Effect of Cyclodextrins Formulated in Liposomes and Gold and Selenium Nanoparticles on siRNA Stability in Cell Culture Medium
Abstract
1. Introduction
2. Results and Discussion
2.1. Serum Stability of siRNA Fragments
2.2. Binding Thermodynamics
2.3. Nanocarrier Synthesis and Characterization
2.4. Nanocarrier Viability and Aqueous Solution Stability Studies
3. Materials and Methods
3.1. Optimization of the Synthesis of SeβCD and AuβCD Nanoparticles
3.2. Optimized Synthesis of β-Cyclodextrin-Modified Selenium Nanoparticles (SeβCD)
3.3. Optimized Synthesis of β-Cyclodextrin-Modified Gold Nanoparticles (AuβCD)
3.4. Characterization and Water Stability of SeβCD and AuβCD Nanoparticles by DLS
3.5. Determination of the βCD Concentrations on the AuβCD and SeβCD Surfaces via NMR Spectroscopy
3.6. Determination of the Au and Se Concentrations in the AuβCD and SeβCD Nanoparticles via Atomic Absorption Spectroscopy (AA)
3.7. Cell Culture and Drug Treatment
3.8. Liposome Preparation
3.9. Serum Stability Measurements
3.10. Thermodynamic Studies
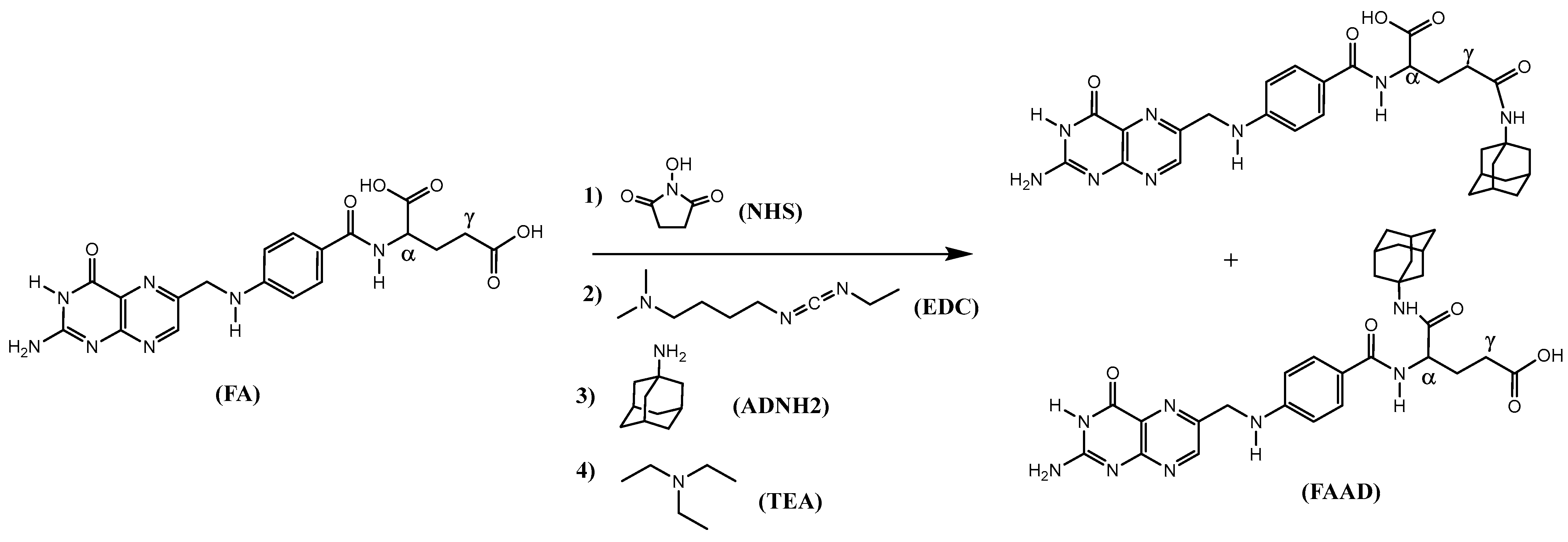
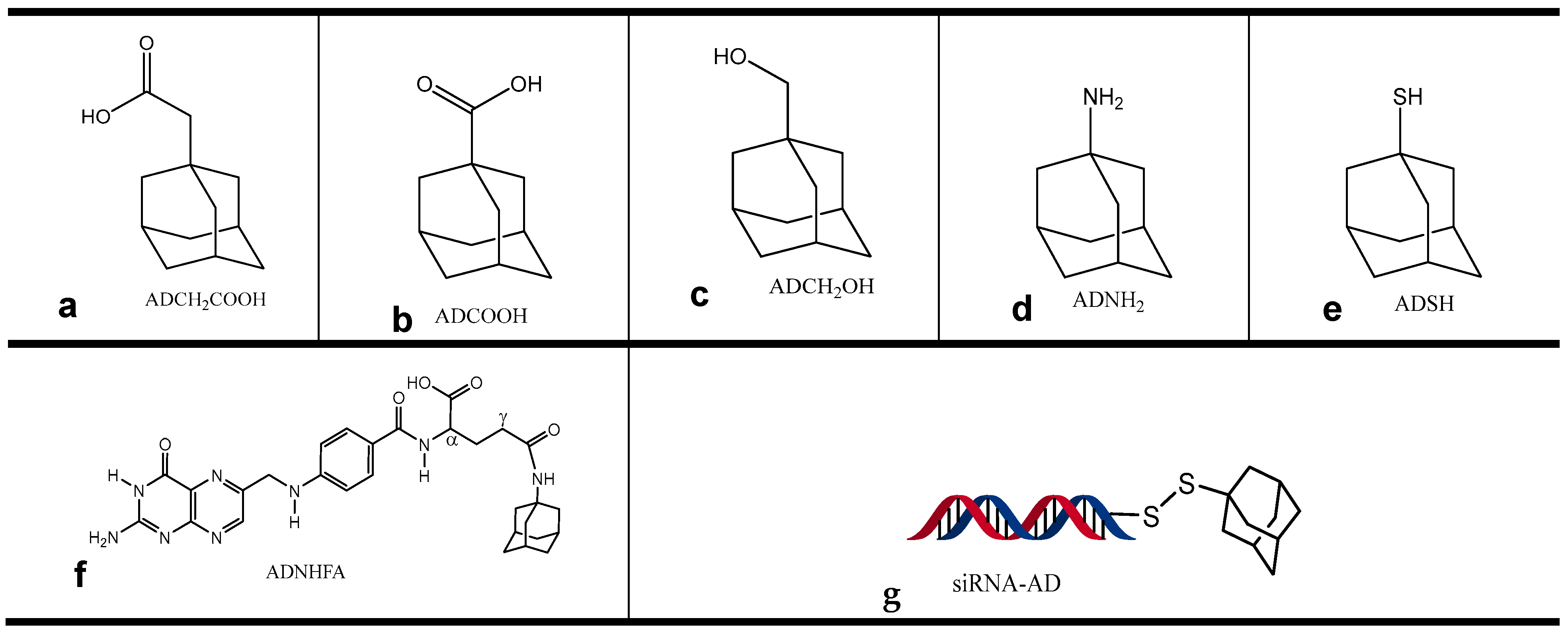
3.11. Synthesis of Adamantane Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali Zaidi, S.S.; Fatima, F.; Ali Zaidi, S.A.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: Challenges and strategies. J. Nanobiotechnol. 2023, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Reyes-González, J.M.; Vivas-Mejía, P.E. c-MYC and Epithelial Ovarian Cancer. Front. Oncol. 2021, 11, 601512. [Google Scholar] [CrossRef]
- Echevarría-Vargas, I.M.; Valiyeva, F.; Vivas-Mejía, P.E. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS ONE 2014, 9, e97094. [Google Scholar] [CrossRef]
- Hickerson, R.P.; Vlassov, A.V.; Wang, Q.; Leake, D.; Ilves, H.; Gonzalez-Gonzalez, E.; Contag, C.H.; Johnston, B.H.; Kaspar, R.L. Stability study of unmodified siRNA and relevance to clinical use. Oligonucleotides 2008, 18, 345–354. [Google Scholar] [CrossRef]
- Hong, J.; Huang, Y.; Li, J.; Yi, F.; Zheng, J.; Huang, H.; Wei, N.; Shan, Y.; An, M.; Zhang, H.; et al. Comprehensive analysis of sequence-specific stability of siRNA. FASEB J. 2010, 24, 4844–4855. [Google Scholar] [PubMed]
- Tai, W. Current Aspects of siRNA Bioconjugate for In Vitro and In Vivo Delivery. Molecules 2019, 24, 2211. [Google Scholar] [CrossRef] [PubMed]
- Morales-Becerril, A.; Aranda-Lara, L.; Isaac-Olivé, K.; Ocampo-García, B.E.; Morales-Ávila, E. Nanocarriers for delivery of siRNA as gene silencing mediator. EXCLI J. 2022, 21, 1028–1052. [Google Scholar]
- Guimaranes, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tian, J.; Chen, X. Effect of surface properties on liposomal siRNA delivery. Biomaterials 2016, 79, 56–68. [Google Scholar] [CrossRef]
- Castillo Cruz, B.; Flores Colón, M.; Rabelo Fernandez, R.J.; Vivas-Mejia, P.E.; Barletta, G.L. A Fresh Look at the Potential of Cyclodextrins for Improving the Delivery of siRNA Encapsulated in Liposome Nanocarriers. ACS Omega 2022, 7, 3731–3737. [Google Scholar] [CrossRef]
- Zappacosta, R.; Cornelio, B.; Pilato, S.; Siani, G.; Estour, F.; Aschi, M.; Fontana, A. Effect of the Incorporation of Functionalized Cyclodextrins in the Liposomal Bilayer. Molecules 2019, 24, 1387. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cruz, F.; García-Gutiérrez, J.L. Molecular size and shape properties of diamondoid molecules occurring in crude oil. Arab. J. Chem. 2020, 13, 8592–8599. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Díaz, M.R.; Vivas-Mejia, P.E. Nanoparticles as Drug Delivery Systems in Cancer Medicine: Emphasis on RNAi-Containing Nanoliposomes. Pharmaceuticals 2013, 6, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Dong, S.D. Biomimetic Reactions Catalyzed by Cyclodextrins and Their Derivatives. Chem. Rev. 1998, 98, 1997–2012. [Google Scholar] [CrossRef]
- Carrazana, J.; Jover, A.; Meijide, F.; Soto, V.H.; Vázquez Tato, J. Complexation of Adamantyl Compounds by β-Cyclodextrin and Monoaminoderivatives. J. Phys. Chem. B 2005, 109, 9719–9726. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mahammad, S.; Parmryd, I. Cholesterol Depletion Using Methyl-β-cyclodextrin. Methods Mol. Biol. 2015, 1232, 91–102. [Google Scholar]
- Geda, O.; Tábi, T.; Lakatos, P.P.; Szökő, É. Differential Ganglioside and Cholesterol Depletion by Various Cyclodextrin Derivatives and Their Effect on Synaptosomal Glutamate Release. Int. J. Mol. Sci. 2022, 23, 9460. [Google Scholar] [CrossRef]
- Fernández, M.A.; Silva, O.F.; Vico, R.V.; De Rossi, R.H. Complex systems that incorporate cyclodextrins to get materials for some specific applications. Carbohydr. Res. 2019, 480, 12–34. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Varillas, S.; Fontanil, T.; Espina Casado, J.; Fernández González, A.; Badía Laíño, R. Surface Modification of Carbon Dots with Cyclodextrins as potential biocompatible photoluminescent delivery/bioimaging nanoplatform. Anal. Chim. Acta 2024, 1318, 342948. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Ennen, F.; Lamponi, S.; Cernescu, M.; Voit, B.; Cappelli, A.; Appelhans, D.; Komber, H. Cyclodextrin-Adamantane Host–Guest Interactions on the Surface of Biocompatible Adamantyl-Modified Glycodendrimers. Macromolecules 2013, 46, 3215–3227. [Google Scholar] [CrossRef]
- Dragomanova, S.; Andonova, V. Adamantane-containing drug delivery systems. Pharmacia 2023, 70, 1057–1066. [Google Scholar] [CrossRef]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef]
- Turnbull, W.B.; Daranas, A.H. On the Value of c: Can Low Affinity Systems Be Studied by Isothermal Titration Calorimetry? J. Am. Chem. Soc. 2003, 125, 14859–14866. [Google Scholar] [CrossRef]
- Schönbeck, C.; Holm, R. Exploring the Origins of Enthalpy-Entropy Compensation by Calorimetric Studies of Cyclodextrin Complexes. J. Phys. Chem. B 2019, 123, 6686–6693. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. [Google Scholar] [CrossRef]
- Bouchemal, K.; Mazzaferro, S. How to conduct and interpret ITC experiments accurately for cyclodextrin-guest interactions. Drug Discov. Today 2012, 17, 623–629. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Wang, Z.-G.; Liu, S.-L. Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules 2022, 27, 5607. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef]
- Yao, L.; Bojic, D.; Liu, M. Applications and safety of gold nanoparticles as therapeutic devices in clinical trials. J. Pharm. Anal. 2023, 13, 960–967. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Q.; Ji, J.-L.; Zheng, S.; Du, L.; Meng, L.; Wu, Y.; Zhao, D.; Wang, X.; Lai, L.; et al. Pharmacokinetic Behaviors of Intravenously Administered siRNA in Glandular Tissues. Theranostics 2016, 6, 1528–1541. [Google Scholar] [CrossRef]
- Hu, B.; Weng, Y.; Xia, X.; Liang, X.; Huang, Y. Clinical advances of siRNA therapeutics. J. Gene Med. 2019, 21, e3097. [Google Scholar] [CrossRef]
- Levet, G.; Krykun, S.; Cornelio, B.; Pilato, S.; Moffa, S.; Fontana, A.; Gouhier, G.; Estour, F. Drugs in Cyclodextrin in Liposomes: How a Suitable Formulation of an Active Substance Can Improve Its Efficiency? Processes 2024, 12, 478. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Zhu, H.; Zhu, Q.; Xia, Y. Three-in-One: Sensing, Self-Assembly, and Cascade Catalysis of Cyclodextrin Modified Gold Nanoparticles. J. Am. Chem. Soc. 2016, 138, 16645–16654. [Google Scholar] [CrossRef]
- Bistri, O.; Mazeau, K.; Auzély-Velty, R.; Sollogoub, M. A hydrophilic cyclodextrin duplex forming supramolecular assemblies by physical cross-linking of a biopolymer. Chem. Eur. J. 2007, 13, 8847–8857. [Google Scholar] [CrossRef]







| Type of siRNA | With or without Liposome Encapsulation | Additive | % siRNA Remaining in the Gel after the Following Incubation Time | Entry | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| NC siRNA | No liposome | * None | 3 | 0 | 1 |
| Liposome | * None | 44 ± 4 ** | 32 ± 3 | 2 | |
| * βCD | 56 ± 6 | 42 ± 4 | 3 | ||
| MeβCD | 91 ± 9 | 78 ± 8 | 4 | ||
| αCD | 76 ± 8 | 9 ± 1 | 5 | ||
| γCD | 52 ± 5 | 15 ± 2 | 6 | ||
| No liposome | βCD | 38 ± 4 | 45 ± 4 | 7 | |
| MeβCD | 80 ± 8 | 30 ± 3 | 8 | ||
| SeβCD | 67 ± 7 | 35 ± 4 | 9 | ||
| AuβCD | 18 ± 2 | 21 ± 2 | 10 | ||
| Liposome | SeβCD | 63 ± 6 | 43 ± 4 | 11 | |
| AuβCD | 15 ± 2 | 20 ± 2 | 12 | ||
| NC siRNA-AD | No liposome | SeβCD | 80 ± 8 | 79 ± 8 | 13 |
| Liposome | SeβCD | 95 ± 10 | 98 ± 10 | 14 | |
| Entry | Syringe | Cell | KA (M−1) | ΔH (kJ/mol) | TΔS (kJ/mol·K) | ΔG (kJ/mol) | KD (M) | C |
|---|---|---|---|---|---|---|---|---|
| 1 | βCD * | ADCH2COOH | 1.2 × 105 | −26 | 0.015 | −30 | 5.4 × 10−6 | 55 |
| 2 | βCD | ADCOOH | 3.4 × 105 | −34 | −0.007 | −32 | 3 × 10−6 | 64 |
| 3 | βCD | ADSH | 2.2 × 105 | −25 | 0.018 | −30 | 4.5 × 10−6 | 15 |
| 4 | βCD | ADNH2 | 2.4 × 104 | −19 | 0.020 | −25 | 4.1 × 10−5 | 10 |
| 5 | βCD | ADCH2OH | 9.0 × 103 | −30 | −0.026 | −23 | 1.1 × 10−4 | 7 |
| 6 | βCD/in serum | ADCH2COOH/ in serum | 1.2 × 105 | −20 | 0.043 | −29 | 8.4 × 10−6 | 50 |
| 7 | MeβCD | ADCH2COOH | 7.8 × 104 | −14 | 0.022 | −28 | 1.3 × 10−5 | 27 |
| 8 | MeβCD | ADCOOH | 2.2 × 105 | −24 | 0.051 | −30 | 4.5 × 10−6 | 69 |
| 9 | MeβCD | ADCH2OH | 6.9 × 104 | −13 | 0.049 | −28 | 1.4 × 10−5 | 36 |
| 10 | MeβCD | ADSH | 1.1 × 105 | −14 | 0.056 | −29 | 9.5 × 10−6 | 11 |
| 11 | MeβCD | ADNH2 | 1.3 × 104 | −7 | 0.015 | −23 | 7.7 × 10−5 | 8 |
| 12 | AuβCD | siRNA | 5.5 × 103 | −10 | 11.0 | −21 | 1.8 × 10−4 | 5 |
| 13 | SeβCD | siRNA | 1.7 × 102 | −2.2 | 10.6 | −13 | 5.7 × 10−3 | 181 |
| 14 | AuβCD | siRNA-AD | 4.8 × 103 | −7.5 | 13.6 | −21 | 2.5 × 10−4 | 159 |
| 15 | SeβCD | siRNA-AD | 1.0 × 103 | −2.8 | 14.5 | −17 | 9.6 × 10−4 | 34 |
| 16 | AuβCD | ADNHFA | 1.0 × 103 | −28 | 0.000 | −17 | 1.0 × 10−3 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo Cruz, B.; Chinapen Barletta, S.; Ortiz Muñoz, B.G.; Benitez-Reyes, A.S.; Amalbert Perez, O.A.; Cardona Amador, A.C.; Vivas-Mejia, P.E.; Barletta, G.L. Effect of Cyclodextrins Formulated in Liposomes and Gold and Selenium Nanoparticles on siRNA Stability in Cell Culture Medium. Pharmaceuticals 2024, 17, 1344. https://doi.org/10.3390/ph17101344
Castillo Cruz B, Chinapen Barletta S, Ortiz Muñoz BG, Benitez-Reyes AS, Amalbert Perez OA, Cardona Amador AC, Vivas-Mejia PE, Barletta GL. Effect of Cyclodextrins Formulated in Liposomes and Gold and Selenium Nanoparticles on siRNA Stability in Cell Culture Medium. Pharmaceuticals. 2024; 17(10):1344. https://doi.org/10.3390/ph17101344
Chicago/Turabian StyleCastillo Cruz, Betzaida, Sandra Chinapen Barletta, Bryan G. Ortiz Muñoz, Adriana S. Benitez-Reyes, Omar A. Amalbert Perez, Alexander C. Cardona Amador, Pablo E. Vivas-Mejia, and Gabriel L. Barletta. 2024. "Effect of Cyclodextrins Formulated in Liposomes and Gold and Selenium Nanoparticles on siRNA Stability in Cell Culture Medium" Pharmaceuticals 17, no. 10: 1344. https://doi.org/10.3390/ph17101344
APA StyleCastillo Cruz, B., Chinapen Barletta, S., Ortiz Muñoz, B. G., Benitez-Reyes, A. S., Amalbert Perez, O. A., Cardona Amador, A. C., Vivas-Mejia, P. E., & Barletta, G. L. (2024). Effect of Cyclodextrins Formulated in Liposomes and Gold and Selenium Nanoparticles on siRNA Stability in Cell Culture Medium. Pharmaceuticals, 17(10), 1344. https://doi.org/10.3390/ph17101344









