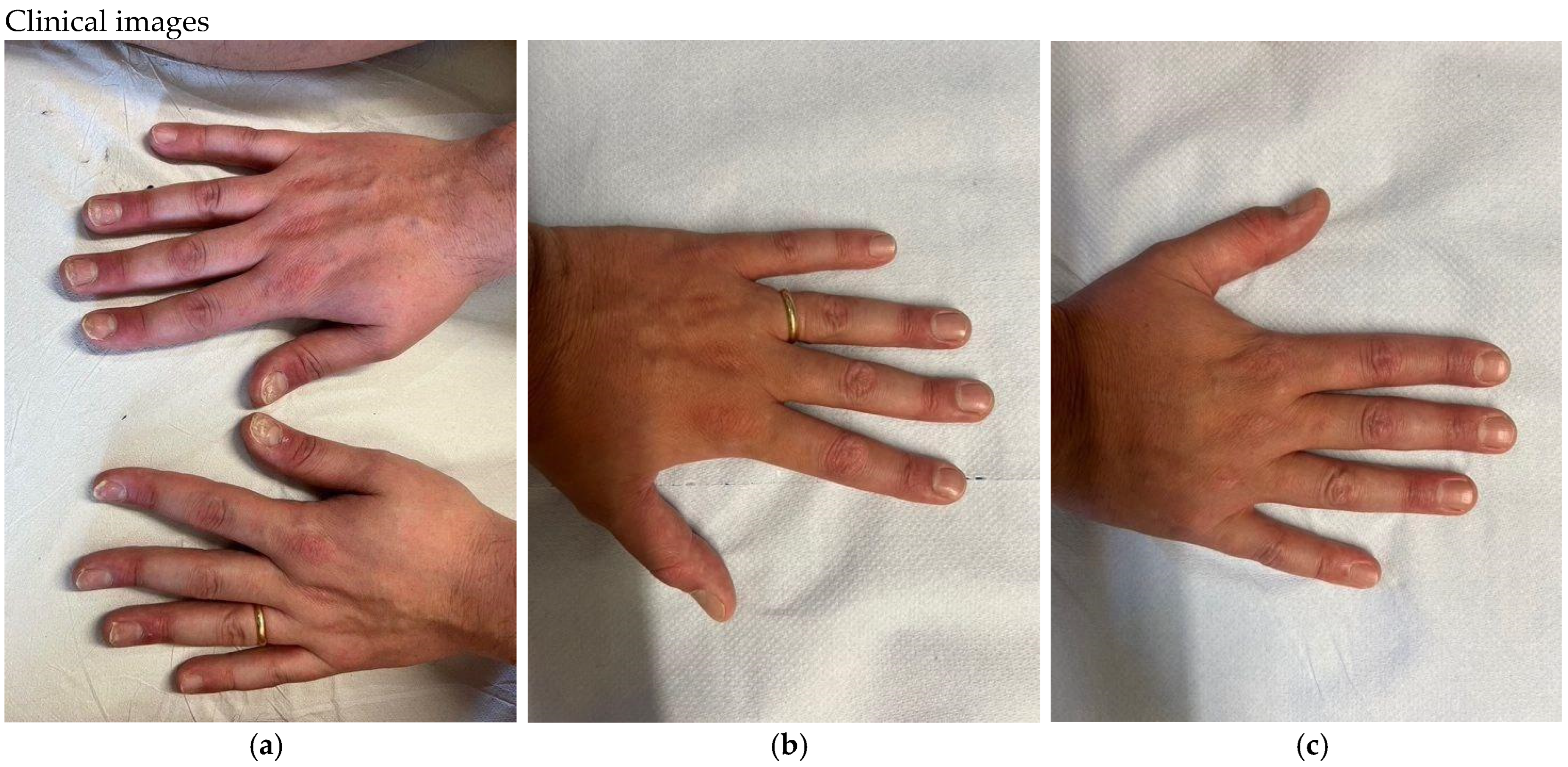
2. Results
3. Materials and Methods
3.1. Assessments and Outcomes
3.2. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventura, A.; Mazzeo, M.; Gaziano, R.; Galluzzo, M.; Bianchi, L.; Campione, E. New insight into the pathogenesis of nail psoriasis and overview of treatment strategies. Drug Des. Dev. Ther. 2017, 11, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N. Psoriasis: Pathogenesis, Comorbidities, and Therapy Updated. Int. J. Mol. Sci. 2021, 22, 2979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Chen, Y.; Yu, Q.; Shi, Y. Biologic and Small-Molecule Therapies for Moderate-to-Severe Psoriasis: Focus on Psoriasis Comorbidities. BioDrugs 2023, 37, 35–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lanna, C.; Mancini, M.; Gaziano, R.; Cannizzaro, M.V.; Galluzzo, M.; Talamonti, M.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Wang, Y.; et al. Skin immunity and its dysregulation in psoriasis. Cell Cycle 2019, 18, 2581–2589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P.; Su, Y.; Li, S.; Chen, H.; Wu, R.; Wu, H. The roles of T cells in psoriasis. Front. Immunol. 2023, 14, 1081256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, Z.; Fanslow, W.C.; Seldin, M.F.; Rousseau, A.M.; Painter, S.L.; Comeau, M.R.; Cohen, J.I.; Spriggs, M.K. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 1995, 3, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Kolbinger, F.; Loesche, C.; Valentin, M.A.; Jiang, X.; Cheng, Y.; Jarvis, P.; Peters, T.; Calonder, C.; Bruin, G.; Polus, F.; et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 2017, 139, 923–932.e8. [Google Scholar] [CrossRef] [PubMed]
- Reich, K. Approach to managing patients with nail psoriasis. J. Eur. Acad. Dermatol. Venereol. 2009, 23 (Suppl. S1), 15–21. [Google Scholar] [CrossRef]
- Prignano, F.; Campione, E.; Parodi, A.; Vegni, E.; Bardazzi, F.; Borroni, R.G.; Burlando, M.; Cinotti, E.; Dini, V.; Giacchetti, A.; et al. EMPATHY Life in Psoriasis: Embracing Patients’ Well-Being in Their Journey of Moderate-to-Severe Psoriasis. J. Clin. Med. 2024, 13, 4469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasch, M.C. Nail psoriasis: A review of treatment options. Drugs 2016, 76, 675–705. [Google Scholar] [CrossRef] [PubMed]
- Pourchot, D.; Bodemer, C.; Phan, A.; Bursztejn, A.; Hadj-Rabia, S.; Boralevi, F.; Miquel, J.; Hubiche, T.; Puzenat, E.; Souillet, A.; et al. Nail Psoriasis: A Systematic Evaluation in 313 Children with Psoriasis. Pediatr. Dermatol. 2017, 34, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Lawry, M. Biological therapy and nail psoriasis. Dermatol. Ther. 2007, 20, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Armesto, S.; Esteve, A.; Coto-Segura, P.; Drake, M.; Galache, C.; Martínez-Borra, J.; Santos-Juanesc, J. Nailpsoriasisinindividuals with psoriasis vulgaris: A study of 661 patients. Actas Dermosifiliogr. 2011, 102, 365–372. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Klaassen, K.M.; van de Kerkhof, P.C.; Pasch, M.C. Nail psoriasis: A questionnaire-based survey. Br. J. Dermatol. 2013, 169, 314–319. [Google Scholar] [CrossRef]
- Jiaravuthisan, M.M.; Sasseville, D.; Vender, R.B.; Murphy, F.; Muhn, C.Y. Psoriasis of the nail: Anatomy, pathology, clinical presentation, and a review of the literature on therapy. J. Am. Acad. Dermatol. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Langenbruch, A.; Radtke, M.A.; Krensel, M.; Jacobi, A.; Reich, K.; Augustin, M. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br. J. Dermatol. 2014, 171, 1123–1128. [Google Scholar] [CrossRef]
- Zaias, N. Psoriasis of the nail. A clinical-pathologic study. Arch. Dermatol. 1969, 99, 567–579. [Google Scholar] [CrossRef]
- Zabotti, A.; De Marco, G.; Gossec, L.; Baraliakos, X.; Aletaha, D.; Iagnocco, A.; Gisondi, P.; Balint, P.V.; Bertheussen, H.; Boehncke, W.-H.; et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann. Rheum. Dis. 2023, 82, 1162–1170. [Google Scholar] [CrossRef]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef] [PubMed]
- Hudgens, S.; Rich, P.; Geng, Z.; Williams, D.; Fleischer, A.; Ganguli, A. Development and validation of the Physician’s Global Assessment of Fingernail Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.; Scher, R.K. Nail psoriasis severity index: A useful tool for evaluation of nail psoriasis. J. Am. Acad. Dermatol. 2003, 49, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Szepietowski, J.C.; Salomon, J. Do fungi play a role in psoriatic nails? Mycoses 2007, 50, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, D.; Papanagiotou, V.; Daniel, R., 3rd; Piraccini, B.M. Onychomycosis in patients with nail psoriasis: A point to point discussion. Mycoses 2017, 60, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E. Onychomycosis: Pathogenesis, diagnosis, and manage-ment. Clin. Microbiol. Rev. 1998, 11, 415–429. [Google Scholar] [CrossRef]
- Zaias, N.; Escovar, S.X.; Zaiac, M.N. Finger and toenail onycholysis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 848–853. [Google Scholar] [CrossRef]
- Zisova, L.; Valtchev, V.; Sotiriou, E.; Gospodinov, D.; Mateev, G. Onycho-mycosis in patients with psoriasis: A multicentre study. Mycoses 2012, 55, 143–147. [Google Scholar] [CrossRef]
- De Luca, A.; Zelante, T.; D’Angelo, C.; Zagarella, S.; Fallarino, F.; Spreca, A.; Iannitti, R.G.; Bonifazi, P.; Renauld, J.-C.; Bistoni, F.; et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010, 3, 361–373. [Google Scholar] [CrossRef]
- Fry, L.; Baker, B.S. Triggering psoriasis: The role of infections and medications. Clin. Dermatol. 2007, 25, 606–615. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-kB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Saulite, I.; Pilmane, M.; Kisis, J. Expression of antimicrobial peptides in nail psoriasis and normal nails. Acta Derm. Venereol. 2017, 97, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Gniadecki, R. Next-generation antipsoriatic drugs: Small molecules join. Br. J. Dermatol. 2015, 173, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.J.; Weinberg, J.M.; Wu, J.J.; Robertson, A.D.; Van Voorhees, A.S.; National Psoriasis Foundation. Treatment of nail psoriasis: Best practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015, 151, 87–94. [Google Scholar] [CrossRef]
- Laheru, D.; Antony, A.; Carneiro, S.; Di Lernia, V.; Garg, A.; Love, T.J.; Garcia, K.d.R.M.; Mendonça, J.A.; Mukherjee, S.; Olteanu, R.; et al. Management of Nail Disease in Patients With Psoriatic Arthritis: An Updated Literature Review Informing the 2021 GRAPPA Treatment Recommendations. J. Rheumatol. 2023, 50, 433–437. [Google Scholar] [CrossRef]
- Marquez Balbas, G.; Sanchez Regana, M.; Umbert Millet, P. Tacalcitol ointment for the treatment of nail psoriasis. J. Dermatol. Treat. 2009, 20, 308–310. [Google Scholar] [CrossRef]
- Reichrath, J. Vitamin D and the skin: An ancient friend, revisited. Exp. Dermatol. 2007, 16, 618–625. [Google Scholar] [CrossRef]
- Fischer-Levancini, C.; Sanchez-Regana, M.; Llambi, F.; Collgros, H.; Exposito-Serrano, V.; Umbert-Millet, P. Nail psoriasis: Treatment with tazarotene 0.1% hydrophilic ointment. Actas Dermosifiliogr. 2012, 103, 725–728. (In Spanish) [Google Scholar] [CrossRef]
- Hadeler, E.; Mosca, M.; Hong, J.; Brownstone, N.; Bhutani, T.; Liao, W. Nail Psoriasis: A Review of Effective Therapies and Recommendations for Management. Dermatol. Ther. 2021, 11, 799–831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papp, K.; Reich, K.; Leonardi, C.L.; Kircik, L.; Chimenti, S.; Langley, R.G.; Hu, C.; Stevens, R.M.; Day, R.M.; Gordon, K.B.; et al. Apremilast, an oral phosphodi- esterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J. Am. Acad. Dermatol. 2015, 73, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Lanna, C.; Cesaroni, G.M.; Mazzilli, S.; Vollono, L.; Gaziano, R.; Marino, D.; Bianchi, L.; Campione, E. Apremilast as a target therapy for nail psoriasis: A real-life observational study proving its efficacy in restoring the nail unit. J. Dermatol. Treat. 2022, 33, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Rich, P.; Menter, A.; Krueger, G.; Goldblum, O.; Dutronc, Y.; Zhu, B.; Wei, H.; Cameron, G.; Heffernan, M. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1763–1770. [Google Scholar] [CrossRef]
- Thaçi, D.; Kimball, A.; Foley, P.; Poulin, Y.; Levi, E.; Chen, R.; Feldman, S.R. Apremilast, an oral phospho-diesterase 4 inhibitor, improves patient-reported outcomes in the treatment of moderate to severe psoriasis: Results of two phase III ran-domized, controlled trials. J. Eur. Acad. Dermatol. Venereol. 2016, 31, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.; Rich, P.; Lain, E.; Soung, J.; Lewitt, G.M.; Jacobson, A. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: Results from three phase 3 trials. J. Dermatol. Treat. 2022, 33, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Kristensen, L.E.; Puig, L.; Rich, P.; Smith, S.D.; Garrelts, A.; See, K.; Holzkaemper, T.; Fotiou, K.; Schuster, C. Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24–28 and 48–52 weeks. J. Dermatol. Treat. 2023, 34, 2263108. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhoi, A.K.; Grover, C.; Singal, A.; Kashyap, B.; Dibyashree, D. Serum levels of tumour necrosis factor (TNF-α) and interleukin-17 (IL-17) in patients with nail psoriasis: A cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2024, 90, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Maroof, A.; Baker, T.; Lawson, A.D.G.; Oliver, R.; Paveley, R.; Rapecki, S.; Shaw, S.; Vajjah, P.; West, S.; et al. Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F. Front. Immunol. 2020, 11, 1894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Medicines Agency. Summary of Product Characteristics: Bimzelx 160 mg Solution for Injection in Pre-Filled Syringe, Bimzelx 160 mg Solution for Injection in Pre-Filled Pen 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf (accessed on 1 January 2020).
- Gargiulo, L.; Narcisi, A.; Ibba, L.; Balato, A.; Bianchi, L.; Brianti, P.; Buononato, D.; Burlando, M.; Caldarola, G.; Campanati, A.; et al. Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: A real-life multicenter study-IL PSO (Italian landscape psoriasis). Front. Med. 2023, 10, 1243843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malakouti, M.; Brown, G.E.; Wang, E.; Koo, J.; Levin, E.C. The role of IL-17 in psoriasis. J. Dermatol. Treat. 2015, 26, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A. Pathogenic role of IL-17 in psoriasis and psoriatic arthritis. Actas Dermosifiliogr. 2014, 105 (Suppl. S1), 9–20. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Langley, R.G.; Armstrong, A.; Warren, R.B.; Gordon, K.B.; Merola, J.F.; Okubo, Y.; Madden, C.; et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021, 397, 487–498, Erratum in Lancet 2021, 397, 670. [Google Scholar] [CrossRef] [PubMed]
- Battista, T.; Scalvenzi, M.; Martora, F.; Potestio, L.; Megna, M. Nail Psoriasis: An Updated Review of Currently Available Systemic Treatments. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1899–1932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, C.; Wang, H.; Bao, C.; Zhang, L.; Ruan, S.; Zhang, J.; Gong, T.; Cheng, B. Challenge of Nail Psoriasis: An Update Review. Clin. Rev. Allergy Immunol. 2021, 61, 377–402. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Soliman, A.M.; Betts, K.A.; Wang, Y.; Gao, Y.; Stakias, V.; Puig, L. Long-Term Benefit-Risk Profiles of Treatments for Moderate-to-Severe Plaque Psoriasis: A Network Meta-Analysis. Dermatol. Ther. 2022, 12, 167–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rompoti, N.; Politou, M.; Stefanaki, I.; Vavouli, C.; Papoutsaki, M.; Neofotistou, A.; Rigopoulos, D.; Stratigos, A.; Nicolaidou, E. Brodalumab in plaque psoriasis: Real-world data on effectiveness, safety and clinical predictive factors of initial response and drug survival over a period of 104 weeks. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Riedl, E.; Pinter, A.; Zaheri, S.; Costanzo, A.; Brnabic, A.; Konicek, B.; McKenzie, R.; Lampropoulou, A.; Rayes, M.E.; Haustrup, N.; et al. Baseline Characteristics and mNAPSI Change from Baseline Scores Through Month 12 for Patients with Moderate-to-Severe Plaque Psoriasis and Concomitant Nail Psoriasis Treated with Biologics from PSoHO. Dermatol. Ther. 2024, 14, 1327–1335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mease, P.; Elaine Husni, M.; Chakravarty, S.D.; Kafka, S.; Parenti, D.; Kim, L.; Hung Lo, K.; Hsia, E.C.; Kavanaugh, A. Evaluation of Improvement in Skin and Nail Psoriasis in Bio-naïve Patients With Active Psoriatic Arthritis Treated With Golimumab: Results Through Week 52 of the GO-VIBRANT Study. ACR Open Rheumatol. 2020, 2, 640–647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Variable | N | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Age at BKZ first dose (years) | 834 | 50.13 | 14.77 | 51.00 | 18.00 | 87.00 |
| Weight (kg) | 792 | 81.01 | 17.63 | 80.00 | 46.00 | 178.00 |
| Height (cm) | 791 | 171.93 | 10.04 | 173.00 | 72.00 | 201.00 |
| BMI | 791 | 27.49 | 6.99 | 26.31 | 16.90 | 125.00 |
| Time from PSO diagnosis (years) | 697 | 14.83 | 12.57 | 12.00 | 0.00 | 57.00 |
| Gender | Frequency | Percent |
|---|---|---|
| Female | 291 | 34.89 |
| Male | 543 | 65.11 |
| Covid-19 | Frequency | Percent |
| NO | 557 | 66.79 |
| YES | 277 | 33.21 |
| Naive to systemic treatment | Frequency | Percent |
| NO | 703 | 84.29 |
| YES | 131 | 15.71 |
| Naive to biologic therapies | Frequency | Percent |
| NO | 385 | 46.1% |
| YES | 359 | 43.05% |
| Undefined | 32 | 3.84% |
| Arthritis | Frequency | Percent |
| NO | 693 | 83.49 |
| YES | 109 | 13.13 |
| Undefined | 28 | 3.37 |
| Other comorbidities | Frequency | Percent |
| None | 370 | 44.36 |
| At least one comorbidity | 464 | 55.64 |
| Cardiovascular disease | 70 | 8.4 |
| Diabetes | 93 | 11.2 |
| Hypertension | 254 | 30.5 |
| Hyperlipidemia | 142 | 17.0 |
| Neoplasias | 31 | 3.7 |
| Other | 198 | 23.7 |
| Variable | n | Mean | SD | Median |
|---|---|---|---|---|
| PASI | 834 | 16.24 | 9.03 | 15.00 |
| DLQI | 830 | 14.62 | 8.81 | 15.00 |
| PASI100 | Frequency | Percent |
|---|---|---|
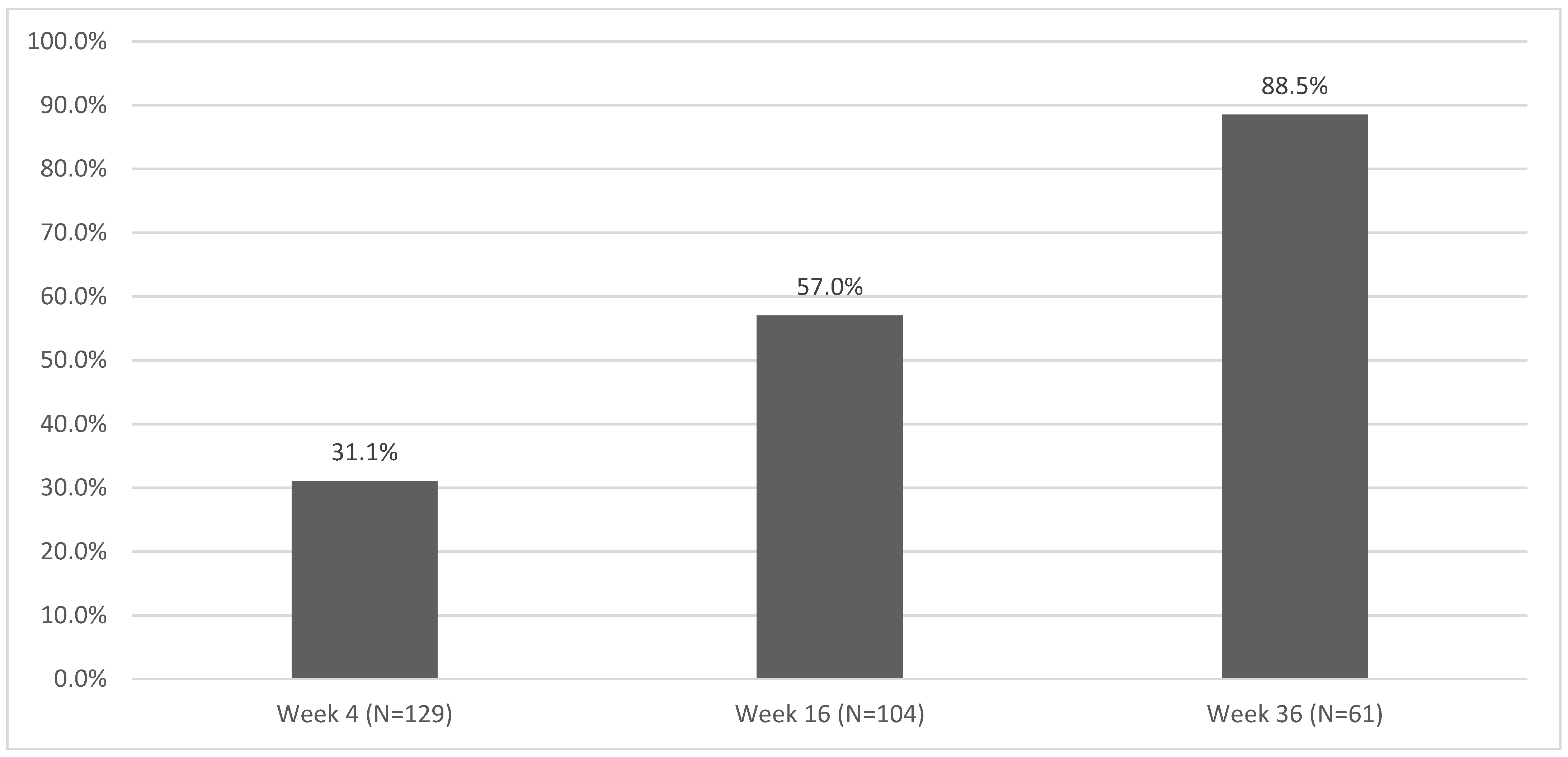
| Week 4 (n = 512) | 164 | 32.03 |
| Week 16 (n = 411) | 254 | 61.80 |
| Week 36 (n = 223) | 176 | 78.92 |
| Nail Involvement | Frequency | Percent |
|---|---|---|
| NO | 579 | 69.76 |
| YES | 232 | 27.95 |
| Undefined | 19 | 2.29 |
| Frequency Missing = 4 | ||
| Variable | n | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Age at BKZ first dose (years) | 232 | 51.52 | 14.21 | 53.00 | 19.00 | 87.00 |
| Weight (kg) | 226 | 82.35 | 16.77 | 80.00 | 46.00 | 178.00 |
| Height (cm) | 225 | 173.13 | 8.48 | 175.00 | 148.00 | 193.00 |
| BMI | 225 | 27.44 | 5.02 | 26.56 | 17.63 | 54.94 |
| Time from diagnosis (years) | 183 | 14.28 | 11.86 | 12.00 | 0.00 | 48.00 |
| Frequency | Percent | |
|---|---|---|
| Female | 66 | 28.45 |
| Male | 166 | 71.55 |
| Covid-19 | Frequency | Percent |
| NO | 135 | 58.19 |
| YES | 97 | 41.81 |
| Systemic therapy | Frequency | Percent |
| NO | 195 | 84.05 |
| YES | 37 | 15.95 |
| Bio-naive | Frequency | Percent |
| NO | 105 | 49.53 |
| YES | 107 | 50.47 |
| Comorbidities | Frequency | Percent |
| None | 93 | 40.1% |
| Comorbidities ≥ 1 | 139 | 59.9% |
| Cardiovascular diseases | 19 | 8.2% |
| Diabetes | 28 | 12.1% |
| Hypertension | 81 | 34.9% |
| Hyperlipidemia | 45 | 19.4% |
| Neoplasias | 10 | 4.3% |
| Other | 71 | 30.6% |
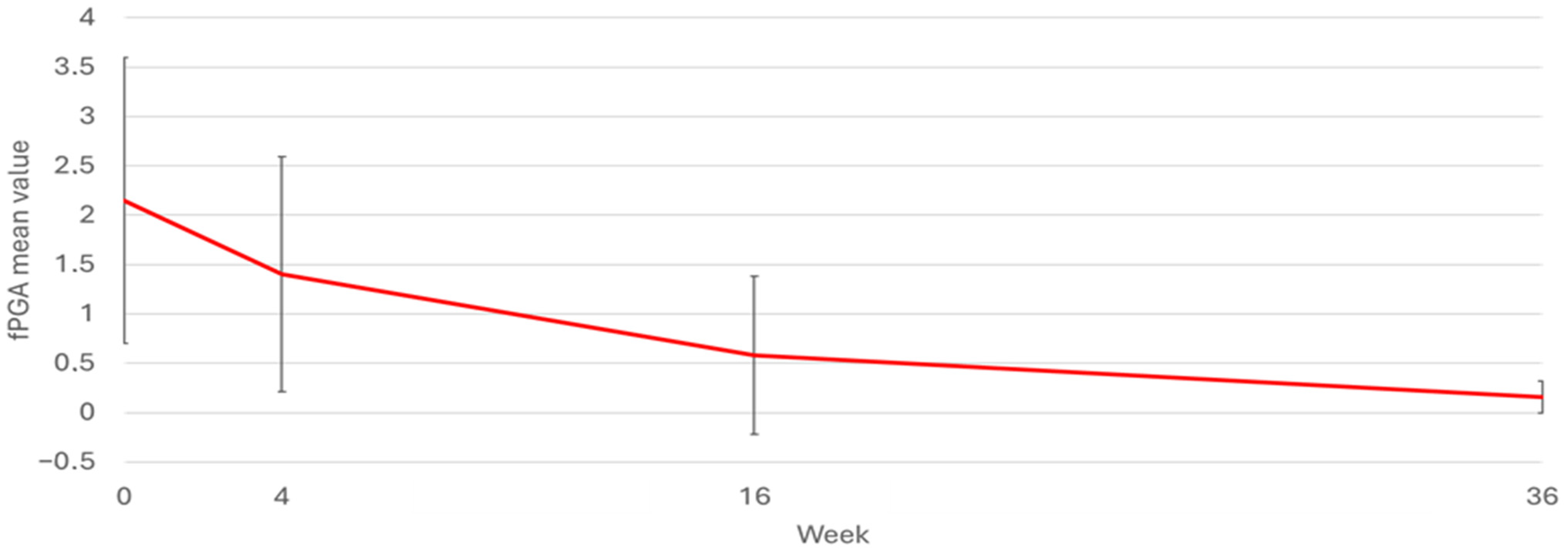
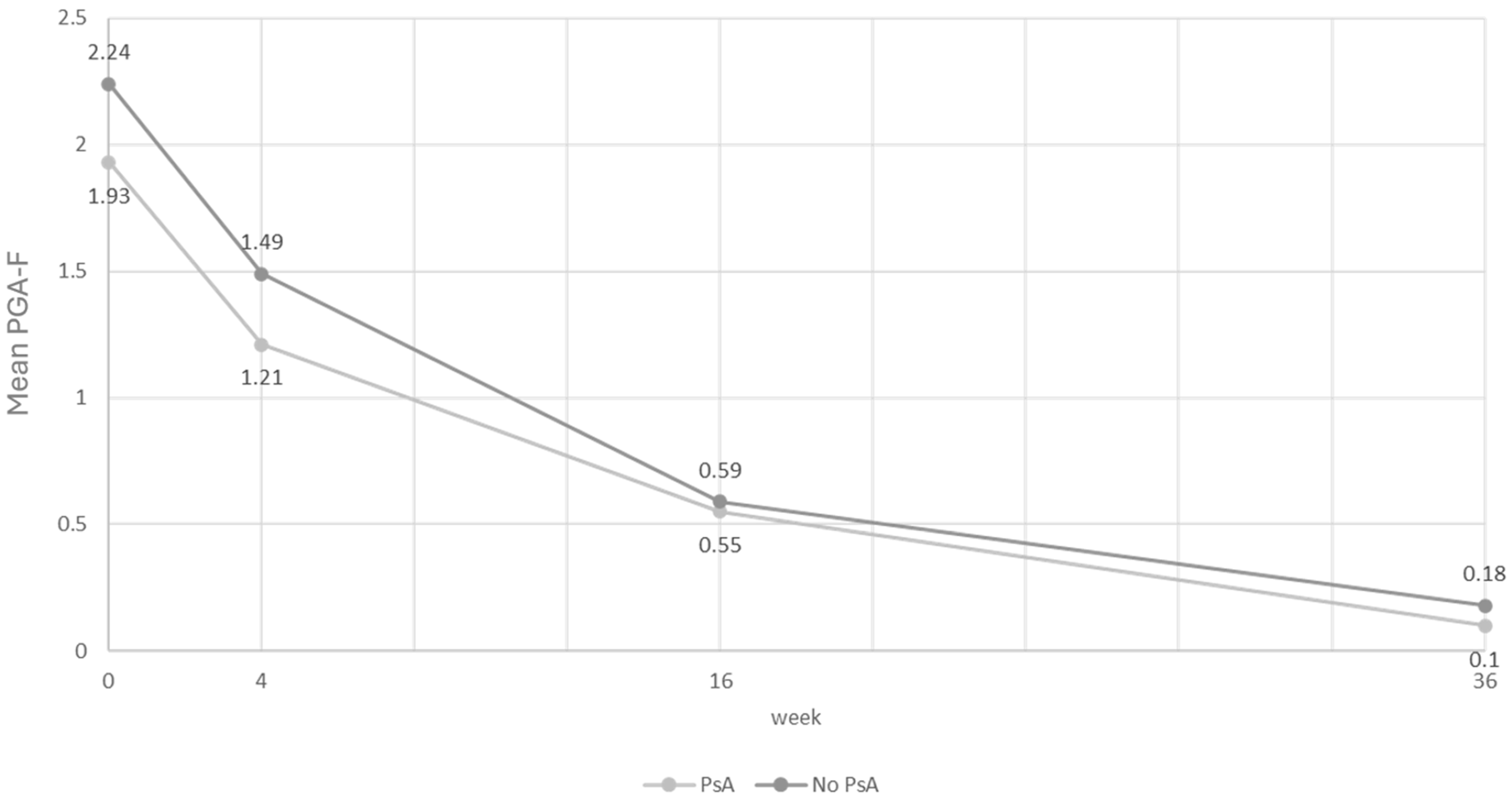
| PsA | Variable: PGA-F | N | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| NO | Baseline | 158 | 2.24 | 1.43 | 3.00 | 0.00 | 4.00 |
| Week 4 | 101 | 1.49 | 1.22 | 2.00 | 0.00 | 4.00 | |
| Week 16 | 83 | 0.59 | 0.84 | 0.00 | 0.00 | 4.00 | |
| Week 36 | 49 | 0.18 | 0.63 | 0.00 | 0.00 | 4.00 | |
| Change w4 vs. baseline | 99 | −0.91 | 1.09 | −1.00 | −4.00 | 1.00 | |
| Change w16 vs. baseline | 81 | −1.72 | 1.25 | −2.00 | −4.00 | 2.00 | |
| Change w36 vs. baseline | 49 | −2.27 | 1.25 | −2.00 | −4 | 0.00 | |
| YES | Baseline | 43 | 1.93 | 1.5 | 2.00 | 0.00 | 4.00 |
| Week 4 | 28 | 1.21 | 1.07 | 1.00 | 0.00 | 3.00 | |
| Week 16 | 22 | 0.55 | 0.67 | 0.00 | 0.00 | 2.00 | |
| Week 36 | 10 | 0.10 | 0.32 | 0.00 | 0.00 | 1.00 | |
| Change w4 vs. baseline | 27 | −1.33 | 1.24 | −1.00 | −4.00 | 0.00 | |
| Change w16 vs. baseline | 21 | −1.81 | 1.17 | −2.00 | −3.00 | 0.00 | |
| Change w36 vs. baseline | 10 | −2.4 | 1.07 | −3.00 | −4.00 | −1.00 |
| Variable | N° | Mean | Std Dev | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Baseline | 830 | 14.62 | 8.81 | 15.00 | 0.00 | 30.00 |
| Week 4 | 512 | 3.02 | 4.06 | 2.00 | 0.00 | 23.00 |
| Week 16 | 411 | 0.83 | 1.87 | 0.00 | 0.00 | 15.00 |
| Week 36 | 225 | 0.50 | 1.66 | 0.00 | 0.00 | 15.00 |
| Variable | N° | Mean | Std Dev | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Baseline | 210 | 15.33 | 9.19 | 17.00 | 0.00 | 30.00 |
| Week 4 | 136 | 3.70 | 4.74 | 2.00 | 0.00 | 23.00 |
| Week 16 | 108 | 1.08 | 2.23 | 0.00 | 0.00 | 11.00 |
| Week 36 | 61 | 0.57 | 1.62 | 0.00 | 0.00 | 8.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campione, E.; Artosi, F.; Shumak, R.G.; Giunta, A.; Argenziano, G.; Assorgi, C.; Balato, A.; Bernardini, N.; Brunasso, A.M.G.; Burlando, M.; et al. Fast Clinical Response of Bimekizumab in Nail Psoriasis: A Retrospective Multicenter 36-Week Real-Life Study. Pharmaceuticals 2024, 17, 1378. https://doi.org/10.3390/ph17101378
Campione E, Artosi F, Shumak RG, Giunta A, Argenziano G, Assorgi C, Balato A, Bernardini N, Brunasso AMG, Burlando M, et al. Fast Clinical Response of Bimekizumab in Nail Psoriasis: A Retrospective Multicenter 36-Week Real-Life Study. Pharmaceuticals. 2024; 17(10):1378. https://doi.org/10.3390/ph17101378
Chicago/Turabian StyleCampione, Elena, Fabio Artosi, Ruslana Gaeta Shumak, Alessandro Giunta, Giuseppe Argenziano, Chiara Assorgi, Anna Balato, Nicoletta Bernardini, Alexandra Maria Giovanna Brunasso, Martina Burlando, and et al. 2024. "Fast Clinical Response of Bimekizumab in Nail Psoriasis: A Retrospective Multicenter 36-Week Real-Life Study" Pharmaceuticals 17, no. 10: 1378. https://doi.org/10.3390/ph17101378
APA StyleCampione, E., Artosi, F., Shumak, R. G., Giunta, A., Argenziano, G., Assorgi, C., Balato, A., Bernardini, N., Brunasso, A. M. G., Burlando, M., Caldarola, G., Campanati, A., Carugno, A., Castelli, F., Conti, A., Costanzo, A., Cuccia, A., Dapavo, P., Dattola, A., ... Bianchi, L. (2024). Fast Clinical Response of Bimekizumab in Nail Psoriasis: A Retrospective Multicenter 36-Week Real-Life Study. Pharmaceuticals, 17(10), 1378. https://doi.org/10.3390/ph17101378















