The Role of Mitochondrial Homeostasis in Mesenchymal Stem Cell Therapy—Potential Implications in the Treatment of Osteogenesis Imperfecta
Abstract
1. Introduction
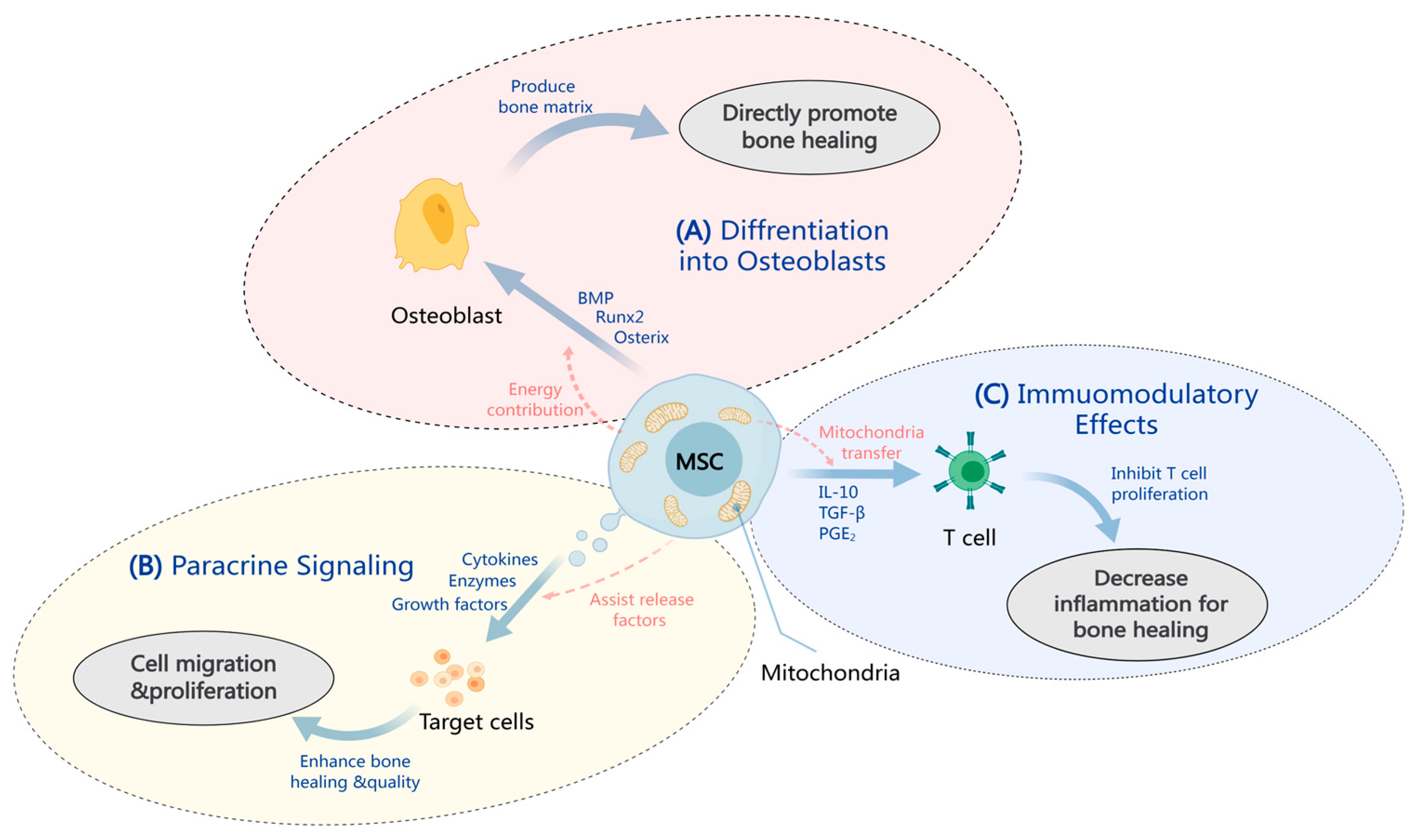
2. MSC Therapy Mechanisms in OI
2.1. MSC Differentiation into Osteoblasts
2.2. Paracrine Effects in Bone Healing
2.3. Immunomodulation by MSCs
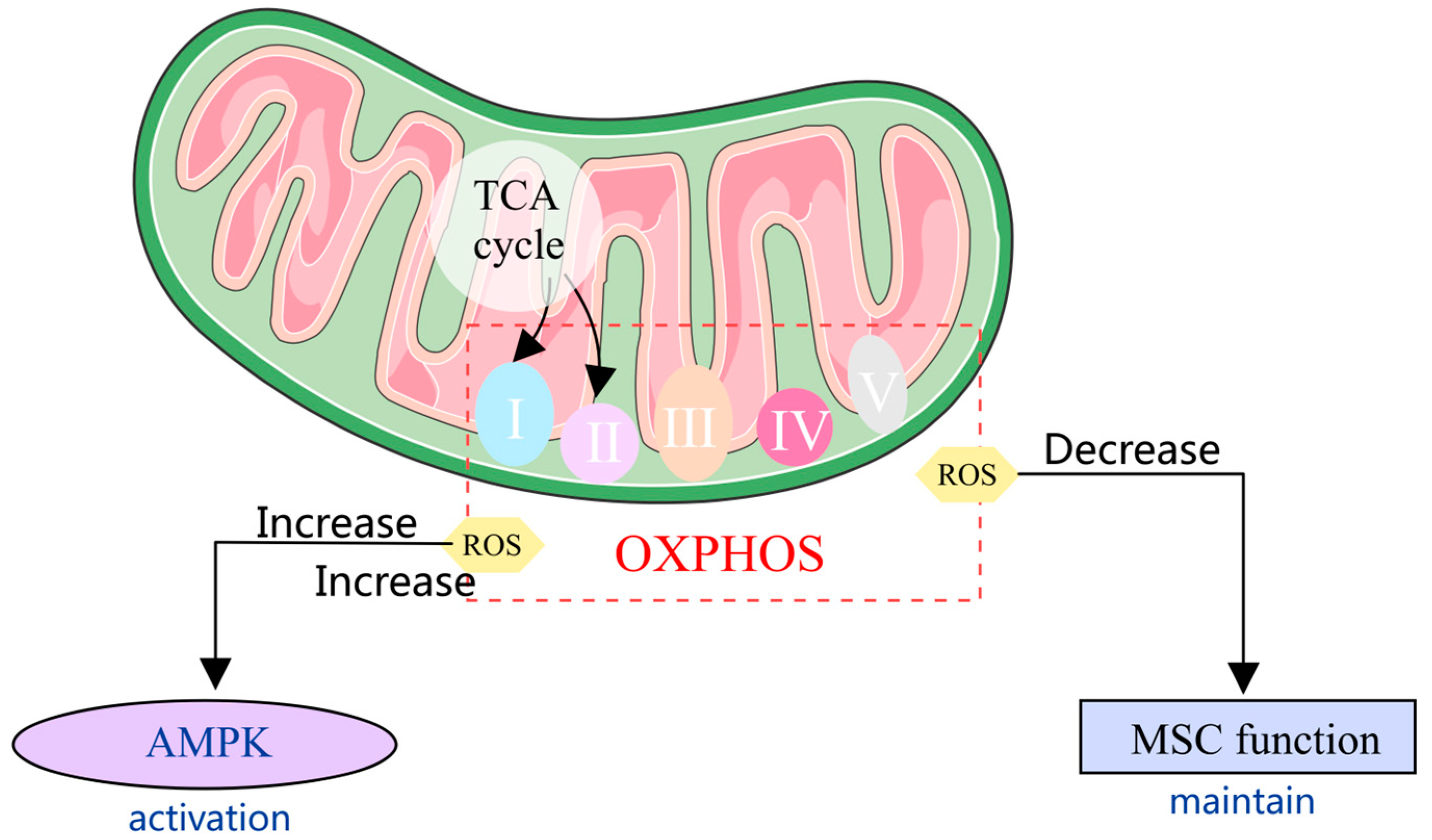
3. Mechanisms of Mitochondrial Homeostasis Regulate the Function of MSCs
3.1. Mitochondrial Metabolism in MSCs
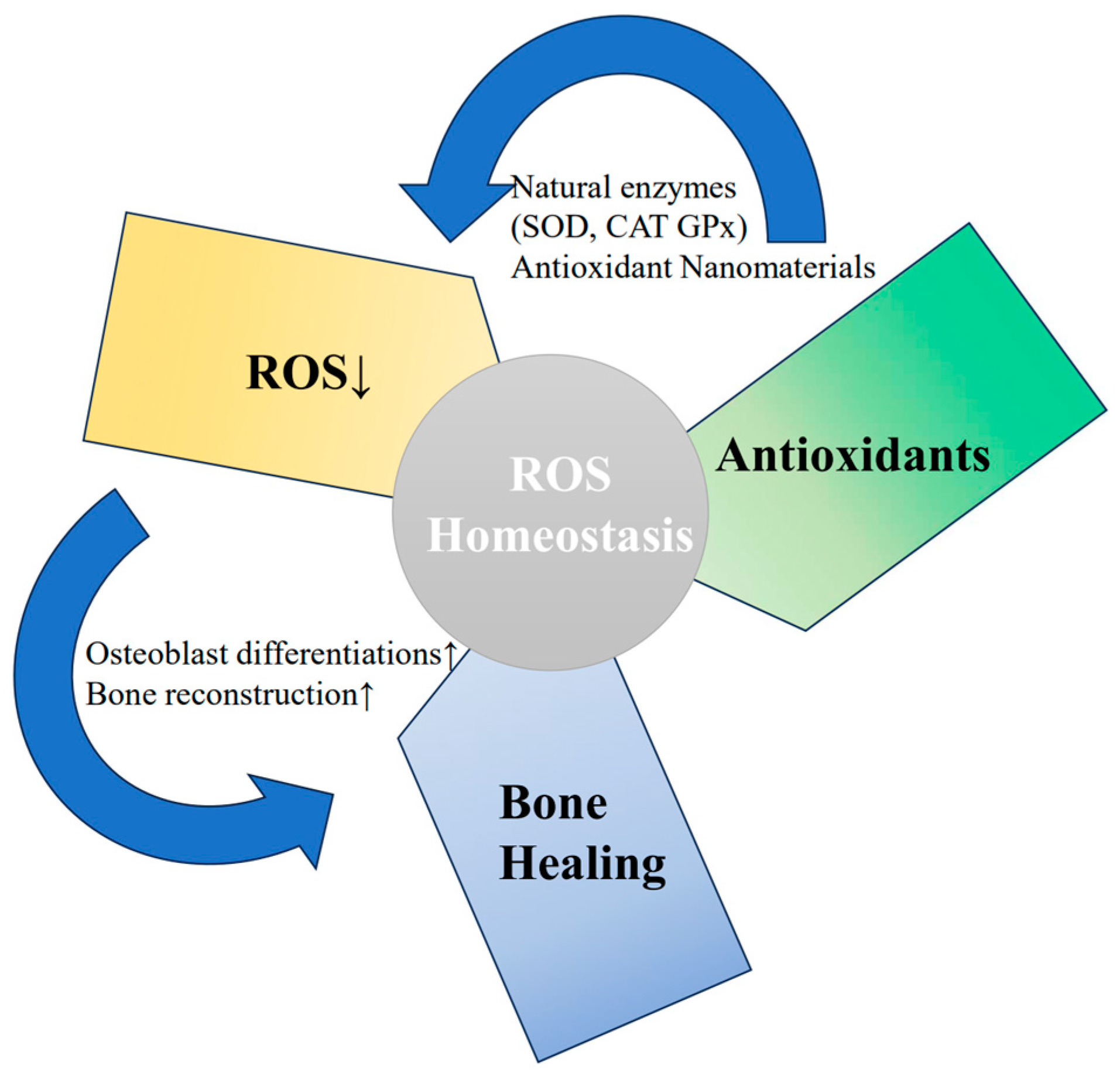
3.2. Mechanism of Mitochondrial Anti-Oxidative Stress
3.3. Mitochondrial Quality Control in MSCs
4. Strategies for Regulating the Function of MSCs through Mitochondrial Homeostasis
4.1. Regulating Mitochondrial Metabolism in MSCs
4.1.1. Resveratrol
4.1.2. NAD+
4.1.3. Alpha-Ketoglutarate (α-KG)
4.2. Mitochondrial Anti-Oxidative Stress Strategy through Antioxidants
4.2.1. N-Acetylcysteine (NAC)
4.2.2. Vitamin C
4.2.3. Alpha-Lipoic Acid (α-LA)
4.3. Mitochondrial Anti-Oxidative Stress Strategy through Biomaterials
4.3.1. Graphene Oxide (GO)
4.3.2. Fullerene Alcohol/Alginate Hydrogels
4.3.3. Polydopamine (PDA)
4.3.4. Cerium Oxide Nanoparticles (CeNPs)
4.3.5. Manganese Dioxide
4.3.6. Iron Oxide
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Etich, J.; Lessmeier, L.; Rehberg, M.; Sill, H.; Zaucke, F.; Netzer, C.; Semler, O. Osteogenesis imperfecta-pathophysiology and therapeutic options. Mol. Cell. Pediatr. 2020, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhytnik, L.; Maasalu, K.; Reimann, E.; Prans, E.; Koks, S.; Martson, A. Mutational analysis of COL1A1 and COL1A2 genes among Estonian osteogenesis imperfecta patients. Hum. Genom. 2017, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Ho Duy, B.; Zhytnik, L.; Maasalu, K.; Kandla, I.; Prans, E.; Reimann, E.; Martson, A.; Koks, S. Mutation analysis of the COL1A1 and COL1A2 genes in Vietnamese patients with osteogenesis imperfecta. Hum. Genom. 2016, 10, 27. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Wang, Y.; Ren, X.; Han, J. Molecular mechanisms and clinical manifestations of rare genetic disorders associated with type I collagen. Intractable Rare Dis. Res. 2019, 8, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Basel, D.; Steiner, R.D. Osteogenesis imperfecta: Recent findings shed new light on this once well-understood condition. Genet. Med. 2009, 11, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.M. Long-term safety of bisphosphonates. J. Clin. Endocrinol. Metab. 2005, 90, 1897–1899. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Majdoub, F.; Ferjani, H.L.; Nessib, D.B.; Kaffel, D.; Maatallah, K.; Hamdi, W. Denosumab use in osteogenesis imperfecta: An update on therapeutic approaches. Ann. Pediatr. Endocrinol. Metab. 2023, 28, 98–106. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Shapiro, J.; Veith, S.; Wang, Y.; Lapidus, J.; Vanek, C.; Reeder, J.L.; Keaveny, T.M.; Lee, D.C.; Mullins, M.A.; et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J. Clin. Investig. 2014, 124, 491–498. [Google Scholar] [CrossRef]
- Rauner, M.; Taipaleenmaki, H.; Tsourdi, E.; Winter, E.M. Osteoporosis Treatment with Anti-Sclerostin Antibodies-Mechanisms of Action and Clinical Application. J. Clin. Med. 2021, 10, 787. [Google Scholar] [CrossRef]
- Botor, M.; Fus-Kujawa, A.; Uroczynska, M.; Stepien, K.L.; Galicka, A.; Gawron, K.; Sieron, A.L. Osteogenesis Imperfecta: Current and Prospective Therapies. Biomolecules 2021, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- Battle, L.; Yakar, S.; Carriero, A. A systematic review and meta-analysis on the efficacy of stem cell therapy on bone brittleness in mouse models of osteogenesis imperfecta. Bone Rep. 2021, 15, 101108. [Google Scholar] [CrossRef] [PubMed]
- Medhat, D.; Rodriguez, C.I.; Infante, A. Immunomodulatory Effects of MSCs in Bone Healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef] [PubMed]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [PubMed]
- Gotherstrom, C.; Walther-Jallow, L. Stem Cell Therapy as a Treatment for Osteogenesis Imperfecta. Curr. Osteoporos. Rep. 2020, 18, 337–343. [Google Scholar] [CrossRef]
- Otsuru, S.; Desbourdes, L.; Guess, A.J.; Hofmann, T.J.; Relation, T.; Kaito, T.; Dominici, M.; Iwamoto, M.; Horwitz, E.M. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy 2018, 20, 62–73. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Prockop, D.J.; Gordon, P.L.; Koo, W.W.; Fitzpatrick, L.A.; Neel, M.D.; McCarville, M.E.; Orchard, P.J.; Pyeritz, R.E.; Brenner, M.K. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001, 97, 1227–1231. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef]
- Jorgensen, C.; Khoury, M. Musculoskeletal Progenitor/Stromal Cell-Derived Mitochondria Modulate Cell Differentiation and Therapeutical Function. Front. Immunol. 2021, 12, 606781. [Google Scholar] [CrossRef]
- Yan, W.; Diao, S.; Fan, Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Akhter, W.; Nakhle, J.; Vaillant, L.; Garcin, G.; Le Saout, C.; Simon, M.; Crozet, C.; Djouad, F.; Jorgensen, C.; Vignais, M.L.; et al. Transfer of mesenchymal stem cell mitochondria to CD4+ T cells contributes to repress Th1 differentiation by downregulating T-bet expression. Stem Cell Res. Ther. 2023, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q.; Hu, H.; Zhao, L.; Zhu, K. Progress in mesenchymal stem cell mitochondria transfer for the repair of tissue injury and treatment of disease. Biomed. Pharmacother. 2022, 153, 113482. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Gener, B.; Vazquez, M.; Olivares, N.; Arrieta, A.; Grau, G.; Llano, I.; Madero, L.; Bueno, A.M.; Sagastizabal, B.; et al. Reiterative infusions of MSCs improve pediatric osteogenesis imperfecta eliciting a pro-osteogenic paracrine response: TERCELOI clinical trial. Clin. Transl. Med. 2021, 11, e265. [Google Scholar] [CrossRef]
- Sinder, B.P.; Novak, S.; Wee, N.K.Y.; Basile, M.; Maye, P.; Matthews, B.G.; Kalajzic, I. Engraftment of skeletal progenitor cells by bone-directed transplantation improves osteogenesis imperfecta murine bone phenotype. Stem Cells 2020, 38, 530–541. [Google Scholar] [CrossRef]
- Kang, I.H.; Baliga, U.K.; Wu, Y.; Mehrotra, S.; Yao, H.; LaRue, A.C.; Mehrotra, M. Hematopoietic stem cell-derived functional osteoblasts exhibit therapeutic efficacy in a murine model of osteogenesis imperfecta. Stem Cells 2021, 39, 1457–1477. [Google Scholar] [CrossRef]
- Guillot, P.V.; Abass, O.; Bassett, J.H.; Shefelbine, S.J.; Bou-Gharios, G.; Chan, J.; Kurata, H.; Williams, G.R.; Polak, J.; Fisk, N.M. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood 2008, 111, 1717–1725. [Google Scholar] [CrossRef]
- Thomas, S.; Jaganathan, B.G. Signaling network regulating osteogenesis in mesenchymal stem cells. J. Cell Commun. Signal. 2022, 16, 47–61. [Google Scholar] [CrossRef]
- Grotheer, V.; Skrynecki, N.; Oezel, L.; Windolf, J.; Grassmann, J. Osteogenic differentiation of human mesenchymal stromal cells and fibroblasts differs depending on tissue origin and replicative senescence. Sci. Rep. 2021, 11, 11968. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.; Brusgard, J.L.; Chumsri, S.; Bhandary, L.; Zhao, X.F.; Lu, S.; Goloubeva, O.G.; Polster, B.M.; Fiskum, G.M.; Girnun, G.D.; et al. The RUNX2 Transcription Factor Negatively Regulates SIRT6 Expression to Alter Glucose Metabolism in Breast Cancer Cells. J. Cell. Biochem. 2015, 116, 2210–2226. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yan, J.; Yao, Z.; Zhang, C.; Li, X.; Mao, H.Q. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv. Healthc. Mater. 2021, 10, e2001689. [Google Scholar] [CrossRef] [PubMed]
- Otsuru, S.; Gordon, P.L.; Shimono, K.; Jethva, R.; Marino, R.; Phillips, C.L.; Hofmann, T.J.; Veronesi, E.; Dominici, M.; Iwamoto, M.; et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012, 120, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Daniel, D.; Gotherstrom, C.; Madhuri, V. Trophic effects of multiple administration of mesenchymal stem cells in children with osteogenesis imperfecta. Clin. Transl. Med. 2021, 11, e385. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Whyte, N.; Niyibizi, C. Differentiating multipotent mesenchymal stromal cells generate factors that exert paracrine activities on exogenous MSCs: Implications for paracrine activities in bone regeneration. Biochem. Biophys. Res. Commun. 2012, 426, 475–479. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Heldring, N.; Mager, I.; Wood, M.J.; Le Blanc, K.; Andaloussi, S.E. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum. Gene Ther. 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Xie, M.; Hun, M.; Abdirahman, A.S.; Li, C.; Wu, F.; Luo, S.; Wan, W.; Wen, C.; Tian, J. Immunoregulatory Effects of Mitochondria Transferred by Extracellular Vesicles. Front. Immunol. 2020, 11, 628576. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Sagar, S.; Ravindran, R.; Najor, R.H.; Quiles, J.M.; Chi, L.; Diao, R.Y.; Woodall, B.P.; Leon, L.J.; Zumaya, E.; et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 2023, 14, 5031. [Google Scholar] [CrossRef]
- Mukkala, A.N.; Jerkic, M.; Khan, Z.; Szaszi, K.; Kapus, A.; Rotstein, O. Therapeutic Effects of Mesenchymal Stromal Cells Require Mitochondrial Transfer and Quality Control. Int. J. Mol. Sci. 2023, 24, 15788. [Google Scholar] [CrossRef]
- Cao, Y.J.; Wei, Z.; Zhang, H.; Zhang, Z.L. Expanding the Clinical Spectrum of Osteogenesis Imperfecta Type V: 13 Additional Patients and Review. Front. Endocrinol. 2019, 10, 375. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Yan, K.; Chen, L.; Chen, X.R.; Li, P.; Chen, F.F.; Jiang, X.D. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflamm. 2013, 10, 106. [Google Scholar] [CrossRef]
- Piekarska, K.; Urban-Wojciuk, Z.; Kurkowiak, M.; Pelikant-Malecka, I.; Schumacher, A.; Sakowska, J.; Spodnik, J.H.; Arcimowicz, L.; Zielinska, H.; Tymoniuk, B.; et al. Mesenchymal stem cells transfer mitochondria to allogeneic Tregs in an HLA-dependent manner improving their immunosuppressive activity. Nat. Commun. 2022, 13, 856. [Google Scholar] [CrossRef]
- Li, H.; Dai, H.; Li, J. Immunomodulatory properties of mesenchymal stromal/stem cells: The link with metabolism. J. Adv. Res. 2023, 45, 15–29. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef]
- Vanleene, M.; Saldanha, Z.; Cloyd, K.L.; Jell, G.; Bou-Gharios, G.; Bassett, J.H.; Williams, G.R.; Fisk, N.M.; Oyen, M.L.; Stevens, M.M.; et al. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood 2011, 117, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Prockop, D.J.; Fitzpatrick, L.A.; Koo, W.W.; Gordon, P.L.; Neel, M.; Sussman, M.; Orchard, P.; Marx, J.C.; Pyeritz, R.E.; et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999, 5, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liao, Z.; Xu, P. Mitochondrial control of innate immune responses. Front. Immunol. 2023, 14, 1166214. [Google Scholar] [CrossRef]
- Mohammadalipour, A.; Dumbali, S.P.; Wenzel, P.L. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front. Cell Dev. Biol. 2020, 8, 603292. [Google Scholar] [CrossRef]
- Collier, J.J.; Olahova, M.; McWilliams, T.G.; Taylor, R.W. Mitochondrial signalling and homeostasis: From cell biology to neurological disease. Trends Neurosci. 2023, 46, 137–152. [Google Scholar] [CrossRef]
- Liu, A.R.; Lv, Z.; Yan, Z.W.; Wu, X.Y.; Yan, L.R.; Sun, L.P.; Yuan, Y.; Xu, Q. Association of mitochondrial homeostasis and dynamic balance with malignant biological behaviors of gastrointestinal cancer. J. Transl. Med. 2023, 21, 27. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Chen, E.; Pan, Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020, 382, 457–462. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Wang, F.S.; Wu, R.W.; Chen, Y.S.; Ko, J.Y.; Jahr, H.; Lian, W.S. Biophysical Modulation of the Mitochondrial Metabolism and Redox in Bone Homeostasis and Osteoporosis: How Biophysics Converts into Bioenergetics. Antioxidants 2021, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; An, H.J.; Kim, J.M.; Sung, M.J.; Kim, D.K.; Kim, H.K.; Oh, J.; Jeong, H.Y.; Lee, Y.H.; Yang, T.; et al. PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis. Stem Cell Res. Ther. 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, C.; Pers, Y.M.; Bony, C.; Jorgensen, C.; Noel, D. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Regulate the Mitochondrial Metabolism via Transfer of miRNAs. Front. Immunol. 2021, 12, 623973. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C. Mitochondria pleiotropism in stem cell senescence: Mechanisms and therapeutic approaches. Free Radic. Biol. Med. 2023, 208, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, K.J.; Suomalainen, A.; Hamalainen, R.H. Stem cells, mitochondria and aging. Biochim. Biophys. Acta 2015, 1847, 1380–1386. [Google Scholar] [CrossRef]
- Saeed, K.; Jo, M.H.; Park, J.S.; Alam, S.I.; Khan, I.; Ahmad, R.; Khan, A.; Ullah, R.; Kim, M.O. 17β-Estradiol Abrogates Oxidative Stress and Neuroinflammation after Cortical Stab Wound Injury. Antioxidants 2021, 10, 1682. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, L.; Shen, M.; Liu, Y.; Liu, G.; Wu, Y.; Ding, F.; Ma, K.; Wang, W.; Zhang, Y.; et al. Pioglitazone Protects Compression-Mediated Apoptosis in Nucleus Pulposus Mesenchymal Stem Cells by Suppressing Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 4764071. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, Y.; Han, J. Emerging roles of mitochondrial functions and epigenetic changes in the modulation of stem cell fate. Cell. Mol. Life Sci. 2024, 81, 26. [Google Scholar] [CrossRef]
- Morganti, C.; Bonora, M.; Marchi, S.; Ferroni, L.; Gardin, C.; Wieckowski, M.R.; Giorgi, C.; Pinton, P.; Zavan, B. Citrate Mediates Crosstalk between Mitochondria and the Nucleus to Promote Human Mesenchymal Stem Cell In Vitro Osteogenesis. Cells 2020, 9, 1034. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Wang, Y.; Xia, S.; Zhu, Y.; Xing, C.; Tian, X.; Du, Y. DNA N6-Methyladenine Modification in Eukaryotic Genome. Front. Genet. 2022, 13, 914404. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Y.; Li, X.; Zou, J.; Zou, S. DNA N6-methyladenine demethylase ALKBH1 enhances osteogenic differentiation of human MSCs. Bone Res. 2016, 4, 16033. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.P.; Liu, Y.L.; Luo, L.P.; Xiao, Y.; Jiang, T.J.; Yuan, J.; Wang, M. Alkbh1-mediated DNA N6-methyladenine modification regulates bone marrow mesenchymal stem cell fate during skeletal aging. Cell Prolif. 2022, 55, e13178. [Google Scholar] [CrossRef] [PubMed]
- Gremminger, V.L.; Harrelson, E.N.; Crawford, T.K.; Ohler, A.; Schulz, L.C.; Rector, R.S.; Phillips, C.L. Skeletal muscle specific mitochondrial dysfunction and altered energy metabolism in a murine model (oim/oim) of severe osteogenesis imperfecta. Mol. Genet. Metab. 2021, 132, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Wen, Y.; Song, J.; Chen, T.; Zhai, Q. Bone regeneration strategies based on organelle homeostasis of mesenchymal stem cells. Front. Endocrinol. 2023, 14, 1151691. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.; Niveria, K.; Verma, A.K. Paradoxical role of reactive oxygen species in bone remodelling: Implications in osteoporosis and possible nanotherapeutic interventions. Explor. Med. 2022, 3, 393–413. [Google Scholar] [CrossRef]
- Sheppard, A.J.; Barfield, A.M.; Barton, S.; Dong, Y. Understanding Reactive Oxygen Species in Bone Regeneration: A Glance at Potential Therapeutics and Bioengineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 836764. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Peng, C.; Li, L. Regulation of the mitochondrial reactive oxygen species: Strategies to control mesenchymal stem cell fates ex vivo and in vivo. J. Cell. Mol. Med. 2018, 22, 5196–5207. [Google Scholar] [CrossRef]
- Nugud, A.; Sandeep, D.; El-Serafi, A.T. Two faces of the coin: Minireview for dissecting the role of reactive oxygen species in stem cell potency and lineage commitment. J. Adv. Res. 2018, 14, 73–79. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Shi, Y.; Wang, C.; Ye, L. Targeting reactive oxygen species in stem cells for bone therapy. Drug Discov. Today 2021, 26, 1226–1244. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.K.; Han, Y.S.; Yoon, Y.M.; Yun, C.W.; Sun, H.Y.; Cho, H.W.; Lee, S.H. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol. Med. Rep. 2016, 14, 3777–3784. [Google Scholar] [CrossRef]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Fus-Kujawa, A.; Mendrek, B.; Bajdak-Rusinek, K.; Diak, N.; Strzelec, K.; Gutmajster, E.; Janelt, K.; Kowalczuk, A.; Trybus, A.; Rozwadowska, P.; et al. Gene-repaired iPS cells as novel approach for patient with osteogenesis imperfecta. Front. Bioeng. Biotechnol. 2023, 11, 1205122. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Dalle Carbonare, L.; Mottes, M. Osteogenic Differentiation in Healthy and Pathological Conditions. Int. J. Mol. Sci. 2016, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Dinulescu, A.; Pasarica, A.S.; Carp, M.; Dusca, A.; Dijmarescu, I.; Pavelescu, M.L.; Pacurar, D.; Ulici, A. New Perspectives of Therapies in Osteogenesis Imperfecta-A Literature Review. J. Clin. Med. 2024, 13, 1065. [Google Scholar] [CrossRef]
- Ren, X.; Liu, H.; Wu, X.; Weng, W.; Wang, X.; Su, J. Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front. Bioeng. Biotechnol. 2021, 9, 820468. [Google Scholar] [CrossRef]
- Yan, C.; Shi, Y.; Yuan, L.; Lv, D.; Sun, B.; Wang, J.; Liu, X.; An, F. Mitochondrial quality control and its role in osteoporosis. Front. Endocrinol. 2023, 14, 1077058. [Google Scholar] [CrossRef]
- Roca-Portoles, A.; Tait, S.W.G. Mitochondrial quality control: From molecule to organelle. Cell. Mol. Life Sci. 2021, 78, 3853–3866. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, J.C.; Jiang, Q.; Lee, W.Y. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell 2021, 20, e13301. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Duan, X.; Niu, Y.; Li, M.; Yun, L.; Sun, H.; Ma, Y.; Guo, Y. Sirtuins mediate mitochondrial quality control mechanisms: A novel therapeutic target for osteoporosis. Front. Endocrinol. 2023, 14, 1281213. [Google Scholar] [CrossRef]
- Simic, P.; Zainabadi, K.; Bell, E.; Sykes, D.B.; Saez, B.; Lotinun, S.; Baron, R.; Scadden, D.; Schipani, E.; Guarente, L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol. Med. 2013, 5, 430–440. [Google Scholar] [CrossRef]
- Moon, D.K.; Kim, B.G.; Lee, A.R.; In Choe, Y.; Khan, I.; Moon, K.M.; Jeon, R.H.; Byun, J.H.; Hwang, S.C.; Woo, D.K. Resveratrol can enhance osteogenic differentiation and mitochondrial biogenesis from human periosteum-derived mesenchymal stem cells. J. Orthop. Surg. Res. 2020, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yan, Y.; Zhao, C.; Xu, Y.; Wang, Q.; Xu, N. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep. 2019, 24, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xin, Z.; Cai, M. The role of resveratrol in bone marrow-derived mesenchymal stem cells from patients with osteoporosis. J. Cell. Biochem. 2019, 120, 16634–16642. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, L.; Makareeva, E.; Omari, S.; Otsuru, S.; Leikin, S. ER, Mitochondria, and ISR Regulation by mt-HSP70 and ATF5 upon Procollagen Misfolding in Osteoblasts. Adv. Sci. 2022, 9, e2201273. [Google Scholar] [CrossRef]
- Ruolan, W.; Liangjiao, C.; Longquan, S. The mTOR/ULK1 signaling pathway mediates the autophagy-promoting and osteogenic effects of dicalcium silicate nanoparticles. J. Nanobiotechnol. 2020, 18, 119. [Google Scholar] [CrossRef]
- Chen, J.; Long, F. mTORC1 Signaling Promotes Osteoblast Differentiation from Preosteoblasts. PLoS ONE 2015, 10, e0130627. [Google Scholar] [CrossRef]
- Wan, M.C.; Tang, X.Y.; Li, J.; Gao, P.; Wang, F.; Shen, M.J.; Gu, J.T.; Tay, F.; Chen, J.H.; Niu, L.N.; et al. Upregulation of mitochondrial dynamics is responsible for osteogenic differentiation of mesenchymal stem cells cultured on self-mineralized collagen membranes. Acta Biomater. 2021, 136, 137–146. [Google Scholar] [CrossRef]
- de Melo Pereira, D.; Eischen-Loges, M.; Birgani, Z.T.; Habibovic, P. Proliferation and Osteogenic Differentiation of hMSCs on Biomineralized Collagen. Front. Bioeng. Biotechnol. 2020, 8, 554565. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, D.S.; Lee, K.M.; Choi, S.M.; Lee, M.H.; Park, K.H.; Han, S.H.; Lee, J.W. Enhancement of Mesenchymal Stem Cell-Driven Bone Regeneration by Resveratrol-Mediated SOX2 Regulation. Aging Dis. 2019, 10, 818–833. [Google Scholar] [CrossRef]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, Y.; Quarles, L.D.; Song, T.; Pan, W.; Zhou, H.; Xiao, Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 2007, 14, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Jia, G.-f.; Zhu, F. Resveratrol Alleviates Osteoporosis by Promoting Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells via SITR1/PI3K/AKT Pathway. Int. J. Morphol. 2024, 42, 216–224. [Google Scholar] [CrossRef]
- Kim, H.N.; Ponte, F.; Warren, A.; Ring, R.; Iyer, S.; Han, L.; Almeida, M. A decrease in NAD+ contributes to the loss of osteoprogenitors and bone mass with aging. NPJ Aging Mech. Dis. 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Ding, Z.; Luo, Q.; Ju, Y.; Song, G. Exogenous NAD+ Postpones the D-Gal-Induced Senescence of Bone Marrow-Derived Mesenchymal Stem Cells via Sirt1 Signaling. Antioxidants 2021, 10, 254. [Google Scholar] [CrossRef]
- Li, B.; Shi, Y.; Liu, M.; Wu, F.; Hu, X.; Yu, F.; Wang, C.; Ye, L. Attenuates of NAD+ impair BMSC osteogenesis and fracture repair through OXPHOS. Stem Cell Res. Ther. 2022, 13, 77. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Liu, T.; Tan, Y.; Chen, C.; Zhao, J.; Geng, H.; Ma, C. The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. 2023, 62, 102663. [Google Scholar] [CrossRef]
- Zurek, A.; Mizerska-Kowalska, M.; Slawinska-Brych, A.; Kalawaj, K.; Bojarska-Junak, A.; Kandefer-Szerszen, M.; Zdzisinska, B. Alpha ketoglutarate exerts a pro-osteogenic effect in osteoblast cell lines through activation of JNK and mTOR/S6K1/S6 signaling pathways. Toxicol. Appl. Pharmacol. 2019, 374, 53–64. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Li, Y.; Song, W.; Shao, B.; Li, H.; Lin, W.; Li, Q.; Shuai, X.; Bai, M.; et al. Alpha-ketoglutarate promotes alveolar bone regeneration by modulating M2 macrophage polarization. Bone Rep. 2023, 18, 101671. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, P.; Liu, Y.; Wu, Y.; Chen, Y.; Guo, Y.; Zhang, S.; Zheng, X.; Zhou, L.; Liu, W.; et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat. Commun. 2020, 11, 5596. [Google Scholar] [CrossRef]
- Yamada, M.; Tsukimura, N.; Ikeda, T.; Sugita, Y.; Att, W.; Kojima, N.; Kubo, K.; Ueno, T.; Sakurai, K.; Ogawa, T. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials 2013, 34, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Yamada, M.; Niibe, K.; Zhang, M.; Kondo, T.; Ishibashi, M.; Egusa, H. Preconditioning of bone marrow-derived mesenchymal stem cells with N-acetyl-L-cysteine enhances bone regeneration via reinforced resistance to oxidative stress. Biomaterials 2018, 185, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Duryee, M.J.; Dusad, A.; Hunter, C.D.; Kharbanda, K.K.; Bruenjes, J.D.; Easterling, K.C.; Siebler, J.C.; Thiele, G.M.; Chakkalakal, D.A. N-Acetyl Cysteine Treatment Restores Early Phase Fracture Healing in Ethanol-Fed Rats. Alcohol Clin. Exp. Res. 2018, 42, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, J.; Feng, Z.; Guo, S.; Wang, M.; Wang, Z.; Li, Z.; Li, H.; Sui, L. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res. Ther. 2022, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Sandukji, A.; Al-Sawaf, H.; Mohamadin, A.; Alrashidi, Y.; Sheweita, S.A. Oxidative stress and bone markers in plasma of patients with long-bone fixative surgery: Role of antioxidants. Hum. Exp. Toxicol. 2011, 30, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.; Khani, F.; Sturmlechner, I.; Dehghani, S.S.; Denbeigh, J.M.; Zhou, X.; Pichurin, O.; Dudakovic, A.; Jerez, S.S.; Zhong, J.; et al. Vitamin C epigenetically controls osteogenesis and bone mineralization. Nat. Commun. 2022, 13, 5883. [Google Scholar] [CrossRef] [PubMed]
- Radzki, R.P.; Bienko, M.; Wolski, D.; Oniszczuk, T.; Radzka-Pogoda, A.; Polak, P.; Borzecki, A.; Stasiak, M. Lipoic acid (LA) dose-dependently protects bone losses in the mandible of rats during the development of osteopenia by inhibiting oxidative stress and promoting bone formation. Biomed. Pharmacother. 2022, 146, 112467. [Google Scholar] [CrossRef]
- Aydin, A.; Halici, Z.; Akoz, A.; Karaman, A.; Ferah, I.; Bayir, Y.; Aksakal, A.M.; Akpinar, E.; Selli, J.; Kovaci, H. Treatment with α-lipoic acid enhances the bone healing after femoral fracture model of rats. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 1025–1036. [Google Scholar] [CrossRef]
- Roberts, J.L.; Moreau, R. Emerging role of α-lipoic acid in the prevention and treatment of bone loss. Nutr. Rev. 2015, 73, 116–125. [Google Scholar] [CrossRef]
- Lu, S.Y.; Wang, C.Y.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.H.; Liu, K.X.; Sun, H.J.; Liu, M.Z. The osteogenesis-promoting effects of α-lipoic acid against glucocorticoid-induced osteoporosis through the NOX4, NF-kappaB, JNK and PI3K/AKT pathways. Sci. Rep. 2017, 7, 3331. [Google Scholar] [CrossRef]
- Li, C.J.; Sun, L.Y.; Pang, C.Y. Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Sci. Rep. 2015, 5, 9819. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Xie, Z.; Zeng, H.; Wang, P.; Li, J.; Zheng, G.; Wang, S.; Cao, Q.; Li, M.; Liu, W.; et al. Oxidative stress-mediated mitochondrial dysfunction facilitates mesenchymal stem cell senescence in ankylosing spondylitis. Cell Death Dis. 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; El-Husseny, M.W.A.; Abushouk, A.I.; Salem, A.M.A.; Mamdouh, M.; Abdel-Daim, M.M. Effects of Antioxidant Supplements on the Survival and Differentiation of Stem Cells. Oxid. Med. Cell. Longev. 2017, 2017, 5032102. [Google Scholar] [CrossRef] [PubMed]
- Tangtrongsup, S.; Kisiday, J.D. Differential Effects of the Antioxidants N-Acetylcysteine and Pyrrolidine Dithiocarbamate on Mesenchymal Stem Cell Chondrogenesis. Cell. Mol. Bioeng. 2019, 12, 153–163. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. α-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Voloboueva, L.A.; Liu, J.; Suh, J.H.; Ames, B.N.; Miller, S.S. (R)-α-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4302–4310. [Google Scholar] [CrossRef][Green Version]
- Perez-Araluce, M.; Jungst, T.; Sanmartin, C.; Prosper, F.; Plano, D.; Mazo, M.M. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics 2024, 9, 23. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef]
- Battaglini, M.; Emanet, M.; Carmignani, A.; Ciofani, G. Polydopamine-based nanostructures: A new generation of versatile, multi-tasking, and smart theranostic tools. Nano Today 2024, 55, 102151. [Google Scholar] [CrossRef]
- Shafiq, M.; Chen, Y.; Hashim, R.; He, C.; Mo, X.; Zhou, X. Reactive Oxygen Species-Based Biomaterials for Regenerative Medicine and Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2021, 9, 821288. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, W.; Xu, X.; Nie, Y.; Liu, Y.; Gould, O.E.C.; Ma, N.; Lendlein, A. Biofunction of Polydopamine Coating in Stem Cell Culture. ACS Appl. Mater. Interfaces 2021, 13, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, J.; Shi, H.; Li, Q.; Zhang, S.; Wu, H.; Li, W.; Gan, L.; Brown-Borg, H.M.; Feng, W.; et al. Ultra-small polydopamine nanomedicine-enabled antioxidation against senescence. Mater. Today Bio 2023, 19, 100544. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2020, 11, 607099. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, J.A.A.; Jimenez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Green Synthesis of Fe(x)O(y) Nanoparticles with Potential Antioxidant Properties. Nanomaterials 2022, 12, 2449. [Google Scholar] [CrossRef]
- Yadav, N.; Patel, V.; McCourt, L.; Ruppert, M.; Miller, M.; Inerbaev, T.; Mahasivam, S.; Bansal, V.; Vinu, A.; Singh, S.; et al. Tuning the enzyme-like activities of cerium oxide nanoparticles using a triethyl phosphite ligand. Biomater. Sci. 2022, 10, 3245–3258. [Google Scholar] [CrossRef]
- Estevez, A.Y.; Ganesana, M.; Trentini, J.F.; Olson, J.E.; Li, G.; Boateng, Y.O.; Lipps, J.M.; Yablonski, S.E.R.; Donnelly, W.T.; Leiter, J.C.; et al. Antioxidant Enzyme-Mimetic Activity and Neuroprotective Effects of Cerium Oxide Nanoparticles Stabilized with Various Ratios of Citric Acid and EDTA. Biomolecules 2019, 9, 562. [Google Scholar] [CrossRef]
- Ju, X.; Fucikova, A.; Smid, B.; Novakova, J.; Matolinova, I.; Matolin, V.; Janata, M.; Belinova, T.; Hubalek Kalbacova, M. Colloidal stability and catalytic activity of cerium oxide nanoparticles in cell culture media. RSC Adv. 2020, 10, 39373–39384. [Google Scholar] [CrossRef]
- Chen, M.; Wang, D.; Li, M.; He, Y.; He, T.; Chen, M.; Hu, Y.; Luo, Z.; Cai, K. Nanocatalytic Biofunctional MOF Coating on Titanium Implants Promotes Osteoporotic Bone Regeneration through Cooperative Pro-osteoblastogenesis MSC Reprogramming. ACS Nano 2022, 16, 15397–15412. [Google Scholar] [CrossRef]
- Filippova, A.D.; Sozarukova, M.M.; Baranchikov, A.E.; Kottsov, S.Y.; Cherednichenko, K.A.; Ivanov, V.K. Peroxidase-like Activity of CeO2 Nanozymes: Particle Size and Chemical Environment Matter. Molecules 2023, 28, 3811. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-containing bioceramics inhibit osteoclastogenesis and promote osteoporotic bone regeneration via scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.S.; Kim, D.S.; Suhito, I.R.; Choo, S.S.; Kim, S.J.; Song, I.; Kim, T.H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.M.; Do, H.D.K.; Nam, N.N.; Tran, N.K.S.; Dan, T.T.; Trinh, K.T.L. Antioxidant Nanozymes: Mechanisms, Activity Manipulation, and Applications. Micromachines 2023, 14, 1017. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Z.; Chu, L. The Antioxidant Effect of the Metal and Metal-Oxide Nanoparticles. Antioxidants 2022, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Kim, D.-S.; Hun Park, K. Evaluating antioxidant activity of phenolic mediated Fe3O4 nanoparticles using Usnea Longissimma methanol extract. Results Chem. 2022, 4, 100661. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Cormode, D.P.; Gao, L.; Koo, H. Emerging Biomedical Applications of Enzyme-Like Catalytic Nanomaterials. Trends Biotechnol. 2018, 36, 15–29. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, F.; Wang, Y.; Li, N.; Ding, B. Enzyme Mimic Nanomaterials and Their Biomedical Applications. Chembiochem 2020, 21, 2408–2418. [Google Scholar] [CrossRef]
- Ren, X.; Chen, D.; Wang, Y.; Li, H.; Zhang, Y.; Chen, H.; Li, X.; Huo, M. Nanozymes-recent development and biomedical applications. J. Nanobiotechnol. 2022, 20, 92. [Google Scholar] [CrossRef]
- Ikram, R.; Shamsuddin, S.A.A.; Mohamed Jan, B.; Abdul Qadir, M.; Kenanakis, G.; Stylianakis, M.M.; Anastasiadis, S.H. Impact of Graphene Derivatives as Artificial Extracellular Matrices on Mesenchymal Stem Cells. Molecules 2022, 27, 379. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Arsalan Iqbal, M.; Qasim, M.; Park, C.H.; Yoo, H.; Hwang, J.H.; Uhm, S.J.; Song, H.; Park, C.; Do, J.T.; et al. Evaluation of Graphene Oxide Induced Cellular Toxicity and Transcriptome Analysis in Human Embryonic Kidney Cells. Nanomaterials 2019, 9, 969. [Google Scholar] [CrossRef]
- Carmignani, A.; Battaglini, M.; Sinibaldi, E.; Marino, A.; Vighetto, V.; Cauda, V.; Ciofani, G. In Vitro and Ex Vivo Investigation of the Effects of Polydopamine Nanoparticle Size on Their Antioxidant and Photothermal Properties: Implications for Biomedical Applications. ACS Appl. Nano Mater. 2022, 5, 1702–1713. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Paulraj, R. Iron Oxide Nanoparticles Induced Oxidative Damage in Peripheral Blood Cells of Rat. J. Biomed. Sci. Eng. 2015, 08, 274–286. [Google Scholar] [CrossRef]
- Mehta, K.J. Iron Oxide Nanoparticles in Mesenchymal Stem Cell Detection and Therapy. Stem Cell Rev. Rep. 2022, 18, 2234–2261. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, T.; Jiang, X.; Li, A.; Su, Y.; Bian, Q.; Wu, H.; Lin, R.; Li, N.; Cao, H.; et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci. Adv. 2021, 7, eabj0534. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Lv, H.; Wu, L.; Cui, Y.; Yao, H.; Li, J.; Zhang, H.; Yang, B.; Jiang, J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int. J. Nanomed. 2019, 14, 573–589. [Google Scholar] [CrossRef]
| Metabolic Modulation | Summary | Key Mechanism | Biological Effect | Ref. |
|---|---|---|---|---|
| Resveratrol | Resveratrol can maintain the therapeutic potential of MSCs during long-term culture by acting through the SIRT1-SOX2 axis | SIRT1-SOX2 Axis | Improved bone regeneration | [74] |
| Resveratrol promotes osteogenic differentiation and mitochondrial biogenesis in periosteum-derived MSCs | Mitochondrial Biogenesis | Enhanced osteogenesis | [75] | |
| Runx2 acetylation/deacetylation is a main mechanism during osteogenic differentiation in MSCs in vitro | Sirt-1/Runx2 Pathway | Promoted osteogenic differentiation | [76] | |
| Resveratrol enhances osteogenesis in human MSCs by upregulating the expression of the RUNX2 gene through the SIRT1/FOXO3A pathway | SIRT1/FOXO3A Axis | Enhanced osteogenesis | [77] | |
| Resveratrol enhances the proliferation and osteoblastic differentiation of human MSCs through ER-dependent ERK1/2 activation | ERK1/2 Activation | Increased proliferation and differentiation | [78] | |
| Resveratrol can attenuate osteoporosis by promoting the osteogenic differentiation of bone marrow MSCs through the SIRT1/PI3K/AKT pathway | SIRT1/PI3K/AKT Pathway | Attenuation of osteoporosis | [80] | |
| NAD+ | NAD+ levels affect osteoblastogenesis in cells from old mice, showing that reduced NAD+ impairs mineralization under osteogenic conditions | NAD+ Level Impact | Impaired mineralization | [81] |
| NAD+ levels impair mitochondrial fusion, leading to mitochondrial dysfunction and reduced activity of OXPHOS, which subsequently blocks osteogenesis and diminishes bone fracture healing | Mitochondrial Dysfunction | Blocked osteogenesis and fracture repair | [82] | |
| Exogenous NAD+ can delay senescence in bone marrow-derived MSCs through the activation of the Sirt1 signaling pathway | Sirt1 Signaling Activation | Delayed senescence in MSCs | [83] | |
| miR-34a uses the NAD+-Sirt1 pathway to further mediate its role in MSC replicative senescence and natural senescence by targeting Nampt | NAD+-Sirt1 Pathway | Ameliorated MSC senescence | [84] | |
| α-KG | α-KG promotes alveolar bone regeneration following jawbone injury by modulating macrophage polarization towards an M2 phenotype, which is conducive to healing and tissue repair | Modulation of Macrophage Polarization | Enhanced bone regeneration | [85] |
| α-KG supplementation increases bone mass in aged mice and accelerates bone regeneration by decreasing histone methylations and upregulating BMP signaling and Nanog expression | Regulation of Histone Modifications | Accelerated bone regeneration | [86] | |
| α-KG influences stem cell fate and promotes osteogenic differentiation through mitochondrial nuclear signaling | Mitochondrial Signaling | Promoted osteogenic differentiation | [68] |
| Antioxidants | Summary | Main Mechanism | Biological Effect | Ref. |
|---|---|---|---|---|
| NAC | NAC and ascorbic acid protect MSCs from oxidative stress-induced mitochondrial dysfunction by enhancing mitochondrial fusion and reducing fragmentation | Mitochondrial Protection | Enhanced mitochondrial function | [121] |
| NAC inhibit ROS production and rescue MSCs from senescence by improving mitochondrial function and reducing oxidative stress | Mitochondrial Protection | Rescued MSCs from senescence | [122] | |
| NAC and mitochondria-targeted ubiquinone can reduce oxidative damage and improve the survival and differentiation of MSCs | Reduction in ROS | Improved survival and differentiation | [123] | |
| NAC and pyrrolidine dithiocarbamate reduce intracellular ROS and their effects on MSC chondrogenesis | Reduction in ROS | Reduced oxidative stress in chondrogenesis | [124] | |
| α-LA | α-LA has potential effects on MSCs by protecting them from oxidative stress | Enhancement of Antioxidant Mechanisms | Protected MSCs from oxidative stress | [125] |
| α-LA can protect mitochondria from oxidative stress by enhancing cellular antioxidant mechanisms | Enhancement of Antioxidant Mechanisms | Protected mitochondria from oxidative stress | [126] | |
| Vitamin C | Vitamin C hydrogel scaffolds enhance cell survival and minimize ROS levels under H2O2-induced oxidative stress conditions | Free Radical Scavenging | Improved cell survival under oxidative stress | [127] |
| Vitamin C can protect MSCs from oxidative stress-induced mitochondrial dysfunction | Mitochondrial Protection | Protected MSCs from oxidative stress-induced mitochondrial dysfunction | [128] |
| Biological Material | Summary | Main Mechanism | Biological Effect | Ref. |
|---|---|---|---|---|
| GO | GO has potential to mitigate cadmium-induced cytotoxicity and oxidative stress | Mitigation of cadmium-induced cytotoxicity | Reduced oxidative stress | [151] |
| GO exposure leads to significant decreases in mitochondrial membrane potential and ATP production | Mitochondrial dysfunction and ATP reduction | Decreased mitochondrial membrane potential and ATP generation | [152] | |
| Fullerenol/Alginate Hydrogel | Fullerenol/alginate hydrogel can effectively scavenge superoxide anion and hydroxyl radicals, improving the survival of stem cells under oxidative stress | Antioxidant activity and cell delivery | Suppression of oxidative stress damage in MSCs | [129] |
| Fullerene/alginate hydrogels in bone regeneration strategies bond to modulation of mitochondrial function and redox homeostasis | Organelle homeostasis and bone regeneration | Improved bone regeneration through organelle homeostasis of MSCs | [74] | |
| PDA | PDA-coated substrate can reduce oxidative stress and mitochondrial damage in mesenchymal stem cells, enhancing their expansion and reducing senescence | Antioxidant properties and cellular protection | Reduction in oxidative stress and mitochondrial damage in MSCs | [133] |
| PAD nanoparticles have enhanced antioxidant properties and cellular uptake, which could be beneficial for protecting MSCs from oxidative stress | Antioxidant effects and mitochondrial health | Enhancement of antioxidant properties and cellular uptake | [153] | |
| CeNPs | CeNPs support the mitochondrial health of MSCs in regenerative contexts | Antioxidant and anti-inflammatory effects | Potential applications in wound healing and tissue regeneration | [154] |
| CeNPs has potential in mitigating oxidative stress and protecting mitochondrial function in various cell types | Biocompatibility and cytotoxicity | Reduction in ROS levels and protection against oxidative stress | [155] | |
| Fe3O4 nanoparticles | Iron oxide nanoparticles could augment intercellular mitochondrial transfer from MSCs | Oxidative stress and cytotoxicity | Enhanced intercellular mitochondrial transfer from MSCs to diseased cells | [156] |
| Fe3O4 nanoparticles can be used for magnetic targeting and delivery of mesenchymal stem cells, improving their retention and therapeutic effects | Magnetic targeting and cell delivery | Improved cell retention and therapeutic effects in various disease models | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Zhai, Q.; Ji, P. The Role of Mitochondrial Homeostasis in Mesenchymal Stem Cell Therapy—Potential Implications in the Treatment of Osteogenesis Imperfecta. Pharmaceuticals 2024, 17, 1297. https://doi.org/10.3390/ph17101297
Guo Q, Zhai Q, Ji P. The Role of Mitochondrial Homeostasis in Mesenchymal Stem Cell Therapy—Potential Implications in the Treatment of Osteogenesis Imperfecta. Pharmaceuticals. 2024; 17(10):1297. https://doi.org/10.3390/ph17101297
Chicago/Turabian StyleGuo, Qingling, Qiming Zhai, and Ping Ji. 2024. "The Role of Mitochondrial Homeostasis in Mesenchymal Stem Cell Therapy—Potential Implications in the Treatment of Osteogenesis Imperfecta" Pharmaceuticals 17, no. 10: 1297. https://doi.org/10.3390/ph17101297
APA StyleGuo, Q., Zhai, Q., & Ji, P. (2024). The Role of Mitochondrial Homeostasis in Mesenchymal Stem Cell Therapy—Potential Implications in the Treatment of Osteogenesis Imperfecta. Pharmaceuticals, 17(10), 1297. https://doi.org/10.3390/ph17101297






